Introduction
The morbidity of gastric cancer(GC) has reached the fifth place in all tumors, and has become the third leading cause of cancer related death. The high-risk factors for gastric cancer mainly include age at the pylorus, high salt intake, Helicobacter pylori infection, and low intake of fruits and vegetables.[
1] The standard treatment for GC patients in traditional chemotherapy regimens includes a combination of platinum, fluoropyrimidine, and paclitaxel with two or three drugs. However, the median survival of sufferers with late stage gastric cance is fewer than 2 years, and new treatment methods need to be introduced. The chief mechanism of immunotherapy is to enhance body’s own immune response and make it fight against tumor cell. Immune checkpoint inhibitors (ICIs) have become an indispensable part of treatment plans for certain cancers, including GC, and have achieved good therapeutic effects [
5,
6]. Although immunotherapy has achieved good efficacy in treating cancer patients, there are still many patients who experience tumor progression during immunotherapy, and immunotherapy resistance has become a huge challenge affecting the effectiveness of immunotherapy. However, the complete mechanism has not been sufficiently explored, it is widely believed that the joint action of immune cells and immune molecules, as well as the heterogeneity of tumor cells themselves, are significant components causing to immune therapy resistance [
7]. Therefore, the lurking mechanisms of resistance to GC immunotherapy need to be clarified. It is also crucial to search for new biomarkers that predict the emergence of immune therapy resistance. Through the review, we discuss the possible mechanisms of immunotherapy resistance in GC patients, biomarkers for predicting immune therapy resistance, and methods that can be applied clinically to improve immune therapy efficacy.
Immunotherapies for GC
Immune Checkpoint Inhibitors (ICIs)
Usually, when T cell receptors (TCRs) bind to major histocompatibility complexes (MHC) delivered by antigen-presenting cells (APCs), CD8+ T cells are activated and transformed into cells that can effectively kill tumor cells. Immunosuppression can protect normal cells during immune response activation, preventing the body from prolonged inflammation and autoimmune damage. The key link of immunosuppression is the immediate immune checkpoint. PD-1 is widely present on the cell membranes of almost all types of immune cells, are the most common immune checkpoints in T cell sensitization. While it combines to PD-L1, the inhibitory signaling pathway of T lymphocytes is switched on and the function of T lymphocytes is inhibited [
8,
9,
10]. Tumor cells expressing PDL1 can can be protected from cytotoxic T lymphocyte lysis. While PD-L1 is involved, CD80 can act as a receptor for transmitting inhibitory signals and be expressed in activated T cells, ultimately leading to T cell dysfunction. Moreover, prolonged stimulation of tumor antigens can upregulate negative regulatory factors such as PD-1 in T lymphocytes, ultimately leading to their functional failure [
11,
12,
13].
Interdicting the PD-1/PD-L1 signaling pathway is a promising immunotherapy for treating tumors. Clinical experiment have proven that nivolumab (anti-PD-1 antibody) can effectively improve median overall survival ( 5.12 months vs. 4.14 months) and median disease progression time (1.61 months vs. 1.45 months) in gastric cancer patients compared to placebo [
14]. In the Phase 2 clinical trial, 223 patients underwent imaging evaluation after receiving regular treatment with Pembrolizumab (anti-pd-1 monoclonal antibody), 95 cases (42.6%) showed tumor volume reduction, with a median response period of 8.4 months [
15]. Other studies have also proven that, compared with chemotherapy, the use of monotherapy avilumab (anti-PD-1 antibody) as third line treatment in GC sufferers didn’t lead to significant improvements in median OS (4.6 months vs 5.0 months) or median PFS (1.4 months vs 2.0 months). However, in the avelumab group, only 90 cases (48.9%) experienced any level of treatment-related adverse events (TRAEs), while in the group receiving chemotherapy alone, 131 cases (74.0%) of patients experienced TRAEs. The avilumab shows more controllable safety than chemotherapy [
16]. In another clinical study, this combination drug group of Toripalimab (anti-PD-1 monoclonal antibody) and chemotherapy showed higher efficacy than the monotherapy group. The median objective response rate (66.7% vs. 12.1%) and median disease progression time (5.8 months vs. 1.9 months) of the joint therapy group were evidently better than those of the immunotherapy group alone [
17]. Proving that the joint application of chemotherapy and anti-PD-1 monoclonal antibodies may provide better prognosis for the treatment of GC patients.
Countless clinical researchs have proven that the use of ICIs targeting PD-1 or PD-L1 may have better efficacy as well as less adverse reactions than traditional chemotherapy alone. ICIs have demonstrated their superiority and may be a prospective option for advanced GC patients.
Chimeric Antigen Receptor -T Cell Therapy (CAR-T)
Tumor cells have high immunogenicity, and the key to activating immune response to combat tumors is the specific expression of tumor associated antigens (TAA). Based on TAA molecules, tumor cells can be recognized and attacked by T lymphocyte. Chimeric antigen receptor (CAR), the key of CAR-T, enables T lymphocyte to recognize antigens in a mode that is not limited by MHC, thereby enabling T cells to recognize more extensive target antigens. The extracellular target hinge region, transmembrane domain, antigen binding domain, and intracellular signaling domain constitute CAR. Some fragments of the antigen binding domain target the extracellular surface TAA, while CAR imparts target antigen specificity through the antigen binding domain. CAR anchors itself to the cell membrane of T cells through a transmembrane domain, and the expression level and stability of CAR are mainly determined by the transmembrane domain [
18,
19].
