Abbreviations: ACLY, ATP citrate lyase; CSE, cystathionine gamma lyase; CBS, cystathionine beta synthase; DMG, dimethylglycine; DNMT, DNA methyl transferase; FOCM, folate 1-carbon metabolism; FTO, fat-mass obesity associated protein; Hcy, homocysteine; H3K9, Histone H3 Lysine 9 (methylated gene-off; acetylated gene on); HDAC, histone de-acetylase; HFD, high fat diet; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LDH, lactate dehydrogenase; 3-MST, 3-mercaprtopyruvate sulfur transferase; Met, methionine; MMP, matrix metalloproteinase; MTHFS/R, methyl tetrahydrofolate synthase/reductase; OCR, oxygen consumption rate; PC, phosphatidylcholine; PCP, phosphatidylcholine phosphatase; Pi, phosphate; PGC1a, peroxisome proliferator gamma co-activator1alpha; ROS, reactive oxygen species; SAHH, S-adenosine homocysteine hydrolase; SAM, S-adenosine methionine; SAH, S-adenosine homocysteine; SOD, superoxide dismutase; TCA, tricarboxylic acid; TET, ten eleven translocator. TFAM, transcription factor mitochondrial.
Introduction
Chronic heart failure (CHF) patients face with formidable challenge of maintaining cardiac function. The chronic high fat dysbiosis diet (HFD) instigates cardiac remodeling, dysfunction and CHF [
1,
2,
3,
4], however, the mechanism(s) are unclear. The therapeutic approaches focusing on oxidative stress, inflammation and hyperlipidemia have not yielded significant positive clinical outcomes for CHF. Interestingly, the recent studies report that the ATP citrate lyase (ACLY) supports cardiac function and decreases more in HFpEF than HFrEF. Paradoxically, the ACLY creates hyperlipidemia. Because the inhibition of ACLY (ACLYi) mitigates hyperlipidemia and fatty acid declines ACLY expression [
5]. Epigenetically ACLY acetylates histone [
6]. The mechanism(s) are unknown. It is known that the transcription is controlled by on/off promoters by rhythmic methylation/de-methylation during development, health and disease. The chromatin maturation, adaption and accessibility are regulated by acetylation
(Figure 1). The epigenetic folate 1-carbon metabolism (FOCM) pathways recycle Hcy back to methionine. The epigenetic DNA methylation by 1-carbon metabolism) [
1,
2,
3,
4,
7] is the hallmark of epigenetic memory i.e., rhythmic gene imprinting, and off-printing during embryogenesis, development, health, remodeling, and diseases [
8]. One
hypothesis is that the HFD induces epigenetic folate 1-carbon metabolism (FOCM) and heart failure. The probiotics utilizes the fat/lipids (25-27), post-biotically increases the mitochondrial bioenergetics, and attenuates the HFD induced heart failure. Here we review a novel and
paradigm-shift epigenetic mitochondrial sulfur transsulfuration pathways that selectively target HFD-induced CHF (
Figs. 1 and 2). The HFD via
transport and metabolism pathways instigates cardiac remodeling.
Discussion:
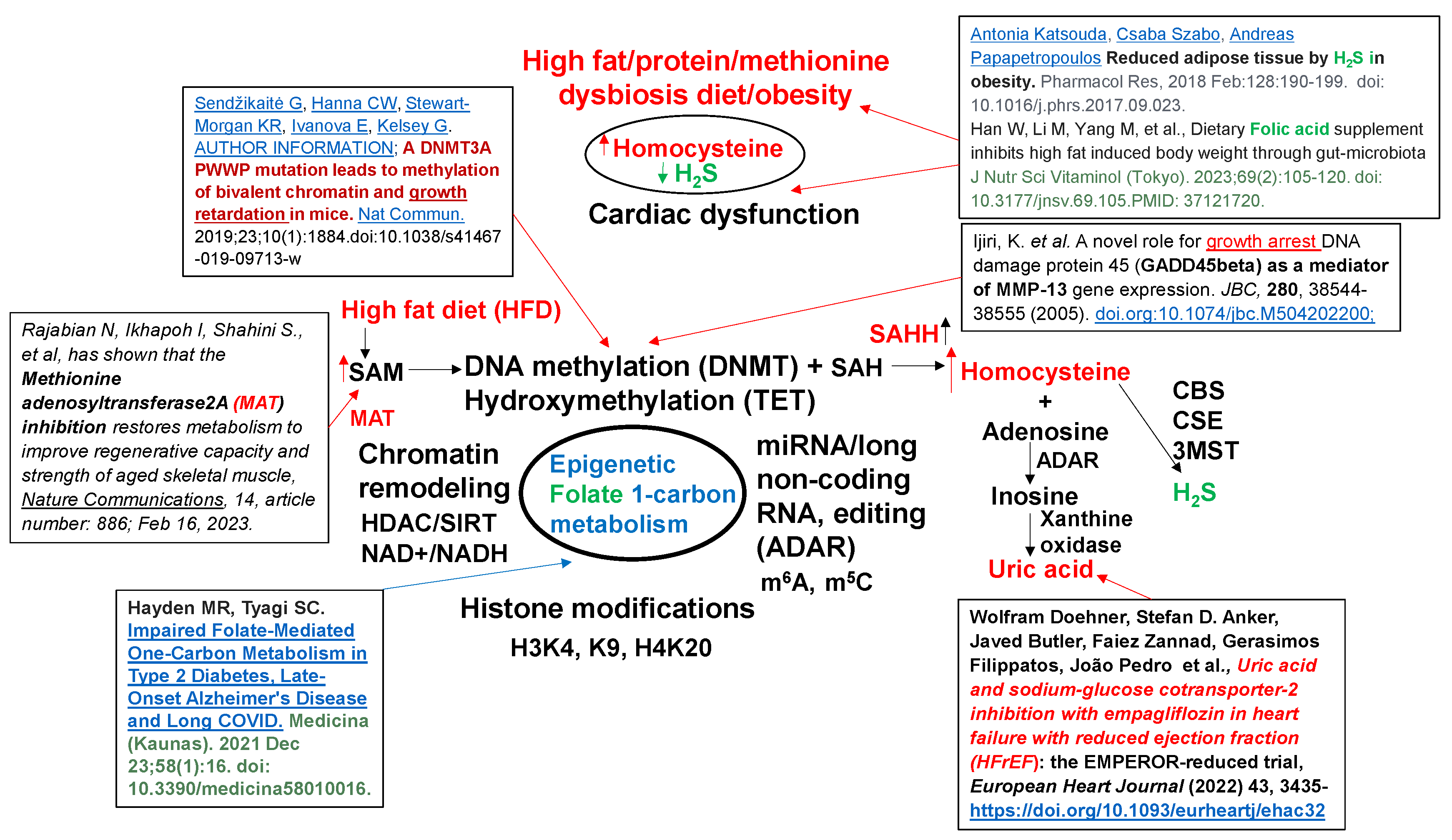
The methionine (Met) is a substrate for epigenetic DNA/RNA/protein, histone methylation and generates homocysteine (Hcy) via SAM/SAH/SAHH pathways. Hcy then get converted to H2S via transsulfuration 3MST/CBS/CSE pathways [
9,
10,
11]
. The inhibition of methyl transferase restores metabolism and improves regenerative capacity of the muscle [
12]. The mutation in DNMT causes hypermethylation and causes growth retardation [
13]. The accumulation of by-product uric acid causes HFrEF [
14]. The activation of growth arrest DNA damage (GADD) is associated with activation of nuclear metalloproteinases [
15] (
Figure 2).
