Submitted:
18 September 2024
Posted:
19 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. The Anemia Control Model (ACM)
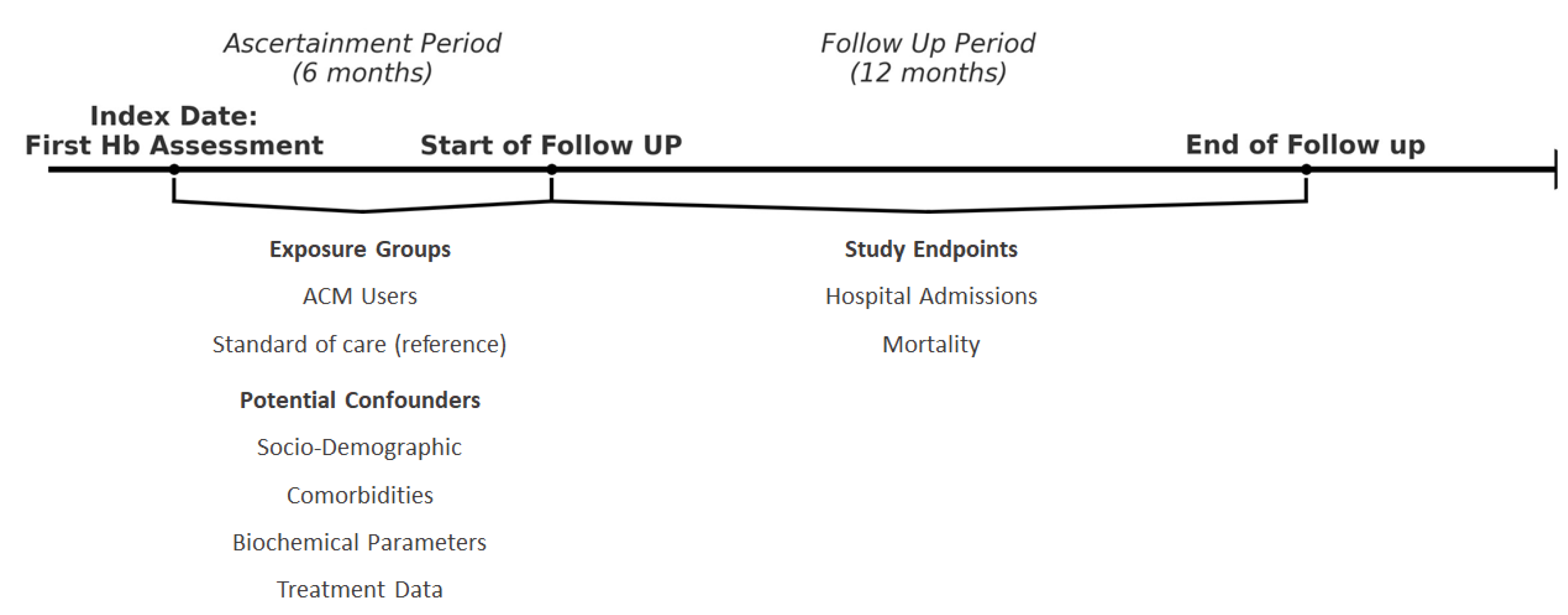
2.2. Study Design & Participants
2.3. Definition of Exposure Groups
2.3.1. ACM Adherent Patients
2.3.2. Reference group
2.4. Covariates
2.5. Outcome Definition
2.6. Statistical Analysis
2.6.1. Primary Analysis
Propensity Score (PM) Estimation
Matching Strategy
Outcomes estimation
2.6.2. Secondary Analysis
3. Results
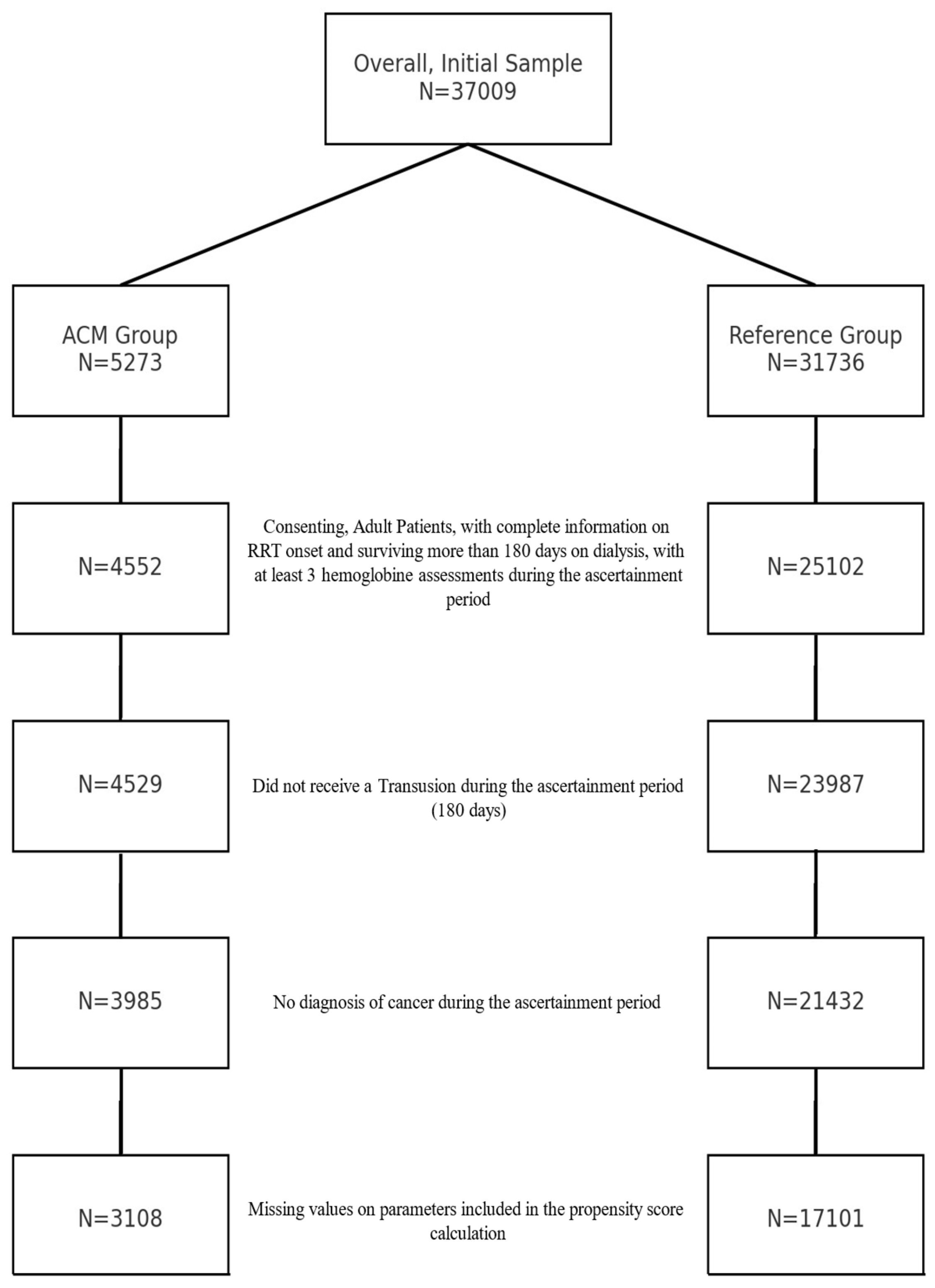
3.1. Study Sample before Matching
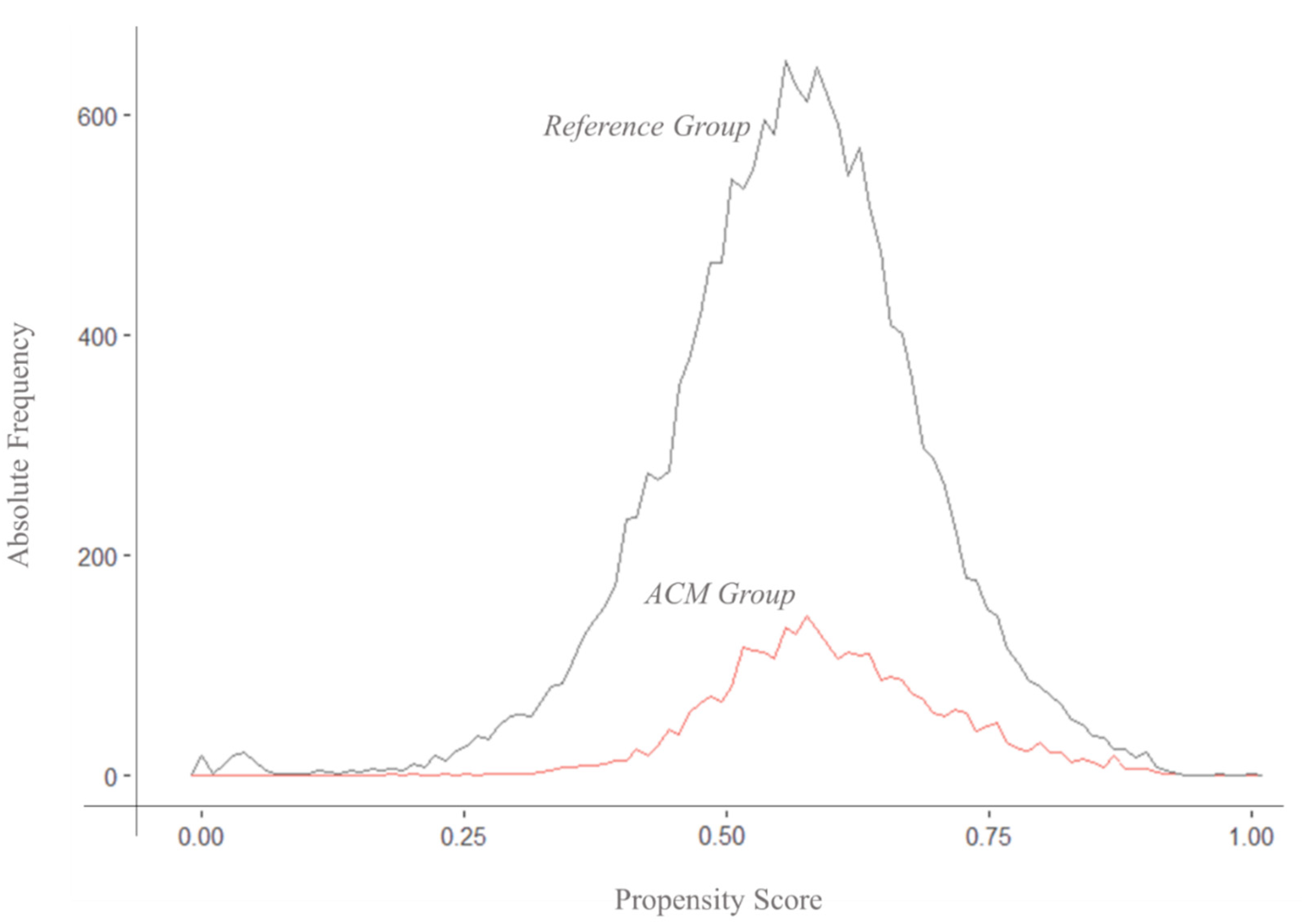
3.2. Propensity Score Estimation
3.3. Study Sample after Matching
3.4. Hospitalization and Mortality Rate
3.5. Secondary Analysis
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl. Published online 2012. [CrossRef]
- Hanna RM, Streja E, Kalantar-Zadeh K. Burden of Anemia in Chronic Kidney Disease: Beyond Erythropoietin. Adv Ther. 2021;38(1):52-75. [CrossRef]
- KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am J Kidney Dis. 2006;47(5 Suppl 3).
