Submitted:
18 September 2024
Posted:
18 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations:
| TMDs: | temporomandibular disorders |
| RH: | The Rhesus |
| RBCS: | Red Blood Cells |
| TMJ: | Temporomandibular Joint |
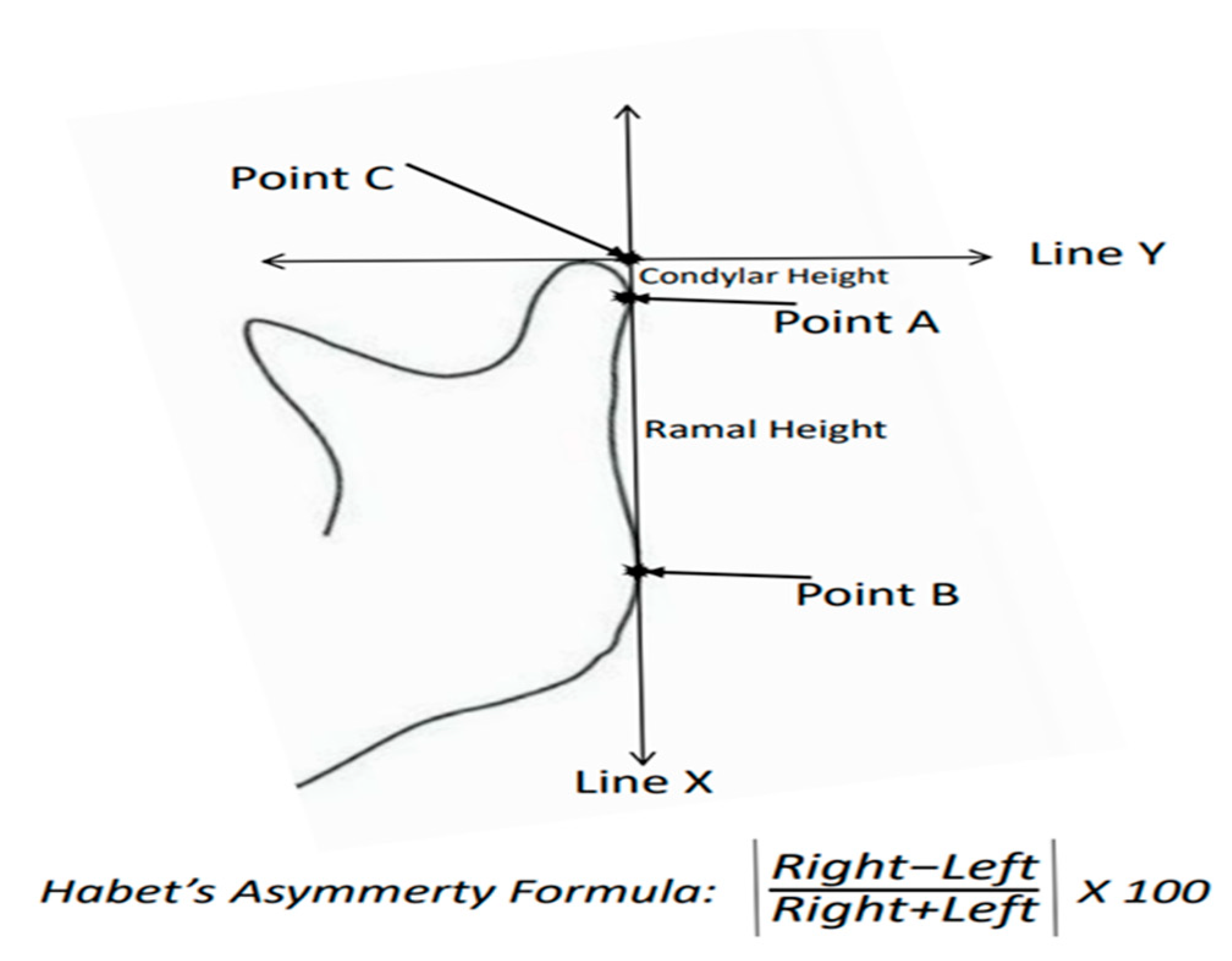
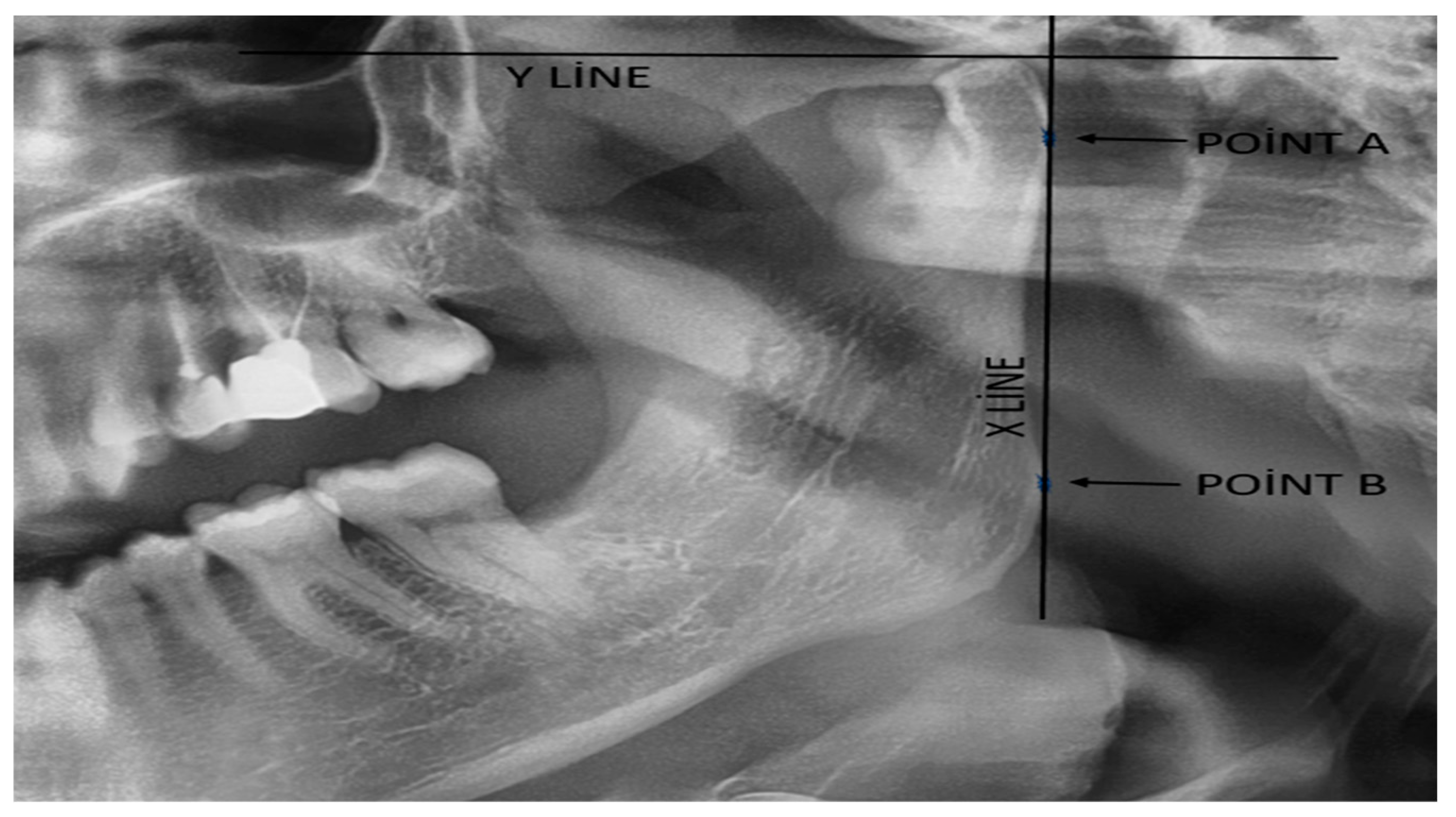
| CH: | Condyle Height |
| RH: | Ramal Height |
| CAI: | Condylar Asymmetry Index |
| RAI: | Ramal Asymmetry Index |
References
- Sfondrini, M.F.; Bolognesi, L.; Bosco, M.; Gandini, P.; Scribante, A. Skeletal divergence and condylar asymmetry in patients with temporomandibular disorders (TMD): A retrospective study. BioMed Res. Int. 2021, 1, 8042910. [Google Scholar] [CrossRef]
- Halicioglu, K.; Celikoglu, M.; Buyuk, S.K.; Sekerci, A.E.; Candirli, C. Effects of early unilateral mandibular first molar extraction on condylar and ramal vertical asymmetry. Eur. J. Dent. 2014, 8, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Dikson, C.; Traebert, J. Malocclusion, dental aesthetic self-perception and quality of life in a 18 to 21 year-old population: A cross section study. BMC Oral. Health 2013, 13, 1–6. [Google Scholar]
- Sezgin, O.S.; Celenk, P.; Arici, S. Mandibular asymmetry in different occlusion patterns. Angle Orthod. 2007, 77, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Habets, L.L.; Bezuur, J.N.; Naeiji, M.; Hansson, T.L. The orthopantomogram, an aid in diagnosis of temporomandibular joint problems. II. The vertical symmetry. J. Oral. Rehabil. 1988, 15, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, Y.; Koizumi, S.; Kim, Y.I.; Adel, M.; Nadim, M.; Hikita, Y.; Yamaguchi, T. Comparison of Mandibular Volume and Linear Measurements in Patients with Mandibular Asymmetry. Diagnostics 2023, 13, 1331. [Google Scholar] [CrossRef]
- Mostafa, D.; Elkhatat, E.I.; Koppolu, P.; Mahgoub, M.; Dhaifullah, E.; Hassan, A.H. Correlation of ABO Blood Groups and Rh Factor with The Severity of Generalized Chronic Periodontitis: Across Sectional Study in Riyadh, Saudi Arabia. Open Access Maced. J. Med. Sci. 2019, 7, 617–622. [Google Scholar] [CrossRef]
