Submitted:
14 September 2024
Posted:
16 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animal Management and Treatment
2.1. Cheese Making
2.2. Chemical, Polyphenols, and Fatty Acid Analyses
2.3. Microbiological Analyses
2.4. Total DNA Extraction and PCR-DGGE Analyses
2.5. Volatile Aroma Compound Analysis
2.5. Qualitative Descriptive Analysis
2.6. Consumer Acceptability Test
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition
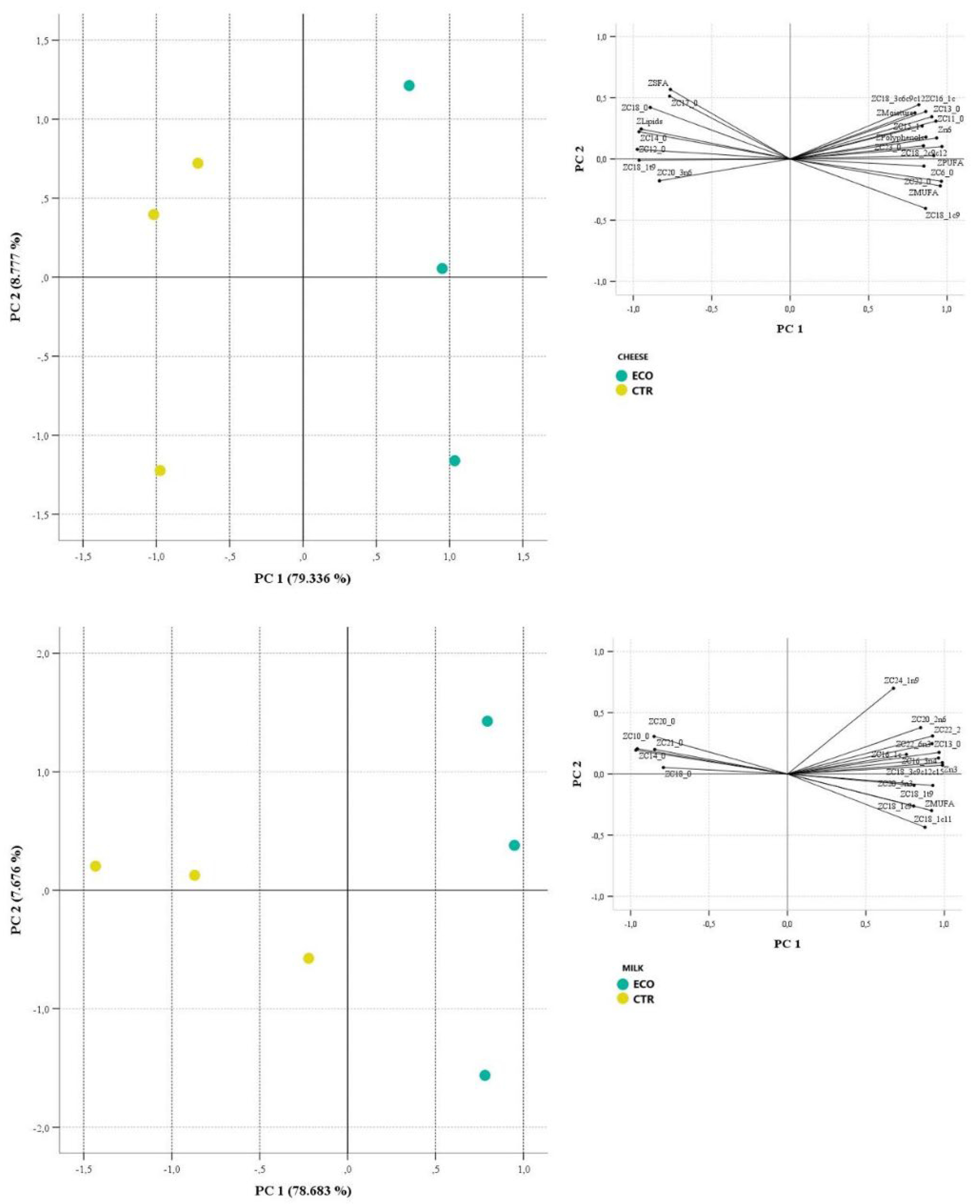
3.2. Principal Component Analysis
3.3. Microbiological Analysis
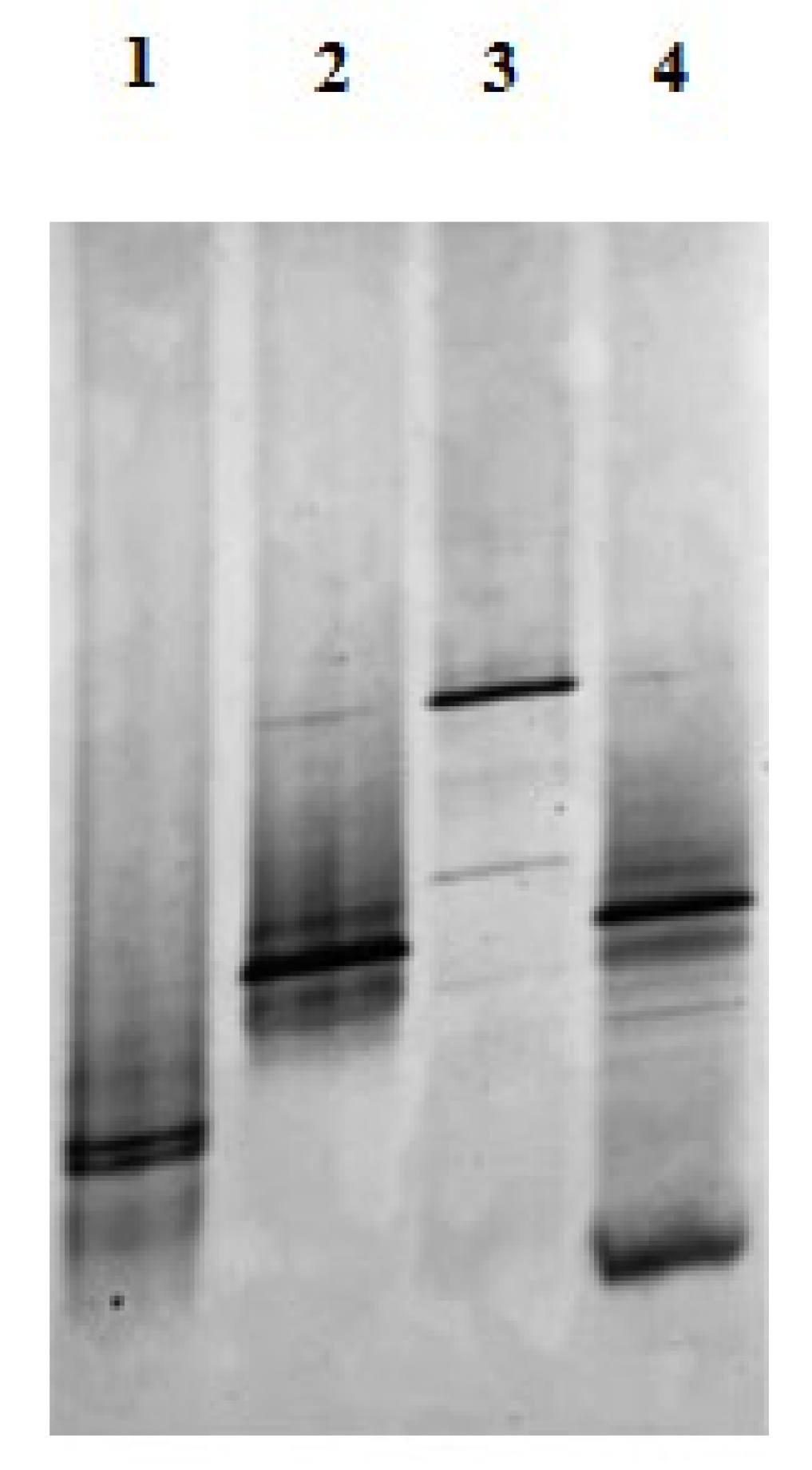
3.4. PCR-DGGE Analyses
3.4. Volatile Constituents
3.5.1. Feeds and Olive Cake
3.5.2. Milk and Cheese
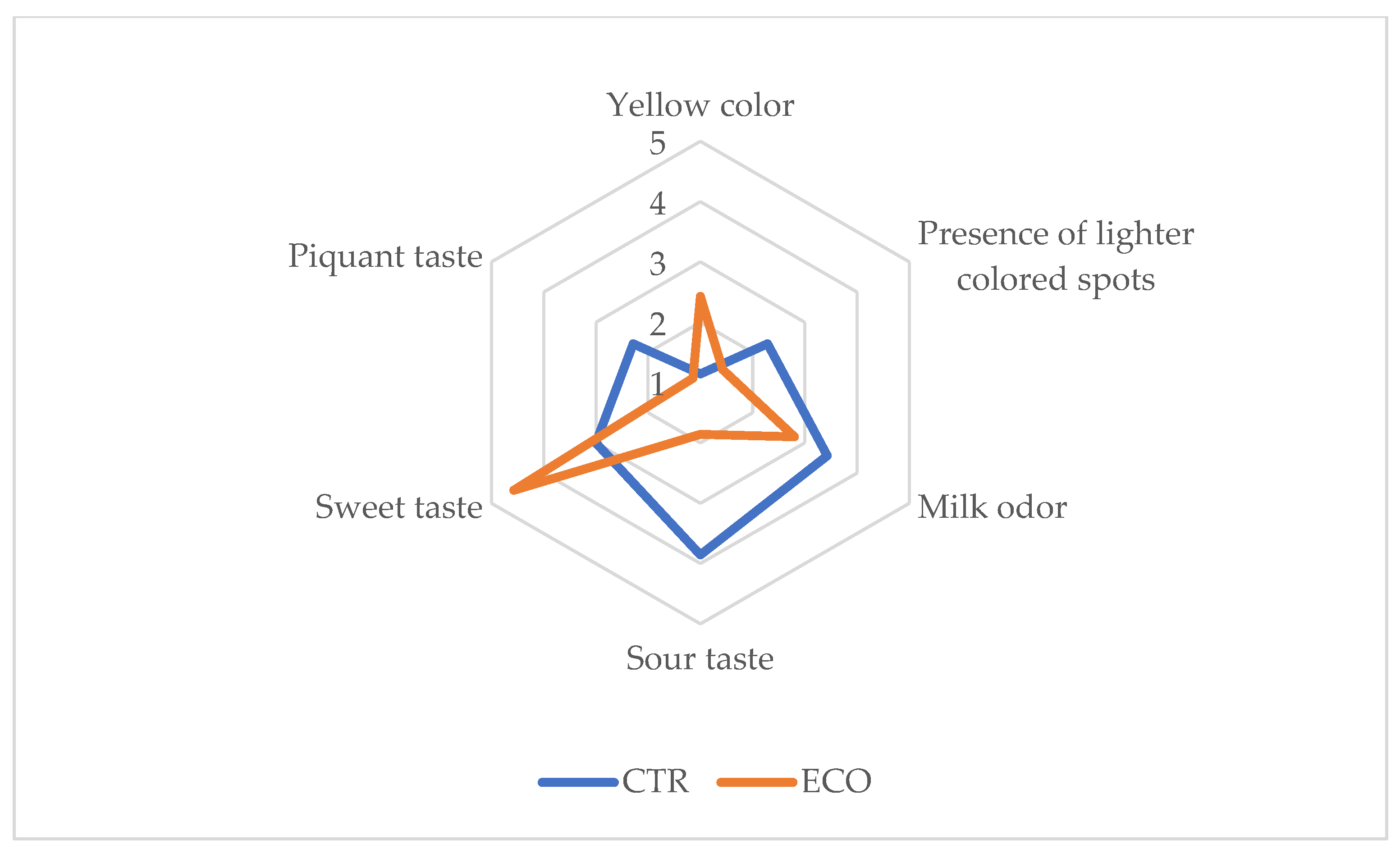
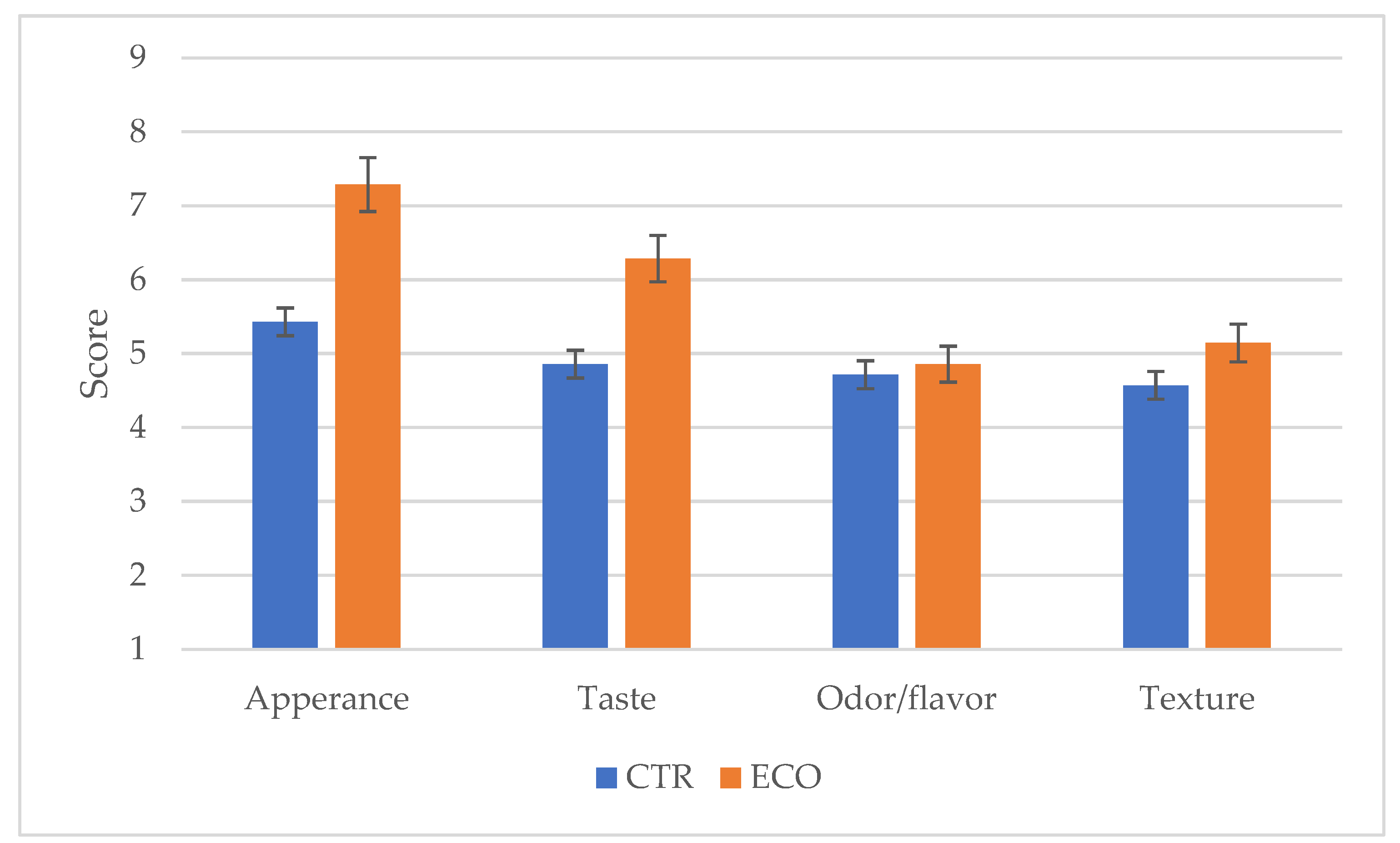
3.5. Sensory Analysis and Acceptability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CLAL UE-27: Produzioni Formaggi Di Latte Vaccino. 2024.