- Toft G, Heide-Jørgensen U, van Haalen H, et al. Anemia and clinical outcomes in patients with non-dialysis dependent or dialysis dependent severe chronic kidney disease: a Danish population-based study. J Nephrol. 2020;33(1):147-156. [CrossRef]
- Quon P, Gitlin M, Isitt JJ, et al. Cost-effectiveness of treating chronic anemia with epoetin alfa among hemodialysis patients in the United States. Health Outcomes Res Med. 2012;3(2):e79-e89. [CrossRef]
- Toida T, Iwakiri T, Sato Y, Komatsu H, Kitamura K, Fujimoto S. Relationship between hemoglobin levels corrected by interdialytic weight gain and mortality in japanese hemodialysis patients: Miyazaki dialysis cohort study. PLoS One. 2017;12(1):1-15. [CrossRef]
- KIDGO Anaemia work group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl. 2012;2(4).
- McMurray JJV, Parfrey PS, Adamson JW, et al. Kidney disease: Improving global outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2(4). [CrossRef]
- Locatelli F, Nissenson AR, Barrett BJ, et al. Clinical practice guidelines for anemia in chronic kidney disease: Problems and solutions. A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). In: Kidney International. Vol 74. ; 2008. [CrossRef]
- Borawski B, Malyszko JS, Kwiatkowska M, Malyszko J. Current status of renal anemia pharmacotherapy—what can we offer today. J Clin Med. 2021;10(18). [CrossRef]
- Drüeke TB, Parfrey PS. Summary of the KDIGO guideline on anemia and comment: Reading between the (guide)line(s). Kidney Int. 2012;82(9):952-960. [CrossRef]
- UK Renal Registry (UKRR). Adults on In-Centre Haemodialysis ( ICHD ) in the UK at the End of 2020.; 2020. https://ukkidney.org/sites/renal.org/files/24th_UKRR_ANNUAL_REPORT_ICHD_Ch5.pdf.
- Zhao X, Niu Q, Gan L, et al. Baseline data report of the China Dialysis Outcomes and Practice Patterns Study (DOPPS). Sci Rep. 2021;11(1):1-10. [CrossRef]
- Chait Y, Nathanson BH, Germain MJ. Individualized anemia management enhanced by ferric pyrophosphate citrate protocol. Sci Rep. 2022;12(1):1-7. [CrossRef]
- United States Renal Data System. Clinical indicators & preventive care. In: 2022 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2022. https://usrds-adr.niddk.nih.gov/2022/end-stage-renal-disease/3-clinical-indicators-and-preventive-care.
