Submitted:
15 September 2024
Posted:
17 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
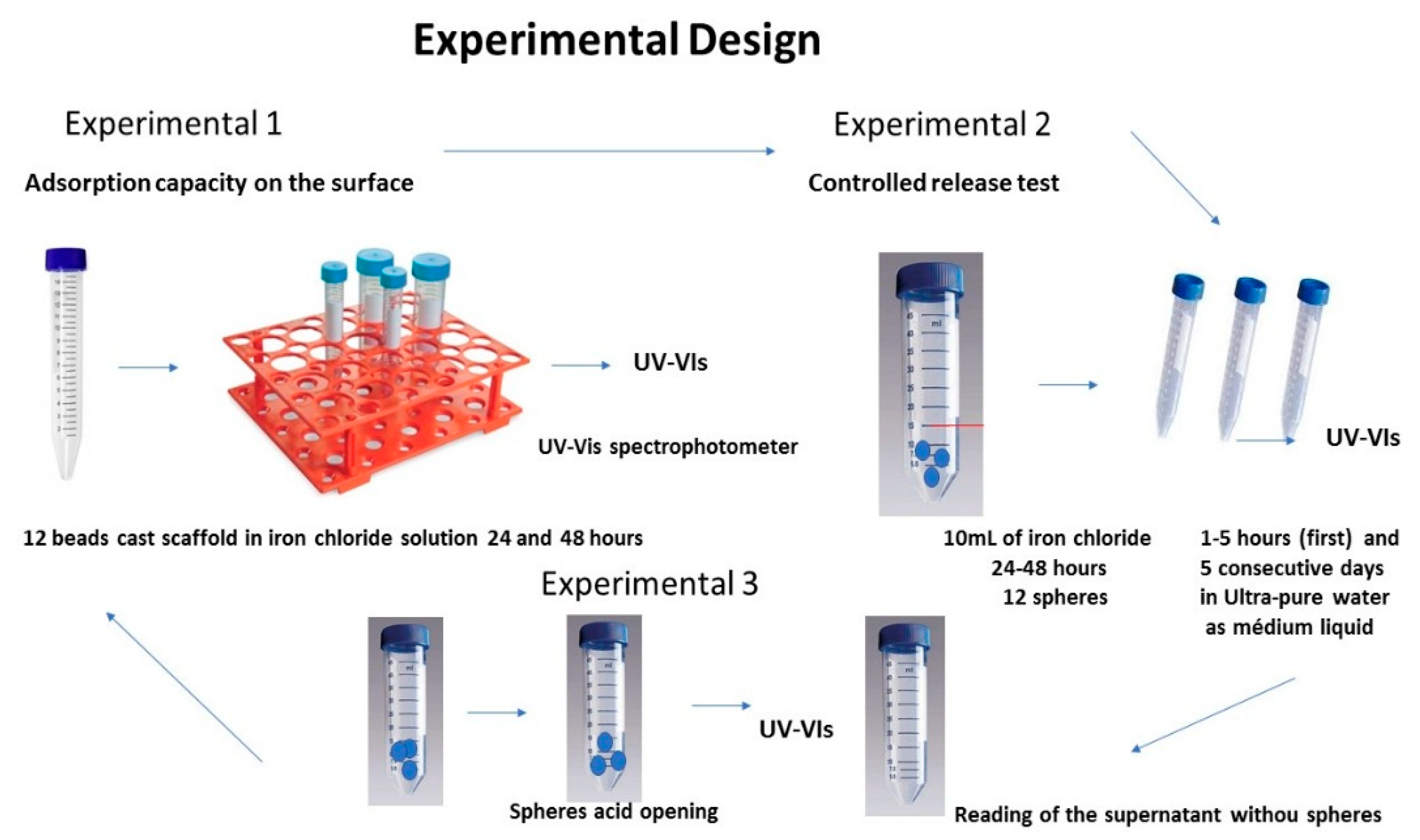
Production of Chitosan Spherical Carriers (Scaffold)
Experimental-Physico–Chemical Characterization of Chitosan Carrier Beads (Spherical Scaffold)- before the Controlled Release Test
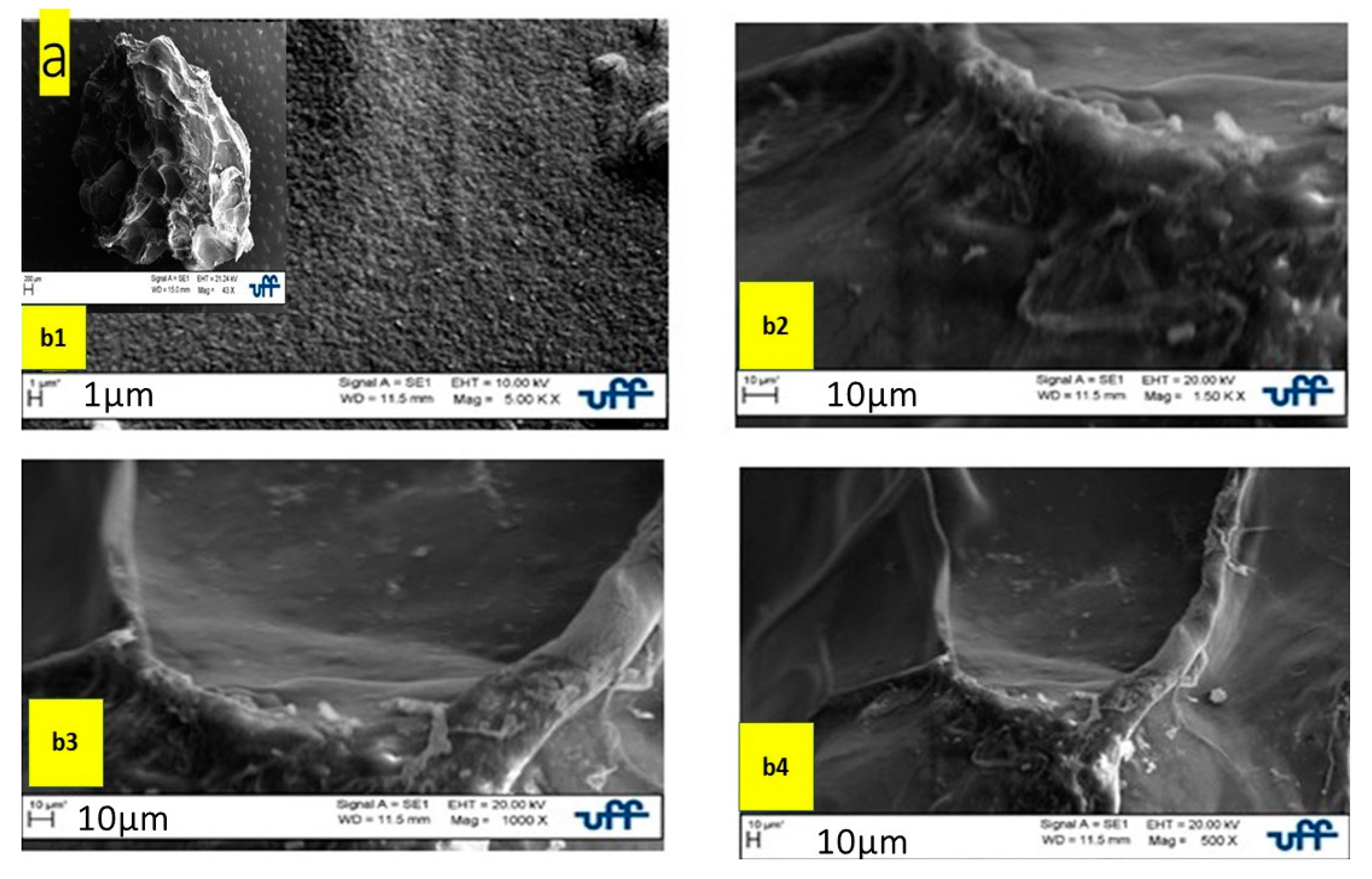
Scanning Electron Microscopy
Fourier Transform Infrared Spectroscopy (FTIR)
X-ray Diffraction Spectroscopy (XRD)
Raman Spectroscopy (RS)
Degradation and Physical–Chemical Characterization of Chitosan (ISO 10993)
Evaluation of Degradation Parameters
