1. Introduction
Coppicing, one of the oldest silvicultural systems in the world, involves regenerating new trees from surviving stumps [
1,
2,
3]. This practice offers significant cost savings compared to establishing new forests, as it reduces expenses related to site preparation, planting, and weed control [
4,
5]. Coppicing not only provides economic benefits but also enhances the resilience of forest ecosystems by maintaining genetic diversity and promoting faster recovery after disturbances, making it a sustainable option for forest management.
Sprouting ability varies significantly among species [
6], as well as provenances and genotypes within species like
Eucalyptus [
7]. Genetic factors, including the number of epicormic buds and lignotuber, the development of the root system, and susceptibility to fungal infection, can influence the species’ ability to sprout [
8,
9,
10]. Environmental factors also influence sprouting responses to silvicultural treatments. Typically, yields decline across coppice rotations, with variations influenced by climate conditions, where higher rainfall may lead to higher yields in coppice rotations compared to initial rotations [
11]. Few studies have tracked individual growth across rotations, with some reporting no correlation between growth in different rotations even across regions with different tropical climates [
12].
Stocking levels, in conjunction with site and genotype characteristics, can influence root system development, subsequently impacting wood growth and survival [
13]. While many studies have explored stocking effects in short rotation planted forests, research on this aspect in coppice rotation is limited, with few exceptions such as a study with willow stands [
14]. The response to changes in stocking may differ in coppice rotations due to preexisting root system, potentially resulting in faster regeneration post-harvest [
15].
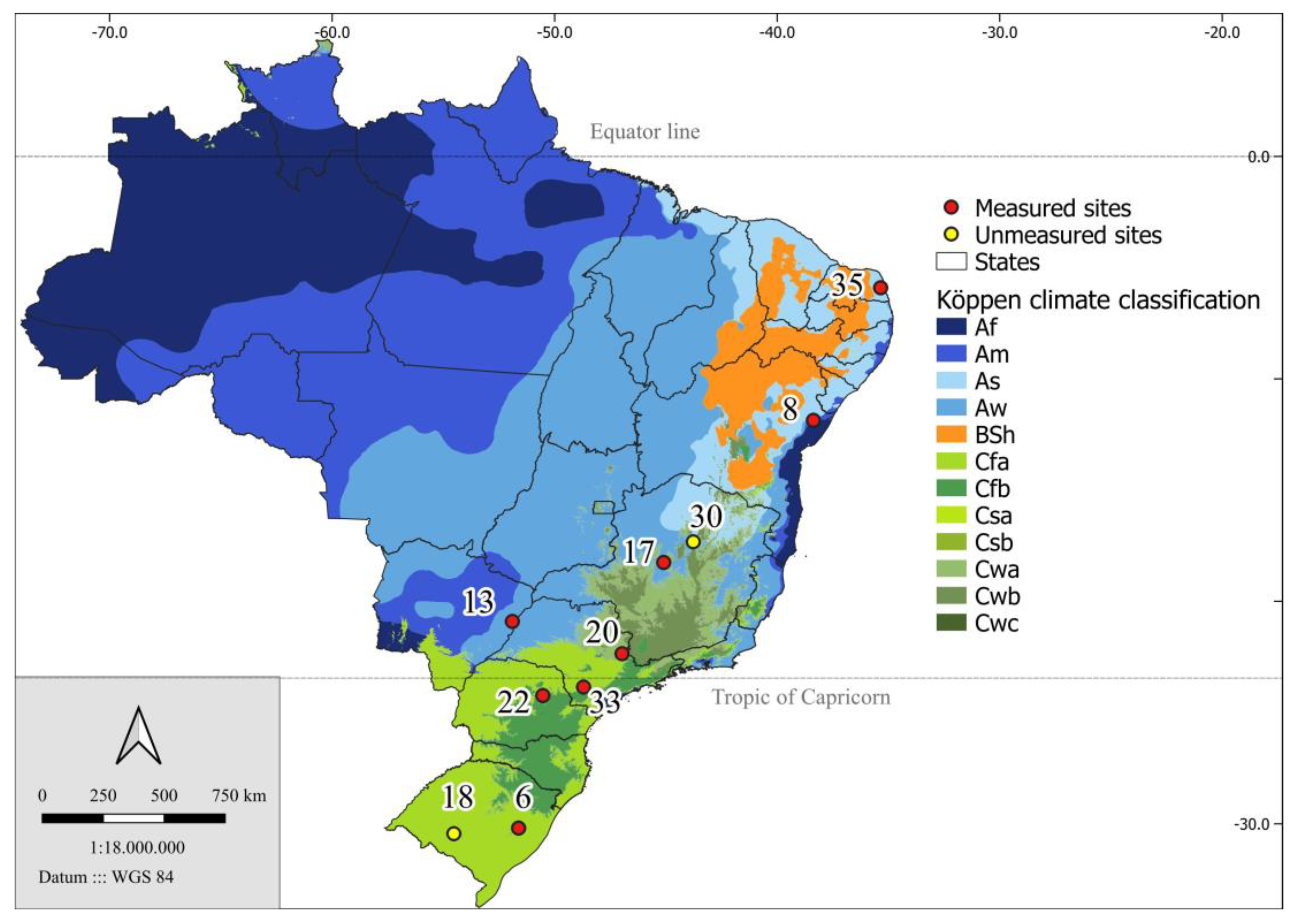
To gain comprehensive insights into why growth patterns differ between coppice and planted rotations, a wide range of experimental treatments and site factors must be considered. Our investigation is focused on a coppice rotation at eight of the original 35 sites of the TECHS project – Tolerance of
Eucalyptus Clones to Hydric, Thermal and Biotic Stresses [
16]. The second phase of this project, known as PCoppice project (Cooperative Program on Sprouting Productivity of
Eucalyptus Clones,
https://www.ipef.br/pcoppice/), specifically investigates into the influence of climate and stocking levels on sprouting and productivity in coppice rotation of the most commonly planted
Eucalyptus clonal genotypes in Brazil.
Therefore, as part of the PCoppice project, this paper aims to address the following 5 questions:
- (1)
Do tropical clones exhibit greater sprouting biomass and wood biomass compared to subtropical clones?
- (2)
Are the most productive clones in the initial rotation also the most productive at the beginning of the coppice rotation?
- (3)
Do dominant trees from the in the initial rotation maintain dominance in the coppice rotation?
- (4)
What is the impact of climate on sprouting and initial growth of genotypes in coppice rotation?
- (5)
How does the interaction between climate and stocking affect the growth of clones at the beginning of the coppice rotation?
By exploring these questions, this study seeks to provide valuable insights into the factors influencing the early stages of coppice growth, ultimately contributing to more effective management strategies for Eucalyptus plantations in varying climatic conditions.