- Gilbertson DT, Ebben JP, Foley RN, Weinhandl ED, Bradbury BD, Collins AJ. Hemoglobin level variability: Associations with mortality. Clin J Am Soc Nephrol. 2008;3(1):133-138. [CrossRef]
- Ishani A, Solid CA, Weinhandl ED, Gilbertson DT, Foley RN, Collins AJ. Association between number of months below K/DOQI haemoglobin target and risk of hospitalization and death. Nephrol Dial Transplant. 2008;23(5):1682-1689. [CrossRef]
- Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007;369(9559):381-388. [CrossRef]
- Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D. Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J Am Soc Nephrol. 2005;16(7):2180-2189. [CrossRef]
- Singh AK, Szczech L, Tang KL, et al. Correction of Anemia with Epoetin Alfa in Chronic Kidney Disease. N Engl J Med. 2006;355(20):2085-2098. [CrossRef]
- Lau JH, Gangji AS, Rabbat CG, Brimble KS. Impact of haemoglobin and erythropoietin dose changes on mortality: A secondary analysis of results from a randomized anaemia management trial. Nephrol Dial Transplant. 2010;25(12):4002-4009. [CrossRef]
- Besarab A, Bolton WK, Browne JK, et al. The Effects of Normal as Compared with Low Hematocrit Values in Patients with Cardiac Disease Who Are Receiving Hemodialysis and Epoetin. N Engl J Med. 1998;339(9):584-590. Accessed May 6, 2024. [CrossRef]
- Pfeffer MA, Burdmann EA, Chen CY, et al. A Trial of Darbepoetin Alfa in Type 2 Diabetes and Chronic Kidney Disease. N Engl J Med. 2009;361(21):2019-2032. [CrossRef]
- Solomon SD, Uno H, Lewis EF, et al. Erythropoietic Response and Outcomes in Kidney Disease and Type 2 Diabetes. N Engl J Med. 2010;363(12):1146-1155. [CrossRef]
- Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-a dose and achieved hemoglobin outcomes. Kidney Int. 2008;74(6):791-798. [CrossRef]
- Food and Drug Administration. FDA Drug Safety Communication: Modified Dosing Recommendations to Improve the Safe Use of Erythropoiesis-Stimulating Agents in Chronic Kidney Disease.; 2011.
- Koulouridis I, Alfayez M, Trikalinos TA, Balk EM, Jaber BL. Dose of Erythropoiesis-Stimulating Agents and Adverse Outcomes in CKD: a metaregression analysis. Am J Kidney Dis. 2013;61(1):44-56. [CrossRef]
- Jeong HY, Ko EJ, Kim SH, et al. Administration of a high-dose erythropoietin-stimulating agent in hemodialysis patients is associated with late arteriovenous fistula failure. Yonsei Med J. 2017;58(4):793-799. [CrossRef]
- Kuragano T, Matsumura O, Matsuda A, et al. Association between hemoglobin variability, serum ferritin levels, and adverse events/mortality in maintenance hemodialysis patients. Kidney Int. 2014;86(4):845-854. [CrossRef]
- Pérez-García R, Varas J, Cives A, et al. Increased mortality in haemodialysis patients administered high doses of erythropoiesis-stimulating agents: a propensity score-matched analysis. Nephrol Dial Transplant. 2018;33(1). [CrossRef]
- Filho NS, Lages JS, de Araújo Brito DJ, et al. Variability in hemoglobin levels and the factors associated with mortality in hemodialysis patients: A 78-month follow-up study. Int J Environ Res Public Health. 2021;18(3):1-11. [CrossRef]
- Streja E, Park J, Chan TY, et al. Erythropoietin Dose and Mortality in Hemodialysis Patients: Marginal Structural Model to Examine Causality. Int J Nephrol. 2016;2016. [CrossRef]
- Cizman B, Smith HT, Camejo RR, et al. Clinical and Economic Outcomes of Erythropoiesis-Stimulating Agent Hyporesponsiveness in the Post-Bundling Era. Kidney Med. 2020;2(5):589-599.e1. [CrossRef]
- Ebben JP, Gilbertson DT, Foley RN, Collins AJ. Hemoglobin level variability: associations with comorbidity, intercurrent events, and hospitalizations. Clin J Am Soc Nephrol. 2006;1(6):1205-1210. [CrossRef]
- Lin FJ, Zhang X, Huang LS, et al. Impact of hemoglobin variability on cardiovascular mortality in maintenance hemodialysis patients. Int Urol Nephrol. 2018;50(9):1703-1712. [CrossRef]
