1. Introduction
Encephalitis is a complex clinical problem due to myriad of aetiologies and pathogenesis with high mortality rate. It is often complicated by prolonged and permanent neurologic deficits. Not only infectious but also immune mediated causes can present with encephalitis. The incidence of the disease can vary depending on the economic status of the country but it is generally between 3.5 and 7.4 per 100,000 patient per year [
1]. The incidence of the disease in the population is not high but the morbidity and mortality rate is high, Thus, it is still a public health problem worldwide. The mortality rate of the encephalitis cases ranges from 3.8% to 7.4% [
2].
The aetiologies of encephalitis are mainly classified into two groups such as: infectious and immune mediated causes. Although infectious causes are the commonest causes, the reported number of autoimmune encephalitis cases were increased in both adults and pediatric patients nowadays due to the availability of the diagnostic facilities and treatment options [
3]. There are various types of autoimmune encephalitis and for young adults, particularly women, anti-N-methyl-D-aspartate receptor antibody (anti-NMDAR Ab) encephalitis was common and in late adulthood, anti–leucine-rich glioma-inactivated 1 (anti-LGI1) encephalitis, are the most prevalent autoimmune encephalitis [
4] Diagnosis is essential for the autoimmune encephalitis cases for the prompt start of effective and correct treatment [
5].
Among the infectious group, viral causes are the commonest and both Deoxyribonucleic Acid (DNA) and Ribonucleic acid (RNA) viruses can present with encephalitis[
6]. Herpes virus group, Varicella-Zoster Virus (VZV), Japanese Encephalitis (JE), Zika (ZKV), Dengue (DENV), Chikungunya (CHIKV), Influenza, Adeno and Human Immunodeficiency (HIV) viruses are common viruses that can cause encephalitis. Some bacterial infections,
Haemophilus influenzae, Escherichia coli, Streptococcus pneumoniae, Streptococcus pyogenes, Neisseria meningitidis,
Mycobacterium tuberculosis,
Mycoplasma pneumoniae,
Listeria monocytogenes,
Leptospira and
Salmonella typhi, can also present as encephalitis.
Cryptococcus neoformans and
Aspergillus should also be considered in the aetiology of encephalitis. The common cause of microorganisms can vary with the seasons, geographical regions and immunity of the patients.
Even in developed countries, diagnostic and management guidelines are based on the prevalence of the diseases causing encephalitis. Genetic backgrounds, immunization status, geographical location are the essential and crucial facts to consider for making diagnostic and management guidelines such as adding of ampicillin in the empiric antibiotic therapy for the high prevalence areas of Listeria monocytogenes, arboviral screening tests at the tropical countries. To make the priority lists of laboratory tests, surveillance was conducted to identify the aetiology, clinical profiles, laboratory investigations and outcomes of the encephalitis patients admitted at Neuro-medical Ward, Yangon General Hospital during 2023 in Myanmar.
2. Materials and Methods
A prospective analytic study was conducted at a tertiary care center, Neuro-medical Ward, Yangon General Hospital (YGH) during March to August, 2023.
2.1. Patients’ Recruitment
Acute encephalitis syndrome (AES) is defined as a patient with acute onset of fever (>38°C) in the preceding 7 days with one or more of the following clinical features: altered mental status or seizure [
7]. All patients presenting with AES admitted to the Neuro-medical ward during the study period were recruited.
2.2. Study Procedures
Demographic profiles, clinical presentations, outcome of the patients at discharge from the hospital, immunocompromised or not, electroencephalogram (EEG), neuroimaging (Computed Tomography (CT) / Magnetic Resonance Imaging (MRI)) findings were recorded. CSF samples were analyzed for molecular tests, and biochemical and microscopic investigations. If the results were negative for infectious diseases, anti-NMDAR Ab were checked.
