Submitted:
13 September 2024
Posted:
16 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods:
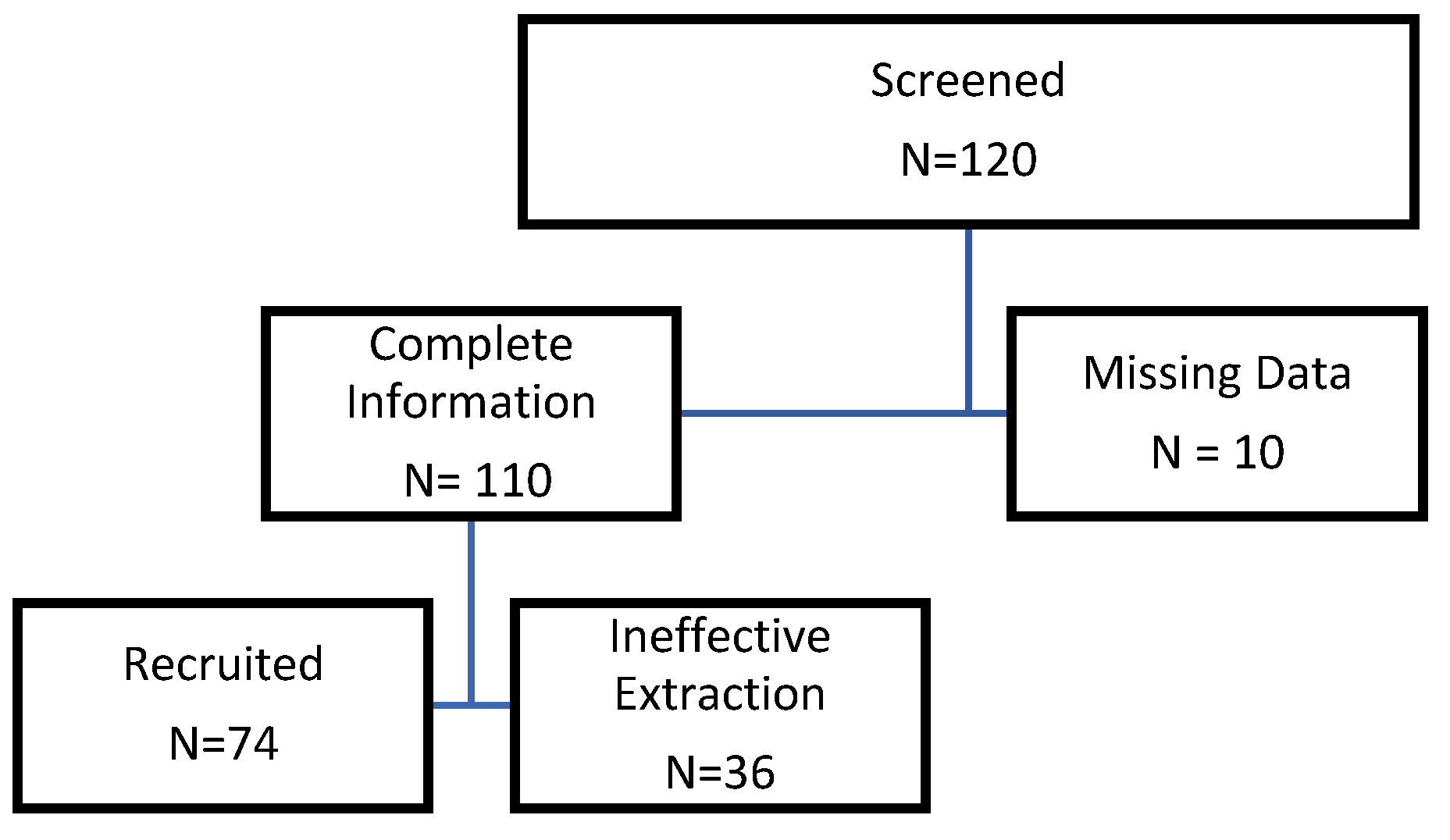
2.1. Patient Recruitment
2.2. Oxytocin Extraction
2.3. Data Analysis
2.4. Statistical Analysis
2.5. Human Fetal RPE Cell Cultures
2.6. RNA Sequencing and Data Analysis
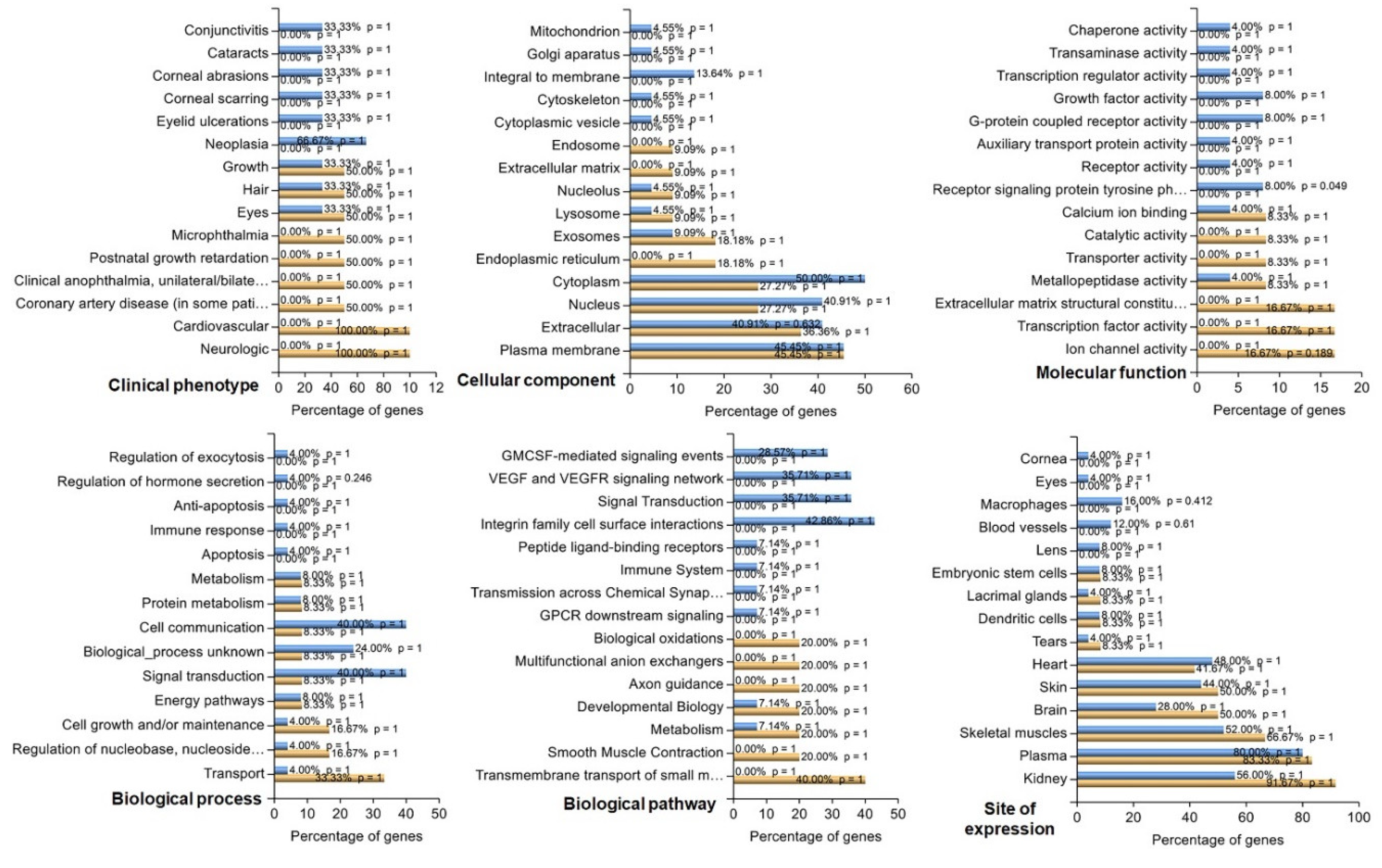
2.7. Functional Analysis of Differentially Expressed Genes
3. Results:
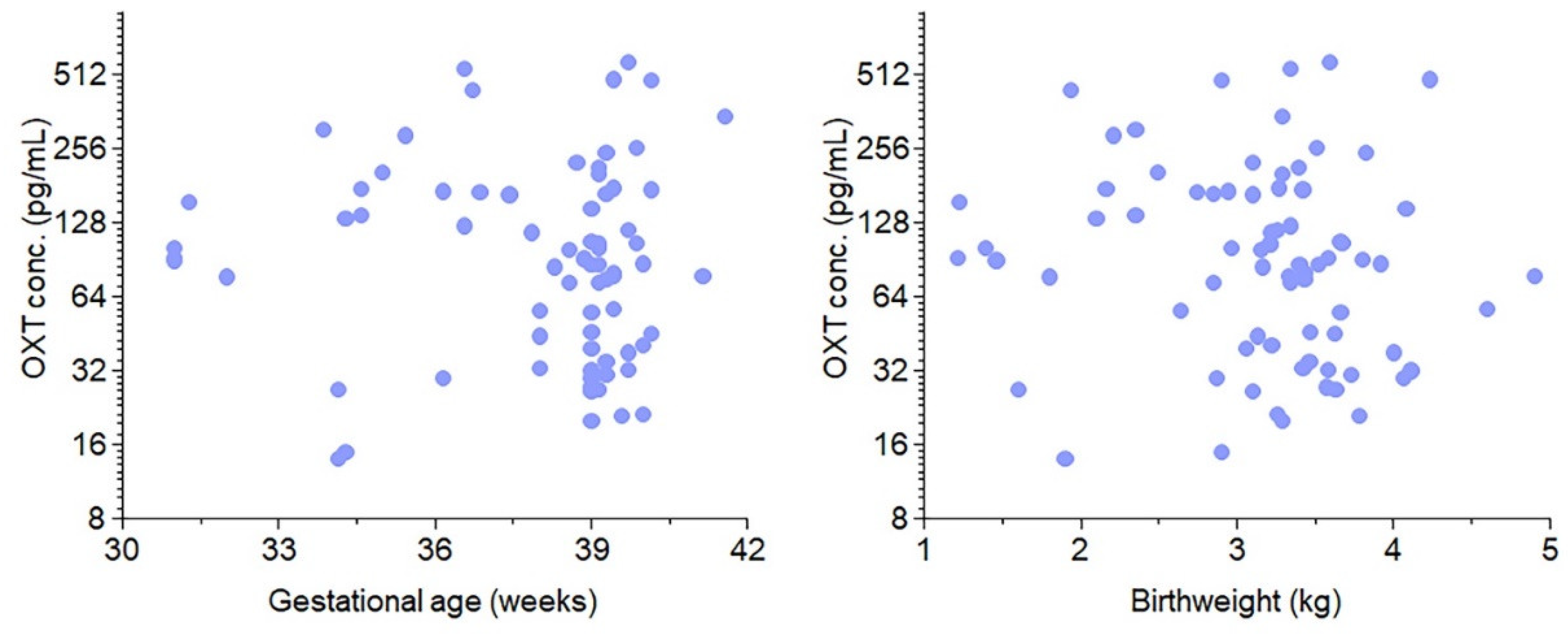
3.1. Study characteristics
