1. Introduction
Nosocomial infections, which are a major concern in healthcare settings, are also examined in this study. These infections, caused by drug-resistant pathogens like Gram-negative bacteria and methicillin-resistant
Staphylococcus aureus (MRSA), are prevalent in Trinidad and Tobago. They lead to increased morbidity, mortality, and healthcare costs, underlining the importance of effective infection control measures and the prudent use of antibiotics [
1,
2].
Another critical issue addressed in this paper is foodborne illnesses, particularly Salmonella infections linked to poultry. The study evaluates the prevalence of Salmonella in Jamaica’s poultry industry, examining its implications for food safety and public health. Effective control of Salmonella requires stringent biosecurity measures and public education.
In addition to bacterial infections, the safety of blood transfusions is of paramount concern.
The paper explores novel approaches in antibody production and vaccine development, particularly using IgM antibodies in egg whites, highlighting their potential for immunological research and applications in the West Indies.
Peptide candidate vaccines to chicks induces specific anti-HIV gp120 and gp41 antibodies that neutralize the original HIV antigens [
3].
This multidisciplinary study provides insights into tackling infectious diseases through microbiological and immunological advancements [
2]. Infectious diseases continue to pose significant public health challenges worldwide, particularly in resource-limited regions like the Caribbean. Microbiology and immunology play a crucial role in understanding the mechanisms of disease transmission, pathogenesis, and host immune responses. In the Caribbean, the dual burden of tuberculosis (TB) and HIV is especially concerning, with TB remaining one of the leading causes of morbidity and mortality in HIV-infected individuals. This paper explores the epidemiology of TB and HIV co-infection in Jamaica, among other themes, highlighting the urgent need for early diagnosis and integrated care [
4].
2. Nosocomial Infections Globally
Nosocomial infections, particularly in intensive care, surgical, and medical wards, are a recurrent issue globally, with rates varying by region. In Trinidad and Tobago, limited data exist, but between 1992-1995, 7,158 infections were recorded, primarily urinary tract infections, postoperative wound infections, pneumonia, and bloodstream infections. Gram-negative bacilli, especially Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus were prevalent. Bloodstream infections significantly increased mortality, length of hospital stays, and costs. Skin and soft tissue infections (SSTIs) are also common, with management depending on severity, involving antibiotics like penicillin, cephalosporins, and aminoglycosides. Antibiotic resistance remains a concern [
5,
6,
7,
8,
9].
The study by Amaral et al. (2005) examines the characteristics of a predominant variant within the Brazilian epidemic clonal complex of methicillin-resistant Staphylococcus aureus (MRSA). This research focuses on the enhanced abilities of this MRSA variant to produce biofilm and to adhere to and invade airway epithelial cells. The researchers characterised the variant by evaluating its biofilm production, which is a key factor in its pathogenicity. They found that this Brazilian MRSA variant exhibits a significantly increased capacity to form biofilms compared to other strains. Biofilms are dense clusters of bacteria that adhere to surfaces and are protected by a matrix, making infections harder to treat. Additionally, the study assessed the variant's ability to adhere to and invade airway epithelial cells, which are crucial in respiratory infections. The findings demonstrated that this MRSA variant has an enhanced capacity to attach to and penetrate these cells, potentially leading to more severe respiratory infections. The results highlight the variant's aggressive nature and its potential impact on patient outcomes, particularly in respiratory infections. The study underscores the need for targeted strategies to address infections caused by this MRSA variant, including novel therapeutic approaches and improved infection control measures [
10].
Gram-negative bacterial infections pose a global health challenge, with beta-lactamase production being a key factor in beta-lactam resistance. Extended spectrum beta-lactamases (ESBLs), particularly in
Escherichia coli and
Klebsiella pneumoniae, are widespread, with over 200 ESBLs characterised worldwide [
12,
13]. ESBLs, such as TEM, SHV, and CTX-M, cause resistance to beta-lactam antibiotics, including cephalosporins and monobactams, but not cephamycins or carbapenems [
14]. ESBL-producing organisms contribute to nosocomial infections, especially urinary tract infections, respiratory infections, and bloodstream infections [
15,
16]. Risk factors for ESBL-associated UTIs include antibiotic use, previous hospitalisations, and underlying conditions. Preventive measures, such as hand hygiene and environmental cleaning, are crucial to controlling the spread of these resistant organisms [
15].
3. Salmonellosis
The Salmonella pandemic, first noted in the late 1980s, has been linked to contaminated eggs, with a rising proportion of
S. serovar
Enteritidis infections globally [
18,
19,
20]. Control of
Salmonella is challenging due to multiple contamination sources in poultry operations, such as chicks, feed, rodents, and farm environments [
21]. Reducing
Salmonella in poultry and livestock could significantly reduce human exposure [
22], as seen in Denmark, where treated animal feed decreased Salmonella infections [
23]. Early detection and understanding of cross-contamination points are critical to controlling salmonellosis outbreaks. Limited data exist on the prevalence of
Salmonella in Jamaica’s poultry industry, prompting this study, which involved 6,693 specimens collected from poultry farms, processing plants, and supermarkets across six parishes. The study revealed a low prevalence of
Salmonella (1%), with environmental specimens, such as pig faeces, showing higher contamination rates [
24,
25]. Notably,
Salmonella Montevideo and
Salmonella Kentucky were isolated from abattoir specimens, marking their first identification in Jamaica [
26,
27].
Fly specimens also tested positive for
Salmonella, raising concerns about their potential as vectors in food contamination. Despite these findings, no
Salmonella was detected in poultry products from marketing outlets, offering some reassurance to consumers. Improved hygiene and surveillance in poultry farms, coupled with public education, are essential to controlling foodborne salmonellosis in Jamaica [
28]. This study provides valuable insights into
Salmonella prevalence and highlights the need for continued preventive measures in the poultry industry as a specific DNA into a cationic liposome to protect against salmonellosis in the Poultry industry, which show to be an effective as an attenuated vaccine [
29].
Salmonella is a common cause of foodborne illness, particularly linked to poultry. This study investigated the effectiveness of three antibiotics—Trisulvitrim, Menorox, and Neochlore—in preventing
Salmonella Typhimurium infection in growing chickens. Four groups of 60 chicks were studied, with three groups receiving one of the antibiotics and the fourth serving as a control. Results showed that chickens treated with antibiotics had a significantly lower prevalence of
Salmonella infection (1.6%) compared to the control group (23.3%), demonstrating the effectiveness of the medications.
Salmonella was predominantly found in the caeca and crops of infected birds. The study emphasises the need for effective antibiotic use in reducing
Salmonella in poultry, although it acknowledges that medication may not provide 100% protection. It also highlights the increased risk of infection in organic farming practices [
30]. Antibiotic resistance remains a concern, particularly due to the widespread use of antibiotics in both human and animal treatment [
31].
4. The Risk of Transfusion-Transmitted Infections
Blood transfusions are critical in medical treatments, saving lives by replenishing blood lost during surgery, injury, or illness. However, transfusion carries risks, including the transmission of bacterial, viral, and parasitic pathogens. Among these, the risk of transfusion-transmitted bacterial infections (TTBI) is notably higher than that of viral infections, particularly in platelet transfusions, which present the highest risk of bacterial contamination. This makes blood safety a major concern in healthcare systems worldwide [
32,
33,
34,
35,
36,
37].