- Finnegan, W.; Yan, M.; Holden, N.M.; Goggins, J. A Review of Environmental Life Cycle Assessment Studies Examining Cheese Production. Int J Life Cycle Assess 2018, 23, 1773–1787. [CrossRef]
- Tarchi, I.; Boudalia, S.; Ozogul, F.; Câmara, J.S.; Bhat, Z.F.; Hassoun, A.; Perestrelo, R.; Bouaziz, M.; Nurmilah, S.; Cahyana, Y.; et al. Valorization of Agri-Food Waste and by-Products in Cheese and Other Dairy Foods: An Updated Review. Food Bioscience 2024, 58, 103751. [CrossRef]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [CrossRef]
- Calabrese, F.M.; Russo, N.; Celano, G.; Pino, A.; Lopreiato, V.; Litrenta, F.; Di Bella, G.; Liotta, L.; De Angelis, M.; Caggia, C.; et al. Effect of Olive By-Products Feed Supplementation on Physicochemical and Microbiological Profile of Provola Cheese. Front. Microbiol. 2023, 14, 1112328. [CrossRef]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can Agro-Industrial by-Products Rich in Polyphenols Be Advantageously Used in the Feeding and Nutrition of Dairy Small Ruminants? Animals 2020, 10. [CrossRef]
- Vastolo, A.; Calabrò, S.; Cutrignelli, M.I. A Review on the Use of Agro-Industrial CO-Products in Animals’ Diets. Italian Journal of Animal Science 2022, 21, 577–594. [CrossRef]
- Steyn, L.; Meeske, R.; Cruywagen, C.W. The Effect of Replacing Maize with Dried Apple Pomace in the Concentrate on Performance of Jersey Cows Grazing Kikuyu Pasture. Animal Feed Science and Technology 2018, 239, 85–93, doi:10.1016/j.anifeedsci.2018.02.012.
- Santos, G.T.; Lima, L.S.; Schogor, A.L.B.; Romero, J.V.; De Marchi, F.E.; Grande, P.A.; Santos, N.W.; Santos, F.S.; Kazama, R. Citrus Pulp as a Dietary Source of Antioxidants for Lactating Holstein Cows Fed Highly Polyunsaturated Fatty Acid Diets. Asian Australas. J. Anim. Sci 2014, 27, 1104–1113. [CrossRef]
- Abdollahzadeh, F.; Pirmohammadi, R.; Farhoomand, P.; Fatehi, F.; Pazhoh, F.F. The Effect of Ensiled Mixed Tomato and Apple Pomace on Holstein Dairy Cow. Ital J Animal Sci 2010, 9, e41. [CrossRef]
- Attard, G.; Bionda, A.; Litrenta, F.; Lopreiato, V.; Di Bella, G.; Potortì, A.G.; Lo Turco, V.; Liotta, L. Using Olive Cake as a Sustainable Ingredient in Diets of Lactating Dairy Cows: Effects on Nutritional Characteristics of Cheese. Sustainability 2024, 16, 3306. [CrossRef]
- Potortì, A.G.; Lopreiato, V.; Nava, V.; Litrenta, F.; Lo Turco, V.; Santini, A.; Liotta, L.; Di Bella, G. The Use of Olive Cake in the Diet of Dairy Cows Improves the Mineral Elements of Provola Cheese. Food Chemistry 2024, 436, 137713. [CrossRef]
- Amato, A.; Liotta, L.; Cavallo, C.; Randazzo, C.L.; Pino, A.; Bonacci, S.; Frisina, M.; Procopio, A.; Litrenta, F.; Floridia, V.; et al. Effects of Feeding Enriched-Olive Cake on Milk Quality, Metabolic Response, and Rumen Fermentation and Microbial Composition in Mid-Lactating Holstein Cows. Italian Journal of Animal Science 2024, 23, 1069–1090. [CrossRef]
- Carpino, S.; Randazzo, C.L.; Pino, A.; Russo, N.; Rapisarda, T.; Belvedere, G.; Caggia, C. Influence of PDO Ragusano Cheese Biofilm Microbiota on Flavour Compounds Formation. Food Microbiology 2017, 61, 126–135. [CrossRef]
- The European Agency for the Evaluation of Medicinal Product Guideline on Good Clinical Practices. Emea 2000, 0–29.
- Council of The European Union Enhancing the Protection of Animals Used for Scientific Purposes. Environmental Law and Management 2011, 23, 75–82.
- Licitra, G.; Portelli, G.; Campo, P.; Longombardo, G.; Farina, G.; Carpino, S.; Barbano, D.M. Technology to Produce Ragusano Cheese: A Survey. Journal of Dairy Science 1998, 81, 3343–3349. [CrossRef]
- AOAC Official Methods of Analysis of AOAC International. Association of Official Analysis Chemists International 2000.
- Di Bella, G.; Litrenta, F.; Pino, S.; Tropea, A.; Potortì, A.G.; Nava, V.; Lo Turco, V. Variations in Fatty Acid Composition of Mediterranean Anchovies (Engraulis Encrasicolus) after Different Cooking Methods. Eur Food Res Technol 2022, 248, 2285–2290. [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Elsevier, 1999; Vol. 299, pp. 152–178 ISBN 978-0-12-182200-2.
- Sik, B.; Buzás, H.; Kapcsándi, V.; Lakatos, E.; Daróczi, F.; Székelyhidi, R. Antioxidant and Polyphenol Content of Different Milk and Dairy Products. Journal of King Saud University - Science 2023, 35, 102839. [CrossRef]
- Pino, A.; Liotta, L.; Caggia, C.; Chiofalo, V.; De Nardo, F.; Zumbo, A.; Todaro, A.; Randazzo, C.L. Effect of Seasonality on Physico-Chemical and Microbiological Properties of Nicastrese Milk and Artisanal Cheese. FEMS Microbiology Letters 2021, 368, fnab055. [CrossRef]
- Randazzo, C.L.; Liotta, L.; Angelis, M.D.; Celano, G.; Russo, N.; Hoorde, K.V.; Chiofalo, V.; Pino, A.; Caggia, C. Adjunct Culture of Non-Starter Lactic Acid Bacteria for the Production of Provola Dei Nebrodi PDO Cheese: In Vitro Screening and Pilot-Scale Cheese-Making. Microorganisms 2021, 9, 179. [CrossRef]
- Nübel, U.; Engelen, B.; Felske, A.; Snaidr, J.; Wieshuber, A.; Amann, R.I.; Ludwig, W.; Backhaus, H. Sequence Heterogeneities of Genes Encoding 16S rRNAs in Paenibacillus Polymyxa Detected by Temperature Gradient Gel Electrophoresis. J Bacteriol 1996, 178, 5636–5643. [CrossRef]
- Sanguinetti, C.J.; Dias Neto, E.; Simpson, A.J. Rapid Silver Staining and Recovery of PCR Products Separated on Polyacrylamide Gels. Biotechniques 1994, 17, 914–921.