Cytotoxicity Assay of Chitosan Beads in Cell Culture
Development
Results
Physical–Chemical Characterization of Chitosan Spheres
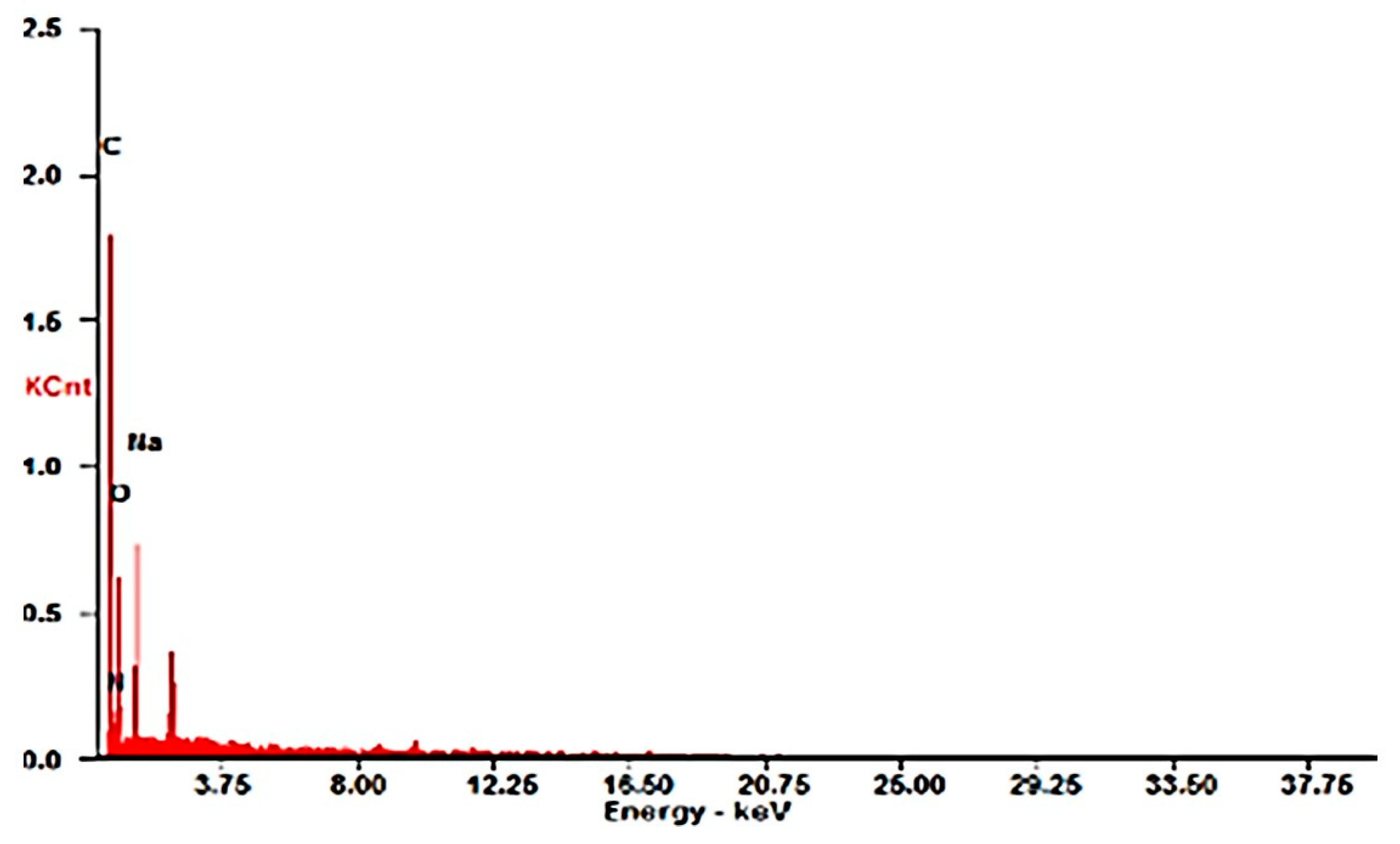
Scanning Electron Microscopy Analysis
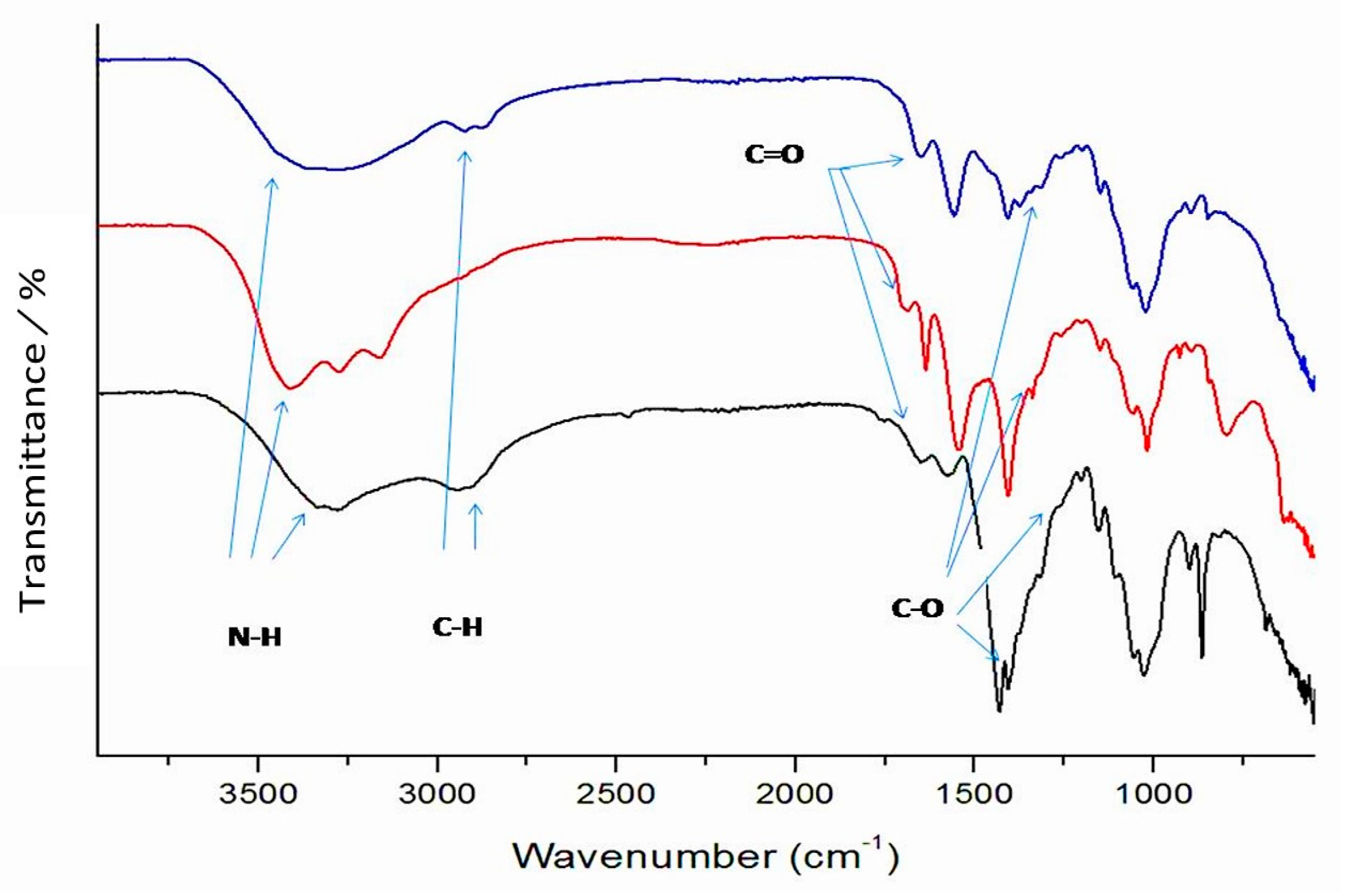
Analysis of Fourier Transform Infrared Spectroscopy (FTIR)
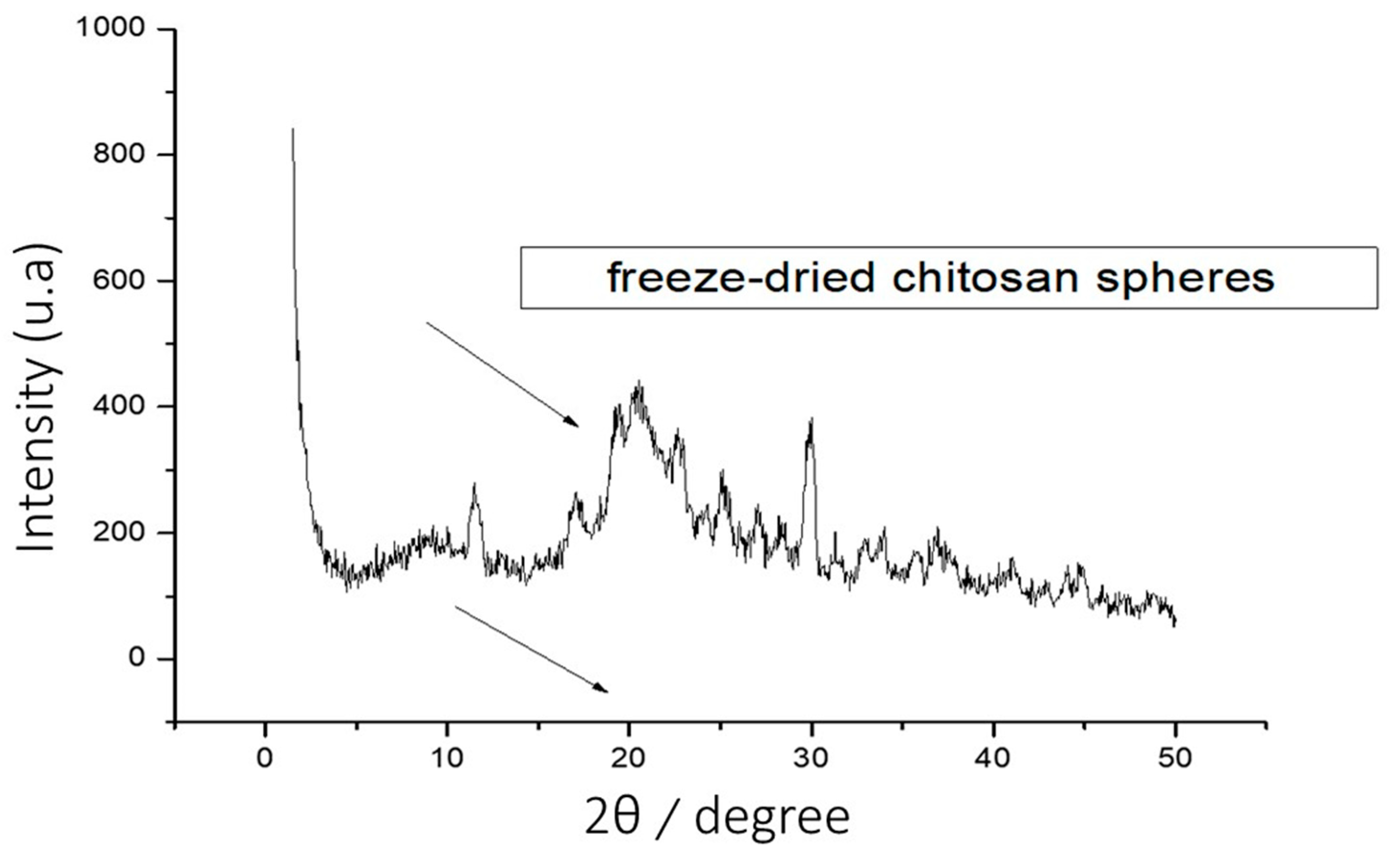
X-Ray Diffraction Characterization (XRD)
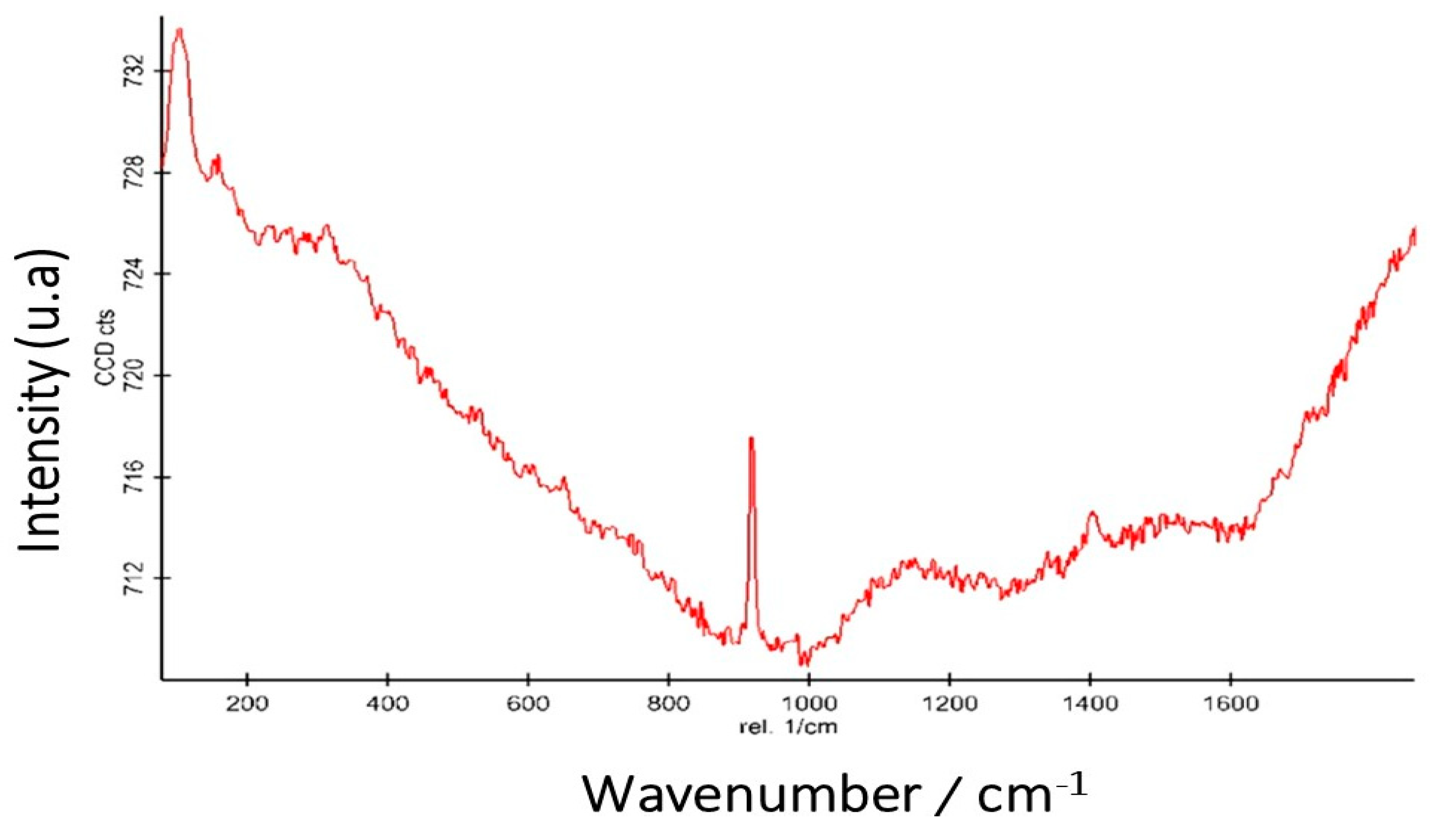
Raman Microscopy Characterization (RS)
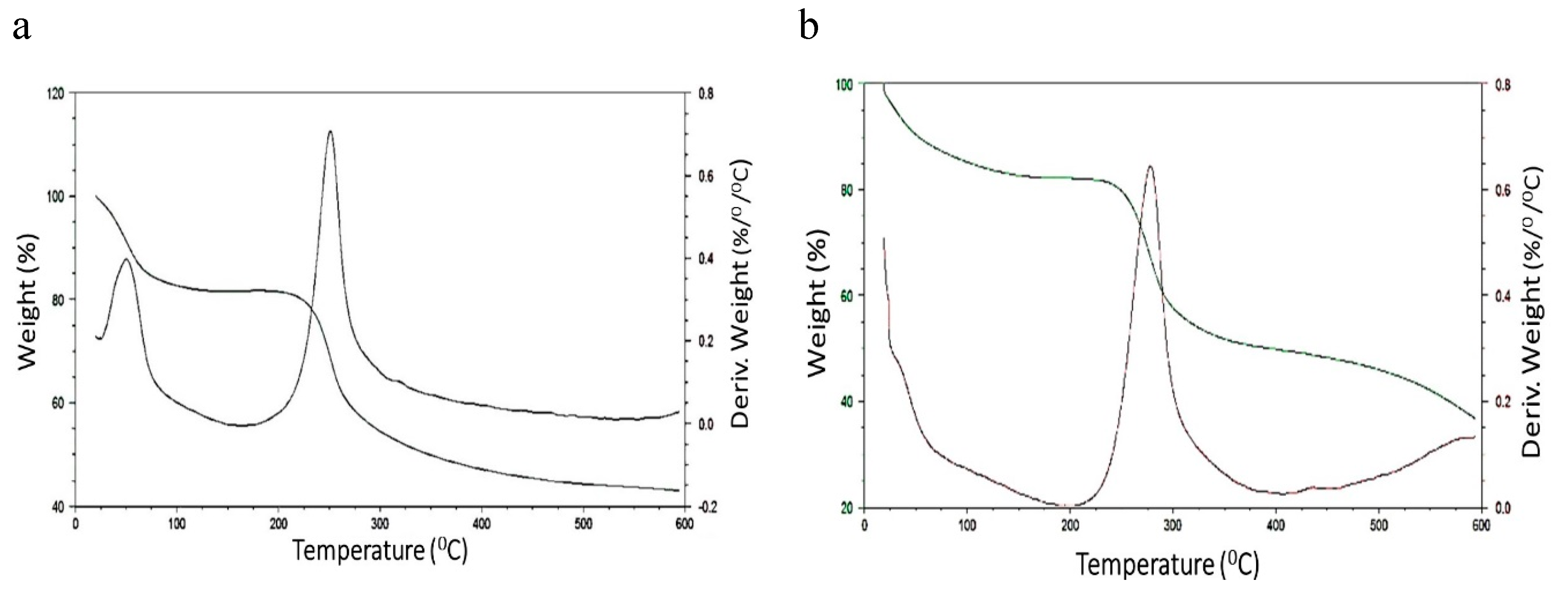
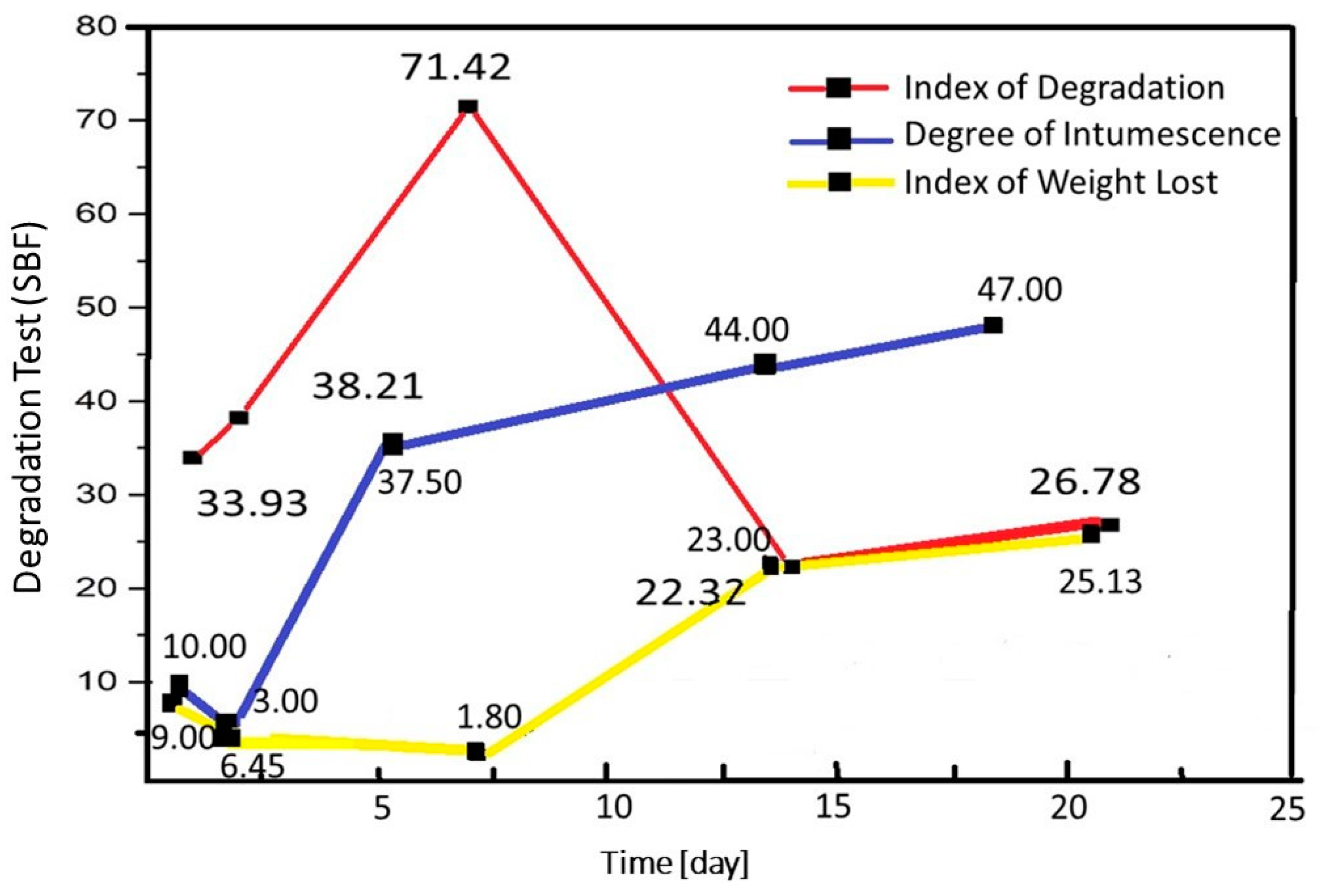
Chitosan Spheres Degradation Assay and Characterization of the Beads after the Tests
