Submitted:
14 September 2024
Posted:
17 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Genetic Diversity and Species Management
2.3. Genetic Bias in Founder Sperm Collection
2.4. Assisted Gene Flow
3. Reproduction Biotechnologies
3.1. Life Stages and Sample Collection
3.2. Reproduction, Gamete Collection, Donor Stress, and Pathogens
3.3. Sperm Qualities
3.4. Refrigerated Storage of Sperm
3.5. Sperm Cryopreservation and Freeze Drying
3.6. Oocyte Storage
3.7. Artificial Fertilisation; Sperm Concentrations, Fertilisation Periods, and Rates
4. Advanced Reproduction Biotechnologies (aARBs)
4.1. Cloning
4.2. Assisted Evolution
5. Ethics and Communication
6. Current and Future Application
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC, 2023: Summary for Policymakers. In: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, H. Lee and J. Romero (eds.)]. IPCC, Geneva, Switzerland, pp. 1-34, Available online:. [CrossRef]
- UNEP. UN Biodiversity Conference (COP 15). December 7-19, 2022. Available online: https://www.unep.org/un-biodiversity-conference-cop-15 (accessed on 23 September 2023).
- Browne, R.K.; Luo, Q.; Wang, P.; Mansour, N.; Kaurova, S.A.; Gakhova, E.N.; Shishova, N.V.; Uteshev, V.K.; Kramarova, L.I. .; Venu, G.; Vaissi, S.; Taheri-Khas, Z.; Heshmatzad, P.; Bagaturov, M.F.; Janzen, P.; Aguilar, R.E.N.; Swegen, A.; Strand, J.; McGinnity, D.; Dunce, I. Ecological Civilization COP 15, COP 28, and Amphibian Sustainability through Reproduction Biotechnologies, Biobanking, and Conservation Breeding Programs (RBCs). Animals 2024, 14, 1455. [Google Scholar] [CrossRef]
- Conradi, T.; Eggli, U.; Kreft, H.; Schweiger, A.H.; Weigelt, P.; Higgins, S.I. Reassessment of the Risks of Climate Change for Terrestrial Ecosystems. Nat Ecol. Evol. 2024, 8, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Grant, E.H.; Amburgey, S.M.; Gratwicke, B.; Chaves, V.A.; Belasen, A.M.; Bickford, D.; Bruhl, C.A.; Calatayud, N.E.; Clemann, N.; Clulow, S.; et al. Amphibian Conservation in the Anthropocene. Biol. Conserv. 2023, 236, 543–547. [Google Scholar] [CrossRef]
- Willcock, S.; Cooper, G.S.; Addy, J. Dearing, J.A. Earlier Collapse of Anthropocene Ecosystems Driven by Multiple Faster and Noisier Drivers. Nat. Sustain. 2023, s41893-023-01157-x. [CrossRef]
- Finn, C.; Gratarolla, F.; Pincheira-Donoso, D. More Losers than Winners: Investigating Anthropocene Defaunation through the Diversity of Population Trends. Biol. Rev. Camb. Philos. Soc. 2023, 98, 1732–1748. [Google Scholar] [CrossRef]
- Bradshaw, C.J.A.; Ehrlich, P.R.; Beattie, A.; Ceballos, G.; Crist, E.; Diamond, J.; Dirzo, R.; Ehrlich, A.H.; Harte, J.; Harte, M.E.; Graham, P.; et al. Underestimating the Challenges of Avoiding a Ghastly Future. Front. Conserv. Sci. 2021, 1, e615419. [Google Scholar] [CrossRef]
- The IUCN Red List of Threatened Species. Version 2022-2. Available online: https://www.iucnredlist.org (accessed on 27 September 2023).
- Silla, A.J.; Byrne, P.G. The Role of Reproductive Technologies in Amphibian Conservation Breeding Programs. Annu. Rev. Anim. Biosci. 2019, 15, 499–519. [Google Scholar] [CrossRef] [PubMed]
- AmphibiaWeb. Available online: https://amphibiaweb.org (accessed on 27 September 2023).
- 157. Smith, D.; Abeli, T.; Bruns, E.B.; Dalrymple, S.E.; Foster, J.; Gilbert, T.C.; Hogg, C.J.; Lloyd, N.A.; Meyer, A.; Moehrenschlager, A. et al. Extinct in the wild: The precarious state of Earth’s most threatened group of species. Science 2023, 379, eadd2889. [Google Scholar] [CrossRef]
- UNFCCC COP 28. November 30th -December 12th, 2023. UN Climate Change Conference. Available online: https://unfccc.int/cop28; (accessed on 29 December 2023).
- Hansen, J.E.; Sato, M.; Simons, L.; Nazarenko, L.S.; Sangha, I.; Kharecha, P.; Zachos, J.C.; von Schuckmann, K.; Loeb, N.G.; Osman, M.B.; et al. Global Warming in the Pipeline, Oxford Open Climate Change, 2023, 3, kgad008. [CrossRef]
- Global Tipping Points. Available online: https://global-tipping-points.org/ (accessed on 29 December 2023).
- CAT. Climate Action Tracker IMF. Available online: https://climateactiontracker.org/ (accessed on 6 August 2024).
- Smith, S M; Geden, O; Gidden, M J; Lamb, W F; Nemet, G F; Minx, J C; Buck, H; Burke, J; Cox, E; Browne, M.R. et al. The State of Carbon Dioxide Removal. 2nd Edition. TI - The State of Carbon Dioxide Removal. 2024. Available online: https://osf.io/f85qj/https://www.stateofcdr.org/ (accessed on 8 August 2024).
- GHR. Global Hydrogen Review 2023, IEA, Paris. Available online: https://www.iea.org/reports/global-hydrogen-review-2023 (accessed on 8 August 2024).
- Juárez-Orozco, S.M.; Siebe, C.; Fernández y Fernández, D. Causes and Effects of Forest Fires in Tropical Rainforests: A Bibliometric Approach. Trop. Conserv. Sci. 2017, 2017, 10. [Google Scholar] [CrossRef]
- Zheng, B.; Ciais, P.; Chevallier, F.; Yang, H.; Canadell, J.G.; Chen, Y.; van der Velde, I.R.; Aben, I.; Chuvielco, E.; et al. Record-high CO2 emissions from boreal fires in 2021. Science 2023, 379, 912–917. [Google Scholar] [CrossRef]
- Hong, X.; Liu, C.; Zhang, C.; Tian, Y.; Wu, H.; Yin, H.; Zhu, Y.; Yafang Cheng, Y. Vast ecosystem disturbance in a warming climate may jeopardize our climate goal of reducing CO2: a case study for megafires in the Australian ‘black summer’. Sci. Total Environ. 2023, 866, 161387. [Google Scholar] [CrossRef] [PubMed]
- Chikamoto, M.O.; DiNezio, P.; Lovenduski, N. Long-Term Slowdown of Ocean Carbon Uptake by Alkalinity Dynamics. Geophys. Res. Lett. 2023, 50, e2022GL101954. [Google Scholar] [CrossRef]
- World Ocean Review. The role of the ocean in the global carbon cycle. Available online: https://worldoceanreview.com/en/wor-8/the-role-of-the-ocean-in-the-global-carbon-cyclee/how-the-ocean-absorbs-carbon-dioxide/ (accessed on 2 August, 2024).
- Smolders, E.J.V.; Western, R.M.; Dijkstra, H.A. Probability Estimates of a 21st Century AMOC Collapse. Available online: https://arxiv.org/html/2406.11738v1 (accessed on 3 August 2024).
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Borzée, A.; Hamidy, A.; Aowphol, A.; Jean, A.; et al. Ongoing Declines for the World’s Amphibians in the Face of Emerging Threats. Nature 2023, 622, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Guirguis, J.; Goodyear, L.E.B.; Finn, C.; Johnson, J.V.; Pincheira-Donoso, D. Risk of Extinction Increases Towards Higher Elevations Across the World's Amphibians. Glob. Ecol. Biogeogr. 2023, 32, 1–12. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, D.; Wang, Liu, J. ; Du N. Altitudinal Patterns of Species Diversity and Phylogenetic Diversity across Temperate Mountain Forests of Northern China. PLOS ONE 2026, 1, e0159995. [Google Scholar] [CrossRef]
- Khatiwada, J.R.; Zhao, T.; Chen, Y.; Wang, B.; Xei, F.; Cannatella, D.C.; Jiang, J. Amphibian Community Structure along Elevation Gradients in Eastern Nepal Himalaya. BMC Ecol. 2019, 19, 1–11. [Google Scholar] [CrossRef]
- Rastegar-Pouyani, N; Gholamifard, A. ; Karamiani, R; Bahmani, Z.; Mobaraki, A.; Abtin E.; Faizi, H.; Heidari, N.; Takesh, M.; Sayyadi. F. et al. Browne RK. Sustainable Management of the Herpetofauna of the Iranian Plateau and coastal Iran. Amphib. Rept. Conser. 2015, 9, 1–15. [Google Scholar]
- Trew, B.T.; Edwards, D.P.; Klinges, D.H.; Early, R.; Svátel, M.; Plichta, R.; Mayula, R.; Okello, J.; Niessner, A.; Barthel, M.; et al. Novel Temperatures are Already Widespread Beneath the World’ Tropical Rainforests. Nat. Clim. Chang. 14, 753–759. [CrossRef]
- Blake, J.G.; Loiselle, B.A. Sharp Declines in Observation and Capture Rates of Amazon Birds in Absence of Human Disturbance. Glob. Ecol. Conserv. 2024, 51, e02902. [Google Scholar] [CrossRef]
- Campbell, L.G.; Anderson, K.A.; Marcec-Greaves, R.; Cardoso, P.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; et al. Scientists' Warning to Humanity on Insect Extinctions. Biol. Conserv. 2020, 242, 108426:1–108426:1. [Google Scholar] [CrossRef]
- Precautionary Principle. International Institute for Sustainable Development. Available online: https://www.iisd.org/articles/deep-dive/precautionary-principle 9acessed 13 August 2024).
- Gergis, J. An Intergenerational Crime Against Humanity What will it Take for Political Leaders to Start Taking Climate Change Seriously. Available online: https://theconversation.com/an-intergenerational-crime-against-humanity-what-will-it-take-for-political-leaders-to-start-taking-climate-change-seriously-231383. (accessed on 14 June 2024).
- Barry, B. Sustainability and Intergenerational Justice. Theoria 1997, 89, 43–65. [Google Scholar] [CrossRef]
- Spanning, R. Youth in the Anthropocene: Questions of Intergenerational Justice and Learning in a More-Than-Human World. In Youth Cultures in a Globalized World.; Knapp, G., Krall, H. Eds.; Springer, Cham, ZG, 2021; pp 113–133, ISBN 3030651762.
- Global Tipping Points. Section 4.6. Risks, Equity and Justice in the Governance of Positive Tipping Points. Pereira, L., Achieng, T., Castro, A., Sara M. Constantino, S.A., Ghadiali, A., Gifford, L., Newell, P., Smith, B., Smith, S.R., Villasante, S., Zimm, C. Eds.; Available online: https://global-tipping-points.org/ (accessed on 29 December 2023).
- Radchuk, V.; Reed, T.; Teplitsky, C.; van de Pol, M.; Charmantiet, A.; Hassall, C.; Adamik, P.; Adriaensen, F.; Ahola, M.P.; Arcese, P.; et al. Adaptive Responses of Animals to Climate Change are Most Likely Insufficient. Nat. Commun. 2019, 10, 3109. [Google Scholar] [CrossRef] [PubMed]
- Treves, A.; Artelle, K.A.; Darimont, C.T.; Lynn, W.S.; Paquet, P.; Santiago-Ávila, F.J.; Shaw, R.; Wood, M.C. Intergenerational Equity can Help to Prevent Climate Change and Extinction. Nat. Ecol. Evol. 2018, 2, 204–207. [Google Scholar] [CrossRef]
- Méjean, A.; Pottier, A.; Zuber, S.; Fleurbaey, M. Intergenerational Equity Under Catastrophic Climate Change. Global Priorities Institute Working Paper No. 5-2020. Availaable online: https://globalprioritiesinstitute.org/wp-content/uploads/M%C3%A9jean-et-al_Intergenerational-equity-under-catastrophic-climate-change.pdf (accesses 7 August 2024).
- Fedele, G.; Donatti, C.; I. , Harvey, C.A.; Hannah, L.; Hole, D.G. (2019). Transformative Adaptation to Climate Change for Sustainable Social-ecological Systems. Environ. Sci. Policy, 2019, 101, 116–125. [Google Scholar] [CrossRef]
- O’Brien, K. (2012). Global Environmental Change II: From Adaptation to Deliberate Transformation. Hum. Geogr. J. 2012, 36, 667–676. [Google Scholar] [CrossRef]
- Bolam, F.C.; Ahumada, J.; Akçakaya, H.R.; Brooks, T.M.; Elliott, W.; Hoban, S.; Mair, L.; Mallon, D.; McGowan, P.J.K.; Raimondo, D.; et al. Preventing Extinctions Post-2020 Requires Recovery Actions and Transformative Change. BioRx 2020. [Google Scholar] [CrossRef]
- Bowman, M. Chapter 1 Law, Legal Scholarship and the Conservation of Biological Diversity: 2020 Vision and Beyond. In Research Handbook on Biodiversity and Law; Bowman, M., Davies, P., Goodwin, E. Eds.; Edward Elgar Publishing, Cheltenham, United Kingdom, 2016. [CrossRef]
- Senior, R.A.; Bagwyn, R.; Leng, D.; Killion, A.K.; Jetz, W.; Wilcove, D.S. Global Shortfalls in Documented Actions to Conserve Biodiversity. Nature 2024, 630, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Bateman, I.; Balmford, A. Current Conservation Policies Risk Accelerating Biodiversity Loss. Nature 2023, 618, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Palacio, R.D.; Abarca, M.; Armenteras, D.; Balza, U.; Dollar, L.; Froese, G.Z.L.; Galligan, M.P.; Gula, J.; Giordano, A.J.; Jacobson, A.P.; et al. The Global Influence of the IUCN Red List can Hinder Species Conservation Efforts. Authorea 2023, preprint. [Google Scholar] [CrossRef]
- Crutzen, P.J.; Stoemer, E.F. The “Anthropocene”. The International Geosphere–Biosphere Programme (IGBP): A Study of Global Change of the International Council for Science (ICSU) Newsletter. Glob. Change Newsl. 2000, 41, 17–18. [Google Scholar]
- United Nations Convention on Biodiversity. Available online: https://www.un.org/en/observances/biological-diversity-day/convention (accessed on 27 September 2023).
