1. Introduction
Citrus farming plays a crucial role in global agriculture, with Brazil standing out as one of the leading citrus producers. In 2022, Brazil ranked as the fourth-largest global producer of limes and lemons, with an estimated production of 1.06 million tons [
1]. Brazilian production is significant for the domestic fresh fruit market and a major exporter of frozen concentrated orange juice in volume and value [
2]. On the other hand, Brazil exported 2023 about 167 thousand tons of Tahiti acid lime, mainly to Europe in 2023, which represents an increase of 113% in the last ten years [
3]. However, India leads the global market, producing 3.77 million tons of limes and lemons in the same year [
1].
In citrus, post-harvest diseases are a significant factor that reduces the quantity of fruit available to consumers, resulting in a decline in fruit quality and quantity from harvest to the point of sale [
4]. Sour rot and green and blue molds, caused by
Geotrichum citri-aurantii,
Penicillium digitatum, and
Penicillium italicum, respectively, are the central infections affecting citrus fruit post-harvest, leading to losses ranging from 25% to 50% of total production [
5,
6].
Sour rot is one of the most prevalent citrus diseases globally, affecting all cultivated varieties, including Tahiti acid lime, and primarily infecting fruit through wounds. Therefore, careful harvesting, handling, and storage are crucial to reducing the incidence of this disease. Many chemical fungicides used in post-harvest treatments, such as Thiabendazole and Imazalil, commonly used to control other post-harvest diseases (green or blue mold), are ineffective against
G. citri-aurantii [
7]. Moreover, some of these products face restrictions in certain countries, contribute to water and soil pollution, and promote increased pathogen resistance [
8]. As a result, it is essential to find new, natural, safe, and environmentally friendly compounds to control sour rot during the post-harvest phase.
Studies indicate that essential oils extracted from plants act as natural fungicides, inhibiting fungal growth without leaving harmful toxic residues [
9,
10]. These oils work directly by inhibiting mycelial growth and spore germination and stimulating the production of phytoalexins in plants, acting as elicitors [
11,
12].
Essential oils can be extracted from plants through steam distillation, the most common method, and by cold pressing the peel of citrus fruits. These oils have perfumery, cosmetics, and food applications and are also used as herbicides, insecticides, and fungicides [
13]. The compounds in essential oils can act directly on pathogens, altering the transmembrane potential, reducing ATP synthesis, and causing significant damage to DNA and mitochondria [
14,
15].
Due to the lack of effective commercial fungicides for controlling G. citri-aurantii, citrus essential oils are expected to replace traditional fungicides in the management of post-harvest diseases due to their excellent multifaceted properties. In this study, we investigated the inhibitory efficacy of citrus essential oils against G. citri-aurantii in vitro, their effectiveness in controlling sour rot in vivo in Tahiti acid lime fruits, and their impact on fruit quality.
2. Results
2.1. Yield of Essential Oils
The essential oil extraction yield from each sample investigated was quantified and expressed as a percentage. The sample with the highest extraction yield was Late IAC 585 (0.16 ml 100g⁻¹), followed by BRS Rainha willowleaf mandarins (0.13 ml 100g⁻¹) and Pera IAC sweet orange (0.11 ml 100g⁻¹). Murcott IAC 221 tangor and Rio IAC 194 willowleaf mandarins had lower extraction yields, with 0.08 and 0.06 ml 100g⁻¹, respectively.
2.2. Bioactivity of Essential Oils on the Mycelial Growth of G. citri-aurantii
The treatments with essential oils on the mycelial growth of G. citri-aurantii through direct contact showed that Late IAC 585 willowleaf mandarin and Pera IAC sweet orange oils at a concentration of 32 µl ml⁻¹ achieved average inhibition rates of approximately 44% and 25%, respectively (
Table 1). Additionally, the oil from mandarin (32 µl ml⁻¹) resulted in a reduction of approximately 57% in growth rate compared to the control. These results suggest that essential oils of both varieties affect G. citri-aurantii growth in a dose-dependent manner in vitro, making both samples viable for in vivo trials for disease control in fruit.
Although the other oil samples reduced the growth rate index compared to the control and showed slight inhibition on the plates, they were less effective at inhibiting the pathogen’s mycelial growth.
For the mycelial growth rate index, in the evaluation of antifungal activity, none of the oils evaluated showed a statistically significant difference from the control (
Table 2).