Although chronic high-animal fat diet (HFD) is associated with “single hit” heart failure [
16,
17,
18], the mechanism is unclear. There is ~1% of methionine (Met) in HFD [
19], which is significantly high for a single amino acid. Interestingly, Studies from our laboratory [
1,
2,
3,
4] observed increase in dysbiosis by HFD (i.e., increase in ratio between polyunsaturated fatty acid/monounsaturated fatty acid in WT mice+HFD). The treatment with lactobacillus-probiotic (PB, a folate and lactone-ketone body producer [
20,
21,
22] mitigated this increase in poly unsaturated fatty acid [
1,
2,
3,
4]. Due to high methionine, the gut-dysbiosis instigates de-arrangement in epigenetic rhythmic methylation/demethylation ratio on the DNA/RNA, and protein/histones, via DNMT1/TET2 ratio [
23] consequently, creates hyperhomocysteinemia (HHcy) [
1,
2,
3,
4,
8]. Previous studies from our laboratory, using a single cell analysis, revealed increase in
SLC25A (transporter of s-adenosine-methionine (SAM)) during elevated levels of homocysteine (Hcy) [
24], caused by impaired recycling of homocysteine back to methionine by increase in epigenetic methylation on H3K4, K9, H4K20) via folate 1-carbon
metabolism (FOCM) pathway by gene writer (DNMT) and decrease in eraser (TET/FTO) [Figure 2].
Although clinical trial shows that microbiome metabolizes cholesterol/lipids [
25,
26] and lactobacillus elicited cardiac benefits [
27], the mechanism is unclear. It is unclear whether treatment with
Lactobacillus rhamnosus, a ketone body fuel for mitochondria [