CAR-T has become an important method for combating the progression of hematological malignancies, such as B-cell acute lymphoblastic leukemia, B-cell non Hodgkin lymphoma, chronic lymphocytic leukemia, and Hodgkin lymphoma [
20]. Lately, CAR-T immunotherapy has also been considered as a possible treatment option and attempted to be applied in combating the progression of solid tumors, including GC. CLDN18.2 is the proteide that exhibits high expression levels on the cell membrane of certain gastric cancer cells. Research has shown that about 10% of GC sufferers respond to therapy with CLDN18.2 specific monoclonal antibody IMAB362, and it has no significant toxicity to normal tissues outside the stomach. Therefore, we can consider CLDN18.2 as the therapeutic with enormous potential target for CAR-T therapy in the anticancer therapy of GC [
21,
22]. Jiang et al. have successfully developed humanized CLDN18.2 specific HU8E5 and HU8E5-2i single chain fragment variables (scFv). And use it as a targeted fragment to develop CLDN18.2 specific CAR-T cells. This CAR-T cells can partially or completely eliminate tumors in the CLDN18.2 positive gastric cancer model (P<0.001), persist in the body and effectively infiltrate tumor tissues, without significant harmful effects on normal organs [
23].
Although many preclinical experiments have proven great potential for CAR-T cells in treating GC, clinical experimental of CAR-T cell therapy in GC patients have not yet begun on a large scale. There are still many challenges to overcome in applying CAR-T cell therapy to the treatment of advanced GC patients.
Tumor Vaccines
Protective vaccination is one of the most effective health measures for preventing infectious diseases, while developing therapeutic vaccines for cancer is clearly more attractive. Cancer vaccines are developed based on antigens that can trigger selective immune responses against cancer cells. At present, the targets of tumor therapeutic vaccines are primarily differentiation antigens, cancer testicular antigens, and/or overexpression antigens, and their applications are extremely limited [
24]. Most cancers have neoantigen specific T cells, which provide specific and highly immunogenic targets for vaccine administration due to somatic mutations [
25]. Previous studies have shown that the most common antigens, such as MAGE-A, CTAG2, NY-ESO-1 and TTK are in the cytomembrane of GC cells [
26]. The CD8+T cells stimulated by these antigens can specifically react with the antigen, so certain tumor cells can be specifically killed by these CD8+T cells.
Some kinds of tumor vaccines have been applied to clinical experiment. MAGE-3 peptide is a tumor vaccine based on the MAGE gene. In experiments, it was used to treat 12 cases of advanced gastrointestinal cancer, including 6 cases of gastric cancer, without any toxic side effects. Ultimately, only four patients experienced peptide specific cytotoxic reactions after vaccination. The physical condition of 4 patients has improved. The tumor markers of 7 patients decreased. Meanwhile, imaging examinations of three patients showed mild tumor regression. Experiments have shown that vaccination with MAGE-3 peptide vaccine may be a safe treatment option for gastrointestinal cancer and has great potential [
27]. In Liu et al.‘s study, the disease-free survival of GC patients can be effectively prolonged through PNVAC (a personalized neoantigen vaccine without complex chemical attachments) treatment PNVAC can induce CD4+ T lymphocytel response, CD8+T lymphocyte response, and memory T lymphocyte activation. Moreover, this immune response is long-lasting and can still be clearly observed one year after vaccination [
28].
Although only a small number of tumor vaccines have been used in clinical experiment for GC, we firmly believe that tumor vaccines are a highly promising, safe, and effective immunotherapy for treating gastric cancer.
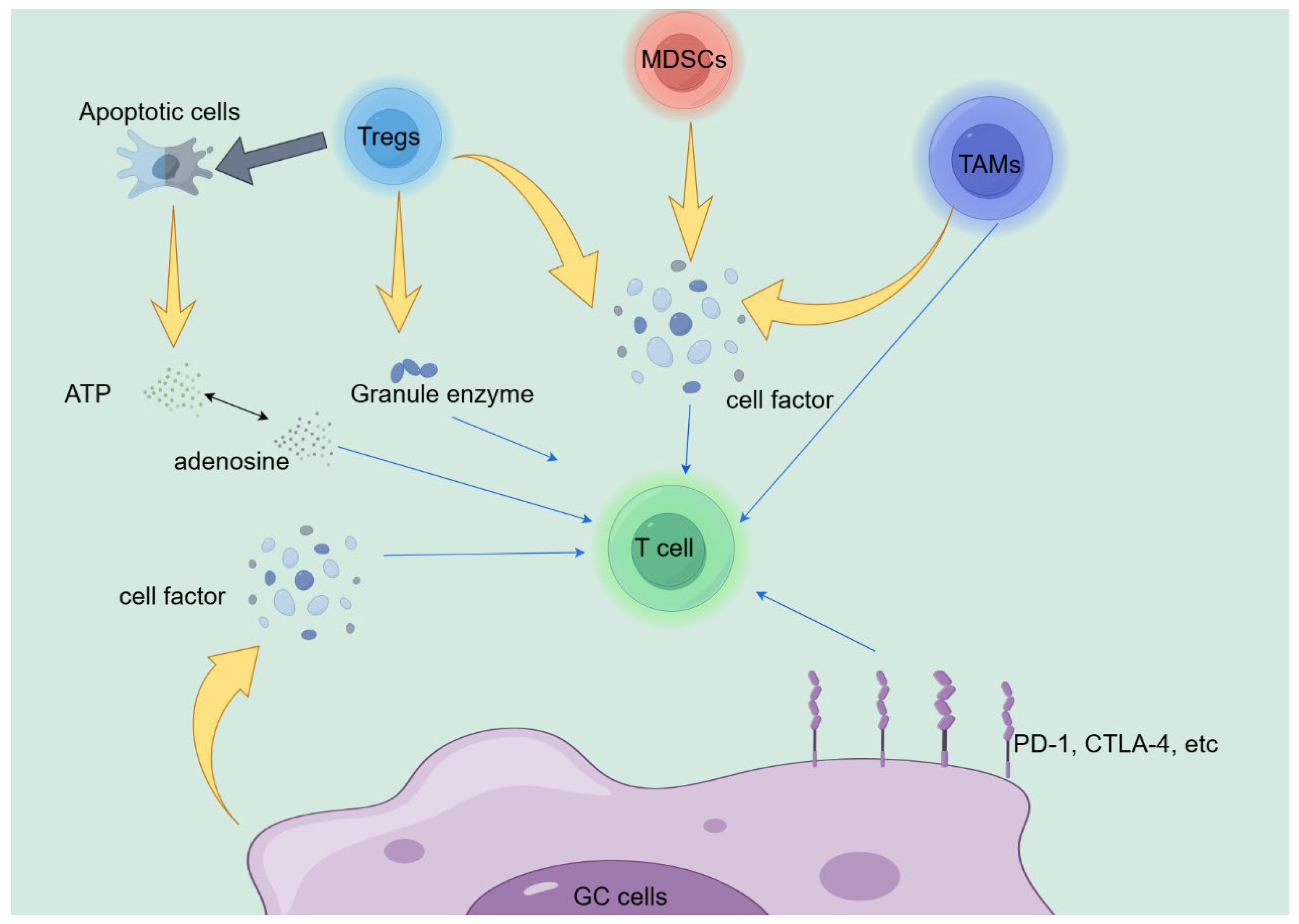
Possible Mechanism of Immune Therapy Resistance in GC