2. Results
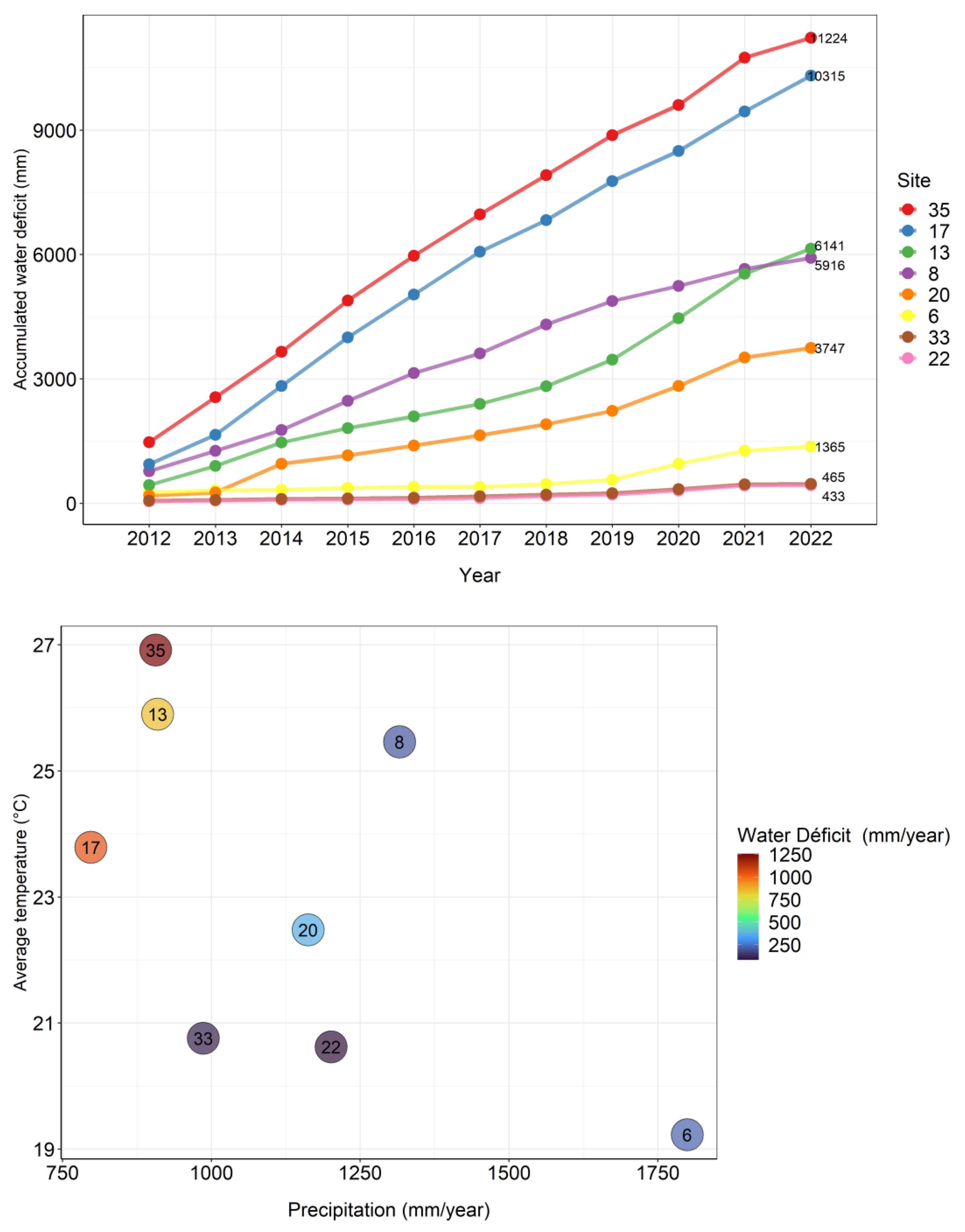
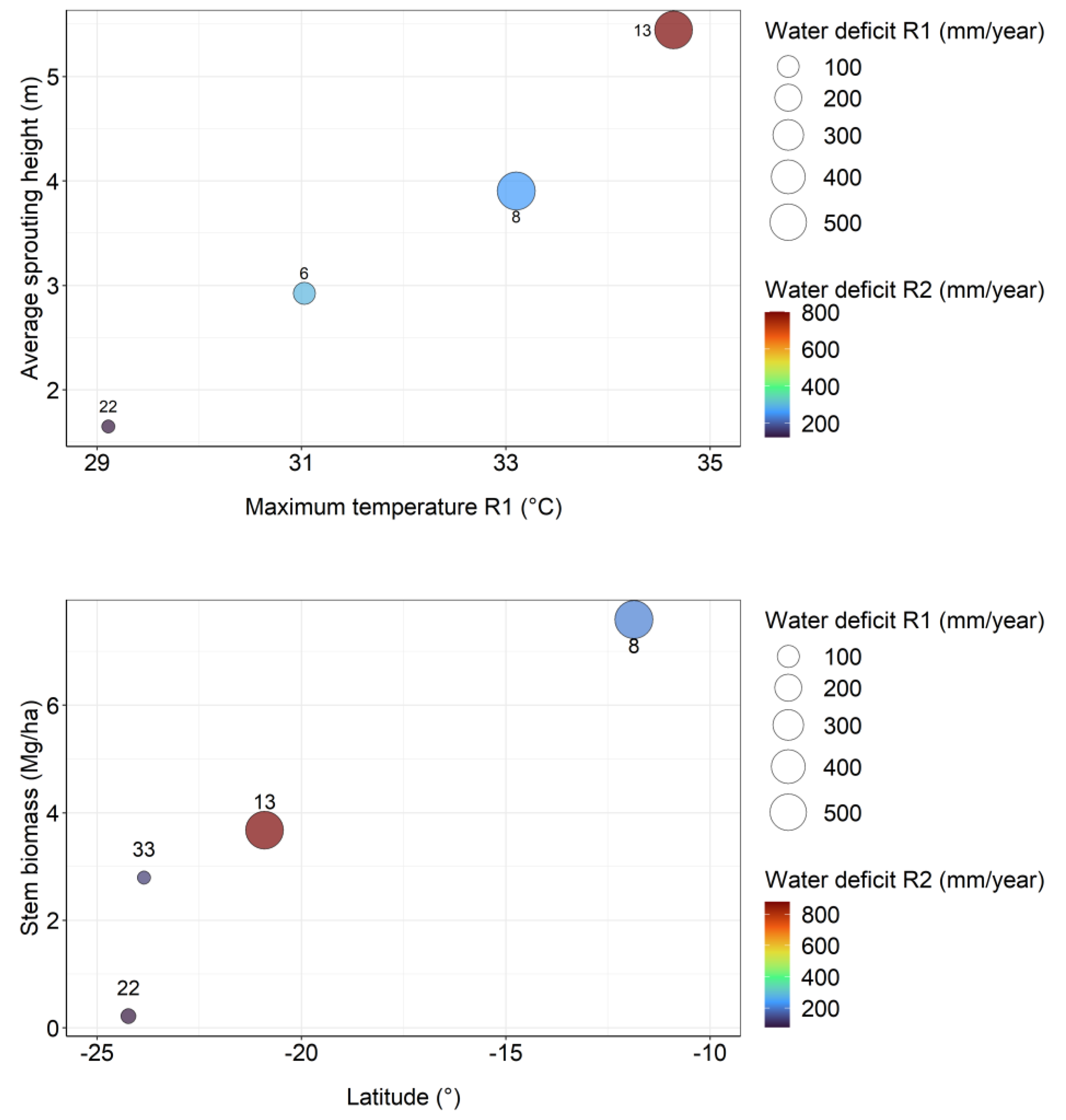
The experimental site with the lowest accumulated water deficit since planting in 2012 was site 22 with 433 mm, while the largest deficit occurred at site 35, with 1,224 mm. Sites 35, 17 and 13 are the hottest and driest in the coppice rotation. Site 8 had a higher water deficit in the initial rotation, but in the coppice rotation is wetter so far. (
Figure 1).
The average sprouting rate across all sites and all genotypes measured at 6 months was 88%. Of the 17 clones tested, 15 showed a sprouting rate above 80%. Clones O6 and L5 had lower sprouting rates, 63.5 and 29.3%, respectively.
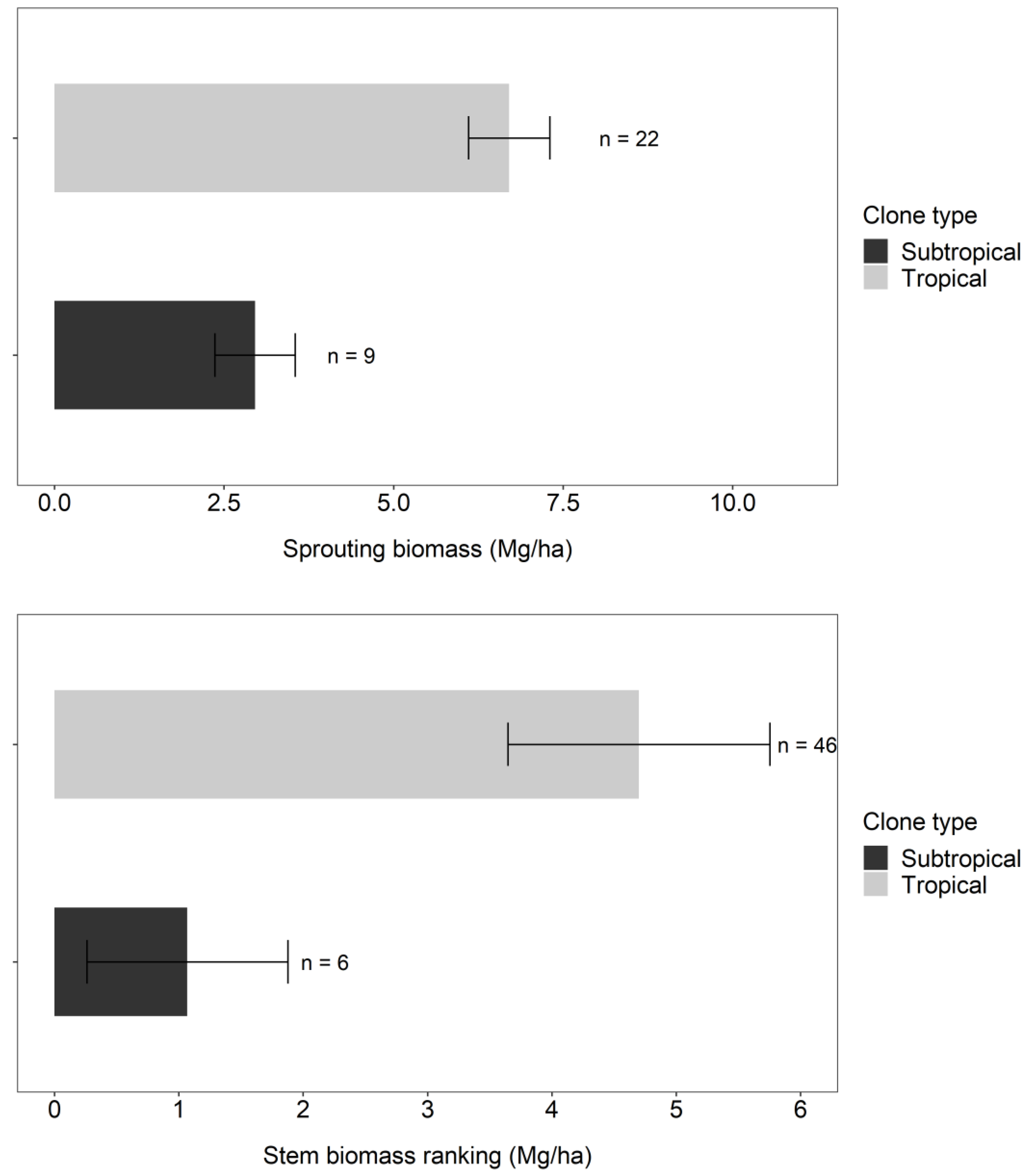
Tropical clones, especially those with
E. urophylla in their genetics, had higher sprouting and woody biomass than subtropical clones. Sprout biomass at 6 months for tropical clones (6.7 Mg ha
-1) was more than double that of subtropical clones (2.9 Mg ha
-1,
Figure 2). The difference between these classes of clones increased to 4-fold at 12 months (4.7 Mg ha
-1 for tropical, 1.1 Mg ha
-1 for subtropical,
Figure 2).