Degradation Test of Chitosan Beads in Blood Plasma Simulator (SBF)
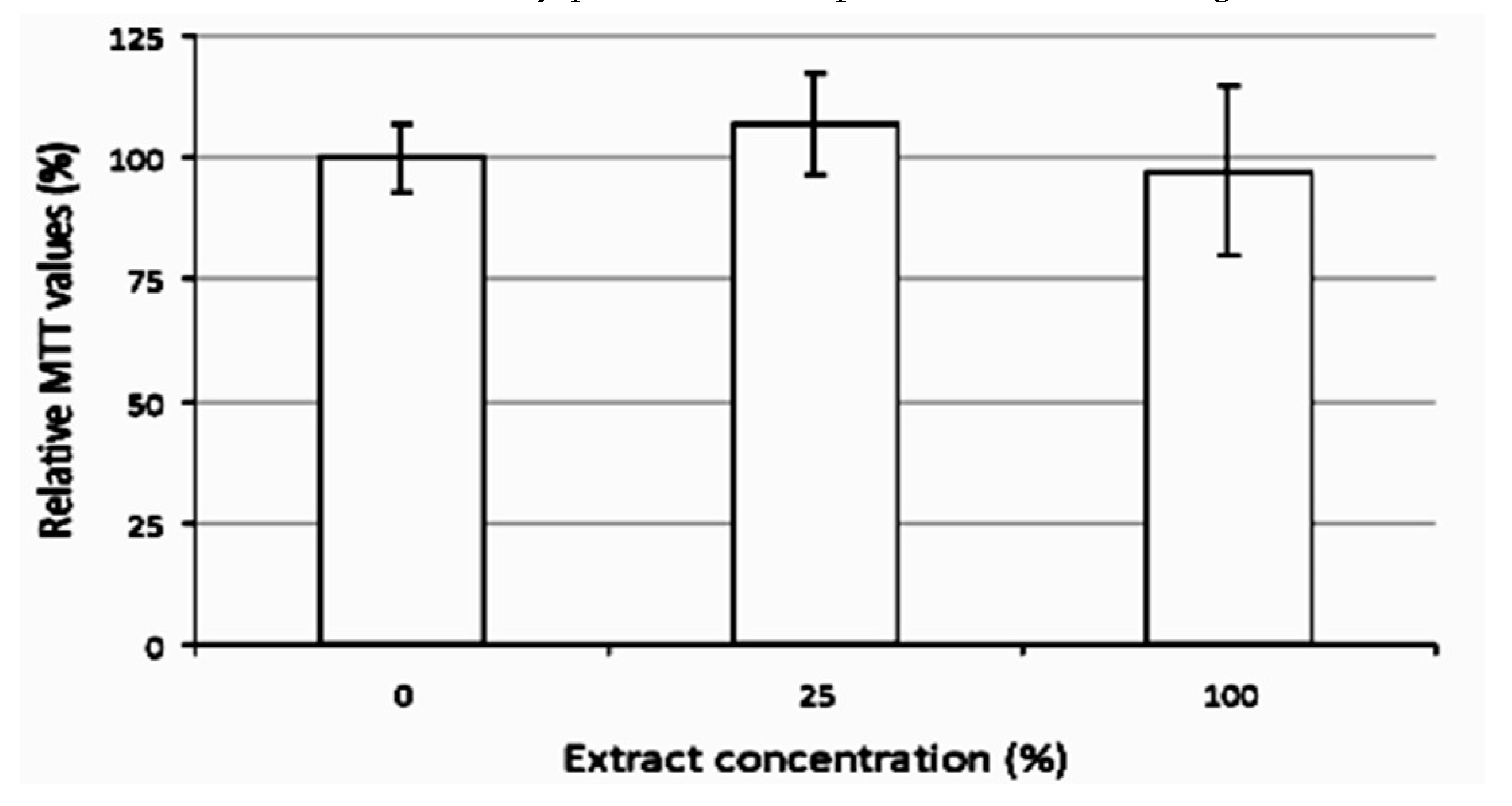
Cytotoxicity Test of Chitosan Spheres in Cell Culture
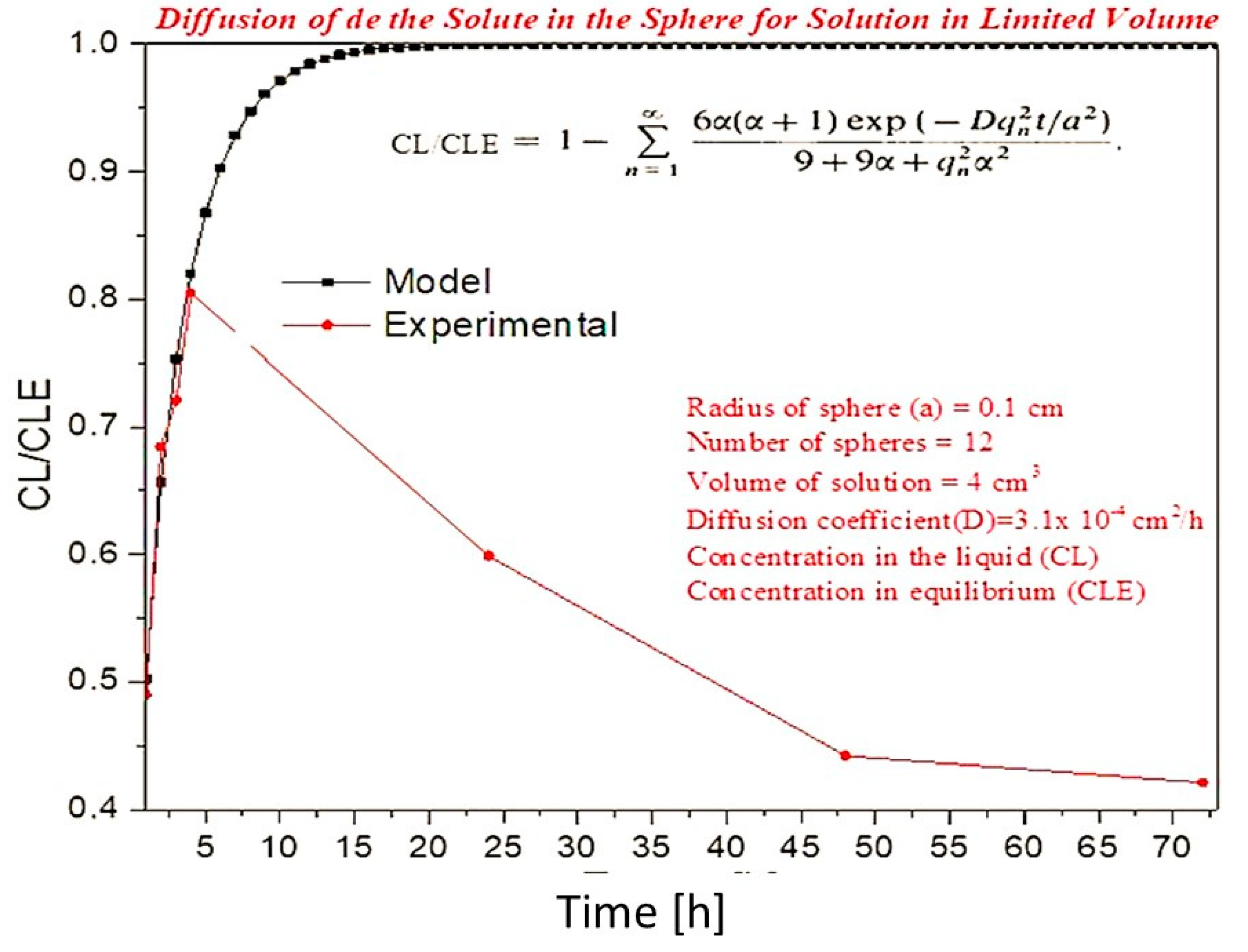
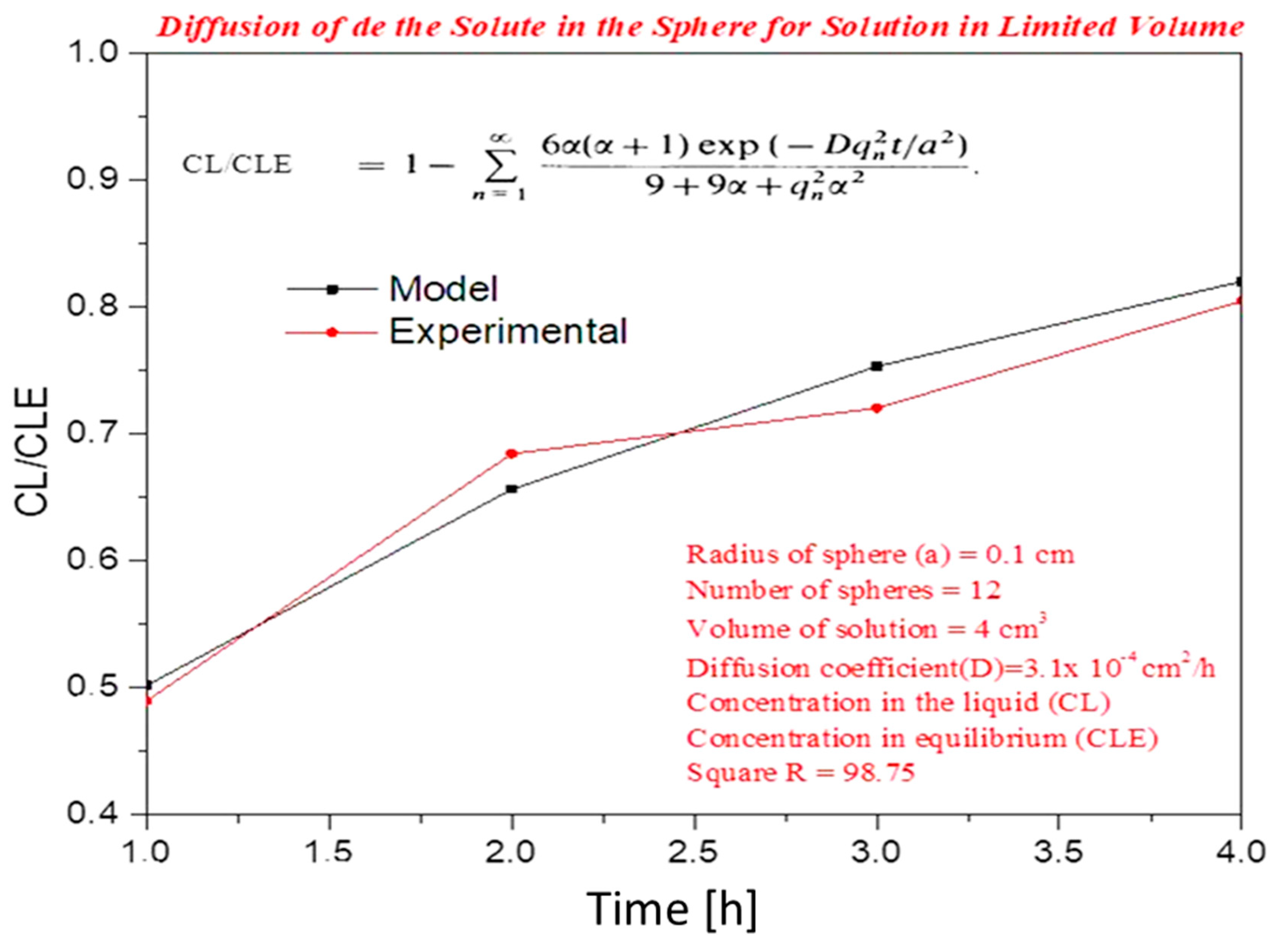
Controlled Release Test
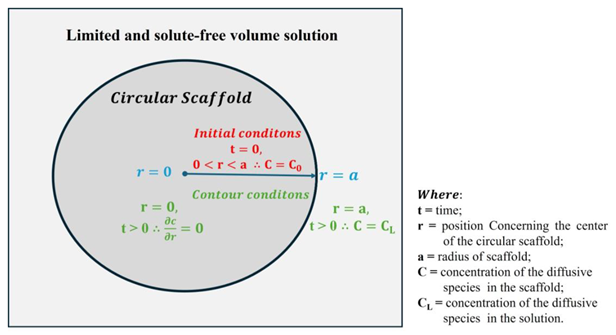
Diffusion Model in Spheres with Known Solution Concentration for Solution
Discussion
Degradation
4. Conclusions
Acknowledgments
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oryan, SS.; Sahvie, S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. International Journal of Biological Macromolecules 2017, 06, 1003–1011. [Google Scholar] [CrossRef]