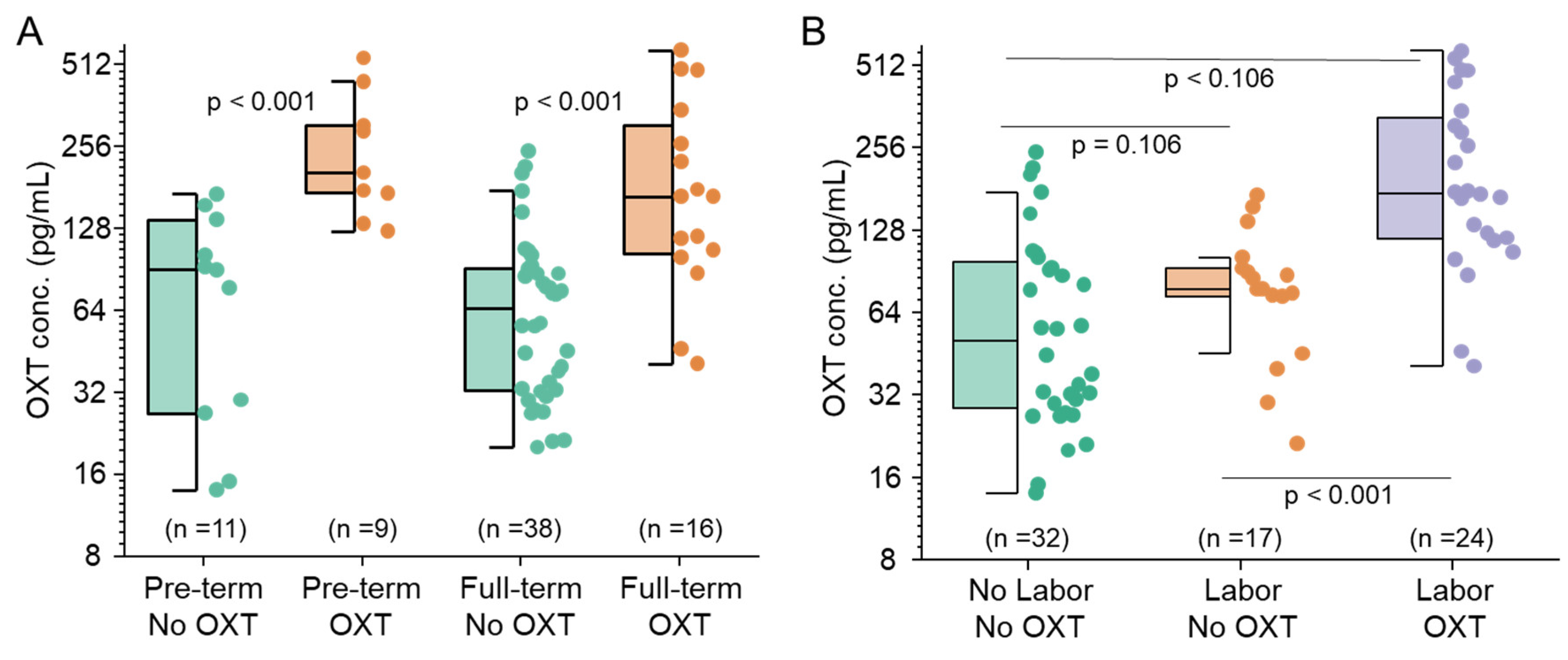
3.2. Maternal Oxytocin Administration Crosses the Placenta
3.3. Maternal Labor Affects Fetal OXT Concentrations
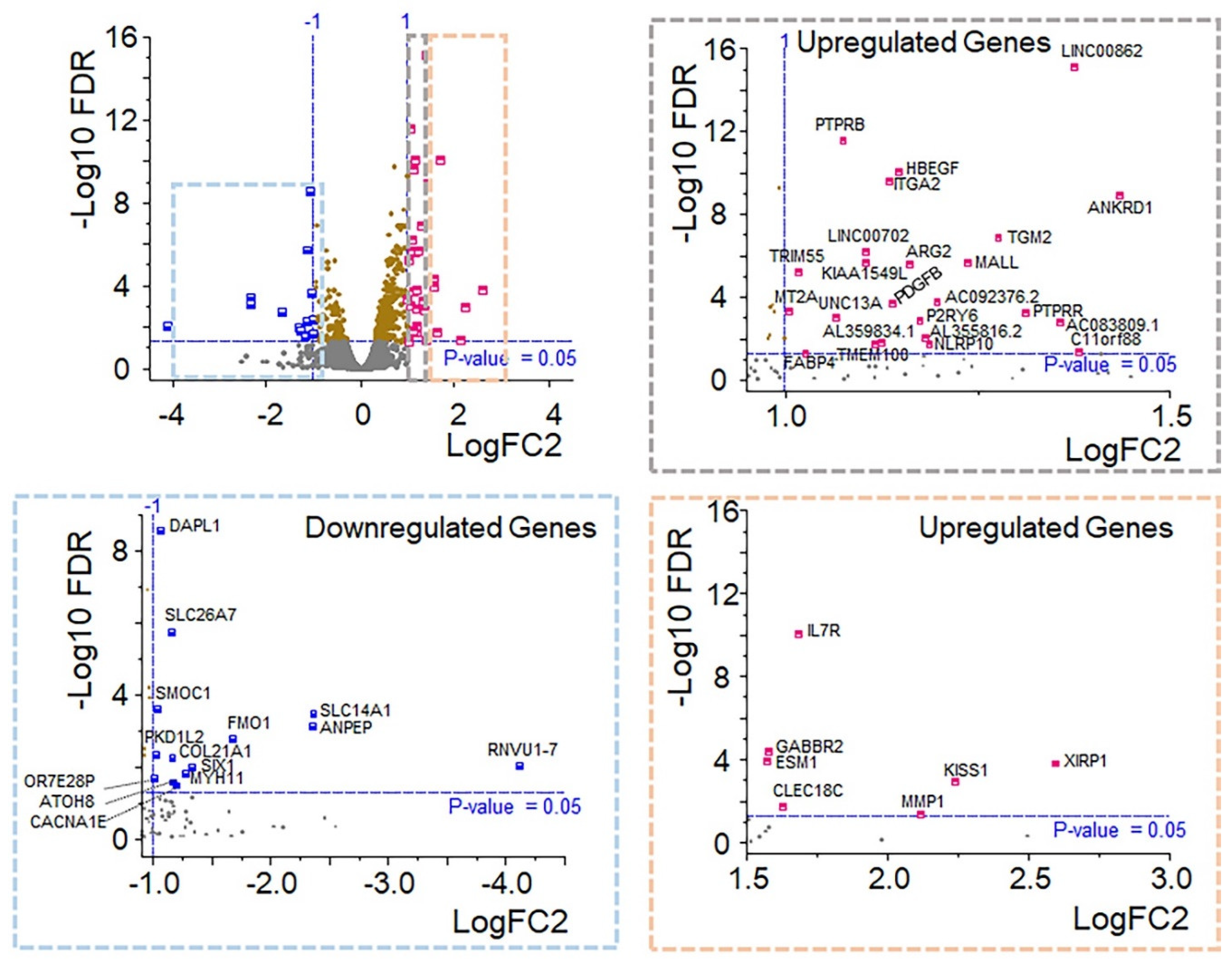
3.4. Identification of Key Pathway-Associated Genes by GO and KEGG Term Enrichment
3.5. Protein Function Network Identified by the Cytoscape plug-in GeneMANIA
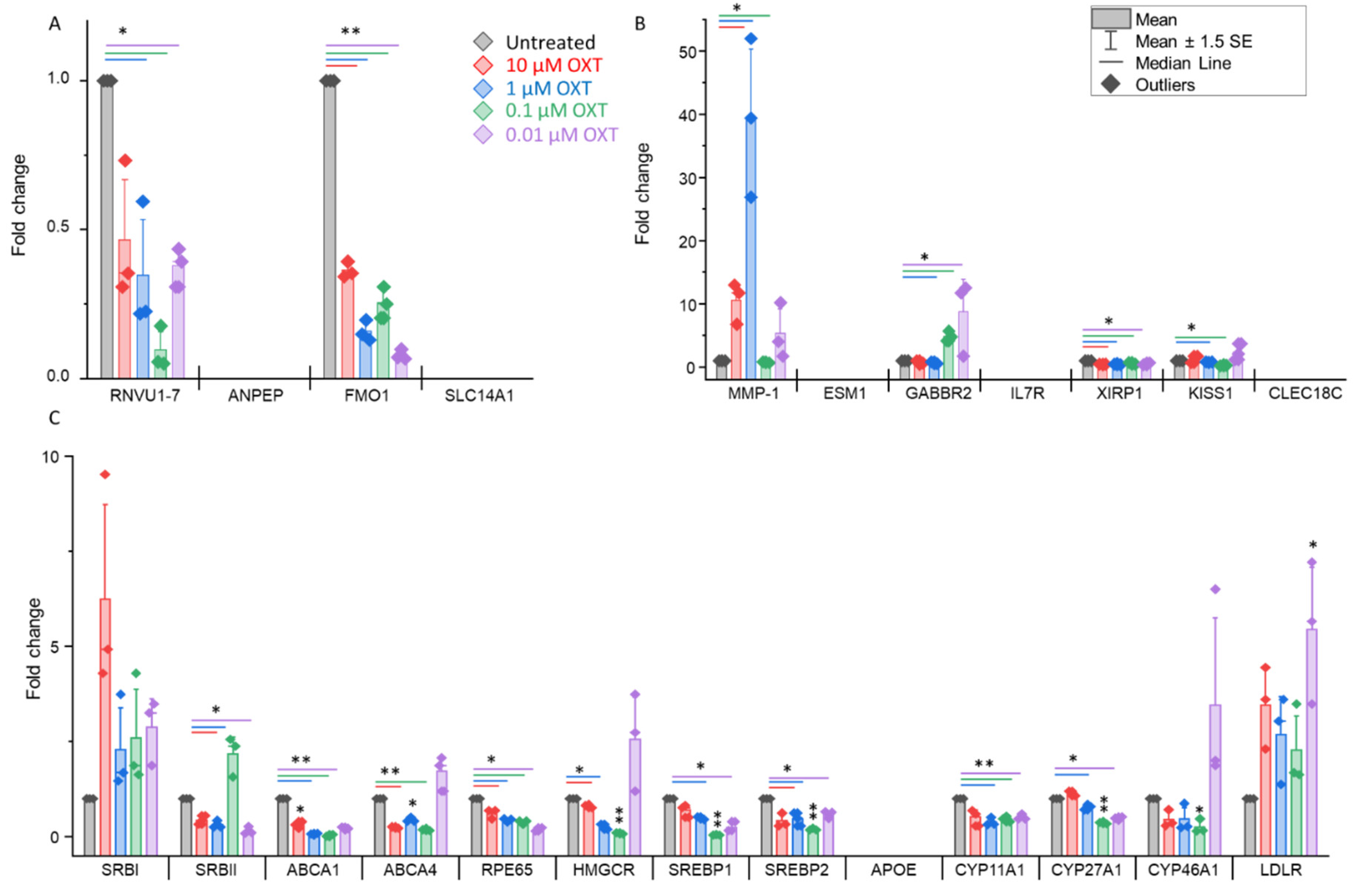
3.6. OXT Treatment of HfRPE Cells Induces Dynamic Modifications in the Expression Profile of Genes