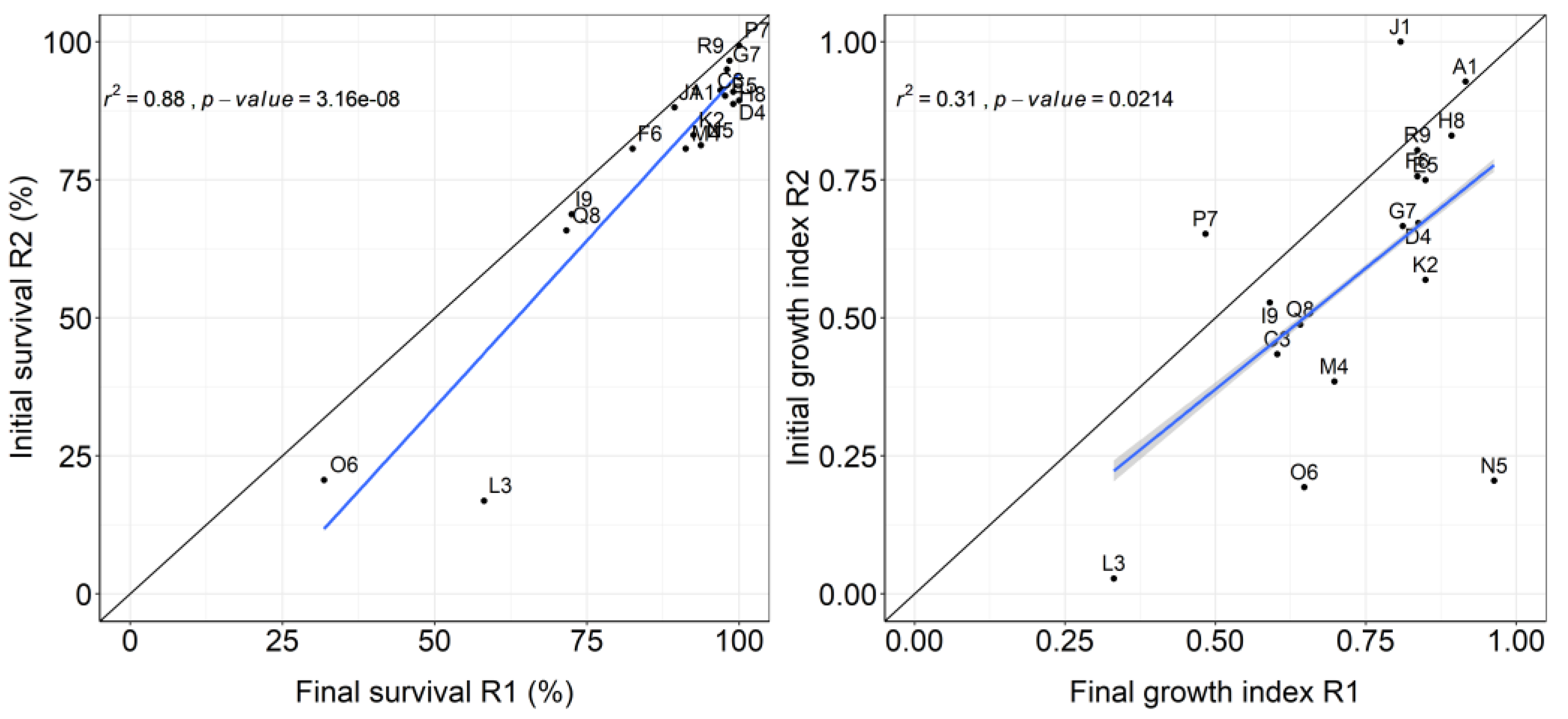
Clones with high survival and growth rates in initial rotation also tended have high rates in the beginning of coppice rotation. This relationship is stronger for survival than for growth (
Figure 3).
Some clones exhibited considerable variations in growth between the initial and coppice rotations. Clone P7 and J1 exhibited a notable increase in growth, with an increase of 35% and 23%, respectively. In contrast, clone N5 and O6 exhibited a significant decrease in growth rate, with a reduction of approximately 75% and 70%, respectively. (
Figure 3).
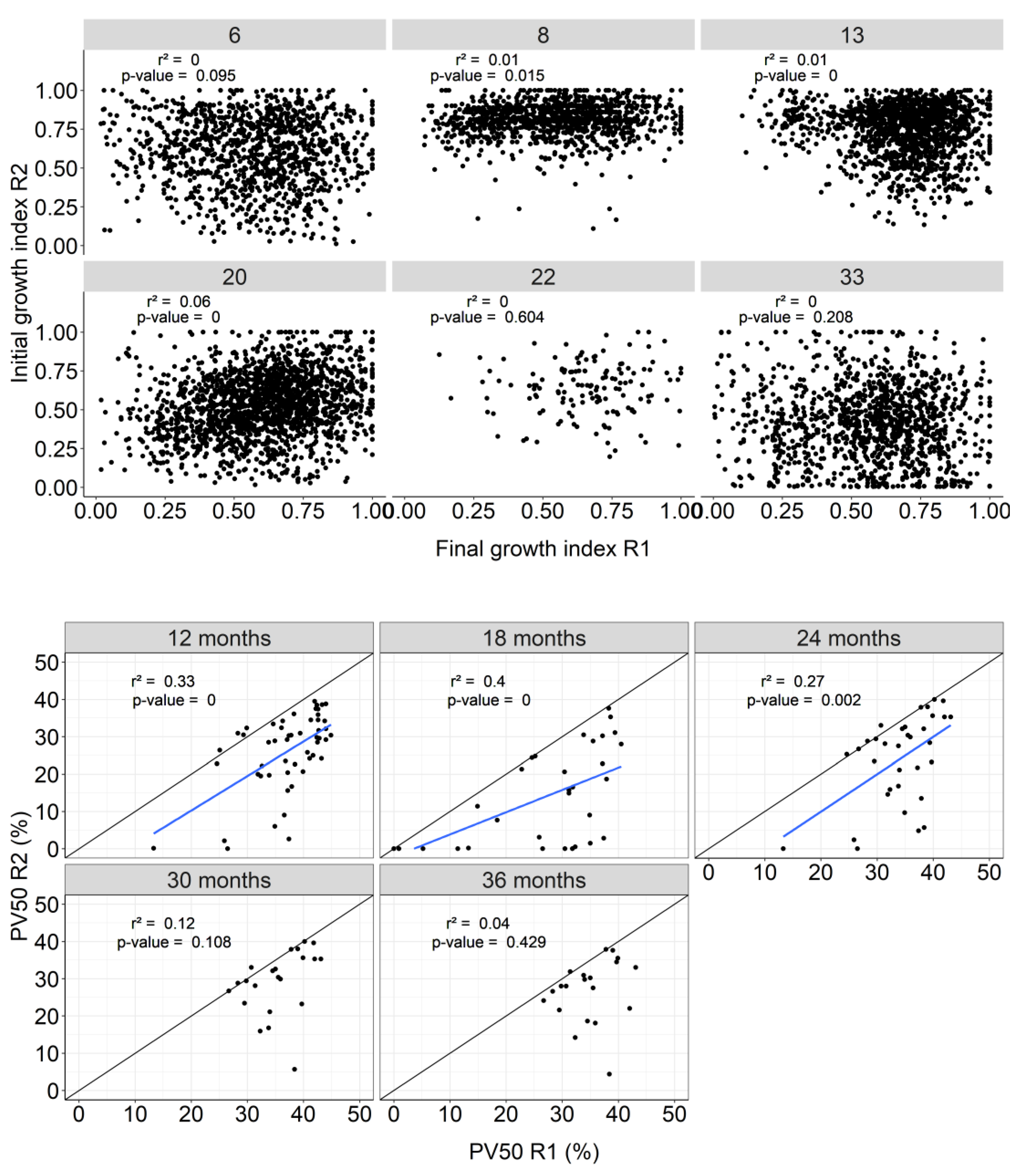
Growth of individual trees in the initial rotation was generally not a clear predictor of growth in the coppice rotation. Therefore, dominant trees in the initial rotation can become dominated in the coppice rotation, indicating that factors led to relative success of individual trees in the initial rotation generally did not carry over into the coppice rotation (
Figure 4A). Changing growth at the individual level affects plot uniformity in coppice rotation. The initial similarity in uniformity (PV50) between the two rotations decreased with advancing age in the coppice rotation. In the first 2 years of the coppice rotation, within-plot uniformity correlated moderately with uniformity in the initial rotation (r² between 0.27 and 0.4), but the correlation declined substantially in the next year. The plots that showed highest uniformity in the initial rotation tended to show lower uniformity in the coppice rotation (
Figure 4B).