2.3. Laboratory Tests
Total eight bacterial pathogens (Neisseria meningitis, Haemophilus inflenzae, Streptococcus pneumoniae, Streptococcus agalactiae, Sterptococcu pyogens, Listeria moncytogenes, Escherichia coli and Mycoplasma pneumoniae), six viral pathogens (Herpes simplex virus 1 (HSV-1), Herpes Simplex Virus (HSV-2), Human Herpes Virus 6 (HHV-6), Entero virus Human Parechovirus and Varicella Zoster Virus (VZV) and one fungal pathogen (Cryptococcus neoformans) were checked on CSF specimens by Real-time PCR based QIAstat-Dx ME Panel (Qiagen, Germany) using QIAstat-Dx Analyzer 1.0.To identify four arboviruses (Japanese Encephalitis, West Nile, Dengue and Chikungunya Viruses), viral RNA was extracted from CSF sample using Qiagen Mini Viral RNA extraction kits (Qiagen, Germany) according to the manufacturer’s instruction. Conventional one step Reverse Transcription RT-PCR (Takara one step RT-PCR, Takara, Japan) was done to detect JEV, DENV, ZIKV and CHIKV genome using specific primers. All the experiments were done according to the procedures described in previous studies [
8,
9,
10]. The molecular tests were done at Pathology Research Division, Department of Medical Research, Yangon.
In this study, laboratory confirmed, probable and possible diseases were defined according to the criteria for the diagnosis of acute encephalitis by an infectious agent [
6]. For the diagnosis of autoimmune encephalitis, cell based indirect immunofluorescence (EUROIMMUNE, Lubeck) assay for anti-NMDAR Ab was measured from either serum or CSF samples[
11]. Statistical analysis