- Watins, W.M. ABO blood group system: Historical background. Transfus. Med. 2001, 11, 243–265. [Google Scholar] [CrossRef] [PubMed]
- Salem, G.I.; Gamal, N.M.; Talaat, E.A.; El-Hammady, D.H.; Hammam, N.; Gheita, T.A. Clinical Impact of the ABO Blood Type in Patients with Rheumatic Diseases: Is there a Link to the ABO and Rhesus? Mediterr J Rheumatol 2021, 32, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.L.; Brodsky, I.E.; Mangalmurti, N.S. The Evolving Erythrocyte: Red Blood Cells as Modulators of Innate Immunity. J. Immunol. Baltim. Md. 1950 2018, 201, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Mittal, N.; Singh, T.B.; Srivastava, R.; Verma, P.K. Correlation of ABO blood group phenotype and rhesus factor with periodontal disease: An observational study. Contemp. Clin. Dent. 2017, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Gheisari, R.; Ghoreishian, M.; Movahedian, B.; Roozbehi, A. The association between blood groups and maxillofacial deformities. Indian. J. Plast. Surg. 2008, 41, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Tortarolo, A.; Rotolo, R.; Nucci, L.; Tepedino, M.; Crincoli, V.; Piancino, M.G. Condylar asymmetry in children with unilateral posterior crossbite malocclusion: A comparative cross-sectional study. Children 2022, 9, 1772. [Google Scholar] [CrossRef] [PubMed]
- Polat, M.; Odabası, O. Can cystic lesions of the jaws be considered as the cause of mandibular asymmetry? Med. Oral. Patol. Oral. Cir. Bucal. 2022, 27, e159–e163. [Google Scholar] [CrossRef] [PubMed]
- Pirttiniemi, P.; Kantomaa, T. Relation of glenoid fossa morphology to mandibulofacial asymmetry, studied in dry human Lapp skulls. Acta Odontol. Scand. 1992, 50, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Almășan, O.; Leucuța, D.C.; Buduru, S. Disc Displacement of the Temporomandibular Joint and Facial Asymmetry in Children and Adolescents: A Systematic Review and Meta-Analysis. Child. (Basel) 2022, 9, 1297. [Google Scholar] [CrossRef]
