Submitted:
16 September 2024
Posted:
18 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
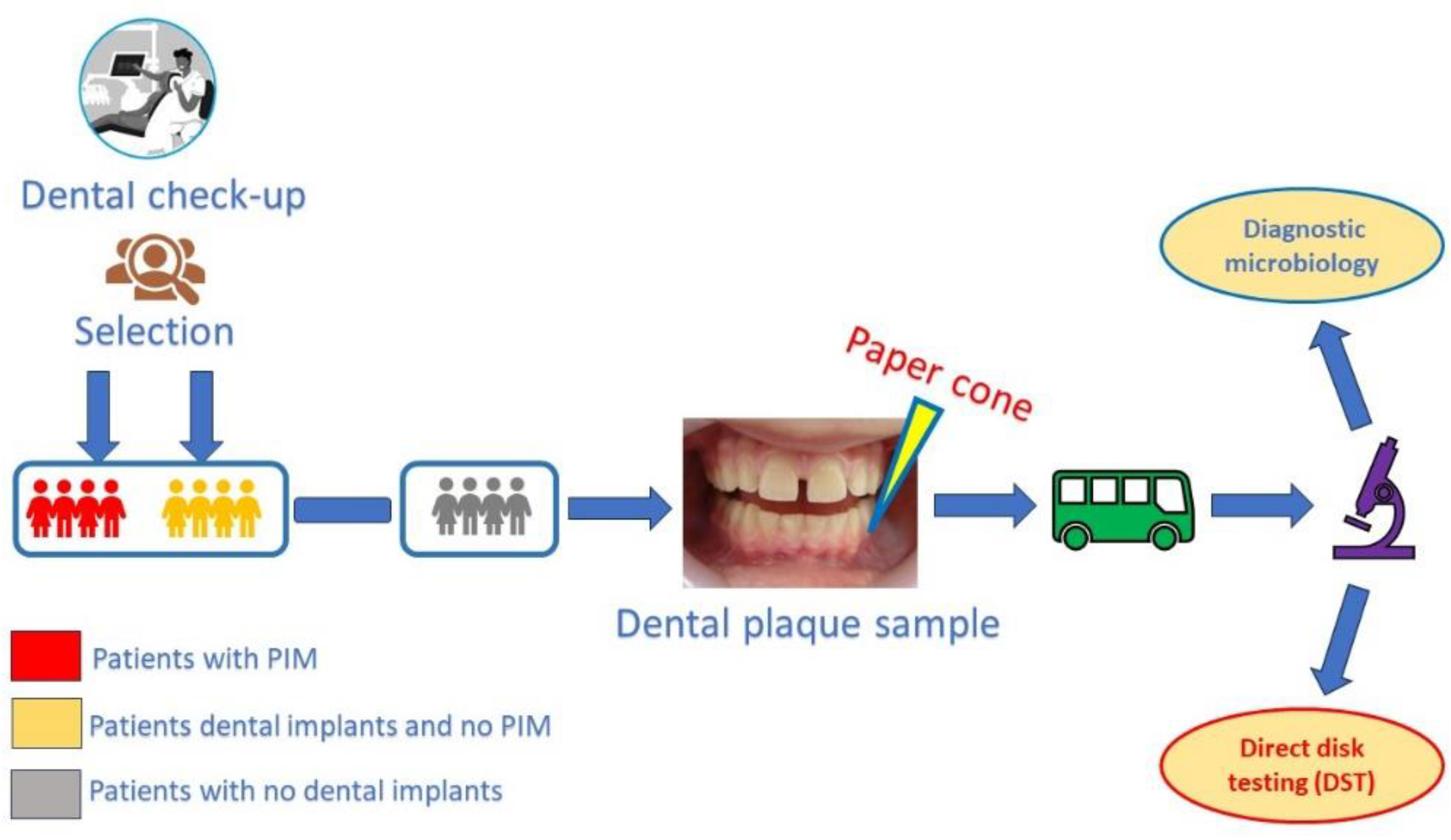
2.1. Selection of Patients and Creating the Study Groups:
- 0.
- - healthy peri-implant mucosa / dental gingiva. No color modification, no swelling, no bleeding on probing;
- 1.