High stress from limited water and high temperature in initial rotation seemed to increase height at 6 months and wood biomass at 12 months in coppice rotation. At 6 months the water stress of the coppice rotation does not seem to affect sprouting growth. However, at 12 months the stem biomass starts to obtain a weak positive correlation with the rainfall of coppice rotation (0.29) and zero with the water deficit (0.14). Correlations with the average temperature in the coppice rotation remain high, indicating that warmer and rainier sites are performing better in the early growth phase of coppice rotation (
Table 1).
The initial rotation of the TECHS Project showed that cooler and moister sites had greater yields at the end of the rotation than drier and warmer sites, but the early growth of the plantations was indeed greater on the water-stressed sites. The coppice rotation showed the same pattern in the first three years. Sites with the highest water deficit in initial rotation were the most productive at the beginning of coppice rotation. However, as age advances, water deficit in coppice rotation can reduce site productivity (
Figure 5).
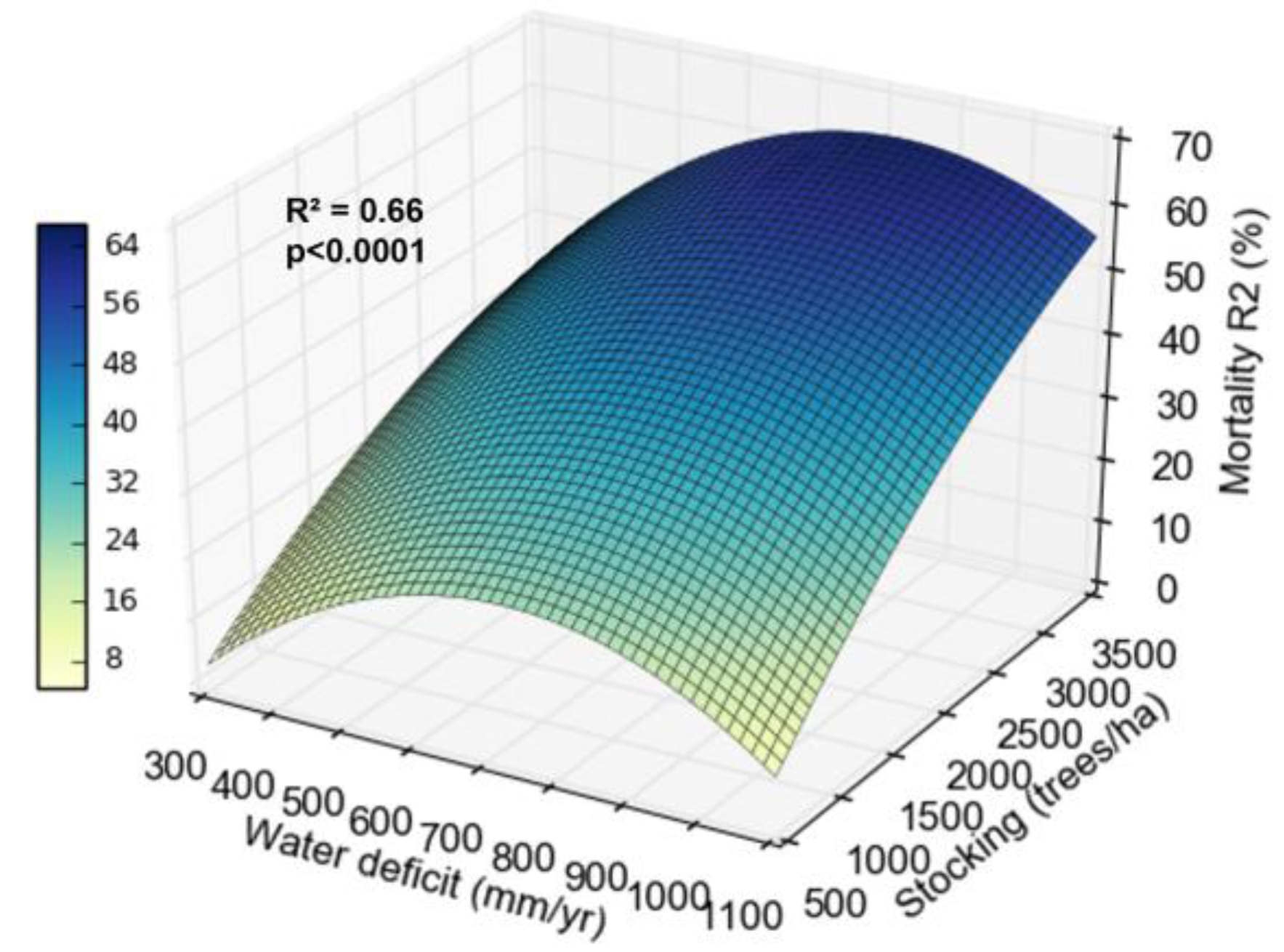
Mortality in coppice rotation was influenced by stocking and water deficit. On sites with 300 mm year
-1 water deficit, mortality was 0 to 30% between 500 and 3300 trees ha
-1. On sites with 1000 mm year
-1 the mortality went from 0 to 70% between 500 and 3300 trees ha
-1 (
Figure 6).