- Byers, O.; Lees, C.; Wilcken, J.; Schwitzer, C. The One Plan Approach: The Philosophy and Implementation of CBSG’s Approach to Integrated Species Conservation Planning. WAZA Mag. 2013, 14, 2–5. [Google Scholar]
- Ziegler, T. The IUCN/SSC CPSG's One Plan Approach and the Role of Progressive Zoos in Conservation: Case Studies from Herpetology. In Proceedings of the 14th national congress of the Italian Society for Herpetology, Torino; 2023; pp. 195–222. [Google Scholar]
- IUCN SSC Amphibian Specialist Group (2024). Amphibian Conservation Action plan: A Status Review and Roadmap for Global Amphibian Conservation. Wren, S., Borzée. A., Marcec-Greaves, R. & Angulo, A. (Eds.). IUCN SSC Occasional Paper, No 57. Gland, Switzerland: IUCN. Available online: https://portals.iucn.org/library/sites/library/files/documents/SSC-OP-057-En.pdf (accessed on 6 August 2024).
- AArk. Amphibian Ark. Available online: www.amphibianark.org/ (accessed on 27th September 2023).
- Zippel, K.; Johnson, K.; Agliardo, R.G.; Gibson, R.; McFadden, M.; Browne, R.; Martinez, C.M.; Townsend, E. The Amphibian ARK: A Global Community for Ex situ Conservation of Amphibians. Herpetol. Conserv. Biol. 2011, 6, 340–352. [Google Scholar]
- Browne, R.K.; Janzen, P.; Bagaturov, M.F.; van Houte, D.K. Amphibian Keeper Conservation Breeding Programs. J. Zoo. Res. 2018, 2, 29–46. [Google Scholar] [CrossRef]
- Responsible Herpetological Project. conservation breeding programs. Available online: https://responsibleherpetoculture.foundation/ (accessed on 27 September 2023).
- Thomas-Walters, L.; McCallum, J.; Montgomery, R.; Petros, C.; Wan, A.K.Y.; Veríssimo, D. Systematic review of conservation interventions to promote voluntary behavior change. Conserv. Biol. 2023, 37, e14000. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, M.; Parenti, L.R.; Craddock, R.A.; Comizzoli, P.; Mabee, P.; Meinke, B.; Wolf, S.M.; Bischof, J.C.; Sandlin, R.D.; Tessier, S.N.; et al. Safeguarding Earth's biodiversity by creating a lunar biorepository. BioScience 2024, biae058. [Google Scholar] [CrossRef] [PubMed]
- Della-Tonga, G.; Howell, L.G.; Clulow, J.; Langhorne, C.J.; Marcec-Greaves, R.; Calatayud, N.E. Evaluating Amphibian Biobanking and Reproduction for Captive Breeding Programs According to the Amphibian Conservation Action Plan Objectives. Theriogenology 2020, 150, 412–431. [Google Scholar] [CrossRef]
- Clulow, J.; Mahony, M.; Browne, R.; Pomering, M.; Clark, A. Applications of Assisted Reproductive Technologies (ART) to Endangered Anuran Amphibians. In Declines and disappearances of Australian frogs; Campbell, A., Ed.; Environment Australia: Canberra, Australia, 1999; pp. 219–225. ISBN 0 642 54656 8. [Google Scholar]
- Browne, R.K.; Kaurova, S.A.; Vasudevan, K.; McGinnity, D.; Venu, G.; Gonzalez, M.; Uteshev, V.K.; Marcec-Greaves, R. Reproduction Technologies for the Sustainable Management of Caudata (salamander) and Gymnophiona (Caecilian) biodiversity. Reprod. Fert. Devel. 2022, 34, 479–497. [Google Scholar] [CrossRef]
- Ananjeva, N.B.; Uteshev, V.K.; Orlov, N.L.; Ryabov, S.A.; Gakhova, E.N.; Kaurova, S.A. , Kramarova L.I., Shishova N.V., Browne R.K. Comparison of the Modern Reproductive Technologies for Amphibians and Reptiles. Russ. J. Herpetol. 2017, 24, 275–290. [Google Scholar] [CrossRef]
- Clulow, J.; Upton, R.; Trudeau, V.L.; Clulow, S. Amphibian Assisted Reproductive Technologies: Moving from Technology to Application. Adv. Exp. Med. Biol. 2019, 1200, 413–463. [Google Scholar] [CrossRef] [PubMed]
- Uteshev, V.K.; Gakhova, E.N.; Kramarova, L.I.; Shishova, N.V.; Kaurova, S.A.; Kidova, E.A.; Kidov, A.A.; Browne, R.K. Russian Collaborative Development of Reproduction Technologies for the Sustainable Management of Amphibian Biodiversity. Asian Herpetol. Res. 2023, 14, 103–115. [Google Scholar] [CrossRef]
- Anastas, Z.M.; Byrne, P.G.; O’Brien, J.K.; Hobbs, R.J.; Upton, R.; Silla, A.J. The Increasing Role of Short-Term Sperm Storage and Cryopreservation in Conserving Threatened Amphibian Species. Animals 2023, 13, 2094:1–2094:40. [Google Scholar] [CrossRef]
- Browne, R.K.; Silla, A.J.; Upton, R.; Della-Togna, G.; Marcec-Greaves, R.; Shishova, N.V.; Uteshev, V.K.; Proano, B.; Pe-rez, O.D.; Mansour, N.; et al. Sperm Collection and Storage for the Sustainable Management of Amphibian Biodiversity. Theriogenology 2019, 133, 187–200. [Google Scholar] [CrossRef]
- Bolton, R.L.; Mooney, A.; Pettit, M.T.; Bolton, A.E.; Morgan, L.; Drake, G.J.; Appeltant, R.; Walker, S.L.; Gillis, J.D.; Hvilsom, C. Resurrecting Biodiversity: Advanced Assisted Reproductive Technologies and Biobanking. Reprod. Fertil. 2022, 3, R121–R146. [Google Scholar] [CrossRef] [PubMed]
- Seddon, P.J.; Griffiths, C.J.; Soorae, P.S.; Armstrong, D.P. Reversing Defaunation: Restoring Species in a Changing World. Science 2014, 345, 406–412. [Google Scholar] [CrossRef]
- Strand, J.; Thomsen, H.; Jensen, J.B.; Marcussen, C.; Nicolajsen, T.B.; Skriver, M.B.; Søgaard, I.M.; Ezaz, T.; Purup, S.; Callesen, H.; et al. Biobanking in Amphibian and Reptilian Conservation and Management: Opportunities and Challenges. Conserv. Gen. Res. 2020, 12, 709–725. [Google Scholar] [CrossRef]
- Strand, J.; Fraser, B.; Houck, M.L.; Clulow, S. Culturing and Biobanking of Amphibian Cell Lines for Conservation Applications. In Reproductive Technologies and Biobanking for the Conservation of Amphibians; Silla, A.J., Kouba, A.J., Heatwole, H., Eds.; CRC Press: London, UK, 2022; pp. 156–177. ISBN 9781486313334. [Google Scholar]
- Mooney, A.; Ryder, O.A.; Houck, M.L.; Staerk, J.; Conde, D.A.; Buckley, Y.M. Maximizing the Potential for Living Cell Banks to Contribute to Global Conservation Priorities. Zoo Biol. 2023, 42, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Cowl, V.B.; Comizzoli, P.; Appeltant, R.; Bolton, R.L.; Browne, R. Holt, W.V.; Penfold, L.M.; Swegen, A. et al. Cloning for the Twenty-First Century and Its Place in Endangered Species Conservation. Annu. Rev. Anim. Biosci. 2024, 12, 91–112. [Google Scholar] [CrossRef]
- Lermen, D.; Blömeke, B.; Browne, R.K.; Clarke, A.; Dyce, P.W.; Fixemer, T.; Fuhr, G.R.; Holt, W.V.; Jewgenow, K.; Lloyd, R.E.; et al. Meeting Review. Cryobanking of Viable Biomaterials: Implementation of New Strategies for Conservation Purposes. Molecular Ecology 2009, 18, 1030–1033. [Google Scholar] [CrossRef]
- Bowgen, K.M.; Kettel, E.F.; Butchart, S.H.M.; Carr, J.A.; Foden, W.B.; Magin, G.; Morecroft, M.D.; Smith, R.K.; Stein, B.A.; Sutherland, W.J.; et al. Conservation interventions can benefit species impacted by climate change, Biol. Conserv. 2022, 269, 109524. [Google Scholar] [CrossRef]
- Bolton, R.L.; Mooney, A.; Pettit, M.T.; Bolton, A.E.; Morgan, L.; Drake, G.J.; Appeltant, R.; Walker, S.L.; Gillis, J.D.; Hvilsom, C. Resurrecting Biodiversity: Advanced Assisted Reproductive Technologies and Biobanking. Reprod. Fertil. 2022, 3, R121–R146. [Google Scholar] [CrossRef]
- Upton, R.; Clulow, S.; Calatayud, N.E.; Colyvas, K.; Seeto, R.G.Y.; Wong, L.A.M.; Mahony, M.J.; Clulow, J. Generation of Reproductively Mature Offspring from the Endangered Green and Golden Bell Frog Litoria aurea using Cryopreserved Spermatozoa. Reprod. Fertil. Dev. 2021, 6. [Google Scholar] [CrossRef]
- Lampert, S.S.; Burger, I.J.; Julien, A.R.; Gillis, A.B.; Kouba, A.J.; Barber, D.; Kouba, C.K. Sperm Cryopreservation as a Tool for Amphibian Conservation: Production of F2 Generation Offspring from Cryo-Produced F1 Progeny. Animals 2023, 13, 53. [Google Scholar] [CrossRef]
- Holmes, B.; Ziermann, J.M.; Strzelecki, A.; Springer, S.; Zieger, M. Who notices Gymnophiona? Google Trends data reveal interesting trends for recent amphibian species. Ecol. Complex. 2024, 58, 101080. [Google Scholar] [CrossRef]
- Venu, G.; Raju, N.G.; Wilkinson, M.; Browne, R.K.; Varadh, K.; Balakrishna, G.N. , Ramakrishna, S.; Venkatachalaiah, G. First records of the Long-headed Caecilian, Ichthyophis longicephalus Pillai, 1986 (Gymnophiona: Ichthyophiidae) from the states of Karnataka and Tamil Nadu, India with comments on its conservation status. JAD 2020, 2, 5–10. Available online: https://jad.lu.ac.ir/article-1-93-en.html.
- Reinhard, S.; Kupfer, A. Maternal investment in the viviparous caecilian amphibian Typhlonectes natans (Gymnophiona: Typhlonectidae). Zool. Anz. 2022, 296, 33–36. [Google Scholar] [CrossRef]
- Kuehnel, S.; Kupfer, A. Sperm Storage in Caecilian Amphibians. Front Zool. 2012, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Parmley, D. General Husbandry of Terrestrial Caecilians. Available online: https://www.caudata.org/cc/articles/caecilian_care_Parmley.pdf, (accessed on 8 August 2024).
- Kaurova, S.A.; Chekurova, N.R.; Melnikova, E.V.; Uteshev, V.K.; Gakhova, E.N. 1996. Cryopreservation of Frog Rana temporaria Sperm Without Loss of Fertilizing Capacity. In Gakhova E. N., Karnaukhov V. N. Eds. Proceedings of the 14th Working Meeting, Pushchino, 13–15 May 1996, Pushchino: Pushchino Press, 106–108 (In Russian).
- Kaurova, S.A.; Uteshev, V.K.; Chekurova, N.R.; Gakhova, E.N. Cryopreservation of Testis of Frog Rana temporaria. Infusionsther Transfusionsmed 1997, 24, 379. [Google Scholar]
- Browne, R.K.; Clulow, J.; Mahony, M.; Clark, A. Successful Recovery of Motility and Fertility of Cryopreserved Cane Toad (Bufo marinus) sperm. Cryobiology 1998, 37, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.K.; Clulow, J.; Mahony, M. Short-term Storage of Cane Toad (Bufo marinus) Gametes. Reproduction 2001, 121, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Karamura, T.; Nishioka, M. Reciprocal Diploid Nucleocytoplasmic Hybrids Between Two Species of Japanese Pond Frogs and their Offspring. J. Sci. Hiroshima Univ. 1963, 21, Ser.B. Div. 1., 65–84. [Google Scholar]
- Karamura, T.; Nishioka, M. Nucleo-cytoplasmic Hybrid Frog between Two Species of Japanese Brown Frogs and their Offspring. J. Sci. Hiroshima Univ. 1963, 21, Ser. B. Div. 1, 107–134. [Google Scholar]
- Kouba, C.K. ; Julien. A.R. Linking In situ and Ex situ Populations of Threatened Amphibian Species using Genome Resource Banks. In Reproductive Technologies and Biobanking for the Conservation of Amphibians, Silla, A.J., Kouba, A.J., H Heatwole, H., Eds.: CRC Press; London, 2022, pp. 188-203, ISBN 9781486313334.
- Taronga Conservation Society Australia. 2023, Available Online: https://taronga.org.au/conservation-and-science/current-research/frog-conservation-biobanking [accessed 20 September 2023).
- Panama Amphibian Conservation and Rescue Project. Available online: http://amphibianrescue.org/ (accessed on 13 August 2024).
- Burger, I.; Julien, A.R.; Kouba, A.J.; Councell, K.R.; Barber, B.; Pacheco, C.; Kouba, C.K. Linking In Situ and Ex Situ Populations of the Endangered Puerto Rican Crested Toad. Conserv. Sci. Pract. 2021, 3, e525. [Google Scholar] [CrossRef]
- Wyoming toad sees Recovery in the Southwest. Wyoming Game and Fish Department. Available online: https://wgfd.wyo.gov/News/Wyoming-toad-recovery-sees-success-in-southeast (accessed on 20 January 2024).
- Northern Corroboree Frog Recovery Program. Australia’s Nature Hub. Available online: https://www.australiasnaturehub.gov.au/action-inventory/northern-corroboree-frog-recovery-program (accessed on 20 January 2024).