However, regarding the AUMGC, it was noted that Late IAC 585 willowleaf mandarin and Pera IAC sweet orange oils, at doses of 16 and 32 µl ml⁻¹, significantly differed from the control, with a mycelial inhibition percentage of G. citri-aurantii of approximately 31% for both doses. BRS Rainha mandarin oil showed an inhibition percentage of around 25.7% compared to the control treatment, with no significant differences among the evaluated dosages. The other tested oils did not inhibit the pathogen’s mycelial growth.
2.3. Effectiveness of Essential Oils in Controlling Sour Rot in Post-Harvest Citrus
The results for disease severity showed that the contrast between the additional treatment (Control) and the triple factorial (Treatment x Oil x Dose) was not significant (p-value = 0.298), indicating that the means of the two groups are statistically equal. The interaction was insignificant when analyzing the triple factorial (Treatment x Oil x Dose) (p-value = 0.934). In this case, the breakdown of factors was examined: Treatment x Oils, Treatment x Doses, and Oils x Dose, where only the interactions Treatment x Doses and Oils x Dose were significant (p-value = 0.011 and p-value = 0.0068, respectively), as shown in
Table 3 and
Table 4. The data suggest that fruits treated curatively with Pera IAC sweet orange oil at a concentration of 32 µl ml⁻¹ statistically differed from the other treatments, showing the minor average lesion diameters of sour rot in Tahiti acid lime fruits stored at 25°C ± 2.
It can also be inferred that in the preventive treatment with Late IAC 585 and Pera IAC oils, the 64 µl ml⁻¹ dose resulted in a higher AUDPC compared to the 32 µl ml⁻¹ dose, leading to a 48.84% reduction in average lesion diameter compared to the lower dose. In evaluating preventive and curative treatments at doses of 32 and 64 µl ml⁻¹, there were no statistically significant differences compared to the control group for the average lesion diameter of sour rot. In the curative treatment, it was found that Pera IAC mandarin oil at 32 µl ml⁻¹ resulted in a lower AUDPC than the higher dose, reducing disease severity by 99.3%.
Regarding the incidence of sour rot, there was no significant difference between the additional treatment and the triple factorial (p-value = 0.2178). The triple interaction of factors (Treatment x Oil x Dose) was insignificant (p-value = 0.2231). However, the two-way interactions Treatment x Dose (p-value = 1x10⁻⁴) and Oil x Dose (p-value = 0.0023) were significant (
Table 5 and
Table 6). Notably, fruits treated curatively with orange oil at 32 µl ml⁻¹ showed a positive effect in controlling sour rot.
Tahiti acid lime fruits were preventively treated with Late IAC 585 oil at a concentration of 64 µl ml-1, and despite reducing the percentage of diseased fruits by 31.7%, there was no statistical difference from the control. We found that Tahiti acid lime fruits curatively treated with Pera IAC sweet orange oil at a concentration of 32 µl ml-1 showed a control efficiency of approximately 96% of sour rot about the control (
Figure 1).
2.4. Fruit Quality in Citrus after Oleo Essential Treatment
The application of Late IAC 585 willow leaf mandarin and Pera IAC sweet orange essential oils at concentrations of 32 and 64 µl ml⁻¹ did not significantly change the fruit quality parameters (average juice yield, total soluble solids (SS), total titratable acidity (TTA), and SS/TTA ratio) under commercial conditions when compared to the reference values determined by Pio et al. [
16]. This indicates that the treated Tahiti acid lime fruits have commercial potential after applying the treatments (
Table 7).
3. Discussion
In this study, essential oils from Late IAC 585 willowleaf and Pera IAC sweet orange exhibited a slight inhibitory effect on mycelial growth in vitro using the agar diffusion method and volatile compound exposure assay, as well as a reduction in
Geotrichum citri-aurantii sporulation, a pathogen that causes sour rot disease in citrus, one of the most challenging post-harvest diseases in citrus, leading to significant economic losses, exacerbated by the lack of effective fungicides [
17]. The increasing resistance of fungi to synthetic fungicides, such as propiconazole and thiabendazole, necessitates alternative strategies that are both sustainable and environmentally safe [
18]. In this context, essential oils have gained attention due to their antifungal properties and broad acceptance for controlling agricultural pathogens.