- - reduced peri-implant mucosal / dental gingival inflammation. Reduced color modification and/or swelling, no bleeding on probing;
- 2.
- - mild peri-implant mucosal / dental gingival inflammation. Obvious color modification and/or swelling, bleeding on probing;
- 3.
- - severe peri-implant mucosal / dental gingival inflammation. Obvious color modification and/or swelling, spontaneous bleeding or bleeding on air dry.
2.2. Selection of Peri-Implant/Periodontal Sites
2.3. Workflow procedure:
- - in cases of PIM, we collected from sites that were most likely to be the starting point of peri-implant infection, where the scores for assessing the peri-implant mucosal or dental gingival health were higher;
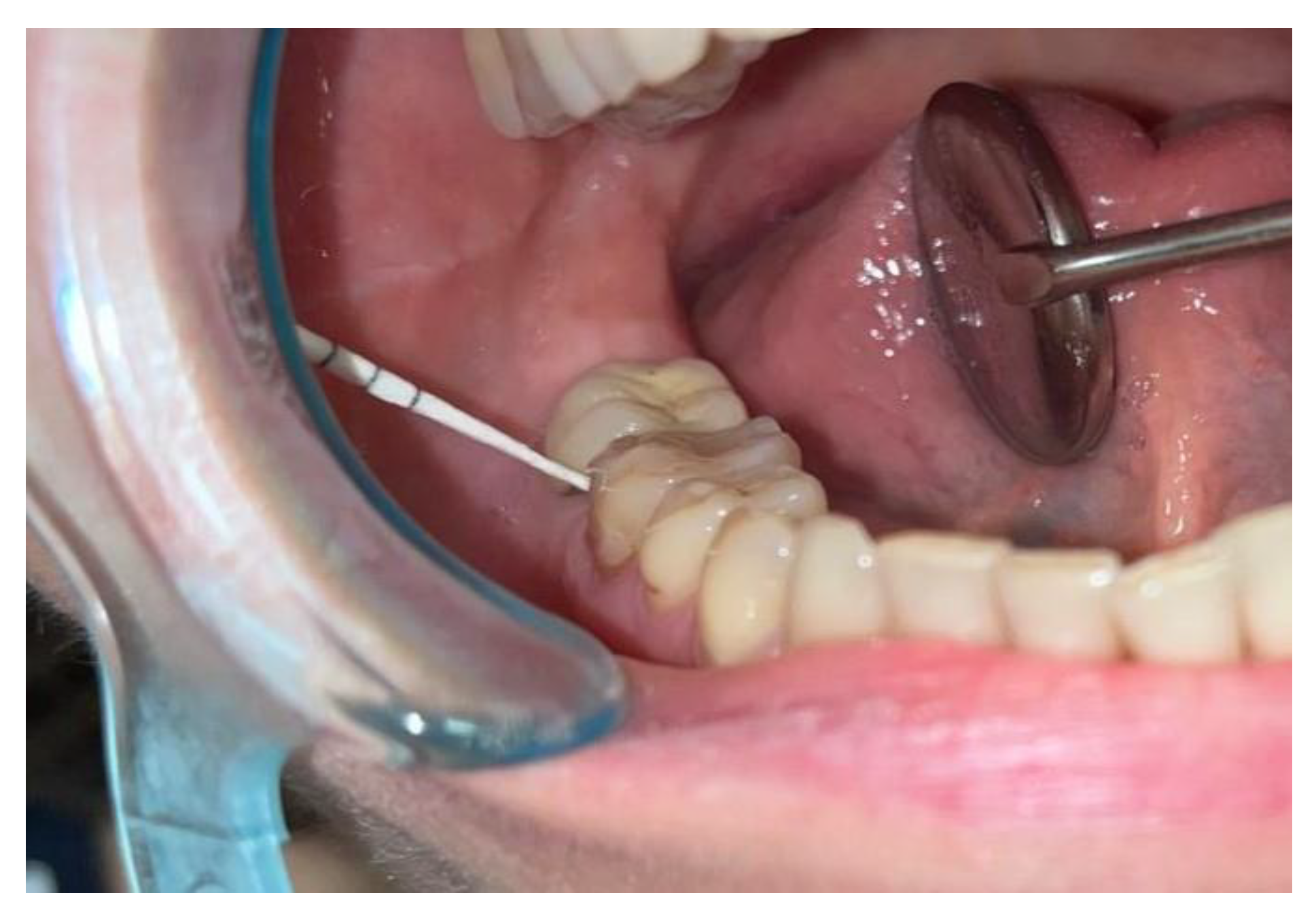