A quantity of GC sufferers have chosen immunotherapy as an anti-tumor treatment option. With the widespread apply of immunotherapy, more and more GC patients have an extended survival period. However, after a period of immunotherapy, some patients may experience tumor recurrence, volume growth, and distant metastasis. The mechanism that leads to this situation has not been fully explored and understood. Traditionally, it is believed that it is often associated with interferon - γ (IFN - γ) signaling, upregulation of escape mutants, downregulation of tumor antigens and abnormal recognition by T cells [
29,
30].
Self Resistance of GC Cells to Immunotherapy
The upregulation of the levels immune checkpoint expressed and downregulation of tumor antigens are the two main mechanisms that lead to tumor cells evading immune responses. The outside of the cytomembrane is the location where immune checkpoints such as PD-1 and CTLA-4 are commonly highly expressed. The activation of immune checkpoints can avoid immune cells to trigger destruction, inflammation, and autoimmunity. The signal transduction within T cells can be blocked by them. These checkpoint may exhibit high expression levels the surface of the cytomembrane of tumor cells, thereby protecting cells from cytotoxic T lymphocyte damage and avoiding spontaneous death [
31]. Multiple studies have shown that the surface of GC cells highly expresses PD-I and CTLA-4 molecules [
32,
33]. The expression levels of immune checkpoints on the cell membrane of different pathological subtypes of tumor cells may be completely different [
34]. Research has discovered that the secretion of PD-L1 by extracellular vesicles is a necessary way for certain tumor cells to develop T cell immunity. Moreover, this immune suppression is often difficult to recover through ICIs [
35]. Therefore, determining a reliable immunotherapy targeting specific immune checkpoints remains a daunting challenge.
Certain factors secreted by tumor cells can upregulate immune checkpoints in various ways. GC cells can also secrete cytokines, including growth factors, to reduce anti-tumor immune responses and facilitate tumour proliferation. The cytokine called transforming growth factorβ (TGF-β) is secreted spontaneously by tumor cells and can regulate the proportion of immune cells, playing an indispensable part in immune tolerance. It is the main mediator that enhances immune suppression in the tumor microenvironment (TME). And the main use of TGF - β in immunosuppression is to reduce the antigen presentation ability of DC cells and inhibit the cytotoxicity of T cells and NK cells. Moreover, TGF - β also has the ability to interdict the process of primitive T lymphocytes differentiating into effector T lymphocytes. At the same time, the transformation process of M0 and M1 phenotype macrophages to M2 phenotype can also be promoted by it [
36,
37]. The most significant and characteristic T cell response in cancer is mediated by Th1 subpopulation cells, and immature T cells cultured in medium rich in TGF - β cannot differentiate into Th1 phenotype. On the contrary, T cells in mice lacking TGF - β specificity exhibit a strong Th1 phenotype [
38]. Meanwhile, many anti-inflammatory reactions can be inhibited by TGF - β, such as those mediated by transcription factor NF kB. All Toll like receptors (TLRs) except TLR3 are linked by MYD88, which plays an important role in activating NF kB signaling. TGF - β can facilitate the degradation of MYD88. SMAD6 induces polyubiquitination of MYD88 by recruiting the ubiquitin ligase SMURF1/2 in macrophages stimulated by TGF - β.(39,40)Therefore, TME rich in TGF - β may promote immune evasion by inhibiting the inflammatory function of macrophages. Other cytokines, such as Dickkopf-1 (DKK1) secreted by GC cells, can promote the upregulation of PD-1 immune checkpoint in gastric cancer by inducing immunosuppressive macrophages, further leading to tumor immune escape [
40].