- Lima, PC.; Gazoni, I. ; Carvalho, AMG. ; Bresolin, D.; Cavalheiro, D.; Oliveira, D.; Rigo, E. β-galactosidase from Kluyveromyces lactis in genipin-activated chitosan: An investigation on immobilization, stability, and application in diluted UHT milk. 2021, 1, 129050. [Google Scholar] [CrossRef]
- Levengood, SL.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. Jornal of Material Chemistry B, 2014, 2, 3161–3184. [Google Scholar] [CrossRef]
- Lima, PA.; Resende, CX.; Soares, GG.; Anselme, K.; Almeida, LE. ; Preparation, characterization and biological test of 3D-scaffolds based on chitosan fibroin and hydroxyapatite for bone tissue engineering. Materials Science & Engineering, Biomimetic Materials. Sensors and Systems, 2013, 33, 3389–3395. [Google Scholar] [CrossRef]
- Aswathy, Fw. Chitosan stabilized platinum nanoparticles: Synthesis, characterization and cytotoxic impacts on human breast cancer cells. Materials chemistry and Physics. [CrossRef]
- Onichimowski, D.; Bedzkowska, A.; Ziólkowski, H.; Jaroszewski, J.; Borys, M.; Czuczwar, M.; Wiczling, P. Population pharmacokinetics off standard-dose meropenem in critically ill patients on continuous renal replacement therapy: a prospective observational trial. Pharmacological reports.