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38] and a folic acid producing probiotic [
20,
21,
22] reverses the dysbiosis-induced cardiac complications, in part, by increasing mitochondrial transsulfuration, H
2S and bioenergetics [
39] (Fig 3). This study will determine for the first time that a probiotic can increase mitochondrial sulfur metabolism, transsulfuration and bioenergetics and mitigates HFD-induced obesity and heart failure.
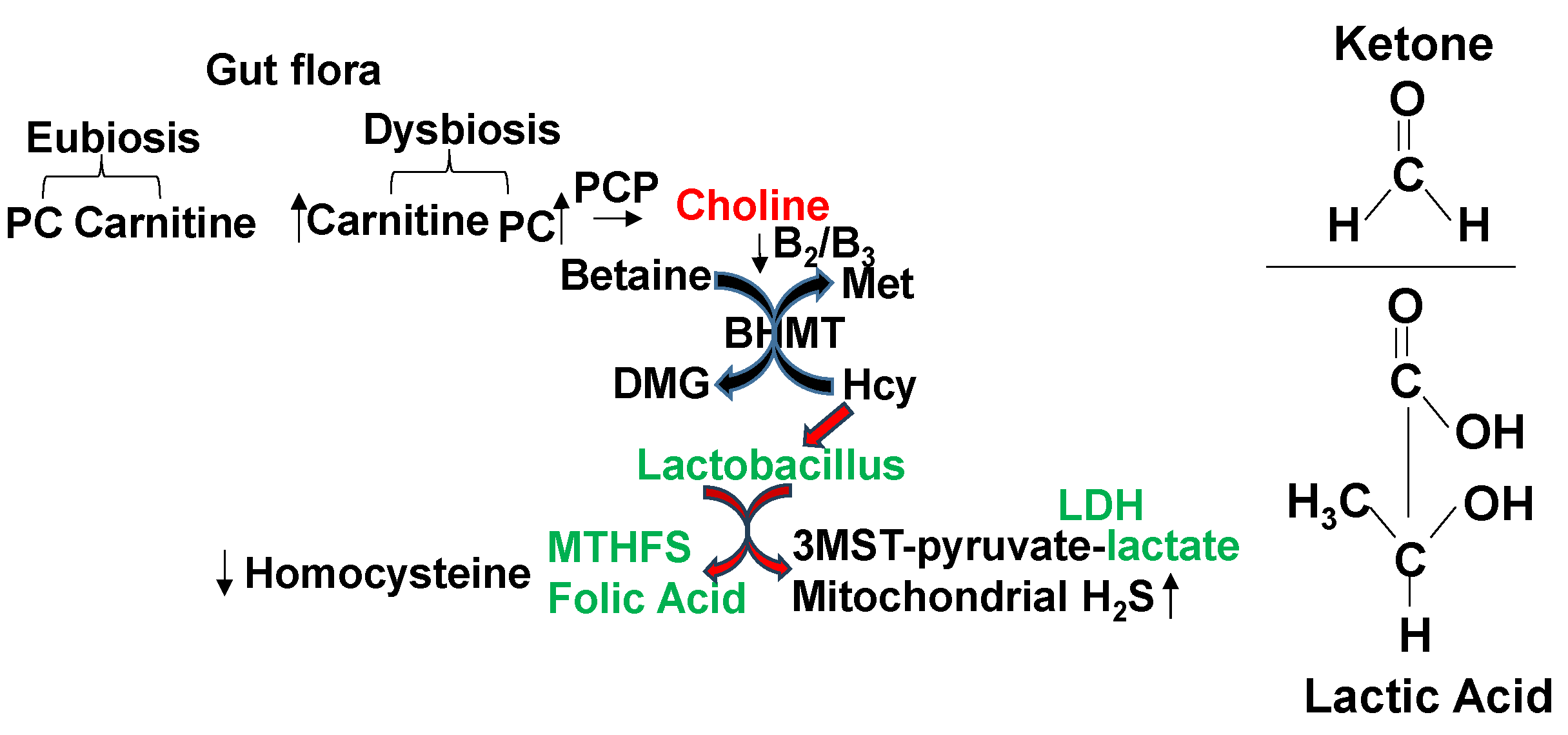
Gut dysbiosis and FOCM, methylation, and HHcy, via DNMT, BHMT, and PEMT and link to microbiome. We suggested an increase in DNMT and PEMT and decrease in BHMT in HFD fed mice [
1,
2,
3,
4]. The treatment with probiotic decreased levels of DNMT and PEMT, but there was no change in BHMT [
1,
2,
3,
4]. These results suggested differential mechanisms i.e., betaine versus folate pathways of re-methylation of Hcy [
40,
41,
42] (
Figure 4). Interestingly, this suggested a prominent central role of DNMT1 in Hcy formation by HFD. This incited us to employ DNMT1knock out mice. Therefore, the
premise of this proposal is that gut-dysbiosis, and altered Hcy metabolism represents the dominant mechanism wherein HFD induces DNMT1 causing detrimental cardiac remodeling and mitochondrial metabolic dysfunction (i.e., transsulfuration and bioenergetics).
Figure 3.
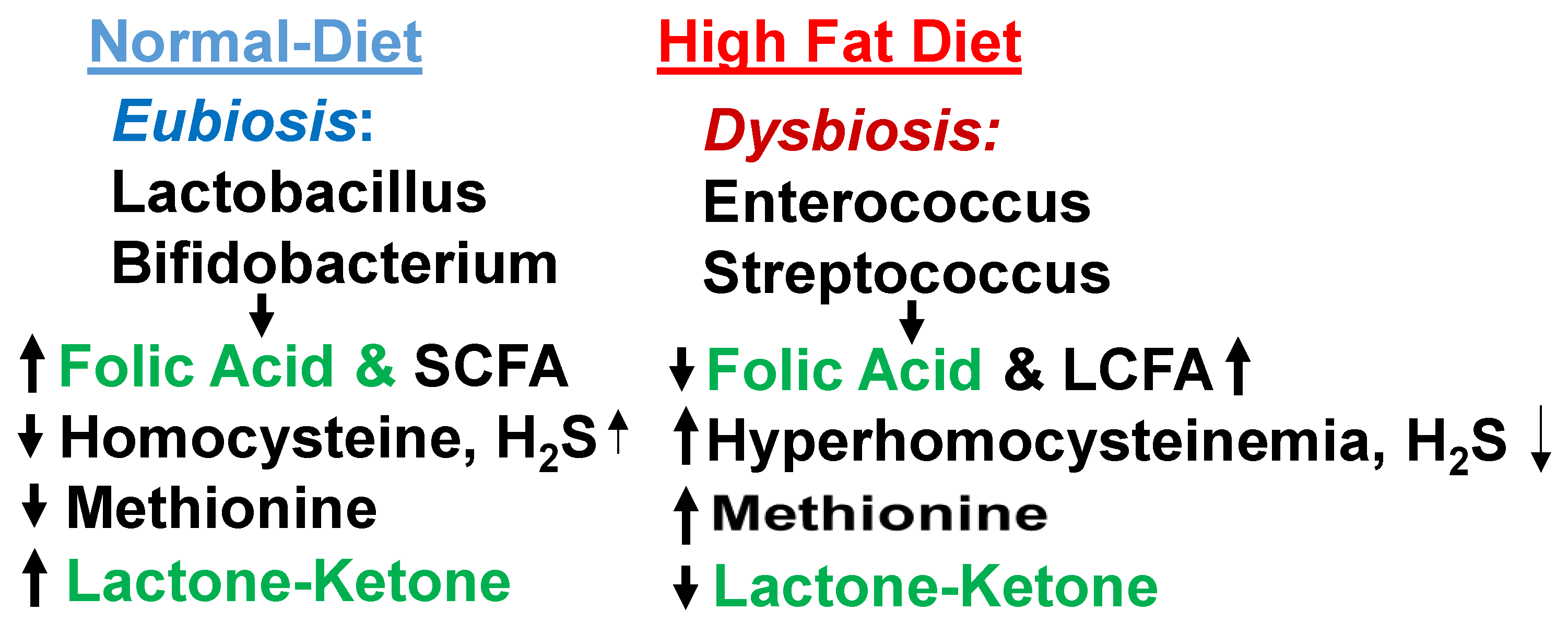
Normal eubiosis contains beneficial microbiome, lactobacillus, SCFA. Whereas dysbiosis contains enterococcus and LCFA. Therefore, it is important to increase eubiosis by lactobacillus.