- Yanez-Vico, R.M.; Iglesias-Linares, A.; Torres-Lagares, D.; Gu tierrez-Perez, J.L.; Solano-Reina, E. Three-dimensional evaluation of craniofacial asymmetry: An analysis using computed tomography. Clin. Oral. Investig. 2011, 15, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Celik, S.; Celikoglu, M.; Buyuk, S.K.; Sekerci, A.E. Mandibular vertical asymmetry in adult orthodontic patients with different vertical growth patterns: A cone beam computed tomography study. Angle Orthod. 2016, 271–277. [Google Scholar] [CrossRef]
- Sanders, D.A.; Rigali, P.H.; Neace, W.P.; Uribe, F.; Nanda, R. Skeletal and dental asymmetries in Class II subdivision malocclusions using cone-beam computed tomography. Am. J. Orthod. Dentofac. Orthop. 2010, 138, e1–e20. [Google Scholar] [CrossRef]
- Lundstrom, A. Some asymmetries of the dental arches, jaws, and skull, and their etiological signifi cance. Am. J. Orthod. Dentofac. Orthop. 1961, 47, 81–106. [Google Scholar] [CrossRef]
- Staniszewski, K.; Lygre, H.; Berge, T.; Rosén, A. Serum Analysis in Patients with Temporomandibular Disorders: A Controlled Cross-Sectional Study in Norway. Pain. Res. Manag. 2019, 1360725. [Google Scholar] [CrossRef]
- Islamoğlu, Z.G.K.; Unal, M. Is there an association of ABO blood groups and Rhesus factor with alopecia areata? J. Cosmet. Dermatol. 2018, 17, 1271–1274. [Google Scholar] [CrossRef]
- Abegaz, S.B. Human ABO Blood Groups and Their Associations with Different Diseases. Biomed. Res. Int. 2021, 6629060. [Google Scholar] [CrossRef]
- Abdulganiyu. Distribution of ABO and Rh (D) blood groups and associated traits: A study of the College of Nursing and Midwifery. Msc Thesis, Dissertation, Kogi State, Obangede, 2016.
- Chandra, T.; Gupta, A. Association and distribution of hypertension, obesity and ABO blood groups in blood donors Iranian. J. Paediatr. Haematol. Oncol. 2012, 2, 140–145. [Google Scholar]
- Ewaldand, R.; Sumner, S. Blood type biochemistry and human disease,” Wiley Interdisciplinary Reviews. Syst. Biol. Med. 2016, 8, 571–35. [Google Scholar]
- Daniels, G. Human Blood Groups. In Blackwell Science, Oxford, 2nd edition, 2002.