- Pennesi, C.; Totti, C.; Beolchini, F. Removal of Vanadium (III) and Molybdenum (V) from wastewater using possidônia oceânica (tracheophyta) biomass. Plos One 2013, 25. [Google Scholar] [CrossRef]
- Duque, MA.; Tiozon, RN.; Espana, N. Chitosan from Portunus Pelagicus in the synthesis of reduced gold nanoparticle as potential carrier for the delivery of erythropoietin. Ed. bioRxiv 2018. [CrossRef]
- Varun, TK.; Senani, S.; Jayapal, N.; Chikkerur, J.; Roy, S.; Tekulapally, VB. Extraction of chitosan and its oligomers from shrimp shell waste, their characterization and antimicrobial effect. Veterinary World 2017, 10, 170–175 1014202/vetworld2017170. [Google Scholar] [CrossRef]
- Zokhrab, ST. The bonding nature of the chemical interaction between trypsin and chitosan based carriers in immobilization process depends n entrapped method: A review. International Journal of Biological Macromolecules 2021, 183, 1676–1696. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J. et. al. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and their derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef]
- Guilherme, CC.; Coiffard, L. ; Applications for marine resources in cosmetics. Cosmetics, 2017, 4, 35. [Google Scholar] [CrossRef]
- Uppala, L. A. Review on active ingredients from marine sources used in cosmetics. SOJ. Pharmacy & Pharmaceutical Sciences 2015, 2. [Google Scholar]
- Ghaffarian, R.; Herrero, EP.; Hyuntaek, D. Chitosan-alginate microcapsules provide gastric protection and intestinal release of icam-1targeting nanocarriers, enabling gi targeting in vivo. Advanced Function Material, 2016, 26, 3382–3393 101002/adfm201600084. [Google Scholar] [CrossRef]
- Lenka, S.; Marián, Z.; Maria, C. In vitro liberation of indomethacin from chitosan gels containing microemulsion in different dissolution mediums. Journal of Pharmaceutical Sciences, Pharmaceutics, Drug and Pharmaceutical technology,.
- Zhi-Xiang, T.; Wen-Da, O. ; The role of chitosan in promoting the catalytic activity of bismuth ferrite as peroxymonosulfate activator for antibiotics removal, International Journal of Biological Macromolecules, 2024, 277, 3.
- Higashi, V. ; Theodoro, JDP.; Pereira, ER.; Theodoro, OS. Congresso Técnico Científico da Engenharia e da Agronomia CONTECC. Uso de coagulantes químico (cloreto) e orgânico no tratamento de águas provenientes de sistema lêntico, Foz do Iguaçu - PR 29 de agosto a 1 de setembro, 2016.
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. European Journal of Pharmaceutics and Biopharmaceutics. [CrossRef]
- Bezerra, A; Silva, V. Sistemas de liberação controlada: Mecanismos e aplicações. Revista Saúde e Meio Ambiente – RESMA, Três Lagoas, 2016, 3, 1–12, ISSN: 2447. [Google Scholar]
- Lisboa, CD.; Silva, LD.; Matos, GC. Investigação da administração de medicamentos por cateteres em terapia intensiva, 2014, 23, 573-80. [CrossRef]
- El Mehdi; Mouad, E. ; Badreddine, R. Review of current trends in chitosan based controlled and slow-release fertilizer: From green chemistry to circular economy. International Journal of Biological Macromolecules, 2024. [CrossRef]
- Nygaard, JN.; Strand, SP.; Varum, KM. Review chitosan: Gels and interfacial properties. Polymers. [CrossRef]
- Luo, Y.; Wang, Q. ; Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. International Journal of Biological Macromolecules. [CrossRef]
- Sæther, HV.; Holme, HK.; Maurstad, G.; Smidsrød, O.; Stokke, BT. Polyelectrolyte complex formation using alginate and chitosan. Carbohydrate Polymers. [CrossRef]
- Filho, MSG.; Modelagem da liberação controlada de fármacos através de modelos em rede, Faculdade UnB Planaltina Licenciatura em Ciências Naturais da Universidade de Brasília-UnB, Dezembro-2013.
- Anirudhan, TS.; Parvathy, J. Design of biopolymeric architecture with thiolated chitosan-poly (lactic acid) blend\methionine modified clay for the controlled release of amoxicillin. Polymer-Plastics Technology and Engineering 2014, 53, 1339–1343. [Google Scholar] [CrossRef]
- Chen, CY.; Chien, Y, Chung. Antibacterial effect of water-soluble chitosan on representative dental pathogens Streptococcus mutans and Lactobacilli brevis. Journal Applied oral science 2012, 20, 620–627 PMID:23329243, PMCID:PMC3881855. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferreira, JGJ. ; Flores, VG.; Marco, MR., Fraga, BB., Zorzo, RR., Morais, PF., Eds.; Morisso, FDP, Diazepam nanocasules as na alternative for sleep induction: Development study and toxicity assessment. Food and Chemical Toxicology, 2024, V.192, 114962. [Google Scholar] [CrossRef]
- Ferraz, LRM. Desenvolvimento e avaliação da liberação in vitro de drug delivery system em pH-dependente à base de benznidazol e ZIF-8 visando a obtenção de uma terapia alternativa. Tese de Doutorado do programa de Pós-Graduação em Ciências farmacêuticas da Universidade Federal de Pernambuco, 2017.
- Neto, BPC. Micropartículas com quitosana estruturadas com aerosil: Estabilidade, adsorção, encapsulação e liberação de substâncias ativas. Tese de Doutorado apresentado ao programa de Engenharia Química da Universidade federal do rio Grande do Norte, 2014.
- Benvenutti, T. Avaliação da eletrodiálise no tratamento de efluentes de processos de eletrodeposição de níquel., Dissertação de mestrado em Engenharia do Programa de Pós-Graduação em engenharia de Minas, Metalúrgica e de Materiais, PPGE3M, 2012.
- Lima, IR.; Costa, AM.; Bastos, IN.; Granjeiro, JM.; Soares, GA. Development and characterization of 5% mol Zn bioceramic in granular form, Material Research 2006, 9, 399-103. [CrossRef]
- Lima, PA.; Resende, CX.; Soares, GD.; Anselme, K.; Almeida, LE. Preparation, characterization and biological test of 3D-scaffolds based on chitosan, fibroin and hydroxyapatite for bone tissue engineering. Materials Science and Engineering 2013, 33, 3389–95. [Google Scholar] [CrossRef]
- Bezerra, A; Silva, V; Sistemas de liberação controlada: Mecanismos e aplicações. Revista Saúde e Meio Ambiente – RESMA, Três Lagoas. 2016, 3, 1–12 ISSN: 2447.
- Lima, IR.; Alves, GG.; Campaneli, AP.; Gasparoto, TH. ; Junior, ESR.; Sena, LA. Understanding the impact of divalent cation substitution on hydroxyapatite: An in vitro multiparametric study on biocompatibility*. Journal of Biomedical Materials Research Part A. [CrossRef]
- Crank. The Mathematics of diffusion by J. Crank Brunel University Uxbridge second edition, Clarendon press Oxford. 1975, University Press, Ely House, London. ISBN 0 19 853344 6.
- Tanaka, H.; Matsumura, M.; Veliky, IA. Diffusion characteristics of substrates in Ca-alginate gel beads. Biotechnology and Bioengineeering. [CrossRef]
- Nunes Nunes, AR. Liberação controlada de curcumina ancorada em sílica hexagonal mesoporosa. Dissertação apresentada ao Instituto de Química da Universidade de Brasília como exigência parcial para obtenção do título de Mestre em Química do Instituto de Química da Universidade de Brasília -UnB, 2013.
- Silva*, MC.; Fideles, TB.; Fook, MVL. Esferas de quitosana e quitosana/curcumina pelo método de gelificação ionotrópica: influência da incorporação do fármaco, Revista de acesso livre no site www.ufcg.edu.br. Revista Eletrônica de Materiais e Processos 2, 1809; -28. [Google Scholar]
- Siepmann, J.; Siepmann, F. Mathematical Modeling of drug delivery. International Journal of Pharmaceutics.
