Submitted:
16 September 2024
Posted:
16 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Molecular Classification
3. Microsatellite Instability
4. The Mismatch Repair System
5. Deficiency of Mismatch Repair/Microsatellite Instability-High (dMMR/MSI-H) as Biomarkers for Immunotherapy in Endometrial Cancer
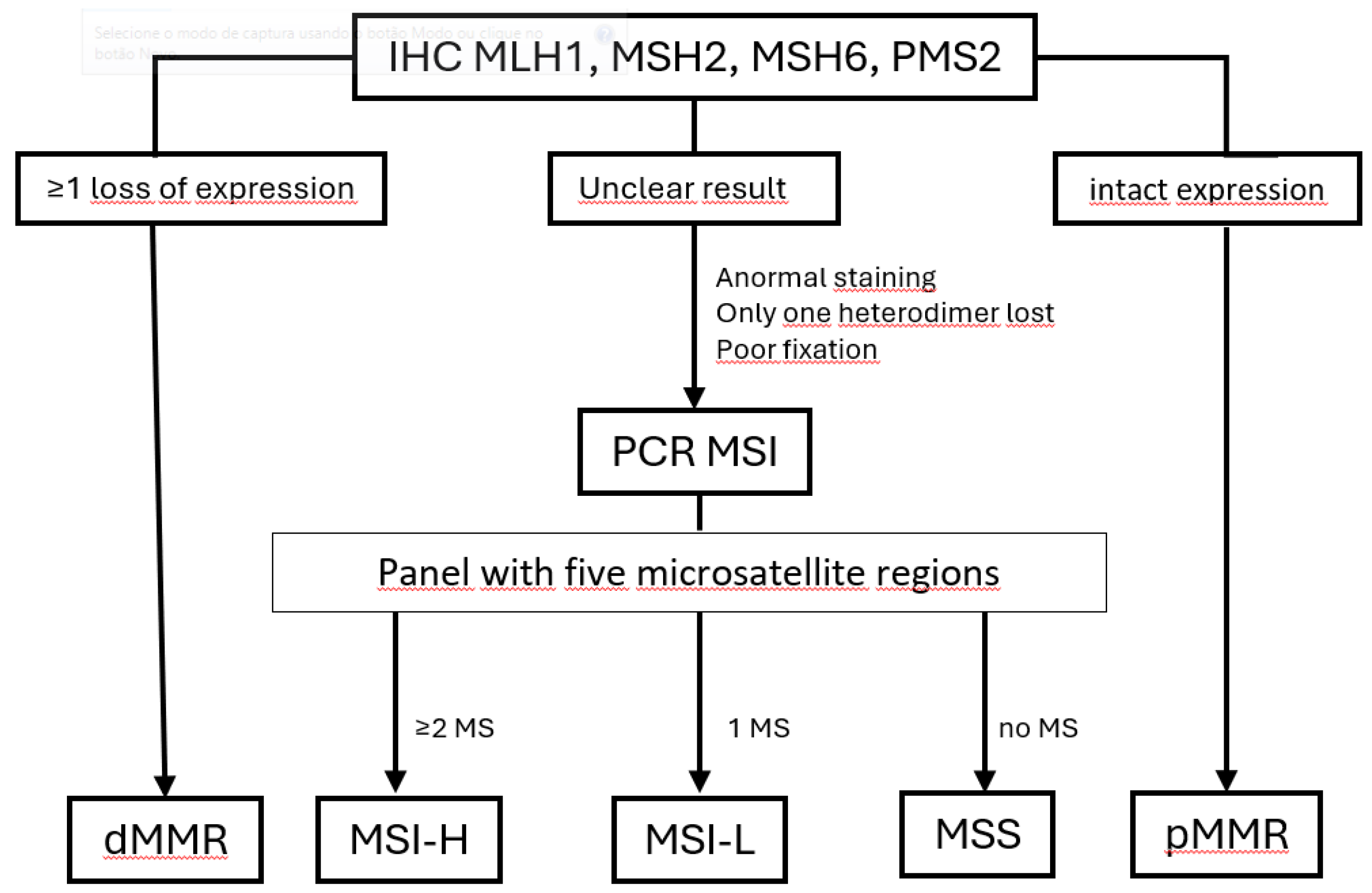
6. Methods Used to Identify dMMR/MSI-H
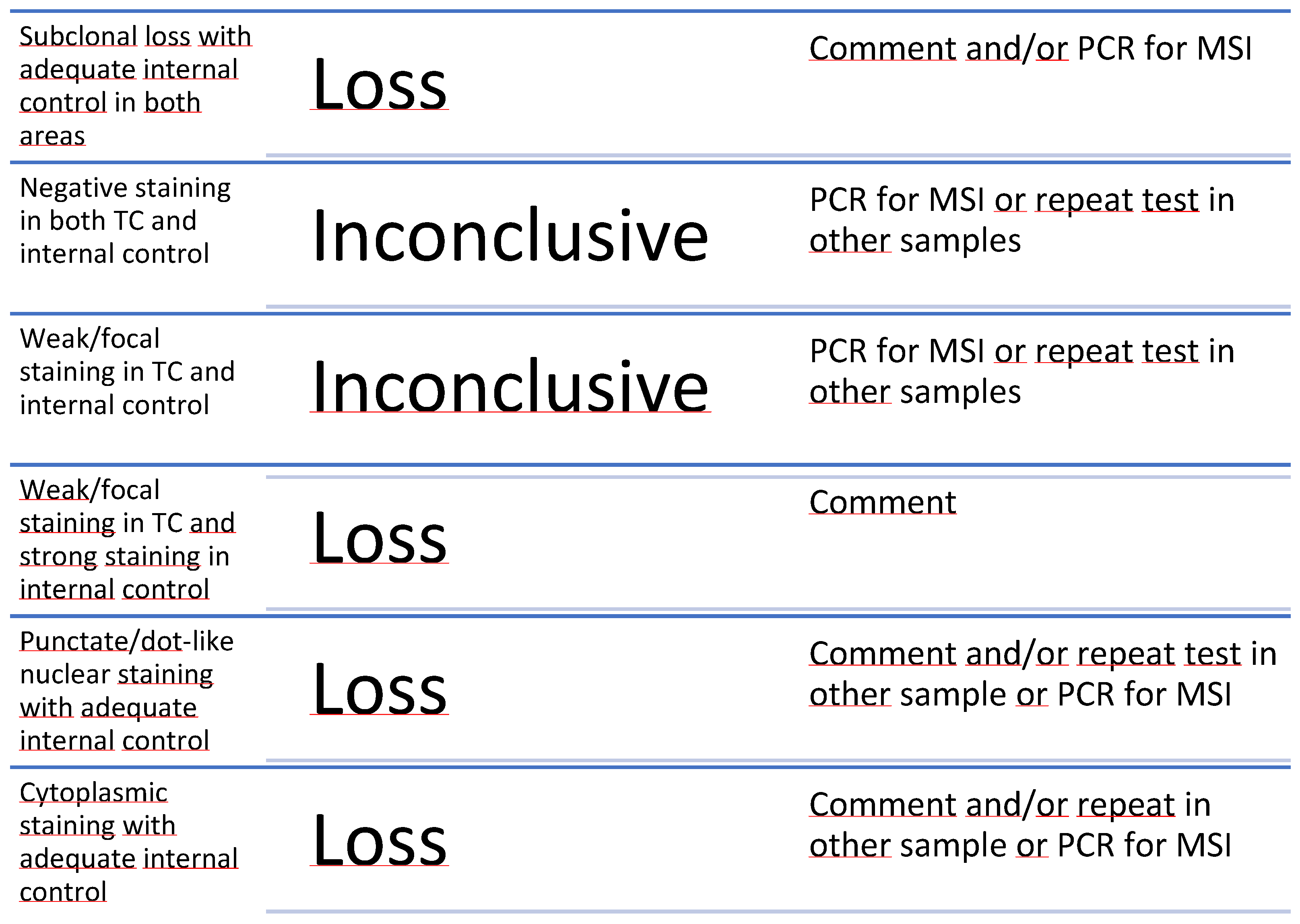
6.1. Challenges in Immunohistochemical for MMR Assessment
6.1.1. Tissue Processing and Handling
6.1.2. Interpretation
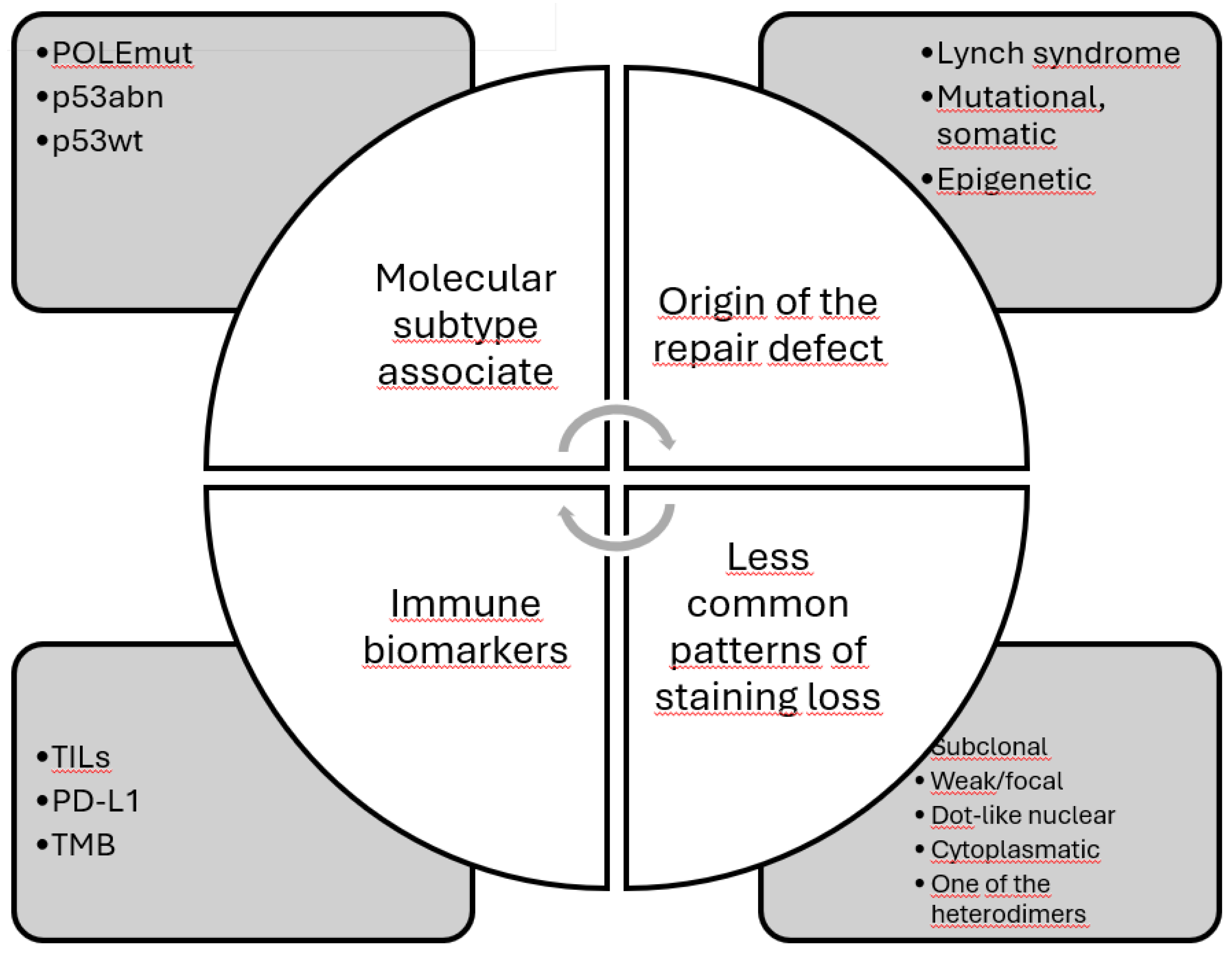
7. Heterogeneity of dMMR
7.1. Origin of the Defect
7.2. Mismatch Repair/Microsatellite Instability Discordance