Potential Biomarkers of GC Immunotherapy
The onset and rapid growth of tumors primarily are decided by the interaction of various cells in TME, such as the multiplication and invasion ability of cancer cells, the interplay of immune cells induced by regulatory factors produced by various cells in TME, etc. Meanwhile, the immune resistance of GC is believed to be the result of specific molecular abnormalities, such as stimulating and inhibitory factors, ultimately leading to abnormal T cell function. Therefore, identifying reliable biomarkers before immunotherapy is crucial for forecasting tthe efficacy of Immune therapy in GC patients.
Immune Checkpoint Proteins
PD-L1, a transmembrane protein expressed on the extracellular membrane of GC cells, sometimes known as CD274. Its ligand, PD-1, typically exhibits high expression levels on the surface of T lymphocytes. When this two proteins specifically combine, the ability to combat tumors of T lymphocytes is reduced. That is to say, the initiation of immune escape mainly relies on the combination of this two proteins. ICIs with the function to reduce their combination can promote T lymphocyte identify and inactivatel GC cells. Most researchers hold the view, the level of PD-L1 or PD-1 expressed can predict the prognosis of GC immunotherapy. Numerous experiments have proven that high leve of PD-L1 expressed of GC cells is often significantly correlated with good therapeutic outcomes [
63]. Clinical experiment have proven that the curative effect of chemotherapy joint application with Cadonilimab in treating HER2 negative gastric or gastroesophageal junction adenocarcinoma was shown positive correlation with the level of PD-L1 expressed. The median OS of patients with high PD-L1 expression was 20.32 months, while it was only 17.64 months in the sufferers with low level of PD-L1 expressed [
64]. Another research proven that the curative effect of Pembrolizumab monotherapy in the PD-L1 low expression group was not better than that in the chemotherapy group, while in the PD-L1 high expression group, the overall survival rate of Pembrolizumab treatment group was significantly better than that of the chemotherapy group [
65]. Meanwhile, Shitara et al.’s study found that compared to traditional paclitaxel chemotherapy, Pembrolizumab as a second-line treatment for advanced GC patients with PD-L high expression did not significantly improve overall survival [
66]. These controversial research findings may be due to various laboratory testing methods, heterogeneity of PD-L1 in different patients, and complicated interactions between anti-tumor immune responses and GC cells. Furthermore, psychological factors of GC patients and treatment levels in different regions may also significantly affect the results. This suggests that the expression grade of PD-L1 may be an effective marker for predicting the efficacy of immunotherapy in GC sufferers, and the merit of PD-L1 in GC needs more clinical data to prove.
Other biomarkers that may predict the prognosis of immunotherapy, such as CTLA-4, IDO1, TGF - β, IL-8, and IL-10, have been shown to be correlated with the treatment response and staging of GC [
67,
68]. Nevertheless, the research on their relevance with the prognosis of GC immunotherapy is still insufficient in terms of quantity. Therefore, it is essential to apply multitudinous basic experiments and extensive clinical data to explore more biomarkers that can predict tumor prognosis.
Tumor-Infiltrating Lymphocytes
Tumor-infiltrating lymphocytes (TILs) have displayed significant value in evaluating the prognosis of various solid tumors, including gastrointestinal tumors [
69]. The prognosis of tumor immunotherapy largely depends on ton the degree of permeation of immune cells like T lymphocytes in the tumour. Increasing the infiltration of CD8+ T cells and CD4+ T cells in the TME of GC patients often leads to prolonged overall survival and improved prognosis [
70]. Due to the indispensable role of TILs in the production of TME anti-tumor ability, researchers have proposed a conception called “Immunoscore”, that combines tumor TNM staging and tumor lymphocyte infiltration as basic parameters for cancer classification. Research has found that cancer patients with high “Immunoscore” are often associated with good clinical outcomes from several different immune therapies [
71]. However, in order to clarify the specific mechanism of the involvement of “immune scoring” in GC tumor immunotherapy, extensive experimental research is essential.
Non-Coding RNA
A kind of RNA that can perform its biological function at the RNA level is a non-coding RNA, although it cannot be translated into proteins. Non-coding RNA includes various RNAs such as rRNA, tRNA, snRNA, snoRNA, and microRNA. MicroRNA (miRNA) is a non-coding RNA containing 20-24 nucleotides. After gene transcription, miRNA binds itself to the 3’UTR region of the corresponding mRNA through the principle of base pairing, causing inhibition of mRNA translation into protein or degradation of mRNA [
72]. So that, miRNAs act as oncogenes or tumor suppressor genes in tumors by regulating the transcription and translation of object genes, regulating various aspects such as tumor growth, metastasis, and angiogenesis [
73]. Multiple experiments have demonstrated that the immune therapy resistance of tumors is regulated by miRNA. The case in point is miR-BART5-5p can promote high expression of PD-L1 through the miR-BART5/PIAS3/pSTAT3/PD-L1 axis. High expression of PD-L1 helps enhance the multiplication, transfer, and invasive ability of tumor cells, resist cell apoptosis, and lead to immune escape, further leading to clinical results of cancer progression and poor prognosis [
74]. Circular RNAs (circRNAs) are widely present in the cytoplasm of almost all types of cells and are endogenous non coding RNAs composed of exons and introns. These circular RNAs have gained stronger stability than linear RNAs through their conserved covalent fixation of closed loop structures [
75,
76,
77]. Tumor occurrence, cancer progression, and immune therapy resistance are usually regulated by circular RNAs. For example, circUSP7 in extracellular vesicles secreted by cancer cells can induce a decrease in cytotoxic T lymphocytes function in tumor TME by adjusting the miR-934/SHP2 axis, ultimately causing to drug-fast to anti-PD-1 immunotherapy [
78].