- Browne, R.K. Book Review of ‘Reproductive Technologies and Biobanking for the Conservation of Amphibians’ by Silla, A. J., Kouba, A.J., Heatwole, H. (eds.); CSIRO Publishing: Victoria, Australia, 2022. 238 pp.; Herpetol. Rev. 2023, 54, 332–335. [Google Scholar]
- Martinez, A.; Mammola, S.S. Specialized Terminology Reduces the Number of Citations of Scientific Papers. Proc. R. Soc. B, Biol. Sci. 2021, 288. [Google Scholar] [CrossRef]
- Textor, M.; Rami, D. ; Proper Names: Philosophical and Linguistic Perspectives. Erkenn 2015, 80 (Suppl 2), 191–194. [Google Scholar] [CrossRef]
- Chala, D.; Endresen, D.; Demissew, S.; Slaughter, L. A.; Johnsen, E. B.; Stenseth, N. C. Address Social Injustices in Taxonomy: Implement Extended Revisions of Names with Ethical Issues and Persistent Identifiers for Tracing Name Changes. Preprints 2024, 2024071673. [Google Scholar] [CrossRef]
- Mc Cartney, A.M.; Head, M.A.; Tsosie, K.S.; Sterner, B.; Glass, J.R.; Paez, S.; Geary, J.; Hudson, M. Indigenous Peoples and Local Communities as Partners in the Sequencing of Global Eukaryotic Biodiversity. npj Biodivers. 2023, 2, 8. [Google Scholar] [CrossRef]
- ABS. Nagoya Protocol on Access and Benefit-sharing. 2014. Available online: https://www.cbd.int/abs/ (accessed on 6 August 2024).
- Khoday, K. Decolonising the Environment: Third World Approaches to the Planetary Crisis. Indones. J. Int. Law, 2022, 19, 189–211. [Google Scholar]
- Abiddin, N.Z.; Ibrahim, I.; Abdul Aziz, S.A. Non-Governmental Organisations (NGOs) and their Part towards Sustainable Community Development. Sustainability 2022, 14, 4386. [Google Scholar] [CrossRef]
- Adams, W.; Mulligan, M. Decolonising Nature; Strategies for Conservation in a Post-colonial Era. 2002. Routledge London. 2002, 320p, ISBN 9781853837494.
- Frankham, R. Genetics and Extinction. Biol. Conserv. 2005, 126, 131–140. [Google Scholar] [CrossRef]
- Williams, R.N.; Bos, D.H.; Gopurenko, D; Dewoody, J. A. Amphibian Malformations and Inbreeding. Biol. Lett. 2008, 4, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Brennan, R.S.; Garrett, A.D.; Huber, K.E.; Hargarten, H.; Pespeni, M.H. Rare Genetic Variation and Balanced Polymorphisms are Important for Survival in Global Change Conditions. Proc. R. Soc. B. 2019, 286, 20190943:1–20190943:9. [Google Scholar] [CrossRef] [PubMed]
- Ralls, K.; Ballou, J.D.; Dudash, M.R.; Eldridge, M.D.B.; Fenster, C.B.; Lacy, R.C.; Sunnucks, P.; Frankham, R. Call for a Paradigm Shift in the Genetic Management of Fragmented Populations. Conserv. Lett. 2018, 11, 1–6. [Google Scholar] [CrossRef]
- AArk Founder Numbers. Available online: https://www.amphibianark.org/conservation-programs/captive-programs/founder-animals/ (accessed on 2nd January 2023).
- Johnston, L.A.; Lacy, R.C. Genome Resource Banking for Species Conservation: Selection of Sperm Donors. Cryobiology 1995, 32, 68–77. [Google Scholar] [CrossRef]
- Amphibian Population Management Guidelines. Created at an Amphibian Population Management Workshop; 2007 December 10-11; San Diego, CA, USA. Schad, K., Ed.; Amphibian Ark, 2008, 31 p. Available online: https://www.amphibianark.org/wp-content/uploads/2018/08/AArk-Amphibian-Population-Management-Guidelines.pdf.
- Beebee, T.J.C. Conservation Genetics of Amphibians. Heredity 2005, 95, 423–427. [Google Scholar] [CrossRef]
- Johnson, W.E. Koepfli, K. The Role of Genomics in Conservation and Reproductive Sciences. In Reproductive Sciences in Animal Conservation. Holt, W., Brown, J., Comizzoli, P. Eds.; Springer, New York, NY. 2014, Vol 753, p 533.
- Farquharson, K.A.; Hogg, C.J.; Grueber, C.E. Offspring Survival Changes over Generations of Captive Breeding. Nat. Commun. 2021, 12, 3045. [Google Scholar] [CrossRef]
- Hinkson, K.M.; Poo, S. Inbreeding Depression in Sperm Quality in a Critically Endangered Amphibian. Zoo. Biol. 2020, 39, 197–204. [Google Scholar] [CrossRef]
- Hinkson, K.M.; Poo, S. Inbreeding Depression in Sperm Quality in a Critically Endangered Amphibian. Zoo Biol. 2020, 39, 197–204. [Google Scholar] [CrossRef]
- Conde, D.A.; Colchero, F.; Gusset, M.; Pearce-Kelly, P.; Byers, O.; Flesness, N.; Browne, R.K.; Jones, O.R. Zoos through the Lens of the IUCN Red List: A Global Metapopulation Approach to Support Conservation Breeding Pro-grams. PLOS One, 2013, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kardos. M.; Armstrong, E.E.;, Fitzpatrick, S.W.; Hauser, S.; Hedrick, P.W.; Miller, J.M.; Tallmon, D.A.; Funk, W.C. The Crucial Role of Genome-wide Genetic Variation in Conservation. Proc. Natl. Acad. Sci USA, 2021, 118, e2104642118. [Google Scholar] [CrossRef]
- Kouba, A.J. Genome Resource Banks as a Tool for Amphibian Conservation. In Reproductive Technologies and Biobanking for the Conservation of Amphibians; Silla A.J., Kouba A.J., Heatwole H., Eds.; CSIRO, Melbourne, Australia, 2022; pp. 204–220. ISBN 9781032372075.
- Onley, I.R.; Moseby, K.E.; Austin, J.J. Genomic Approaches for Conservation Management in Australia under Climate Change. Life 2021, 11, 653. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, V.; Barkha, S.; Venu, G.; Gowri, M.; Lisa, G. ; Kishor. G.B. Ex-situ Management of Amphibians in Indian Zoos. Central Zoo Authority, New Delhi, 2022; pp.102.
- Eskew, E.A.; Shock, B.C.; LaDouceur, E.E.B.; Keel, K.; Miller, M.R.; Foley, J.E.; Todd, B.D. Gene Expression Differs in Susceptible and Resistant Amphibians Exposed to Batrachochytrium dendrobatidis. R. Soc. Open. Sci. 2018, 5, 170910. [Google Scholar] [CrossRef] [PubMed]
- Hantak, M.M.; Kuchta, S.R. Predator Perception Across Space and Time: Relative Camouflage in a Colour Polymorphic Salamander, Biol. J. Linnean Soc. 2018, 123, 21–33. [Google Scholar] [CrossRef]
- Mahony, M.J.; Clulow, J. Appendix 2. Cryopreservation and Reconstitution Technologies: A Proposal to Establish A Genome Resource Bank For Threatened Australian Amphibians. In Guidelines for minimising disease risks associated with captive breeding, raising and restocking programs for Australian frogs; Murray, K., Skerratt, L.F., Marantelli, G., Berger, L., Hunter, D., Eds.; Canberra: Australian Government, Department of Sustainability, Environment, Water, Population and Communities, 2011, ID 1011-1151. [Google Scholar]
- FAO. 2012. Cryoconservation of Animal Genetic Resources. UN Food and Agriculture Organization. No. 12. Rome.
- Harnal, V.K.; Wildt, D.E.; Bird, D.M.; Monfort, S.L.; Ballou, J.D. Computer Simulations to Determine the Efficacy of Different Genome Resource Banking Strategies for Maintaining Genetic Diversity. Cryobiology, 2002, 44, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Witzenberger, K.A.; Hochkirch, A. Ex situ Conservation Genetics: a Review of Molecular Studies on the Genetic Consequences of Captive Breeding Programs for Endangered Animal Species. Biodivers. Conserv. 2011, 20, 1843–1861. [Google Scholar] [CrossRef]
- Crates, R.; Stojanovic, D.; Heinsohn, R. The Phenotypic Costs of Captivity. Biol. Rev. Camb. Philos. Soc. 2023, 98, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, J.; Do, Y. Importance and Application of Amphibian Sperm Cryopreservation. J. Wetl. Res. 2023, 25, 257–266. [Google Scholar] [CrossRef]
- Howell, L.G.; Frankham, R.; Rodger, J.C.; Witt, R.R.; Clulow, S.; Upton, R.M.O.; Clulow, J. Integrating Biobanking Minimises Inbreeding and Produces Significant Cost Benefits for a Threatened Frog Captive Breeding Programs. Conserv. Lett. 2021, 14, e12776. [Google Scholar] [CrossRef]
- Howell, L.G.; Mawson, P.R.; Frankham, R.; Rodger, J.C.; Upton, R.M.O.; Witt, R.W.; Calatayud, N.E.; Clulow, S.; Clulow, J. Integrating Biobanking could Produce Significant Cost Benefits and Minimise Inbreeding for Australian Amphibian Captive Breeding Program. Reprod. Fert. Dev. 2021, 33, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, R.E.; Naydenova, E.; Proaño-Bolaños, C.; Vizuete, K.; Debut, A.; Arias, M.T.; Coloma, L.A. Development of Assisted Reproductive Technologies for the Conservation of Atelopus sp. (spumarius complex). Cryobiology 2022, 105, 20–31. [Google Scholar] [CrossRef]
- AArk Conservation Needs Assessments. 2023. Available online: https://www.conservationneeds.org/default.aspx (accessed on 23 September 2023).
- Rehberg-Besler, N.; Doucet, S.M.; Mennill, D.J. Vocal Behavior of the Explosively Breeding Neotropical Yellow Toad, Incilius luetkenii J. Herpetol. 2016, 50, 502–508. [Google Scholar] [CrossRef]
- Kupfer, A. Ch 5, Sexual size dimorphism in amphibians: an overview In Sex, Size and Gender Roles: Evolutionary Studies of Sexual Size Dimorphism Fairbairn, D.J., Wolf U. Blanckenhorn, W.U., Székely, T. Ed Oxford Academic Books, 2007, pp 50–59. [CrossRef]
- Liao, W.B.; Zeng, Y.; Yang, J.D. Sexual Size Dimorphism in Anurans: Roles of Mating System and Habitat Types. Front Zool. 2013, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.C.; Zamudio, K.R. Sexual Dichromatism in Frogs: Natural Selection, Sexual Selection and Unexpected Diversity. Proc. Biol. Sci. 2012, 279, 4687–4693. [Google Scholar] [CrossRef]
- Christe, P.; Keller, L.; Roulin, A. The Predation Cost of Being a Male: Implications for Sex-specific Rates of Ageing. Oikos 2006, 114, 381–384. [Google Scholar] [CrossRef]
- Han, X.; Fu, J. Does Life History Shape Sexual Size Dimorphism in Anurans? A Comparative Analysis. BMC Evol. Biol. 2013, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.J.; Tuttle, M.; Taft, LK. The Costs and Benefits of Frog Chorusing Behavior. Behav. Ecol. Sociobiol. 1981, 8, 273–278. [Google Scholar] [CrossRef]
- Watt, A.M.; Marcec-Greaves, R.; Hinkson, K.M.; Poo, S.; Roberts, B.; Pitcher, T.E. Effects of Age on Sperm Quality Metrics in Endangered Mississippi Gopher Frogs (Lithobates sevosus) from Captive Populations Used for Controlled Propagation and Reintroduction Efforts. Zoo Biol. 2021, 40, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Vieites, D.R.; Nieto-Román, S.; Barluenga, M.; Palanca, A.; Vences, M.; Meyer, A. Post-mating Clutch Piracy in an Amphibian. Nature 2004, 431, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Borowsky, R.; Luk, A.; He, X.; Kim, R.S. Unique Sperm Haplotypes are Associated with Phenotypically Different Sperm Subpopulations in Astyanax Fish. BMC Biol. 2018, 16, 72. [Google Scholar] [CrossRef]
- Scheltinga, D.M.; Jamieson, B.G.M. Spermatogenesis and the Mature Spermatozoon: Form, Function and Phylogenetic Implications, In Reproductive biology and phylogeny of Anura; Jamieson, G.M., Ed.; Science Publishers Inc.: NH, USA, 2013; Volume 2, pp. 119–252. ISBN 1-57808-288-9. [Google Scholar]
- Firman, R.C.; Gasparini, C.; Manier, M.K.; Pizzari, T. Postmating Female Control: 20 Years of Cryptic Female Choice. Trends Ecol. Evol. 2017, 32, 368–382. [Google Scholar] [CrossRef]
- Poo, S.; Bogisich, A.; Mack, M.; Lynn, B.K.; Devan-Song, A. Post-release Comparisons of Amphibian Growth reveal Challenges with Sperm Cryopreservation as a Conservation Tool. Conserv. Sci. Pract. 2022, 4, 5721–5729. [Google Scholar] [CrossRef]
- Hughes, DP.; Libersat, F. Parasite Manipulation of Host Behavior, Curr. Biol. 2019, 29, 45–47. [Google Scholar] [CrossRef]
- Browne, R.K.; Li, H.; Vaughan, M. Sexually Mediated Shedding of Myxobolus fallax Spores During Spermiation of Litoria fallax (anura). Dis. Aquat. Org. 2006, 72, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Brannelly, L.A.; Webb, R.; Skerratt, L.F.; Berger, L. Amphibians with Infectious Disease Increase their Reproductive Effort: Evidence for the Terminal Investment Hypothesis. Open Biol. 2016, 6, 150251. [Google Scholar] [CrossRef] [PubMed]
- Hartigan, A.; Phalen, D.N.; Slapeta, J. Myxosporean Parasites in Australian Frogs: Importance, Implications and Future Directions. Int. J. Parasitol Parasites Wildl. 2013, 2, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Eiras, J.C. An Overview on the Myxosporean Parasites in Amphibians and Reptiles. Acta Parasitol. 2005, 50, 267–275. [Google Scholar]
- Hartigan, A.; Phalen, D.N.; Šlapeta, J. Museum Material Reveals a Frog Parasite Emergence after the Invasion of the Cane Toad in Australia. Parasites Vectors, 2010, 3, 50. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.R.; Scheele, B.C.; Silla, A.J.; Keogh, S.; Hunter, D.A.; Endler, J.A.; Byrne, P.G. Disease influences male advertisement and mating outcomes in a critically endangered amphibian. Anim. Behav. 2021, 173, 145–157. [Google Scholar] [CrossRef]
- Narayan, E. 2022. Chapter 5. Non-invasive Monitoring of Stress Physiology During Management and Breeding of Amphibians in Captivity. In Reproductive Technologies and Biobanking for the Conservation of Amphibians; Silla, A.J., Kouba, A.J., Heatwole, H., Eds.; CRC Press: London, UK, 2022; pp. 49–68. ISBN 9781032372075. [Google Scholar]
- 94. Grummer, J.A.; Booker, T.R.; Matthey-Doret, R.; Nietlisbach, P.; Thomaz, A.T.; Whit-lock, M.C. The Immediate Costs and Long-term Benefits of Assisted Gene Flow in Large Populations. Conserv. Biol. 2022, 36, 13911. [Google Scholar] [CrossRef]
- Burger, I.J. The ART of Amphibian Conservation: Linking In-situ and ex-situ Populations of Endangered Species Through Genome Banking, 2021. MS Theses and Dissertations. 5379. https://scholarsjunction.msstate.edu/td/5379.