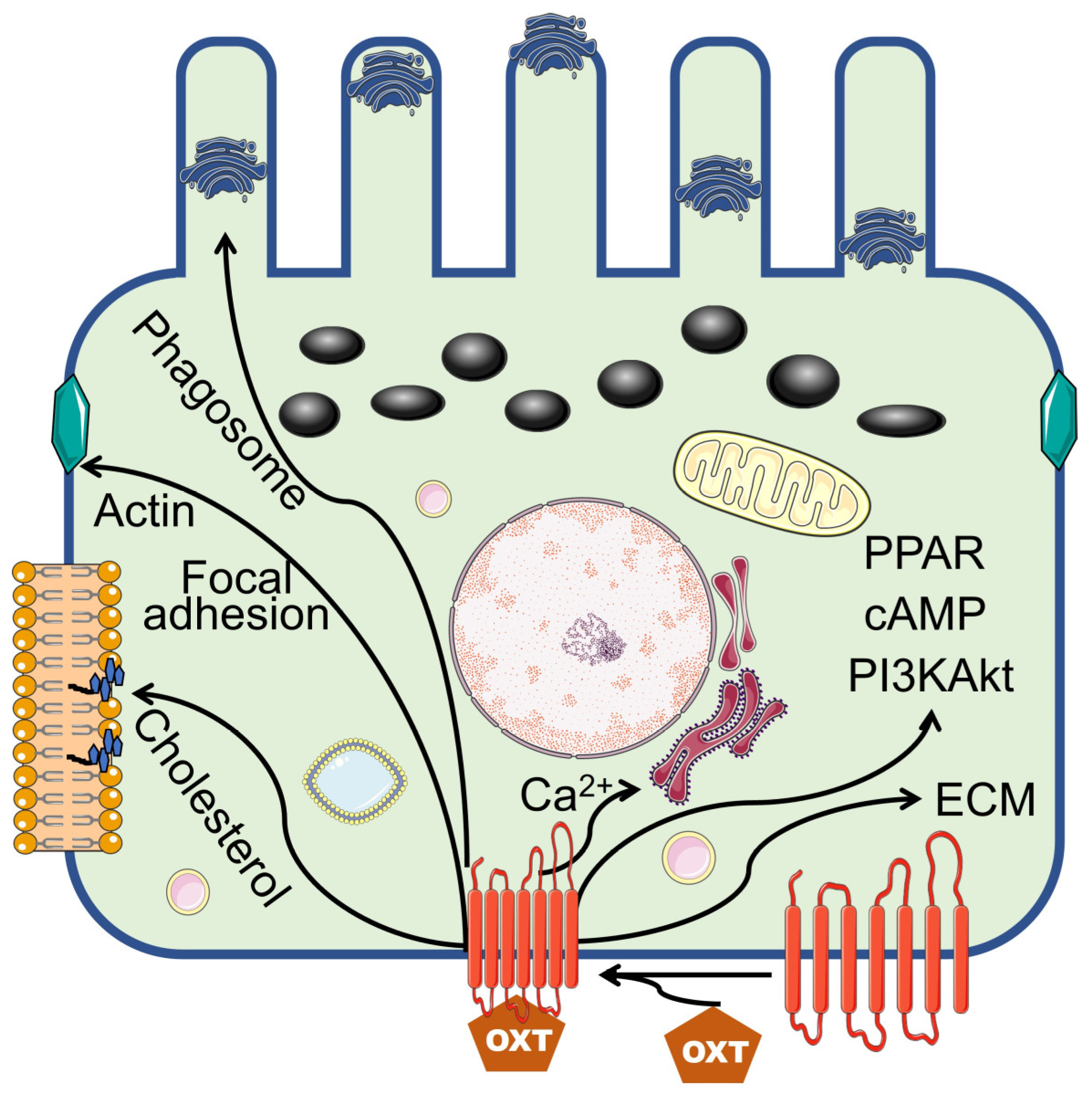
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Acknowledgments
References
- Prevost, M. et al. Oxytocin in pregnancy and the postpartum: relations to labor and its management. Front Public Health 2, 1 (2014). [CrossRef]
- Malek, A., Blann, E. & Mattison, D. R. Human placental transport of oxytocin. J Matern Fetal Med 5, 245-255 (1996). [CrossRef]
- Schubert, F., George, J. M. & Bhaskar Rao, M. Vasopressin and oxytocin content of human fetal brain at different stages of gestation. Brain Research 213, 111-117 (1981). [CrossRef]