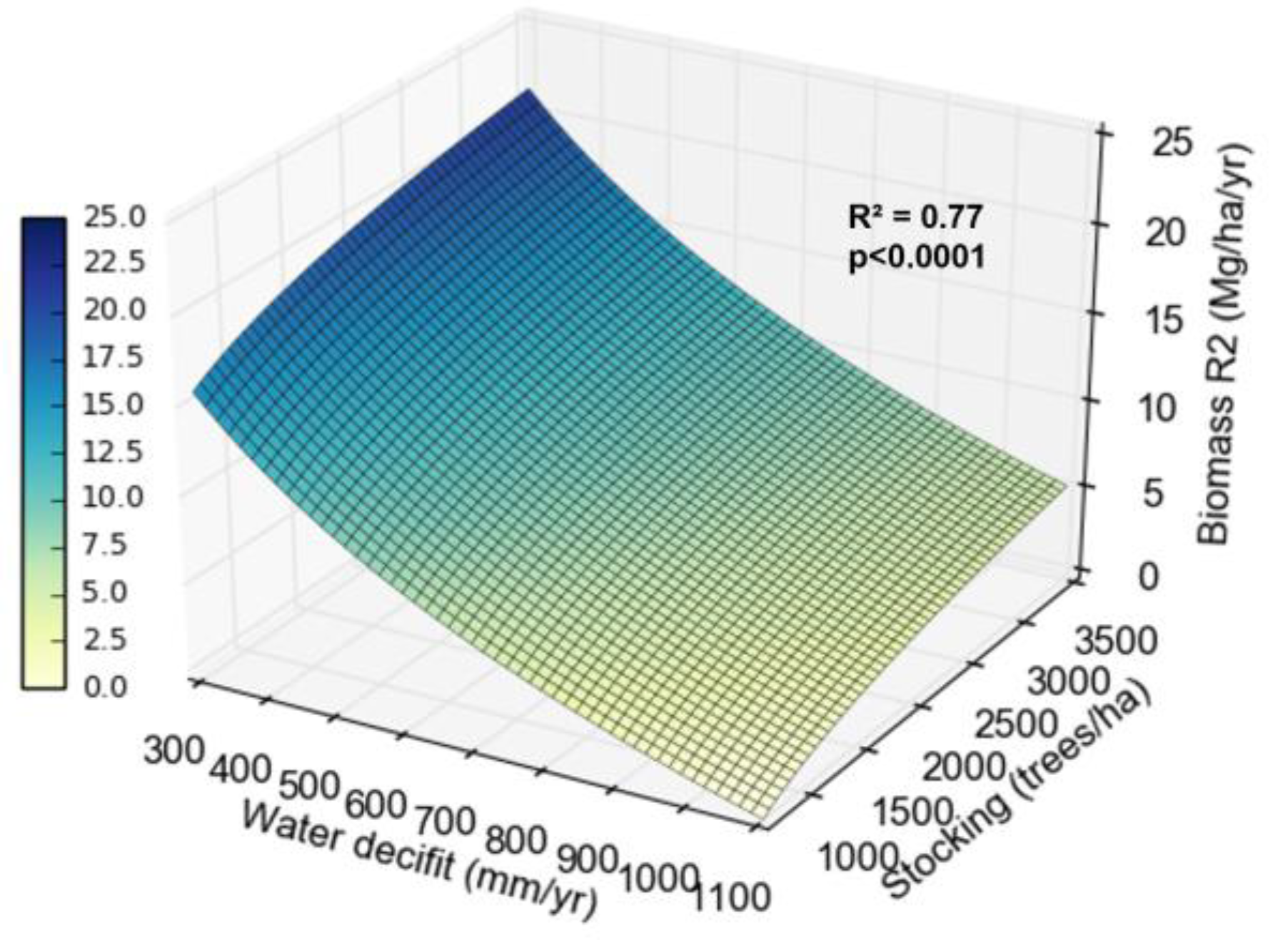
Biomass production at the beginning of the coppice rotation was also influenced by water deficit and stocking. On sites with lower water deficit (300 mm yr
-1), biomass increased by about 7 Mg ha
-1 yr
-1 between widest and tightest stockings. On sites with greater water deficit (1,000 mm yr
-1), the biomass increased less, only about 3 Mg ha
-1 yr
-1 between the widest and tightest stockings (
Figure 7).
3. Discussion
3.1. Do Tropical Clones Exhibit Greater Sprouting Biomass and Wood Biomass Compared to Subtropical Clones?
Tropical clones produced more sprout biomass and faster initial growth, especially those with
E. urophylla in their genetics (
Figure 2). We speculate that tropical clones allocated more C3 belowground in the initial rotation, supporting better coppice growth. [
17] documented that the carbon allocation to the aboveground and belowground parts of 5 TECHS clones at the end of the initial rotation followed patterns consistent with the region where they were developed. According to the author, the higher the water stress in the environment, the higher the proportion of belowground biomass. The P7 clone, for example, developed in regions with high temperatures and water deficit, allocates more carbon to the roots than subtropical clones developed in regions of low temperatures and high rainfall.
Species with high regrowth capacity tend to have around 5 times more carbohydrates in the roots than those that do not regrow [
18,
19]. As a consequence of this strategy, these species tend to produce less biomass in the aboveground part and present lower growth rates. This general trend across species and sites was similar to the pattern found in clone P7. This clone was the least productive in the initial rotation, with an average MAI of 21 m³ ha
-1 year
-1. Its low productivity was a reflection of its greater carbon allocation to the root system [
17]. Perhaps clone P7 stumps and root systems had larger stores of carbohydrates to invest in new sprouts compared to other clones with lower belowground C allocation from the initial rotation. This speculation would warrant direct measurements of carbohydrates stores belowground (across clones and sites), as well as the duration of these stores in supporting sprouts that are increasing their own photosynthesis over time.
3.2. Will the Most Productive Clones in the Initial Rotation Be the Most Productive at the Beginning of the Coppice Rotation?
Overall, the results showed a tendency for the most productive clones in the initial rotation were the most productive in the early growth stage of the coppice rotation (
Figure 3). [
20] obtained similar results when comparing several clones of
E. grandis,
E. urophylla x
E. grandis,
E. saligna and
E. urophylla in first and coppice rotation in a breeding program. About ¾ of the best clones in their initial rotation continued to be best in the coppice rotation. The relationship between survival in the first and coppice rotation was very strong, because naturally survival at the beginning of coppice rotation is directly related to the number of surviving strains at the end of initial rotation.
The growth index reflects the ranking and distances between the yields of the clones at each site, and it showed a moderate correlation between the first and second rotations (
Figure 3B). This is because some clones showed large variations in their positioning between the end of initial rotation and beginning of coppice rotation. Two clear examples are clones P7 and N5, which may represent the extremes of the effect of carbon allocation to the root in initial rotation on the potential for sprouting and initial growth in coppice rotation. Clone P7 was developed in a tropical region with high temperatures and dry summer (As) and N5 was developed in a subtropical region with high rainfall and low temperature (Cfb). Another 4 subtropical clones follow N5 in the group of clones that lost more than 30% growth rate between the two rotations: L3, O6, M4 and K2.
We found some stability in the ranking of the clones between the 2 rotations, those these early rankings may not persist to the end of the rotation. The ranking of clones in the initial rotation shifted over time [
21], so results from the coppice rotation may still change.
3.3. Will the Dominant Trees in the Initial Rotation Remain Dominant in the Coppice Rotation?
Dominance status of individual trees and plot uniformity did not generally continue into the coppice rotation (
Figure 4), in line with results from [
12]. Natural or operational factors, such as shading or damage to the stumps at harvest time, can alter dominance relationships at the individual level. A stronger relationship between root and shoot growth in smaller trees, as opposed to dominant ones, could also account for the shifting hierarchy observed among trees. By applying the equation developed by [
22] to our dataset, we discovered that trees that are 20% smaller would have a root-to-shoot ratio of 25%, in comparison to the 21% found in trees that are 20% larger. This altered relationship would lead to a greater accumulation of carbohydrates below ground, ultimately resulting in similar growth between dominated and dominant trees.
3.4. How Does Climate Affect Sprouting and Initial Growth of Genotypes in Coppice Rotation?
The tropical sites, which suffered the most water and heat stresses during the initial rotation had better initial sprouting and growth in the coppice rotation (
Figure 5).
Throughout the growth of the coppice rotation, water deficit can reduce the biomass production of the clones. At 6 months site 13 were the most productive, with a high value of water deficit in initial rotation (562 mm year
-1) and in coppice rotation (762 mm year
-1). At 12 months site 13 lost the position of most productive site to site 8, which also had a high water deficit in the initial rotation (570 mm year
-1), but not in the coppice rotation (265 mm year
-1). The fact that at 6 months site 13 was the most productive site, and at 12 months it lost this position to site 8, indicates that throughout the growth of the coppice rotation will depend in large part on current rainfall than on water stress in the prior rotation. On the other hand, site 33 was one of the most productive in the initial rotation, showing the lowest water deficit (34 mm year
-1), and even with a low water deficit in the coppice rotation (76 mm year
-1) showed a lower average wood biomass than the sites with higher water stress (
Figure 5). It will be very interesting to see if the whole-rotation yields of the coppice rotation indicate higher growth in cooler, moister sites (as in the initial rotation), or in the warmer, drier sites (as in the first half of the coppice rotation).
The justifications for the faster initial growth of the tropical sites may be related to the survival strategies of the genotypes in more stressful environments. During the initial rotation, in sites with greater water deficit, the trees allocated more carbon to the roots than in sites with greater rainfall and milder temperatures [
17]. [
23] found that individuals of
E. globulus developed less lignotuber in more humid environments, indicating that water stress stimulates the development of this structure. So, it is important to investigate the root biomass of the clones at the PCoppice sites, to confirm the hypothesis that drier sites in the initial rotation are more productive at the beginning of the second rotation due to the higher amount of carbon in the root system.
3.5. How Does the Interaction between Climate and Stocking Affect the Growth of Clones at the Beginning of the Coppice Rotation?