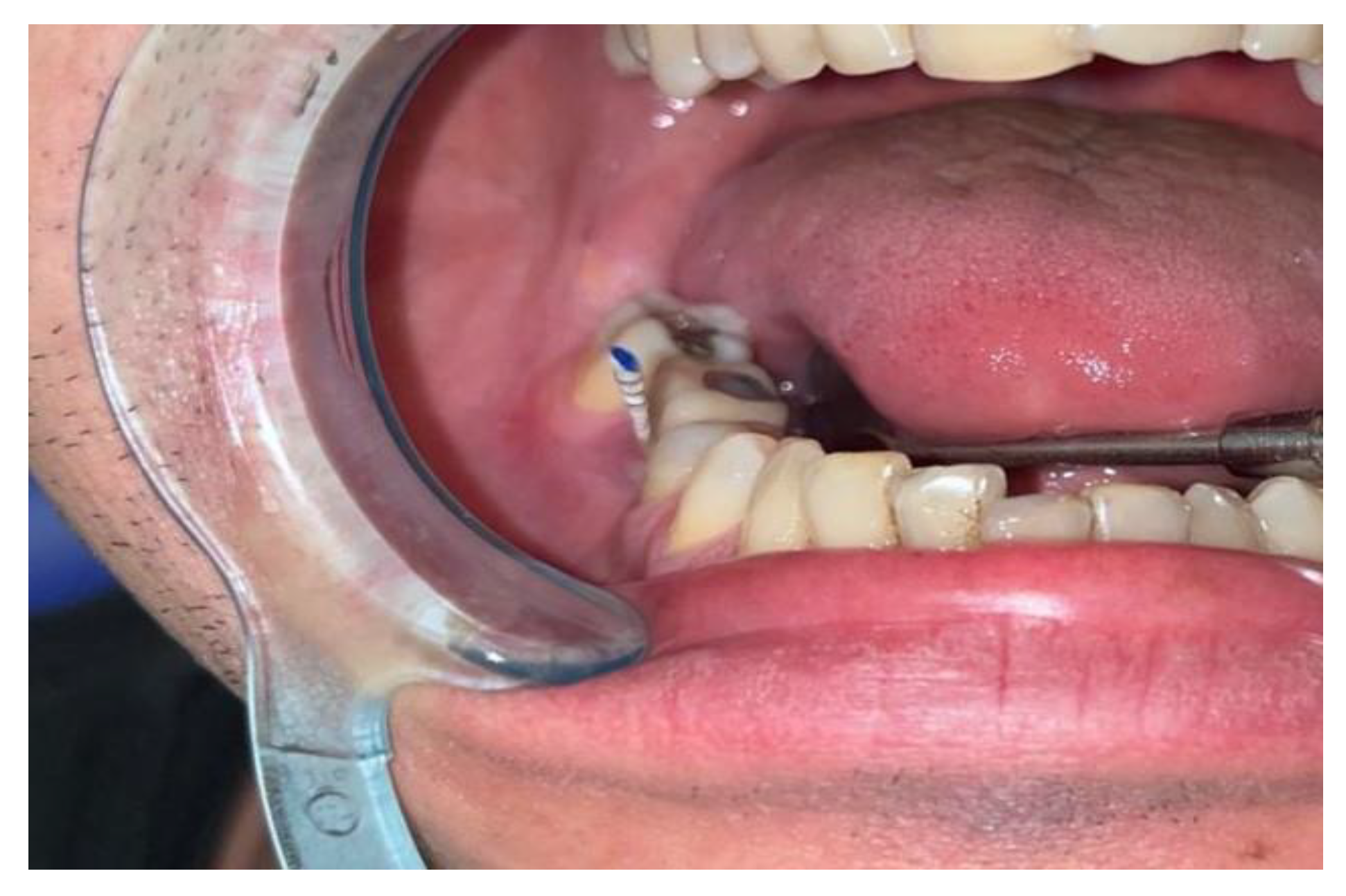
- - the paper cones were inserted into the gingival / mucosal sulcus as deep as possible, without generating pain or discomfort for the patient and removed after approximatively 5 second in order to have enough time to collect bacteria and crevicular fluid;
- - we always tried to collect a sufficient amount of fluid; the paper cones had to be obviously wet on the whole depth of insertion before removing and being further processed;
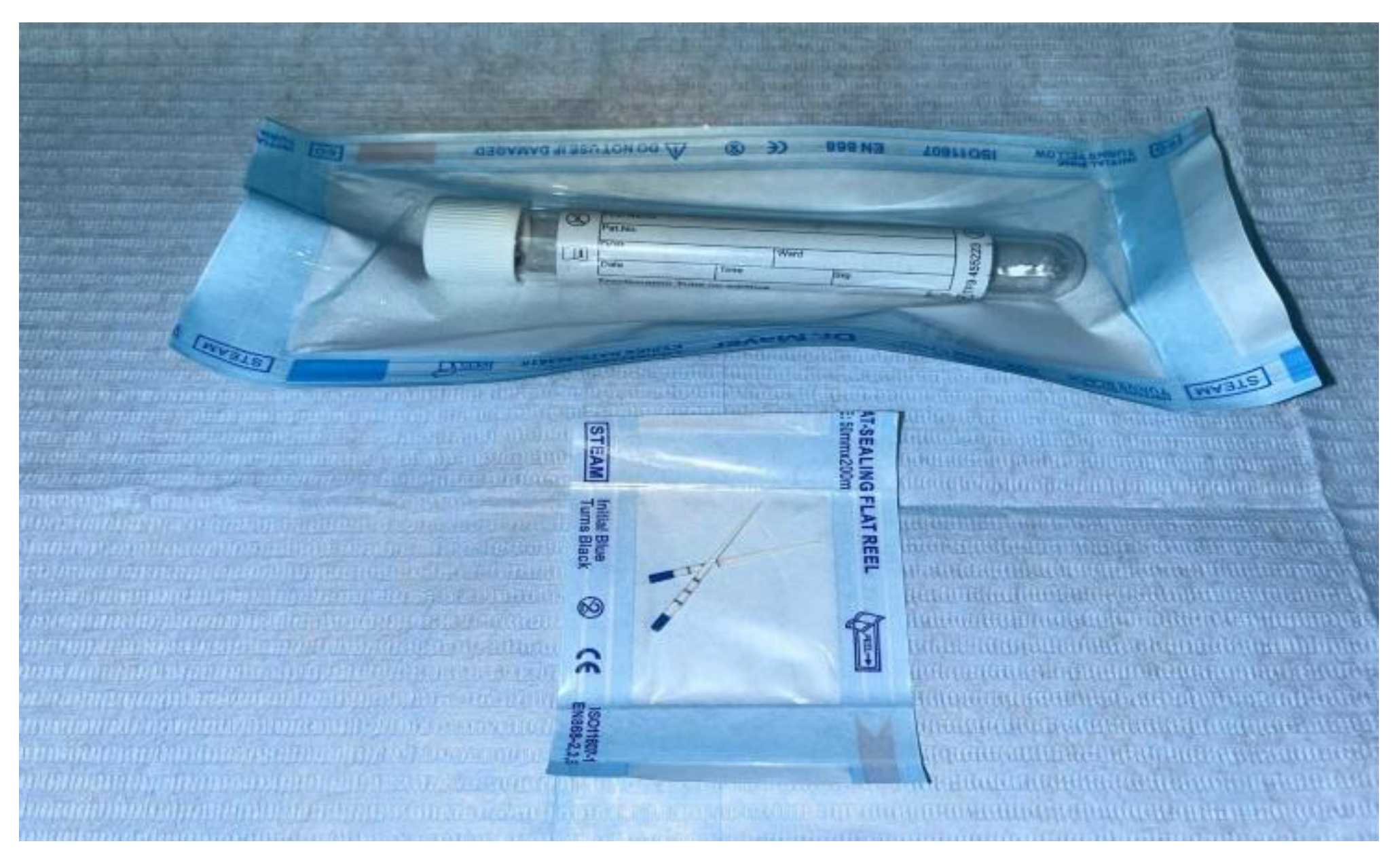
- - every container received a label with the patient and site numbers (e.g. “patient no. 3, site no. 1”); the study was simple-blind – so, the laboratory technician did not receive any information about the patient or about the origin of the sample (implants, dental crowns or natural teeth);
- - the external surface of the containers was disinfected after they have been used and introduced in single use plastic bags; then, they were stored in safe, clean place until were picked up for sending to the laboratory;
- - the transport took place in the same day of collecting;
- - we used specific procedures for the transport of microbiological samples; also, the transports were processed as fast as possible in order to prevent the samples degradation;
- - each sample received a unique serial number;
- - the laboratory staff established the correct procedure for sowing, cultivating and analyzing the samples;
- - an antibiogram was performed for each of the 30 bacterial cultures.