Bacterial proteins such as staphylococcal protein A, peptostreptococcal protein L, and streptococcal protein G have been utilized in laboratory diagnostic studies to substitute diagnostic antibodies in tests such as ELISA, western blot, immunofluorescence, flow cytometry, and molecular biology assays. These proteins bind to immunoglobulins, enabling detection and identification of pathogens without the need for disease-specific antibodies. This application is especially useful in diagnosing infections, improving the accuracy and speed of results, and enhancing the overall effectiveness of diagnostic tools in transfusion safety [
38].
Viral organisms transmitted through blood transfusions include HIV, hepatitis C (HCV), hepatitis B (HBV), hepatitis A (HAV), West Nile virus (WNV), cytomegalovirus (CMV), severe acute respiratory syndrome coronavirus (SARS-CoV-1), human T-cell lymphotropic viruses (HTLV), Zika virus, and parvovirus B19. These viruses pose significant risks, particularly for immunocompromised patients, and may cause long-term health complications if transmitted through contaminated blood products [
39,
40,
41,
42,
43,
44,
45].
Screening and pathogen reduction technologies have significantly reduced the incidence of viral infections transmitted through transfusions. For example, nucleic acid amplification tests (NAT) are now standard in blood screening processes to detect viral RNA or DNA in blood samples. However, despite advances in viral detection, bacterial infections continue to be a significant concern. There is association between some cancers and ABO groups. Bacteria can thrive in stored blood products, particularly platelets, which are stored at room temperature. This creates a breeding ground for bacterial growth, making the timely detection and prevention of TTBI essential for ensuring the safety of blood transfusions [
38,
46,
47]
5. Egg Antibody Technology
The study focuses on investigating IgM antibodies in egg whites, a topic with limited prior research. Antibodies targeting bacterial antigens were developed, utilizing ELISA for detection and affinity chromatography for purification. By day 9 post-immunization, large amounts of anti-protein A antibodies were detected in egg whites, confirmed through affinity chromatography. Samples containing anti-SpA antibodies demonstrated agglutination inhibition, whereas negative samples exhibited agglutination. This indicates the successful production of IgM anti-protein A antibodies in egg whites, along with the inhibition of bacterial growth observed in vitro. Protein A-affinity chromatography played a crucial role in characterizing these antibodies, underscoring its value in antibody production and research [
48,
49,
50,
51].
The research also explores the complexities of experimental vaccine development, specifically focusing on immunogenicity outcomes. A key aspect highlighted is the innovative concept of idiotypic-antiidiotypic interactions, which can modulate antibody responses against bacterial and viral proteins. Three experimental vaccines targeting HIV, Salmonella, and Staphylococcus aureus were examined methodologically [
52]. The author proposes that immunized chicken eggs could provide a valuable platform for producing IgY antibodies against these pathogens. This research holds significant relevance for epidemiology, particularly in regions such as the West Indies, where infectious diseases are a major public health concern. By outlining vaccine development methods and potential applications, the study offers the groundwork for future clinical trials aimed at combating important infectious microorganisms affecting human populations. Additionally, other researchers have reported the production of IgY antibodies with similar results [
53,
54,
55,
56,
57].
6. Confronting Tuberculosis
This retrospective study examined 537 hospital records of patients with pulmonary tuberculosis at Jamaica's National Chest Hospital between 1995 and 2001. Of the 406 eligible patients, 11.6% were HIV-1 positive, primarily males aged 30-39. The mortality rate in HIV-positive patients (23.4%) was significantly higher than in HIV-negative patients (3.9%). All patients received standard quadruple drug therapy, with no cases of multiple drug resistance. Early HIV diagnosis and antiretroviral therapy are critical in reducing mortality [
58].
This study aimed to determine the seroprevalence of HIV-1 infection among patients with pulmonary tuberculosis (TB) in Jamaica and assess the epidemiological links, characteristics, and mortality associated with coinfection. HIV infection is the strongest risk factor for TB, with individuals over 100 times more likely to develop TB than non-infected persons. Pulmonary TB remains a leading cause of HIV-related morbidity and mortality globally. By 2003, an estimated 22,000 people were living with HIV/AIDS in Jamaica. The study’s findings are essential for developing strategies and policies to control the spread of HIV and TB in resource-limited settings [
58].
This study examined 132 culture-positive Mycobacterium tuberculosis cases in Trinidad and Tobago over a year. The findings revealed a high male-to-female ratio of 4:1 and a significant 30% HIV-TB co-infection rate. African descendants, who make up 37.5% of the population, accounted for 69.7% of TB cases (P < 0.001). Spoligotyping identified 25 patterns and 12 clusters, with the SIT566 clone being the most prevalent [
59].
A year-long investigation in Trinidad and Tobago characterized Mycobacterium tuberculosis strains using molecular and phylogenetic methods. The SIT566 clone, an "evolutionary modern" strain, represented over half of the cases, predominantly affecting younger populations, particularly in Port-of-Spain. Unique strain distributions, such as LAM-10CAM and EAI, set Trinidad and Tobago apart from other Caribbean countries. SIT566, also identified in Trinidadian patients in the United States, exhibited a distinct MIRU profile. Phylogenetic data suggest SIT566 evolved from X lineage strains, potentially linked to Anglo-Saxon ancestry. This study provides insights into the genetic diversity and origins of TB strains in the region [
60].
Another study compared the QuantiFERON®-TB Gold (QFT-G) assay with the tuberculin skin test (TST) for latent tuberculosis infection (LTBI) screening among high-risk individuals in Trinidad & Tobago. Of 560 participants (including TB contacts, HIV patients, healthcare workers, inmates, and TB patients), QFT-G detected LTBI in 51% compared to TST’s 39.4% (P = 0.001), except in the control group, where TST was preferred. Although QFT-G provided faster results, it was more expensive (
$18.60 vs.
$3.70 per subject). Importantly, QFT-G demonstrated lower sensitivity in immunocompromised individuals, raising concerns about its use in resource-limited settings with high HIV prevalence, such as Trinidad & Tobago [
61,
62].
In another investigation, spoligotyping and 15-loci MIRU-VNTR were used to characterize Mycobacterium tuberculosis strains in 74 Guyanese and 80 Surinamese patients. The average age in both countries was around 38 years, and male-to-female ratios were 2.25 in Guyana and 4.27 in Suriname. The techniques revealed 41 and 65 distinct patterns, with clustering rates of 83.8% and 68.8%, respectively. Combined analyses identified 18 clusters (2-41 isolates), with an overall clustering rate of 67.5% and a recent transmission rate of 55.8%. Clustering was significantly higher in Guyana compared to Suriname (79.7% vs. 56.3%; p=0.0019), and shared clusters between the two countries suggest potential transmission routes, highlighting the SIT53/15-MIT861 clone as a key target for intervention [
63].
Mincle, a major C-type lectin receptor in macrophages, plays a vital role in the immune response against mycobacteria. Computational tools like Discovery Studio and Molegro Virtual Studios predict ligand-receptor binding positions, while ChemDraw assists in ligand preparation due to the time-consuming nature of protein preparation. Mincle's involvement in mycobacterial-induced inflammatory signaling, particularly in tuberculosis, is of growing interest. However, Molegro’s inability to depict metallic atom interactions is a noted limitation. Mincle detects mycobacterial components like trehalose dimycolate (TDM) and trehalose dibenzenate (TDB), activating CARD9 signaling and prompting the release of pro-inflammatory cytokines and chemokines. This research shows potential for therapeutic applications, particularly in tuberculosis research in the West Indies [
64].
