1. Introduction
Canine Distemper Virus (CDV) is the etiological agent of canine distemper (CD), one of the most common infectious multisystem diseases in domestic canines and wildlife. It also affects other members of the orders Carnivora, Rodentia, Primata, Artiodactyla, and Proboscidea, being a potential threat to wild and endangered species [
27].
It spreads through the respiratory tract, induces immunosuppression, which favors secondary infections [
5,
12,
37]. Despite the fact that this disease has reduced its prevalence due to the use of vaccines, numerous infectious outbreaks have been described in various parts of the world [
31]. Although the disease has been known for many years, there is still no effective treatment, which has led CD to become an endemic disease throughout the world [
34].
Some of the neurological signs that can occur in cases of CD are neck stiffness, vestibular and cerebellar signs such as ataxia and nystagmus, paresis, paraplegia, seizures and myoclonus, being the latter highly suggestive of the disease. Visual deficits such as chorioretinitis and optic neuritis have also been described [
2,
3,
17,
29,
43].
CD has been proposed as a model for different demyelinating conditions in humans [
47]. In particular, CD shares several neuropathological similarities with Multiple Sclerosis (MS) [
5,
46]. Similarly, Amude et al. [
2,
3] propose neurological CD as “acute MS in canines”. Some of the shared clinical signs of both diseases are optic neuritis, retinal alterations, paresis, motor incoordination, instability, and involuntary movements such as myoclonus [
15,
35,
40,
41].
In MS, evoked potentials (EP) have been proposed as solid biomarkers since abnormalities on them can be found in early stages of the disease, prior to manifestation of clinical signs and functional impairment. Multimodal EPs have been shown to be highly correlated with, and useful predictors of the disability that the disease will cause [
4,
19,
36].
These techniques allow the early detection of functional alterations prior to their clinical manifestation and even in the absence of deficit signs [
6,
13,
20,
24,
36].
In addition, EP can help differentiate between demyelination and axonal damage [
23,
35,
48,
49].
One of the most employed EP in MS diagnosis are visual evoked potentials, and have been shown to be useful to detected MS-associated optic neuritis, which occurs in more than half of MS patients.
VEPs are a commonly used method for the electrophysiological diagnosis of MS-associated optic neuritis [
8,
9,
10]. More than 50% of patients develop optic neuritis (ON) in MS. ON occurs as the first manifestation in 20 to 30% of cases [
7,
14,
15,
41]. In addition to this, it has been demonstrated that MS can cause direct damage to the retina, including thinning of the inner and outer nuclear layers, as well as alterations in the retinal nerve fiber layer and the ganglion cell layer. The function of the retina can be evaluated by means of another neurophysiological technique, the electroretinogram (ERG).
To date, there are few studies that describe how VEPs and ERG are affected in canines with CDV infection.
Ochikubo et al. [
30] studied visual evoked potentials in squirrel monkeys infected with DCV, finding alterations in the subacute stage of the disease, as well as finding alterations in an animal without clinical signs, but which showed the histopathological changes typical of the disease at autopsy. Richards et al. [
38] reported a case of a 9-year-old Jack Russell terrier dog with seizures, turning in circles and blindness, who underwent ERG without finding any alterations.
Consequently, the present work proposes to study the damage that this neurodegenerative disease causes naturally in domestic dogs, using neurophysiological techniques, and to evaluate whether there is functional impairment of the visual pathways in naturally infected dogs with CDV using both VEP and ERG.
2. Materials and Methods
2.1. Study Population
For the present work, two groups of dogs were established, no distinction between sex, breed, or vaccinal status was made. Group 1 (control) consisted of ten healthy animals that did not show any signs of general disease and had a normal clinical examination. Group 2 (clinical cases) consisted of thirty-five animals with confirmed diagnosis of CD. Diagnosis was based on clinical signs and the qualitative detection of antigens of the canine distemper virus (CDV) using an analytical kit, in ocular, nasal and urine discharge (Fast test Distemper®, Megacor, Germany). A real-time RT-PCR assay was also performed for detection and quantitation of CDV. Animals were excluded if the presence of myoclonus was either so extensive or intense that the muscle contraction induced artifacts on the EPs, making their interpretation unreliable.