- Kelly, E.; Phillips, B.L. Targeted gene flow for conservation. Conserv. Biol. 2016, 30, 259–267. [Google Scholar] [CrossRef]
- Keller, L. F.; Waller, D. M. Inbreeding Effects in Wild Populations. Trends Ecol. Evol. 2002, 17, 230–241. [Google Scholar] [CrossRef]
- Brook, B.W.; Tonkyn, D.W.; O'Grady, J.J.; Frankham, R. Contribution of Inbreeding to Extinction Risk in Threatened Species. Conserv. Ecol. 2002, 6, 16. Available online: http://www.consecol.org/vol6/iss1/art16/138. [CrossRef]
- Stock, S.E.; Klop-Toker, K.; Wallace, S.; Kelly, O.; Callen, A.; Seeto, R.; Mahony, S.V.; Hayward, M.W.; Mahony, M.J. Uncovering Inbreeding, Small Populations, and Strong Genetic Isolation in an Australian Threatened Frog, Litoria littlejohni. Conserv. Genet. 2023, 24, 575–588. [Google Scholar] [CrossRef]
- Reed, D.H.; Frankham, R. Correlation Between Fitness and Genetic Diversity. Conserv. Biol. 2003, 17, 230–237. [Google Scholar] [CrossRef]
- Cheptou, P.O.; Hargreaves, A.L.; Bonte, D.; Jacquemyn, H. Adaptation to Fragmentation: Evolutionary Dynamics Driven by Human Influences. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160037. [Google Scholar] [CrossRef] [PubMed]
- Westram, A.M.; Stankowski, S.; Surendranadh, P.; Barton, N. What is Reproductive Isolation? J. Evol. Biol. 2022, 35, 1143–1164. [Google Scholar] [CrossRef] [PubMed]
- Nistelberger, H.M.; Roycroft, E.; Macdonald, A.J.; McArthur, S.; White, L.C.; Grady, P.G.S.; Pierson, J.; Sims, C.; Cowen, S.; Moseby, K.; et al. Genetic Mixing in Conservation Translocations Increases Diversity of a Keystone Threatened Species, Bettongia lesueur. Mol. Ecol. 2023, Epub ahead of print. [CrossRef]
- Albert, E.M.; Ferna´ndez-Beaskoetxea, S.; Godoy, J.A.; Tobler, U.; Schmidt, B.R.; Bosch, J. Genetic Management of an Amphibian Population after Chyridiomycosis Outbreak. Conserv. Genet. 2015, 16, 103–111. [Google Scholar] [CrossRef]
- Frankham, R.; Ballou, J.D.; Eldridge, M.D.; Lacy, R.C.; Ralls, K.; Dudash, M.R.; Fenster, C.B. Predicting the Probability of Outbreeding Depression. Conserv. Biol. 2011, 25, 465–475. [Google Scholar] [CrossRef]
- Lindsay, W.R.; Madsen, T.; Wapstra, E.; Lillie, M.; Loeb, L.; Ujvari, B.; Olsson, M. Long Term Effects of Outbreeding: Experimental Founding of Island Population Eliminates Malformations and Improves Hatching Success in Sand Lizards. Biol. Conserv. 2020, 249, 108710. [Google Scholar] [CrossRef]
- Frankham, R.; Bradshaw, C.J.A.; Brook, B.W. Genetics in Conservation Management: Revised Recommendations for the 50/500 rules, Red List Criteria and Population Viability Analysis. Biol. Conserv. 2014, 170, 56–63. [Google Scholar] [CrossRef]
- Silla, A.J.; Byrne, P.G. The importance of quantifying fitness-determining traits throughout life to assess the application of reproductive technologies for amphibian species recovery. Front. Conserv. Sci. 2024, 5. [Google Scholar] [CrossRef]
- Scheele, B.C.; Hunter, D.A.; Skerratt, L.F.; Brannelly, L.A.; Driscoll, D.A. Low Impact of Chytridiomycosis on Frog Recruitment Enables Persistence in Refuges Despite High Adult Mortality. Biol. Conserv. 2015, 182, 36–43. [Google Scholar] [CrossRef]
- Sgrò, C.M.; Lowe, A.J.; Hoffmann, A.A. Building Evolutionary Resilience for Conserving Biodiversity under Climate Change. Evol. Appl. 2011, 4, 326–337. [Google Scholar] [CrossRef]
- Carvalho, CS.; Lanes ECM. ; Silva AR.; Caldeira, CF.; Carvalho-Filho, N.; Gastauer, M.; Imperatriz-Fonsecaa, VL, Nascimento Jr, W.; Oliveeira, G.; Siqueira, J.O.; Viana, P.L. Jaffe, R. Habitat Loss Does Not Always Entail Negative Genetic Consequences. Front. Genet. 2019, 10, 1011. [Google Scholar] [CrossRef]
- Willi, Y.; Hoffmann, A.A. Demographic Factors and Genetic Variation Influence Population Persistence under Environmental Change. J. Evol. Biol. 2009, 22, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.; Phillips, B.L. Targeted Gene Flow for Conservation. Conserv. Biol. 2016, 30, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Tigano, A.; Friesen, V.L. ; Genomics of Local Adaptation with Gene Flow. Molec. Ecol. 2016, 25, 2144–2164. [Google Scholar] [CrossRef] [PubMed]
- Robert, A. Captive Breeding Genetics and Reintroduction Success. Biol. Conserv. 2009, 142, 2915–2922. [Google Scholar] [CrossRef]
- Calatayud, N.E.; Jacobs, L.; Della Togna, G.; Langhorne, C.J.; Mullen, A.C.; Upton, R. Hormonal Induction, Quality Assessments and the Influence of Seasonality on Male Reproductive Viability in a Long-term Managed Ex Situ Breeding Colony of Southern Rocky Mountain Boreal Toads, Anaxyrus boreas boreas. bioRxrv preprint, 2023. [CrossRef]
- Edmands, S. Between a Rock and a Hard Place: Evaluating the Relative Risks of Inbreeding and Outbreeding for Conservation and Management. Molec. Ecol. 2007, 16, 463–475. [Google Scholar] [CrossRef]
- Earnhardt, J.M. Reintroduction Programmes: Genetic Trade-offs for Populations. Anim. Conserv. 2006, 2, 279–286. [Google Scholar] [CrossRef]
- Byrne, P.G.; Silla, A.J. Genetic Management of Threatened Amphibians: Using Artificial Fertilisation Technologies to Facilitate Genetic Rescue and Assisted Gene Flow. In Reproductive Technologies and Biobanking for the Conservation of Amphibians; Silla A.J., Kouba A.J., Heatwole H., Eds.; CSIRO, Melbourne, Australia, 2022; pp. 124–146, ISBN 9781032372075.
- Byrne, P.G.; Keogh, J.S.; O'Brien, D.M.; Gaitan-Espitia, J.D.; Silla, A.J. Evidence that genetic compatibility underpins female mate choice in a monandrous amphibian. Evolution 2021, 75, 529–541. [Google Scholar] [CrossRef]
- Spielman, D.; Brook, B. W.; Frankham, R. Most Species are not Driven to Extinction before Genetic Factors Impact Them. PNAS, 2004, 101, 15261–15264. [Google Scholar] [CrossRef]
- Teixeira, J.C.; Huber, C.D. The Inflated Significance of Neutral Genetic Diversity in Conservation Genetics. Proc. Nat. Acad. Sci. 2021, 118, e2015096118. [Google Scholar] [CrossRef] [PubMed]
- Walter, H.S. The Mismeasure of Islands: Implications for Biogeographic Theory and the Conservation of Nature. J. Biogeog. 2004, 31, 177–197. [Google Scholar] [CrossRef]
- Revive and Restore. The Black-footed Ferret Project. Available online: https://reviverestore.org/projects/black-footed-ferret/ (accessed on 23 September 2023).
- Scheele, B.C.; Guarino, F.; Osborne, W.; Hunter, D.A.; Skerratt, L.F.; Driscoll, D.A. Decline and Re-expansion of an Amphibian with High Prevalence of Chytrid Fungus. Biol. Conserv. 2014, 170, 86–91. [Google Scholar] [CrossRef]
- O'Hanlon, S.J.; Rieux, A.; Farrer, R.A.; Rosa, G.M.; Waldman, B.; Bataille, A.; Kosch, T.A.; Murray, K.A.; Brankovics, B.; Fumagalli, M.; et al. Recent Asian Origin of Chytrid Fungi Causing Global Amphibian Declines. Science 2018, 360, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Dreitz, V.J. Issues in Species Recovery: An Example Based on the Wyoming Toad: Forum. BioScience 2006, 56, 765–771. [Google Scholar] [CrossRef]
- Scheele, B.C.; Hunter, D.A.; Skerratt, L.F.; Brannelly, L.A.; Driscoll, D.A. Low Impact of Chytridiomycosis on Frog Recruitment Enables Persistence in Refuges Despite High Adult Mortality. Biol. Conserv. 2015, 182, 36–43. [Google Scholar] [CrossRef]
- Burger, I.; Julien, A.R.; Kouba, A.J.; Councell, K.R.; Barber, B.; Pacheco, C.; Kouba, C.K. Linking In Situ and Ex Situ Populations of the Endangered Puerto Rican Crested Toad (Peltophryne lemur) through Genome Banking. Conserv. Sci. Pract. 2021, 3, e525. [Google Scholar] [CrossRef]
- Viggers, K.L.; Lindenmayer, D.B.; Spratt, D.M. The Importance of Disease in Reintroduction Programmes. Wildlife Research 1993, 20, 687–698. [Google Scholar] [CrossRef]
- Ballou, J.D. Assessing the risks of infectious diseases in captive breeding and reintroduction programs. J. Zoo. Wildl. Med. 1993, 24, 327–335. [Google Scholar]
- Chaudhary, V.; Oli, M.K. A Critical Appraisal of Population Viability Analysis. Conserv. Biol. 2019, 34, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Willi, Y.; Kristensen, T.N.; Sgrò, C.M.; Weeks, A.R.; Ørsted, M.; Hoffmann, A.A. Conservation genetics as a management tool: The Five Best-supported Paradigms to Assist the Management of Threatened Species. Proc Natl Acad Sci USA. 2022, 119, e2105076119. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R. Do Island Populations Have Less Genetic Variation than Mainland Populations? Heredity 1997, 78, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Fauvel, C.; Suquet, M.; Cosson, J. Evaluation and Determinism of Fish Sperm Quality. J. Appl. Ichthyol. 2010, 26, 636e43. [Google Scholar] [CrossRef]
- EDGE. Evolutionary Distinct and Globally Endangered. Zoological Society of London. Available online: https://www.edgeofexistence.org/species/ (accessed on 1 August 2023).
- Khosla, K.; Kangas, J.; Liu, Y.; Zhan, L.; Daly, J.; Hagedorn, M.; Bischof, J. Cryopreservation and Laser Nanowarming of Zebrafish Embryos Followed by Hatching and Spawning. Adv. Biosyst. 2020, 4, e2000138. [Google Scholar] [CrossRef]
- de Siqueira-Silva, D.H.; Saito, T.; dos Santos-Silva, A.P.; da Silva Costa, R.; Psenicka, M.; Yasui, G.S. Biotechnology Applied to Fish Reproduction: Tools for Conservation. Fish Physiol. Biochem. 2018, 44, 1469–1485. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.K. Figiel, C. Chapter 2. Crypreservation in Amphibians In: Cryopreservation of Aquatic Species. Vol 8. Tiersch, T., Mazik, P. Eds.: World Aquaculture Society, Baton Rouge, La Hardcover, 2011, Vol 8, ~450 pages.
- Behera, B.J. Surrogacy Technology in Fisheries and Aquaculture. In: Current Trends in Fisheries Biotechnology. Behera, B.K. Ed.; Springer, 2024. [CrossRef]
- Browne, R.K.; Zippel, K.; Odum, A.R.; Herman, T. Physical Facilities and Associated Services. Use of Amphibians in Research, Laboratory, or Classroom Settings. Inst. Lab. Anim. Res. (ILAR), 2007, 48, 188–202. [Google Scholar] [CrossRef]
- AArk Reproduction Technologies videos. Available online: https://www.amphibianark.org/art-videos/ (accessed on 23 September 2023).
- Hamer, A.J.; Mahony, M.J. 2007. Life History of an Endangered Amphibian Challenges the Declining Species Paradigm. Australian J. Zool. 2007, 55, 79–88. [Google Scholar] [CrossRef]
- Richter, S.C.; Seigel, R.A. Annual Variation in the Population Ecology of the Endangered Gopher Frog, Rana sevosa Goin and Netting. Copeia 2002, 2002, 962–972. [Google Scholar] [CrossRef]
- McGinnity, D.; Reinsch, S.R.; Schwartz, H.; Trudeau, V.; Browne, R.K. Semen and Egg Collection, Sperm Cryopreservation, and in vitro Fertilisation with Threatened North American Giant Salamanders (Cryptobranchus alleganiensis). Reprod. Fertil. Dev. 2021, 34, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Lötters, S.; Plewnia, A.; Catenazzi, A.; Neam, K.; Acosta-Galvis, A.R.; Vela, Y.A.; Allen, J.P.; Segundo, J.O.A.; de Lourdes Almendáriz Cabezas, A. Barboza, G.A.; et al. Ongoing Harlequin Toad Declines Suggest the Amphibian Extinction Crisis is Still an Emergency. Commun. Earth Environ. 2023, 4, 412. [Google Scholar] [CrossRef]
- Valencia, L.M.; Fonte, L.F. The Fate of Harlequin Toads – Help Through a Synchronous Approach and the IUCN ‘Amphibian Conservation Action Plan? Oryx 2007, 39, 343–346. [Google Scholar] [CrossRef]
- Valencia, L.M.; Fonte, L.F. Atelopus Survival Initiative IUCN SSC ASG Atelopus Task Force. 2021. Available online: https://www.atelopus.org/_files/ugd/9db650_60f3e6095cbf4b1dabb7376a4fb88366.pdf (accessed on 7 August 2024).