Although numerous experiments have proven that non-coding RNAs are relevant to immune resistance in cancer therapy, the selection of non-coding RNAs as biomarkers for GC immunotherapy is still uncertain due to the abundance of non-coding RNAs from various sources in the tumor microenvironment, and more research is indispensable.
Strategies to Enhance the Efficacy of Immunotherapy
Chemotherapy Combined with Immunotherapy
The use of chemotherapy is often accompanied by severe bone marrow suppression and leukopenia, therefore chemotherapy is considered to suppress the efficacy of immunotherapy [
79]. But more and more recent studies have proven that chemotherapy combined with immunotherapy has a significant synergistic effect. In a clinical trial, hemotherapy combined with neoadjuvant immunotherapy was used for the first three cycles of surgical resection of gastric cancer tissue. Sintilimab (3 mg/kg in cases with less than 60 kg or 200 mg on the first day in cases with ≥ 60 kg) was combined with CapeOx (oxaliplatin 130 mg/m2 combined with capecitabine 1000 mg/m2 twice a day) for D1-D14, with a 21 day interval between cycles. After surgical resection, CapeOx was used for three cycles of adjuvant therapy at the same dose. Sintilimab combined with CapeOx showed superior pathological complete response (PCR) rates and good safety in neoadjuvant therapy [
80]. This synergistic effect may be due to chemotherapy can induce immunogenic cell death (ICD) in tumors, followed by widespread innate and adaptive immune responses. According to reports, the classic chemotherapy regimens FOLFOX or FOLFIRI can induce damage associated molecular models (DAMPs) in tumor cell lines including GC cells. DAMPs are intense signals for tumor antigen presentation and promotion of DC maturity, which may further lead to tumor ICD, thereby enhancing the function of cytotoxic T lymphocytes to lyse tumor cells [
81,
82].
Immunoadjuvants
Immunoadjuvants are the auxiliary substance that, when injected into the body in advance or together with an antigen, have ability to strengthen the intensity of the immune response of the organism or change the type of immune response. Currently, clinically proven Immunoadjuvants typically activate and enhance humoral immunity, rather than cellular immunity. Therefore, Immunoadjuvants are currently commonly used in various vaccines to prevent viral and bacterial infections [
83]. Fortunately, research has found that manganese salts may become adjuvants to enhance the efficacy of immunotherapy. It promotes immune response by promoting the formation of germinal centers, antigen uptake, and presentation. In the experiment, the manganese salt combined with immunotherapy significantly inhibited the proliferation and metastasis of melanoma tumors implanted subcutaneously in mice, and the survival rate of mice in the combination therapy group was obvious improved [
84]. Although there is currently no research indicating that immune adjuvants such as manganese salts can counteract immune suppression in GC patients, immunoadjuvants still have unlimited potential in improving the efficacy of immunotherapy for GC.
Antiangiogenics
Most solid tumors have the characteristic of vascular abnormalities, which often promote the progression and treatment resistance of tumors such as gastric cancer. (85-87)These abnormalities are caused by increased expression of angiogenic factors, like angiopoietin 2 (ANG2) and VEGF ; The use of drugs targeting these molecules can promote the normalization of abnormal tumor vascular systems, thereby improving therapeutic efficacy. Clinical trials have shown that anlotinib (an anti angiogenic drug targeting VEGF) can dramatically inhibit local progression and distant metastasis of gastric cancer. Moreover, joint application can reduce the level of PD-L1 expression and acquire better therapeutic outcomes, when used together with anti-PD-1 antibody [
88]. The second-line treatment of GC often includes anti vascular therapy, so it is crucial to determine whether its combination with immunotherapy can have a synergistic effect.
Tumor Microenvironment
The direct or indirect interactions between various types of cells in TME are considered as an indispensable part in the formation of tumor immunotherapy resistance. Therefore, targeting TME has great potential in enhancing the therapeutic effect of immunotherapy. The level of Tregs infiltration often negatively correlates with the overall survival after treatment of solid tumors, including gastric cancer [
89,
90]. For example, Tregs with high expression of CCR4 are considered as an important factor in suppressing anti-tumor immune responses in TME [
91]. In a clinical study, researchers have found that the joint application of Mogamulizumab (anti-CCR4 antibody) and nivolumab (anti-PD-1 antibody) can effectively reduce effector Tregs infiltration in tumor TME of solid tumor patients, including gastric cancer, increase CD8+T cell infiltration, and ultimately enhance immune response [
92].