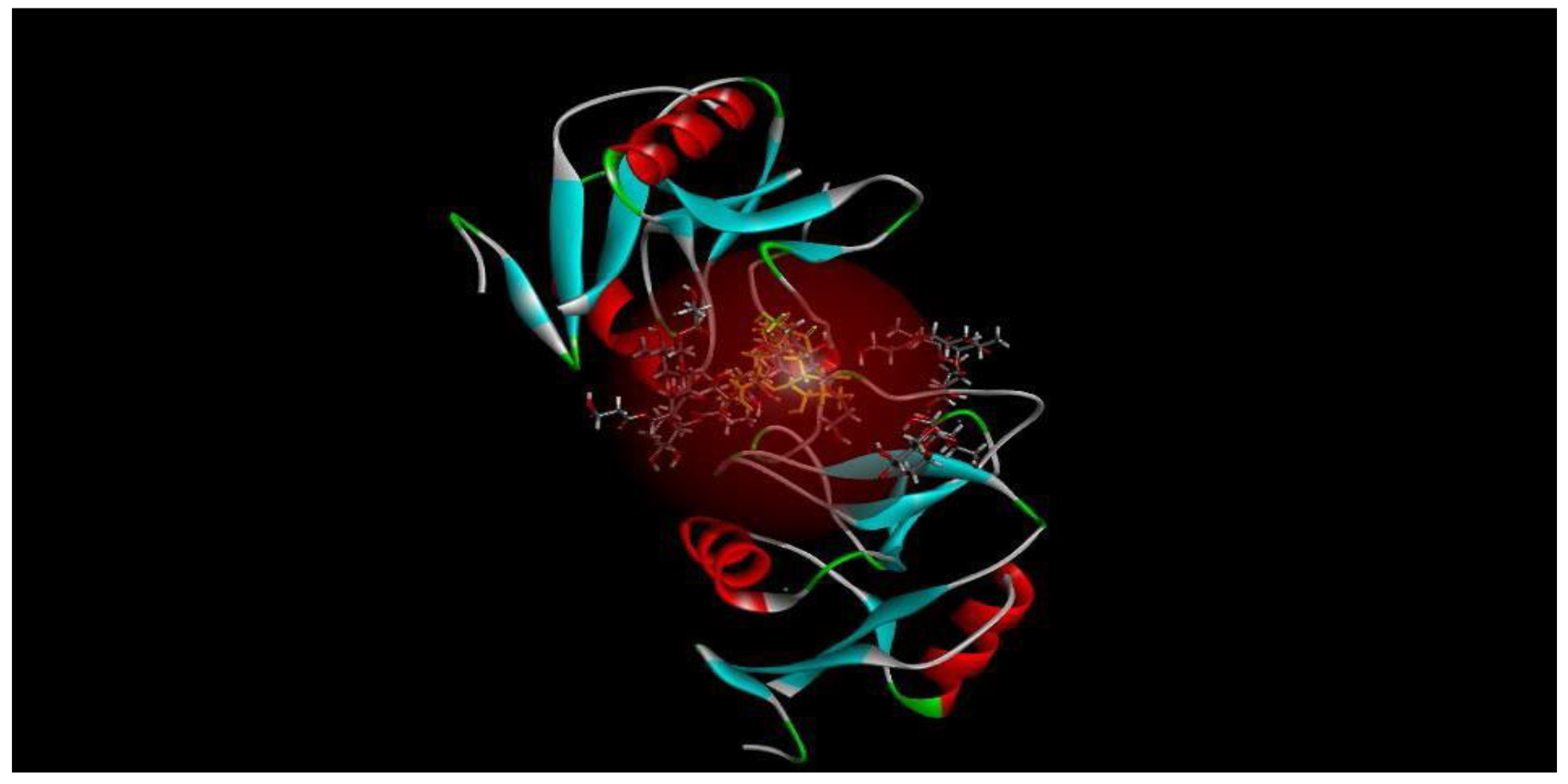
Figure 1 presents a diagram of the five most feasible poses for TDB, a derivative of TDM, binding to mincle [
64].
7. Infections in the Course of Immunological Disorders
7.1. Severe Combined Immunodeficiency Disorders (SCID)
Patients with severe combined immunodeficiency disorders (SCID) have profound defects in both their T and B cells, leaving them highly vulnerable to a broad spectrum of infections. These individuals are susceptible to bacterial, viral, fungal, and parasitic infections caused by both common and rare microorganisms. Among bacteria, patients often experience infections from
Staphylococcus aureus,
Pseudomonas aeruginosa, and
Streptococcus pneumoniae. However, they are also vulnerable to rare organisms such as
Nocardia and
Mycobacterium avium complex. Viral infections are frequent, with cytomegalovirus (CMV), Epstein-Barr virus (EBV), and adenovirus being common culprits. Unusual viral pathogens, such as the measles virus and certain strains of enterovirus, can cause severe complications in these patients. Fungal infections are a significant threat, particularly from
Candida species and
Aspergillus. Less common fungal pathogens, such as
Cryptococcus neoformans and
Pneumocystis jirovecii, may also lead to life-threatening infections. In terms of parasitic infections,
Toxoplasma gondii and
Cryptosporidium are frequently seen. Additionally, these patients are susceptible to rare parasites like
Strongyloides stercoralis. In SCID patients, even microorganisms that are relatively harmless to healthy individuals can cause severe, recurrent, or disseminated infections, making prompt diagnosis and treatment critical [
66].
7.2. Chronic Granulomatous Disease (CGD)
Chronic granulomatous disease (CGD) is a primary immunodeficiency disorder characterised by the inability of phagocytes, particularly neutrophils, to effectively kill certain bacteria and fungi. This defect results from mutations affecting the production of reactive oxygen species, essential for pathogen destruction. As a consequence, individuals with CGD are highly susceptible to recurrent and severe infections caused by specific pathogens. Common bacterial infections in CGD include those caused by
Staphylococcus aureus, Serratia marcescens, and
Nocardia. Fungal infections, particularly from
Aspergillus and
Candida species, are also frequent and often life-threatening. In addition,
Burkholderia cepacia and
Salmonella are known pathogens in these patients. Chronic infections and the formation of granulomas in organs such as the lungs, liver, and lymph nodes are typical features of CGD, requiring lifelong antimicrobial prophylaxis and, in some cases, stem cell transplantation for treatment [
67].
7.3. Transient Hypogammaglobulinemia of Infancy (THI)
In transient hypogammaglobulinemia of infancy (THI), infants are prone to recurrent infections due to their low levels of immunoglobulin G (IgG), which plays a crucial role in the immune response. The most common infections in THI are upper and lower respiratory tract infections, including sinusitis, otitis media (ear infections), and bronchitis. These infections are often caused by bacteria such as
Haemophilus influenzae, Streptococcus pneumoniae, and
Moraxella catarrhalis. Children with THI may also experience gastrointestinal infections, although less frequently. Viral infections like respiratory syncytial virus (RSV) and adenovirus can be more severe in infants with THI, further complicating their clinical course. However, severe or life-threatening infections are uncommon in THI compared to other primary immunodeficiencies. Management of infections in THI usually involves the use of antibiotics to treat active infections or, in some cases, prophylactic antibiotics to prevent recurrent infections. Immunoglobulin replacement therapy is rarely needed, as most infants outgrow the condition by age 2 to 4 [
68].