- Al-Askar, M. Is there an association between periodontal diseases and ABO blood group? Systematic review and meta-analysis. Quintessence Int. 2022, 53, 404–412. [Google Scholar] [CrossRef]
- Kundu, D.; Bandyopadhyay, P.; Nair, V.; Chowdhury, M.; Mukherjee, S.; Nayek, M. Aggressive periodontitis: A clinico-hematological appraisal. J. Indian. Soc. Periodontol. 2014, 18, 166–171. [Google Scholar] [CrossRef]
- Solanki, J.; Dileep, C.L.; Adyanthaya, B.R.; Mishra, P.; Yadav, O. Association betweengn different blood groups, depression and oral health status of dental students. Clujul Med. 2018, 91, 317–321. [Google Scholar] [CrossRef]
- Alsadat-Hashemipour, M.; Tahmasbi-Arashlow, M.; Fahimi-Hanzaei, F. Incidence of impacted mandibular and maxillary third molars-a radiographic study in a Southeast Iran population. Medicina Oral, Patologia Oral y Cirurgia Bucal 2013, 18, e140–5. [Google Scholar] [CrossRef] [PubMed]
- Koregol, A.; Raghavendra, M.; Nainegali, S.; Kalburgi, N.; Varma, S. ABO blood groups and Rhesus factor: An exploring link to periodontal diseases. Indian J. Dent. Res. 2010, 21, 364. [Google Scholar] [CrossRef] [PubMed]
- Aditi, P. Assessment of Relationship of ABO-RH Blood Group in Oral Cancer Patients: A Prospective Cohort Study of Hospital Patients. Int. J of Life Sci., Biotechnology and Pharma Research 2023, 2, 1661–1671. [Google Scholar]
- Than, N.G.; Romero, R.; Meiri, H.; Erez, O.; Xu, Y.; Tarquini, F.; Barna, L.; Szilagyi, A.; Ackerman, R.; Sammar, M.; et al. PP13, Maternal ABO Blood Groups and the Risk Assessment of Pregnancy Compli cations. PLoS ONE 2011, 7, e21564. [Google Scholar]
- Naeini, A.E.; Rostami, M.; Naeini, S.E. Chronic viral hep atitis and their relation to ABO blood groups and rhesus (Rh) factor. Med. Case Stud. 2010, 1, 5–7. [Google Scholar]
- Anna, S.; Stelcer, B.; Roy, M. Is there a relationship between psychological factors and TMD? Brain Behav. 2019, 9, e01360. [Google Scholar]
- Pisk, S.V.; Vuk, T.; Ivezic, E.; Jukic, I.; Bingulac-Popovic, J.; Filipcic, I. ABO blood groups and psychiatric disorders: A Croatian study. Blood Transfus. 2019, 17, 66–71. [Google Scholar] [CrossRef]