7.3. Association with POLE Mutation and/or p53-Mutated
7.4. Association with Other Immune Biomarkers
8. Conclusions
9. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel RL, Miller KD, Wagle NS, Jemal A: Cancer statistics, 2023. CA Cancer J Clin. 2023, 73:17-48. [CrossRef]
- Bray F, Laversanne M, Sung H, et al.: Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024, 74:229-263. [CrossRef]
- SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. (2024). Accessed: 2024/07/14: https://seer.cancer.gov/statistics-network/explorer/.
- Anton C, Kleine RT, Mayerhoff E, et al.: Ten years of experience with endometrial cancer treatment in a single Brazilian institution: Patient characteristics and outcomes. PLoS One. 2020, 15:e0229543. [CrossRef]
- Miller DS, Filiaci VL, Mannel RS, et al.: Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209). J Clin Oncol. 2020, 38:3841-3850. [CrossRef]
- Kandoth C, Schultz N, Cherniack AD, et al.: Integrated genomic characterization of endometrial carcinoma. Nature. 2013, 497:67-73. [CrossRef]
- O’Malley DM, Bariani GM, Cassier PA, et al.: Pembrolizumab in Patients With Microsatellite Instability-High Advanced Endometrial Cancer: Results From the KEYNOTE-158 Study. J Clin Oncol. 2022, 40:752-761. [CrossRef]
- Bokhman JV: Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983, 15:10-17.
- Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R: Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982, 6:93-108. [CrossRef]
- Cancer Genome Atlas Research N, Kandoth C, Schultz N, et al.: Integrated genomic characterization of endometrial carcinoma. Nature. 2013, 497:67-73. [CrossRef]
- Kommoss S, McConechy MK, Kommoss F, et al.: Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol. 2018, 29:1180-1188. [CrossRef]
- Jamieson A, Thompson EF, Huvila J, et al.: Endometrial carcinoma molecular subtype correlates with the presence of lymph node metastases. Gynecol Oncol. 2022, 165:376-384. [CrossRef]
- Raffone A, Travaglino A, Gabrielli O, et al.: Clinical features of ProMisE groups identify different phenotypes of patients with endometrial cancer. Arch Gynecol Obstet. 2021, 303:1393-1400. [CrossRef]
- Raffone A, Travaglino A, Mascolo M, et al.: Histopathological characterization of ProMisE molecular groups of endometrial cancer. Gynecol Oncol. 2020, 157:252-259. [CrossRef]
- Léon-Castillo A: Update in the molecular classification of endometrial carcinoma. Int J Gynecol Cancer. 2023, 33:333-342. [CrossRef]
- Oaknin A, Bosse TJ, Creutzberg CL, et al.: Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022, 33:860-877. [CrossRef]
- Concin N, Matias-Guiu X, Vergote I, et al.: ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021, 31:12-39. [CrossRef]
- Berek JS, Matias-Guiu X, Creutzberg C, et al.: FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet. 2023, 162:383-394. [CrossRef]
- HUGO Gene Nomenclature Committee. Last update: 2024-07-09 edition. University of Cambridge.
- Gupta D, Heinen CD: The mismatch repair-dependent DNA damage response: Mechanisms and implications. DNA Repair (Amst). 2019, 78:60-69. [CrossRef]
- Bateman AC: DNA mismatch repair proteins: scientific update and practical guide. J Clin Pathol. 2021, 74:264-268. [CrossRef]
- FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. (2017). Accessed: 28/07/2024: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication.
- Marabelle A, Le DT, Ascierto PA, et al.: Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020, 38:1-10. [CrossRef]
- Ott PA, Bang YJ, Berton-Rigaud D, et al.: Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1-Positive Endometrial Cancer: Results From the KEYNOTE-028 Study. J Clin Oncol. 2017, 35:2535-2541. [CrossRef]
- Maio M, Ascierto PA, Manzyuk L, et al.: Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann Oncol. 2022, 33:929-938. [CrossRef]
- FDA approves pembrolizumab for advanced endometrial carcinoma. (2022). Accessed: 28/07/2024: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-advanced-endometrial-carcinoma.
- FDA grants regular approval to dostarlimab-gxly for dMMR endometrial cancer. (2023). Accessed: July 28, 2024, 2024: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-dostarlimab-gxly-dmmr-endometrial-cancer.
- Oaknin A, Tinker AV, Gilbert L, et al.: Clinical Activity and Safety of the Anti-Programmed Death 1 Monoclonal Antibody Dostarlimab for Patients With Recurrent or Advanced Mismatch Repair-Deficient Endometrial Cancer: A Nonrandomized Phase 1 Clinical Trial. JAMA Oncol. 2020, 6:1766-1772. [CrossRef]
- Oaknin A, Gilbert L, Tinker AV, et al.: Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET-a phase I, single-arm study. J Immunother Cancer. 2022, 10. [CrossRef]
- Makker V, Colombo N, Herráez AC, et al.: Lenvatinib Plus Pembrolizumab in Previously Treated Advanced Endometrial Cancer: Updated Efficacy and Safety From the Randomized Phase III Study 309/KEYNOTE-775. J Clin Oncol. 2023, 41:2904-2910. [CrossRef]
- Mirza MR, Chase DM, Slomovitz BM, et al.: Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N Engl J Med. 2023, 388:2145-2158. [CrossRef]
- Eskander RN, Sill MW, Beffa L, et al.: Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N Engl J Med. 2023, 388:2159-2170. [CrossRef]
- Wan X, Huang J, Huang L, et al.: Effectiveness and safety of PD-1/PD-L1 inhibitors monotherapy in patients with endometrial cancer. Discov Oncol. 2024, 15:168. [CrossRef]
- Bartley AN, Mills AM, Konnick E, et al.: Mismatch Repair and Microsatellite Instability Testing for Immune Checkpoint Inhibitor Therapy: Guideline From the College of American Pathologists in Collaboration With the Association for Molecular Pathology and Fight Colorectal Cancer. Arch Pathol Lab Med. 2022, 146:1194-1210. [CrossRef]
- Vikas P, Messersmith H, Compton C, et al.: Mismatch Repair and Microsatellite Instability Testing for Immune Checkpoint Inhibitor Therapy: ASCO Endorsement of College of American Pathologists Guideline. J Clin Oncol. 2023, 41:1943-1948. [CrossRef]
- Interpretation and reporting terminology for mismatch repair protein immunohistochemistry in endometrial carcinoma. (2020). Accessed: 08/09/2024 2024: https://www.thebagp.org/resources/bagp-guidance-documents/.