- Lopes, CM.; Lobo, JMS. ; Costa, P. Modified release of drug delivery systems: hydrophilic polymers. Revista Brasileira de Ciências farmacêuticas; 2005, 41, 143–154. [Google Scholar] [CrossRef]
- Hopfenberg, HB. Controlled release from erodible slabs, cylinders, and spheres, Department of Chemical Engineering, North Carolina State University. Controlled Release Polymeric Formulations, Copyright. American Chemical Society, 2: 3. [CrossRef]
- Aparicio, G.; Garrido, Q.; Garrido, L. Diffusion of small molecules in a chitosan\water gel determined by proton localized NMR spectroscopy. Journal Colloid Interface Science. [CrossRef]
- Valadão, ICR. ; Silva, AJ.; Castro, JA Ritter, E. Simulação computacional da difusão molecular de poluentes líquidos de aterros em solos organo argilos- Aterro Metropolitano de Gramacho. Revista estudos tecnológicos 2006, 2, 55–64, ISSN 1808. [Google Scholar]
- Chandy, DL. ; Mooradian, Rao, G. H. R. Journal of Applied Polymer Science, 2143. [Google Scholar]
- Lima, HA.; Lia, F, MV. ; Preparation and Characterization of Chitosan-Insulin-Tripolyphosphate Membrane for Controlled Drug Release: Effect of Cross Linking Agent. JBNB 2014, 5, 211–219. [Google Scholar] [CrossRef]
- Da Silva, ARPS. ; Macedo, T.; Coletta, D. J. Feldman, S., Pereira, M. M., Synthesis, characterization and cytotoxicity of Chitosan/Polyvinyl Alcohol/Bioactive Glass hybrid scaffolds obtained by lyophilization. Revista Matéria 2016, 21, 964–973 ISSN 1517. [Google Scholar] [CrossRef]
- Tavaria, FK.; Costa, EM.; Pina-Vaz, I.; Carvalho, M.F.; Pintad, M.M. 2013; 29. [CrossRef]
- Demadis, KD.; Pachis, K.; Ketsetzi, A. Stathoulopoulou. Advances in Colloid and Interface Science. [CrossRef]
- Negrea, P.; Caunib, A.; Sarac, I.; Butnariu, M. The study of infrared spectrum of chitin and chitosan extract as potential sources of biomass. Digest Journal of Nanomaterials and biostructures 2015, 10, 1129. [Google Scholar]
- Resende, CX, Desenvolvimento a base de dupla camada de Quitosana para a reconstrução tecidual. Tese de Doutorado COPPE-Universidade Federal do Rio de Janeiro. Rio de Janeiro. Brasil, 2010.
- Puente, C.; Sánchez-Domínguez, M. ; Brosseau, C L.; López, I. Silver-chitosan and gold-chitosan substrates for surface-enhanced Raman Spectroscopy (SERS): Effect of nanoparticle morphology on SERS performance. Materials Chemistry and Physics 2021, 260: 124107, 2021. [Google Scholar] [CrossRef]
- Zajac, A.; Hanuza, J.; Wandas, M.; Dyminska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. [CrossRef]
- Castrejón-Flores, J L.; Reyna-Luna, J.; Flores-Martinez, Y M.; García-Ventura, M I.; Zamudio-Medina, A.; Franco-Pérez, M. Characterizing the thermal degradation mechanism of two bisphosphoramidates by TGA, DSC, mass spectrometry and first-principle theoretical protocols. Journal of Molecular Structure, 1287.
- Luyen, DV, Huong, DM. Chitin and derivatives. In: J.C. Salamone (Ed.), Polymeric Materials Encyclopedia. 1996, 2:1208-1217.
- Cruz, J B. ; Catão, CDS. Synthesis and characterization of chitosan scaffolds with antineoplastic agent. Matéria 2016, 21, 129–140 ISSN 1517. [Google Scholar] [CrossRef]
- Kumar, S.; Kumari, M.; Mallick, A.; Swain, BS.; Sobral, AJFN. ; Dutta, P.K. Preparation and characterization of microporousnanocomposites for biomedical applications. K. Preparation and characterization of microporousnanocomposites for biomedical applications. Asian Chitian Journal 2015, 26–28. [Google Scholar]
- Souza, NLGD. ; Salles, TF.; Brandão, HM.; Edwards, HGM.; Oliveira, LFC. Synthesis, Vibration spectroscopic and thermal properties of oxocarbon cross-linked chitosan. Journal of the Brazilian Chemical Society 2015, 26, 1247–1256. [Google Scholar] [CrossRef]
- Jameela, SR.; Misra, A.; Javakrishnan, A. Cross-linked chitosan microspheres as carriers for prolonged delivery of macromolecular drugs. Journal of Biomaterials Science Polymer Edition. [CrossRef]
- Silva. Santa catarina. 2015.
- Miculescu, F.; Maidaniuc, A.; Miculescu, M.; Batalu, ND.; Ciocoiu, C.; Voicu, SI.; Stan, GE.; Thakur, VK. Synthesis and characterization of jellified composites from bovine bone-derived hydroxyapatite and starch as precursors for robocasting. ACS Omega 2018, 3, 1338–1349. [Google Scholar] [CrossRef]
- Santana, IL. ; Gonçalves, L, M. ; Ribeiro, JJS, Filho.; JRM., Júnior, AAC. Thermal behavior of direct resin composites: glass transition temperature and initial degradation analyses, Revista Odonto Ciência. 2011, 26, 50–55 http://wwwscielobr/pdf/roc/v26n1/a12v26n1pdf. [Google Scholar]
- Thürmer, MB.; Diehl, CE.; Brum, FJB. ; Santos, LA. Preparation and characterization of hydrogels with potential for use as biomaterials. Materials Research, 2014, 17, 109–113. [Google Scholar] [CrossRef]
- Elbanna, AE.; Carlson, J M. . Dynamics of polymer molecules with sacrificial bond and hidden length systems: Towards a physically-based mesoscopic constitutive law. Mechanics and Physics of Soft Materials. 2013, 8, 56118. [Google Scholar] [CrossRef] [PubMed]
- Yuhang, C. ; Zhou, S, Li, Q. Mathematical modeling of degradation for bulk-erosive polymers: Applications in tissue engineering scaffolds and drug delivery systems. Acta Biomaterialia. 1140. [Google Scholar] [CrossRef]
- Gualandi, C.; Soccio, M.; Saino, E.; Visai, L. Easily synthesized novel biodegradable copolyesters with adjustable properties for biomedical applications. Soft Matter V. 2012, 8, 5466–5476. [Google Scholar] [CrossRef]
- Li, G.; Huang, J.; Chen, T.; Wang, X.; Zhang, H.; Chen, Q. Insight into the interaction between chitosan and bovine serum albumin. Carbohydrate Polymers. [CrossRef]
- Schütz, CA.; Juillerat-Jeanneret, J.; Käuper, P.; Wandrey, C. Cell Response to the exposure to chitosan–TPP//alginate nanogels. Biomacromolecules. 2011, 12, 4153–4161. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Chang, Y.; Liu, RH.; Chen, C.; Lee, I.; Yang, N. Resveratrol can be stable in a medium containing fetal bovine serum with pyruvate but shortens the lifespan of human fibroblastic Hs68 cells. Oxidative Medicine and Cellular Longevity, 2371. [Google Scholar] [CrossRef]
- Júnior, ADC.; Vieira, FP.; Melo, VJ.; Lopes, MTP.; Silveira, JN.; Ramaldes, GA.; Garnier-Suillerot, A.; Pereira-Maia, EC.; Oliveira, MC. Preparation and cytotoxicity of cisplatin-containing liposomes. Brazilian Journal of Medical and Biological Research. 2007, 40, 1149–1157. [Google Scholar] [CrossRef]
- Lima, IR.; Alves, GG.; Soriano, CA.; Campaneli, AP. ; Gasparoto, T H. Understanding the impact of divalent cation substitution on hydroxyapatite: an in vitro multiparametric study on biocompatibility. Journal Biomedical Materials Research. 3: 98A, 2011. [Google Scholar] [CrossRef]
- Pessoa, P.; Neto, L.; Almeida, S.; Farias, F.; Vieira, L. R.; Medeiros, L.; Boligon, A. Polyphenol composition, antioxidant activity and cytotoxicity of seeds from two underexploited wild licania species: L. rigida and L. tomentosa. Molecules, 2: 21, 1755. [Google Scholar] [CrossRef]
- Faria, DM.; Dourado Júnior, SM.; Nascimento, JPLD. ; Nunes, EDS.; Marques, RP.; Rossino, LS.; and Moreto, JA.; Development and evaluation of a controlled release system of TBH herbicide using alginate microparticles. Materials Research. 2017, 20, 225–235. [Google Scholar] [CrossRef]
- Douglas, KL.; Tabrizian, M. Effect of experimental parameters on the formation of alginate-chitosan nanoparticles and evaluation of their potential application as DNA carrier. Journal of Biomaterials Science. Polymer Edition. 2005, 16(1), 43–56. [Google Scholar] [CrossRef] [PubMed]
- Torres, MA.; Beppu, MM.; Santana, CC. Characterization of chemically modified chitosan microspheres as adsorbents using standard Proteins (bovine serum albumin and lysozyme). Brazilian Journal of Chemical Engineering. 2007, 24, . 325–336. [Google Scholar] [CrossRef]
- Wan Ngah, WS. ; Endud, CS; Mayanar, R. Reactive and Functional Polymers, 1: 50.
- Dias, FS.; Queiroz, DC.; Nascimento, RF.; Lima, M. B. Um sistema simples para preparação de microesferas de quitosana. Quím. Nova. 2008, 31, 160–163. [Google Scholar] [CrossRef]
- Schnürch, AB,; Dünnhaupt, SS. Chitosan-based drug delivery systems. European Journal of Pharmaceutics and Biopharmacetics. 2012, 81, 463–469. [Google Scholar] [CrossRef]
- Pereira, FAR. Quitosana e seus compósitos como adsorventes e sistemas de liberação controlada de fármacos.[tese]. Universidade Federal da Paraíba em Química Inorgânica.2014.