Data entry was done using Microsoft excel and analysis was done using STATA Software, Version 15 (STATA Corp., College Station, TX, USA). Descriptive statistics for demographic features, clinical findings, CSF analysis and radiological features were presented as frequencies and percentages. The classification of causes of acute encephalitis were presented using proportions and 95% confidence intervals. Differences in demographic profiles, clinical presentations, laboratory parameters and neuroimaging results were compared by classification of causes of acute encephalitis using chi-square test. Univariable analyses were applied to identify the factors associated with survival of patients with encephalitis. A p-value of <0.05 was considered statistically significant.
3. Results
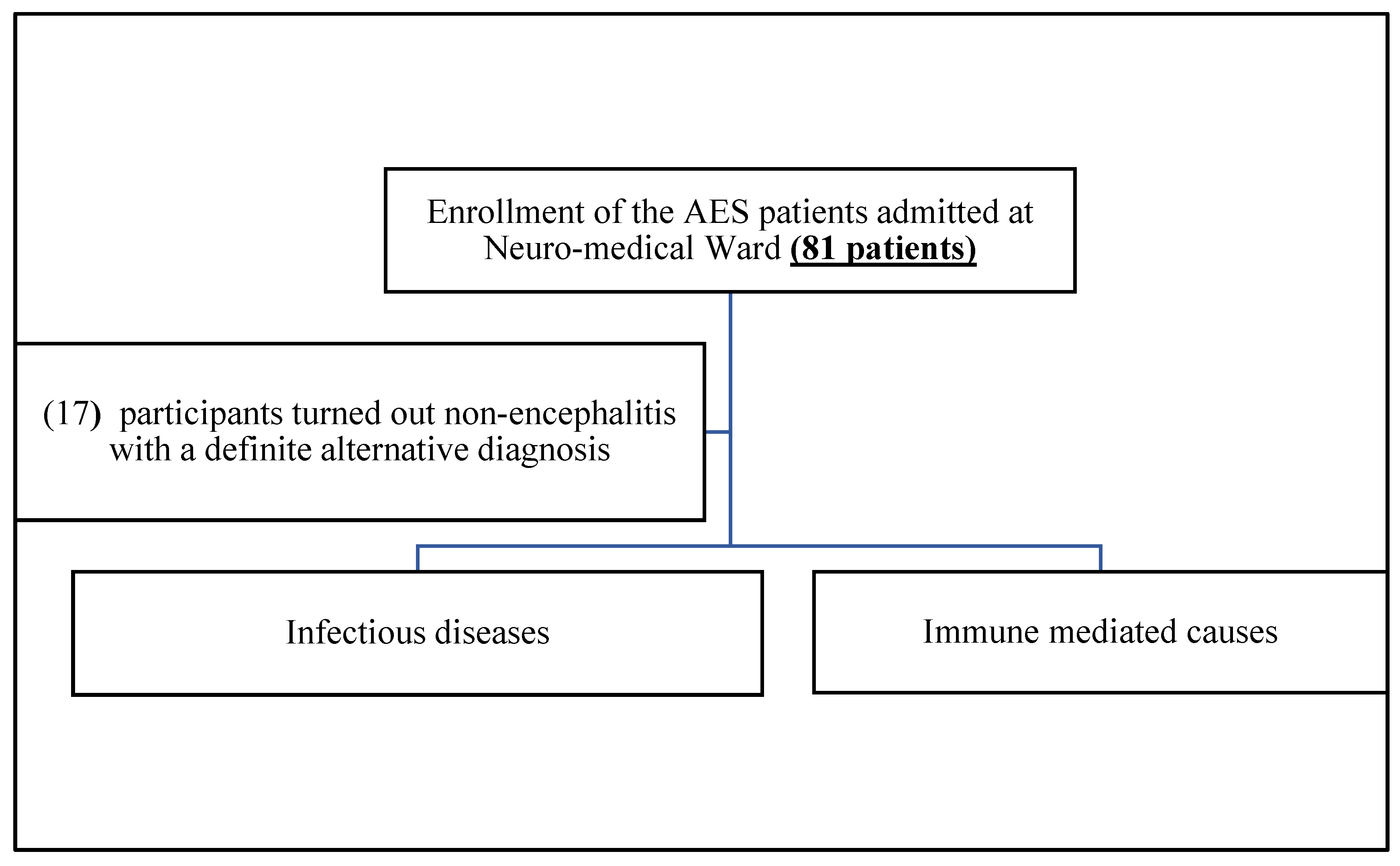
In this study, total 81 AES cases were enrolled, and 40/81 (49.4%) cases were males and 41/81 (50.6%) cases were females. Among 81 cases, 29 patients (35.8%) were less than 35 years old, 32 were (39.5%) were 35-59 years old and 20 cases (20.7%) were more than 60 years old. Of 81 total patients, 17 cases turned out non-encephalitis with a definite alternative diagnosis such as metabolic encephalopathy, septic encephalopathy, cerebral malaria, stroke, epilepsy, brain metastasis.
Figure 1.
Flow chart of the study.
Figure 1.
Flow chart of the study.
Out of the remaining 64 encephalitis cases, viral encephalitis cases constitute 32, pyogenic meningoncephalitis 16, tuberculous meningoencephalitis 8, immune encephalitis 6, fungal 3,. Six patients were expired among 64, and the mortality rate of encephalitis in this study was 9.37%, (95% CI, 3.3, 15.8). Among these 64 encephalitis cases, only 31/64 (48.44%) cases got exact aetiological diagnosis. Of 31 aetiology identified cases, infectious encephalitis accounted for 83.87% (26/31) and immune causes accounted for 16.13% (5/31), of the latter, four were anti-NMDAR ab autoimmune encephalitis and one was systemic lupus erythematosus (SLE) cerebritis. The proportion of aetiology undiagnosed encephalitis cases was 33/64 (51.56%).