- Raffone A, Travaglino A, Cerbone M, et al.: Diagnostic Accuracy of Immunohistochemistry for Mismatch Repair Proteins as Surrogate of Microsatellite Instability Molecular Testing in Endometrial Cancer. Pathol Oncol Res. 2020, 26:1417-1427. [CrossRef]
- Aiyer KTS, Doeleman T, Ryan NA, et al.: Validity of a two-antibody testing algorithm for mismatch repair deficiency testing in cancer; a systematic literature review and meta-analysis. Mod Pathol. 2022, 35:1775-1783. [CrossRef]
- Olave MC, Graham RP: Mismatch repair deficiency: The what, how and why it is important. Genes Chromosomes Cancer. 2022, 61:314-321. [CrossRef]
- Stelloo E, Jansen AML, Osse EM, et al.: Practical guidance for mismatch repair-deficiency testing in endometrial cancer. Ann Oncol. 2017, 28:96-102. [CrossRef]
- Ryan NAJ, McMahon R, Tobi S, et al.: The proportion of endometrial tumours associated with Lynch syndrome (PETALS): A prospective cross-sectional study. PLoS Med. 2020, 17:e1003263. [CrossRef]
- Addante F, d’Amati A, Santoro A, et al.: Mismatch Repair Deficiency as a Predictive and Prognostic Biomarker in Endometrial Cancer: A Review on Immunohistochemistry Staining Patterns and Clinical Implications. Int J Mol Sci. 2024, 25. [CrossRef]
- Compton CC, Robb JA, Anderson MW, et al.: Preanalytics and Precision Pathology: Pathology Practices to Ensure Molecular Integrity of Cancer Patient Biospecimens for Precision Medicine. Arch Pathol Lab Med. 2019, 143:1346-1363. [CrossRef]
- Parente P, Grillo F, Vanoli A, et al.: The Day-To-Day Practice of MMR and MSI Assessment in Colorectal Adenocarcinoma: What We Know and What We Still Need to Explore. Dig Dis. 2023, 41:746-756. [CrossRef]
- Grillo F, Paudice M, Gambella A, et al.: Evaluating mismatch repair deficiency in colorectal cancer biopsy specimens. Histochem Cell Biol. 2023, 160:113-125. [CrossRef]
- Matias-Guiu X, Stanta G, Carneiro F, et al.: The leading role of pathology in assessing the somatic molecular alterations of cancer: Position Paper of the European Society of Pathology. Virchows Arch. 2020, 476:491-497. [CrossRef]
- Riedinger CJ, Esnakula A, Haight PJ, et al.: Characterization of mismatch-repair/microsatellite instability-discordant endometrial cancers. Cancer. 2024, 130:385-399. [CrossRef]
- Watkins JC, Nucci MR, Ritterhouse LL, Howitt BE, Sholl LM: Unusual Mismatch Repair Immunohistochemical Patterns in Endometrial Carcinoma. Am J Surg Pathol. 2016, 40:909-916. [CrossRef]