Figure 3.
Normal eubiosis contains beneficial microbiome, lactobacillus, SCFA. Whereas dysbiosis contains enterococcus and LCFA. Therefore, it is important to increase eubiosis by lactobacillus.
Figure 4.
A link between gut-dysbiosis and decrease in betaine homocysteine methyltransferase (BHMT)-dependent re-methylation of homocysteine. Interestingly, lactobacillus produces folate and ketone/lactate, post-biotically, therefore decreases Hcy and increases mitochondrial bioenergetics, because lactate/ketone fuel for mitochondria increases H2S and mitigates HFD-induced cardiac dysfunction.
Figure 4.
A link between gut-dysbiosis and decrease in betaine homocysteine methyltransferase (BHMT)-dependent re-methylation of homocysteine. Interestingly, lactobacillus produces folate and ketone/lactate, post-biotically, therefore decreases Hcy and increases mitochondrial bioenergetics, because lactate/ketone fuel for mitochondria increases H2S and mitigates HFD-induced cardiac dysfunction.
Others [
7,
43] have also supported our hypothesis that there is a rhythmic methylation/demethylation during mitochondrial TCA cycle by epigenetic gene writer (DNMT) and erasers (TET and FTO). The premise of this study is also supported by Kay et al [
44,
45], and Engler et al [
46,
47,
48] that reveal the role of transforming fibroblasts, and fibrosis in cardiac remodeling. The study by Dees et al [
49] suggests that DNA-methyltransferase 3A (DNMT3A), and DNMT1 in fibroblasts in a SMAD-dependent manner silence the expression of suppressor of cytokine signaling 3 (SOCS3) by promoter hypermethylation. Downregulation of SOCS3 facilitated activation of signal transducers, and activators of transcription 3 (STAT3) to promote fibroblast-to–myofibroblast transition, collagen release, and fibrosis
in vitro and
in vivo. Re-establishment of the epigenetic control of STAT3 signaling by genetic or pharmacological inactivation of DNMT3A reversed the phenotype of fibroblasts in tissue culture, inhibited TGFb-dependent fibroblast activation, and ameliorated experimental fibrosis in murine models [
49,
50,
51]. Felisbino, and McKinsey suggested the role of epigenetics in cardiac fibrosis with emphasis on inflammation, and fibroblast activation [
52,
53]. The authors demonstrated the role of histone acetylation, and reader proteins using small molecule inhibitor of bromo-domain containing protein 4 (BRD4) for mitigating cardiac fibrosis [
54]. Interestingly, along the same line, we demonstrated that Hcy induces collagen expression in a dose- and time-dependent manner as measured by Northern blot analysis [
55]. Hcy causes fibroblast activation, and myofibroblast differentiation in murine aortic endothelial cells [
56]. Previously, in human, we show the role of a dis-integrin and metalloproteinase (ADAM) in connexin-43 (Cx43) degradation in human end stage heart failure [
57]. Others have shown that Cx-43 synchronizes myocyte-mitochondrial function [
58,
63]. Here, we focus on the significance of epigenetics via gene writer, eraser, and reader function along with direct consequences of Hcy in cardiac remodeling.
Homocysteine and Lipid Connection:
Most of the human population is mildly HHcy, and asymptomatic, however, if on an HFD, especially red meat rich diet that is high in methionine (a substrate for Hcy generation during epigenetically regulated DNA methylation) [
1,
2,
3,
4], develop severe HHcy, and that will ultimately cause symptomatic cardiovascular diseases. Interestingly, although folic acid and H
2S reduce obesity and brown the adipose fat [
59,
60,
61,
62], the mechanism is unclear.
The high fat dysbiosis diet leads to cardiovascular diseases (CVD). Interestingly, according to the homocysteine theory, the diet one high in animal protein and low in B-vitamins, which occur in many foods but are very easily destroyed by processing – a dirt of meat, cheese, milk, white flour, and foods that are canned, boxed, refined, processed, or preserved. This suggests a strong connection between diet and CVD, but one that is different path from cholesterol/lipids. The homocysteine theory considers CVD of what McCully call protein intoxication. The cholesterol theory (sometimes called the lipid theory) instead demonizes fats. Since proteins and fats often occur in the same foods, the potential dietary treatments for high homocysteine and high cholesterol are similar, with this distinction: the anti-homocysteine diet focuses on what should be eaten, as a preventive, while the anti-cholesterol diet focuses on what should be avoided; as a precipitator. Thus, a diet of lower homocysteine would include many natural sources of B-vitamins like fresh fruits and vegetables and would limit animal protein. The cholesterol-reducing diet would limit foods high in saturated fats and cholesterol, like eggs, meat, and butter. Unfortunately, the latter is more commercial popular. Therefore, we use lactobacillus that produces folic acid and lactone-ketone bodies, food for mitochondria, post-biotically.
The probiotic, post-biotically producing folic acid and lactone-ketone body, mitigate HFD-induced heart failure (
Figure 4) is therapeutically innovative. (i.e., killing two pathologies by one strike). Interestingly, some probiotics also lower cholesterol [
26], however, the mechanism is unclear. In this novel proposal we will address that the lactobacillus eats lipids and post-biotically produces folic acid and lactone-ketone body, a bi-directional probiotic that mitigates HFD-induced mitochondrial remodeling and heart failure
Conclusions and Future Directions:
This review presents an innovative hypothesis to test the role of “single hit” HFD in the development of CHF through intestinal dysbiosis-induced HHcy, which is a consequence of the disruption of “folate 1-carbon metabolism” by HFD via modulation of epigenetics (DNA hypermethylation and HHcy). In addition, the therapeutic effect of probiotic treatment will be tested to reverse these effects. Elucidation of the mechanisms through which diet (and particularly HFD) and dysbiotic events in distant organs such as the intestine affect heart health is important, and positive findings will support the relevance and impact of systemic health in heart health. Although use of probiotics as a therapeutic alternative for heart disease has been previously studied in pre-clinical models and clinical trials, however, there is no mechanism-based simple and safe therapy of probiotics and here we propose that a probiotic, that produces folic acid (a Hcy lowering agent) and lactate (ketone-body, fuel for mitochondria) post-biotically, can eat fat and increases mitochondrial bioenergetics.