7.4. Neuropsychiatric Systemic Lupus Erythematosus (NPSLE)
Neuropsychiatric systemic lupus erythematosus (NPSLE) refers to the involvement of the central and peripheral nervous systems in patients with systemic lupus erythematosus (SLE), leading to a range of neurological and psychiatric manifestations. These may include cognitive dysfunction, mood disorders, seizures, psychosis, and even stroke. Patients with NPSLE are particularly vulnerable to infections due to a combination of immunosuppressive therapy, disease-related immune dysfunction, and the involvement of the central nervous system. Infections in these patients can complicate the clinical picture, mimicking or exacerbating neuropsychiatric symptoms, which makes diagnosis challenging. Common pathogens include
Staphylococcus aureus, Escherichia coli, and
Pneumocystis jirovecii, as well as viral infections like herpes simplex virus (HSV) and cytomegalovirus (CMV). The dual burden of neuropsychiatric manifestations and infection increases morbidity in NPSLE patients, highlighting the need for careful monitoring and management to differentiate between lupus flares and infection-related complications [
69,
70]. Systemic Lupus Erythematosus (SLE) is an autoimmune disease characterized by the formation of immune complexes that trigger inflammation. It affects multiple organs, leading to conditions such as nephritis, arthritis, and vasculitis. Inflammatory cytokines and complement activation play key roles in tissue damage and disease progression [
71,
72,
73].
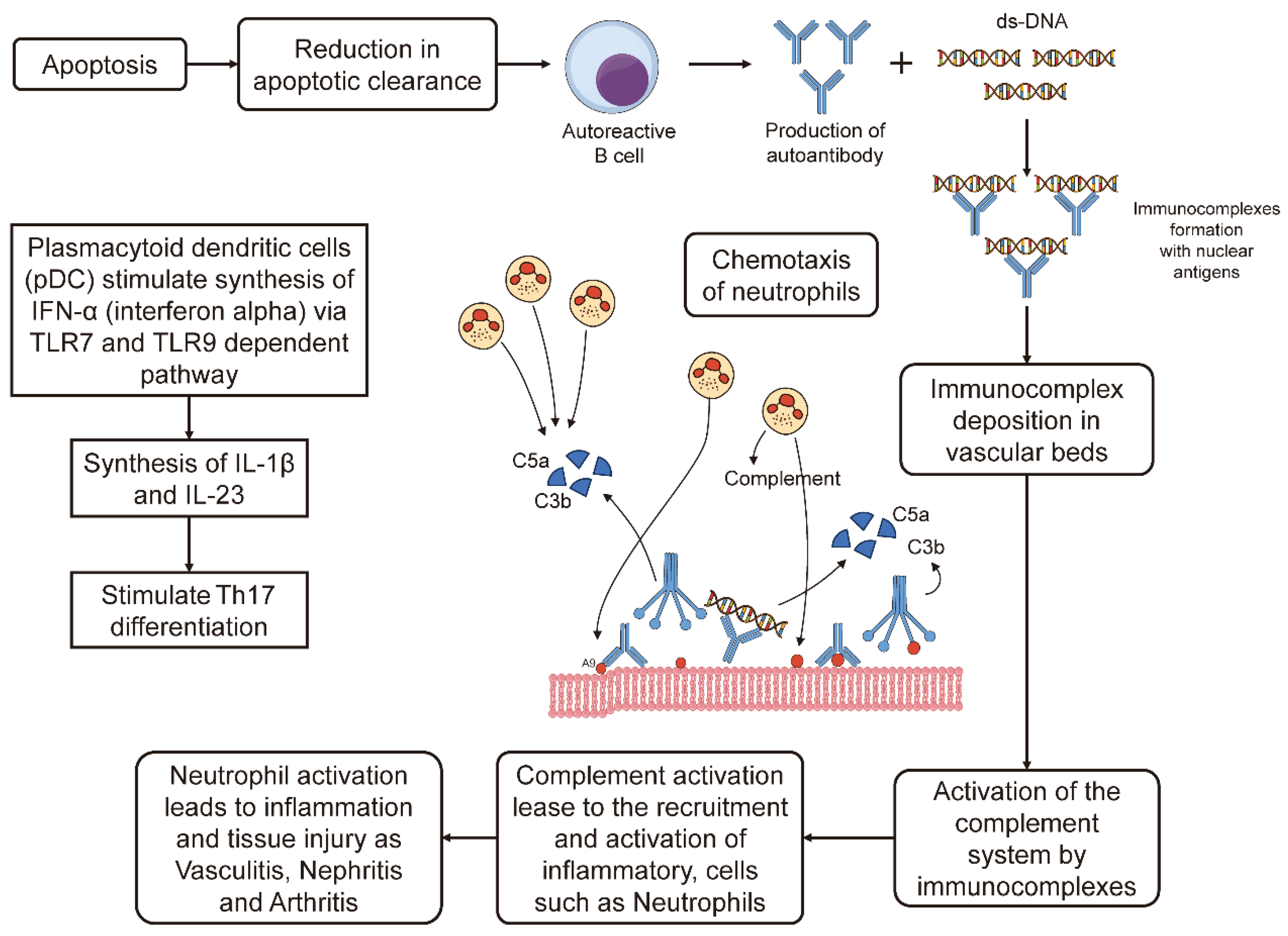
Figure 2 shows the mechanism of SLE pathogenesis. Taken from [
69].
7.5. Interleukin-2 Receptor Alpha (IL-2Rα) Deficiency
CD25 immunodeficiency, also known as interleukin-2 receptor alpha (IL-2Rα) deficiency, is a rare form of severe combined immunodeficiency (SCID). This condition results from mutations in the
IL2RA gene, which impairs the function of regulatory T cells (Tregs) that play a critical role in controlling immune responses. Without functional CD25, Tregs cannot effectively modulate immune activity, leading to dysregulated immune responses, autoimmunity, and an increased susceptibility to infections. Individuals with CD25 immunodeficiency are prone to a wide range of infections caused by bacterial, viral, fungal, and parasitic pathogens. Common bacterial infections include those caused by
Staphylococcus aureus and
Streptococcus pneumoniae. Viral infections, such as those from cytomegalovirus (CMV), Epstein-Barr virus (EBV), and enteroviruses, are frequent and can lead to severe, life-threatening complications. Fungal infections, particularly by
Candida and
Aspergillus species, are also prevalent. Due to the severe immune dysregulation, infections in CD25 immunodeficiency are often persistent and recurrent, requiring aggressive antimicrobial treatment. Hematopoietic stem cell transplantation is the only curative option for restoring immune function in these patients, as it replaces the defective immune system [
74].
7.6. Multiple Sclerosis (MS)
Multiple sclerosis (MS) is a chronic autoimmune disease in which the immune system attacks the myelin sheath surrounding nerve fibres in the central nervous system. This leads to a range of neurological symptoms, including motor and sensory impairments, vision problems, and cognitive dysfunction. While MS itself does not directly cause infections, the disease and its treatments can increase a person's susceptibility to infections. Patients with MS are often treated with immunomodulatory or immunosuppressive therapies, such as corticosteroids, disease-modifying drugs (DMDs), or biologics like natalizumab and ocrelizumab. These treatments can weaken the immune system, increasing the risk of infections. Respiratory tract infections, such as those caused by
Streptococcus pneumoniae and
Haemophilus influenzae, are common in MS patients. Urinary tract infections (UTIs) are also prevalent, particularly in those with bladder dysfunction, a common MS complication. Viral infections, such as those caused by herpes simplex virus (HSV) and varicella-zoster virus (VZV), can be more frequent or severe in MS patients, particularly when on immunosuppressive therapies. Furthermore, certain infections, like Epstein-Barr virus (EBV), have been associated with an increased risk of developing MS, although this connection is still being studied. Careful infection monitoring and management are crucial in MS patients, especially during immunosuppressive treatment [
75].