- Mendoza RP, Wang P, Schulte JJ, et al.: Endometrial Carcinomas With Subclonal Loss of Mismatch Repair Proteins: A Clinicopathologic and Genomic Study. Am J Surg Pathol. 2023, 47:589-598. [CrossRef]
- Dondi G, Coluccelli S, De Leo A, et al.: An Analysis of Clinical, Surgical, Pathological and Molecular Characteristics of Endometrial Cancer According to Mismatch Repair Status. A Multidisciplinary Approach. Int J Mol Sci. 2020, 21. [CrossRef]
- de Freitas D, Aguiar FN, Anton C, et al.: Clinicopathological characteristics of endometrial carcinomas according to DNA mismatch repair protein status. Heliyon. 2023, 9:e17495. [CrossRef]
- How JA, Jazaeri AA, Westin SN, et al.: Translating biological insights into improved management of endometrial cancer. Nat Rev Clin Oncol. 2024. [CrossRef]
- Ercan AB, Aronson M, Fernandez NR, et al.: Clinical and biological landscape of constitutional mismatch-repair deficiency syndrome: an International Replication Repair Deficiency Consortium cohort study. Lancet Oncol. 2024, 25:668-682. [CrossRef]
- Underkofler KA, Ring KL: Updates in gynecologic care for individuals with lynch syndrome. Front Oncol. 2023, 13:1127683. [CrossRef]
- Ramchander NC, Ryan NAJ, Walker TDJ, et al.: Distinct Immunological Landscapes Characterize Inherited and Sporadic Mismatch Repair Deficient Endometrial Cancer. Front Immunol. 2019, 10:3023. [CrossRef]
- Bellone S, Roque DM, Siegel ER, et al.: A phase 2 evaluation of pembrolizumab for recurrent Lynch-like versus sporadic endometrial cancers with microsatellite instability. Cancer. 2022, 128:1206-1218. [CrossRef]
- Manning-Geist BL, Liu YL, Devereaux KA, et al.: Microsatellite Instability-High Endometrial Cancers with MLH1 Promoter Hypermethylation Have Distinct Molecular and Clinical Profiles. Clin Cancer Res. 2022, 28:4302-4311. [CrossRef]
- Khushman MM, Toboni MD, Xiu J, et al.: Differential Responses to Immune Checkpoint Inhibitors are Governed by Diverse Mismatch Repair Gene Alterations. Clin Cancer Res. 2024, 30:1906-1915. [CrossRef]
- Salem ME, Bodor JN, Puccini A, et al.: Relationship between MLH1, PMS2, MSH2 and MSH6 gene-specific alterations and tumor mutational burden in 1057 microsatellite instability-high solid tumors. Int J Cancer. 2020, 147:2948-2956. [CrossRef]