The identified bacterial causes were higher than identified viral causes in this study. Herpes simplex encephalitis (HSE) was the commonest cause among laboratory confirmed cases. Regarding tuberculous meningitis, only one bacteriologically confirmed case by nucleic acid amplification test and seven cases were probable tuberculous meningitis cases. A case of VZV infection (Ramsay Hunt syndrome) with meningoencephalitis was detected in this study. The organisms detected in this study were shown in
Table 1.
Among the aetiology identified encephalitis, seven cases were immunocompromised patients. Three probable tuberculous meningitis patients were with retroviral infection and one was end stage renal disease (ESRD) due to prolonged diabetes mellitus. In this study, a case of plasmacytoma with post autologous stem cell transplant on immunosuppressant, presented with acute meningoencephalitis and
Listeria monocytogenes was detected. Interestingly, one dual pathogen infected case was immunocompetent. The causes of encephalitis cases among immunocompetent and immunosuppressed patients were shown in
Table 2.
Demographic profiles, clinical presentations, laboratory parameters and neuroimaging results of aetiologically identified encephalitis cases were compared among bacterial, viral, fungal and autoimmune causes and described in
Table 3. Male were more affected than female among bacterial cause and female were more at viral cause (P < 0.05). CSF pleocytosis and abnormal CSF protein levels were significantly high at bacterial cause (P < 0.05).
Among the neuroimaging results of 31 aetiology identified encephalitis cases, five cases (two HSE, one tuberculous, one anti-NMDAR encephalitis and one SLE cerebritis) showed encephalitis, two tuberculous cases showed infection related infarcts, one cryptococcal case showed meningitis one aspergillous case had venous sinus thrombosis and sinusitis, and the rest 22 cases were normal. EEG was done in 71.6% (58/81); normal in 39.7% (23/58), diffuse slowing in 24.1% (14/58), focal slowing in 17.2% (10/58), focal epileptiform discharges 13.8% (8/58), electrographic seizures/status epilepticus in 8.6% (5/58) and periodic lateralized epileptiform discharges (PLEDs) in 3.4% (2/58). Two cases of PLEDs were HSE confirmed cases.
Regarding the clinical outcomes of encephalitis, the mortality rate was 6/64, 9.4% [95%CI: 4.2, 19.7] and 2/31 (6.45%) of patients with identified causes had poor outcome. One was herpes encephalitis who could not afford the cost for the treatment and the another one was plasmacytoma case (post stem cell transplant) with
Listeria infection. On the other hand, 4/34 (11.8%) of encephalitis without identified aetiology had bad outcome. Regression analysis was done to predict the fatal outcome, demographic factors, clinical presentations and laboratory parameters (
Table 4). There was no significant variables for determining the fatal outcomes in this study.
4. Discussion
Central Nervous System (CNS) infection is the second commonest among Neuro-medical ward inpatients at YGH, and morbidity and mortality are also high for these patients [
12]. In this study, the mortality rate was 9.38% (6 out of 64 encephalitis cases) and it was still high mortality. If the exact aetiology is known, early diagnosis and effective treatment can be given such as anti-viral drugs for herpes virus, antibiotics for bacterial causes, antifungals for fungal causes. Subsequently, morbidity and mortality of the disease can be reduced. And it also avoids unnecessary antimicrobials [
13]. Unlike other infections, CNS infections need parenteral and prolonged antivirals, antibiotics and antifungals (at least 10-14 days) [
13]. By knowing the exact organisms, use of antimicrobials can be organism-directed therapy and this reduces cost significantly, avoids use of unnecessary expensive antimicrobials, posing lesser risk of adverse effects.