Visual evoked potentials and ERGs were performed in every dog. In the animals whose clinical condition allowed it, the studies were carried out under sedation with Xylazine (Xilased®, Vetcross, Argentina) (0.5-1.0 mg / kg) per intramuscular via (I / M) [
18,
32,
33].
2.2. Evoked Potential Recordings
The bioelectric signals were recorded with a digitized signal amplification, averaging and filtering system (Sistema Bio-PC Potentiales Evocados® V.9, Akonic S.A. Argentina). Subdermal stainless steel needle electrodes were used; one active or recording (+) close to the generating area of the electrical responses, another reference (-) far from the previous one, and a third ground electrode. For VEPs, the recording electrode was placed on the midline of the occipital protuberance (Oz), the reference electrode on the midline of the frontal bone between both eyes (Fpz), and the ground electrode on the midline of the vertex (Cz) between both ears [
21,
42,
45]. For the ERG, the same type of electrode was used; the recording electrode was placed half a centimeter below the lower eyelid, while the reference electrode was placed half a centimeter caudal to the lateral edge of the eye to be stimulated. The ground electrode was placed on the midline of the vertex (Cz).
As stimuli to evoke the responses, flashes of white light generated by a stroboscope at a frequency of 1 Hz were used, placed close to the eye to be stimulated without touching the eyelids. A hundred and twenty-eight monocular stimulations were performed for the VEPs and 64 for the ERGs, covering the contralateral eye with an opaque patch to isolate the responses of each eye and, therefore, to detect functional asymmetries between both eyes. For each technique, two complete sets of stimuli were performed on each eye. The two consecutive recordings in each eye confirmed the reproducibility of the responses. The signal was filtered using a 1-100 Hz passband, with an analysis time of 300 milliseconds. The impedance for all electrodes was checked to be less than 5 kOhm.
2.3. Statistical Methods
Latency (time elapsed between the application of the stimulus and the maximum peak of the positive or negative wave, in ms) and amplitude (measured from the maximum positive or negative value at the peak of the previous or subsequent wave, in microvolts -µV-) values were evaluated. Normality was evaluated by means of the Shapiro-Wilk test, and since the data did not showed a normal distribution, the differences between groups were evaluated using the non-parametric Mann-Whitney- Wilcoxon U test for independent samples. Values were considered significant at p ≤ 0.05.
3. Results
3.1. Population
Dogs’ age ranged between 3 to 144 months, with an average of 22.78 months.
3.2. Visual Evoked Potentials
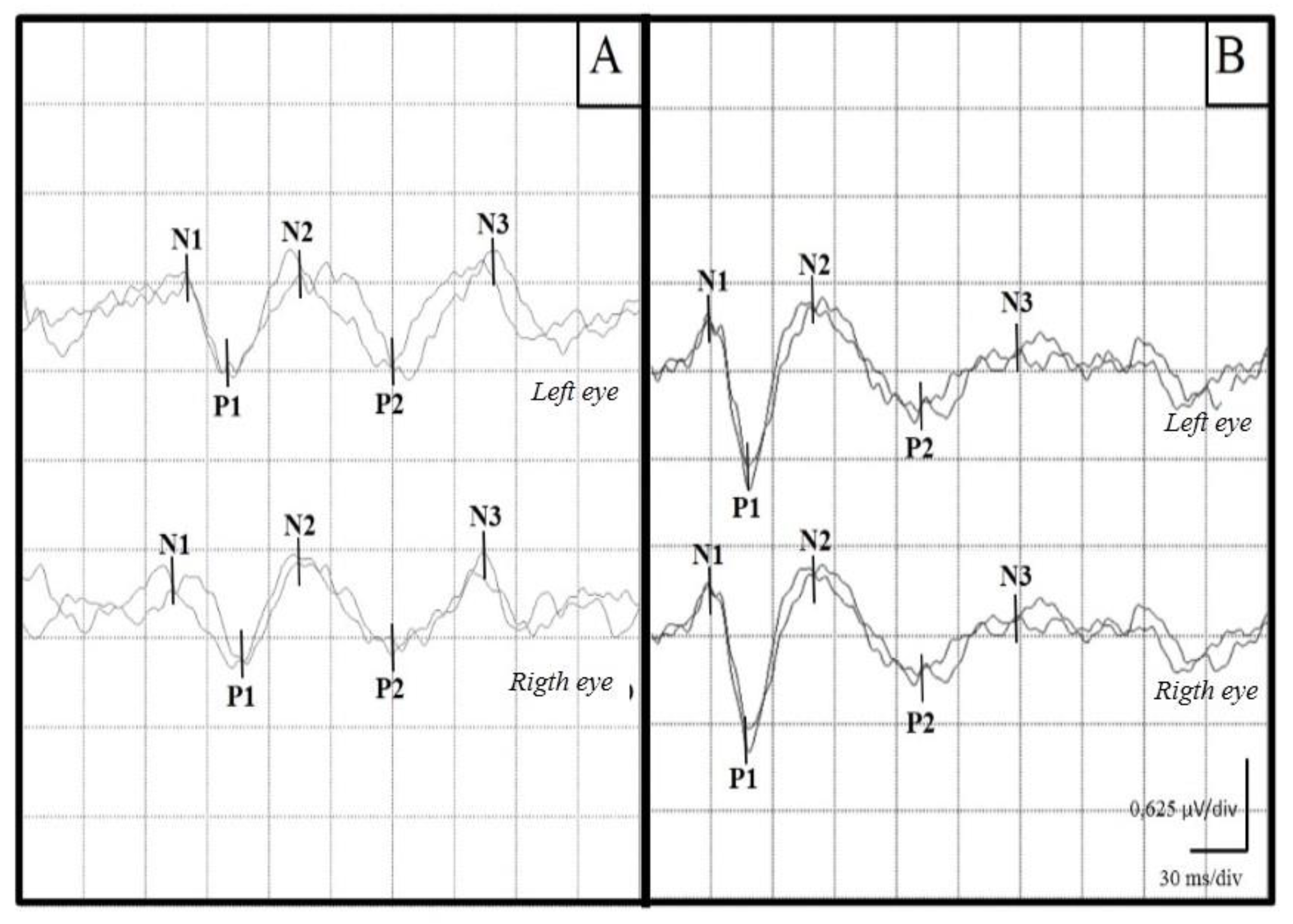