7.7. Ataxia-Telangiectasia (A-T)
Ataxia-telangiectasia (A-T) is a rare, inherited disorder that affects the nervous and immune systems, leading to progressive neurological problems, weakened immune function, and increased susceptibility to infections. A-T is caused by mutations in the
ATM gene, which is responsible for DNA repair, immune regulation, and cellular responses to stress. Individuals with A-T typically present with ataxia (loss of coordination), telangiectasia (small dilated blood vessels), and immunodeficiency. The immunodeficiency in A-T mainly affects the production and function of immunoglobulins (particularly IgA and IgG), leading to recurrent respiratory infections. These infections are primarily caused by bacteria such as
Haemophilus influenzae,
Streptococcus pneumoniae, and
Staphylococcus aureus, which result in frequent sinusitis, bronchitis, and pneumonia. Viral infections, particularly from respiratory syncytial virus (RSV) and adenovirus, can also occur more frequently and with greater severity. Due to the weakened immune system, infections in A-T patients tend to be persistent and can lead to long-term complications, such as chronic lung disease. Management of infections in A-T typically includes regular monitoring, antibiotic treatment, and sometimes immunoglobulin replacement therapy to prevent severe or recurrent infections [
76,
77].
7.8. Interleukin-12 Receptor Deficiency
Interleukin-12 receptor deficiency is a primary immunodeficiency that impairs the immune system’s ability to effectively combat intracellular infections. This deficiency disrupts the signalling pathway of interleukin-12, a cytokine critical for activating natural killer (NK) cells and promoting the differentiation of T cells into Th1 cells. Without proper IL-12 receptor function, individuals are more susceptible to infections caused by intracellular pathogens, including mycobacteria, Salmonella, and certain viruses. Patients often experience recurrent or severe infections, as their immune system cannot mount an adequate cell-mediated immune response. Treatment typically involves antimicrobial therapy and, in some cases, cytokine replacement [
78].
7.9. Allergic Bronchial Asthma
Allergic bronchial asthma is a chronic respiratory condition characterised by airway inflammation and narrowing, often triggered by allergens and microorganisms. Allergic asthma, the most common form, occurs when the immune system overreacts to allergens such as pollen, dust mites, and mould, leading to airway inflammation. Microorganisms, particularly respiratory viruses like rhinovirus and influenza, can worsen asthma by increasing inflammation and disrupting the balance of the airway microbiome. Some bacteria, such as
Haemophilus influenzae, have also been linked to asthma exacerbations. Additionally, the hygiene hypothesis suggests that reduced exposure to microorganisms early in life may contribute to the development of asthma by impairing immune system regulation [
79,
80].
8. Conclusions
This comprehensive study delves into the critical challenges of infectious diseases in the Caribbean and South America, particularly tuberculosis and nosocomial infections, and their intersections with immunodeficiency and antibiotic resistance. The high HIV-TB co-infection rates in Jamaica and Trinidad and Tobago underscore the need for early detection and integrated treatment strategies. Furthermore, the spread of antibiotic-resistant pathogens, such as Gram-negative bacteria and MRSA, poses a significant threat in healthcare settings, necessitating stringent infection control measures and prudent antibiotic use. The findings related to Salmonella infections in poultry in Jamaica highlight the importance of biosecurity measures and public education to reduce foodborne disease risks.
The paper also brings attention to the potential of IgM antibodies in egg whites for immunological research, offering innovative approaches to vaccine development and bacterial antigen detection. Additionally, the study emphasises the vulnerability of immunodeficient patients, such as those with SCID, CGD, and other immunological disorders, to various infections. In conclusion, a multidisciplinary approach involving molecular characterisation, immunological research, and effective public health strategies is essential for controlling infectious diseases in the region, particularly in resource-limited settings.
Author Contributions
The manuscript was conceptualized by A.J.-V. and O.A.-M, and planning and discussion were conducted by all authors. All authors investigated, reviewed and edited the final manuscript, and agreed to the final version of the manuscript.
Funding
This study did not receive any external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The dataset supporting the findings of this study is included within the manuscript and its referenced sources, ensuring comprehensive access to the relevant data for further examination and analysis.
Acknowledgments
The authors sincerely thank the West Indian Immunology Society (WIIS) for their invaluable assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elliott, C.; Vaillant, A. Antimicrobials and Enterobacterial Repetitive Intergenic Consensus (ERIC) Polymerase Chain Reaction (PCR) Patterns of Nosocomial Serratia Marcescens Isolates: A One-Year Prospective Study (June 2013–May 2014) in a Rural Hospital in the Republic of Trinidad and Tobago. Prog. Chem. Biochem. Res. 2020, 3, 105–120. [CrossRef]
- Vire, F.P.; Akpaka, P.E.; Unakal, C. Molecular Characterization of Methicillin-Resistant Staphylococcus Aureus Isolates from Rural Community Settings in Trinidad and Tobago. Niger. J. Clin. Pract. 2018, 21, 1596–1601. [CrossRef]
- Vaillant, A.J.; Cosme, F.; Smikle, F.; Pérez, O. Feeding Eggs from Hens Immunized with Specific KLH-Conjugated HIV Peptide Candidate Vaccines to Chicks Induces Specific Anti-HIV gp120 and gp41 Antibodies that Neutralize the Original HIV Antigens. Vaccine Research (VACRE). 2020, 7, 92–96.
- Zwyer, M.; Rutaihwa, L.K.; Windels, E.; Hella, J.; Menardo, F.; Sasamalo, M.; Sommer, G.; Schmülling, L.; Borrell, S.; Reinhard, M.; Dötsch, A.; Hiza, H.; Stritt, C.; Sikalengo, G.; Fenner, L.; De Jong, B.C.; Kato-Maeda, M.; Jugheli, L.; Ernst, J.D.; Niemann, S.; Brites, D. Back-to-Africa Introductions of Mycobacterium tuberculosis as the Main Cause of Tuberculosis in Dar Es Salaam, Tanzania. PLoS Pathog. 2023, 19, e1010893. [CrossRef]
- Kutlesa, M.; Santini, M.; Krajinovic, V.; et al. Nosocomial Blood Stream Infections in Patients Treated with Venovenous Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. Minerva Anestesiol. 2017, 83, 493–501. [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; et al. Practice Guidelines for the Diagnosis and Management of Skin and Soft Tissue Infections: 2014 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–52. [CrossRef]
- Nadimpalli, M.; Stewart, J.R.; Pierce, E.; et al. Livestock-Associated, Antibiotic-Resistant Staphylococcus Aureus Nasal Carriage and Recent Skin and Soft Tissue Infection among Industrial Hog Operation Workers. PLoS ONE. 2016, 11, e0165713. [CrossRef]
- Elliott, C.; Justiz-Vaillant, A. Nosocomial Infections: A 360-Degree Review. Int. Biol. Biomed. J. 2018, 4, 72–81.