Since HSE is the commonest cause identified as in literature, in places where there is no testing facilities, empirical acyclovir should be given. However, acyclovir nephropathy can occur up to 48% [
14]. In this study, HSE was also the commonest cause. A case of HSE actually presented with stroke like presentation (sudden onset left hemiparesis with extreme right gaze deviation) with fever started only on day 2, for which the diagnosis at first point was stroke. CT (Head) was normal. MRI (brain) was also reported as right temporal infarct. However, because of confusion and EEG showing PLEDs and electrographic seizures, CSF study was proceeded. At molecular test, HSV-1 was detected, completely changing the line of management saving doctors and patient from missing appropriate treatment of HSE. HSE is curable but if untreated, it has grave consequences. According to literature, empiric acyclovir therapy should be started at presentation and the dose can be increased for VZV encephalitis cases. In this study, a case with typical features of Ramsay Hunt syndrome (right lower motor neuron facial palsy with right ear ache with redness in right ear) was recruited because of subtle right sixth cranial neuropathy with mild sensorial change. CSF VZV PCR turned out positive, indicating CNS extension, which has led us to giving parenteral acyclovir instead of per oral acyclovir for just Ramsay Hurt syndrome[
15]. The nucleic acid amplification testing from CSF specimens has greatly increased the ability to diagnose infections of the CNS, especially viral infections caused by the herpesviruses [
16]. Thus, it is essential to establish the HSV PCR testing or ME panel testing in public sector laboratories.
Tubercular meningitis (TBM) is one of the predominant causes of acute febrile encephalopathy in developing countries including Myanmar [
17]. In this study, one lab confirmed cases and seven probable cases of TB meningoencephalitis were included, and TB was one of the commonest causes of encephalitis in this study. Based on the fourth national tuberculosis survey in 2017-2018, the estimated adult pulmonary TB cases was 468/100,000 populations [
18]. Thus, TB should be considered at the first differential diagnosis of encephalitis and meningitis cases. Among our 8 tuberculous meningoencephalitis patients, 50% were immunocompromised but all 8 patients had good outcome. This might be because of our AES criteria which has recruited only early cases of CNS tuberculosis patients, and early diagnosis and immediate treatment improves outcome of TBM [
19].
Although a small sample size in this study,
Sphingomonas paucimobillis meningitis,
Listeria monocytogenes and
Burkholderia cepacia and
Aspergillus meningitis/encephalitis cases were identified. Although
Sphingomonas paucimobillis infection mostly occurred among immunocompromised patients, few studies reported that
Sphingomonas paucimobillis can also infect in immunocompetent individuals [
20] and even about coinfection of
Sphingomonas paucimobillis and Listeria monocytogenes was also noted in an immunocompetent individual [
21].
Burkholderia cepacia is a rare cause worldwide but now becoming emerging cause as a bacterial meningitis [
22,
23].
Burkhodelria cepacia and Aspergillus were isolated from serum samples and Sphingomonas paucimobilis was isolated from CSF samples of routine culture and antibiotic sensitivity assays. Two patients got positive CSF culture results (Staphylococcus hominis and Staphylococcus epidemidis). But their CSF biochemical and microscopic assay results and clinical features were not compatible with pyogenic meningitis and those cases were determined as contaminated result in this study. Therefore, only routine CSF culture was not enough to get the diagnosis of encephalitis cases.
During the study period, a case of cryptococcal meningoencephalitis (CM) was totally unexpected since the case is immunocompetent. Previous researches described that CM cases in immunocompetent patients is underestimation and it is not as rare as previously estimated. Many studies showed that up to 43.5 % of cases occur in immunocompetent young individuals, non-HIV/non-organ transplant patients [
24,
25,
26] Furthermore, among immunocompetent patients, 67% of whom presented with CM had auto-antibodies [
27]In this study, we came across two cases of
Haemophilus influenzae encephalitis, which we did not have any experience before, since it was nearly never tested before in our country. This case has alerted the clinicians to have a high index of suspicion since it might be one of the commonest identifiable organisms of encephalitis in Myanmar. Myanmar is an endemic country for many arboviruses such as DENV, JEV, ZKV and CHIKV[
9,
10]. Unfortunately, no cases were identified as the causes in this study.