Several studies have shown the effects of essential oils on the mycelial growth of phytopathogens. For example, mint oil inhibited
P. digitatum and
G. citri-aurantii for one week [
19]. Essential oils from Mentha piperita, Mentha spicata, and
Mentha suaveolens completely or nearly inhibited
Botryotinia fuckeliana at 400 µg/ml, with 92–100% mycelial growth inhibition. The same authors noted that reducing the dose of
M. suaveolens oil to 200 µg/ml resulted in significantly lower inhibition levels [
20]. Volatile citral applied at 60 ml/L showed potential for controlling sour rot; however, high concentrations of volatile citral may cause phytotoxicity symptoms in fruits [
21]. According to Bhandari et al. [
22], the mode of action of essential oils is not fully understood. Still, their effects on post-harvest phytopathogens are mainly attributed to their direct impact on spore germination and mycelial growth, disrupting cellular metabolism.
Previous studies have demonstrated the potential of essential oils as antimicrobial agents, likely due to their main volatile components or combinations [
22,
23,
24,
25,
26]. Citrus peels can extract essential oils, with yields ranging from 0.2% to 1.0% depending on variety, agronomic conditions, and extraction methods [
26,
27]. Additionally, orange peel essential oil mainly consists of limonene, β-pinene, and myrcene [
28].
Studies have shown that the volatile compounds in essential oils can cause cell membrane disruption, cytoplasmic disorganization, and inhibition of fungal reproduction, which are mechanisms that control
G. citri-aurantii [
5,
29]. Cai et al. [
18] reported that compounds such as D-limonene, citral, and eugenol in essential oils disrupt fungal cell membranes and induce reactive oxygen species (ROS) formation, leading to cell death. These findings highlight the potential of essential oils for citrus citrus’s post-harvest treatment.
The present research demonstrated that Tahiti acid lime fruits treated curatively were more effective in controlling sour rot, reducing lesion diameter by 99.3% and achieving 96% control efficacy. Serna-Escolano et al. [
30] found that the incidence and severity of sour rot in treated mature lemons were significantly higher in curative experiments than in preventive ones, with values of 58.67%, 40.33%, and 62.33% for 25, 50 mM HP-β-CD-thymol, and propiconazole, respectively. Similarly, Regnier et al. [
31] demonstrated that essential oils from
Cymbopogon citratus,
Cymbopogon martinii,
Origanum vulgare, and
Geranium graveolens roseum Bourbon (1000 µl/L), incorporated into coatings or applied as curative dips, resulted in a 90% reduction in sour rot compared to the negative control.
Mandarin essential oils, such as those studied by Devite et al. [
32], show great potential as alternatives to chemical fungicides for controlling phytopathogens. In their study, different concentrations of essential oils extracted from mandarin varieties effectively inhibited the mycelial growth of Alternaria alternata, a fungus that causes brown spot in citrus. The authors observed that essential oil from the IAC 2019 Maria variety, applied at 16 µL·mL⁻¹, demonstrated the highest inhibition of fungal growth, significantly reducing disease severity in curative and preventive treatments.
The large-scale applicability of these essential oils has shown promise, mainly due to their biodegradability and low toxicity. However, challenges remain concerning their volatility and the need for microencapsulation to ensure controlled and prolonged release during storage [
30,
33,
34]. Additionally, studies have shown that fruits treated with different essential oils maintain their quality parameters, underscoring the importance of understanding optimal concentrations and the antagonistic effects against post-harvest pathogens to enhance fruit quality and shelf life [
35]. Future research should focus on optimizing formulations and exploring combinations of these oils to maximize their efficacy in controlling citrus pathogens. However, further research is required to fully elucidate the mechanisms of action and optimize the application methods, including microencapsulation, to enhance efficacy and longevity during storage. Adopting essential oils in commercial citrus operations could reduce reliance on chemical fungicides, addressing fungicide resistance issues and environmental pollution.