- Cosgrove CM, Cohn DE, Hampel H, et al.: Epigenetic silencing of MLH1 in endometrial cancers is associated with larger tumor volume, increased rate of lymph node positivity and reduced recurrence-free survival. Gynecol Oncol. 2017, 146:588-595. [CrossRef]
- Kaneko E, Sato N, Sugawara T, et al.: promoter hypermethylation predicts poorer prognosis in mismatch repair deficiency endometrial carcinomas. J Gynecol Oncol. 2021, 32:e79. [CrossRef]
- Ma J, Lin J, Lin X, et al.: Assessment of Immune Status in Patients with Mismatch Repair Deficiency Endometrial Cancer. J Inflamm Res. 2024, 17:2039-2050. [CrossRef]
- Smithgall MC, Remotti H, Hsiao SJ, Mansukhani M, Liu-Jarin X, Fernandes H: Investigation of discrepant mismatch repair immunohistochemistry and microsatellite instability polymerase chain reaction test results for gynecologic cancers using next-generation sequencing. Hum Pathol. 2022, 119:41-50. [CrossRef]
- Ta RM, Hecht JL, Lin DI: Discordant loss of mismatch repair proteins in advanced endometrial endometrioid carcinoma compared to paired primary uterine tumors. Gynecol Oncol. 2018, 151:401-406. [CrossRef]
- León-Castillo A, Gilvazquez E, Nout R, et al.: Clinicopathological and molecular characterisation of ‘multiple-classifier’ endometrial carcinomas. J Pathol. 2020, 250:312-322. [CrossRef]
- De Vitis LA, Schivardi G, Caruso G, et al.: Clinicopathological characteristics of multiple-classifier endometrial cancers: a cohort study and systematic review. Int J Gynecol Cancer. 2024, 34:229-238. [CrossRef]
- Kato MK, Fujii E, Asami Y, et al.: Clinical features and impact of p53 status on sporadic mismatch repair deficiency and Lynch syndrome in uterine cancer. Cancer Sci. 2024, 115:1646-1655. [CrossRef]
- León-Castillo A, Britton H, McConechy MK, et al.: Interpretation of somatic POLE mutations in endometrial carcinoma. J Pathol. 2020, 250:323-335. [CrossRef]
- Oaknin A, Pothuri B, Gilbert L, et al.: Safety, Efficacy, and Biomarker Analyses of Dostarlimab in Patients with Endometrial Cancer: Interim Results of the Phase I GARNET Study. Clin Cancer Res. 2023. [CrossRef]
- Favier A, Varinot J, Uzan C, Duval A, Brocheriou I, Canlorbe G: The Role of Immunohistochemistry Markers in Endometrial Cancer with Mismatch Repair Deficiency: A Systematic Review. Cancers (Basel). 2022, 14. [CrossRef]