Mortality increased at the beginning of the coppice rotation with increasing stocking and water deficit (
Figure 6). The effect of stocking on water relations is well studied in
Eucalyptus in the initial rotation, but scarce in the coppice rotation. Because the root system is already established, the influence of water deficit might be weaker. Sites with the highest water deficit showed lower productivity gains between wider and tighter stocking, indicating that in these locations, increasing stocking does not reflect a significant increase in biomass production per hectare. The yield difference between the tighter and wider stockings dropped by 50% for every 200 mm more water deficit (
Figure 7). This pattern may result from greater reduction in water availability per tree with tighter stocking, increasing mortality and reducing the increase in productivity. In sites with high water availability, the increase in stocking reflected in significant increases in biomass. [
16] and Hakamada et al. (in. prep) also observed that subtropical sites (with lower water deficits) showed greater productivity responses to stocking than tropical sites in the initial rotation.
[
24] analyzed the effect of stocking on growth and distribution of biomass in individuals of
E. camaldulensis,
E. pellita and
E. urophylla. The authors concluded that, in addition to affecting growth, stocking had an effect on biomass partitioning between above and below ground. At wider stockings (833 trees ha
-1),
E. camaldulensis and
E. urophylla showed increases of around 10% in the proportion of root biomass compared to tighter stockings (2,222 trees ha
-1). So, in the wider stockings the trees are larger and the biomass partitioning to the roots is greater, resulting in root systems with higher amounts of carbon. The authors end their paper by concluding that “since larger root systems may increase future coppice growth yields, the effect of differences in allocation to the root system on future productivity needs to be evaluated.” Considering the positive effect of increased amount of carbon in the root system on sprouting vigor, the results of this work may provide insights to the hypothesis raised by [
24]. The lower amount of carbon in roots at the tighter stockings may be the explanation for the high mortality rates at sites with high water deficit, making it necessary to plant 3,000 more trees ha
-1 at these sites to achieve a productivity increase of only 3 Mg ha
-1. Therefore, although the trees begin the coppice rotation with a developed root system, it is not sufficient to guarantee the survival of very tight stands in sites with high water deficit.
4. Materials and Methods
4.1. Site Description
The eight experimental sites (
Figure 8) are distributed between latitudes 6° S and 30° S (
Table 2), with mean annual temperatures differing by up to 6.5 °C, and mean annual precipitation ranging from 600 mm. Each experimental site comprised plots designed for testing clones with 120 trees, planted in 8 rows of 15 trees at the standard plantation spacing of 3 x 3 m (1,100 trees ha
-1) in tropical Brazilian sites. The first five trees in each row were used for destructive sampling in the initial rotation, so the useful plot consists of 80 trees (8 rows x 10 trees). Additionally, three of the sites continued a spacing trial with plots of 7 rows by 27 trees, with varying distances between trees and stand densities ranging 450 to 14,000 trees ha
-1 (
Figure 9). For this study, we considered the trees planted at stand densities ranging from 500 to 3,500 trees ha
-1 as useful plots.
4.2. Genotypes
Seventeen commercial
Eucalyptus clones were evaluated, nine tropical clones (A1, C3, D4, E5, G7, H8, P7, Q8, R9), and eight subtropical clones (F6, I9, J1, K2, L3, M4, N5, O6), according to its genotype and climate of the breeding pipeline site (
Table 3). The genotypes were grouped by the breeders of the TECHS group, as shown in the paper published by [
16] (
refer to Figure 2 on page 3). Genotypes previously classified as plastic in the TECHS study (A1, C3, K2, and Q8) were reclassified as tropical or subtropical in this study. This reclassification was necessary because they did not exhibit truly plastic behavior during the initial rotation.
4.3. Climatic Data and Water Balance
The climate data were sourced from the NASA/POWER platform - NASA Langley Research Center POWER Project, providing daily estimates of climate variables derived from predictive models utilizing images from the MERRA-2 satellite, at a 0.5 x 0.625-degree resolution [
26]. Daily scale series of maximum, minimum, and average temperature (°C), dew point temperature (°C), precipitation (mm), relative humidity (%), wind speed at 2 meters height (m s
-1), surface pressure (kPa), and incident radiation (MJ m
-2 day
-1) were collected from 01/01/2010 (2 years prior to the planting of the initial rotation) to 12/31/2022. Accumulated degree days were calculated [
27] as:
where DD Accumulated = accumulated degree days (°C),
Tmin = daily minimum temperature (°C),
Tmax = maximum daily temperature (°C),
Ti = lower basal temperature of the crop (10 °C) [
28], n = number of days.
The soil water balance was calculated using an adapted version of the Thornthwaite and Mather (TM) method [
29], proposed by [
30]. The water holding capacity of each site was taken from [
16] (
Table 2). Reference evapotranspiration (ETo) was calculated using the Penman-Montheith (PM) method [
31]. This calculation employed the using the PM function from the SPEI package [
32] whitin the R software [
33]. The computation of Eto relied on data for minimum temperature, maximum temperature, dew point temperature, wind speed, latitude, incident radiation, relative humidity, atmospheric pressure, and altitude. The main results obtained from TM water balance method include estimates of soil water storage, surplus, and water deficit.
4.4. Establishment of the Coppice Rotation
The original rotation of the TECHS project was harvested between 2019 to 2021. Different companies employed various harvesting system, including long log systems (Feller Buncher + Skidder), short log systems (Harvester + Forwarder, or Feller Buncher + tracer claw + Forwarder), and semi-mechanized cutting (chainsaw). Despite the system used, all stumps were cut at a height of 10 to 15 cm to avoid damage.
Ant control baits were applied approximately 2 months before harvest, adhering to local company protocols. Harvested stems were removed from the sites within a month, along with residues that could potentially shade or impede the growth of the new sprouts. Fertilizer applications followed the patterns used by each company in their commercial coppice areas, typically consisting of 1,950 kg ha-1 of limestone, 66 kg N ha-1, 65 kg P ha-1, and 120 kg K ha-1 on average. Weed control was performed approximately twice until canopy closure, employing glyphosate at a rate of 2.88 kg of active ingredient ha-1, applied manually with care to avoid contact with trees leaves. After 12 months, weed control with the same method was performed in average once a year, depending on the degree of weed infestation.
Multiple shoots per stump were pruned at 6 months post-harvest. However, pruning was delayed till about 12 months on sites 22 and 35, due to concerns regarding potential strong winds, allowing the sprouts to develop greater resistance. Across all sites, only the most vigorous sprout was retained, and in the event of mortality, two sprouts were maintained on the subsequent tree to uphold stand density (
Figure 10).
4.5. Growth Measurements
The initial evaluation, conducted at 6 months pre-pruning, aimed to determine survival rates and estimate sprouting biomass for each genotype. Sprouting rate was defined as the percentage of stumps from the initial rotation that sprouted. Given that not all initially planted trees survived until the end of the initial rotation, survival was calculated based on the number of sprouted stumps relative to the original planting density. Sprout biomass estimation relied on allometries equations adjusted in this study, utilizing total height and the number of sprouts per stump. Allometric equations were developed with destructively sampled sprouts was carried out to adjust the specific equations for each plot, and general equations applicable to the genus
Eucalyptus:
where
= shoot dry biomass in kg DM individual
-1 and
= total individual height. R² = 0.86.
To capture the variability in the height of individuals, four height classes were determined in each plot using the mean and standard deviation.1. Eight individuals per plot were sampled, with two individuals per height class. These selected individuals underwent uniform sprouting, where one or two main shoots were kept and the rest were removed. The removed shoots were separated into leaves, twig, and wood, and each compartment had its wet weight measured in the field. Samples of each component were weighted and then dried in the laboratory. To factor in the biomass of the retained shoots on the stumps, an average dry biomass per removed shoot was calculated for each individual, and this average was added to the dry biomass of the removed shoots.
After pruning, forest inventories were conducted every six months until 24 months of age, and annually thereafter. However, only the site 20 was old enough to carry out annual measurements. Therefore, they were not used in comparative analyzes between sites, only in the comparisons between growth in the initial rotation and in the coppice rotation on the same site. Height and DBH of the trees were measured using a Haglof clinometer and a diametric tape.
At sites that have not yet reached the expected age for destructive biomass sampling (36 months), dry mass (B, in kg tree-¹) of wood was predicted using the linearized model of Schumacher and Hall [
34], with a clone-specific intercept fitted by [
35]:
where
is the dry wood mass in kg tree
-1 of clone
,
is the specific intercept of clone
,
is the diameter at breast height in centimeters, and
is the total height of the tree in meters.
Due to the differences in age between the sites, a growth index was generated to represent the ranking of the clones in each site and allow for comparison between them even with different ages. The growth index was defined as a value that can range from 0 to 1, where 1 represents the productivity of the most productive plot of the site.
Thus, the indices of the other plots are defined by dividing their productivity by the productivity of the most productive plot on the site:
where
is the growth index of clone j at site
s,
is the productivity of clone j on site
s, and
is the maximum productivity (yield of the most productive clone) of site
s.
Thus, this index represents the position of a clone in relation to the most productive clone on the site, allowing comparison between clones regardless of the age of measurement.