- Zhao L, Hu C, Cheng J, Zhang P, Jiang H, Chen J. Haemoglobin variability and all-cause mortality in haemodialysis patients: A systematic review and meta-analysis. Nephrology. 2019;24(12):1265-1272. [CrossRef]
- Yang W, Israni RK, Brunelli SM, Joffe MM, Fishbane S, Feldman HI. Hemoglobin variability and mortality in ESRD. J Am Soc Nephrol. 2007;18(12):3164-3170. [CrossRef]
- Fishbane S, Berns JS. Evidence and implications of haemoglobin cycling in anaemia management. Nephrol Dial Transplant. 2007;22(8):2129-2132. [CrossRef]
- Schneider A, Asmus G, Biggar P, et al. Hemoglobin Cycling in Hemodialysis Patients. Nephrol Res Rev. 2010;2(1):1-5. [CrossRef]
- Barbieri C, Mari F, Stopper A, et al. A new machine learning approach for predicting the response to anemia treatment in a large cohort of End Stage Renal Disease patients undergoing dialysis. Comput Biol Med. 2015;61:56-61. [CrossRef]
- Barbieri C, Molina M, Ponce P, et al. An international observational study suggests that artificial intelligence for clinical decision support optimizes anemia management in hemodialysis patients. Kidney Int. 2016;90(2). [CrossRef]
- Bucalo ML, Barbieri C, Roca S, et al. The anaemia control model: Does it help nephrologists in therapeutic decision-making in the management of anaemia? Nefrologia. 2018;38(5). [CrossRef]
- Garbelli M, Bellocchio F, Baro Salvador ME, et al. The use of Anemia Control Model is Associated with Improved Hemoglobin Target Achievement, Lower Rates of Inappropriate Erythropoietin Stimulating Agents and Severe Anemia Among Dialysis Patients. Blood Purif. Published online February 21, 2024. [CrossRef]
- Barbieri C, Bolzoni E, Mari F, et al. Performance of a predictive model for long-Term hemoglobin response to darbepoetin and iron administration in a large cohort of hemodialysis patients. PLoS One. 2016;11(3). [CrossRef]
- Escandell-Montero P, Chermisi M, Martínez-Martínez JM, et al. Optimization of anemia treatment in hemodialysis patients via reinforcement learning. Artif Intell Med. 2014;62(1):47-60. [CrossRef]
- Barbieri C, Molina M, Ponce P, et al. An international observational study suggests that artificial intelligence for clinical decision support optimizes anemia management in hemodialysis patients. Kidney Int. 2016;90(2):422-429. [CrossRef]
- Barbieri C, Molina M, Ponce P, et al. An international observational study suggests that artificial intelligence for clinical decision support optimizes anemia management in hemodialysis patients. Kidney Int. 2016;90(2):422-429. [CrossRef]
- Garbelli M, Bellocchio F, Salvador MEB, et al. The Use of Anemia Control Model Is Associated with Improved Hemoglobin Target Achievement, Lower Rates of Inappropriate Erythropoietin Stimulating Agents, and Severe Anemia among Dialysis Patients. Blood Purif. 2024;53(5):310-322. [CrossRef]
- Garbelli M, Ion Titapiccolo J, Bellocchio F, Stuard S, Brancaccio D, Neri L. Prolonged patient survival after implementation of a continuous quality improvement programme empowered by digital transformation in a large dialysis network. Nephrol Dial Transplant. 2022;37(3):469-476. [CrossRef]
- Pellicer-Valero OJ, Cattinelli I, Neri L, Mari F, Martín-Guerrero JD, Barbieri C. Enhanced prediction of hemoglobin concentration in a very large cohort of hemodialysis patients by means of deep recurrent neural networks. Artif Intell Med. 2020;107:101898. [CrossRef]
- Palmer SC, Navaneethan SD, Craig JC, et al. Meta-analysis: Erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med. 2010;153(1):23-33. [CrossRef]
- Solomon SD, Uno H, Lewis EF, et al. Erythropoietic Response and Outcomes in Kidney Disease and Type 2 Diabetes. N Engl J Med. 2010;363(12):1146-1155. [CrossRef]
- Besarab A, Bolton WK, Browne JK, et al. The Effects of Normal as Compared with Low Hematocrit Values in Patients with Cardiac Disease Who Are Receiving Hemodialysis and Epoetin. N Engl J Med. 1998;339(9):584-590. [CrossRef]