In the group of healthy animals (group 1) the flash VEPs consisted, according to their polarity, of 3 negative and 2 positive components, recorded in the first 150-200 ms after visual stimulation.
Figure 1 shows the records of both a healthy and an animal infected with VDC.
In most of the waves of VEPs performed on group 2 (n = 35), a significant increase was observed bilaterally in all latencies of the potential.
Table 1 shows the mean latencies of each wave for each group. In one animal no response was obtained bilaterally, while in 4 dogs responses were only obtained unilaterally.
The latency of the N1 component was higher in dogs with DC, by 76.5% on left eye, and by 64.7% on the right eye. In four of the animals, no response was obtained unilaterally. For P1 an increase in latency was found in 76.5% of the animals in the left eye and 70.6% in the right eye. Four of the animals did not respond unilaterally. N2 latency in the left eye was prolonged in 70.6% of the cases, while the values for the right eye were prolonged in 58.8%. Six of the dogs with CD did not present this wave bilaterally, and one dog presented it only on the left eye. P2 component had an increase in latency in 32.3% of the animals in the left eye and in 35.3% of the animals in the right eye. Five of the dogs did not present this wave bilaterally, while in two dogs the latency increase was unilateral. Regarding N3 wave, consistent and reproducible records were only obtained in nine of the thirty-five animals studied (26.4%). A bilateral increase in latency was found in 11.7% of the animals.
No differences in amplitudes were found between groups, however the N2-P2 amplitude tended to be higher in the group of CD for the left eye, with the mean and standard deviation of the controls being 1.02 ± 0.58 and 1.66 ± 1.06 that of the affected animals (p = 0.08).
3.3. Electroretinograms
In all animals ERG by flash was characterized by the presence of a positive wave (wave a) followed by a wave of great negative amplitude (wave b), without the presence of wave c. The latencies corresponding to waves a and b in the ERG were not different between groups. Likewise, the amplitudes were not affected.
Of the eighteen CD infected animals that underwent ERG, six presented altered VEP (five with increased latencies and one with no response).
4. Discussion
Considering that there is no consensual nomenclature of the waves, in this work it was decided to name them descriptively according to their polarity, taking into account the inputs of the differential amplifier and how the polarity was determined according to it [
25].
The development of this work provides new data, that can be used both in the diagnosis and in the follow-up of patients with CD, as well as for other demyelinating, neurological and ophthalmological diseases.