- Xu, F.; Yin, J.W.; Xiong, E.F.; He, H.; Zhang, Q.T.; Fan, S.W.; Qin, X.L.; Wang, S. Correlation between Preoperative Anxiety and ABO Blood Types: Evidence from a Clinical Cross-Sectional Study. Disease Markers 2019, 1761693. [Google Scholar] [CrossRef]
| Intrusion Criteria | Exclusuion Criteria |
|---|---|
| Age ≥ 18 years | Age < 18 years |
| Suitable panoramic film quality (for asymmetry index calculation) | Unsuitable panoramic film quality (for asymmetry index calculation) |
| Complete demographic information (age, gender, blood type) | Inomplete demographic information (age, gender, blood type) |
| systemic disease free | Presence of systemic diseases (e.g., diabetes, autoimmune diseases). |
| craniofacial deformity free | craniofacial deformity presence (congenital or acquired) |
| No orthodontic treatment history. | History of orthodontic treatment or current orthodontic appliances. |
| TMJ complaint free | TMJ-related complaint presence |
| Inconsistent or incomplete patient records. |
| ABO groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | AB | O | P value | |||||
| Sex distrubution | Male | Female | Male | Female | Male | Female | Male | Female | ,613 a |
| 26 | 28 | 12 | 11 | 6 | 12 | 23 | 31 | ||
| Age distrubution | 26,12±7.38 | 26,69±7,81 | 31,33±11,66 | 28,12±7,32 | ,148 b | ||||
| Condylar asymmetry score | 9,82±11,86 x y | 7,33±6,46 y | 13,60±9,79 x | 9,40±9,95 x y | ,044 c | ||||
| Ramal asymmetry score | 5,45±13,51 | 3,15±3,00 | 3,45±2,35 | 2,79±3,51 | ,344 b | ||||
| a Chi square test b Kruskal Wallis test c Kruskal Wallis test with pairwise comparison inculding Bonferroni correction | |||||||||
| RH Groups | |||||
|---|---|---|---|---|---|
| Rh + | Rh - | P value | |||
| Sex distrubution | Male | Female | Male | Female | ,744 a |
| 43 | 76 | 4 | 6 | ||
| Age distrubution | 27,81±8,13 | 30,30±7,97 | ,109 b | ||
| Condylar asymmetry score | 9,99±10,56 | 6,18±3,78 | ,371 b | ||
| Ramal asymmetry score | 3,97±8,84 | 2,79±2,16 | ,952 b | ||
| a Chi square test b Mann Whitney U test | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