- Pfeffer MA, Burdmann EA, Chen CY, et al. A Trial of Darbepoetin Alfa in Type 2 Diabetes and Chronic Kidney Disease. N Engl J Med. 2009;361(21):2019-2032. [CrossRef]
- Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-.alpha dose and achieved hemoglobin outcomes. Kidney Int. 2008;74(6):791-798. [CrossRef]
- Bachmann F, Koch G, Pfister M, Szinnai G, Schropp J. OptiDose: Computing the Individualized Optimal Drug Dosing Regimen Using Optimal Control. J Optim Theory Appl. 2021;189(1):46-65. [CrossRef]
- Yang JY, Lee TC, Liao WT, Hsu CC. Multi-head self-attention mechanism enabled individualized hemoglobin prediction and treatment recommendation systems in anemia management for hemodialysis patients. Heliyon. 2023;9(2):e12613. [CrossRef]
- Rogg S, Fuertinger DH, Volkwein S, Kappel F, Kotanko P. Optimal EPO dosing in hemodialysis patients using a non-linear model predictive control approach. J Math Biol. 2019;79(6-7):2281-2313. [CrossRef]
- Jörg DJ, Fuertinger DH, Kotanko P. Mechanisms of hemoglobin cycling in anemia patients treated with erythropoiesisstimulating agents. PLoS Comput Biol. 2023;19(1). [CrossRef]
- Kalicki RM, Uehlinger DE. Red Cell Survival in Relation to Changes in the Hematocrit: More Important than You Think. Blood Purif. 2008;26(4):355-360. [CrossRef]
- Gilbertson DT, Hu Y, Peng Y, Maroni BJ, Wetmore JB. Variability in hemoglobin levels in hemodialysis patients in the current era: A retrospective cohort study. Clin Nephrol. 2017;88(5):254-263. [CrossRef]
- Portolés J, Martín L, Broseta JJ, Cases A. Anemia in Chronic Kidney Disease: From Pathophysiology and Current Treatments, to Future Agents. Front Med. 2021;8(March):1-14. [CrossRef]
- Gaweda AE, Goldsmith LJ, Brier ME, Aronoff GR. Iron, inflammation, dialysis adequacy, nutritional status, and hyperparathyroidism modify erythropoietic response. Clin J Am Soc Nephrol. 2010;5(4):576-581. [CrossRef]
- Karimi Z, Raeisi Shahraki H, Mohammadian-Hafshejani A. Erythropoiesis-stimulating agents and cardiovascular mortality: A systematic review and meta-analysis of 17 studies and 372,156 hemodialysis patients. Int J Cardiol Cardiovasc risk Prev. 2023;19:200220. [CrossRef]
- Suttorp MM, Hoekstra T, Mittelman M, et al. Treatment with high dose of erythropoiesis-stimulating agents and mortality: Analysis with a sequential Cox approach and a marginal structural model. Pharmacoepidemiol Drug Saf. 2015;24(10):1068-1075. [CrossRef]
- Weinhandl ED, Gilbertson DT, Collins AJ. Association of mean weekly epoetin alfa dose with mortality risk in a retrospective cohort study of medicare hemodialysis patients. Am J Nephrol. 2011;34(4):298-308. [CrossRef]
- Streja E, Park J, Chan TY, et al. Erythropoietin Dose and Mortality in Hemodialysis Patients: Marginal Structural Model to Examine Causality. Int J Nephrol. 2016;2016. [CrossRef]
- Pérez-García R, Varas J, Cives A, et al. Increased mortality in haemodialysis patients administered high doses of erythropoiesis-stimulating agents: a propensity score-matched analysis. Nephrol Dial Transplant. 2018;33(1):690-699. [CrossRef]
- Handelman GJ, Kotanko P, Cisternas MG, et al. Hospitalization and mortality in hemodialysis patients: Association with hemoglobin variability. Blood Purif. 2013;35(4):247-257. [CrossRef]
- Eckardt KU, Kim J, Kronenberg F, et al. Hemoglobin variability does not predict mortality in european hemodialysis patients. J Am Soc Nephrol. 2010;21(10):1765-1775. [CrossRef]
- Goodkin DA, Fuller DS, Robinson BM, et al. Naturally occurring higher hemoglobin concentration does not increase mortality among hemodialysis patients. J Am Soc Nephrol. 2011;22(2):358-365. [CrossRef]
- Ofsthun N, LaBrecque J, Lacson E, Keen M, Lazarus JM. The effects of higher hemoglobin levels on mortality and hospitalization in hemodialysis patients. Kidney Int. 2003;63(5):1908-1914. [CrossRef]
- Weinhandl ED, Peng Y, Gilbertson DT, Bradbury BD, Collins AJ. Hemoglobin variability and mortality: Confounding by disease severity. Am J Kidney Dis. 2011;57(2):255-265. [CrossRef]