Although the use of evoked potentials in canines has been primarily focused on research rather than on clinical applications, the normal response of VEP and ERGs in canines has long been described by several authors [
25,
42,
44].
In the present work, no distinction was made between sex, age and vaccinal status. There are no reports that justify making a distinction between sexes; Human studies indicated that VEP values in men versus women were not clinically significant [
9,
10].
Regarding the changes that occurred in VEP in relation to age; Kimotsuki et al. [
22] report a prolongation in the latencies of the N2, P2 and N3 waves (adequate nomenclature according to the polarity used in this work) in the VEP of canines older than 15 years when compared with the PEV of 1-year-old animals. On the other hand, there are reports that mention that the maturation of the visual pathway in canines occurs between 11 and 15 weeks, when amplitudes increase and latencies decrease [
16,
45].
In this work, VEPs are altered in animals infected with DC as compared to the control group. According to the hypothesis, the latencies in the CD group were prolonged VEPs. These findings are consistent with reports regarding MS given the neuropathological similarities between both diseases [
4,
26].
Regarding the alterations found in the VEPs performed on canines with CD, the latencies of all components were increased, while the amplitudes did not undergo changes except for N2-P2 for the left eye.
On the other hand, in MS it has been shown that an increase in the latency of the VEP waves occurs (performed by pattern), with an increase in P100 latency being the main alteration described. This has been associated with disorders of myelination [
11]. A demyelinating lesion in the optic nerve approximately 10 mm in length causes a conduction delay of approximately 25 ms [
19]. MRI studies in patients with MS have shown lesions in optical radiation in 70% of individuals [
1], which explains the visual dysfunction present in them.
In our study group, it was observed that the increases in latencies were more marked in the P1, N1 and P2 waves. This increase in latency is consistent with what is expected in connection with the pathophysiology of DC.
Infection by DCV leads to lesions in the myelin sheaths and formation of vacuoles in the white matter [
39]. With regard to neuropathological lesions that could influence the functioning of the visual pathway, Amude et al. [
2,
3] described that the occipital cortex is mainly affected in old dog encephalitis. Mike and Carithes [
28] observed histopathological lesions in the eye of infected canines as well as signs of demyelination of the optic nerve and its tracts. This set of alterations is in agreement with our findings, since the prolongation of VEP latency has long been considered an indicator of demyelination. Moreover, the degree of demyelination within the visual pathway of animal models correlates with the magnitude of delay [
48].
Regarding the increase in the N2-P2 amplitude in the VEP for the left eye, these results were not expected given that in the reports of EP alterations due to demyelinating lesions with nerve conduction involvement, an increase in latency and a decrease in amplitude usually occur.
Increased amplitude of the VEP, seen in our animals infected with CDV, could only be observed on the left side, which can be explained by having always started the stimulations in the same eye (right) while the other remained occluded. This allows for an adaptation to darkness in the second eye, with the consequent increase in amplitude.
Regarding the ERG results, although, Mike and Carithes [
28] found in canines infected with CDV lesions at the histopathological level of the following type: necrosis, degeneration of the retinal ganglion cells, edema and peri-vascular cuffings, proliferation pigment epithelial cells, retinal inclusion bodies, and rod and cone atrophy. In our work, the absence of specific alterations in the electroretinography in diseased animals agrees with what has been demonstrated for this technique in patients with MS, given its low specificity [
4].
Klistoner et al. [
23] found alterations in patients with MS, which are presumably due to the thinning of the retinal ganglion cells. However, these lesions are more evident by optical coherence tomography. Given its low specificity and the additional time involved in performing the electroretinogram, it would not provide any clinical benefit in the routine management of CD.
5. Conclusions
The existence of alterations in the VEP (prolonged latencies) was verified. The increase in VEP latencies indicates that neurological lesions can be detected before neurological signs appear. These techniques have demonstrated to be a very useful tool in the detection of silent lesions, since signs related to nervous system involvement in patients with CD often appear later than signs in other organ systems.
Thus, we propose VEPs as a good tool to evaluate the effectiveness of different treatments against CD in future studies.