4. Material e Methods
4.1. Essential Oils
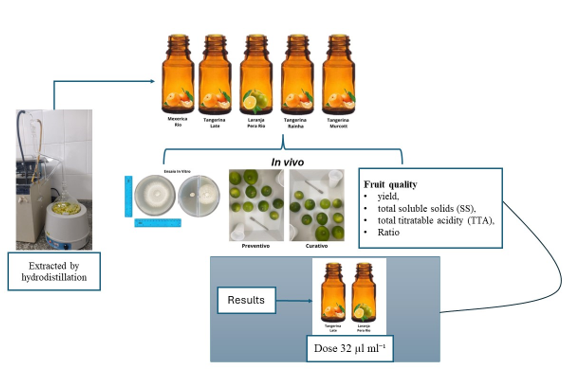
The essential oils were extracted by hydrodistillation using a modified Clevenger apparatus, divided into two stages: steam distillation and cohobation [
36]. A 400g sample of peel cut with approximately 1 cm
2 of fresh fruit was used with 800ml of distilled water, heated to 100°C on a heating mantle until the mixture reached boiling. The vapor and volatile compounds were directed to the condenser for heat exchange, condensing the vapors with cooling water. At this stage, the liquid forms of essential oil and water were visualized in the separator tube of the extractor, maintaining a continuous four-hour extraction cycle [
36]. The oil was stored in an amber vial, protected from light, and kept at 4°C.
Five essential oils were used in the experiments, from the following varieties: Late IAC 585, BRS Rainha, and Rio IAC 194 willowleaf mandarins (Citrus X deliciosa), Murcott IAC 221 tangor (C. X sinensis x C. reticulata), and Pera IAC sweet orange (Citrus X sinensis). Ripe fruit peels were used for the oil extraction, except for Late IAC 585 mandarin and Pera sweet orange, where the extraction was performed using green fruit peels.
The essential oil yield, expressed as a percentage, was calculated according to the following equation:
4.2. Bioactivity of Essential Oils on Mycelial Growth of Geotrichum citri-aurantii
a) Antifungal Activity of Essential Oils by Direct Contact
Essential oils were incorporated into molten Potato Dextrose Agar - PDA medium (50°C), supplemented with Tween 80 (0.5% v/v), and poured into 90 mm Petri dishes. The tested oil concentrations were 0 (control), 2, 4, 8, 16, and 32 µl ml⁻¹. After 24 hours, a 5 mm disc from the edge of a 7-day-old pathogen colony was placed in the center of each Petri dish, ensuring the fungal structures were in contact with the culture medium. The plates were incubated in a BOD chamber at 27°C with a 12-hour light/dark photoperiod for seven days.
The evaluation was performed by measuring the fungal colony diameter (two orthogonal measurements) daily until one of the treatments reached the total plate diameter. The values obtained were used to calculate the percentage inhibition of mycelial growth (PIMG) about the 0 µl ml⁻¹ (control), the mycelial growth rate index (MGRI), and the area under the mycelial growth curve (AUMGC), as shown in the equations below:
where Yi and Yi-1 are the colony growth values observed in two consecutive evaluations, and (Ti+1 - Ti) is the time interval between evaluations.
b) Antifungal Activity of Essential Oil Volatile Compounds
To assess the effect of volatile compounds, the concentrations of 0 (control), 2, 4, 8, 16, and 32 µl ml⁻¹ of each essential oil were tested. The essential oil solution was prepared by adding Tween 80 (0.5% v/v) and distilled water. A 20 µL aliquot of each concentration was deposited onto a sterile filter paper disc (5 mm), which was then placed in one compartment of a two-compartment Petri dish. In the opposite compartment, a 5 mm disc of pathogen mycelium was placed on the PDA with the mycelium facing upward. The plates were sealed with PVC film and incubated at 27°C with a 12-hour light/dark photoperiod for four days. The colony diameter was measured daily. The values obtained were used to calculate the percentage inhibition of mycelial growth relative to the 0 µl ml⁻¹ (control), the mycelial growth rate index, and the area under the mycelial growth curve (AUMGC), as described in the previous section.
4.3. Effectiveness of Essential Oils in Controlling Sour Rot in Post-Harvest Citrus
Tahiti acid lime fruits were collected, sanitized with neutral detergent, and surface disinfected with 0.1% sodium hypochlorite for two minutes. After drying, the fruits were wounded at two equidistant points on the equatorial region of the fruits using sterilized needles to a depth of 3 mm. The fruits were then inoculated at the wound site with 20 µl of a G. citri-aurantii spore suspension containing a concentration of 1x10⁴ conidia ml⁻¹ 24 hours before (preventive treatment) and 24 hours after (curative treatment) the application of essential oil from Late willowleaf or Pera IAC sweet orange, at doses of 32 and 64 µl ml⁻¹, selected through in vitro assays. The essential oil solution was prepared by adding Tween 80 (0.5% v/v) and distilled water. In the control treatment, the fruits were treated only with water. The fruits were stored under ambient conditions (25°C ± 2 and 70% relative humidity).