- Leylabadlo, H.E.; Pourlak, T.; Bialvaei, A.Z.; Aghzadeh, M.; Asgharzadeh, M.; Samadi Kafil, H. Extended-Spectrum Beta-Lactamase Producing Gram Negative Bacteria in Iran: A Review. Afr. J. Infect. Dis. 2017, 11, 39–53. [CrossRef]
- Amaral, M.M.; Coelho, L.R.; Flores, R.P.; Souza, R.R.; Silva-Carvalho, M.C.; Teixeira, L.A.; Ferreira-Carvalho, B.T.; Figueiredo, A.M. The Predominant Variant of the Brazilian Epidemic Clonal Complex of Methicillin-Resistant Staphylococcus Aureus Has an Enhanced Ability to Produce Biofilm and to Adhere to and Invade Airway Epithelial Cells. J. Infect. Dis. 2005, 192, 801–810. [CrossRef]
- Paterson, D.L.; Bonomo, R.A. Extended-Spectrum β-Lactamases: A Clinical Update. Clin. Microbiol. Rev. 2005, 18, 657–686. [CrossRef]
- Tillekeratne, L.G.; Vidanagama, D.; Tippalagama, R.; et al. Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae as a Common Cause of Urinary Tract Infections in Sri Lanka. Infect. Chemother. 2016, 48, 160–165. [CrossRef]
- Briongos-Figuero, L.S.; Gómez-Traveso, T.; Bachiller-Luque, P.; et al. Epidemiology, Risk Factors and Comorbidity for Urinary Tract Infections Caused by Extended-Spectrum Beta-Lactamase (ESBL)-Producing Enterobacteria. Int. J. Clin. Pract. 2012, 66, 891–896. [CrossRef]
- Pilmis, B.; Delory, T.; Groh, M.; et al. Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae (ESBL-PE) Infections: Are Carbapenem Alternatives Achievable in Daily Practice? Int. J. Infect. Dis. 2015, 39, 62–67. [CrossRef]
- Denis, B.; Lafaurie, M.; Donay, J.-L.; et al. Prevalence, Risk Factors, and Impact on Clinical Outcome of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli Bacteraemia: A Five-Year Study. Int. J. Infect. Dis. 2015, 39, 1–6. [CrossRef]
- Akpaka, P.E.; Vaillant, A.; Wilson, C.; Jayaratne, P. Extended Spectrum Beta-Lactamase (ESBL) Produced by Gram-Negative Bacteria in Trinidad and Tobago. Int. J. Microbiol. 2021, 5582755. [CrossRef]
- Galanis, E.; Lo Fo Wong, D.M.A.; Patrick, M.; Binsztein, N.; Cieslik, A.; Chalemchaikit, T.; Aidara-Kane, A.; et al. Web-Based Surveillance and Global Salmonella Distribution. Emerg. Infect. Dis. 2006, 12, 381–388. [CrossRef]
- Bailey, S.J.; Stern, P.; Ferorka-Cray, Craven, S.E.; Cox, N.A.; Cosby, D.E.; Ladely, S.; Musgrove, M.T. Source and Movement of Salmonella through Integrated Poultry Operations: A Multistate Epidemiological Investigation. J. Food Prot. 2001, 64, 1690–1697. [CrossRef]
- Fang, G.; Arauja, V.; Guerrant, R.L. Enteric Infections Associated with Exposure to Animals or Animal Products. Infect. Dis. Clin. N. Am. 1991, 5, 681–701. [CrossRef]
- Giannella, R.A. Importance of the Intestinal Inflammatory Reaction in Salmonella 277 Mediated Intestinal Secretion. Infect. Immun. 1979, 23, 140–145. [CrossRef]
- Korsak, N.; Degeye, J.N.; Etienne, G.; China, B.; Daube, G. Comparison of Four Different Methods for Salmonella Detection in Fecal Samples of Porcine Origin. J. Food Prot. 2004, 67, 2158–2164. [CrossRef]
- Seepersadsingh, N.; Adesiyun, A.A. Prevalence and Antimicrobial Resistance of Salmonella spp. in Pet Mammals, Reptiles, Fish Aquarium Water, and Birds in Trinidad. J. Vet. Med. 2003, 50, 488–493. [CrossRef]
- Browning, L.M.; Brown, D.J.; Coia, J.E.; Mather, H.; Relly, W.J.; Cowden, J.M. Salmonella in Scotland. SCIEH Wkly. Rep. Health Prot. Scotl. 2003, 306–312.
- Weill, F.X.; Bertrand, S.; Guesnier, F.; Baucheron, S.; Cloeckaert, A.; Grimont, P.A. Ciprofloxacin-Resistant Salmonella Kentucky in Travellers. Emerg. Infect. Dis. 2006, 12, 1611–1612. [CrossRef]
- Skov, M.N.; Angen, O.; Chriel, M.; Olsen, J.E.; Bisgaard, M. Risk Factors Associated with Salmonella enterica Serovar Typhimurium Infection in Danish Broiler Flocks. Poult. Sci. 1999, 78, 848–854. [CrossRef]
- Curtello, S.; Vaillant, A.A.J.; Asemota, H.; Akpaka, P.E.; Smikle, M.P. Prevalence of Salmonella Organisms in Poultry and Poultry Environments in Jamaica. J. Food Prot. 2013, 76, 461–469. [CrossRef]
- Curtello, S.; Vaillant, A.A.J.; Asemota, H.; Akpaka, P.E.; Smikle, M.P. The Effectiveness of Antibiotics in the Prevention of Salmonella Typhimurium in Growing Chickens. J. Food Prot. 2013, 76, 849–856. [CrossRef]
- Zwe, Y.H.; Yuk, H.G. Prior Exposure of Agriculture Cephalosporin Ceftiofur Impaired Conjugation of blaCTX-M-65 Gene-Bearing Plasmid in Salmonella Saintpaul. J. Appl. Microbiol. 2020, 129, 1552–1565. [CrossRef]
- Curtello, S.; Justiz Vaillant, A.A.; Asemota, H.; Smikle, M.P.; Akpaka, P.E. A DNA Vaccine versus Attenuated Vaccine to Protect against Salmonella Infection in Chickens. Br. J. Med. Med. Res. 2013, 4, 828–842. [CrossRef]
- Justiz-Vaillant, A.A.; Akpaka, P.E.; McFarlane-Anderson, N.; Smikle, M.P. Anti-Salmonella Antibodies: An Immunoepidemiological Study. J. Infect. Public Health 2019, 12, 194–196.
- Zuo, W.; Yang, D.; Wu, X.; Zhang, B.; Wang, X.; Hu, J.; Qi, J.; Tian, M.; Bao, Y.; Wang, S. The aroA and luxS Double-Gene Mutant Strain Has Potential to Be a Live Attenuated Vaccine against Salmonella Typhimurium. Vaccines. 2024, 12, 162. [CrossRef]
- Zwittnig, K.; Mukaddam, K.; Vegh, D.; Herber, V.; Jakse, N.; Schlenke, P.; Zrnc, T.A.; Payer, M. Platelet-Rich Fibrin in Oral Surgery and Implantology: A Narrative Review. Transfus. Med. Hemother. 2022, 50, 348–359. [CrossRef]
- Zwiers, C.; Scheffer-Rath, M.E.; Lopriore, E.; de Haas, M.; Liley, H.G. Immunoglobulin for Alloimmune Hemolytic Disease in Neonates. Cochrane Database Syst. Rev. 2018, 3, CD003313. [CrossRef]
- Zweitzig, D.R.; Riccardello, N.M.; Pester, J.M.; Jeanmonod, R.; Kopnitsky, M.J.; O'Hara, S.M. A Novel Approach for Rapid Detection of Bacterially Contaminated Platelet Concentrates via Sensitive Measurement of Microbial DNA Polymerase Activity. Transfusion 2014, 54, 1642–1651. [CrossRef]
- Justiz-Vaillant, A.A.; Akpaka, P.E.; McFarlane-Anderson, N.; Smikle, M.P. Comparison of Techniques of Detecting Immunoglobulin-Binding Protein Reactivity to Immunoglobulin Produced by Different Avian and Mammalian Species. West Indian Med. J. 2013, 62, 12–20.