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakka S, Baroudi K, Nassani MZ. Factors associated with early and late failure of dental implants. J Investig Clin Dent. 2012 Nov;3(4):258-61. [CrossRef]
- Basson AA, Mann J, Findler M, Chodick G. Correlates of Early Dental Implant Failure: A Retrospective Study. Int J Oral Maxillofac Implants 2023 Oct 17;38(5):897-906. [CrossRef]
- Do TA, Le HS, Shen YW, Huang HL, Fuh LJ. Risk Factors related to Late Failure of Dental Implant-A Systematic Review of Recent Studies. Int J Environ Res Public Health. 2020 Jun 2;17(11):3931. [CrossRef] [PubMed] [PubMed Central]
- Howe MS, Keys W, Richards D. Long-term (10-year) dental implant survival: A systematic review and sensitivity meta-analysis. J Dent. 2019 May:84:9-21. [CrossRef]
- Wittneben JG, Buser D, Salvi GE, Bürgin W, Hicklin S, Brägger U. Complication and failure rates with implant-supported fixed dental prostheses and single crowns: a 10-year retrospective study. Clin Implant Dent Relat Res. 2014 Jun;16(3):356-64. [CrossRef]
- Wang Y, Fan Y, Lin Z, Song Z, Shu R, Xie Y. Survival rate and potential risk indicators of implant loss in non-smokers and systemically healthy periodontitis patients: An up to 9-year retrospective study. J Periodontal Res. 2021 Jun;56(3):547-557. [CrossRef] [PubMed]
- Jemt, T. Jemt T. A retro-prospective effectiveness study on 3448 implant operations at one referral clinic: A multifactorial analysis. Part II: Clinical factors associated to peri-implantitis surgery and late implant failures. Clin Implant Dent Relat Res. 2017 Dec;19(6):972-979. [CrossRef] [PubMed]
- Staedt H, Rossa M, Lehmann KM, Al-Nawas B, Kämmerer PW, Heimes D. Potential risk factors for early and late dental implant failure: a retrospective clinical study on 9080 implants. Int J Implant Dent. 2020 Nov 30;6(1):81. [CrossRef] [PubMed] [PubMed Central]
- Takamoli J, Pascual A, Martinez-Amargant J, Garcia-Mur B, Nart J, Valles C. Implant failure and associated risk indicators: A retrospective study. Clin Oral Implants Res. 2021 May;32(5):619-628. [CrossRef] [PubMed]
- Marcantonio C, Nicoli LG, Marcantonio Junior E, Zandim-Barcelos DL. Prevalence and Possible Risk Factors of Peri-implantitis: A Concept Review. J Contemp Dent Pract. 2015 Sep 1;16(9):750-7. [CrossRef] [PubMed]
- Zhou N, Huang H, Liu H, Li Q, Yang G, Zhang Y, Ding M, Dong H, Mou Y. Microbiota analysis of peri-implant mucositis in patients with periodontitis history. Clin Oral Investig. 2022 Oct;26(10):6223-6233. [CrossRef] [PubMed] [PubMed Central]
- Alves CH, Russi KL, Rocha NC, Bastos F, Darrieux M, Parisotto TM, Girardello R. Host-microbiome interactions regarding peri-implantitis and dental implant loss. J Transl Med. 2022 Sep 23;20(1):425. [CrossRef] [PubMed] [PubMed Central]
- Sato J, Gomi K, Makino T, Kawasaki F, Yashima A, Ozawa T, Maeda N, Arai T. The evaluation of bacterial flora in progress of peri-implant disease. Aust Dent J. 2011 Jun;56(2):201-6. [CrossRef]
- Pokrowiecki R, Mielczarek A, Zaręba T, Tyski S. Oral microbiome and peri-implant diseases: where are we now? Ther Clin Risk Manag. 2017 Nov 29:13:1529-1542. eCollection 2017. [CrossRef]
- Rahnama-Hezavah M, Mertowska P, Mertowski S, Skiba J, Krawiec K, Łobacz M, Grywalska E. How Can Imbalance in Oral Microbiota and Immune Response Lead to Dental Implant Problems? Int J Mol Sci. 2023 Dec 18;24(24):17620. [CrossRef] [PubMed] [PubMed Central]
- Cuculescu, M. Indici utilizați în medicina dentară. In Prevenție primară în carie și parodontopatii. Publisher: Editura didactică și pedagogică R.A. Bucharest, Romania, 2010, pp. 96-97.