To compare the uniformity between individuals in the plots, the PV50 index proposed by [
36] was used, which consists of the percentage of total biomass present in 50% of the smallest trees in the plot, including planting gaps. PV50 requires the ordering of trees from the smallest to the largest tree in individual volume and is calculated according to the following equation:
where PV50 = Accumulated percentage of the individual volume of the 50% smallest trees planted, V = individual volume of plot i at age j and n = number of trees planted in order (from smallest to largest).
4.6. Data Analysis
Comparisons between clones were made with descriptive graphs of sprout and woody biomass grouped by clone type. The averages were calculated using the available plots from each group, with a larger n for the group of tropical clones due to the greater presence of tropical sites in the experiment. The error bars indicate the standard error of the mean.
Growth differences from the initial rotation into the coppice rotation were compared at the individual level, at the plot level, and at the clone level, with graphical analysis and linear regression. The averages of the variables analyzed (survival, growth rate and PV50) were calculated in the first measurement of the plots in the second rotation, and in the last measurement of the same plots in the initial rotation. For the results shown at the clone level, the averages of all the plots available for each genotype were calculated.
The relationships between climate variables and growth variables in the first and coppice rotation were examined with graphical analysis and Pearson correlation. The average sprouting height at 6 months and stem biomass at 12 months were calculated for the 4 sites with measurements at these ages, taking into account all the plots planted at each site. A correlation matrix was generated with the climatic variables, and the strongest correlations are shown in the results.
Finally, to verify the interaction between climate and stocking on the growth of the clones, three-dimensional plots were generated by Curve Expert to describe the behavior of mortality and biomass production at different stockings and water deficit ranges. All the other analyses were performed in R software [
33].
5. Conclusions
The development of the root system in the initial rotation has a direct effect on the initial sprouting. Sites with high water stress promote a more pronounced development of the root system than humid sites. Furthermore, clones developed in drier regions tend to allocate proportionally more C to the roots.
This study presents the hypothesis that water stress conditions in the initial rotation may favor the sprouting of materials at the beginning of the coppice rotation. This is because sprout growth is mostly determined by the accumulation of reserves in the roots, especially in clones adapted to water stress conditions. However, after the first year of coppice rotation, rainfall is expected to have more of an effect on growth, favoring wetter sites.
We evaluated the survival and initial growth of the interaction of 17 genotypes, 8 contrasting climate sites, and densities between 450 and 14 thousand trees ha
-1 in
Eucalyptus clone sprouts. Despite the wide variation in factors, which makes the study unprecedented, we recognize some weaknesses in the methods. The existence of hybrid clonal materials from contrasting species, such as clone C3, a hybrid of
E. grandis and
E. camaldulensis, precludes the possibility of broadening the discussion about the response of a particular species, since other clones with the same species can have completely different results [
37]. The absence of replications at the same site and the lack of standardization of treatments make it challenging to identify independent variables, as there are sites that do not have precisely the same genotypes. Nevertheless, this analysis with repetitions along the climatic gradient, in which the predictor variable becomes the site’s water deficit, has been employed in forestry and ecology studies and allows the population of interest to be broadened [
38,
39]. Finally, it should be acknowledged that the evaluations were conducted at an early age, and the results may undergo significant changes over time. However, some studies conducted on fast-growing stands in Brazil have demonstrated that early growth results (up to one year) are highly correlated with the final outcomes of the study.
Author Contributions
P.G.F. created a first version of the manuscript, which was further improved in cooperation with all co-authors, conceptualized the study, conducted the data processing, model application, statistical analysis; P.V.P. in cooperation with T.Q., J.S.B., J.S., F.S.O., F.T.M., R.A., G.M.S. and G.G.C.S. supervised the work and designed the field campaign, accompanying data collection and validation; C.A.A. designed the project methodology and data validation; SBBM and RRS assisted in the preparation of the published work; R.E.H. provided statistical advice, supported the data processing, analysis, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the CAPES (Higher Education Personnel Improvement Coordination).
Acknowledgments
We thank PCoppice group (Cooperative Program on Sprouting Productivity of
Eucalyptus clones) for supporting the project, which was possible only through the coordination provided by Forestry Science and Research Institute (IPEF,
https://www.ipef.br/pcoppice/). The following companies support PCoppice: Aço Verde do Brasil, Bracell, CMPC, Dexco, Gerdau, Klabin, Suzano, Sylvamo and Vallourec. Several universities and institutes also contributed to PCoppice: University of Sao Paulo, Sao Paulo State University, Federal Rural University of Pernambuco and Federal University of Rio Grande do Norte.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Hawley, R.C. The Practice of Silviculture: With Particular Reference to Its Pplication in the United States; John Wiley & Sons, Inc: New York, 1921;
- Andrade, E.N. O Eucalipto; 2a.; Typografia Brasil Rothschild: São Paulo, 1961;
- Ford-Robertson, F.C. Terminology of Forest Science, Technology, Practice and Procedures; Society of American Foresters: Washington D.C., 1971;
- Camargo, F.R.A.; Silva, C.R.; Stape, J.L. Resultados Experimentais Da Fase de Emissão de Brotação Eucalyptus Manejado Por Talhadia. Série Técnica IPEF 1997, 11, 115–122.
- Silva, N.F. da; Barros, N.F. de; Neves, J.C.L.; Schulthais, F.; Novais, R.F. de; Mattiello, E.M. Yield and Nutrient Demand and Efficiency of Eucalyptus under Coppicing Regime. Forests 2020, 11, 852 . [CrossRef]
- Higa, R.C.V.; Sturion, J.A. Capacidade de Brotação Em Subgêneros e Espécies de Eucalyptus. Série Técnica IPEF 1997, 11, 23–30.
- Little, K.M.; Gardner, R.A. Coppicing Ability of 20 Eucalyptus Species Grown at Two High-Altitude Sites in South Africa. Canadian Journal of Forest Research 2003, 33, 181–189. [CrossRef]
- Barnard, E.L.; Geary, T.; English, J.T.; Gilly, S.P. Basal Cankers and Coppice Failure of Eucalyptus Grandis in Florida. Plant Dis 1987, 71, 358–361. [CrossRef]
- Burrows, G.E. Epicormic Strand Structure in Angophora, Eucalyptus and Lophostemon (Myrtaceae): Implications for Fire Resistance and Recovery. New Phytologist 2002, 153, 111–131.
- Teixeira, P.C.; Novais, R.F.; Barros, N.F.; Neves, J.C.L.; Teixeira, J.L. Eucalyptus Urophylla Root Growth, Stem Sprouting and Nutrient Supply from the Roots and Soil. For Ecol Manage 2002, 160, 263–271. [CrossRef]
- Gonçalves, J.L. de M.; Alvares, C.A.; Behling, M.; Alves, J.M.; Pizzi, G.T.; Angeli, A. Productivity of Eucalypt Plantations Managed under High Forest and Coppice Systems, Depending on Edaphoclimatic Factors. Scientia Forestalis/Forest Sciences 2014, 40, 411–419.