Author Contributions
Conceptualization, M.G.; L.D.; methodology, M.G.; L.D.; software, M.G.; L.D.; validation, M.G.; L.D.; formal analysis, M.G.; L.D.; A.B.; investigation, M.G.; resources, L.D.; data curation, M.G.; L.D.; A.B.; J.M.V.; writing—original draft preparation, M.G.; L.D.; A.B.; J.M.V.; writing—review and editing, M.G.; L.D.; A.B.; J.M.V.; visualization, M.G.; L.D.; supervision, L.D.; A.B.; J.M.V.; project administration, L.D.; J.M.V.; funding acquisition, L.D.; J.M.V.
Funding
This research was partially funded by National Agency of Research and Innovation (ANII, Fondo María Viñas 2019), Grant FMV-1-2019-1-155934), M.G. was MSc fellowship recipient of National Agency of Research and Innovation (ANII). A.B. and J.M.V. are Research Career Members of the National Research System (SNI-ANII), Uruguay.
Acknowledgments
The authors would like to thank all the owner's that agreed to have their dogs participate in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alshowaeir, D.; Yiannikas, C.; Garrick, R.; Parratt, J.; Barnett, M.; Graham, S.; Klistorner, A. Latency of multifocal visual evoked potentials in nonoptic neuritis eyes of multiple sclerosis patients associated with optic radiation lesions. Invest Ophthalmol & Vis Sci 2014, 55, 3758–3764. [Google Scholar]
- Amude, A.; Alfieri, A.; Alfieri, A. The nervous form of canine distemper. Vet Zootec 2006, 13, 125–136. [Google Scholar]
- Amude, A.; Alfieri, A.; Alfieri, A. Canine distemper virus and multiple sclerosis: A real or an anecdotal association. Appl Microbiol 2010, 737–745. [Google Scholar]
- Barton, J.; Garber, J.; Klistorner, A.; Barnett, M. The electrophysiological assessment of visual function in Multiple Sclerosis. Clin Neurophysiol Prac 2019, 4, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Beineke, A.; Puff, C.; Seehusen, F.; Baumgärtner, W. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet Immunol Immunopathol 2009, 127, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cambron, M.; D'Haeseleer, M.; Laureys, G.; Clinckers, R.; Debruyne, J.; De Keyser, J. White-matter astrocytes, axonal energy metabolism, and axonal degeneration in multiple sclerosis. J Cereb Blood Flow Metab 2012, 32, 413–424. [Google Scholar] [CrossRef]
- Cantore, A. Optic neuritis. Penn Med 1996, 99, 96–98. [Google Scholar]
- Chiappa, K. H. Pattern shift visual, brainstem auditory, and short-latency somatosensory evoked potentials in multiple sclerosis. Neurology 1980, 30, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Chiappa, K. Pattern-Shift Visual Evoked Potential: Methodology. In: Chiappa K. Ed. Evoked Potential in Clinical Medicine. Ed. Lippincott-Raven 3rd. ed. Philadelphia, USA, 1997, Chapter 2, pp. 30–94.
- Chiappa, K.; Hill, R. Pattern-Shift Visual Evoked Potential: Interpretation. In: Chiappa K. Ed. Evoked Potential in Clinical Medicine. Ed. Lippincott-Raven 3rd. ed. Philadelphia, USA, 1997, Chapter 3, pp. 95–130.