The severity of the disease was evaluated daily by measuring the diameter of the lesions formed. The obtained measurements were used to determine the area under the disease progress curve (AUDPC) using the equation proposed by Shaner and Finney [
37]:
where Yi is the lesion diameter at time Ti (in days), and Yi+1 is the lesion diameter at time Ti+1.
The percentage of healthy fruits determined the incidence. The experimental design for the severity and incidence measurements was completely randomized in a triple factorial scheme with additional treatment (2x2x2 + 1), where factor 1 refers to the fruit treatment (preventive and curative), factor 2 to the essential oils evaluated (Late IAC 585 willowleaf mandarin and Pera IAC sweet orange), and factor 3 to the doses (32 and 64 µl ml⁻¹), with the additional treatment being the control. Each treatment consisted of 3 replications, with ten fruits per replication.
4.4. Fruit Quality in Citrus After Oleo Essential Treatment
The fruits were pre-processed as described in
Section 2.3. Tahiti acid lime fruits were treated by spraying with different concentrations (0 (control), 32, and 64 µl ml⁻¹) of essential oils from Late IAC 585 willowleaf mandarin and Pera IAC sweet orange. The quality parameters evaluated were average juice yield, total soluble solids (SS), total titratable acidity (TTA), and the SS/TTA ratio. The mean values were determined from 10 fruits per treatment 24 hours post-treatment, followed by evaluations after 7 and 14 days of storage under ambient conditions (25°C ± 2 and 70% relative humidity).
4.5. Analyses Statistical
The data obtained were initially subjected to analysis of variance (ANOVA), and subsequently, the means were compared using Tukey’s test at a 5% significance level using the R software [
38]. Data normality was verified using the Shapiro-Wilk test at a 5% significance level.
5. Conclusion
The study demonstrated that essential oils from Pera IAC sweet orange and Late IAC 585 willowleaf offer promising potential as natural antifungal agents for controlling sour rot in post-harvest citrus, specifically in Tahiti acid lime fruits. Pera IAC sweet orange essential oil, at a concentration of 32 µl ml⁻¹, effectively reduced disease severity by approximately 96% in curative treatments, with no adverse effects on fruit quality. These results suggest that citrus essential oils could be a viable alternative to synthetic fungicides, contributing to more sustainable and eco-friendly post-harvest management practices.
Author Contributions
Conceptualization, V.S.M., F.T.D. F.A.d.A and M.B.; methodology, V.S.M., F.T.D. and M.B.; formal analysis, V.S.M., J.R., M.B., L.D.O. and B.S.M.; investigation, V.S.M, F.T.D. and M.B.; resources, F.T.D. and F.A.d.A.; data curation, F.T.D.; writing—original draft preparation, V.S.M., F.A.d.A., F.T.D, M.B., L.D.O. and B.S.M writing—review and editing, V.S.M., F.A.d.A., J.R., F.T.D and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Fundação de Amparo à Pesquisa do Estado de São Paulo—Fapesp (proc. nº 2014/1450880-0 andnº2017/24564-1) and CNPq (proc. nº 465440/2014-2).
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAOSTAT - Food and Agriculture Organization of the United Nations. Data Production and Trade. Disponível em: http://www.fao.org/faostat/en/#data/. Acesso em: 03 set. 2024.
- Carvalho, S.A.; Girardi, E.A.; Mourão Filho, F.D.A.A.; Ferrarezi, R.S.; Coletta Filho, H.D. Avanços na propagação dos Citros no Brasil. Rev. Bras. Frutic., 2019, 41. [CrossRef]
- Gomes, F.G. Limão em Foco 2024: Ano 4. Porto Ferreira: CCSH, 2024, p. 4. Disponível em: https://ccsm.br/wp-content/uploads/2024/04/LIMAO_EM_FOCO_2024_ano4.pdf. Acesso em: 10 set. 2024.
- Fischer, I.H.; Toffano, L.; Lourenço, S.A.; Amorim, L. Caracterização dos danos pós-colheita em citros procedentes de” packinghouse”. Fitopatol. Bras., 2007, 32, 304–310.