- Merlino, M.; Tripodi, G.; Cincotta, F.; Prestia, O.; Miller, A.; Gattuso, A.; Verzera, A.; Condurso, C. Technological, Nutritional, and Sensory Characteristics of Gnocchi Enriched with Hemp Seed Flour. Foods 2022, 11, 2783. [CrossRef]
- Chiofalo, B.; Liotta, L.; Zumbo, A.; Chiofalo, V. Administration of Olive Cake for Ewe Feeding: Effect on Milk Yield and Composition. Small Ruminant Research 2004, 55, 169–176. [CrossRef]
- Castellani, F.; Vitali, A.; Bernardi, N.; Marone, E.; Palazzo, F.; Grotta, L.; Martino, G. Dietary Supplementation with Dried Olive Pomace in Dairy Cows Modifies the Composition of Fatty Acids and the Aromatic Profile in Milk and Related Cheese. Journal of Dairy Science 2017, 100, 8658–8669. [CrossRef]
- Lock, A.L.; Bauman, D.E. Modifying Milk Fat Composition of Dairy Cows to Enhance Fatty Acids Beneficial to Human Health. Lipids 2004, 39, 1197–1206. [CrossRef]
- Sundram, K.; French, M.A.; Clandinin, M.T. Exchanging Partially Hydrogenated Fat for Palmitic Acid in the Diet Increases LDL-Cholesterol and Endogenous Cholesterol Synthesis in Normocholesterolemic Women. European Journal of Nutrition 2003, 42, 188–194. [CrossRef]
- Loften, J.R.; Linn, J.G.; Drackley, J.K.; Jenkins, T.C.; Soderholm, C.G.; Kertz, A.F. Invited Review: Palmitic and Stearic Acid Metabolism in Lactating Dairy Cows. Journal of Dairy Science 2014, 97, 4661–4674. [CrossRef]
- Smith, S.B.; Lunt, D.K.; Chung, K.Y.; Choi, C.B.; Tume, R.K.; Zembayashi, M. Adiposity, Fatty Acid Composition, and Delta-9 Desaturase Activity during Growth in Beef Cattle. Animal Science Journal 2006, 77, 478–486. [CrossRef]
- Vargas-Bello-Pérez, E.; Vera, R.R.; Aguilar, C.; Lira, R.; Peña, I.; Fernández, J. Feeding Olive Cake to Ewes Improves Fatty Acid Profile of Milk and Cheese. Animal Feed Science and Technology 2013, 184, 94–99. [CrossRef]
- Terramoccia, S.; Bartocci, S.; Taticchi, A.; Di Giovanni, S.; Pauselli, M.; Mourvaki, E.; Urbani, S.; Servili, M. Use of Dried Stoned Olive Pomace in the Feeding of Lactating Buffaloes: Effect on the Quantity and Quality of the Milk Produced. Asian-Australasian Journal of Animal Sciences 2013, 26, 971–980. [CrossRef]
- Chiofalo, B.; Di Rosa, A.R.; Lo Presti, V.; Chiofalo, V.; Liotta, L. Effect of Supplementation of Herd Diet with Olive Cake on the Composition Profile of Milk and on the Composition, Quality and Sensory Profile of Cheeses Made Therefrom. Animals 2020, 10. [CrossRef]
- Tzamaloukas, O.; Neofytou, M.C.; Simitzis, P.E. Application of Olive By-Products in Livestock with Emphasis on Small Ruminants: Implications on Rumen Function, Growth Performance, Milk and Meat Quality. Animals 2021, 11, 1–14. [CrossRef]
- Symeou, S.; Miltiadou, D.; Constantinou, C.; Papademas, P.; Tzamaloukas, O. Feeding Olive Cake Silage up to 20% of DM Intake in Sheep Improves Lipid Quality and Health-Related Indices of Milk and Ovine Halloumi Cheese. Trop Anim Health Prod 2021, 53, 229. [CrossRef]
- Neofytou, M.C.; Miltiadou, D.; Sfakianaki, E.; Constantinou, C.; Symeou, S.; Sparaggis, D.; Hager-Theodorides, A.L.; Tzamaloukas, O. The Use of Ensiled Olive Cake in the Diets of Friesian Cows Increases Beneficial Fatty Acids in Milk and Halloumi Cheese and Alters the Expression of SREBF1 in Adipose Tissue. Journal of Dairy Science 2020, 103, 8998–9011. [CrossRef]
- Rubió, L.; Macià, A.; Castell-Auví, A.; Pinent, M.; Blay, M.T.; Ardévol, A.; Romero, M.-P.; Motilva, M.-J. Effect of the Co-Occurring Olive Oil and Thyme Extracts on the Phenolic Bioaccesibility and Bioavailability Assessed by in Vitro Digestion and Cell Models. Food Chemistry 2014, 149, 277–284, doi:10.1016/j.foodchem.2013.10.075.
- Pérez-Mañá, C.; Farré, M.; Pujadas, M.; Mustata, C.; Menoyo, E.; Pastor, A.; Langohr, K.; De La Torre, R. Ethanol Induces Hydroxytyrosol Formation in Humans. Pharmacological Research 2015, 95–96, 27–33, doi:10.1016/j.phrs.2015.02.008.
- Vasta, V.; Nudda, A.; Cannas, A.; Lanza, M.; Priolo, A. Alternative Feed Resources and Their Effects on the Quality of Meat and Milk from Small Ruminants. Animal Feed Science and Technology 2008, 147, 223–246, doi:10.1016/j.anifeedsci.2007.09.020.
- Parr, A.J.; Bolwell, G.P. Phenols in the Plant and in Man. The Potential for Possible Nutritional Enhancement of the Diet by Modifying the Phenols Content or Profile. J. Sci. Food Agric. 2000, 80, 985–1012, doi:10.1002/(SICI)1097-0010(20000515)80:7<985::AID-JSFA572>3.0.CO;2-7.
- Kable, M.E.; Srisengfa, Y.; Laird, M.; Zaragoza, J.; McLeod, J.; Heidenreich, J.; Marco, M.L. The Core and Seasonal Microbiota of Raw Bovine Milk in Tanker Trucks and the Impact of Transfer to a Milk Processing Facility. mBio 2016, 7, e00836-16. [CrossRef]
- Wouters, J.T.M.; Ayad, E.H.E.; Hugenholtz, J.; Smit, G. Microbes from Raw Milk for Fermented Dairy Products. International Dairy Journal 2002, 12, 91–109, doi:10.1016/S0958-6946(01)00151-0.
- Doyle, C.J.; Gleeson, D.; O’Toole, P.W.; Cotter, P.D. Impacts of Seasonal Housing and Teat Preparation on Raw Milk Microbiota: A High-Throughput Sequencing Study. Appl Environ Microbiol 2017, 83, e02694-16, doi:10.1128/AEM.02694-16.