- Comi, G.; Leocani, L.; Medaglini, S.; Locatelli, T.; Martinelli, V.; Santuccio, G.; Rossi, P. Measuring evoked responses in multiple sclerosis. Mult Scler 1999, 5, 263–267. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R.D.; Duprex, W.P.; de Swart, R.L. Morbillivirus infections: an introduction. Viruses 2015, 7, 699–706. [Google Scholar] [CrossRef]
- Di Maggio, G.; Santangelo, R.; Guerrieri, S.; Bianco, M.; Ferrari, L.; Medaglini, S.; Rodegher, M.; Colombo, B.; Moiola, L.; Chieffo, R.; Del Carro, U.; Martinelli, V.; Comi, G.; Leocani, L. Optical coherence tomography and visual evoked potentials: which is more sensitive in multiple sclerosis? Mult Scler 2014, 20, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Fernández, O.; Arroyo-González, R.; Rodríguez-Antigüedad, A.; García-Merino, A.; Comabella, M.; Villar, M.; Meca-Lallana, E. Biomarkers in multiple sclerosis. Rev Neurol 2013, 56, 375–390. [Google Scholar]
- Fernández, O.; Fernández, V.; Guerrero, V. Enfermedades desmielinizantes del sistema nervioso central. Rev Ed Sup 2015, 11, 4601–4609. [Google Scholar] [CrossRef]
- Fox, M. Neuronal development and ontogeny of evoked potentials in auditory and visual cortex of the dog. Electroencephalogr Clin Neurophysiol 1968, 24, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Galán, A.; Gamito, A.; Carletti, B.E.; Guisado, A.; de las Mulas, J. M.; Pérez, J.; Martín, E. M. Uncommon acute neurologic presentation of canine distemper in 4 adult dogs. Can Vet J 2014, 55, 373–378. [Google Scholar]
- Gutiérrez, M.; Feijóo, G.; Delucchi, L. J. Neurophysiological evaluation of canine congenital hydrocephalus in three dogs. Vet Rec Case Rep 2020. [Google Scholar] [CrossRef]
- Hardmeier, M.; Leocani, L.; Fuhr, P. A new role for evoked potentials in MS? Repurposing evoked potentials as biomarkers for clinical trials in MS. Mult Scler 2017, 23, 1309–1319. [Google Scholar] [CrossRef]
- Kiiski, S.; Riada, S. N.; Lalor, C.; Goncalves, R.; Nolan, H.; Whelan, R.; Bramham, J. Delayed P100-Like latencies in multiple sclerosis: A preliminary investigation using visual evoked spread spectrum analysis. Plos One 2016, 11, e0146084. [Google Scholar] [CrossRef]
- Kimotsuki, T.; Yasuda, M.; Tamahara, S.; Matsuki, N.; Ono, K. Topographic analysis of flash visual evoked potentials in dogs. J Vet Med Sci 2005, 67, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Kimotsuki, T.; Yasuda, M.; Tamahara, S.; Tomihari, M.; Matsuki, N.; Ono, K. Age associated changes on flash visual evoked potentials in dogs. J Vet Med Sci 2006, 68, 79–82. [Google Scholar] [CrossRef]
- Klistorner, A.; Graham, S.; Fraser, C.; Garrick, R.; Nguyen, T.; Paine, M.; Billson, A. Electrophysiological evidence for heterogeneity of lesions in optic neuritis. Investig Ophthalmol Vis Sci 2007, 48, 4549–4556. [Google Scholar] [CrossRef] [PubMed]
- Kraft, H. Evoked potentials in multiple sclerosis. Phys Med Rehabil Clin N Am 2013, 24, 717–720. [Google Scholar] [CrossRef]
- Lee, E.; Seok, H.; Park, K.; Seo, D. Evoked potential: basic requirements and guidelines for writing reports. Ann Clin Neuriphysiol 2018, 20, 18–25. [Google Scholar] [CrossRef]
- Leocani, L.; Guerrieri, S.; Comi, G. Visual evoked potentials as a biomarker in multiple sclerosis and associated optic neuritis. Neuro-ophthalmol 2018, 38, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gutiérrez, M.; Ruiz-Sáenz, J. Diversity of susceptible hosts in canine distemper virus infection: a systematic review and data synthesis. BMC Vet Res 2016, 12, 78. [Google Scholar] [CrossRef]
- Mike, B.; Carithers, W. Chorioretinitis and detached retina as post-distemper lesions in the canine. Iowa State University Veterinarian 1975, 37, 1. [Google Scholar]