Immune encephalitis accounted for 16.13% of aetiology identified encephalitis cases (5/31) and only 9.38% (6/64) among all presumed encephalitis cases. Four were anti-NMDAR ab positive, one was SLE case and one was presumed autoimmune encephalitis [
28]. 26 % of paediatric encephalitis cases were diagnosed as presumed autoimmune encephalitis in a study conducted in Myanmar during 2017-2018 [
29]. In our study, because of limited resources and sample size, only anti-NMDAR Ab was tested and this would have underestimated the actual number of autoimmune encephalitis.
In this study only 31/64 encephalitis cases could have the definite aetiology confirmed. Even in developed countries, the proportion of undiagnosed encephalitis cases were still high[
30]. However, many advanced diagnostic tests are available nowadays and both infectious and non-infectious causes can be identified and the mortality rate of the patients will be reduced.
5. Conclusions
Herpes and tuberculous was the commonest causes and followed by the autoimmune encephalitis and the proportion of patients who did not get accurate diagnosis was high. Not only molecular testing but also routine laboratory tests together with clinical and radiological findings should be used to get the early diagnosis of encephalitis cases. Furthermore, many emerging and reemerging pathogens are found to cause encephalitis and annual surveillance should be conducted.
Author Contributions
Conceptualization, AKK, PPL, HMT and ZTH methodology, AKK, and OM; formal analysis, KLS.; investigation, AKK, NATO, MTW, KZCA.; resources, WPPN, HMT and ZTH data curation, ZNW, SKW, ZMS, OM and PPL.; writing—original draft preparation-AKK writing—review and editing, AKK, OM and ZTH project administration, HMT and ZTH.; funding acquisition, AKK and ZTH All authors have read and agreed to the published version of the manuscript.”
Funding
This research was funded by Department of Medical Research Grant, Ministry of Health, Republic of the Union of Myanmar
Institutional Review Board Statement
This study was approved by the Institutional Review Board of the Department of Medical Research with the approval number (Ethics/DMR/2022/20). The study was conducted in accordance with the Declaration of Helsinki,
Informed Consent Statement
Due to vulnerability and incapacity to give an informed consent, immediate family members or caretakers were well informed to give a surrogate informed consent on behalf of the eligible patients.
Conflicts of Interest
“The authors declare no conflicts of interest.
References
- Granerod J, Crowcroft NS. The epidemiology of acute encephalitis. Neuropsychol Rehabil 2007; 17. [CrossRef]
- Wang H, Zhao S, Wang S, et al. Global magnitude of encephalitis burden and its evolving pattern over the past 30 years. Journal of Infection 2022; 84. [CrossRef]
- Venkatesan, A. Epidemiology and outcomes of acute encephalitis. Curr Opin Neurol. 2015; 28. [CrossRef]
- Rao, S.S. Chapter 1 Fundamentals of Vibrations. 2011.
- van Sonderen A, Thijs RD, Coenders EC, et al. Anti-LGI1 encephalitis: Clinical syndrome and long-term follow-up. Neurology 2016; 87. [CrossRef]
- Granerod J, Cunningham R, Zuckerman M, et al. Causality in acute encephalitis: Defining aetiologies. Epidemiol Infect. 2010; 138. [CrossRef]
- Standards, S. Japanese Encephalitis Japanese Encephalitis Vaccine-Preventable Diseases.
- Morita K, Tanaka M, Igarashi A. Rapid identification of dengue virus serotypes by using polymerase chain reaction. J Clin Microbiol 1991; 29. [CrossRef]
- Tun MMN, Kyaw AK, Hmone SW, et al. Detection of zika virus infection in Myanmar. American Journal of Tropical Medicine and Hygiene 2018; 98.
- Ngwe Tun MM, Thant KZ, Inoue S, et al. Detection of East/Central/South African genotype of chikungunya virus in Myanmar, 2010. Emerg Infect Dis 2014; 20. [CrossRef]
- Lee SK, Lee S-T. The Laboratory Diagnosis of Autoimmune Encephalitis. Available online: www.kes.or.kr.