- François, G.A.; Pontes, J.G.de M.; Pereira, A.K.; Fill, T.P. Exploring the Citrus Sour Rot pathogen: biochemical aspects, virulence factors, and strategies for disease management - a review, Fungal Biology Reviews, 2022, 41, 70–83.
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci Technol, 2016, 47, 39–49.
- Kupper, K.C.; Moura, V.S.; de Paula, F.B.F. Leveduras como agentes de controle biológico de patógenos de pós-colheita em citros. Rapp, 2023, 29.
- Wang, W.; Liu, S.; Deng, L.; Ming, J.; Yao, S.; Zeng, K. Control of citrus post-harvest green molds, blue molds, and sour rot by the Cecropin A-Melittin hybrid peptide BP21. Front. Microbiol., 2018, 9, 2455.
- Pereira, M.C.; Vilela, G.R.; Costa, L.M.A.S.; Silva, R.F.D.; Fernandes, A.F.; Fonseca, E.W.N.D.; Piccoli, R.H. Inibição do desenvolvimento fúngico através da utilização de óleos essenciais de condimentos. Ciênc. agrotec., Lavras, 2006, 30, 731–738. [CrossRef]
- Araújo, P.C.; de Souza Neto, A.C.A.; W.C.; Medeiros, J.G.; de Aguiar, A.V. Óleo essencial de anis na incidência e controle de patógenos em sementes de erva-doce (Foeniculum vulgare Mill.). Ver. Verde, Mossoró, 2012, 7, 170–176.
- Brum, R.B.C.S. Efeito de óleos essenciais no controle de fungos fitopatogênicos. 135 f. Dissertação, Mestrado em Produção Vegetal, Universidade Federal do Tocantins, Gurupi, 2012.
- Bigaton, D.; Bacchi, L; M.A.; Formagio, A.S.N.; Gavassoni, W.L.; Zanella, C.D.S. Avaliação da atividade fungicida de extratos e óleos essenciais sobre ferrugem asiática da soja. Rev. Ciênc. Agron., 2013, 44, 757–763.
- Bizzo, H.R.; Hovell, A. M. C., Rezende, C. M. Óleos essenciais no Brasil: aspectos gerais, desenvolvimento e perspectivas. Quím. Nova, 2009, 32, 588–594.
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils - A review. Food Chem Toxicol., 2008, 46, 446–475.
- Mazaro, S.M.; Citadin, I.; De Gouvêa, A.; Luckmann, D.; Guimarães, S.S. Indução de fitoalexinas em cotilédones de soja em resposta a derivados de folhas de pitangueira. Cienc. Rural, 2008, 38, 1824-1829.
- Pio, R.M.; Figueiredo, J.O.; Stuchi, E.S.; Cardoso, S.A.B. Variedades copas. In: Citros. Eds. Mattos, D. J. R., Negri, J. D., Pio, R. M., Pompeu, J. J. R. Campinas: IAC e Fundag. 2005, 37-60.
- McKay, A.H.; Förster, H.; Adaskaveg, J. E. Efficacy and application strategies for propiconazole as a new postharvest fungicide for managing sour rot and green mold of citrus fruit. Plant Dis. 2012, 96, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Xu, Z.; Li, X.; Wang, D.; Ren, X.; Kong, Q. Underlying mechanism of menthol on controlling postharvest citrus sour rot caused by Geotrichum citri-aurantii. Postharvest Biol. Technol, 2023, 196, 112160.
- Phala, K.; Mapossa, A.B.; Augustyn, W.; Combrinck, S.; Botha, B. Development of EVA and LLDPE polymer-based carvone and spearmint essential oil release systems for citrus postharvest diseases applications. Arab. J. Chem., 2023, 16, 2, 104458.
- Giménez-Santamarina, S.; Llorens-Molina, J.A.; Sempere-Ferre, F.; Santamarina, C.; Roselló, J.; Santamarina, M.P. Chemical composition of essential oils of three Mentha species and their antifungal activity against selected phytopathogenic and post-harvest fungi. All Life, 2022, 15(1), 64–73.
- Wuryatmo, E.; Able, A.J; Ford, C.M; Scott, E.S. Efeito do citral volátil no desenvolvimento de mofo azul, mofo verde e podridão azeda em laranja-baía. Australasian Plant Pathol., 2014, 43(4), 403–411. [CrossRef]
- Bhandari, N.; Bika, R.; Subedi, S.; Pandey, S. Essential oils amended coatings in citrus postharvest management. J. Agric. Food Res, 2022, 10, 100375.