DNA Damage Response and Repair
Tumor tissue often exhibits genomic instability, and tumor cells often have significant defects in DNA damage response and repair (DDR) [
93]. The DDR pathway affects the resistance and susceptiveness of tumor cells to radiotherapy, chemotherapy, and immunotherapy. In theory, the joint application of ICIs and DNA damage repair therapy has the ability to improve the efficacy of ICIs treatment. Drugs targeting the DDR pathway have been used in anti-cancer therapy. For example, Cerabastertib (AZD6738) is an effective selective ATR pathway inhibitor that can inhibit the replication stress response caused by DNA damage during tumor cells proliferation cycle and promote DDR. Joint application of ceralasterib and durvalumab (anti-PD-L1 antibody) can significantly extend the overall survival and disease-free survival of melanoma patients [
94]. Although there is limited research on the joint application of drugs targeting DDR and immunotherapy for GC, we are confident that this is a strategy with enormous potential to enhance the curative effect of immunotherapy.
Conclusion
We discussed the present situation of GC immunotherapy, in this review. At the same time, the possible mechanisms of the resistance in patients undergoing immunotherapy and described potential biomarkers with prognostic value for GC patients receiving immunotherapy were described. Meanwhile, we introduced some auxiliary methods that that may have the ability to improve the effectiveness of immunotherapy. In clinical practice, immunotherapy act as a significant part in rescuing advanced gastric cancer patients. Due to the differences in immune environments, it is necessary to study the possible mechanisms of immune suppression in GC in order to develop precise targeted therapy methods, overcome immune therapy resistance in GC, and improve the prognosis of GC patients. More clinical experiment are essential to clarify the mechanisms of immune resistance and find strategies to combat resistance and improve the efficacy of immunotherapy.
Acknowledgments
The Figure 1 is drawn by Figdraw
References
- Smyth EC, Nilsson M, Grabsch HI, van Grieken NCT, Lordick F. Gastric cancer. The Lancet. 2020;396:635-48.
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. The New England journal of medicine. 2008;358:36-46. [CrossRef]
- Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA: A Cancer Journal for Clinicians. 2021;71:264-79. [CrossRef]
- Li S, Yu W, Xie F, Luo H, Liu Z, Lv W, et al. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nature Communications. 2023;14.
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. The New England journal of medicine. 2016;375:1823-33. [CrossRef]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443-54.
- Rieth J, Subramanian S. Mechanisms of Intrinsic Tumor Resistance to Immunotherapy. International journal of molecular sciences. 2018;19. [CrossRef]
- Li B, Chan HL, Chen P. Immune Checkpoint Inhibitors: Basics and Challenges. Current medicinal chemistry. 2019;26:3009-25. [CrossRef]
- Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y, et al. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Frontiers in immunology. 2022;13:964442.
- Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Molecular cancer. 2018;17:129.
- Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116:1291-8.
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321-30. [CrossRef]
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Science translational medicine. 2016;8:328rv4. [CrossRef]
- Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England). 2017;390:2461-71. [CrossRef]
- Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA oncology. 2018;4:e180013.
- Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Annals of oncology : official journal of the European Society for Medical Oncology. 2018;29:2052-60.
- Wang F, Wei XL, Wang FH, Xu N, Shen L, Dai GH, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Annals of oncology : official journal of the European Society for Medical Oncology. 2019;30:1479-86. [CrossRef]
- Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood cancer journal. 2021;11:69.
- Ma S, Li X, Wang X, Cheng L, Li Z, Zhang C, et al. Current Progress in CAR-T Cell Therapy for Solid Tumors. Int J Biol Sci. 2019;15:2548-60. [CrossRef]
- Wang Z, Wu Z, Liu Y, Han W. New development in CAR-T cell therapy. Journal of hematology & oncology. 2017;10:53. [CrossRef]
- Trarbach T, Schuler M, Zvirbule Z, Lordick F, Krilova A, Helbig U, et al. Efficacy and Safety of Multiple Doses of Imab362 in Patients with Advanced Gastro-Esophageal Cancer: Results of a Phase Ii Study. Annals of Oncology. 2014;25. [CrossRef]
- Niimi T, Nagashima K, Ward JM, Minoo P, Zimonjic DB, Popescu NC, et al. claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Molecular and cellular biology. 2001;21:7380-90. [CrossRef]
- Jiang H, Shi Z, Wang P, Wang C, Yang L, Du G, et al. Claudin18.2-Specific Chimeric Antigen Receptor Engineered T Cells for the Treatment of Gastric Cancer. Journal of the National Cancer Institute. 2019;111:409-18. [CrossRef]
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nature medicine. 2004;10:909-15. [CrossRef]
- Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nature medicine. 2013;19:747-52. [CrossRef]
- Ku GY. The Current Status of Immunotherapies in Esophagogastric Cancer. Hematology/oncology clinics of North America. 2019;33:323-38. [CrossRef]
- Sadanaga N, Nagashima H, Mashino K, Tahara K, Yamaguchi H, Ohta M, et al. Dendritic cell vaccination with MAGE peptide is a novel therapeutic approach for gastrointestinal carcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7:2277-84.
- Liu Q, Chu Y, Shao J, Qian H, Yang J, Sha H, et al. Benefits of an Immunogenic Personalized Neoantigen Nanovaccine in Patients with High-Risk Gastric/Gastroesophageal Junction Cancer. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2022:e2203298.
- Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. British Journal of Cancer. 2018;118:9-16.
- Schoenfeld AJ, Hellmann MD. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer cell. 2020;37:443-55. [CrossRef]
- Ishida, Agata, Shibahara. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. Honjo J The EMBO journal. 1992.