- Buckner, J.C.; Sanders, R.C.; Faircloth, B.C.; Chakrabarty, P. The Critical Importance of Vouchers in Genomics. Elife 2021, 10, e68264. [Google Scholar] [CrossRef]
- Andreone, D.; Raselimanana, A.P. Crottini, A, Vouchering, Integrative Taxonomy and Natural History Collections: a Case Study with the Amphibians of Madagascar. Boll. Mus. Reg. Sci. Nat. Toronto 2023, 40(1–2), 41–57.
- Rocha, L.A.; Aleixo, A.; Allen, G.; Almeda, F.; Baldwin, C.C.; Barclay, M.V.L.; Bates, M.; Bauer, A.M.; Benzoni, F.; Berns, C.M.; et al. Specimen Collection: An Essential Tool. Science 2014, 344, 814–815. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hopkins, A.J.M.; Davis, R.A. Going, Going, Gone The Diminishing Capacity of Museum Specimen Collections to Address Global Change Research: A Case Study on Urban Reptiles. Animals 2023, 13, 1078. [Google Scholar] [CrossRef]
- Powell, D.M.; Meyer, T.G. , Duncan, M. By Bits and Pieces: The Contributions of Zoos and Aquariums to Science and Society via Biomaterials. J. Zool. Bot. Gard. 2023, 4, 277–287. [Google Scholar] [CrossRef]
- Goncharov, B.F.; Shubravy, O.I.; Serbinova, I.A.; Uteshev, V.K. The USSR programme for breeding amphibians, including rare and endangered species. Int. Zoo Yearbook, 1989, 28, 10–21. [Google Scholar] [CrossRef]
- World Animal Protection. Available online: https://www.worldanimalprotection.org/ (accessed on 27 July 2024).
- Rakesh, R.K. Seasonal Cycle in Anuran (Amphibia) Testis: The Endocrine and Environmental Controls. Italian J. Zool. 1976, 43(1-2), 151–172.
- Rastogi, R.K.; Tammaro, L.; Di Meglio, M.; Lela, L.; Di Matteo, l.; Giovanni Chieffi, G. Circannual Testicular Rhythm in the Green Frog, Rana esculenta. Italian J. Zool. 1981, 48, 97–105. [Google Scholar] [CrossRef]
- Ulloa, J.S.; Aubin, T.; Llusia, D.; Courtois, E.A.; Fouquet, A.; Gaucher, P.; Pavoine, S.; Sueur, J.; et al. Explosive Breeding in Tropical Anurans: Environmental Triggers, Community Composition and Acoustic Structure. BMC Ecol. 2019, 19, 28. [Google Scholar] [CrossRef]
- Kaurova, S.A.; Uteshev, V.K.; Gapeyev, A.B.; Shishova, N.V.; Gakhova, E.N.; Browne, R.K.; Kramarova, L.I. Cryopreservation of Spermatozoa Obtained Postmortem from the European Common Frog Rana temporaria. Reprod. Fert. Dev. 2021, 33. [Google Scholar] [CrossRef]
- Figiel Jr., C. R. Effects of Water Temperature on Gonads Growth in Ambystoma mexicanum Axolotl Salamanders. Animals 2023, 13, 874. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D. M.; Silla, A. J.; Forsythe, P. S.; Byrne, P. G. Sex Differences in Response to Environmental and Social Breeding Cues in an Amphibian. Behaviour 2021, 158, 397–426. [Google Scholar] [CrossRef]
- Edwards, D.L.; Mahony, M.J.; Clulow, J. Effect of Sperm Concentration, Medium Osmolality and Oocyte Storage on Artificial Fertilisation Success in a Myobatrachid Frog (Limnodynastes tasmaniensis). Reprod. Fertil. Dev. 2004, 16, 347–354. [Google Scholar] [CrossRef]
- Upton, R.; Clulow, S.; Colyvas, K.; Mahony, M.; Clulow, J. Paradigm Shift in Frog Sperm Cryopreservation: Reduced Role for Non-penetrating Cryoprotectants. Reproduction 2023, 165, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Upton, R.; Clulow, S.; Mahony, M.J.; and Clulow, J. Generation of a Sexually Mature Individual of the Eastern Dwarf Tree Frog Litoria fallax, from Cryopreserved Testicular Macerates: Proof of Capacity of Cryopreserved Sperm Derived Offspring to Complete Development. Conserv. Physiol. 2018, 6, coy043. [Google Scholar] [CrossRef] [PubMed]
- Silla, A.J.; Keogh, L.M.; Byrne, P.G. Sperm Motility Activation in the Critically Endangered Booroolong Frog: the Effect of Medium Osmolality and Phosphodiesterase Inhibitors. Reprod. Fertil. Dev. 2017, 29, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Germano, J.M.; Cree, A; Molinia, F. C; Arregui, L.; Bishop, P.J. Hormone Treatment does not Reliably Induce Spermiation or Mating in Hamilton’s frog from the Archaic Leiopelmatid Lineage. Reprod. Fert. Devel. 2021, 34, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Hormone therapy. Available online: https://en.wikipedia.org/wiki/Hormone_therapy (accessed on 17th July, 20240 (accessed on 7 August 2024).
- Miyamoto, K.; Simpson, D.; Gurdon, J.B. Manipulation and in vitro Maturation of Xenopus laevis Eggs, Followed by Intracytoplasmic Sperm Injection, to Study Embryonic Development. J. Vis. Exp. 2015, 96, 52496. [Google Scholar] [CrossRef]
- Herbert, D. Studies of Assisted Reproduction in the Spotted Grass Frog Limnodynastes tasmaniensis: Ovulation, Early Development and Microinjection (ICSI). Masters by Research thesis, 2004, The University of Newcastle, Australia.
- Ishibashi, S.; Kroll, K.L.; Amaya, E. Generation of Transgenic Xenopus laevis: III. Sperm Nuclear Transplantation. Cold Spring Harb. Protoc. 2007, 2007:pdb.prot4840. [CrossRef]
- Parmar, M.S.; Pant, C.; Karuppanasamy, K.; Mili, B.; Upadhyay, D.; Kant, V. Intracytoplasmic Sperm Injection (ICSI) and its Applications in Veterinary Sciences: An Overview. Sci. Int. 2013, 1, 266–270. [Google Scholar]
- Silla, A.J.; Byrne, P.G. Hormone-induced Ovulation and Artificial Fertilisation in Four Terrestrial-breeding Anurans. Reprod. Fertil. Dev. 2021, 33, 615–618. [Google Scholar] [CrossRef]
- Browne, R.K.; Seratt, J.; Vance, C.; Kouba, A. Hormonal Priming, Induction of Ovulation and In-vitro Fertilization of the Endangered Wyoming Toad (Bufo baxteri). Reprod. Biol. Endocrinol. 2006, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Marcec-Greaves, R.M.; Kouba, C.K.; Willard, S.T.; Andy, J. Kouba, A.J. Ovarian Ultrasound Analysis for Developing Temporal and Spatially Explicit Hormone Regimens for Induced Ovulation and Egg Deposition in the Tiger Salamander (Ambystoma tigrinum), Theriogenology Wild, 2023, 2,2023, 100038. [CrossRef]
- Shishova, N.V.; Uteshev, V. K.; Sirota, N.P.; Kuznetsova, E.A.; Kaurova, S.A.; Browne, R.K.; Gakhova, E.N. The Quality and Fertility of Sperm Collected from European Common Frog (Rana temporaria) Carcasses Refrigerated for up to 7 Days. Zoo Biol. 2013, 32, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Silla, A.J.; Roberts, J.D.; Byrne, P.G. The Effect of Injection and Topical Application of hCG and GnRH Agonist to Induce Sperm-release in the Roseate Frog, Geocrinia rosea. Conserv. Physiol. 2020, 8, coaa104. [Google Scholar] [CrossRef] [PubMed]
- Marcec, R.M. Development of Assisted Reproductive Technologies for Endangered North American Salamanders by Ruth Marie Marcec. Faculty of Mississippi State University, Degree of Doctor of Philosophy in Animal Physiology, 2016; Mississippi, MS, USA.
- Coxe, N.; Liu, Y.; Arregui, L.; Upton, R.; Bodenstein, S.; Voss, S.R.; Gutierrez-Wing, M.T.; Tiersch, T.R. Establishment of a Practical Sperm Cryopreservation Pathway for the Axolotl (Ambystoma mexicanum): A Community-Level Approach to Germplasm Repository Development. Animals 2024, 14, 206. [Google Scholar] [CrossRef] [PubMed]
- Guy, E.L.; Gillis, A.B.; Kouba, A.J.; Barber, D.; Poole, V.; Marcec-Greaves, R.M.; Kouba, C.K. Sperm Collection and Cryopreservation for Threatened Newt Species. Cryobiology 2020, 94, 80–88. [Google Scholar] [CrossRef]
- Chen, D.M.; Kouba, C.K.; Songsasen, N.; Roth, T.L.; Allen, P.J.; Kouba, A.J. Comparing novel sperm extenders for the internally-fertilizing tiger salamander (Ambystoma tigrinum). Front. Amphib. Reptile Sci. 2023, 1, 1320803. [Google Scholar] [CrossRef]
- Julien, A.R.; Kouba, A.J.; Kabelik, D.; Feugangm, J.M.; Willard, S.T.; Kouba, C.K. Nasal administration of gonadotropin releasing hormone (GnRH) elicits sperm production in Fowler’s toads (Anaxyrus fowleri). BMC Zool. 2019, 4, doi. [Google Scholar] [CrossRef]
- Campbell, L.G.; Anderson, K.A.; Marcec-Greaves, R.M. Topical Application of Hormone Gonadotropin-releasing Hormone (GnRH-A) Stimulates Reproduction in the Endangered Texas Blind Salamander (Eurycea rathbuni). Conserv. Sci. Pract. 2021, 4, e609. [Google Scholar] [CrossRef]
- Toledo RC, Jared C. Cutaneous adaptations to water balance in amphibians. Comp. Biochem. Physiol. B Biochem. Physiol. 1993, 105, 593–608. [Google Scholar] [CrossRef]
- Chen, D.M.; Chen, L.D.; Kouba, C.K.; Songsasen, N.; Roth, T.L.; Allen, P.J.; Kouba, A.J. Oral Administration of GnRH via a Cricket Vehicle Stimulates Spermiation in Tiger Salamanders (Ambystoma tigrinum). PLoS ONE, 2024, 19, e0289995. [Google Scholar] [CrossRef]
- John Clulow, Melissa Pomering, Danielle Herbert, Rose Upton, Natalie Calatayud, Simon Clulow, Michael J. Mahony, Vance L. Trudeau. Differential success in obtaining gametes between male and female Australian temperate frogs by hormonal induction: A review. Gen. Comp. Endocrinol. 2028, 265, 141–148. [CrossRef]
- Trudeau, V.L.; Raven, B.H.; Pahuja, H.K.; Narayan, E. J 2022. Chapter 4. Hormonal Control of Amphibian Reproduction. In Reproductive Technologies and Biobanking for the Conservation of Amphibians; Silla, A.J., Kouba, A.J., Heatwole, H., Eds.; CRC Press: London, UK, 2022; pp. 49–68. ISBN 9781032372075. [Google Scholar]
- Browne, R.K.; Li, H.; Seratt, J.; Kouba. A. Progesterone Improves the Number and Quality of Hormone Induced Fowler toad (Bufo fowleri) Oocytes. Reprod. Biol. Endocrinol. 2006, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mansour, N.; Lahnsteiner, F.; Patzner, R.A. Collection of Gametes from Live Axolotl, Ambystoma mexicanum, and Standardization of In Vitro Fertilization. Theriogenology 2011, 75, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Uteshev, V.K.; Kaurova, S.A.; Shishova, N.V.; Stolyarov, S.D.; Browne, R.K.; Gakhova, E.N. In Vitro Fertilization with Hormonally Induced Sperm and Eggs from Sharp-ribbed Newt Pleurodeles waltl. Russ. J. Herpetol. 2015, 22, 35–40. [Google Scholar] [CrossRef]
- Graham, K.M.; Langhorne, C.J.; Vance, C.K.; Willard, S,T. ; Kouba, A.J. Ultrasound Imaging Improves Hormone Therapy Strategies for Induction of Ovulation and In Vitro Fertilization in the Endangered Dusky Gopher Frog (Lithobates sevosa). Conserv. Physiol. 2018, 6, coy020. [Google Scholar] [CrossRef]
- Bogolyubova, I.O.; Bogolyubov, D.S. ; Effect of Hormonal Stimulation on the Oocyte Chromosomal Apparatus in the Common Frog. J. Evol. Biochem. Phys. 2023, 59, 1229–1236. [Google Scholar] [CrossRef]
- Browne, R.K.; Wang, Z.; Okada, S.; McGinnity, D.; Luo, Q.; Taguchi Y.; Kilpatrick, D.; Hardman, R.; Janzen, P.; Zhang, Z, Geng Y. The Sustainable Management of Giant Salamanders (Cryptobranchoidea). Report, Sustainability America: Belize, 2020.
- Silla, A.J.; Langhorne, C.J. Protocols for Hormonally Induced Spermiation, and the Cold Storage, Activation, and Assessment of Sperm. In Reproductive Technologies and Biobanking for the Conservation of Amphibians; Silla, A.J., Kouba, A.J., H Heatwole, H., Eds.: London: CRC Press; London, 2022; pp. 106-123.