- Tao, N.; Jia, L.; Zhou, H. Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem, 2014, 153, 265–271.
- Chutia, M.; Bhuyan, P.D.; Pathak, M.G.; Sarma, T.C.; Boruah, P. Antifungal activity and chemical composition of Citrus reticulata Blanco essential oil against phytopathogens from North East India. LWT-Food Sci. Technol, 2009, 42, 777–780.
- Varano, A.; Shirahigue, L.D.; Azevedo, F.A.; Altenhofen da Silva, M., Ceccato-Antonini, S.R. Mandarin essential oil as an antimicrobial in ethanolic fermentation: Effects on Limosilactobacillus fermentum and Saccharomyces cerevisiae. Lett. Appl. Microbiol, 2022, 74, 6, 981-991.
- Khamsaw P.; Lumsangkul C.; Karunarathna A.; Onsa N.E.; Kawichai S.; Chuttong B.; Sommano S.R. Recovery of orange peel essential oil from ‘Sai-Namphaung’ tangerine fruit drop biomass and its potential use as citrus fruit postharvest diseases control. Agriculture, 2022, 12, 701.
- Kamal, G.; Anwar, F.; Hussain, A.; Sarri, N.; Ashraf, M. Yield and chemical composition of Citrus essential oils as affected by drying pretreatment of peels. Int. Food Res. J, 2011, 18, 1275.
- Ahmad, M.M.; Iqbal, Z.; Anjum, F.M.; Sultan, J.I. Genetic variability to essential oil composition in four citrus fruit species. Pak. J. Bot, 2006, 38, 319.
- Liu, X.; Li, Y. C.; Li, H. Y.; Yu, T.; Zheng, X.D. Antifungal activity of thyme oil against Geotrichum citri-aurantii in vitro and in vivo. J. Appl. Microbiol, 2009; 107, 1450-1456.
- Serna-Escolano V.; Serrano M.; Valero D.; Isabel Rodríguez-López M.; Gabaldón J. A.; Castillo S.; Valverde J.M.; Zapata P.J.; Guillén F.; Martínez-Romero D. Thymol encapsulated into HP-β-Cyclodextrin as an alternative to synthetic fungicides to induce lemon resistance against sour rot decay. Moléculas, 2020, 25(18), 4348.
- Regnier, T.; Combrinck, S.; Veldman, W.; Du Plooy, W. Application of essential oils as multi-target fungicides for the control of Geotrichum citri-aurantii and other postharvest pathogens of citrus. Ind Crops Prod., 2014, 61, 151–159.
- Devite F.T.; Azevedo F.A.d; Bastianel M.; Schinor E.H.; Conceição P. M. d. Mandarin essential oils as an alternative method of controlling the fungus Alternaria alternata (Fr.: Fr.) Keissler. Horticulturae, 2023, 9(6), 613.
- Tian, Y.; Zhou, L.; Liu, J.; Yu, K.; Yu, W.; Jiang, H.; Zhong, J.; Zou, L.; Liu, W. Effect of sustained-release tea tree essential oil solid preservative on fresh-cut pineapple storage quality in modified atmospheres packaging. Food Chem. 2023, 417, 135898. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Zhang, Z.; Li, H. Advances in controllable release essential oil microcapsules and their promising applications. Molecules. 2023, 28, 4979. [Google Scholar] [CrossRef] [PubMed]
- Ju J.; Xie Y.; Guo Y.; Cheng Y.; Qian H.; Yao W. Application of edible coating with essential oil in food preservation. Crit Rev Food Sci Nutr., 2019, 59, 2467 – 2480.
- Santos, A.S.; Alves, S.D.M.; Figueiredo, F.J.C.; Da Rocha Neto, O.G. Descrição de Sistema e de Métodos de Extração de Óleos Essenciais e Determinação de Umidade de Biomassa em Laboratório: Descrição do Sistema de Extração de Óleos Essenciais. Belém: EMBRAPA, 2004, n. 99, 6. ISSN 1517-2244.
- Shaner G.; Finney R.E. The Effect of Nitrogen Fertilization on the Expression of Slow-Mildewing Resistance in Knox Wheat. Phytopathology, 1977, 67, 8, 1051-1056.
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna. Austria, 2024. www.R-project.org/.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).