- Ohnmar, Kyaw M, Shwe ZM, et al. The pattern and burden of neurological disorders: A systemic review of neurology department, Yangon General Hospital, Myanmar. Neurol Asia. 2020; 25.
- Bookstaver PB, Mohorn PL, Shah A, et al. Management of Viral Central Nervous System Infections: A Primer for Clinicians. J Cent Nerv Syst Dis 2017; 9. [CrossRef]
- Lam NN, Weir MA, Yao Z, et al. Risk of acute kidney injury from oral acyclovir: A population-based study. American Journal of Kidney Diseases 2013; 61. [CrossRef]
- Chan TLH, Cartagena AM, Bombassaro AM, Hosseini-Moghaddam SM. Ramsay hunt syndrome associated with central nervous system involvement in an adult. Canadian Journal of Infectious Diseases and Medical Microbiology 2016; 2016. [CrossRef]
- Tunkel AR, Glaser CA, Bloch KC, et al. The management of encephalitis: Clinical practice guidelines by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2008; 47. [CrossRef]
- Ary KA, Singh H, Suri V, et al. Changing Clinical Profile and Predictors of Mortality in Patients of Acute Febrile Encephalopathy from North India. J Glob Infect Dis 2023; 15. [CrossRef]
- Aung ST, Nyunt WW, Moe MM, Aung HL, Lwin T. The fourth national tuberculosis prevalence survey in Myanmar. PLOS Global Public Health 2022; 2. [CrossRef]
- Chiang SS, Khan FA, Milstein MB, et al. Treatment outcomes of childhood tuberculous meningitis: A systematic review and meta-analysis. Lancet Infect Dis 2014; 14. [CrossRef]
- Mehmood H, Khan N, Ullah S, Ullah A, Marwat A. A rare case of Sphingomonas paucimobilis meningitis in the absence of cerebrospinal fluid pleocytosis. J Investig Med High Impact Case Rep 2018; 6. [CrossRef]
- Bae SW, Lee JH. Coinfection of Sphingomonas paucimobilis meningitis and Listeria monocytogenes bacteremia in an immunocompetent patient: a case report. Journal of Yeungnam Medical Science 2022; 39. [CrossRef]
- Sanchez PG, Guiot H, Febles A, et al. Burkholderia cepacia: a rare but emerging cause of bacterial meningitis. Am J Med Sci 2023; 365. [CrossRef]
- H N, Varad Rajesh. Burkholderia Cepacia: A Rare Cause of Bacterial Meningitis. International Journal of Contemporary Medical Research [IJCMR] 2020; 7.
- Brizendine KD, Baddley JW, Pappas PG. Predictors of Mortality and Differences in Clinical Features among Patients with Cryptococcosis According to Immune Status. PLoS One 2013; 8. [CrossRef]
- Yuchong C, Fubin C, Jianghan C, et al. Cryptococcosis in China (1985-2010): Review of Cases from Chinese Database. Mycopathologia 2012; 173. [CrossRef]
- Stack M, Hiles J, Valinetz E, Gupta SK, Butt S, Schneider JG. Cryptococcal Meningitis in Young, Immunocompetent Patients: A Single-Center Retrospective Case Series and Review of the Literature. Open Forum Infect Dis 2023; 10. [CrossRef]
- Lui G, Lee N, Ip M, et al. Cryptococcosis in apparently immunocompetent patients. QJM 2006; 99. [CrossRef]
- Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016; 15. [CrossRef]
- Galardi MM, Sowa GM, Crockett CD, et al. Pathogen and Antibody Identification in Children with Encephalitis in Myanmar. Ann Neurol 2023; 93. [CrossRef]
- Liem B, Liem M, Anderson N. Clinical characterisation of encephalitis of unknown cause and ‘mimics’ of encephalitis. (P4-5.004). 2023.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).