Based on this review it is important to determine whether the lactobacillus downregulates SLC25A and epigenetic gene writer DNMT1, upregulates eraser TET2/FTO, and attenuates cardiac dysfunction by lowering homocysteine. The lactobacillus post biotically produces folic acid and attenuates HHcy by decrease in epigenetic gene writer (DNMT1) and increase in eraser (TET2/FTO. Also, it will be novel to determine whether the lactobacillus increases SLC7A and mitochondrial H
2S production by transsulfuration. Lactobacillus post-biotically produces lactone/ketone body and improves mitochondrial bioenergetics via transsulfuration
(Figure 5). The use of DNMT1KO and mitigation of HFD-induced epigenetic de-arrangement is novel. The use of transgenic mice over expressing TET2, FTO (erasers), CBS (transsulfuration) and TFAM (mitochondrial) is mechanistically innovative.
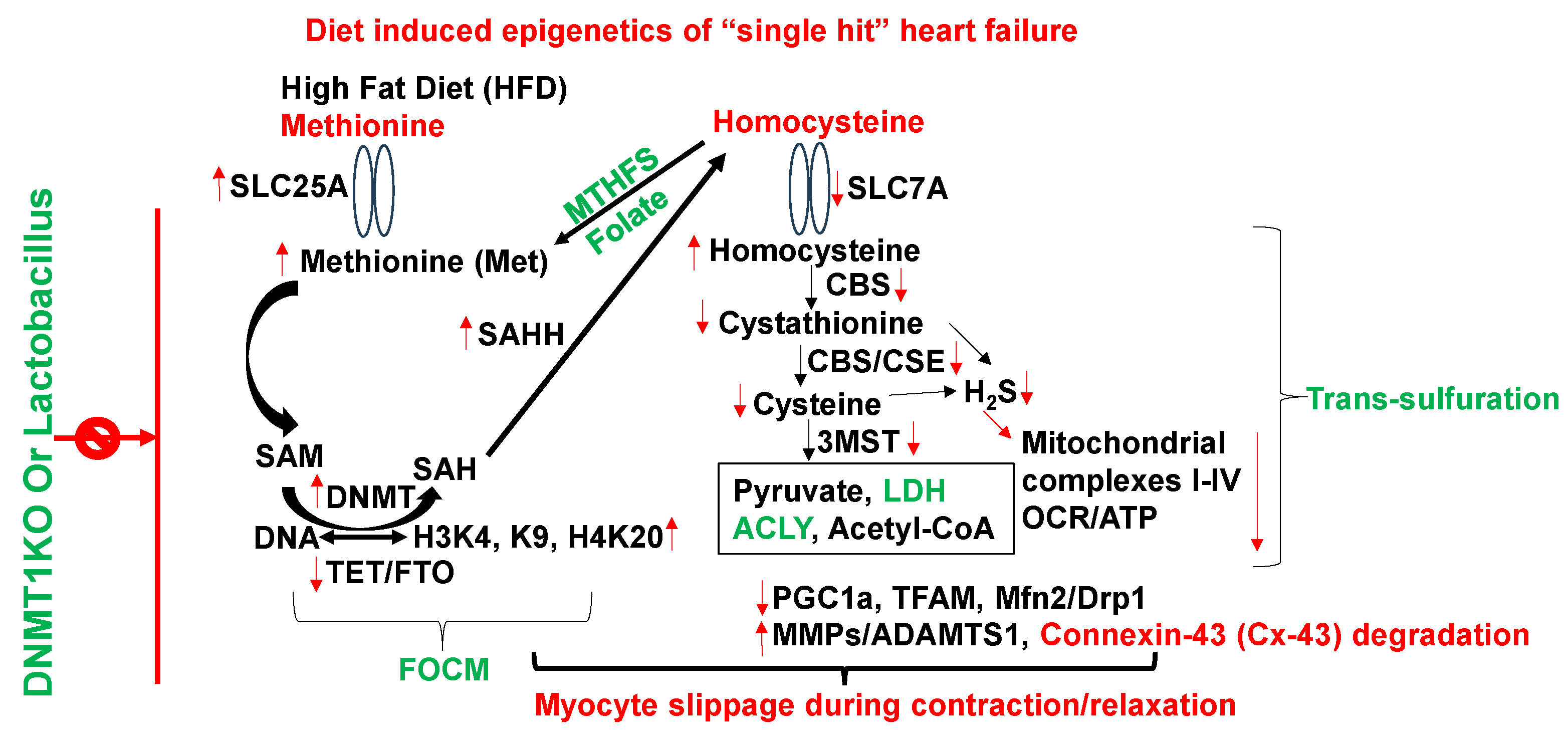
Figure 5.
The hypothesis is that dysbiotic HFD increases transporter SLC25A and rhythmic i.e., methylation of histone lysine by gene writer, eraser, creating hyperhomocysteinemia (HHcy). The transporter SLC7A is decreased, causing disruption in mitochondrial sulfur metabolism H2S. The probiotic lactobacillus post-biotically produces folic acid and lactone-ketone body, converting Hcy back to methionine, and lactone (fuel for mitochondria), respectively, improving mitochondrial bioenergetics.
Figure 5.