- Justiz-Vaillant, A.A. A Protocol and Detailed Methodological Study on Immunogenicity of Various Experimental Vaccines. Int. Biol. Biomed. J. 2021, 7, 1–10. Available online: http://ibbj.org/article-1-252-fa.html (accessed on Sept 11, 2024]).
- Zhu, N.; Xu, R.; Tang, W.; Wang, H.; Wan, Z.; Wu, X.; Fu, Y.; Tang, S.; Yu, S. Correlation between Serum Hepatitis B Virus DNA Levels and Liver Pathology in Chronic Hepatitis B Patients: Analysis of Liver Biopsy Findings. Nan Fang Yi Ke Da Xue Xue Bao. 2018, 38, 842–849. [CrossRef]
- Zhou, P.; Ouchari, M.; Xue, Y.; Yin, Q. In Vitro Generation of Red Blood Cells from Stem Cells and Targeted Therapy. Cell Transplant. 2020, 29, 963689720946658. [CrossRef]
- Justiz-Vaillant, A.A. Multiple Myeloma Update. Int. Biol. Biomed. J. 2018, 4, 136–141. Available online: http://ibbj.org/article-1-177-en.html (accessed on Sept 12, 2024]).
- Zheng, X.; Ye, X.; Du, P.; Zeng, J.; Zhu, W.; Yang, B.; Li, C.; Allain, J.P. High Prevalence of Anti-Hepatitis B Core Antigen in Hepatitis B Virus-Vaccinated Chinese Blood Donors Suggests Insufficient Protection but Little Threat to the Blood Supply. Transfusion. 2015, 55, 890–897. [CrossRef]
- Justiz-Vaillant, A.A. Insights in the Management of Human T Cell-Lymphotropic Virus-1 Associated Adult-T Cell Leukaemia/Lymphoma (ATL). Int. Biol. Biomed. J. 2020, 6, 1–10. Available online: http://ibbj.org/article-1-246-fa.html (accessed on August 18, 2024).
- Weisberg, S.P.; Staley, E.M.; Williams, L.A.; Pham, H.P.; Bachegowda, L.S.; Cheng, Y.H.; Schwartz, J.; Shaz, B.H. Survey on Transfusion-Transmitted Cytomegalovirus and Cytomegalovirus Disease Mitigation. Arch. Pathol. Lab. Med. 2017, 141, 1705–1711. [CrossRef]
- Datta, S.; Khillan, K.; Ranjan, V.; Wattal, C. Nucleic Acid Amplification Test: Bridging the Gap in Blood Safety and Re-Evaluation of Blood Screening for Cryptic Transfusion-Transmitted Infection among Indian Donors. Indian J. Med. Res. 2019, 149, 389–395. [CrossRef]
- Hans, R.; Marwaha, N.; Sharma, S.; Sachdev, S.; Sharma, R.R. Initial Trends of Individual Donation Nucleic Acid Testing in Voluntary and Replacement Donors from a Tertiary Care Centre in North India. Indian J. Med. Res. 2019, 149, 633–640. [CrossRef]
- Zhang, J.; Yan, X.; Li, Y.; Gao, R.; Wang, P.; Mo, W. Reactive Plasmacytosis Mimicking Multiple Myeloma Associated with SFTS Virus Infection: A Report of Two Cases and Literature Review. BMC Infect. Dis. 2018, 18, 528. [CrossRef]
- Vaillant, A.J.; Bazuaye, P.; McFarlane-Anderson, N.; Smikle, M.P.; Fletcher, H.; Akpaka, P.E. Association Between ABO Blood Type and Cervical Dysplasia/Carcinoma in Jamaican Women. Br. J. Med. Med. Res. 2013, 3, 2017–2021. [CrossRef]
- Charles, K.S.; Chisholm, K.; Gabourel, K.; Philip, K.; Ramdath, S.; Abdul-Hakeem, H.; Vaillant, A.; Pooransingh, S.; Legall, G.; Chantry, A. A Follow-up Survey of Knowledge, Attitudes and Practices Surrounding Blood Donation in Trinidad and Tobago. ISBT Sci. Ser. 2017, 12, 349–356. [CrossRef]
- Vaillant, A.J.; Ferrer-Cosme, B.; Vuma, S. Production of Antibodies in Egg Whites of Chickens. CJAST. 2021, 40, 17–22. [CrossRef]
- Kovacs-Nolan, J.; Mine, Y. Avian Egg Antibodies: Basic and Potential Applications. Avian Poult. Biol. Rev. 2004, 15(1), 25–46. [CrossRef]
- Goudswaard, J.; Noordzij, A.; van Dam, R.H.; åvander Donk, J.A.; Vaerman, J.P. The Immunoglobulins of the Turkey (Meleagris gallopavo): Isolation and Characterization of IgG, IgM and IgA in Body Fluids, Eggs and Intraocular Tissues. Poult. Sci. 1977, 56(6), 18471851. [CrossRef]
- Tizard, I. The Avian Antibody Response. Semin. Avian Exot. Pet Med. 2002, 11(1), 2–14. [CrossRef]
- Vega, C.; Bok, M.; Chacana, P.; Saif, L.; Fernandez, F.; Parreño, V. Egg Yolk IgY: Protection against Rotavirus-Induced Diarrhea and Modulatory Effect on the Systemic and Mucosal Antibody Responses in Newborn Calves. Vet. Immunol. Immunopathol. 2011, 142, 156–169. [CrossRef]
- Mohammadi, M.; Zangooei, M.; Abbasi, E.; Ebrahimi Fana, S.; Aminian, M. Production of Anti-Tetanus Toxin IgY and Study of Its Protective Effects in a Mouse Model. J. Immunoassay Immunochem. 2023, 44, 283–295. [CrossRef]
- Bentes, G.A.; Lanzarini, N.M.; Guimarães, J.R.; Heinemann, M.B.; Volotão, E.M.; da Silva, A.D.S.; Heneine, L.G.D.; de Oliveira, J.M.; Pinto, M.A. Production and Evaluation of Chicken Egg Yolk Immunoglobulin (IgY) against Human and Simian Rotaviruses. Viruses. 2022, 14, 1995. [CrossRef]
- Vega, C.G.; Bok, M.; Vlasova, A.N.; Chattha, K.S.; Fernández, F.M.; Wigdorovitz, A.; Parreño, V.G.; Saif, L.J. IgY Antibodies Protect against Human Rotavirus-Induced Diarrhea in the Neonatal Gnotobiotic Piglet Disease Model. PLoS ONE. 2012, 7, e42788. [CrossRef]
- Diraviyam, T.; Zhao, B.; Wang, Y.; Schade, R.; Michael, A.; Zhang, X. Effect of Chicken Egg Yolk Antibodies (IgY) against Diarrhea in Domesticated Animals: A Systematic Review and Meta-Analysis. PLoS ONE. 2014, 9, e97716. [CrossRef]
- Gadde, U.; Rathinam, T.; Lillehoj, H.S. Passive Immunization with Hyperimmune Egg-Yolk IgY as Prophylaxis and Therapy for Poultry Diseases—A Review. Anim. Health Res. Rev. 2015, 16(2), 163–176. [CrossRef]
- Akpaka, P.E.; Tulloch-Reid, M.; Justiz-Vaillant, A.; Smikle, M.F. Prevalence of Human Immunodeficiency Virus Infection in Patients with Pulmonary Tuberculosis at the National Chest Hospital in Jamaica. Rev. Panam. Salud Pública. 2006, 19, 38–43. [CrossRef]
- Baboolal, S.; Millet, J.; Akpaka, P.E.; Ramoutar, D.; Rastogi, N. First Insight into Mycobacterium Tuberculosis Epidemiology and Genetic Diversity in Trinidad and Tobago. J. Clin. Microbiol. 2009, 47, 1911–1914. [CrossRef]
- Millet, J.; Baboolal, S.; Akpaka, P.E.; Ramoutar, D.; Rastogi, N. Phylogeographical and Molecular Characterization of an Emerging Mycobacterium Tuberculosis Clone in Trinidad and Tobago. Infect. Genet. Evol. 2009, 9, 1336–1344. [CrossRef]
- Baboolal, S.; Ramoutar, D.; Akpaka, P.E. Comparison of the QuantiFERON®-TB Gold Assay and Tuberculin Skin Test to Detect Latent Tuberculosis Infection among Target Groups in Trinidad and Tobago. Rev. Panam. Salud Pública. 2010, 28, 36–42.
