Submitted:
18 September 2024
Posted:
19 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
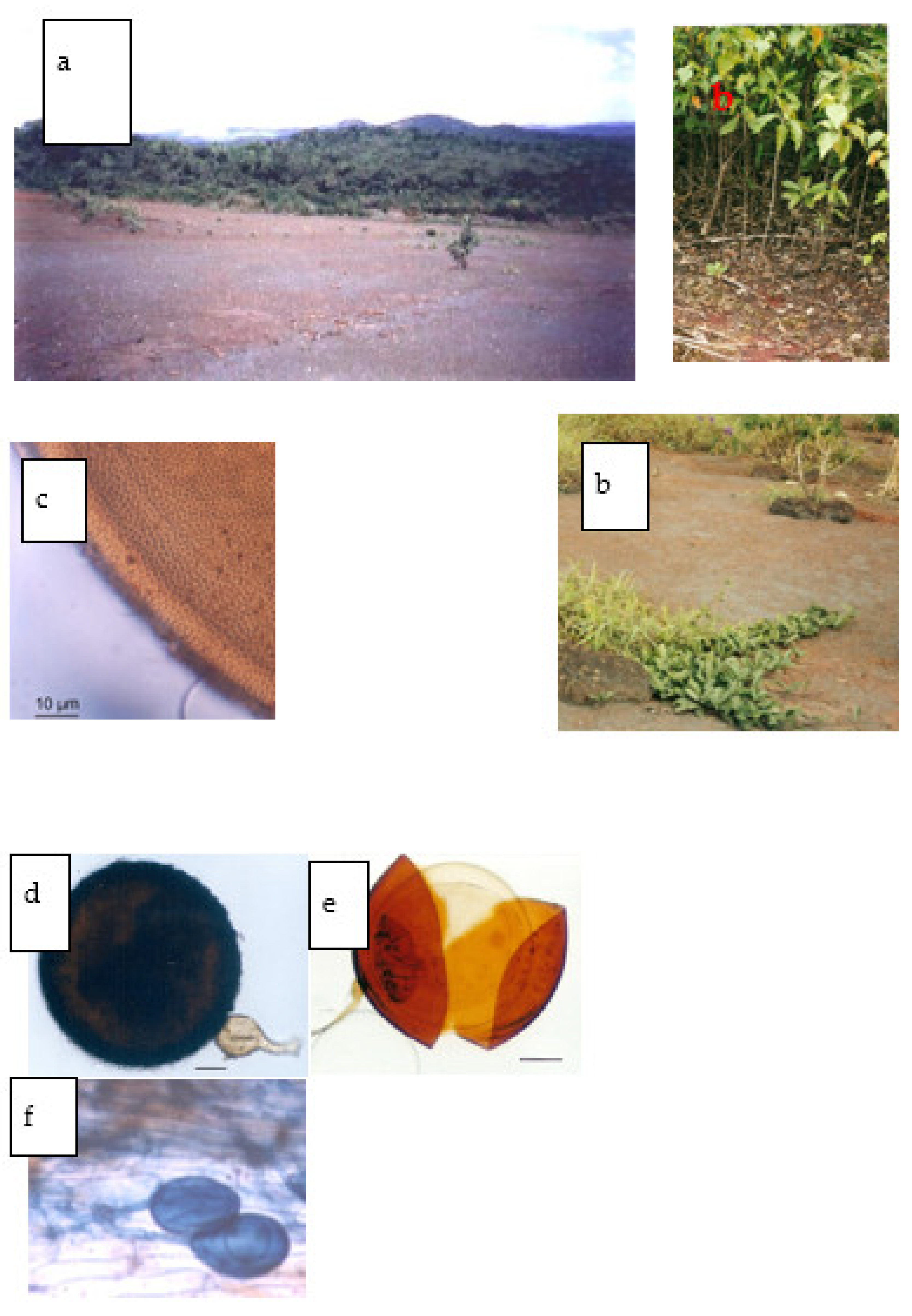
2. Material and Methods
2.1. Experimental Design
2.2. Site and Soil Characteristics
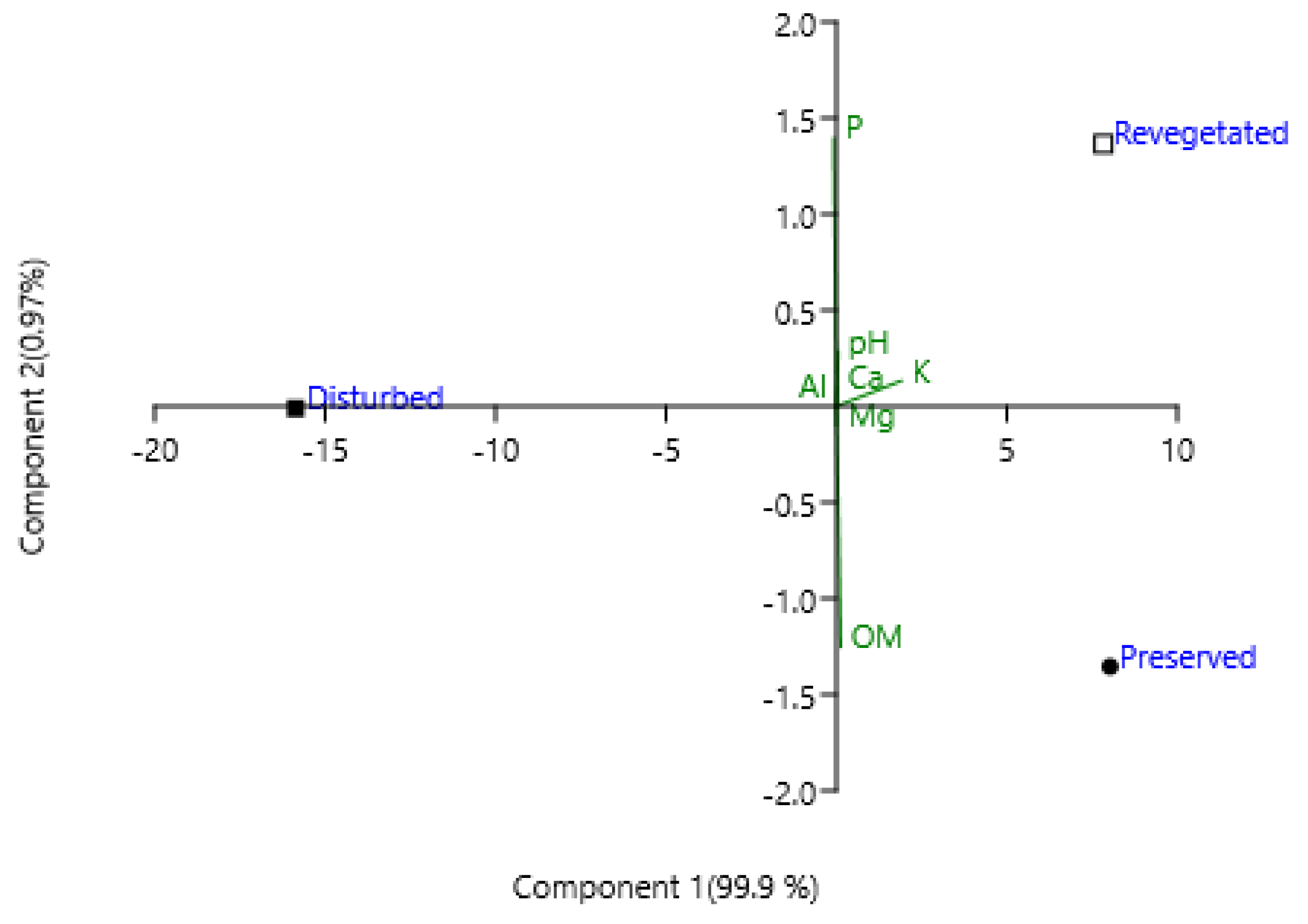
3. Results
| AMF plant restoration | Country | AMF | Reference |
|---|---|---|---|
| History of restoration | Review | [1] | |
| Restoration of riparian forest. | Brazil | Eight species | [5] |
| Soil microbiome | Review | NI | [28] |
| Riparian forest | Brazil-Review | NI | [23] |
| Mined ecosystems | Brazil | Twenty-one species | [34] |
| Mined ecosystems | Brazil | seven species | [30] |
| AMF managed environments | Review | NI | [31] |
| Mycorrhizal inoculation | [29] | ||
| Brazil | Succession of AMF | [34] | |
| Grassy biomes | NI | [20] | |
| Site | pH | Al3+ | Mg2+ | K | P (dag kg-1 | OM (dag kg-1) | Ca+2 |
|---|---|---|---|---|---|---|---|
| Preserved | 5.4 | 0.86 | 0.16 | 31.7 | 2.0 | 2.5 | 0.46 |
| Revegetated | 5.8 | 0.2 | 0.08 | 8 | 4.0 | 0.71 | 0.3 |
| Disturbed | 4.9 | 0.19 | 0.18 | 5.14 | 10 | 0.04 | 0.16 |
| Site | Acaulospora delicata | Acaulospora laevis | Acaulospora mellea | Acaulospora spinosa | Gigaspora margarita | Scutellospora cerradensis | Scutellospora verrucosa |
|---|---|---|---|---|---|---|---|
| Preserved | + | + | + | + | + | - | - |
| Revegetated | - | - | + | + | + | + | + |
| Disturbed | - | - | - | - | - | - | - |
4. Discussion
5. Conclusions
References
- Vaughn, K. J., Porensky, L. M., Wilkerson, M. L., Balachowski, J., Peffer, E., Rig nos, C. & Young, T. P. (2010) Restoration Ecology. Nature Education Knowledge 3,10, 66.