The hypothesis is that dysbiotic HFD increases transporter SLC25A and rhythmic i.e., methylation of histone lysine by gene writer, eraser, creating hyperhomocysteinemia (HHcy). The transporter SLC7A is decreased, causing disruption in mitochondrial sulfur metabolism H2S. The probiotic lactobacillus post-biotically produces folic acid and lactone-ketone body, converting Hcy back to methionine, and lactone (fuel for mitochondria), respectively, improving mitochondrial bioenergetics.
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
*A part of this study was supported by NIH grants AR-71789, HL139047, and DK116591.
References
- Veeranki, S. & Tyagi, S. C. Dysbiosis and Disease: Many Unknown Ends, Is It Time to Formulate Guidelines for Dysbiosis Research? Journal of cellular physiology 232, 2929-2930 (2017). [CrossRef]
- Singh, M. et al. Epigenetics, 1-Carbon Metabolism, and Homocysteine During Dysbiosis. Front Physiol 11, 617953 (2020). [CrossRef]
- George, A. K. et al. Dysbiotic 1-carbon metabolism in cardiac muscle remodeling. Journal of cellular physiology 235, 2590-2598 (2020). [CrossRef]
- Singh M, Pushpakumar S, Zheng Y, Homme RP, Smolenkova I, Mokshagundam SPL, Tyagi SC. Hydrogen sulfide mitigates skeletal muscle mitophagy-led tissue remodeling via epigenetic regulation of the gene writer and eraser function. Physiol Rep. 2022 Aug;10(16):e15422. [CrossRef] [PubMed]
- Meddeb M, Koleini N, Kass DA, et al., ATP citrate lyase supports cardiac function and NAD+/NADH balance and os depressed in human heart failure., June 10, 2024, bioRxiv. [CrossRef]
- Liu S, Gammon ST, Tan L, Karlstaedt A, ATP-dependent citrate lyase drives LV dysfunction by metabolic remodeling of the heart. bioRxiv, 2024 June 21:2024.06.21.600099. [CrossRef]
- Arnold, P. K. & Finley, L. W. S. Regulation and function of the mammalian tricarboxylic acid cycle. The Journal of biological chemistry 299, 102838 (2023). [CrossRef]
- Greco, C. M. et al. S-adenosyl-l-homocysteine hydrolase links methionine metabolism to the circadian clock and chromatin remodeling. Sci Adv 6 (2020). [CrossRef]
- Hayden MR, Tyagi SC.Impaired Folate-Mediated One-Carbon Metabolism in Type 2 Diabetes, Late-Onset Alzheimer's Disease and Long COVID. Medicina (Kaunas). 2021 Dec 23;58(1):16. [CrossRef]
- Antonia Katsouda, Csaba Szabo, Andreas Papapetropoulos Reduced adipose tissue by H2S in obesity. Pharmacol Res, 2018 Feb:128:190-199. [CrossRef]
- Han W, Li M, Yang M, et al., Dietary Folic acid supplement inhibits high fat induced body weight through gut-microbiota J Nutr Sci Vitaminol (Tokyo). 2023;69(2):105-120. [CrossRef] [PubMed]
- Rajabian, N. et al. Methionine adenosyltransferase2A inhibition restores metabolism to improve regenerative capacity and strength of aged skeletal muscle. Nat Commun 14, 886 (2023). [CrossRef]
- Sendžikaitė G, Hanna CW, Stewart-Morgan KR, Ivanova E, Kelsey G. AUTHOR INFORMATION; A DNMT3A PWWP mutation leads to methylation of bivalent chromatin and growth retardation in mice. Nat Commun. 2019;23;10(1). [CrossRef]
-
Wolfram Doehner, Stefan D. Anker, Javed Butler, Faiez Zannad, Gerasimos Filippatos, João Pedro et al., Uric acid and sodium-glucose cotransporter-2 inhibition with empagliflozin in heart failure with reduced ejection fraction (HFrEF): the EMPEROR-reduced trial, European Heart Journal (2022) 43, 3435-
. [CrossRef]
- Ijiri, K.; et al. Ijiri, K. et al. A novel role for growth arrest DNA damage protein 45 (GADD45beta) as a mediator of MMP-13 gene expression. JBC, 280, 38544-38555 (2005). [CrossRef]
- Saleem, T. H., Algowhary, M., Kamel, F. E. M. & El-Mahdy, R. I. Plasma amino acid metabolomic pattern in heart failure patients with either preserved or reduced ejection fraction: The relation to established risk variables and prognosis. Biomed Chromatogr 35, e5012 (2021). [CrossRef]
- Liu, B. et al. A novel rat model of heart failure induced by high methionine diet showing evidence of association between hyperhomocysteinemia and activation of NF-kappaB. Am J Transl Res 8, 117-124 (2016).
- Joseph, J. et al. Associations of methyl donor and methylation inhibitor levels during anti-oxidant therapy in heart failure. J Physiol Biochem 77, 295-304 (2021). [CrossRef]
- Service, N. H. Meat in your diet, (2021) & Tyagi SC. Clinical & Exper Hypertension, 21:181-198. 1999.