- Franciosi, E.; Settanni, L.; Cologna, N.; Cavazza, A.; Poznanski, E. Microbial Analysis of Raw Cows’ Milk Used for Cheese-Making: Influence of Storage Treatments on Microbial Composition and Other Technological Traits. World J Microbiol Biotechnol 2011, 27, 171–180. [CrossRef]
- Frétin, M.; Martin, B.; Rifa, E.; Isabelle, V.-M.; Pomiès, D.; Ferlay, A.; Montel, M.-C.; Delbès, C. Bacterial Community Assembly from Cow Teat Skin to Ripened Cheeses Is Influenced by Grazing Systems. Sci Rep 2018, 8, 200. [CrossRef]
- De Pasquale, I.; Di Cagno, R.; Buchin, S.; De Angelis, M.; Gobbetti, M. Microbial Ecology Dynamics Reveal a Succession in the Core Microbiota Involved in the Ripening of Pasta Filata Caciocavallo Pugliese Cheese. Appl Environ Microbiol 2014, 80, 6243–6255. [CrossRef]
- Barbaccia, P.; Francesca, N.; Gerlando, R.D.; Busetta, G.; Moschetti, G.; Gaglio, R.; Settanni, L. Biodiversity and Dairy Traits of Indigenous Milk Lactic Acid Bacteria Grown in Presence of the Main Grape Polyphenols. FEMS Microbiology Letters 2020, 367, fnaa066. [CrossRef]
- Kleerebezem, M.; Bachmann, H.; van Pelt-KleinJan, E.; Douwenga, S.; Smid, E.J.; Teusink, B.; van Mastrigt, O. Lifestyle, Metabolism and Environmental Adaptation in Lactococcus Lactis. FEMS Microbiology Reviews 2020, 44, 804–820. [CrossRef]
- Busetta, G.; Garofalo, G.; Claps, S.; Sardina, M.T.; Franciosi, E.; Alfonzo, A.; Francesca, N.; Moschetti, G.; Settanni, L.; Gaglio, R. The Wooden Shelf Surface and Cheese Rind Mutually Exchange Microbiota during the Traditional Ripening Process. International Journal of Food Microbiology 2024, 409, 110478. [CrossRef]
- Foulquié Moreno, M.R.; Sarantinopoulos, P.; Tsakalidou, E.; De Vuyst, L. The Role and Application of Enterococci in Food and Health. International Journal of Food Microbiology 2006, 106, 1–24. [CrossRef]
- Licitra, G.; Ogier, J.C.; Parayre, S.; Pediliggieri, C.; Carnemolla, T.M.; Falentin, H.; Madec, M.N.; Carpino, S.; Lortal, S. Variability of Bacterial Biofilms of the “Tina” Wood Vats Used in the Ragusano Cheese-Making Process. Appl Environ Microbiol 2007, 73, 6980–6987. [CrossRef]
- Randazzo, C.L.; Torriani, S.; Akkermans, A.D.L.; De Vos, W.M.; Vaughan, E.E. Diversity, Dynamics, and Activity of Bacterial Communities during Production of an Artisanal Sicilian Cheese as Evaluated by 16S rRNA Analysis. Appl Environ Microbiol 2002, 68, 1882–1892. [CrossRef]
- Tufariello, M.; Durante, M.; Veneziani, G.; Taticchi, A.; Servili, M.; Bleve, G.; Mita, G. Patè Olive Cake: Possible Exploitation of a By-Product for Food Applications. Front. Nutr. 2019, 6, 3. [CrossRef]
| Milk | ||||
|---|---|---|---|---|
| Groups | SEM1 | p-value | ||
| Item | CTR | ECO | ||
| PH | 6.68 | 6.65 | 0.07 | 0.26 |
| SH° | 3.2 | 3.2 | 0.01 | 0.31 |
| Moisture (%) | 86.82 | 88.21 | 0.09 | 0.51 |
| Total proteins (%) | 3.36 | 3.30 | 0.02 | 0.27 |
| Casein (%) | 2.64 | 2.59 | 0.02 | 0.21 |
| Total lipids (%) | 3.76 | 3.59 | 0.08 | 0.06 |
| Lactose (%) | 4.54 | 4.49 | 0.03 | 0.33 |
| Total polyphenols (mg/kg) | 62.13 | 109.25 | 0.12 | 0.04 |
| Fatty acids (g/100g FA) | ||||
| C4:0 | 2.47 | 2.51 | 0.13 | 0.70 |
| C6:0 | 2.06 | 1.96 | 0.18 | 0.85 |
| C8:0 | 0.97 | 1.02 | 0.05 | 0.26 |
| C10:0 | 2.77 | 2.60 | 0.04 | 0.55 |
| C11:0 | 0.06 | 0.13 | 0.45 | 0.05 |
| C12:0 | 3.52 | 3.15 | 0.11 | 0.09 |
| C13:0 | 0.09 | 0.13 | 0.07 | 0.04 |
| C14:0 | 12.92 | 12.10 | 0.04 | 0.05 |
| C15:0 iso* | 0.13 | 0.14 | 0.07 | 0.19 |
| C15:0 anteiso* | 0.01 | 0.01 | 0.0001 | 1.00 |
| C15:0 | 1.53 | 1.48 | 0.04 | 0.51 |
| C16:0 | 28.14 | 27.02 | 0.02 | 0.06 |
| C17:0 | 1.22 | 0.93 | 0.16 | 0.05 |
| C18:0 | 13.50 | 12.17 | 0.06 | 0.05 |
| C20:0 | 0.56 | 0.30 | 0.38 | 0.09 |
| C21:0 | 0.07 | 0.05 | 0.19 | 0.06 |
| C22:0 | 0.04 | 0.09 | 0.16 | 0.05 |
| C23:0 | 0.08 | 0.09 | 0.09 | 0.05 |
| C24:0 | 0.02 | 0.01 | 0.05 | 0.20 |
| SFA | 70.17 | 65.92 | 0.04 | 0.05 |
| C14:1 | 0.91 | 0.95 | 0.06 | 0.50 |
| C15:1 | 0.12 | 0.11 | 0.17 | 0.66 |
| C16:1 | 1.79 | 1.93 | 0.05 | 0.05 |
| C16:1 trans* | 0.01 | 0.01 | 0.000 | 1.00 |
| C16:1 n-5 | 0.01 | 0.01 | 0.01 | 1.00 |
| C17:1 | 0.27 | 0.30 | 0.11 | 0.27 |
| C18:1 cis9 | 15.91 | 18.26 | 0.08 | 0.05 |
| C18:1 trans9 | 0.68 | 0.78 | 0.09 | 0.05 |
| C18:1 cis11* | 3.08 | 3.64 | 0.11 | 0.07 |
| C18:1 trans11* | 0.25 | 0.31 | 0.17 | 0.13 |
| C20:1 n-11 | 0.06 | 0.06 | 0.08 | 0.98 |
| C22:1 n-9 | 0.01 | 0.01 | 0.36 | 0.94 |
| C24:1 n-9 | 0.03 | 0.04 | 0.05 | 0.11 |
| MUFA | 23.14 | 26.42 | 0.07 | 0.05 |
| C16:3 n-4* | 0.04 | 0.07 | 0.30 | 0.06 |
| C18:2 cis9 cis12 | 1.38 | 1.60 | 0.21 | 0.09 |
| C18:2 trans9 trans12 | 0.67 | 0.66 | 0.09 | 0.98 |
| C18:3 cis6 cis9 cis12 | 0.05 | 0.05 | 0.03 | 0.19 |
| C18:3 cis9 cis12 cis15 | 0.71 | 1.06 | 0.24 | 0.05 |
| C20:2 n-6 | 0.05 | 0.06 | 0.15 | 0.09 |
| C20:3 n-6 | 0.04 | 0.03 | 0.16 | 0.45 |
| C20:3 n-3 | 0.01 | 0.01 | 0.28 | 0.32 |
| C20:4 n-6 | 0.04 | 0.05 | 0.19 | 0.09 |
| C20:5 n-3 | 0.06 | 0.07 | 0.12 | 0.09 |