- Morena M, Jaussent A, Chalabi L, et al. Treatment tolerance and patient-reported outcomes favor online hemodiafiltration compared to high-flux hemodialysis in the elderly. Kidney Int. 2017;91(6):1495-1509. [CrossRef]
- Ok E, Asci G, Toz H, et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF Study. Nephrol Dial Transplant. 2013;28(1):192-202. [CrossRef]
- Blankestijn PJ, Vernooij RWM, Hockham C, et al. Effect of Hemodiafiltration or Hemodialysis on Mortality in Kidney Failure. N Engl J Med. 2023;389(8):700-709. [CrossRef]
- Mitchell CR, Hornig C, Canaud B. Systematic review to compare the outcomes associated with the modalities of expanded hemodialysis (HDx) versus high-flux hemodialysis and/or hemodiafiltration (HDF) in patients with end-stage kidney disease (ESKD). Semin Dial. 2023;36(2):86-106. [CrossRef]
| Exposure Groups | Significance* | ||||
|---|---|---|---|---|---|
| Characteristics |
Whole Sample (n= 20209) |
ACM Group (n=3108) |
Reference Group (n= 17101) |
p-value | Effect Size |
| N (%), mean (St.D), or Median (IQR) | |||||
| Age | 65.3 (14.5) | 67.8 (14.4) | 64.8 (14.4) | <0.001 | 0.0107 |
| Men | 11962 (59.2) | 1945 (62.6) | 10017 (58.6) | <0.001 | 0.0234 |
| BMI | 26.8 (6.5) | 26.4 (5.4) | 26.9 (6.7) | <0.001 | 0.0015 |
| Dialysis Vintage (years) | 2,03 (4,7) | 2,8 (5,26) | 1,87 (4,59) | <0.001 | 0,2672 |
| Vascular Access | <0.001 | 0.1280 | |||
| Arteriovenous Fistula | 5578 (28.2) | 592 (19.0) | 4986 (29.9) | ||
| Catheter or Graft | 14188 (71.8) | 2517 (81.0) | 11671 (70.1) | ||
| Missing | 419 (2.1) | 0 (0) | 419 (2.5) | ||
| Kt/V | 1.6 (0.4) | 1.9 (0.4) | 1.6 (0.4) | <0.001 | 0.2554 |
| Treatment Time (minutes) | 241.8 (13.1) | 243.7 (13.1) | 241.5 (13.1) | <0.001 | 0.0169 |
| Hemoglobin (g/dL) | 11.1 (1.2) | 11.4 (1.1) | 11.0 (1.2) | <0.001 | 0.0188 |
| Albumin (g/dL) | 3.9 (0.4) | 4.0 (0.5) | 3.9 (0.4) | <0.001 | 0.0075 |
| Ferritin (ng/mL) | 558.8 (436.5) | 567.0 (349.7) | 557.3 (450.8) | 0.176 | 0.0037 |
| Phosphate (mg/dL) | 4.7 (1.4) | 4.3 (1.1) | 4.8 (1.4) | <0.001 | 0.0269 |
| Leukocytes (10^3/µL) | 7883.2 (41545.4) | 6582.5 (1824.9) | 8123.0 (45203.6) | <0.001 | 0.0286 |
| C-Reactive Protein (mg/L) | 13.6 (22.3) | 12.1 (17.0) | 13.8 (23.1) | <0.001 | 0.0030 |
| Transferrin Saturation (%) | 29.9 (12.8) | 32.2 (11.9) | 29.5 (12.9) | <0.001 | 0.0007 |
| MCV (fL) | 94.3 (6.5) | 95.1 (5.9) | 94.1 (6.6) | <0.001 | 0.0288 |
| MCH (pg/cell) | 32.9 (43.1) | 32.9 (0.8) | 32.9 (46.9) | 0.874 | 0.0044 |
| Serum Sodium (mmol/L) | 138.1 (4.0) | 138.3 (2.6) | 138.1 (4.1) | 0.0042 | 0.0042 |
| Serum Potassium (meq/L) | 4.9 (0.9) | 5.0 (0.6) | 4.9 (0.9) | 0.0066 | 0.0066 |
| Serum Calcium (mg/dL) | 8.8 (1.3) | 9.0 (0.6) | 8.8 (1.4) | 0.0060 | 0.0059 |
| Cerebrovascular disease | 2750 (13.6) | 486 (15.6) | 2264 (13.2) | <0.001 | 0.0401 |
| Chronic pulmonary disease | 2197 (10.9) | 398 (12.8) | 1799 (10.5) | <0.001 | 0.0417 |
| Congestive heart failure | 4376 (21.7) | 696 (22.4) | 3680 (21.5) | 0.287 | 0.0075 |
| Connective tissue disorder | 332 (1.6) | 43 (1.4) | 289 (1.7) | 0.246 | 0.0105 |
| Coronary artery disease | 4199 (20.8) | 621 (20.0) | 3578 (20.9) | 0.243 | 0.0152 |
| Dementia | 313 (1.5) | 53 (1.7) | 260 (1.5) | 0.491 | 0.0115 |
| Diabetes without complication | 6155 (30,5) | 214 (6.9) | 871 (5.1) | <0.001 | 0.0052 |
| Diabetes with organ damage | 5070 (25.1) | 921 (29.6) | 4149 (24.3) | <0.001 | 0.0560 |
| Hemiplegia | 157 (0.8) | 20 (0.6) | 137 (0.8) | 0.418 | 0.0157 |
| Mild liver disease | 2002 (9.9) | 385 (12.4) | 1617 (9.5) | <0.001 | 0.0419 |
| Moderate/severe liver disease | 111 (0.5) | 16 (0.5) | 95 (0.6) | 0.880 | 0.0179 |
| Peptic ulcer disease | 1026 (5.1) | 152 (4.9) | 874 (5.1) | 0.638 | 0.0125 |
| Peripheral Vascular Disease | 3769 (18.7) | 740 (23.8) | 3029 (17.7) | <0.001 | 0.0506 |
| Exposure Groups | Significance* | |||||