- Weiss, G. Endocrinology of parturition. J Clin Endocrinol Metab 85, 4421-4425 (2000). [CrossRef]
- Gimpl, G. & Fahrenholz, F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81, 629-683 (2001). [CrossRef]
- O’Rahilly, R. The prenatal development of the human eye. Exp Eye Res 21, 93-112 (1975). [CrossRef]
- Gauquelin, G. et al. Presence of vasopressin, oxytocin and neurophysin in the retina of mammals, effect of light and darkness, comparison with the neuropeptide content of the neurohypophysis and the pineal gland. Peptides 4, 509-515 (1983). [CrossRef]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol Rev 85, 845-881 (2005). [CrossRef]
- Sparrow, J. R., Hicks, D. & Hamel, C. P. The retinal pigment epithelium in health and disease. Curr Mol Med 10, 802-823 (2010). [CrossRef]
- Halbach, P. et al. Oxytocin expression and function in the posterior retina: a novel signaling pathway. Invest Ophthalmol Vis Sci 56, 751-760 (2015). [CrossRef]
- Zhong, S. et al. U-shaped relation between plasma oxytocin levels and behavior in the trust game. PLoS One 7, e51095 (2012). [CrossRef]
- Szeto, A. et al. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom Med 73, 393-400 (2011). [CrossRef]
- Leng, G. & Sabatier, N. Measuring Oxytocin and Vasopressin: Bioassays, Immunoassays and Random Numbers. J Neuroendocrinol 28 (2016). [CrossRef]
- Aitkin, M., Francis, B. & J., H. Statistical Modeling in GLIM4. 123-124 (2005).