| C22:2 | 0.10 | 0.14 | 0.24 | 0.06 |
| C22:6 n-3 | 0.02 | 0.04 | 0.31 | 0.07 |
| PUFA | 3.15 | 3.85 | 0.13 | 0.05 |
| n3 | 0.80 | 1.18 | 0.23 | 0.05 |
| n6 | 2.22 | 2.45 | 0.14 | 0.51 |
| n6/n3 | 2.82 | 2.10 | 0.25 | 0.27 |
| Caciocavallo cheese | ||||
|---|---|---|---|---|
| Groups | SEM1 | p-value | ||
| Item | CTR | ECO | ||
| PH | - | - | - | - |
| SH° | - | - | - | - |
| Moisture (%) | 37.74 | 39.96 | 0.01 | 0.04 |
| Total proteins (%) | 30.24 | 31.57 | 0.02 | 0.27 |
| Casein (%) | - | - | - | - |
| Total lipids (%) | 27.49 | 25.03 | 0.02 | 0.05 |
| Lactose (%) | - | - | - | - |
| Total polyphenols (mg/kg) | 110.91 | 232.85 | 0.18 | 0.03 |
| Fatty acids (g/100g FA) | ||||
| C4:0 | 2.73 | 2.65 | 0.07 | 0.27 |
| C6:0 | 2.37 | 2.58 | 0.02 | 0.05 |
| C8:0 | 1.80 | 1.74 | 0.02 | 0.27 |
| C10:0 | 3.58 | 3.65 | 0.01 | 0.51 |
| C11:0 | 0.03 | 0.06 | 0.13 | 0.04 |
| C12:0 | 3.70 | 3.58 | 0.01 | 0.05 |
| C13:0 | 0.09 | 0.11 | 0.05 | 0.04 |
| C14:0 | 11.11 | 10.37 | 0.02 | 0.05 |
| C15:0 iso* | 0.25 | 0.23 | 0.03 | 0.10 |
| C15:0 anteiso* | 0.01 | 0.01 | 0.000 | 1.00 |
| C15:0 | 1.00 | 1.01 | 0.01 | 0.66 |
| C16:0 | 27.93 | 27.85 | 0.003 | 0.83 |
| C17:0 | 0.55 | 0.46 | 0.06 | 0.05 |
| C18:0 | 15.03 | 14.45 | 0.01 | 0.04 |
| C20:0 | 0.94 | 1.04 | 0.03 | 0.51 |
| C21:0 | 0.04 | 0.03 | 0.08 | 0.09 |
| C22:0 | 0.05 | 0.07 | 0.07 | 0.03 |
| C23:0 | 0.04 | 0.05 | 0.09 | 0.04 |
| C24:0 | 0.04 | 0.04 | 0.07 | 0.19 |
| SFA | 71.29 | 69.98 | 0.005 | 0.05 |
| C14:1 | 0.97 | 0.98 | 0.01 | 0.38 |
| C15:1 | 0.21 | 0.28 | 0.07 | 0.05 |
| C16:1 | 1.35 | 1.71 | 0.07 | 0.05 |
| C16:1 trans* | 0.01 | 0.01 | 0.000 | 1.00 |
| C16:1 n-5 | 0.01 | 0.01 | 0.000 | 1.00 |
| C17:1 | 0.59 | 0.52 | 0.06 | 0.51 |
| C18:1 cis9 | 16.43 | 18.12 | 0.03 | 0.04 |
| C18:1 trans9 | 1.67 | 0.98 | 0.12 | 0.05 |
| C18:1 cis11* | 2.10 | 2.41 | 0.05 | 0.38 |
| C18:1 trans11* | 0.01 | 0.02 | 0.15 | 0.11 |
| C20:1 n-11 | 0.10 | 0.10 | 0.02 | 0.19 |
| C22:1 n-9 | 0.01 | 0.01 | 0.000 | 1.00 |
| C24:1 n-9 | 0.02 | 0.03 | 0.11 | 0.09 |
| MUFA | 23.48 | 25.19 | 0.02 | 0.03 |
| C16:3 n-4* | 0.01 | 0.01 | 0.000 | 1.00 |
| C18:2 cis9 cis12 | 0.75 | 0.93 | 0.05 | 0.05 |
| C18:2 trans9 trans12 | 0.40 | 0.38 | 0.02 | 0.37 |
| C18:3 cis6 cis9 cis12 | 0.27 | 0.31 | 0.04 | 0.05 |
| C18:3 cis9 cis12 cis15 | 0.41 | 0.43 | 0.04 | 0.83 |
| C20:2 n-6 | 0.05 | 0.05 | 0.05 | 0.19 |
| C20:3 n-6 | 0.06 | 0.04 | 0.07 | 0.07 |
| C20:3 n-3 | 0.01 | 0.01 | 0.000 | 1.00 |
| C20:4 n-6 | 0.02 | 0.03 | 0.16 | 0.09 |
| C20:5 n-3 | 0.06 | 0.07 | 0.08 | 0.09 |
| C22:2 | 0.26 | 0.22 | 0.05 | 0.27 |
| C22:6 n-3 | 0.01 | 0.02 | 0.15 | 0.11 |
| PUFA | 2.30 | 2.50 | 0.02 | 0.05 |
| n3 | 0.48 | 0.53 | 0.03 | 0.12 |
| n6 | 1.55 | 1.74 | 0.03 | 0.05 |
| n6/n3 | 3.22 | 3.30 | 0.03 | 0.83 |
| Microbial groups | CTR | ECO | p-values |
|---|---|---|---|
| Enterococcus spp. | 3.04±0.13 | 3.15±0.15 | 0.34 |
| Enterobacteriaceae | 2.70±0.35 | 2.43±0.23 | 0.24 |
| S. aureus | 0.93±1.60 | 0.95±0.1.64 | 0.11 |
| Total mesophilic bacteria | 4.54±0.25 | 4.42±0.26 | 0.13 |
| Yeasts&moulds | 2.57±0.20 | 2.42±0.10 | 0.84 |
| Lactococcus spp. | 3.11±0.02 | 3.20±0.02 | 0.41 |
| Thermophilic lactococci | 3.77±0.04 | 5.09±0.75 | 0.01 |
| Lactic acid bacteria | 4.55±0.18 | 4.45±0.08 | 0.41 |
| E. coli | 1.47±1.29 | 0.67±1.15 | 0.01 |
| Total coliforms | 1.80±1.56 | 1.33±1.15 | 0.41 |
| Salmonella spp. | Absent | Absent | --- |
| L. monocytogenes | Absent | Absent | --- |
| Microbial groups | CTR | ECO | P-values |
|---|---|---|---|
| Enterococcus spp. | 5.04±0.19 | 5.15±0.15 | 0.65 |
| Enterobacteriaceae | 2.54±0.34 | 2.72±0.17 | 0.33 |
| Coagulase+ staphylococci | 4.48±0.01 | 5.29±0.30 | 0.13 |
| Total mesophilic bacteria | 6.38±0.11 | 6.23±0.11 | 0.62 |
| Yeasts&moulds | 6.57±0.26 | 6.42±0.17 | 0.84 |
| Lactococcus spp. | 6.91±0.02 | 6.68±1.42 | 0.35 |
| Thermophilic lactococci | 2.77±0.04 | 5.29±0.75 | 0.01 |
| Lactic acid bacteria | 7.34±0.48 | 7.03±0.28 | 0.41 |
| E. coli O157 | Absent | Absent | --- |
| E. coli | Absent | Absent | --- |
| Salmonella spp. | Absent | Absent | --- |
| L. monocytogenes | Absent | Absent | --- |
| Compounds | LRI | Control | ECO | Olive cake |
|---|---|---|---|---|
| Acids | ||||
| Acetic acid | 1455 | tra | tra | 0.36±0.02b |
| Propionic acid | 1540 | tra | tra | 0.23±0.01b |
| 2-Methyl-propanoic acid | 1566 | 0.62±0.04b | 0.55±0.04b | 0.28±0.02a |
| Butanoic acid | 1629 | tra | tra | 0.94±0.06b |
| 2-Methyl-butanoic acid | 1666 | 3.14±0.12c | 2.64±0.22b | 0.85±0.05a |
| Hexanoic acid | 1844 | 1.62±0.18a | 5.02±0.41b | 5.58±0.41b |
| Heptanoic acid | 1950 | tra | 0.77±0.06b | 0.85±0.06b |
| Octanoic acid | 2060 | 0.26±0.03a | 0.94±0.07b | 1.06±0.08b |
| Nonanoic acid | 2165 | tra | 0.71±0.03b | 0.74±0.04b |
| Tetradecanoic acid | 2694 | 0.90±0.07a | 0.87±0.07a | 0.39±0.02b |
| Pentadecanoic acid | 2799 | 0.26±0.03b | 0.26±0.03b | tra |
| Hexadecanoic acid | 2906 | 3.23±0.16a | 3.63±0.18b | 3.89±0.31b |
| All | 10.02±0.63a | 15.39±1.11b | 15.18±1.08c | |