- Chazdon, R.L. 2008. Beyond deforestation: Restoring forests and ecosystem services on degraded lands. Science, 320, 1458–1460. [CrossRef]
- Crouzeilles, R. , Ferreira, MS, Chazdon, R.L. Sansevero, J.B. B., Monteiro, L,. Latawiec, A., Strassburg, BN. Ecological restoration success is higher for natural regeneration than for active restoration in tropical forests. Science Advances, 2017 3, 11. [CrossRef]
- Harris, J. 2009. Soil Microbial Communities and Restoration Ecology: Facilitators or Followers? Science, 325, 573–574. [CrossRef]
- Pagano, MC; Correa, EJA; Lugo, MA.; Duarte, NF. 2022. Diversity and benefits of arbuscular mycorrhizae in restored riparian plantations. Diversity 14, 11, 938. [CrossRef]
- Holl, K.D., Aide, T.M. When and where to actively restore ecosystems? Forest Ecol. and Manage., 2011, 1558-1563. [CrossRef]
- Kardol, P., Wardle, D.A. 2010. How understanding aboveground-belowground linkages can assist restoration ecology. Trends in Ecology and Evolution, 25, 670–679. [CrossRef]
- Callaham, M., Rhoades, C. R., Heneghan, L. 2008. A striking profile: soils knowledge in restoration management and science. Restoration Ecology, 16, 604–607. [CrossRef]
- Yadav, A. K., Ahmad, R 2023.Rhizosphere engineering for semiarid tropics: prospects and bottlenecks, Review 2023-09-22. [CrossRef]
- Albrecht, M.A., Guerrant E.O., Maschinski J., Kennedy, K.L. 2011. A long-term view of rare plant reintroduction. Biological Conservation, 144, 2557–2558. [CrossRef]
- Larjavaara A Review on Benefits and Disadvantages of Tree Diversity. M. The Open Forest Science Journal, 2008, 1, 24-26,2005, 310 Science, 1629-1632 www.sciencemag.org.
- Margules, C.R. Pressey R. L. Systematic conservation planning. Nature, 405 (2000), 243–253.20. [CrossRef]
- Fonseca, C.R. Venticinque, E. M. Biodiversity conservation gaps in Brazil: A role for systematic conservation planning Perspectives in Ecology and Conservation, 16, 2, 2018, 61-67.63. [CrossRef]
- Aronson, J., Brancalion, P. H. S., Durigan, G., Rodrigues, R. R., Engel, V. L. et al. 2011. What Role Should Government Regulation Play in Ecological. Restoration? Ongoing Debate in São Paulo State, Brazil. Restoration Ecol, 19, 6, 690–695. [CrossRef]
- Chaer, G. M., Resende, A.S., Campello, E.F.C., de Faria, S.M., Boddey, R.M. 2011. Nitrogen-fixing legume tree species for the reclamation of severely degraded lands in Brazil. Tree Physiol., 31, 139–149. [CrossRef]
- Rodrigues, RR, Lima, RAF, Gandolfi, S. André G. Nave. On the restoration of high diversity forests: 30 years of experience in the Brazilian Atlantic Forest.