- Montane Jaime, L.K.; Akpaka, P.E.; Vuma, S.; Justiz-Vaillant, A.A. A Healthy Patient with Positive Mantoux Test but Negative Quantiferon Gold Assay and No Evidence of Risk Factors—To Treat or Not to Treat? IDCases. 2019, 18, e00658.
- Streit, E.; Baboolal, S.; Akpaka, P.E.; Millet, J.; Rastogi, N. Finer Characterization of Mycobacterium Tuberculosis Using Spoligotyping and 15-Loci MIRU-VNTRs Reveals Phylogeographical Specificities of Isolates Circulating in Guyana and Suriname. Infect. Genet. Evol. 2015, 30, 114–119. [CrossRef]
- Soodeen, S.; Justiz-Vaillant, A.; Jalsa, N. Is Possible Molecular Docking of Carbohydrates to a Mycobacterium Tuberculosis Molecule? Preprints 2023. Available online: https://www.preprints.org (accessed on [date of access]).
- Justiz-Vaillant, A.A.; Qurie, A. Interleukin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Justiz-Vaillant, A.A.; Gopaul, D.; Akpaka, P.E.; Soodeen, S.; Arozarena-Fundora, R. Severe Combined Immunodeficiency—Classification, Microbiology Association and Treatment. Microorganisms. 2023, 11, 1589. [CrossRef]
- Justiz-Vaillant, A.A.; Williams-Persad, A.F.-A.; Arozarena-Fundora, R.; Gopaul, D.; Soodeen, S.; Asin-Milan, O.; Thompson, R.; Unakal, C.; Akpaka, P.E. Chronic Granulomatous Disease (CGD): Commonly Associated Pathogens, Diagnosis and Treatment. Microorganisms. 2023, 11, 2233. [CrossRef]
- Justiz-Vaillant, A.A.; Hoyte, T.; Davis, N.; Deonarinesingh, C.; De Silva, A.; Dhanpaul, D.; Dookhoo, C.; Doorpat, J.; Dopson, A.; Durgapersad, J.; et al. A Systematic Review of the Clinical Diagnosis of Transient Hypogammaglobulinemia of Infancy. Children. 2023, 10, 1358. [CrossRef]
- Justiz-Vaillant, A.A.; Gopaul, D.; Soodeen, S.; Arozarena-Fundora, R.; Barbosa, O.A.; Unakal, C.; Thompson, R.; Pandit, B.; Umakanthan, S.; Akpaka, P.E. Neuropsychiatric Systemic Lupus Erythematosus: Molecules Involved in Its Immunopathogenesis, Clinical Features, and Treatment. Molecules. 2024, 29, 747. [CrossRef]
- Justiz-Vaillant, A.; Gopaul, D.; Soodeen, S.; Akpaka, P.; Pooransingh, S.; Thompson, R.; Gordon, L. Advancements in Immunology and Microbiology Research: A Comprehensive Exploration of Key Areas. Microorganisms. 2024, 12, 1672. [CrossRef]
- Justiz-Vaillant, A.; Akpaka, P.E.; Poonking, P. Systemic Lupus Erythematosus: Some Epidemiological and Clinical Aspects. Am. J. Public Health Res. 2015, 3, 46–50. [CrossRef]
- Justiz-Vaillant, A.; Ferrer Cosme, B. Systemic Lupus Erythematosus and Its Treatment with Intravenous Immunoglobulins (IVIG). Preprints 2021. [CrossRef]
- Justiz-Vaillant, A.; Akpaka, P. Cytokines (IL-17, IL-23, and IL-33) in Systemic Lupus Erythematosus in Trinidad and Tobago. Int. Biol. Biomed. J. 2021, 7, 262. Available online: http://ibbj.org/article-1-262-fa.html (accessed on 19 August 2024).
- Caudy, A.A.; Reddy, S.T.; Chatila, T.; Atkinson, J.P.; Verbsky, J.W. CD25 Deficiency Causes an Immune Dysregulation, Polyendocrinopathy, Enteropathy, X-Linked–Like Syndrome, and Defective IL-10 Expression from CD4 Lymphocytes. J. Allergy Clin. Immunol. 2007, 119, 482–487. [CrossRef]
- Marrodan, M.; Alessandro, L.; Farez, M.F.; Correale, J. The Role of Infections in Multiple Sclerosis. Mult. Scler. J. 2019, 25, 891–901. [CrossRef]
- Rothblum-Oviatt, C.; Wright, J.; Lefton-Greif, M.A.; McGrath-Morrow, S.A.; Crawford, T.O.; Lederman, H.M. Ataxia Telangiectasia: A Review. Orphanet J. Rare Dis. 2016, 11, 1–21. [CrossRef]
- Zielen, S.; Duecker, R.P.; Woelke, S.; Donath, H.; Bakhtiar, S.; Buecker, A.; Kreyenberg, H.; Huenecke, S.; Bader, P.; Mahlaoui, N.; Ehl, S.; El-Helou, S.M.; Pietrucha, B.; Plebani, A.; van der Flier, M.; van Aerde, K.; Kilic, S.S.; Reda, S.M.; Kostyuchenko, L.; McDermott, E.; Schubert, R. Simple Measurement of IgA Predicts Immunity and Mortality in Ataxia-Telangiectasia. J. Clin. Immunol. 2021, 41, 1878–1892. [CrossRef]
- Lee, P.P.; Jiang, L.P.; Wang, X.C.; Chan, K.W.; Tu, W.W.; Lau, Y.L. Severe Mycobacterial Infections in Two Pairs of Chinese Siblings with Interleukin-12 Receptor Beta1 Deficiency. Eur. J. Pediatr. 2008, 167, 231–232. [CrossRef]
- Zhong, Y.; Su, C.; Wu, S.; Miao, C.; Wang, B. Nasal Delivery of an Immunotherapeutic Vaccine in Thermosensitive Hydrogel Against Allergic Asthma. Int. Immunopharmacol. 2023, 116, 109718. [CrossRef]
- Zhao, L.; Luo, J.L.; Ali, M.K.; Spiekerkoetter, E.; Nicolls, M.R. The Human Respiratory Microbiome: Current Understandings and Future Directions. Am. J. Respir. Cell Mol. Biol. 2023, 68(3), 245–255. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).