| Aldehydes | ||||
| Pentanal | 985 | 0.85±0.05a | 1.23±0.07b | 0.66±0.04a |
| Hexanal | 1085 | 9.79±0.57c | 8.45±0.42b | 4.42±0.22a |
| Heptanal | 1188 | 1.21±0.08a | 2.44±0.17b | 2.03±0.14b |
| Octanal | 1292 | 3.54±0.14a | 5.76±0.23b | 6.86±0.27c |
| (Z)-2-Heptenal | 1328 | 0.58±0.03a | 0.50±0.02a | 1.39±0.07b |
| Nonanal | 1395 | 3.71±0.19a | 10.31±0.61b | 24.04±1.44c |
| (E)-2-Octenal | 1431 | 0.96±0.06a | 0.92±0.05a | 0.80±0.04a |
| Decanal | 1499 | 0.37±0.01a | 0.42±0.02a | 1.25±0.05b |
| Benzaldehyde | 1527 | 0.24±0.02a | 0.55±0.04a | 2.01±0.14b |
| (E)-2-Nonenal | 1535 | 0.39±0.02b | 0.26±0.02b | tra |
| (E)-2-Decenal | 1645 | 0.29±0.01a | 0.93±0.05b | 0.80±0.04b |
| (Z)-8-Undecenal | 1750 | tra | 0.30±0.02b | tra |
| All | 21.95±1.18a | 32.07±1.72b | 44.27±2.45c | |
| Alcohols | ||||
| Ethanol | 937 | tra | tra | 0.23±0.01b |
| 5-Ethyl-2-heptanol | 1121 | tra | tra | 0.25±0.02b |
| 1-Butanol | 1143 | 0.32±0.02b | 0.29±0.01b | tra |
| 2-Methyl-1-butanol | 1158 | 0.26±0.01b | tra | tra |
| 3-Methyl-1-butanol | 1205 | 0.39±0.02b | 0.27±0.01a | 0.27±0.02a |
| 1-Pentanol | 1247 | 6.27±0.34c | 4.75±0.19b | tra |
| Heptan-2-ol | 1315 | 0.35±0.02b | tra | tra |
| 3-Methyl-1-pentanol | 1351 | 25.90±1.18c | 13.40±0.40b | 2.06±0.06a |
| 1-Octen-3-ol | 1444 | 5.27±0.26c | 4.04±0.18b | 2.08±0.04a |
| 1-Heptanol | 1450 | 7.57±0.45c | 5.77±0.28b | 0.29±0.01a |
| 6-Methyl-5-hepten-2-ol | 1457 | 0.38±0.04b | 0.34±0.03b | tra |
| 2,4-Dimethyl-cyclohexanol | 1478 | 0.31±0.02b | tra | tra |
| 2-Ethyl-1-hexanol | 1484 | 0.26±0.01b | tra | 0.28±0.02b |
| 1-Octanol | 1553 | 7.21±0.10c | 6.78±0.06b | 0.98±0.01a |
| (Z)-2-Octen-1-ol | 1612 | tra | 0.23±0.01b | 0.26±0.01b |
| 1-Nonanol | 1656 | 0.87±0.04b | 1.17±0.06c | tra |
| Guaiacol | 1859 | tra | tra | 0.29±0.02b |
| Benzyl alcohol | 1875 | tra | 0.47±0.04b | 0.89±0.07b |
| Phenethyl alcohol | 1909 | tra | 0.58±0.05b | 1.10±0.09c |
| Creosol | 1954 | tra | tra | 3.30±0.26b |
| 4-Ethyl-phenol | 2176 | tra | 0.64±0.05b | 6.04±0.48c |
| All | 55.37±2.51c | 38.74±1.37b | 18.33±1.12a | |
| Ketones | ||||
| Heptan-2-one | 1185 | 1.21±0.09b | 0.90±0.07a | 0.78±0.05a |
| 2-Octanone | 1287 | 0.47±0.03a | 0.43±0.02a | 0.72±0.06b |
| 1-Octen-3-one | 1304 | tra | tra | 0.25±0.02b |
| 6-Methyl-5-hepten-2-one | 1336 | 2.27±0.18b | 1.67±0.11a | 2.12±0.09b |
| 4-Ethyl-cyclohexanone | 1344 | 0.51±0.03b | tra | tra |
| 2-Nonanone | 1389 | 0.60±0.04a | 0.66±0.03a | 1.79±0.13b |
| Oct-3-en-2-one | 1409 | 1.96±0.14a | 3.07±0.31b | 3.40±0.45b |
| 6-Methoxy-2-hexanone | 1421 | 0.27±0.03b | tra | tra |
| 2-Decanone | 1492 | 0.29±0.04a | 0.45±0.05b | 0.48±0.05b |
| 3,5-Octadien-2-one | 1520 | tra | 0.52±0.03b | 0.31±0.01b |
| (E,E)-3,5-Octadien-2-one | 1572 | 0.48±0.04b | 0.41±0.03b | tra |
| 6-Methyl-3,5-heptadiene-2-one | 1594 | tra | 0.40±0.02b | 1.02±0.05c |
| Acetophenone | 1652 | tra | tra | 0.35±0.02b |
| Nerylacetone | 1851 | tra | tra | 0.29±0.01b |
| All | 8.06±0.62a | 8.51±0.67a | 11.51±0.94b | |
| Esters | ||||
| Methyl acetate | 833 | tra | tra | 0.68±0.04b |
| Ethyl acetate | 893 | tra | tra | 0.55±0.03b |
| Methyl propionate | 912 | tra | tra | 0.38±0.03b |
| Ethyl propanoate | 961 | tra | tra | 0.29±0.02b |
| Methyl butyrate | 991 | tra | tra | 0.25±0.02b |
| Ethyl butanoate | 1038 | tra | tra | 0.41±0.03b |
| Methyl nonanoate | 1489 | tra | tra | 0.71±0.05b |
| Pentyl hexanoate | 1511 | 0.24±0.02a | 0.50±0.04b | 0.51±0.04b |
| Hexyl hexanoate | 1607 | tra | 0.66±0.05b | Tra |
| All | 0.24±0.02a | 1.17±0.09b | 3.78±0.26c | |
| Lactones | ||||
| γ-Octalactone | 1916 | 0.36±0.02b | 0.76±0.05c | tra |
| γ-Nonalactone | 2028 | 1.09±0.07a | 1.43±0.12b | 6.04±0.47c |
| All | 1.45±0.09a | 2.19±0.17b | 6.04±0.47c | |
| Oxides | ||||
| cis-Linalool oxide | 1439 | 0.95±0.08c | 0.58±0.03b | tra |
| trans-Linalool oxide (furanoid) | 1467 | 0.44±0.02c | 0.31±0.01b | tra |
| All | 1.39±0.10c | 0.89±0.04b | tra | |
| Furans | ||||
| 2-Pentyl-furan | 1230 | 1.52±0.12c | 1.06±0.07b | 0.89±0.05a |
| All | 1.52±0.12c | 1.06±0.07b | 0.89±0.05a |
| Cheese | Milk | ||||||
|---|---|---|---|---|---|---|---|
| Compounds | LRI | Control | Biotrak | Control | Biotrak | ||
| Acids | |||||||
| Acetic acid | 1460 | 0.90±0.06b | 0.33±0.02a | ** | 2.19±0.15b | 0.13±0.01a | *** |
| Propanoic acid | 1543 | tra | 0.46±0.03b | *** | - | - | ns |
| Butanoic acid | 1631 | 3.24±0.16b | 1.14±0.07a | ** | 0.51±0.04a | 0.93±0.03b | * |
| Hexanoic acid | 1740 | 9.09±0.63b | 3.90±0.31a | * | 1.07±0.05a | 1.05±0.04a | ns |
| Heptanoic acid | 1954 | 0.22±0.01a | 0.16±0.01a | * | 0.37±0.02b | Tra | * |
| Octanoic acid | 2062 | 3.79±0.18b | 2.88±0.14a | * | 1.21±0.06a | 1.65±0.08b | ns |
| Nonanoic acid | 2170 | 2.02±0.10a | 1.96±0.08a | ns | 3.49±0.21b | 1.09±0.06a | ** |
| (E)-2-Octenoic acid | 2184 | tra | 0.36±0.02b | ** | - | - | ns |
| Decanoic acid | 2276 | 2.24±0.11b | 1.87±0.07a | * | 5.21±0.36b | 4.00±0.28a | ns |