- Rostamian M, Chegene Lorestani R, Jafari S, Mansouri R, Rezaeian S, Ghadiri K, Akya A. A systematic review and meta-analysis on the antibiotic resistance of Neisseria meningitidis in the last 20 years in the world. Indian J Med Microbiol. 2022 Jul-Sep;40(3):323-329. [CrossRef] [PubMed]
- Lee Y, Kim CK, Kim M, Yong D, Lee K, Chong Y. Detection of mecA in strains with oxacillin and cefoxitin disk tests for detection of methicillin-resistant Staphylococcus. Korean J Lab Med. 2007 Aug;27(4):276-80. Korean. [CrossRef] [PubMed]
- Keleş E, Aral M, Alpay HC. Antibiotic sensitivities of Streptococcus pneumoniae, viridans streptococci, and group A hemolytic streptococci isolated from the maxillary and ethmoid sinuses. Kulak Burun Bogaz Ihtis Derg. 2006;16(1):18-24. [PubMed]
- Lakhssassi N, Elhajoui N, Lodter JP, Pineill JL, Sixou M. Antimicrobial susceptibility variation of 50 anaerobic periopathogens in aggressive periodontitis: an interindividual variability study. Oral Microbiol Immunol. 2005 Aug;20(4):244-52. [CrossRef] [PubMed]
- Li J, Wang H, Li N, Zhang Y, Lü X, Liu B. Antibiotic susceptibility and biofilm-forming ability of Veillonella strains. Anaerobe. 2022 Dec;78:102667. [CrossRef] [PubMed]
- Mareş M, Mareş M, Rusu M. Antifungal susceptibility of 95 yeast strains isolated from oral mycoses in HIV-negative and HIV-positive patients. Bacteriol Virusol Parazitol Epidemiol. 2008 Jan-Mar;53(1):41-2. [PubMed]
- Umar M, Jananni M, Saravana Kumar R, Pratebha B, Peri implantitis - A narrative review. IP Int J Periodontol Implantol 2021;6(4):204-211.
- Ata-Ali J, Candel-Marti ME, Flichy-Fernández AJ, Peñarrocha-Oltra D, Balaguer-Martinez JF, Peñarrocha Diago M. Peri-implantitis: associated microbiota and treatment. Med Oral Patol Oral Cir Bucal. 2011 Nov 1;16(7):e937-43. [CrossRef]
- Berglundh T, Zitzmann NU, Donati M. Are peri-implantitis lesions different from periodontitis lesions? J Clin Periodontol 2011; 38(Suppl. 11): 188-202. [CrossRef]
- Quirynen M, De Soete M, van Steenberghe D. Infectious risks for oral implants: A review of the literature. Clin Oral Implants Res 2002; 13(1): 1-19. [CrossRef]
- Cai Z, Li Y, Wang Y, Chen S, Jiang S, Ge H, Lei L, Huang X. Antimicrobial effects of photodynamic therapy with antiseptics on Staphylococcus aureus biofilm on titanium surface. Photodiagnosis Photodyn Ther. 2019 Mar;25:382-388. [CrossRef] [PubMed]
- Salvi GE, Fürst MM, Lang NP, Persson GR. One-year bacterial colonization patterns of Staphylococcus aureus and other bacteria at implants and adjacent teeth. Clin Oral Implants Res 2008; 19(3): 242-8. [CrossRef]
- Apse P, Ellen RP, Overall CM, Zarb GA. Microbiota and crevicular fluid collagenase activity in the osseointegrated dental implant sulcus: A comparison of sites in edentulous and partially edentulous patients. J Periodontal Res 1989; 24(2): 96-105. [CrossRef]
- Kocar M, Seme K, Hren NI. Characterization of the normal bacterial flora in peri-implant sulci of partially and completely edentulous patients. Int J Oral Maxillofac Implants 2010; 25(4): 690-8.