|---|---|---|---|---|---|---|
| Characteristics | Whole Sample (n= 5051) |
ACM Group (n=1952) |
ACM unmatched (n=1167) |
Reference Group (n= 1952) |
p-value | Effect Size |
| N (%) or mean (St.D) | ||||||
| Age | 66,1 (14,7) | 67,6 (14,5) | 68,3 (14,3) | 63,3 (14,8) | <0.001 | 0.0413 |
| Men | 3165 (62.7) | 1243 (64.0) | 701 (60.1) | 1221 (62.9) | 0.09 | 0.0111 |
| BMI | 26.7 (5.6) | 26.4 (5.3) | 26.3 (5.5) | 27.2 (6.0) | <0.001 | 0.0010 |
| Dialysis Vintage | 2.03 (4.59) | 1.25 (2.98) | 5.29 (5.51) | 1.05 (3.28) | <0.001 | 0.0004 |
| Vascular Access | <0.001 | 0.1410 | ||||
| Catheter or Graft | 1241 (24.8) | 420 (21.6) | 172 (14.7) | 649 (34.2) | ||
| Arteriovenous Fistula | 3766 (75.2) | 1522 (78.4) | 995 (85.3) | 1249 (65.8) | ||
| Missing | 44 (0.9) | 0 (0) | 0 (0) | 44 (2.3) | ||
| Kt/V | 1.8 (0.4) | 1.9 (0.4) | 1.9 (0.4) | 1.5 (0.4) | <0.001 | 0.3300 |
| Treatment Time (minutes) | 242.2 (13.3) | 243.9 (11.8) | 243.2 (15.0) | 239.8 (13.2) | <0.001 | 0.0170 |
| Hemoglobin (g/dL) | 11.3 (1.0) | 11.3 (0.9) | 11.6 (1.2) | 11.2 (0.9) | <0.001 | 0.0061 |
| Albumin (g/dL) | 3.9 (0.4) | 3.9 (0.5) | 4.0 (0.4) | 3.9 (0.4) | <0.001 | 0.0000 |
| Ferritin (ng/mL) | 520.5 (371.9) | 561.0 (362.0) | 577.0 (328.2) | 443.4 (394.3) | <0.001 | 0.0460 |
| Phosphate (mg/dL) | 4.4 (1.1) | 4.3 (1.1) | 4.2 (1.1) | 4.6 (1.1) | <0.001 | 0.0358 |
| Leukocytes (10^3/µL) | 6738.9 (2148.4) | 6685.2 (1833.5) | 6411.6 (1798.3) | 6992.9 (2569.3) | <0.001 | 0.0094 |
| C-Reactive Protein (mg/L) | 11.8 (16.6) | 12.4 (18.0) | 11.8 (15.2) | 11.3 (16.1) | 0.213 | 0.0020 |
| Transferrin Saturation (%) | 30.3 (11.8) | 31.8 (11.6) | 32.9 (12.3) | 27.6 (11.1) | <0.001 | 0.0641 |
| MCV (fL) | 94.3 (6.1) | 95.2 (5.9) | 94.9 (5.9) | 92.9 (6.3) | <0.001 | 0.0663 |
| MCH (pg/cell) | 32.8 (1.0) | 32.9 (0.9) | 32.9 (0.8) | 32.8 (1.1) | <0.001 | 0.0049 |
| Serum Sodium (mmol/L) | 138.1 (3.1) | 138.2 (2.6) | 138.4 (2.7) | 137.9 (3.7) | <0.001 | 0.0044 |
| Serum Potassium (meq/L) | 4.9 (0.6) | 4.9 (0.6) | 5.0 (0.6) | 4.8 (0.6) | <0.001 | 0.0137 |
| Serum Calcium (mg/dL) | 8.9 (0.6) | 8.9 (0.6) | 9.0 (0.7) | 8.8 (0.6) | <0.001 | 0.0137 |
| Cerebrovascular disease | 736 (14.6) | 296 (15.2) | 192 (16.5) | 248 (12.8) | 0.011 | 0.0348 |
| Chronic pulmonary disease | 594 (11.8) | 261 (13.4) | 140 (12.0) | 193 (9.9) | 0.003 | 0.0535 |
| Congestive heart failure | 1117 (22.1) | 425 (21.9) | 275 (23.6) | 417 (21.5) | 0.377 | 0.0043 |
| Connective tissue disorder | 74 (1.5) | 25 (1.3) | 18 (1.5) | 31 (1.6) | 0.703 | 0.0108 |
| Coronary artery disease | 964 (19.1) | 362 (18.6) | 260 (22.3) | 342 (17.6) | 0.005 | 0.0127 |
| Dementia | 84 (1.7) | 37 (1.9) | 16 (1.4) | 31 (1.6) | 0.507 | 0.0098 |
| Diabetes without complication | 332 (6.6) | 141 (7.3) | 73 (6.3) | 118 (6.1) | 0.291 | 0.0226 |
| Diabetes with organ damage | 1454 (28.8) | 612 (31.5) | 311 (26.6) | 531 (27.3) | 0.003 | 0.0450 |
| Hemiplegia | 31 (0.6) | 9 (0.5) | 11 (0.9) | 11 (0.6) | 0.239 | 0.0036 |
| Mild liver disease | 549 (10.9) | 204 (10.5) | 179 (15.3) | 166 (8.5) | <0.001 | 0.0324 |
| Moderate/severe liver disease | 30 (0.6) | 11 (0.6) | 5 (0.4) | 14 (0.7) | 0.578 | 0.0064 |
| Peptic ulcer disease | 239 (4.7) | 88 (4.5) | 66 (5.7) | 85 (4.4) | 0.232 | 0.0025 |
| Peripheral Vascular Disease | 1087 (21.5) | 424 (21.8) | 318 (27.2) | 345 (17.8) | <0.001 | 0.0502 |
| Group | Incidence Rate (events/100 person-years) |
Incidence Rate Difference (events/100 person-years) |
p-value |
|---|---|---|---|
| Before Matching | |||
| Whole Sample | 80.9 (95% CI: 79.6–82.3) | - | - |
| ACM Group | 71.3 (95% CI: 68.0–74.6) | - | - |
| Reference Group | 82.6 (95% CI: 81.2–84.2) | 11.4 (95% CI: 7.6–15.2) | <0.001 |
| After Matching | |||
| Matched Sample | 80.9 (95% CI: 77.8–84.1) | - | - |
| ACM Group | 74.3 (95% CI: 70.2–78.7) | - | - |
| Reference Group | 86.7 (95% CI: 82.4–91.6) | 12.6 (95% CI: 6.3–18.9) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