| (E)-9-Decenoic acid | 2332 | - | - | ns | 0.08±0.01a | Tra | ns |
| Undecanoic acid | 2379 | 0.27±0.02a | 0.23±0.02a | ns | 0.26±0.02a | 0.29±0.02a | ns |
| (E)-2-Decenoic acid | 2399 | 0.15±0.01a | 0.37±0.03a | * | 0.24±0.02a | 0.32±0.03a | ns |
| Benzoic acid | 2430 | - | - | ns | 0.32±0.01a | 0.29±0.02a | ns |
| Dodecanoic acid | 2488 | 1.70±0.12a | 2.03±0.16a | ns | 2.32±0.13a | 3.25±0.23b | ns |
| Tridecanoic acid | 2587 | tra | 0.23±0.01a | * | 0.34±0.02a | 0.54±0.04b | ns |
| Tetradecanoic acid | 2698 | 6.27±0.25a | 11.57±0.81b | * | 22.69±1.34a | 25.67±1.26b | ns |
| Pentadecanoic acid | 2799 | 2.50±0.15a | 3.34±0.23b | ns | 3.13±0.10a | 3.44±0.08b | ns |
| Hexadecanoic acid | 2810 | 24.42±1.22a | 41.36±1.65b | * | 43.50±2.26a | 48.72±2.59b | ns |
| All | 56.81±3.02a | 72.19±3.66b | * | 86.93±4.80a | 91.37±4.77b | ns | |
| Short-Medium | 21.92±1.28b | 13.66±0.80a | * | 14.63±0.94b | 9.46±0.55a | * | |
| Long | 34.89±1.74a | 58.53±2.86b | * | 71.98±3.86a | 81.91±4.22b | * | |
| Ratio S-M/L | 0.63±0.74a | 0.23±0.28a | * | 0.20±0.24b | 0.11±0.13a | * | |
| Alcohols | |||||||
| Ethanol | 938 | 0.74±0.05a | 4.15±0.54b | *** | 0.16±0.01a | tra | * |
| 2-Methyl-propanol | 1096 | 0.27±0.02a | 0.13±0.01a | * | - | - | ns |
| Isoamyl alcohol | 1206 | 1.73±0.12a | 5.39±0.85b | * | - | - | ns |
| 3-Methyl-butanol | 1212 | - | - | ns | 0.04±0.01a | tra | ns |
| Pentanol | 1250 | - | - | ns | 0.15±0.01a | 0.20±0.02a | ns |
| 3-Methyl-2-buten-1-ol | 1319 | tra | 0.07±0.01a | * | - | - | ns |
| Hexanol | 1350 | 0.08±0.01a | tra | * | 0.08±0.01a | tra | ns |
| 2-Ethyl-hexanol | 1482 | 0.61±0.04a | 0.42±0.03a | ns | 0.61±0.04b | 0.42±0.03a | ns |
| Octanol | 1550 | 0.13±0.01a | 0.10±0.01a | ns | 0.13±0.01a | 0.10±0.01a | ns |
| Dodecanol | 1970 | tra | 0.72±0.05b | * | - | - | ns |
| Tetradecanol | 2174 | tra | 0.29±0.03a | * | - | - | ns |
| All | 3.56±0.25a | 11.27±1.53b | ** | 1.17±0.09b | 0.72±0.06a | ns | |
| Aldehydes | |||||||
| Pentanal | 984 | - | - | ns | 0.31±0.02b | 0.21±0.01a | ns |
| Hexanal | 1085 | 1.48±0.07b | 1.06±0.04a | ns | 1.58±0.13b | 0.67±0.04a | * |
| Heptanal | 1189 | 0.82±0.04b | 0.32±0.02a | * | - | - | ns |
| Octanal | 1282 | - | - | ns | 0.35±0.02b | tra | * |
| Nonanal | 1394 | 2.17±0.15a | 1.99±0.11a | ns | 1.26±0.11a | 1.30±0.12b | ns |
| Decanal | 1491 | - | - | ns | 0.78±0.06b | tra | * |
| Undecanal | 1598 | - | - | ns | 0.47±0.03b | tra | * |
| All | 4.47±0.26b | 3.37±0.17a | ns | 4.75±0.37b | 2.18±0.17a | * | |
| Ketones | |||||||
| 2-Butanone | 910 | 0.84±0.06a | 0.52±0.04a | ns | 1.67±0.11b | 1.47±0.09a | ns |
| 2,3-Butanedione | 985 | 8.76±0.35b | 1.61±0.08a | *** | - | - | ns |
| 2,3-Pentanedione | 1062 | 0.24±0.02a | 0.12±0.01a | * | - | - | ns |
| 3-Heptanone | 1155 | 0.08±0.01a | tra | * | - | - | ns |
| 2-Heptanone | 1185 | 10.01±0.48b | 1.54±0.12a | *** | 0.56±0.04a | 0.70±0.05b | ns |
| Acetoin | 1294 | 9.82±0.59b | 5.63±0.22a | * | - | - | ns |
| 2,5-Octanedione | 1316 | - | - | ns | 0.11±0.01b | tra | * |
| 6-Methyl-5-hepten-2-one | 1338 | 0.12±0.01a | 0.10±0.01a | ns | - | - | ns |
| 2-Nonanone | 1389 | 0.48±0.03a | 0.11±0.01a | ** | 0.16±0.01b | 0.09±0.07a | * |
| All | 30.35±1.55b | 9.63±0.49a | *** | 2.50±0.17b | 2.26±0.21a | ns | |
| Esters | |||||||
| Ethyl acetate | 896 | 0.26±0.03a | 0.66±0.05a | * | - | - | ns |
| Ethyl propanoate | 962 | 0.04±0.01a | 0.22±0.01a | *** | - | - | ns |
| Ethyl butanoate | 1039 | 0.31±0.02a | 0.63±0.04a | * | 0.13±0.01b | tra | * |
| Isoamyl acetate | 1122 | 0.32±0.03a | 0.09±0.01a | ** | - | - | ns |
| Methyl propyl butanoate | 1157 | 0.12±0.01a | tra | * | - | - | ns |
| Butyl butanoate | 1218 | 0.15±0.01a | tra | * | - | - | ns |
| Ethyl hexanoate | 1232 | tra | 0.09±0.01a | * | 0.05±0.01a | 0.09±0.01a | ns |
| Isopentyl butanoate | 1264 | 0.32±0.02a | tra | ** | - | - | ns |
| Methyl hexadecanoate | 2216 | - | - | ns | 0.23±0.01a | 0.64±0.04b | * |
| All | 1.52±0.13a | 1.69±0.12a | ns | 0.41±0.03a | 0.73±0.05a | * | |
| Aromatic hydrocarbons | |||||||
| Ethylbenzene | 1128 | 0.18±0.01b | 0.05±0.01a | ** | tra | tra | ns |
| Styrene | 1262 | 0.61±0.04b | 0.22±0.02a | ** | tra | tra | ns |
| All | 0.79±0.05b | 0.27±0.03a | ** | tra | tra | ns | |
| Lactones | |||||||
| δ-Decalactone | 2197 | tra | 0.29±0.03b | * | - | 0.29±0.02b | * |
| All | tra | 0.29±0.03b | * | - | 0.29±0.02b | * | |
| Terpenes | |||||||
| α-Pinene | 1024 | tra | tra | ns | 0.32±0.02a | 0.55±0.04b | * |
| δ-3-Carene | 1146 | 0.02±0.01a | tra | ns | - | - | ns |
| Myrcene | 1160 | 0.16±0.01b | tra | * | - | - | ns |
| α-Terpinene | 1180 | 0.04±0.01a | tra | ns | - | - | ns |
| Limonene | 1201 | 1.21±0.01a | 1.38±0.08a | ns | 0.36±0.03b | tra | * |
| Eucalyptol | 1211 | 0.64±0.05b | 0.13±0.01a | ** | 0.17±0.01a | 0.10±0.01a | ns |
| p-Cymene | 1272 | 0.31±0.03a | 0.33±0.02a | ns | 0.72±0.06b | tra | * |
| All | 2.38±0.12b | 1.84±0.11a | ns | 1.57±0.12b | 0.65±0.05a | * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