- Albano, C., Silvetti, T. & Brasca, M. Screening of lactic acid bacteria producing folate and their potential use as adjunct cultures for cheese bio-enrichment. FEMS Microbiol Lett 367 (2020). [CrossRef]
- Guerrero-Encinas, I. et al. Protective Effect of Lacticaseibacillus casei CRL 431 Postbiotics on Mitochondrial Function and Oxidative Status in Rats with Aflatoxin B(1)-Induced Oxidative Stress. Probiotics Antimicrob Proteins 13, 1033-1043 (2021). [CrossRef]
- Gu, Y. et al. Lactobacillus plantarum dy-1 fermented barley extraction activates white adipocyte browning in high-fat diet-induced obese rats. J Food Biochem 45, e13680 (2021). [CrossRef]
- Hong, T. et al. TET2 modulates spatial relocalization of heterochromatin in aged hematopoietic stem and progenitor cells. Nat Aging 3, 1387-1400 (2023). [CrossRef]
- Singh, M. et al. Circular RNAs profiling in the cystathionine-beta-synthase mutant mouse reveals novel gene targets for hyperhomocysteinemia induced ocular disorders. Exp Eye Res 174, 80-92 (2018). [CrossRef]
- Romão da Silva LF, de Oliveira Y, de Souza EL, de Luna Freire MO, Braga VA, Magnani M, de Brito Alves JL. Effects of probiotic therapy on cardio-metabolic parameters and autonomic modulation in hypertensive women: a randomized, triple-blind, placebo-controlled trial. Food Funct. 2020 Aug 19;11(8):7152-7163. [CrossRef] [PubMed]
- Chenhao Li, Martin Stražar, Ahmed M T Mohamed, Julian A Pacheco, Rebecca L Walker, et al., Gut microbiome and metabolome profiling in Framingham heart study reveals cholesterol-metabolizing bacteria, Cell. 2024 Apr 11;187(8):1834-1852.e19. [CrossRef]
- Romao da Silva, L. F. et al. Effects of probiotic therapy on cardio-metabolic parameters and autonomic modulation in hypertensive women: a randomized, triple-blind, placebo-controlled trial. Food Funct 11, 7152-7163 (2020). [CrossRef]
- Guerrero-Encinas, I. et al. Protective Effect of Lacticaseibacillus casei CRL 431 Postbiotics on Mitochondrial Function and Oxidative Status in Rats with Aflatoxin B(1)-Induced Oxidative Stress. Probiotics Antimicrob Proteins 13, 1033-1043 (2021). [CrossRef]
- Gu, Y. et al. Lactobacillus plantarum dy-1 fermented barley extraction activates white adipocyte browning in high-fat diet-induced obese rats. J Food Biochem 45, e13680 (2021). [CrossRef]
- Aubert, G.; et al. Aubert, G. et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation 133, 698-705 (2016). [CrossRef]
- Panigrahi, P. et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 548, 407-412 (2017). [CrossRef]
- Brooks, G. A. et al. Lactate as a myokine and exerkine: drivers and signals of physiology and metabolism. Journal of applied physiology 134, 529-548 (2023). [CrossRef]
- Matsuura, T. R., Puchalska, P., Crawford, P. A. & Kelly, D. P. Ketones and the Heart: Metabolic Principles and Therapeutic Implications. Circulation research 132, 882-898 (2023). [CrossRef]
- Gumus Balikcioglu, P. et al. Branched-chain alpha-keto acids and glutamate/glutamine: Biomarkers of insulin resistance in childhood obesity. Endocrinol Diabetes Metab 6, e388 (2023). [CrossRef]
- Chu, H. N., Kim, H. R., Jang, K. A., Hwang, Y. J. & Kim, J. S. Anti-obesity effects of yuja (Citrus junos Sieb ex Tanaka) pomace extract fermented with lactic acid bacteria on the hepatocytes and epididymal fat tissue of rats. Appl Microsc 53, 7 (2023). [CrossRef]
- Matsuura TR, Puchalska P, Crawford PA, Kelly DP. Ketones and the Heart: Metabolic Principles and Therapeutic Implications. Circ Res. 2023 Mar 31;132(7):882-898. [CrossRef] [PubMed]
- Gumus Balikcioglu P, Jachthuber Trub C, Balikcioglu M, Ilkayeva O, White PJ, Muehlbauer M, Bain JR, Armstrong S, Freemark M. Branched-chain α-keto acids and glutamate/glutamine: Biomarkers of insulin resistance in childhood obesity. Endocrinol Diabetes Metab. 2023 Jan;6(1):e388. [CrossRef]
- Chu HN, Kim HR, Jang KA, Hwang YJ, Kim JS. Anti-obesity effects of yuja (Citrus junos Sieb ex Tanaka) pomace extract fermented with lactic acid bacteria on the hepatocytes and epididymal fat tissue of rats. Appl Microsc. 2023 Aug 9;53(1):7. [CrossRef] [PubMed]
- Gu, Y. et al. Lactobacillus plantarum dy-1 fermented barley extraction activates white adipocyte browning in high-fat diet-induced obese rats. J Food Biochem 45, e13680 (2021). [CrossRef]
-
Schaevitz L, Berger-Sweeney J, Ricceri L. One-carbon metabolism in neurodevelopmental disorders: using broad-based nutraceutics to treat cognitive deficits in complex spectrum disorders. Neurosci Biobehav Rev. 2014 Oct;46 Pt 2:270-84. [CrossRef]
-
Gumus Balikcioglu P, Jachthuber Trub C, Balikcioglu M, Ilkayeva O, White PJ, Muehlbauer M, Bain JR, Armstrong S, Freemark M. Branched-chain α-keto acids and glutamate/glutamine: Biomarkers of insulin resistance in childhood obesity. Endocrinol Diabetes Metab. 2023 Jan;6(1):e388. [CrossRef] [PubMed]
- Han-Na Chu, Haeng-Ran Kim, Kyeong-A Jang, Yu-Jin Hwang, Jeong-Sang Kim Anti-obesity effects of yuja (Citrus junos Sieb ex Tanaka) pomace extract fermented with lactic acid bacteria on the hepatocytes and epididymal fat tissue of rats, Appl Microsc, 2023 Aug 9;53(1):7. [CrossRef] [PubMed]