- Tavares, JK. Modelagem da liberação controlada de princípios ativos (betacaroteno e lidocaína) de microcápsulas. Tese submetida ao Programa de Pós-Graduação em Engenharia Química da Universidade Federal de Santa Catarina, 2015.
- Maciel, VBV. Complexos dos polieletrólitos quitosana e pectina para obtenção de sistemas carreadores de compostos bioativos. Tese apresentada à Faculdade de Engenharia Química da Universidade Estadual de Campinas como parte dos requisitos exigidos para obtenção do título de Doutor em Engenharia Química da Universidade Estadual de Campinas, 2015.
- Alj, A,; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. International Journal of Biological macromolecules. [CrossRef]
- Lopes, CM. ; Lobo, JMS.; Costa, P. Formas farmacêuticas de liberação modificada: polímeros hidrofílicos.
- Abdelakder, H.; Siti, A.; Abdullah, N. Chitosan potential aspects for drug delivery and pharmaceutical applications using microencapsulation techniques. Applied Engineering and Sciences. 2017, 14–15. [Google Scholar]
- Yang, Y.; Wang, S.; Wang, Y.; Wang, Q.; Chen, M. Advances in self-assembled chitosan nanomaterials for drug delivery. Biotechnology Advances. 2014, 32, 1301–1316. [Google Scholar] [CrossRef]
- Dobrovolskaya, IP.; Yudin, VE.; Popryadukhin, PV.; Morganti, P. Effect of chitin nanofibrils on electrospinning of chitosan-based composite nanofibers. Carbohydrate Polymers, 2: 194. [CrossRef]
- Leite, MV. Microcápsulas de alginato-quitosana contendo nanopartículas magnéticas para liberação controlada de progesterona, Melina Vasconcelos Leite. Universidade Estadual do Norte Fluminense Darcy Ribeiro, UENF, Campos dos Goytacazes, 2014.
- Morais, SJS. ; Devilla, IA.; Ferreira, DA.; Teixeira, IR. Modelagem matemática das curvas de secagem e coeficiente de difusão de grãos de feijão-caupi (Vignaunguiculata (L.) walp. Mathematical modeling of the drying curves and diffusion coefficient of cowpea grains (Vigna unguiculata (L.) Walp. Revista Ciência Agronômica. 2013, 44, 455–463, ISSN 1806. [Google Scholar]
- Kashani-Nejad, MA.; Mortazavi, A.; Safekordi, AG. Thin-layer drying characteristics and modeling of pistachio nuts. Journal of Food Engineering.
- Üzüm, C.; Shahwan, T.; Eroglu, AE.; Lieberwirth, I.; Scott, TB.; Hallan, KR. Application of zero-valent iron nanoparticles for the removal of aqueous Co2+ ions under various experimental conditions. Chemical Engineering Journal. 2008, 144, 213–220. [Google Scholar] [CrossRef]
- Silva. LA. Síntese e caracterização de nanopartículas de hematita e de ferro zerovalente sintetizadas a partir de cloreto férrico, [tese], Doutorado do Programa de Engenharia Química da Universidade de Santa Catarina, 2015.
- Fu, F.; Dinysiou, DD.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. Journal of Hazardous Materials, 1: 267. [CrossRef]
- Bird, RB.; Stewart, WE.; Lightfoot, EN. Transport Phenomena. John Wiley & Sons, 2007.
- Crank, J. The Mathematics of Diffusion. University Brunel Uxbridge Clarendon Press, 1975. [Google Scholar]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. Journal of Controlled Release. 2012, 161(2), 351–362. [Google Scholar] [CrossRef] [PubMed]
- Bryszewska, M. A.; Cobos, L.T.; Gallego, E.; Villalba, M.; Rivera, D. ; Laure. ; D, Saa.; D. L. T. Gianotti, A., In vitro bioaccessibility and bioavailability of iron from breads fortified with microencapsulated iron. LWT-Food Science and Technology. 2019, 99, 431–437. [Google Scholar]
- Cian, RE,; Proano, JL. ; Salgado, PR.; Mauri, NA.; Drago, SR. High iron bioaccessibility from co-microencapsulated iron\ascorbic acid using chelating polypeptides from brewers spent grain protein as wall material. LWT-Food Science and Technology, 2021, 50, 72–78. [Google Scholar]
- Moreira, FR, Moreira, JC.
- Sharma, VM.; Chopra, R.; Ghosh, I.; Ganesan, K. Quantitative target display: a method to screen yeast mutants conferring quantitative phenotypes by ‘mutant DNA fingerprints’. Nucleic Acids Research. 2001, 29, e86. [Google Scholar] [CrossRef]
- Silva, GC. Mecanismo de acumulação de ferro e arsênio em biomassa vegetal fibrosa, Dissertação de Mestrado apresentado à Universidade Federal de Minas como dissertação de mestrado “Mecanismo de acumulação de ferro e arsênio em biomassa vegetal fibrosa, 2008.
- Altaner, B. arXiv:1410.3983v1 [cond-mat.stat-mech] 15 October, 2014.
- Owens, GJ.; Singh, RK.; Foroutan, F.; Alqaysi, M. Sol-gelbased materials for biomedical applications. Progress in Materials Science. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Shubham, K.; Anukiruthika, T.; Sayantani, D.; Kashyap, AV.; Moses, J.A. Iron deficiency anemia: A comprehensive review on iron absorption, bioavailability, and emerging food fortification approaches. Trendes in Food Science and Technology 2020, 99, 58–75. [Google Scholar] [CrossRef]
- Grotto, H ZW. Metabolismo do ferro: uma revisão sobre os principais mecanismos envolvidos em sua homeostase. Revista Brasileira de Hematologia e Hemoterapia, 2008, 30, 390–397.
- Zhang, D.; Wan, H.; Zhao, R.; Zhang, Yu. Eudragit S100 coated iron oxide-chiotsan nanocomposites for colon targeting of 5-aminosalicylic acid ameliorate ulcerative colitis by improving intestinal barrier function and inhibiting NLRP3 inflammasome. International Immunopharmacology. 2024, V. 139, 112661. [Google Scholar] [CrossRef]
- GrottoGrotto, HZW. Fisiologia e metabolismo do ferro. Revista Brasileira de Hematologia e Hemoterapia. 2010, 32:8-17. 2010, 32, 8–17.
- Rathor, G.; Chopra, N.; Adhikari, T. International Journal of Chemical Studies. 2017, 5, 282-285.

| α | q1 | q2 | q3 | q4 | q5 | q6 |
|---|---|---|---|---|---|---|
| ∞ | 3.1416 | 6.2832 | 9.4248 | 12.5664 | 15.7080 | 18.8496 |
| 9.0000 | 3.2410 | 6,3353 | 9.4599 | 12.5928 | 15.7292 | 18.8671 |
| 4.0000 | 3.3485 | 6.3979 | 9.5029 | 12.6254 | 15.7554 | 18.8891 |
| 2.3333 | 3.4650 | 6.4736 | 9.5567 | 12.6668 | 15.7888 | 18.9172 |
| 1.5000 | 3.5909 | 6.5665 | 9.6255 | 12.7205 | 15.8326 | 18.9541 |
| 1.0000 | 3.7264 | 6.6814 | 9.7156 | 12.7928 | 15.8924 | 19.0048 |
| *Description | Symbol | Value | Unit |
|---|---|---|---|
| Scaffold radius | a | 0.1 | cm |
| Volume of solution | V | 10 | cm3 |
| Number of scaffold | n | 5 | --- |
| Tests time | t | 130 | hours |
| Infinite concentration in solution CLE | CLE | 1 | Mol |
| Concentration in time solution | CL | 0.79644876 | Mol |
| The starting point for applying the algorithm | a | 0 | --- |
| The Final point for the application of the algorithm | b | 1 | --- |
| Sphere radius | a | 0.1 | cm |
| Volume of solution | V | 10 | cm3 |
| Number of spheres | n | 5 | --- |
| Tests time | t | 130 | hours |
| Infinite concentration in solution CLE | CLE | 1 | Mol |
| Concentration in time solution | CL | 0.79644876 | Mol |
| Starting point for applying the algorithm | a | 0 | --- |
| Final point for application of algorithm | b | 1 | --- |
| Description | Symbol | Value | Unity |
|---|---|---|---|
| Ray Sphere | a | 0.1 | cm |
| Solution Volume | V | 4 | cm3 |
| Number of Spheres | n | 12 | --- |
| Time [h] | Concentration in the LiquidCL[mol] | Concentration in BalanceCLE [mol] | CL/CLE |
|---|---|---|---|
| 1 | 0.04912 | 0.10000 | 0.4912 |
| 2 | 0.06843 | 0.10000 | 0.6843 |
| 3 | 0.07204 | 0.10000 | 0.7204 |
| 4 | 0.08045 | 0.10000 | 0.8045 |
| 24 | 0.05987 | 0.10000 | 0.5987 |
| 48 | 0.04421 | 0.10000 | 0.4421 |
| 72 | 0.04211 | 0.10000 | 0.4211 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