- Nell, B. Optic neuritis in dogs and cats. Vet Clin North Am Small Anim Pract 2008, 38, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Ochikubo, F.; Nagata, T.; Yoshikawa, Y.; Matsubara, Y.; Kai, C.; Yamanouchi, Y. Electroencephalogram and evoked potentials in the primate model of viral encephalitis. Electroencephalogr Clin Neurophysiol / Evoked Potentials Section 1993, 88, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Panzera, Y.; Sarute, N.; Iraola, G.; Hernández, M.; Pérez, R. Molecular phylogeography of canine distemper virus: Geographic origin and global spreading. Mol Phylogenet Evol 2015, 92, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, F.; Sica, R. Canine electroencephalographic recording technique: findings in normal and epileptic dogs. Clin Neurophysiol 2004, 115, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, F. Neuropatología y síndromes clínicos del virus del moquillo canino: estado actual del conocimiento. NeuroVet 2015, 31, 2–59. [Google Scholar]
- Pinotti, M.; Gollan, A.; Canavesio, M.; Passeggi, C.; Larrateguy, M.; Paz, M.; Formentini, E. Virus de distemper canino: detección molecular de diferentes aislamientos provenientes de perros de la provincia de Santa Fé, Argentina, ente los años 2000 y 2010. In Vet 2016, 18, 349–355. [Google Scholar]
- Pueyo, V.; Ara, J. R.; Martín, J. La retina como marcador biológico de daño neuronal [The retina as a biologicalmarker of neuronal damage]. Arch Soc Esp Oftalmol 2010, 85, 163–164. [Google Scholar] [CrossRef]
- Ramanathan, S.; Prelog, K.; Barnes, E.; Tantsis, E.; Reddel, S.; Henderson, A.; Vucic, S.; Gorman, M.; Benson, L.; Alper, G.; Riney, C.; Barnett, M.; Parratt, J.; Hardy, T.; Leventer, R.; Merheb, V.; Nosadini, M.; Fung, V.; Brilot, F.; Dale, R. The utility of multimodal evoked potentials in multiple sclerosis prognostication. Clin Neurosci 2013, 20, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Rendon-Marin, S.; da Fontoura Budaszewski, R.; Canal, C. W.; Ruiz-Saenz, J. Tropism and molecular pathogenesis of canine distemper virus. Virol J 2019, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Richards, T.; Whelan, N.; Pinard, C.; Alcala, F.; Wolfe, K. Optic neuritis caused by canine distemper virus in a Jack Russell terrier. Can Vet J 2011, 52, 398–402. [Google Scholar] [PubMed]
- Schobesberger, M.; Zurbriggen, A.; Doherr, M. G.; Weissenbóck, H.; Vandevelde, M.; Lassman, H.; Griot, C. Demyelination precedes oligodendrocyte loss in canine distemper virus-induced encephalitis. Acta Neuropathol 2002, 103, 11–19. [Google Scholar] [CrossRef]
- Shaharabani, R.; Ram-On, M.; Talmon, Y.; Beck, R. Pathological transitions in myelin membranes driven by environmental and multiple sclerosis conditions. Proc Natl Acad Sci (PNAS) 2018, 115, 11156–11161. [Google Scholar] [CrossRef]
- Söderström, M. Optic neuritis and multiple sclerosis. Acta Ophthalmol Scand 2001, 79, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Strain, G.; Jackson, M.; Tedford, L. Visual evoked potentials in the clinically normal dog. J Vet Intern Med 1990, 4, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Tipold, A.; Vandevelde, M.; Jaggy, A. Neurological manifestations of canine distemper virus infection. Small Anim Prac 1992, 33, 466–470. [Google Scholar] [CrossRef]
- Torres, D.; Tovar, M. Clinical guideline for assessing flash visual evoked potentials in laboratory dogs and normal data for beagle dogs. Scand J Lab Anim Sci 2016, 42, 1–8. [Google Scholar]
- Tovar-Sahuquillo, M.; Torres-Soriano, D. Protocolo Combinado de PEV y ERG en perros Beagle para evaluar la integridad funcional de las vías visuales. Archivos de Medicina Veterinaria 2014, 46, 289–297. [Google Scholar]
- Ulrich, R.; Puff, C.; Wewetzer, K.; Kalkuhl, A.; Deschl, U.; Baumgärtner, W. Transcriptional changes in canine distemper virus-induced demyelinating leukoencephalitis favor a biphasic mode of demyelination. PLoS One 2014, 9, e95917. [Google Scholar] [CrossRef]
- Vandevelde, M.; Zurbriggen, A. The neurobiology of canine distemper virus infection. Vet Microbiol 1995, 44, 271–280. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Klistorner, A.; Thie, J.; Graham, SL. Latency delay of visual evoked potential is a real measurement of demyelination in a rat model of optic neuritis. Invest Ophthalmol Vis Sci 2011, 52, 6911–6918. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Klistorner, A.; Thie, J.; Gupta, V. K.; Graham, S. L. Axonal loss in a rat model of optic neuritis is closely correlated with visual evoked potential amplitudes using electroencephalogram-based scaling. Invest Ophthalmol Vis Sci 2012, 53, 3662. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).