- Tóth ME, Sárközy M, Szűcs G, Dukay B, Hajdu P, Zvara Á, Puskás LG, Szebeni GJ, Ruppert Z, Csonka C, Kovács F, Kriston A, Horváth P, Kővári B, Cserni G, Csont T, Sántha M. Exercise training worsens cardiac performance in males but does not change ejection fraction and improves hypertrophy in females in a mouse model of metabolic syndrome. Biol Sex Differ. 2022 Jan 31;13(1):5. As there is a close link between MetS and cardiovascular diseases, we aimed to investigate the sex-based differences in MetS-associated heart failure (HF) and cardiovascular response to regular exercise training (ET). METHODS: High-fat diet-fed …. [CrossRef] [PubMed]
- Entcheva, E. & Kay, M. W. Cardiac optogenetics: a decade of enlightenment. Nat Rev Cardiol 18, 349-367 (2021). [CrossRef]
- Zasadny, F. M., Dyavanapalli, J., Dowling, N. M., Mendelowitz, D. & Kay, M. W. Cholinergic stimulation improves electrophysiological rate adaptation during pressure overload-induced heart failure in rats. American journal of physiology. Heart and circulatory physiology 319, H1358-1368 (2020). [CrossRef]
- Whitehead, A. J. & Engler, A. J. Regenerative cross talk between cardiac cells and macrophages. American journal of physiology. Heart and circulatory physiology 320, H2211-H2221 (2021). [CrossRef]
- Yeoman, B. et al. Adhesion strength and contractility enable metastatic cells to become adurotactic. Cell Rep 34, 108816 (2021). [CrossRef]
- Banisadr, A. et al. EGFRvIII uses intrinsic and extrinsic mechanisms to reduce glioma adhesion and increase migration. J Cell Sci 133 (2020). [CrossRef]
- Dees, C. et al. TGF-beta-induced epigenetic deregulation of SOCS3 facilitates STAT3 signaling to promote fibrosis. The Journal of clinical investigation 130, 2347-2363 (2020). [CrossRef]
- Kakoki, M. et al. Cyanocobalamin prevents cardiomyopathy in type 1 diabetes by modulating oxidative stress and DNMT-SOCS1/3-IGF-1 signaling. Commun Biol 4, 775 (2021). [CrossRef]
- Kanai, S. M., Edwards, A. J., Rurik, J. G., Osei-Owusu, P. & Blumer, K. J. Proteolytic degradation of regulator of G protein signaling 2 facilitates temporal regulation of G(q/11) signaling and vascular contraction. The Journal of biological chemistry 292, 19266-19278 (2017). [CrossRef]
- Felisbino, M. B. & McKinsey, T. A. Epigenetics in Cardiac Fibrosis: Emphasis on Inflammation and Fibroblast Activation. JACC Basic Transl Sci 3, 704-715 (2018). [CrossRef]
- Trial, J. & Cieslik, K. A. Changes in cardiac resident fibroblast physiology and phenotype in aging. American journal of physiology. Heart and circulatory physiology 315, H745-H755 (2018). [CrossRef]
- Stratton, M. S. et al. Dynamic Chromatin Targeting of BRD4 Stimulates Cardiac Fibroblast Activation. Circulation research 125, 662-677 (2019). [CrossRef]
- Tyagi, S. C. Homocysteine redox receptor and regulation of extracellular matrix components in vascular cells. The American journal of physiology 274, C396-405 (1998). [CrossRef]
- Sen, U., Moshal, K. S., Tyagi, N., Kartha, G. K. & Tyagi, S. C. Homocysteine-induced myofibroblast differentiation in mouse aortic endothelial cells. Journal of cellular physiology 209, 767-774 (2006). [CrossRef]
- Hunt MJ, Aru GM, Hayden MR, Moore CK, Hoit BD, Tyagi SC. Induction of oxidative stress and disintegrin metalloproteinase in human heart end-stage failure. Am J Physiol Lung Cell Mol Physiol. 2002 Aug;283(2):L239-45. [CrossRef] [PubMed]
- Miro-Casas, E., Ruiz-Meana, M., Agullo, E., Stahlhofen, S., Rodriguez-Sinovas, A., Cabestrero, A., Jorge, I., Torre, I., Vazquez, J., Boengler, K., Schulz, R., Heusch, G., and Garcia-Dorado, D. (2009) Connexin43 in cardiomyocyte mitochondria contributes to mitochondrial potassium uptake. Cardiovascular research 83, 747-756. [CrossRef]
- Katsouda, A., Szabo, C. & Papapetropoulos, A. Reduced adipose tissue H(2)S in obesity. Pharmacol Res 128, 190-199 (2018). [CrossRef]
- Han, W. et al. Dietary Folic Acid Supplementation Inhibits HighFat DietInduced Body Weight Gain through Gut Microbiota-Associated Branched-Chain Amino Acids and Mitochondria in Mice. J Nutr Sci Vitaminol (Tokyo) 69, 105-120 (2023). [CrossRef]
- Antonia Katsouda, Csaba Szabo, Andreas Papapetropoulos Reduced adipose tissue by H2S in obesity. Pharmacol Res, 2018 Feb:128:190-199. [CrossRef]
- Han W, Li M, Yang M, Chen S, Lu Y, Tang T, Wang R, Zhang C, Qi K. Dietary Folic Acid Supplementation Inhibits High Fat Diet Induced Body Weight Gain through Gut Microbiota-Associated Branched-Chain Amino Acids and Mitochondria in Mice. J Nutr Sci Vitaminol (Tokyo). 2023;69(2):105-120. Free article. The effects of folic acid on body weight gain in obesity and gut microbiota-associated branched-chain amino acids (BCAAs) and mitochondrial function were investigated. ...Therefore, dietary folic acid supplementation differentially affected body weight …. [CrossRef] [PubMed]
- Boengler, K., Ruiz-Meana, M., Gent, S., Ungefug, E., Soetkamp, D., Miro-Casas, E., Cabestrero, A., Fernandez-Sanz, C., Semenzato, M., Di Lisa, F., Rohrbach, S., Garcia-Dorado, D., Heusch, G., and Schulz, R. (2012) Mitochondrial connexin 43 impacts on respiratory complex I activity and mitochondrial oxygen consumption. Journal of cellular and molecular medicine 16, 1649-1655. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).