- Jiang, H., Lei, R., Ding, S. W. & Zhu, S. Skewer: a fast and accurate adapter trimmer for next-generation sequencing paired-end reads. BMC Bioinformatics 15, 182 (2014). [CrossRef]
- Bourgon, R., Gentleman, R. & Huber, W. Independent filtering increases detection power for high-throughput experiments. Proc Natl Acad Sci U S A 107, 9546-9551 (2010). [CrossRef]
- Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol 11, R25 (2010). [CrossRef]
- Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15-21 (2013). [CrossRef]
- Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011). [CrossRef]
- Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139-140 (2010). [CrossRef]
- Reiner, A., Yekutieli, D. & Benjamini, Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics 19, 368-375 (2003). [CrossRef]
- Raudvere, U. et al. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res 47, W191-W198 (2019). [CrossRef]
- Franz, M. et al. GeneMANIA update 2018. Nucleic Acids Res 46, W60-W64 (2018). [CrossRef]
- Fuhrmann, S., Zou, C. & Levine, E. M. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Exp Eye Res 123, 141-150 (2014). [CrossRef]
- Stern, J. & Temple, S. Retinal pigment epithelial cell proliferation. Exp Biol Med (Maywood) 240, 1079-1086 (2015). [CrossRef]
- Ramachandra Rao, S. & Fliesler, S. J. Cholesterol homeostasis in the vertebrate retina: biology and pathobiology. J Lipid Res 62, 100057 (2021). [CrossRef]
- Pikuleva, I. A. & Curcio, C. A. Cholesterol in the retina: the best is yet to come. Prog Retin Eye Res 41, 64-89 (2014). [CrossRef]
- Goldstein, J. L. & Brown, M. S. Regulation of the mevalonate pathway. Nature 343, 425-430 (1990). [CrossRef]
- Yoshioka, H. et al. A key mammalian cholesterol synthesis enzyme, squalene monooxygenase, is allosterically stabilized by its substrate. Proc Natl Acad Sci U S A 117, 7150-7158 (2020). [CrossRef]
- Brown, M. S. & Goldstein, J. L. Receptor-mediated endocytosis: insights from the lipoprotein receptor system. Proc Natl Acad Sci U S A 76, 3330-3337 (1979). [CrossRef]
- Li, J., Takaishi, K., Cook, W., McCorkle, S. K. & Unger, R. H. Insig-1 “brakes” lipogenesis in adipocytes and inhibits differentiation of preadipocytes. Proc Natl Acad Sci U S A 100, 9476-9481 (2003). [CrossRef]
- Tsai, Y. T. et al. Impaired cholesterol efflux in retinal pigment epithelium of individuals with juvenile macular degeneration. Am J Hum Genet 108, 903-918 (2021). [CrossRef]
- Zheng, W. et al. Spatial distribution of the pathways of cholesterol homeostasis in human retina. PLoS One 7, e37926 (2012). [CrossRef]
- Zheng, W., Mast, N., Saadane, A. & Pikuleva, I. A. Pathways of cholesterol homeostasis in mouse retina responsive to dietary and pharmacologic treatments. J Lipid Res 56, 81-97 (2015). [CrossRef]
- Ramachandra Rao, S. et al. Compromised phagosome maturation underlies RPE pathology in cell culture and whole animal models of Smith-Lemli-Opitz Syndrome. Autophagy 14, 1796-1817 (2018). [CrossRef]
- Biswas, L., Zhou, X., Dhillon, B., Graham, A. & Shu, X. Retinal pigment epithelium cholesterol efflux mediated by the 18 kDa translocator protein, TSPO, a potential target for treating age-related macular degeneration. Hum Mol Genet 26, 4327-4339 (2017). [CrossRef]
- Kwon, W. & Freeman, S. A. Phagocytosis by the Retinal Pigment Epithelium: Recognition, Resolution, Recycling. Front Immunol 11, 604205 (2020). [CrossRef]
- Roehlecke, C. et al. Stress reaction in outer segments of photoreceptors after blue light irradiation. PLoS One 8, e71570 (2013). [CrossRef]
- Qin, S. & Rodrigues, G. A. Roles of alphavbeta5, FAK and MerTK in oxidative stress inhibition of RPE cell phagocytosis. Exp Eye Res 94, 63-70 (2012). [CrossRef]
- Finnemann, S. C. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J 22, 4143-4154 (2003). [CrossRef]
- Nunes, P. & Demaurex, N. The role of calcium signaling in phagocytosis. J Leukoc Biol 88, 57-68 (2010). [CrossRef]
- Karl, M. O. et al. Endogenous Gas6 and Ca2+ -channel activation modulate phagocytosis by retinal pigment epithelium. Cell Signal 20, 1159-1168 (2008). [CrossRef]
- Müller, C., Charniga, C., Temple, S. & Finnemann, S. C. Quantified F-Actin Morphology Is Predictive of Phagocytic Capacity of Stem Cell-Derived Retinal Pigment Epithelium. Stem Cell Reports 10, 1075-1087 (2018). [CrossRef]
- Eleniste, P. P. & Bruzzaniti, A. Focal adhesion kinases in adhesion structures and disease. J Signal Transduct 2012, 296450 (2012). [CrossRef]
- Wavre-Shapton, S. T., Meschede, I. P., Seabra, M. C. & Futter, C. E. Phagosome maturation during endosome interaction revealed by partial rhodopsin processing in retinal pigment epithelium. J Cell Sci 127, 3852-3861 (2014). [CrossRef]
- Ikarashi, R. et al. Regulation of molecular clock oscillations and phagocytic activity via muscarinic Ca(2+) signaling in human retinal pigment epithelial cells. Sci Rep 7, 44175 (2017). [CrossRef]
- Kovacs, K., Kohidai, L., Pallinger, E. & Csaba, G. Effect of oxytocin and its analogs on the phagocytosis of Tetrahymena: outstanding impact of isotocin. Acta Protozoologica 41, 191-1 (2002).