- Chavez JA, Wei L, Suarez AA, Parwani AV, Li Z: Clinicopathologic characteristics, tumor infiltrating lymphocytes and programed cell death ligand-1 expression in 162 endometrial carcinomas with deficient mismatch repair function. Int J Gynecol Cancer. 2019, 29:113-118. [CrossRef]
- Friedman CF, Manning-Geist BL, Zhou Q, et al.: Nivolumab for mismatch-repair-deficient or hypermutated gynecologic cancers: a phase 2 trial with biomarker analyses. Nat Med. 2024, 30:1330-1338. [CrossRef]
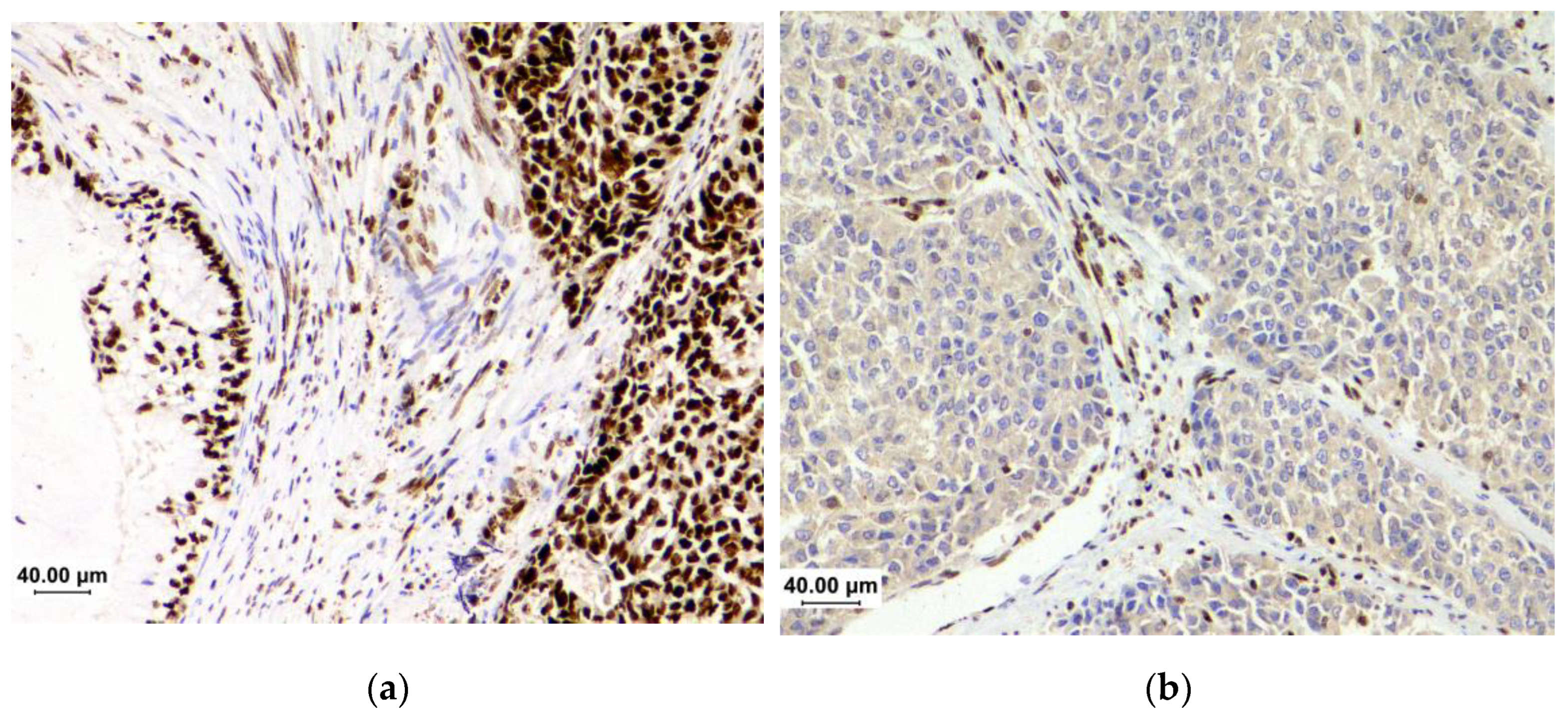
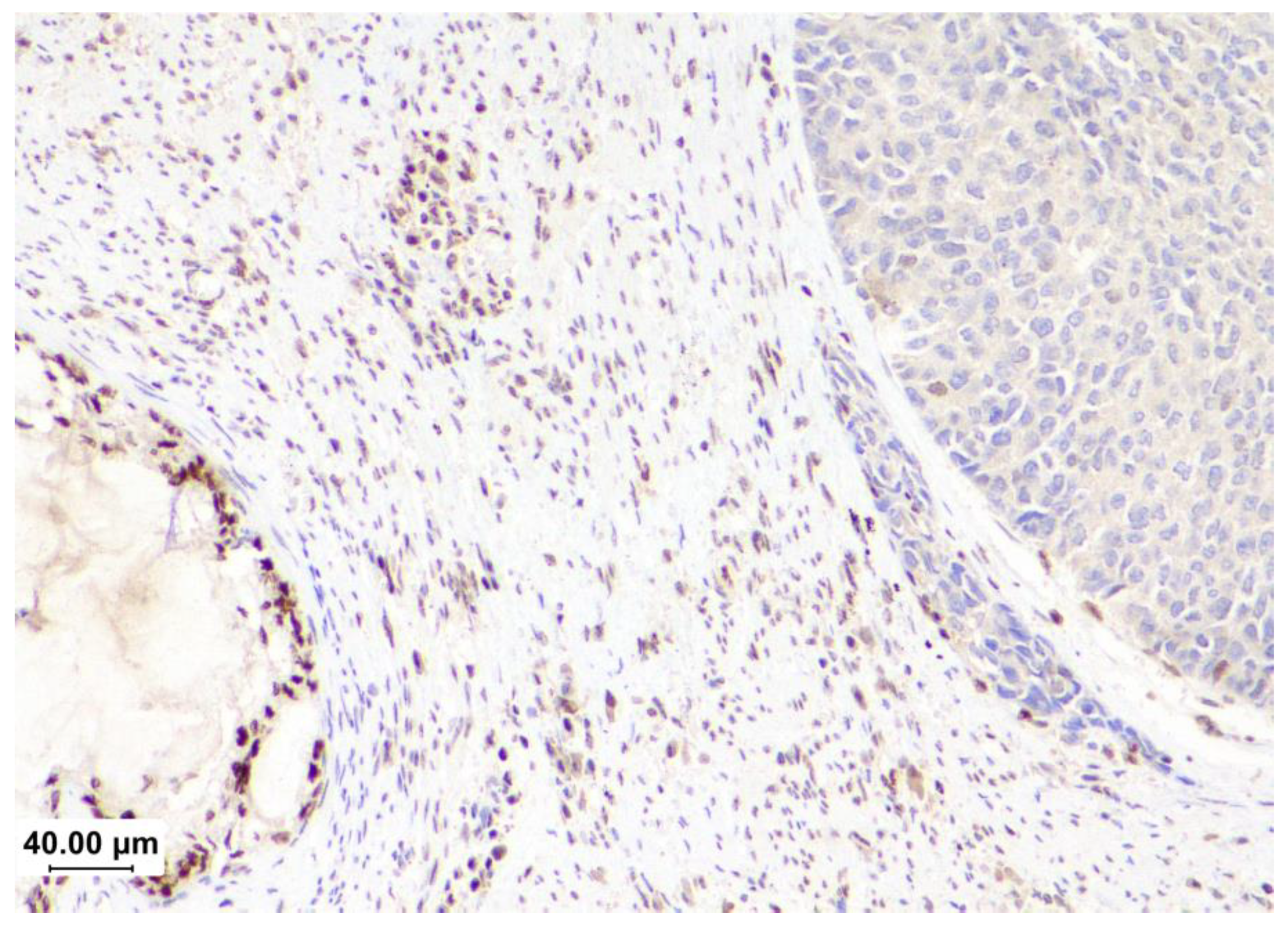
| TCGA subgroups[6] | POLE | MSI1 | CN-H2 | CN-L3 |
|---|---|---|---|---|
| ProMisE4 surrogates [11] | Exons 9-14 mutations | dMMR5 | p53-mutated | p53-wild-type |
| Frequency [6] | 7% | 28% | 26% | 39% |
| Age at diagnosis <60 y [12] | 57.1% | 38.3% | 6.6% | 51.4% |
| BMI [13]6 | 27,2±0.9 | 30.6±1.2 | 29.1±0.5 | 32.3±1.4 |
| High-risk ESMO (2016)7 [11] | 16.7% | 33.9% | 87.3% | 14.5% |
| FIGO8 stage I (2009) [11] | 92.9% | 78% | 52.7% | 86.8% |
| Positive lymph node [12] | 14.2% | 14.9% | 44.8% | 10.8% |
| Endometrioid histology [14] | 86.1% | 85.8% | 27% | 96.7% |
| High-grade tumor (grade 3) [12] | 23.8% | 12.8% | 93.3% | 6.8% |
| TILs9 [15] | high | high | absent | low |
| LVSI10 [12] | 28.6% | 34% | 20.3% | 60% |
| TP53 mutation [6] | 35% | 5% | 1% | >90% |
| Prognosis [6,11] | excellent | intermediate | poor | intermediate |
| Clinical trial | Type of study | n | Treatment | Main result |
|---|---|---|---|---|
| KN-158 NCT02628067 [23] |
Single-arm, phase II study |
49 | Pembrolizumab | ORR 57.1% (95%CI 42.2-71.2) |
| GARNET NCT02715284 [28] | Phase I, single-arm | 104 | Dostarlimab | ORR 42.3% (95%CI 30.6-54.6%) |
| KN-868/NRG-GY018 NCT03914612 [32] |
Phase 3, randomized, placebo control | 222 | Pembrolizumab + carboplatin/paclitaxel followed by pembrolizumab | PFS 74% vs. 38% |
| RUBY NCT03981796 [31] |
Phase 3, randomized, placebo control | 118 | Dostarlimab + carboplatin/paclitaxel followed by dostarlimab | PFS: 61.4% vs. 15.7% OS: 36.1% vs. 18.1% |
| Features | Mutation | No mutation |
|---|---|---|
| Type of defective protein | MSH2/MSH6 | MLH1/PMS2 |
| Age of patient | younger | older |
| Tumor size | smaller | larger |
| Tumor grade | low | high |
| LVSI* | less | more |
| Stage | early | advanced |
| TILs, PD-L1, TMB | high | less |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