- Charalampakis G, Leonhardt Å, Rabe P, Dahlén G. Clinical and microbiological characteristics of peri-implantitis cases: a retrospective multicentre study. Clin Oral Implants Res. 2012 Sep;23(9):1045-54. [CrossRef]
- Tambone E, Marchetti A, Ceresa C, Piccoli F, Anesi A, Nollo G, Caola I, Bosetti M, Fracchia L, Ghensi P, Tessarolo F. Counter-Acting Candida albicans-Staphylococcus aureus Mixed Biofilm on Titanium Implants Using Microbial Biosurfactants. Polymers (Basel). 2021 Jul 23;13(15):2420. [CrossRef] [PubMed] [PubMed Central]
- Kumar PS, Mason MR, Brooker MR, O’Brien K. Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. J Clin Periodontol. 2012 May;39(5):425-33. [CrossRef]
- De Bruyn H, Christiaens V, Doornewaard R, Jacobsson M, Cosyn J, Jacquet W, Vervaeke S. Implant surface roughness and patient factors on long-term peri-implant bone loss. Periodontol 2000. 2017 Feb;73(1):218-227. [CrossRef] [PubMed]
- de Freitas MM, da Silva CH, Groisman M, Vidigal GM Jr. Comparative analysis of microorganism species succession on three implant surfaces with different roughness: an in vivo study. Implant Dent. 2011 Apr;20(2):e14-23. [CrossRef]
- Al-Radha AS, Pal A, Pettemerides AP, Jenkinson HF. Molecular analysis of microbiota associated with peri-implant diseases. J Dent. 2012 Nov;40(11):989-98. [CrossRef]
- Ramanauskaite A, Schwarz F. Current concepts for the treatment of peri-implant disease. Int J Prosthodont. 2023 Nov 21;0(0):1-32. [CrossRef] [PubMed]
- Gomes BP, Jacinto RC, Montagner F, Sousa EL, Ferraz CC. Analysis of the antimicrobial susceptibility of anaerobic bacteria isolated from endodontic infections in Brazil during a period of nine years. J Endod. 2011 Aug;37(8):1058-62. [CrossRef]
- Thomas E Rams, John E Degener, Arie J van Winkelhoff. Antibiotic resistance in human peri-implantitis microbiota. Clin Oral Implants Res. 2014 Jan;25(1):82-90. [CrossRef]
- van Winkelhoff AJ. Antibiotics in the treatment of peri-implantitis. Eur J Oral Implantol. 2012;5 Suppl:S43-50.
| Microorganism | Number of sites | PIMa | MPATb | PATc | HSd |
|---|---|---|---|---|---|
| Candida albicans (Cand. a.) | 7 | 3 | 1 | 2 | 1 |
| Candida krusei (Cand. k.) | 3 | 0 | 0 | 1 | 2 |
| Candida parapsilosis (Cand. p.) | 2 | 1 | 0 | 1 | 0 |
| Neisseria mucosa (Neis. m.) | 2 | 2 | 0 | 0 | 0 |
| Prevotella (Prev.) | 7 | 3 | 1 | 3 | 0 |
| Staphylococcus aureus (Staph. a.) | 3 | 1 | 2 | 0 | 0 |
| Group A beta hemolytic streptococcus (Strept. A.) |
5 | 1 | 0 | 1 | 3 |
| Group C streptococcus (Strept. C.) | 3 | 2 | 0 | 0 | 1 |
| Group G streptococcus (Strept. G.) | 3 | 0 | 1 | 2 | 0 |
| Pyogenic streptococcus (Strept. Pyo.) | 2 | 0 | 0 | 1 | 1 |
| Veillonella (Veil.) | 3 | 1 | 2 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