- Burger, I.J.; Chen, L.; Lampert, S.S.; Kouba, C.K.; Barber, D.; Smith, D.; Cobos, C.; Kouba, A.J. Applying Sperm Collection and Cryopreservation Protocols Developed in a Model Amphibian to Three Threatened Anuran Species Targeted for Biobanking Management. Biol. Conserv. 2023, 277, 109850:1–109850:6. [Google Scholar] [CrossRef]
- Arregui, L.; Martinez-Pastor, F.; Arroyo, F.; Gosálvez, J. Determining the Effects of Sperm Activation in Anuran Cloaca on Motility and DNA Integrity in Epidalea calamita (Bufonidae). Reprod. Fertil. Dev. 2022, 34, 438–446. [Google Scholar] [CrossRef]
- Valencia, L.C.; García, A.; Ramírez-Pinilla, M.P.; Fuentes, J.L. Estimates of DNA Damage by the Comet Assay in the Direct-developing Frog Eleutherodactylus johnstonei (Anura, Eleutherodactylidae). Genet. Mol. Biol. 2011, 34, 681–688. [Google Scholar] [CrossRef]
- Shishova, N.V.; Uteshev, V.K.; Kaurova, S.A.; Browne, R.K.; Gakhova, E.N. Cryopreservation of Hormonally Induced Sperm for the Conservation of Threatened Amphibians with Rana temporaria as a Model Research Species. Theriogenology 2011, 75, 220–232. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Upton, R.; Calatayud, N.E.; Silla, A.J.; Daly, J.; McFadden, M.S.; O’Brien, J.K. Cryopreservation Cooling Rate Impacts Post-thaw Sperm Motility and Survival in Litoria booroolongensis. Animals (Basel) 2023, 13, 3014. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.K.; Venu, G.; Kaurova, S.A. The Case for Considering the Term ‘Mitochondrial Vesicle’ as a Misnomer in Publications about Assisted Reproductive Technologies (ART) for Amphibians. Reprod. Fert. Develop. 2023. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.Y.; Jamieson, B.G.M. The Ultrastructure of the Spermatozoa of Bufonid and Hylid Frogs (Anure, Am-phibia): Implications for Phylogeny and Fertilisation Biology. Zool. Scripta 1993, 22, 309–323. [Google Scholar] [CrossRef]
- Chen, D.M.; Moore, M.G.; Willis, E.L.; Kouba, A.J.; Kouba, C.K. The Impact of Time and Environmental Factors on the Mitochondrial Vesicle and Subsequent Motility of Amphibian Sperm. Comp. Biochem. Physiol. A: Molec. Integ. Physiol. 2022, 111191. [Google Scholar] [CrossRef] [PubMed]
- Figiel Jr., C. R. Cold Storage of Sperm from the Axolotl, Ambystoma mexicanum. Herpetol. Conserv. Biol. 2022, 15, 367–371. [Google Scholar]
- Silla, A.J.; Keogh, L.M.; Byrne, P.G. Antibiotics and Oxygen Availability Affect the Short-term Storage of Spermatozoa from the Critically Endangered Booroolong Frog, Litoria booroolongensis. Reprod. Fertil. Dev. 2015, 27, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Germano, J.M.; Arregui, L.; Kouba, A.J. Effects of Aeration and Antibiotics on Short-term Storage of Fowler’s toad (Bufo fowleri) Sperm. Aquaculture 2013, 396–399, 20–24. [Google Scholar] [CrossRef]
- Kaurova, S.A.; Browne, R.K.; Uteshev, V.K. Antibiotics for the Refrigerated Storage at 4°C of Hormonally Induced European Common Frog (Rana temporaria) Spermatozoa. Theriogenology Wild 2022, 1, 100009. [Google Scholar] [CrossRef]
- Kaurova, S.A.; Shishova, N.V.; Uteshev, V.K. The Effect of Gentamicin on the Motility of Hormonally Induced Spermatozoa of Toad Bufo bufo during Storage at 4°C. Bull. Exp. Biol. Med. 2023, 176, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Kaurova, S.A.; Uteshev, V.K. , Shishova, N.V. Effect of Antibiotics Metranidazole, Streptomycin, and Gentamicin on the Maintenance of Sperm Motility of the European Common Frog (Rana temporaria) During Refrigerated Storage. Russ. J. Herpetol. 2024, 31, 47–55. [Google Scholar] [CrossRef]
- Poo, S.; Hinkson, K.M. Applying Cryopreservation to Anuran Conservation Biology. Conserv. Sci. Pract. 2019, 1, e91. [Google Scholar] [CrossRef]
- Kaneko, T.; Ito, H.; Sakamoto, H.; Onuma, M.; Inoue-Murayama, M. Sperm Preservation by Freeze-drying for the Conservation of Wild Animals. PLoS One 2014, 9, e113381. [Google Scholar] [CrossRef] [PubMed]
- Shishova, N.V.; Kaurova, S.A.; Uteshev, V.K.; Gakhova, E.N. Comparison of Cryoresistance of Testicular and Urinal Spermatozoa of the Toad Bufo bufo (Amphibia, Anura, Bufonidae) during Slow Freezing. Inland Water Biol. 2023, 16, 788–792. [Google Scholar] [CrossRef]
- Liu, M.; Yuan, S.; Zhao, Z.; Liu, M.; Lin, A.; Gong, Q. Non-programmable Cryopreservation of Sperm from Industrial-farmed Murray Cod (Maccullochella peelii). Aquaculture 2021, 541, 736811. [Google Scholar] [CrossRef]
- Arregui, L.; Bóveda, P.; Gosálvez, J.; Kouba, A.J. Effect of Seasonality on Hormonally Induced Sperm in Epidalea calamita (Amphibia, Anura, Bufonidae) and its Refrigerated and Cryopreserved Storage. Aquaculture 2020, 529, e735677. [Google Scholar] [CrossRef]
- Silla, A.J.; Kouba, A.J. Chapter 1. Integrating Reproductive Technologies into the Conservation Toolbox for the Recovery of Amphibian Species. In Reproductive Technologies and Biobanking for the Conservation of Amphibians, Silla, A.J., Kouba, A.J., Heatwole, H., Eds.; CRC Press: London, UK, 2022; pp 1-9, ISBN 9781486313334.172.
- Clulow, J.; Upton, R.; Clulow, S. 2022. Cryopreservation of Amphibian Genomes: Targeting the Holy Grail, Cryopreservation of Maternal-haploid and Embryonic-Diploid Genomes. In Reproductive Technologies and Biobanking for the Conservation of Amphibians; Silla, A.J., Kouba, A.J., H Heatwole, H., Eds.: London: CRC Press; London, 2022; pp. 147-165.
- Uteshev, V.K.; Gakhova, E.N.; Kramarova, L.I.; Shishova, N.V.; Kaurova, S.A.; Browne, R.K. Refrigerated Storage of European Common Frog Rana temporaria Oocytes. Cryobiology 2018, 83, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Wolf, DP.; Hendrick, J.L. A Molecular Approach to Fertilisation. II. Viability and Artificial Fertilisation of Xenopus laevis Gametes. Dev. Biol. 1971, 25, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, TG.; Corton, G.L. Artificial Fertilization of Gametes from the South African Clawed Frog, Xenopus laevis. Gamete Res. 1980, 3, 45–57. [Google Scholar] [CrossRef]
- Gagarinskiy, E.; Uteshev, V.; Fesenko, E.Jr. Prolonged hypothermic storage of oocytes of the European Common Frog Rana temporaria in a Gas Mixture of Oxygen and Carbon Monoxide. PLoS ONE 2023, 18, e0288370:1–e0288370:10. [Google Scholar] [CrossRef] [PubMed]
- Firman, R.C.; Gasparini, C.; Manier, M.K.; Pizzari, T. Postmating Female Control: 20 Years of Cryptic Female Choice. Trends Ecol. Evol. 2017, 32, 368–382. [Google Scholar] [CrossRef]
- Ukita, M.; Itoh, T.; Watanabe, T.; Watanabe, A.; Onitake, K. Substances for the Initiation of Sperm Motility in Egg-jelly of the Japanese Newt, Cynops pyrrhogaster. Zool. Sci. 1999, 16, 793–802. [Google Scholar] [CrossRef]
- Olson, J.H.; Chandler, D.E. Xenopus laevis Egg Jelly contains Small Proteins that are essential to fertilization. Dev. Biol. 1999, 210, 401–410. [Google Scholar] [CrossRef]
- Omata, S. Relative Roles of Jelly Layers in Successful Fertilization of Bufo japonicus. J. Exp. Zool. 1993, 265, 329–335. [Google Scholar] [CrossRef]
- Ukita, M.; Itoh, T.; Watanabe, T.; Watanabe, A.; Onitake, K. Substances for the Initiation of Sperm Motility in Egg-jelly of the Japanese Newt, Cynops pyrrhogaster. Zool. Sci. 1999, 16, 793–802. [Google Scholar] [CrossRef]
- Comizzoli, P. Biotechnologies for Wildlife Fertility Preservation, Anim. Front. 2015, 5, 73–78. [Google Scholar] [CrossRef]
- Kouba, A.; Vance, C.; Willis, E. Artificial Fertilization for Amphibian Conservation: Current Knowledge and Future Considerations. Theriogenology, 2009, 71, 214–227. [Google Scholar] [CrossRef]
- Taniguchi-Sugiura, Y.; Tanaka, E.M. Artificial Insemination in Axolotl. Methods Mol. Biol. 2023, 2562, 417–423. [Google Scholar] [CrossRef]
- Rugh 1962. Experimental Embryology: Techniques and Procedures. 3rd Edn. BurgessPublishing Company, Minneapolis MN, USA.
- Hoitsy, G.A. Woynarovich, T. Moth-Poulsen, T. Guide to the Small-scale Artificial Propagation of Trout. The FAO Regional Office for Europe and Central Asia, Budapest, Hungary. 2012; p 11, Para 3.
- Rugh, R. Induced Ovulation and Artificial Fertilisation in the Frog. Biol. Bull. 1934, 66, 22–29. [Google Scholar] [CrossRef]
- Wolf, D.P.; Hendrick, J.L. 1971. A molecular Approach to Fertilisation: II, Viability and Artificial Fertilisation of Xenopus laevis Gametes. Devel. Biol. 1971, 25, 348–359. [Google Scholar] [CrossRef]
- Hollinger, TG. , Corton GL. Artificial Fertilization of Gametes of the African Clawed Frog, Xenopus laevis. Gamete Res. 1980, 3, 45–57. [Google Scholar] [CrossRef]
- Cabada, M.O. Sperm Concentration and Fertilisation Rate in Bufo arenarum (Amphibia: Anura). J. Exp. Biol. 1975, 62, 481–486. [Google Scholar] [CrossRef]
- Silla, A.J. Artificial Fertilisation in a Terrestrial Toadlet (Pseudophryne guentheri): Effect of Medium Osmolarity, Sperm Concentration and Gamete Storage. Reprod. Fert. Devel. 2013, 25, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Byrne, P.G.; Anastas, Z.M.; Silla, A.J. A test for plasticity in sperm motility activation in response to osmotic environment in an anuran amphibian. Ecol Evol. 2022, 12, e9387. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, T.G.; Corton, G.L. Artificial Fertilization of Gametes from the South African Clawed Frog, Xenopus laevis. Gamete Res. 1980, 3, 45–57. [Google Scholar] [CrossRef]
- Browne, R.K.; Kaurova, S.A.; Uteshev, V.K.; Shishova, N.V.; Kramarova, L.; McGinnity, D.; Figiel, C.R.; Mansour, N.; Agnew, D.; Wu, M.; et al. Sperm Motility of Externally Fertilizing Fish and Amphibians. Theriogenology 2015, 83, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Burger, I.J.; Lampert, S.S.; Kouba, C.K.; Morin, D.J.; Kouba, A.J. Development of an Amphibian Sperm Biobanking Protocol for Genetic Management and Population Sustainability. Conserv. Physiol. 2022, 10, coac032. [Google Scholar] [CrossRef]
- Strand, J.; Callesen, H.; Pertoldi, C.; Purupm, S. 2022. Amphibian cell lines: Usable tissue types and differences between individuals within a species. Amphib. Rept. Conserv. 2022, 16 [General Section]: 245–256.
- Nikitina, L.A. Nuclear Transplantation in Amphibians. Physiol. Gen. Biol. Rev. 1997, 13, 35–72. [Google Scholar]
- Kaurova, S.A.; Nikitina, L.A.; Uteshev, V.K.; Gakhova, E.N. Cryopreservation of Totipotent Embryo Cells and Their use in Reconstruction of Enucleated Eggs, In Proceedings of the 15th Working Meeting, Pushchino, 13–15, October 1998; Gakhova E., Karnaukhov V.N. Eds.; Pushchino Press, 1998; pp. 206–208 (In Russian).
- Briggs, R.; King, T.J. Transplantation of Living Nuclei from Blastula Cells into Enucleated Frogs’ Eggs. PNAS 1952, 38, 455–453. [Google Scholar] [CrossRef]
- Sambuichi, H. The Roles of the Nucleus and the Cytoplasm in Development. I. An Interspecific Hybrid Frog, Developed from a Combination of Rana nigromaculata nigromaculata Cytoplasm and a Diploid Nucleus of Rana nigromacula tabrevipoda. J. Sci. Hiroshima Univ. 1957, Ser. B. Div. 17, 33–41. [Google Scholar]
- Moore, J.A. Transplanation of Nuclei Between Rana pipiens and Rana sylvatica. Exp. Cell. Res. 1958, 14, 532–540. [Google Scholar] [CrossRef]
- Sambuichi, H. The Roles of the Nucleus and the Cytoplasm in Development. III. Diploid Nucleocytoplasmic Hybrids, Derived from Rana nigromaculata brevipoda Cytoplasm and Rana nigromaculata nigromaculata Nuclei. J. Sci. Hiroshima Univ. 1961, 20, 1–5.
- Hennan, S. Nucleocytoplasmic hybrids between Rana pipiens and Rana palustris. I. Analysis of the Developmental Properties of the Nuclei by Means of Nuclear Transplantation. Dev. Biol. 1963, 11, 243–267. [Google Scholar] [CrossRef] [PubMed]
- Toshijiro, K.; Nishioka, M. Viabiity and Abnormalities of the Offspring of Nucleo-cytoplasmic Hybrids between Rana japonica and Rana ornativentris. Sci. Rep. Lab. Amphib. Biol. Hiroshima Univ. 1972, 1, 95–209. [Google Scholar]
- Nishioka, M. Abnormalities of the Offspring of Nucleo-cytoplasmic Hybrids Between Rana nigromaculata and Rana brevipoda. Sci. Rel. Lab. Amphib. Biol. Hiroshima Univ.