- 18, Rillig, MC, Wright, SF, Eviner, VT. 2002. The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: comparing effects of five plant species. Plant soil, 2002. [CrossRef]
- Fynn, R.W.S.; Wragg, D.; Morris, C.D.; Kirkman, K.P.; Naiken, J. Vegetative traits predict grass species’ invasiveness and the invasibility of restored grassland. Afr. J. Range For. Sci. 2009, 26, 59–68. [CrossRef]
- Buisson, A Fidelis, Overbeck, G.E., I. B. Schmidt, G Durigan, G., Young T. P., Swanni T. Alvarado, André J. Arruda, Sylvain Boisson, William Bond10, A et al. A research agenda for the restoration of tropical and subtropical grasslands and savannas 2020 Restoration Ecology, 2021, 29 (S1), pp. e13292. ff10.1111/rec.13292ff. ffhal-02982495f. [CrossRef]
- Guerra, A., L., Reis,K. L. K., Borges,G. F. L., Alves Ojeda, PT., Pineda, DAM., Camila Oliveira Miranda, C. O., Maidana, DPF, TMR. Patrícia Sayuri Shibuya, C.M. Marques, M. Laurance, SGD, Garcia, LC.2020. Ecological restoration in Brazilian biomes: Identifying advances and gaps. Forest Ecology and Management, 2020, 117802. [CrossRef]
- Koziol, L, Bever,J D. The missing link in grassland restoration: arbuscular mycorrhizal fungi inoculation increases plant diversity and accelerates succession.
- Perring, M. P., R. J. Standish, J. N. Price, M. D. Craig, T. E. Erickson, K. X. Ruthrof, A. S. Whiteley, L. E. Valentine, and R. J. Hobbs. 2015. Advances in restoration ecology: rising to the challenges of the coming decades, Ecosphere 6(8):131. Advances in restoration ecology: Rising to the challenges of the coming decades. [CrossRef]
- Braghirolli, F.L., Sgrott A.F., Pescador R., Uhlmann A., Stürmer, S.L. 2012. Arbuscular Mycorrhizal Fungi in Riparian Forest Restoration and Soil Carbon Fixation. R. Bras. Ci. Solo, 36, 733-743. [CrossRef]
- Nannipieri, P., Ascher, J.; Ceccherini, M.T.; Landi, L., Pietramellara, G., Renella, G. 2003. Microbial diversity and soil functions. Eur. J. Soil Sci., 54, 655–670. [CrossRef]
- Subramanian, K.S., Charest, C., Dwyer, L.M., Hamilton, R.I., 1995. Arbuscular mycorrhizas and water relations in maize under drought stress at tasseling. New Phytologist 129, 643–650. [CrossRef]
- Rillig, M.C., Mummey, D.L., 2006. Mycorrhizas and soil structure. New Phytologist, 171, 41–53. [CrossRef]
- Chaudhary, B., Griswold, M. 2001. Mycorrhizal Fungi - A Restoration Practitioner’s Point of View. Ecesis, Newsletter of the California Society for Ecological Restoration – SERCAL. p. 4.
- Jansson, J. K., McClure, R., Egbert., R.G. “Soil microbiome engineering for sustainability in a changing environment.” Nature Biotechnology 41.12 (2023): 1716-1728. 28. [CrossRef]
- Neuenkamp, L., Prober, S.M., Price, J.N, Zobel, M. M., Standish, R.J. 2019. Benefits of mycorrhizal inoculation to ecological restoration depend on plant functional type, restoration context and time. Fungal Ecology,40,2019, 140-149. [CrossRef]
- Matias, SR, MC Pagano, FC Muzzi, CA Oliveira, AA Carneiro, SN Horta, Effect of rhizobia, mycorrhizal fungi and phosphate-solubilizing microorganisms in the rhizosphere of native plants used to recover an iron ore area in Brazil . European Journal of Soil Biol 45 (3), 259-266. [CrossRef]
- Asmelash, F, Bekele, T Birhane, E. The Potential Role of Arbuscular Mycorrhizal Fungi in the Restoration of Degraded Lands. Front. Microbiol., 7.
- Jansson, JK, Mc Clure, R. Egbert, RG.2023. Soil microbiome engineering for sustainability in a changing environment Nature biotechnology Review article. [CrossRef]
- Gelviz-Gelvez,S. M. Numa P. Pavón, Patricia Illoldi-Rangel, Claudia Ballesteros-Barrera, Ecological niche modeling under climate change to select shrubs for ecological restoration in Central Mexico, Ecological Engineering, 74,2015, 302-309. [CrossRef]
- Hoeksema, JD, V. Bala Chaudhary, VB., Gehring,C A. S, Johnson, NC et al. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Letters (2010) 13: 394–407. [CrossRef]
- Souza, RG. , Silva, DKA , Oliveira,JRG., Goto,BT., Silva,FS, EV.S.B. Sampaio Maia,L.C. Use of mycorrhizal seedlings on recovery of mined dunes in northeastern Brazil Pedobiologia, 55, 6, 2012, 303-309. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