- Wang, L., Chen, Y., Sternberg, P. & Cai, J. Essential roles of the PI3 kinase/Akt pathway in regulating Nrf2-dependent antioxidant functions in the RPE. Invest Ophthalmol Vis Sci 49, 1671-1678 (2008). [CrossRef]
- Yang, P., Peairs, J. J., Tano, R. & Jaffe, G. J. Oxidant-mediated Akt activation in human RPE cells. Invest Ophthalmol Vis Sci 47, 4598-4606 (2006). [CrossRef]
- Liao, R. et al. Insulin-like growth factor-1 activates PI3K/Akt signaling to protect human retinal pigment epithelial cells from amiodarone-induced oxidative injury. Br J Pharmacol 175, 125-139 (2018). [CrossRef]
- Sur, A. et al. Pharmacological protection of retinal pigmented epithelial cells by sulindac involves PPAR-alpha. Proc Natl Acad Sci U S A 111, 16754-16759 (2014). [CrossRef]
- Rodrigues, G. A. et al. Differential effects of PPARgamma ligands on oxidative stress-induced death of retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci 52, 890-903 (2011). [CrossRef]
- Plafker, S. M., O’Mealey, G. B. & Szweda, L. I. Mechanisms for countering oxidative stress and damage in retinal pigment epithelium. Int Rev Cell Mol Biol 298, 135-177 (2012). [CrossRef]
- Bellezza, I. Oxidative Stress in Age-Related Macular Degeneration: Nrf2 as Therapeutic Target. Front Pharmacol 9, 1280 (2018). [CrossRef]
- Lu, L., Hackett, S. F., Mincey, A., Lai, H. & Campochiaro, P. A. Effects of different types of oxidative stress in RPE cells. J Cell Physiol 206, 119-125 (2006). [CrossRef]
- Hecquet, C., Lefevre, G., Valtink, M., Engelmann, K. & Mascarelli, F. cAMP inhibits the proliferation of retinal pigmented epithelial cells through the inhibition of ERK1/2 in a PKA-independent manner. Oncogene 21, 6101-6112 (2002). [CrossRef]
- Kokkinaki, M. et al. Klotho regulates retinal pigment epithelial functions and protects against oxidative stress. J Neurosci 33, 16346-16359 (2013). [CrossRef]
- Ardeljan, D. & Chan, C. C. Aging is not a disease: distinguishing age-related macular degeneration from aging. Prog Retin Eye Res 37, 68-89 (2013). [CrossRef]
- Aisenbrey, S. et al. Retinal pigment epithelial cells synthesize laminins, including laminin 5, and adhere to them through alpha3- and alpha6-containing integrins. Invest Ophthalmol Vis Sci 47, 5537-5544 (2006). [CrossRef]
- Sugino, I. K. et al. Cell-deposited matrix improves retinal pigment epithelium survival on aged submacular human Bruch’s membrane. Invest Ophthalmol Vis Sci 52, 1345-1358 (2011). [CrossRef]
- Romero, R., Dey, S. K. & Fisher, S. J. Preterm labor: one syndrome, many causes. Science 345, 760-765 (2014). [CrossRef]
- Weber, A., Harrison, T. M., Sinnott, L., Shoben, A. & Steward, D. Plasma and Urinary Oxytocin Trajectories in Extremely Premature Infants During NICU Hospitalization. Biol Res Nurs 19, 549-558 (2017). [CrossRef]
- Kuwabara, Y., Takeda, S., Mizuno, M. & Sakamoto, S. Oxytocin levels in maternal and fetal plasma, amniotic fluid, and neonatal plasma and urine. Arch Gynecol Obstet 241, 13-23 (1987). [CrossRef]

| Preterm | Full-term | ||
|---|---|---|---|
| Characteristic | (n=20) | (n=54) | p value |
| Gest. Age, Weeks | |||
| Median [IQR] | 34.5 [32.7--36.0] | 39.2 [39.0--39.6] | |
| Birth weight, kg | |||
| Median [IQR] | 2.19 [1.68--2.74] | 3.43 [3.21--3.69] | |
| Labor, n(%) | 0.039 | ||
| No | 5 (25) | 28 (52) | |
| Yes | 15 (75) | 26 (48) | |
| Delivery, n(%) | 0.246 | ||
| C-Section | 10 (50) | 35 (65) | |
| Vaginal | 10 (50) | 19 (35) | |
| HBP/preeclampsia, n(%) | <0.001 | ||
| No | 8 (40) | 48 (89) | |
| Yes | 12 (60) | 6 (11) | |
| Abruption, n(%) | 0.070 | ||
| No | 18 (90) | 54 (100) | |
| Yes | 2 (10) | --- | |
| DM, n(%) | 0.001 | ||
| No | 11 (55) | 49 (91) | |
| Yes | 9 (45) | 5 (9) | |
| Maternal Steroid, n(%) | <0.001 | ||
| No | 11 (55) | 54 (100) | |
| Yes | 9 (45) | --- | |
| 5-min Apgar < 5 | 0.270 | ||
| No | 19 (95) | 54 (100) | |
| Yes | 1 (5) | --- | |
| FiO2 > 21% | 0.003 | ||
| No | 13 (65) | 51 (94) | |
| Yes | 7 (35) | 3 (6) | |
| Maternal OXT, n(%) | 0.214 | ||
| No | 11 (55) | 38 (70) | |
| Yes | 9 (45) | 16 (30) | |
| Cord blood [OXT], pg/mL | 0.106 | ||
| Median [IQR] | 137 [71.1--223] | 82.1 [40.3--146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