- Nishioka, M. Nucleo-cytoplasmic Hybrids between Rana japonica and Rana temporaria temporaria. Sci. Rep. Lab. Amphibian Biol. Hiroshima Univ. 1972, 1, 211–243. [Google Scholar]
- Nishioka, M. Nucleo-cytoplasmic Hybrids between Rana brevipoda and Rana plancyichosenica. Rep. Lab. Amphibian Biol. Hiroshima Univ. 1972, 1, 245–257. [Google Scholar]
- Gallien, L.; Aimar, C. On a New Mode of Gemelarity, Produced by Nuclear Grafting in Amphibians (Urodeles) of the Genus Pleurodeles. Acad. Sci. 1972, 272, 3348–3351. [Google Scholar]
- Gallien, C.L.; Aimar, C.; Guillet, F. Nucleocytoplasmic Interactions During Ontogenesis in Individuals Obtained by Intra- and Interspecific Nuclear Transplantation in the Genus Pleurodeles (Urodele Amphibian). Develop. Biol. 1973, 33, 154–170. [Google Scholar] [CrossRef]
- De-extinction. Available online: https://colossal.com/de-extinction/ (accessed on 16 March 2024).
- Gurdon, J.B. Adult Frogs Derived from the Nuclei of Single Somatic Cells. Devel. Biol. 1962, 4, 250–273. [Google Scholar] [CrossRef]
- "The Nobel Prize in Physiology or Medicine 2012". Available online: https://www.nobelprize.org/prizes/medicine/2012/summary/ (accessed on 8 October 2012).
- Gurdon, J.B.; Byrne, J.A. The First Half-century of Nuclear Transplantation. Proc. Natl. Acad. Sci. USA. 2003, 100, 8048–8052. [Google Scholar] [CrossRef]
- Gurdon, J.B. The Egg and the Nucleus: A Battle for Supremacy. Development 2013, 140, 2449–2456. [Google Scholar] [CrossRef]
- Rangel, G. From Corgis to Corn a Brief Look at the Long History of Gmo-technology, 2015, Available online:. Available online: https://sitn.hms.harvard.edu/flash/2015/from-corgis-to-corn-a-brief-look-at-the-long-history-of-gmo-technology/ (accessed on 27 September 2023).
- Kosch, T.A.; Silva, C.N.S.; Brannelly, L.A.; Roberts, A.A.; Lau, Q. [ Marantelli, G.; Berger, L.F.L.; Skerratt L.F. Genetic Potential for Disease Resistance in Critically Endangered Amphibians Decimated by Chytridiomycosis. Anim. Conserv. 2019, 22, 238–250. [Google Scholar] [CrossRef]
- Eskew, E.A.; Shock, B.C.; LaDouceur, E.E.B.; Keel, K.; Miller, M.R.; Foley, J.E.; Todd, B.D. Gene Expression Differs in Susceptible and Resistant Amphibians Exposed to Batrachochytrium dendrobatidis. R. Soc. Open Sci. 2018, 5, 170910:1–170910:23. [Google Scholar] [CrossRef] [PubMed]
- Silla, A.J.; Calatayud, N.E.; Trudeau, V.L. Amphibian Reproductive Technologies: Approaches and Welfare Considerations. Conserv. Physiol. 2021, 9, coab011. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, M. Capturing Wild Animal Welfare: a Physiological Perspective. Biol. Rev. 2024, 99, 1–22. [Google Scholar] [CrossRef]
- Singer, P. 10th Anniversary Edition of The Life You Can Save. Available online: https://www.thelifeyoucansave.org/the-book/ (accessed on 22 July 2024).
- Irwin, L.N. Renewed Perspectives on the Deep Roots and Broad Distribution of Animal Consciousness. Front. Syst. Neurosci. 2020, 14, 57. [Google Scholar] [CrossRef]
- Lambert, H.; Elwin, A.; D’Cruze, N. Frog in the Well: A Review of the Scientific Literature for Evidence of Amphibian Sentience. Appl. Anim. Behav. Sci. 2022, 247, 105559. [Google Scholar] [CrossRef]
- Winlow, W.; Mather, J.; Cosmo, A.D. Postscript to Invertebrate Welfare: “We Have Met the Enemy and He Is Us”. Animals 2024, 14, 2082. [Google Scholar] [CrossRef] [PubMed]
- Lambert, H.; Elwin, A.; D’Cruze, N. Frog in the well: A Review of the Scientific Literature for Evidence of Amphibian Sentience. Appl. Anim. Behav. Sci. 2022, 247, 105559. [Google Scholar] [CrossRef]
- Offor, I. Second Wave Animal Ethics and (Global) Animal Law: a View from the Margins. J. Hum. Rights Environ. 2020, 11, 268–296. [Google Scholar] [CrossRef]
- Ikegbu, E.A.; Diana-Abasi, F.I. Utilitarianism as a Veritable Vehicle for the Promotion of a Just Society. LWATI: J. Contemp. Res. 2017, 14, 121–137. [Google Scholar]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Universities Federation for Animal Welfare: UFAW, Wheathampstead, UK, 1959; p. 238. ISBN 0-900767-78-2. [Google Scholar]
- Tannenbaum, J.; Bennett, B.T. Russell and Burch's 3Rs Then and Now: The Need for Clarity in Definition and Purpose. J. Am. Assoc. Lab. Anim. Science, 2015, 54, 120–132. [Google Scholar]
- NC3R. National Center for the Replacement, Reduction, and Refinement of Animals in Research. Available online: https://www.nc3rs.org.uk/who-we-are/3rs (accessed on 23 September 2023).
- Woolly Mammoth De-extinction Project & Process | Colossal. Available online: https://colossal.com/mammoth/ (accessed on 27 September 2023).
- van Urk-Costa, E. Introduction - Why (Reformed) Theology Needs Reflection on Biodiversity Loss and Extinction. J. Reform. Theol. 2023, 17, 121–123. [Google Scholar] [CrossRef]
- Link, H.J. Playing God and the Intrinsic Value of Life: Moral Problems for Synthetic Biology? Sci. Eng. Ethics 2013, 19, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Dabrock, P. Playing God? Synthetic Biology as a Theological and Ethical Challenge. Syst. Synth. Biol. 2009, 3, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Peters, T. Playing God” and Germline Intervention, Appl. Anim. Behav. Sci., 1995, 20, 365–386. [Google Scholar] [CrossRef]
- Negi, C.S. Religion and biodiversity conservation: not a mere analogy. Inter. J. Biodiv. Sci. Manag. 2005, 1, 85–96. [Google Scholar] [CrossRef]
- White, T.B.; Petrovan, S.O.; Christie, A.P.; Martin, P.A.; Sutherland, W.J. What is the Price of Conservation? A Review of the Status Quo and Recommendations for Improving Cost Reporting, BioScience 2022, 72, 461–471. [Google Scholar] [CrossRef]
- Rowling, M.; How much will it cost to save nature - and who will pay? World Economic Forum and the Thomas Reuters Foundation Trust.org. Available online: https://www.weforum.org/agenda/2021/05/biodiversity-deforestation-global-investment-inititive/ (accessed on 2 August 2024).
- Gordon, E.R.; Butt, N.; Rosner-Katz, H.; Binley, A.D.; Bennett, J.R. Relative Costs of Conserving Threatened Species Across Taxonomic Groups. Conserv. Biol. 2019, 34, 276–281. [Google Scholar] [CrossRef]
- Balmford, A.; Gaston, K.J.; Rodrigues, A.S.L.; James. A. Integrating Costs of Conservation into International Priority Setting. Conserv. Biol. 2013, 14, 597–605. [Google Scholar] [CrossRef]
- Conde-Pueyo, N.; Vidiella, B.; Sardanyés, J.; Berdugo, M.; Maestre, F.T.; De Lorenzo, V.; Solé, R. Synthetic Biology for Terraformation Lessons from Mars, Earth, and the Microbiome. Life 2020, 10, 14. [Google Scholar] [CrossRef]
- Gualandris-Parisot L, Husson D, Foulquier F, Kan P, Davet J, Aimar C, Dournon C, Duprat AM. Pleurodeles waltl, Amphibian, Urodele, is a Suitable Biological Model for Embryological and Physiological Space Experiments on a Vertebrate. Adv Space Res. 2001, 28, 569–578. [CrossRef]
- Jacobson, S.K.; McDuff, M.D.; Monroe, M.C. Conservation Education and Outreach Techniques. In 1st edn, Techniques in Ecology & Conservation; Oxford Academic, 2007, doi.org/10.1093/acprof:oso/9780198567714.001.0001, (accessed 19 July 2024).
- Meredith, H.M.R.; St. John, F.A.V.; Collen, B.; Black, S.A.; Griffiths R.A. Practitioner and scientist perceptions of successful amphibian conservation. Conserv. Biol. 2018, 32, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Bradfield, K.S.; Tapley, B.; Johnson, B. Amphibians and Conservation Breeding Pro-grammes: How do we Determine Who Should be on the Ark? Biodiver. Conserv. 2023, 32, 885–898. [Google Scholar] [CrossRef]
- The Nature Education Knowledge Project. Saving Endangered Species: A Case Study Using Global Amphibian Declines. Available online: https://www.nature.com/scitable/knowledge/library/saving-endangered-species-a-case-study-using-19445898/ (accessed on 8 August 2024).
- Rothschild, J. Ethical Considerations of Gene Editing and Genetic Selection. J. Gen. Fam. Med. 2020, 21, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Frieze, C. Cloning Wild Life - Zoos, Captivity, and the Future of Endangered Animals. 2013, NYU Press, USA, 258 p. 350.
- Dabrock, P. Playing God? Synthetic Biology as a Theological and Ethical Challenge. Syst. Synth. Biol. 2009, 3, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Link, H.J. Playing God and the Intrinsic Value of Life: Moral Problems for Synthetic Biology? Sci. Eng. Ethics 2013, 19, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Loy, I.; Worley, W. Your jargon-busting climate glossary. 2023, Available online:. Available online: https://www.thenewhumanitarian.org/news-feature/2023/12/01/oh-ffs-guide-climate-change-acronyms. (accessed on 8 August 2024).
- Charo, A. Charo: Bioengineering Can Help Heal the World. https://colossal.com/charo-bioengineering-can-help-heal-the-world/ (accessed 10 August 2024).
- Lann, B. Petition to the United Federation of Planets: Revert the Ban on Gene Editing for Ecological Applications. Available online: https://www.reddit.com/r/startrek/comments/1df045s/petition_to_the_united_federation_of_planets/?rdt=37953 (accessed on 10 August 2024).