- Lote H, Cafferkey C, Chau I. PD-1 and PD-L1 blockade in gastrointestinal malignancies. Cancer Treatment Reviews. 2015;41:893-903. [CrossRef]
- Mimura K, Kua LF, Xiao JF, Asuncion BR, Nakayama Y, Syn N, et al. Combined inhibition of PD-1/PD-L1, Lag-3, and Tim-3 axes augments antitumor immunity in gastric cancer-T cell coculture models. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2021;24:611-23.
- Derks S, Nason KS, Liao X, Stachler MD, Liu KX, Liu JB, et al. Epithelial PD-L2 Expression Marks Barrett’s Esophagus and Esophageal Adenocarcinoma. Cancer immunology research. 2015;3:1123-9.
- Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell. 2019;177:414-27.e13. [CrossRef]
- Batlle E, Massagué J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity. 2019;50:924-40. [CrossRef]
- Chen J, Gingold JA, Su X. Immunomodulatory TGF-β Signaling in Hepatocellular Carcinoma. Trends in molecular medicine. 2019;25:1010-23. [CrossRef]
- Sad S, Mosmann TR. Single IL-2-secreting precursor CD4 T cell can develop into either Th1 or Th2 cytokine secretion phenotype. J Journal of Immunology. 1994;153:3514-22. [CrossRef]
- Lee YS, Park JS, Kim JH, Jung SM, Lee JY, Kim SJ, et al. Smad6-specific recruitment of Smurf E3 ligases mediates TGF-β1-induced degradation of MyD88 in TLR4 signalling. Nat Commun. 2011;2:460. [CrossRef]
- Shi T, Zhang Y, Wang Y, Song X, Wang H, Zhou X, et al. DKK1 Promotes Tumor Immune Evasion and Impedes Anti–PD-1 Treatment by Inducing Immunosuppressive Macrophages in Gastric Cancer. Cancer immunology research. 2022;10:1506-24.
- Tay C, Tanaka A, Sakaguchi S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer cell. 2023;41:450-65. [CrossRef]
- Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Molecular cancer. 2020;19:116. [CrossRef]
- Nakamura K, Smyth MJ. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cellular & molecular immunology. 2020;17:1-12. [CrossRef]
- Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and Metabolism in the Tumor Microenvironment. Cell metabolism. 2019;30:36-50. [CrossRef]
- Damo M, Joshi NS. Treg cell IL-10 and IL-35 exhaust CD8+ T cells in tumors. Nature immunology. 2019;20:674-5. [CrossRef]
- Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. Journal of immunology (Baltimore, Md : 1950). 2009;182:6121-8. [CrossRef]
- Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nature immunology. 2017;18:1332-41. [CrossRef]
- Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589-601. [CrossRef]
- Wu Y, Yi M, Niu M, Mei Q, Wu K. Myeloid-derived suppressor cells: an emerging target for anticancer immunotherapy. Molecular cancer. 2022;21:184. [CrossRef]
- Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. The Journal of experimental medicine. 2003;198:1741-52.
- Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. The Journal of clinical investigation. 2015;125:3356-64. [CrossRef]
- Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. Journal of immunology (Baltimore, Md : 1950). 2002;168:689-95. [CrossRef]
- Smith LK, Boukhaled GM, Condotta SA, Mazouz S, Guthmiller JJ, Vijay R, et al. Interleukin-10 Directly Inhibits CD8+ T Cell Function by Enhancing N-Glycan Branching to Decrease Antigen Sensitivity. Immunity. 2018;48:299-312.e5.
- Dixon KO, Tabaka M, Schramm MA, Xiao S, Tang R, Dionne D, et al. TIM-3 restrains anti-tumour immunity by regulating inflammasome activation. Nature. 2021;595:101-6. [CrossRef]
- Strauss L, Mahmoud MAA, Weaver JD, Tijaro-Ovalle NM, Christofides A, Wang Q, et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Science immunology. 2020;5. [CrossRef]
- Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495-9.
- Thomas DA, Massagué J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer cell. 2005;8:369-80.
- Saraiva M, Vieira P, O’Garra A. Biology and therapeutic potential of interleukin-10. The Journal of experimental medicine. 2020;217. [CrossRef]
- Song X, Traub B, Shi J, Kornmann M. Possible Roles of Interleukin-4 and -13 and Their Receptors in Gastric and Colon Cancer. International journal of molecular sciences. 2021;22. [CrossRef]
- Zhang C, Wei S, Dai S, Li X, Wang H, Zhang H, et al. The NR_109/FUBP1/c-Myc axis regulates TAM polarization and remodels the tumor microenvironment to promote cancer development. Journal for immunotherapy of cancer. 2023;11.
- Qiao J, Liu Z, Dong C, Luan Y, Zhang A, Moore C, et al. Targeting Tumors with IL-10 Prevents Dendritic Cell-Mediated CD8+ T Cell Apoptosis. Cancer cell. 2019;35:901-15.e4. [CrossRef]
- Guo Y, Xie YQ, Gao M, Zhao Y, Franco F, Wenes M, et al. Metabolic reprogramming of terminally exhausted CD8+ T cells by IL-10 enhances anti-tumor immunity. Nature immunology. 2021;22:746-56. [CrossRef]
- Thompson ED, Zahurak M, Murphy A, Cornish T, Cuka N, Abdelfatah E, et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2017;66:794-801. [CrossRef]
- Gao X, Ji K, Jia Y, Shan F, Chen Y, Xu N, et al. Cadonilimab with chemotherapy in HER2-negative gastric or gastroesophageal junction adenocarcinoma: the phase 1b/2 COMPASSION-04 trial. Nature medicine. 2024;30:1943-51. [CrossRef]
- Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA oncology. 2020;6:1571-80. [CrossRef]
- Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet (London, England). 2018;392:123-33. [CrossRef]
- Sun T, Zhou Y, Yang M, Hu Z, Tan W, Han X, et al. Functional genetic variations in cytotoxic T-lymphocyte antigen 4 and susceptibility to multiple types of cancer. Cancer research. 2008;68:7025-34.