- Schwägerl, C. The Anthropocene – The Human Era and How it Shapes the Planet. Synergetic Press, 2015, pp 236. ISBN: 9780907791546. [CrossRef]
- Malhi, Y. The Concept of the Anthropocene. Ann. Rev. Environ. Resour. 2017, 42, 77–104. [Google Scholar] [CrossRef]
- Tapley, B.; Bradfield, B.; Michaels, C.; Bungard, M. Amphibians and Conservation Breeding Programmes: do all Threatened Amphibians Belong on the Ark? Biodivers. Conserv. 2015, 24, 2625–2645. [Google Scholar] [CrossRef]
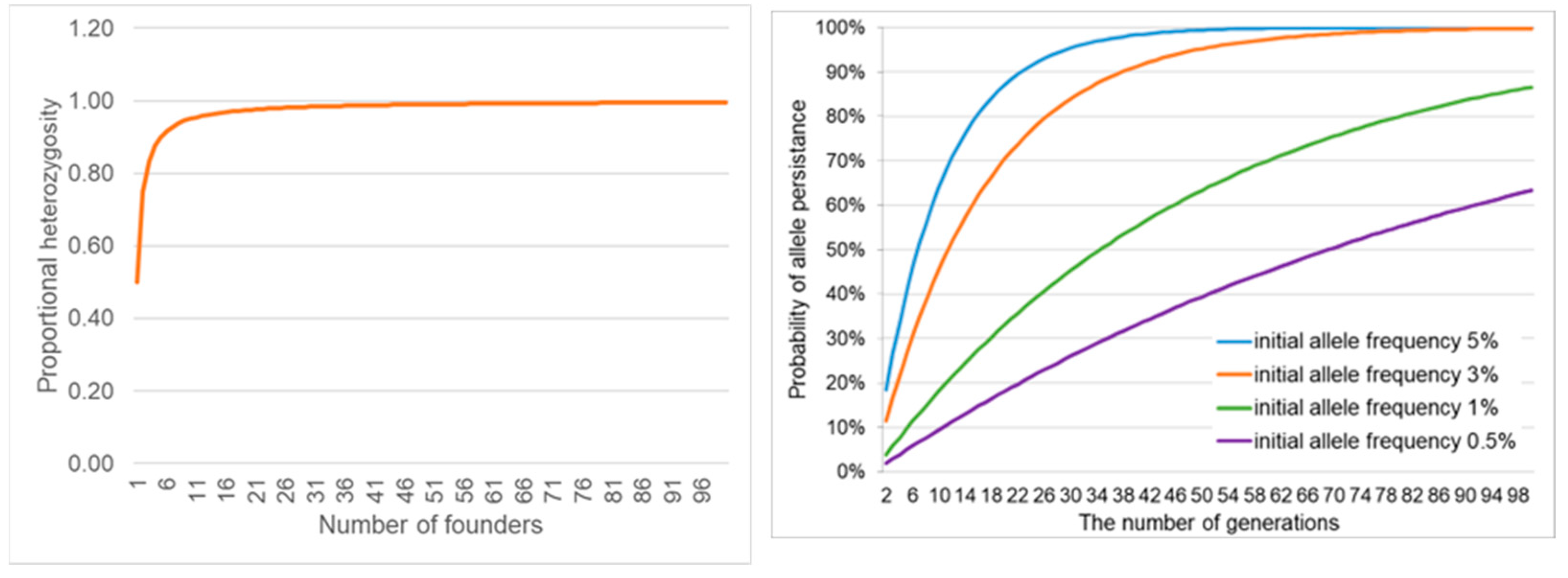


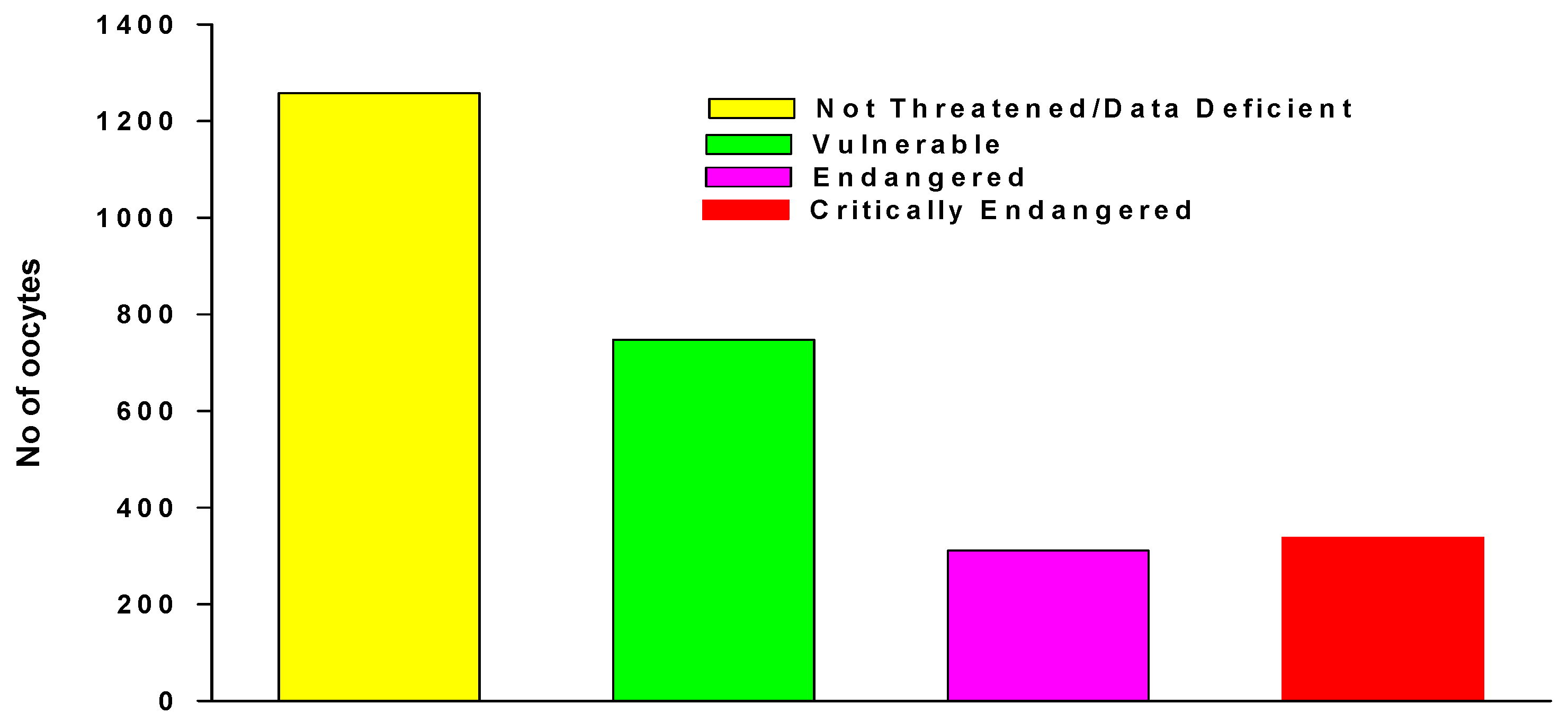


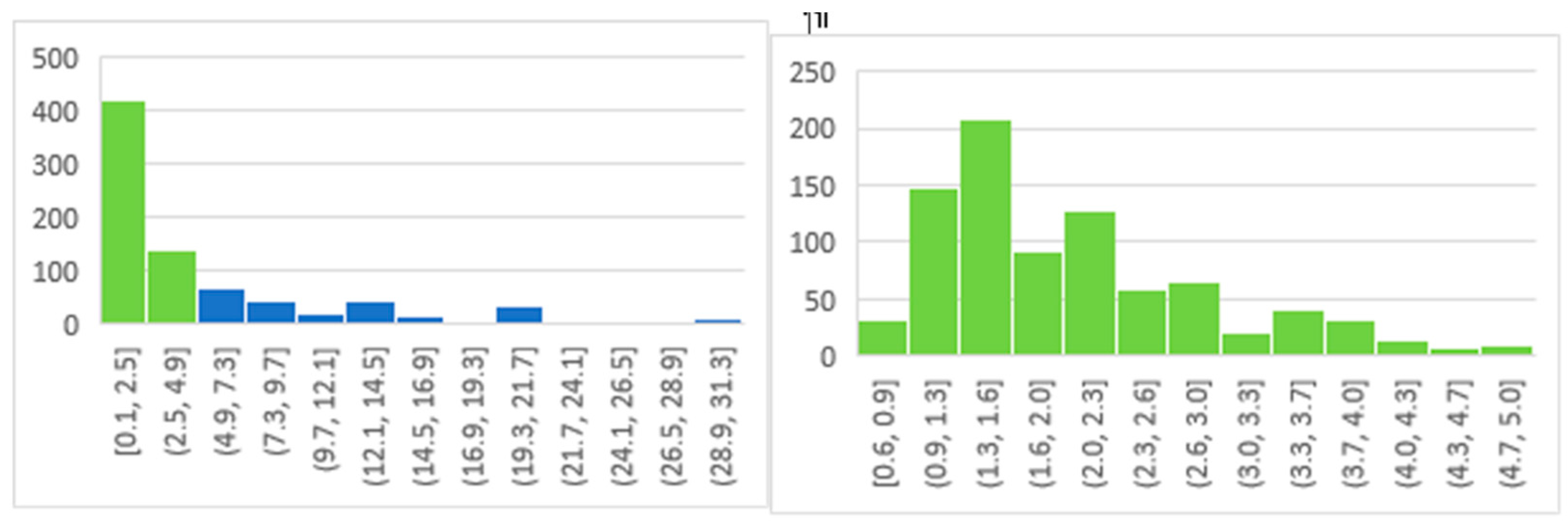
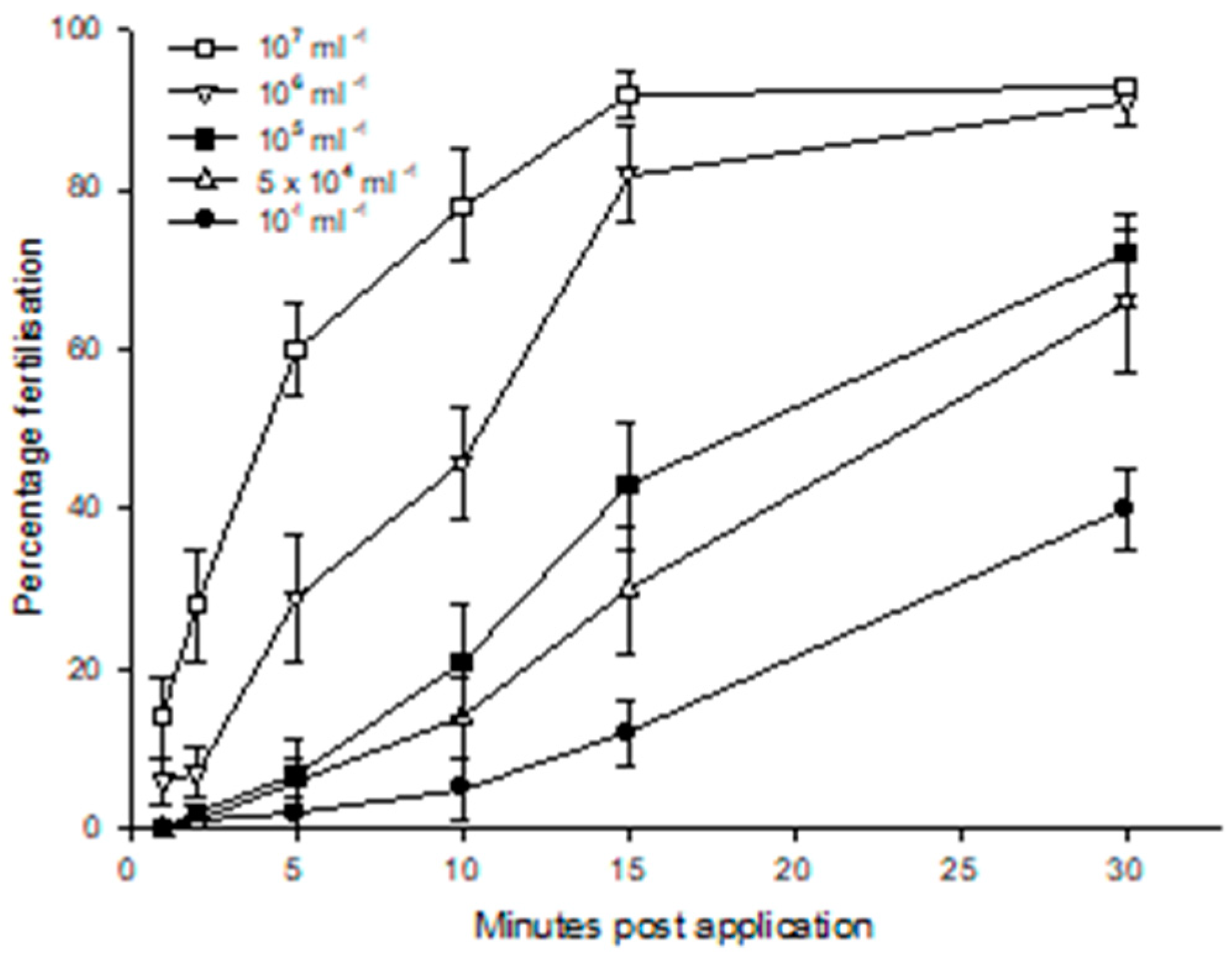
| Population | M | F | Application | Year | Reference |
|---|---|---|---|---|---|
| Metapopulation | 5 | 5 | 27 Threatened species. | 2005 | [123] |
| Metapopulation | 28 | 25 | CBP | 2007 | [110] |
| Metapopulation | 25 | - | CBP | 2012 | [124] |
| Fragmented/sub-pop. | 10 | - | AGF 4 years, depending on kinship | 2022 | [125] |
| Geographic clusters | 10 | 20 | Geographic clusters | 2022 | [120] |
| Metapopulation | 20+ | 20+ | CBP | 2024 | [108] |
| Examples of Genera | Age to maturity | Longevity | Individual | Group |
|---|---|---|---|---|
| Acris | < 1 year | 1 year | 1590 | # |
| Eleutherodactylus, Nectophrynoides, some Hyperoliidae | < 1 year | 2-5 year | 400 | 400 |
| Hylidae, some Hyperoliidae, Scaphiophryne | 1-5 year | < 5 years | 135 | 265 |
|
Dendrobatidae, Typhlonectes, Tylototriton/Echinotriton, Theloderma, Cynops, Leptodactylus, Ceratobatrachus, Mantella, Atelopus |
1-5 year | 5-15 years | 70* | 140 |
| Salamandra, some Ambystoma | 1-5 year | > 15 years | 60* | 80* |
| Cryptobranchus, Andrias | > 5 years | > 15 years | 45* | 80* |
| Range | NoT | n | VU | n | EN | n | CE | n |
|---|---|---|---|---|---|---|---|---|
| 0-10 | 7 | 93 | 4 | 8 | 5 | 8 | 5 | 8 |
| 10-100 | 39 | 361 | 33 | 14 | 27 | 31 | 27 | 14 |
| 100-1000 | 410 | 571 | 483 | 8 | 394 | 4 | 272 | 14 |
| 1000 - 10,000 | 3013 | 328 | 5162 | 5 | 2466 | 5 | 4278 | 2 |
| 10,000 - 100,000 | 17980 | 28 | NA | 0 | NA | 0 | NA | 0 |
| Nucleus donors | Recipients | Results | Year | Ref |
|---|---|---|---|---|
| Rana pipiens | Aquarana (Rana) catesbeiana | Died late blastula | 1952 | [301] |
| R. n. brevipoda | R.n. nigromaculata | Metamorphs | 1957 | [302] |
| R. pipiens | R. sylvatica | Late blastula/early neurula | 1958 | [303] |
| R. n. nigromaculata | R. n. brevipoda | Adults | 1961 | [304] |
| R. pipiens | R. palustris | Post-neurula | 1963 | [305] |
| R. n nigromaculata | R. n. brevipoda | Adults –F1 reproduction | 1963 | [87,88] |
| R. japonica | R. ornativentris | F2 | 1963 | [306] |
|
R. nigromaculata R. temporaria |
R. brevipoda R. japonica |
F3. | 1972 | [307] |
|
R. japonica R. temporaria |
R. temporaria R. japonica |
Adults | 1972 | [308] |
|
R. brevipoda R. plancyi R. brevipoda R. esculenta |
R. plancyi R. brevipoda R. esculenta R. brevipoda |
Adults | 1972 | [309] |
|
Pleurodeles waltlii P. poireti |
P. poireti P. waltlii |
Adults | 1971 | [310] |
|
P. waltlii P. poireti |
P. poireti P. waltlii |
Adults | 1972 | [311] |
| Technique | Injection | Application stress | Collection technique | Collection stress | *Yield | Donor Size limitation | References |
|---|---|---|---|---|---|---|---|
| Mating and spawning | |||||||
| Natural mating and spawning | None | None | Fertilised eggs | None | Very high | None | [214,215] |
| Hormonally induced mating and spawning | Yes | Moderate | Fertilised eggs | None | Very high | None | [64,214] |
| Oocyte collection | |||||||
| Hormonal stimulation oocytes with spawning into solutions | Yes | Moderate | Spawning into physiological saline | Low | High to very high | None | [233,247] |
| Hormonally stimulated ovarian oocytes | Yes | Moderate | Abdominal massage or cannulation | High | Low | Avoid for small species | [64,233,237,245,246,247] |
| Excision of hormonally stimulated ovarian oocytes | Yes | Moderate | Direct sampling | None | Very high | None | [233,247]. |
| Sperm collection | |||||||
| Collection from testicular tissue | Yes | Moderate | Macerating testicular tissue | None | Very High | None | [61,64,66,76,85,86,222,223,224] |
| Hormonal stimulation sperm | Yes | Moderate | Urination | Moderate | High to none | Avoid for small species | [233,247] |
| Hormonal stimulation sperm | Yes | Moderate | Cannulation | High | Moderate - very low | Avoid for small species | [52,65] |
| Hormonal stimulation sperm - topical, nasal, oral, or food item | None | Low | Cannulation | High | Moderate - very low | Possibly for small species | [61,241,242] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