- Lin SJ, Gagnon-Bartsch JA, Tan IB, Earle S, Ruff L, Pettinger K, et al. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut. 2015;64:1721-31. [CrossRef]
- Ammannagari N, Atasoy A. Current status of immunotherapy and immune biomarkers in gastro-esophageal cancers. Journal of gastrointestinal oncology. 2018;9:196-207. [CrossRef]
- Zhuang Y, Peng LS, Zhao YL, Shi Y, Mao XH, Chen W, et al. CD8(+) T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology. 2012;143:951-62.e8. [CrossRef]
- Galon J, Pagès F, Marincola FM, Angell HK, Thurin M, Lugli A, et al. Cancer classification using the Immunoscore: a worldwide task force. Journal of translational medicine. 2012;10:205. [CrossRef]
- Lytle JR, Yario TA, Steitz JA. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5’ UTR as in the 3’ UTR. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9667-72.
- Svoronos AA, Engelman DM, Slack FJ. OncomiR or Tumor Suppressor? The Duplicity of MicroRNAs in Cancer. Cancer research. 2016;76:3666-70. [CrossRef]
- Yoon CJ, Chang MS, Kim DH, Kim W, Koo BK, Yun SC, et al. Epstein-Barr virus-encoded miR-BART5-5p upregulates PD-L1 through PIAS3/pSTAT3 modulation, worsening clinical outcomes of PD-L1-positive gastric carcinomas. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2020;23:780-95.
- Li J, Sun D, Pu W, Wang J, Peng Y. Circular RNAs in Cancer: Biogenesis, Function, and Clinical Significance. Trends in cancer. 2020;6:319-36. [CrossRef]
- Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell-type specific features of circular RNA expression. PLoS genetics. 2013;9:e1003777.
- Wilusz JE, Sharp PA. Molecular biology. A circuitous route to noncoding RNA. Science (New York, NY). 2013;340:440-1. [CrossRef]
- Chen S-W, Zhu S-Q, Pei X, Qiu B-Q, Xiong D, Long X, et al. Cancer cell-derived exosomal circUSP7 induces CD8+ T cell dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC. Molecular cancer. 2021;20.
- Garewal HS, Robertone A, Salmon SE, Jones SE, Alberts DS, Brooks R. Phase I trial of esorubicin (4’deoxydoxorubicin). Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1984;2:1034-9.
- Jiang H, Yu X, Li N, Kong M, Ma Z, Zhou D, et al. Efficacy and safety of neoadjuvant sintilimab, oxaliplatin and capecitabine in patients with locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma: early results of a phase 2 study. Journal for immunotherapy of cancer. 2022;10. [CrossRef]
- Zindel J, Kubes P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annual review of pathology. 2020;15:493-518. [CrossRef]
- Murao A, Aziz M, Wang H, Brenner M, Wang P. Release mechanisms of major DAMPs. Apoptosis : an international journal on programmed cell death. 2021;26:152-62.
- Pulendran B, P SA, O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nature reviews Drug discovery. 2021;20:454-75. [CrossRef]
- Zhang R, Wang C, Guan Y, Wei X, Sha M, Yi M, et al. Manganese salts function as potent adjuvants. Cellular & molecular immunology. 2021;18:1222-34. [CrossRef]
- Abouelnazar FA, Zhang X, Zhang J, Wang M, Yu D, Zang X, et al. SALL4 promotes angiogenesis in gastric cancer by regulating VEGF expression and targeting SALL4/VEGF pathway inhibits cancer progression. Cancer cell international. 2023;23:149.
- Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer cell. 2014;26:605-22. [CrossRef]
- Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:2205-18.
- Zheng W, Sun G, Li Z, Wu F, Sun G, Cao H, et al. The Effect of Anlotinib Combined with anti-PD-1 in the Treatment of Gastric Cancer. Frontiers in surgery. 2022;9:895982. [CrossRef]
- Cheng N, Li P, Cheng H, Zhao X, Dong M, Zhang Y, et al. Prognostic Value of Tumor-Infiltrating Lymphocytes and Tertiary Lymphoid Structures in Epstein-Barr Virus-Associated and -Negative Gastric Carcinoma. Frontiers in immunology. 2021;12:692859. [CrossRef]
- Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cellular & molecular immunology. 2021;18:842-59. [CrossRef]
- Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17945-50.
- Doi T, Muro K, Ishii H, Kato T, Tsushima T, Takenoyama M, et al. A Phase I Study of the Anti-CC Chemokine Receptor 4 Antibody, Mogamulizumab, in Combination with Nivolumab in Patients with Advanced or Metastatic Solid Tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019;25:6614-22.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-74.
- Kim R, Kwon M, An M, Kim ST, Smith SA, Loembé AB, et al. Phase II study of ceralasertib (AZD6738) in combination with durvalumab in patients with advanced/metastatic melanoma who have failed prior anti-PD-1 therapy. Annals of oncology : official journal of the European Society for Medical Oncology. 2022;33:193-203. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).