- Pereira Filho, G.M.; Jacovine, L.A.G.; Schettini, B.L.S.; Paiva, H.N. de; Leite, F.P.; Villanova, P.H.; Rocha, S.J.S.S. da; Leite, H.G. Correlação Entre Dimensões Das Árvores de Eucalipto Em Alto Fuste e Talhadia. Sci For 2020, 48, 1–8. [CrossRef]
- Soares, A.A.V.; Scolforo, H.F.; Forrester, D.I.; Carneiro, R.L.; Campoe, O.C. Exploring the Relationship between Stand Growth, Structure and Growth Dominance in Eucalyptus Monoclonal Plantations across a Continent-Wide Environmental Gradient in Brazil. For Ecol Manage 2020, 474, 118340. [CrossRef]
- Willebrand, E.; Ledin, S.; Verwijst, T. Willow Coppice Systems in Short Rotation Forestry: Effects of Plant Spacing, Rotation Length and Clonal Composition on Biomass Production. Biomass Bioenergy 1993, 4, 323–331. [CrossRef]
- Germon, A.; Laclau, J.-P.; Robin, A.; Jourdan, C. Tamm Review: Deep Fine Roots in Forest Ecosystems: Why Dig Deeper? For Ecol Manage 2020, 466, 118135. [CrossRef]
- Binkley, D.; Campoe, O.C.; Alvares, C.A.; Carneiro, R.L.; Cegatta, Í.R.; Stape, J.L. The Interactions of Climate, Spacing and Genetics on Clonal Eucalyptus Plantations across Brazil and Uruguay. For Ecol Manage 2017, 405, 271–283 . [CrossRef]
- Campoe, O.C.; Alvares, C.A.; Carneiro, R.L.; Binkley, D.; Ryan, M.G.; Hubbard, R.M.; Stahl, J.; Moreira, G.; Moraes, L.F.; Stape, J.L. Climate and Genotype Influences on Carbon Fluxes and Partitioning in Eucalyptus Plantations. For Ecol Manage 2020, 475, 118445. [CrossRef]
- Palacio, S.; Maestro, M.; Montserrat-Martí, G. Relationship between Shoot-Rooting and Root-Sprouting Abilities and the Carbohydrate and Nitrogen Reserves of Mediterranean Dwarf Shrubs. Ann Bot 2007, 100, 865–874. [CrossRef]
- Bond, W.J.; Midgley, J.J. Ecology of Sprouting in Woody Plants: The Persistence Niche. Trends Ecol Evol 2001, 16, 45–51. [CrossRef]
- Amâncio, M.R.; Pereira, F.B.; Paludeto, J.G.Z.; Vergani, A.R.; Bison, O.; Peres, F.S.B.; Tambarussi, E.V. Genetic Control of Coppice Regrowth in Eucalyptus Spp. Silvae Genet 2020, 69, 6–12. [CrossRef]
- Binkley, D.; Campoe, O.C.; Alvares, C.A.; Carneiro, R.L.; Stape, J.L. Variation in Whole-Rotation Yield among Eucalyptus Genotypes in Response to Water and Heat Stresses: The TECHS Project. For Ecol Manage 2020, 462, 117953. [CrossRef]
- Ledo, A.; Paul, K.I.; Burslem, D.F.R.P.; Ewel, J.J.; Barton, C.; Battaglia, M.; Brooksbank, K.; Carter, J.; Eid, T.H.; England, J.R.; et al. Tree Size and Climatic Water Deficit Control Root to Shoot Ratio in Individual Trees Globally. New Phytologist 2018, 217, 8–11.
- Whittock, S.P.; Apiolaza, L.A.; Kelly, C.M.; Potts, B.M. Genetic Control of Coppice and Lignotuber Development in Eucalyptus Globulus. Aust J Bot 2003, 51, 57–67. [CrossRef]
- Bernardo, A.L.; Reis, M.G.F.; Reis, G.G.; Harrison, R.B.; Firme, D.J. Effect of Spacing on Growth and Biomass Distribution in Eucalyptus Camaldulensis, E. Pellita and E. Urophylla Plantations in Southeastern Brazil. For Ecol Manage 1998, 104, 1–13. [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorologische Zeitschrift 2013, 22, 711–728. [CrossRef]
- Stackhouse, P.W.; Westberg, D.; Chandler, W.S.; Zhang, T.; Hoell, J.M. Prediction Of Worldwide Energy Resource (POWER): Agroclimatology Methodology (1.0° Latitude by 1.0° Longitude Spatial Resolution).
- Villa Nova, N.A.; Pedro Junior, M.J.; Pereira, A.R.; Ometto, J.C. Estimativa de Graus-Dia Acumulados Acima de Qualquer Temperatura Base, Em Função Das Temperaturas Máxima e Mínima. Carderno de Ciências da Terra 1972, 30, 1–8.
- Oliveira, A.S. de; Steidle Neto, A.J.; Ribeiro, A.; Rascon, N.Jr.L.; Rody, Y.P.; Almeida, A.Q. de Determinação Do Tempo Térmico Para o Desenvolvimento de Mudas de Eucalipto Na Fase de Enraizamento. Revista Brasileira de Engenharia Agrícola e Ambiental 2012, 16, 1223–1228. [CrossRef]
- Thornthwaite, C.W.; Mather, J.R. The Water Balance.; 8a.; Drexel Institute of Technology – Laboratory of Climatology: Centerton, 1955;
- Pereira, A.R.; Angelocci, L.R.; Sentelhas, P.C. Meteorologia Agrícola; 4a.; Universidade de São Paulo, Escola Superior de Agricultura “Luiz de Queiroz”: Piracicaba, 2007;
- Allen, R.G.; Smith, M.; Pereira, L.S.; Perrier, A. An Update for the Calculation of Reference Evapotranspiration. ICID ICID Bulletin of the International Commission on Irrigation and Drainage 1994, 43, 35–92.
- Beguería, S.; Vicente-Serrano, S.M. Package ‘SPEI’. Calculation of the Standardized Precipitation-Evapotranspiration Index. CRAN 2023.
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. R Foundation for Statistical Computing 2023.
- Schumacher, F.X.; Hall, F.S. Logarithmic Expression of Timber-Tree Volume. J Agric Res 1933, 47, 719–734.
- Mattos, E.M. de; Binkley, D.; Campoe, O.C.; Alvares, C.A.; Stape, J.L. Variation in Canopy Structure, Leaf Area, Light Interception and Light Use Efficiency among Eucalyptus Clones. For Ecol Manage 2020, 463, 118038. [CrossRef]
- Hakamada, R.E.; Stape, J.L.; Lemos, C.C.Z. de; Almeida, A.E.A.; Silva, L.F. Uso Do Inventário Florestal e Da Uniformidade Entre Árvores Como Ferramenta de Monitoramento Da Qualidade Silvicultural Em Plantios Clonais de Eucalipto. Scientia Forestalis/Forest Sciences 2015, 43, 27–39.
- Romão, K. de C.S.; Nunes, A.C.P.; Santos, A.P. dos; Santos, O.P. dos; Valente, B.M. dos R.T. Multi-Site Comparison of Clonal Arrangements for Tropical and Subtropical Hybrids of Eucalyptus. Ind Crops Prod 2023, 197, 116536. [CrossRef]
- Kreyling, J.; Schweiger, A.H.; Bahn, M.; Ineson, P.; Migliavacca, M.; Morel-Journel, T.; Christiansen, J.R.; Schtickzelle, N.; Larsen, K.S. To Replicate, or Not to Replicate – That Is the Question: How to Tackle Nonlinear Responses in Ecological Experiments. Ecol Lett 2018, 21, 1629–1638.
- Hakamada, R.E.; Binkley, D.; Mattos, E.M. Light Use Efficiency Declines with Water Deficit and Age in Eucalyptus Plantations across Brazil. For Ecol Manage 2023, 549, 121477. [CrossRef]
Figure 1.
Accumulated water deficit from 2012 to 2022 (above), precipitation, average temperature and water deficit in the first year of the coppice rotation at the PCoppice sites (below).
Figure 1.
Accumulated water deficit from 2012 to 2022 (above), precipitation, average temperature and water deficit in the first year of the coppice rotation at the PCoppice sites (below).
Figure 2.
Sprouting biomass averages at 6 months (above) and wood biomass averages at 12 months (below) of clones classified as tropical and subtropical, where n represents the number of plots of each type. The error bar indicates the standard error of the mean.
Figure 2.
Sprouting biomass averages at 6 months (above) and wood biomass averages at 12 months (below) of clones classified as tropical and subtropical, where n represents the number of plots of each type. The error bar indicates the standard error of the mean.
Figure 3.
Relationships between final survival in initial rotation and initial survival in coppice rotation grouped by clone (left), final growth index in initial rotation and initial growth index in coppice rotation (right).
Figure 3.
Relationships between final survival in initial rotation and initial survival in coppice rotation grouped by clone (left), final growth index in initial rotation and initial growth index in coppice rotation (right).
Figure 4.
Relationships between final growth index in initial rotation and initial growth index in coppice rotation at individual level by site (above), final uniformity (PV50) in initial rotation and initial uniformity in coppice rotation by age (below).
Figure 4.
Relationships between final growth index in initial rotation and initial growth index in coppice rotation at individual level by site (above), final uniformity (PV50) in initial rotation and initial uniformity in coppice rotation by age (below).
Figure 5.
Relationships between sprouting height at 6 months (above), stem biomass at 12 months (below) and climatic variables, showing that the driest sites in the initial rotation became the most productive at the beginning of the coppice rotation.
Figure 5.
Relationships between sprouting height at 6 months (above), stem biomass at 12 months (below) and climatic variables, showing that the driest sites in the initial rotation became the most productive at the beginning of the coppice rotation.
Figure 6.
Relationship between mortality in the second rotation (R2), water deficit and stocking.
Figure 6.
Relationship between mortality in the second rotation (R2), water deficit and stocking.
Figure 7.
Relationship between biomass production in the second rotation (R2), water deficit and stocking.
Figure 7.
Relationship between biomass production in the second rotation (R2), water deficit and stocking.
Figure 8.
Location of the 10 PCoppice project sites and the climatic types in which they are located, according to Köppen’s climatic classification. The two sites in yellow were not considered in this work.
Figure 8.
Location of the 10 PCoppice project sites and the climatic types in which they are located, according to Köppen’s climatic classification. The two sites in yellow were not considered in this work.
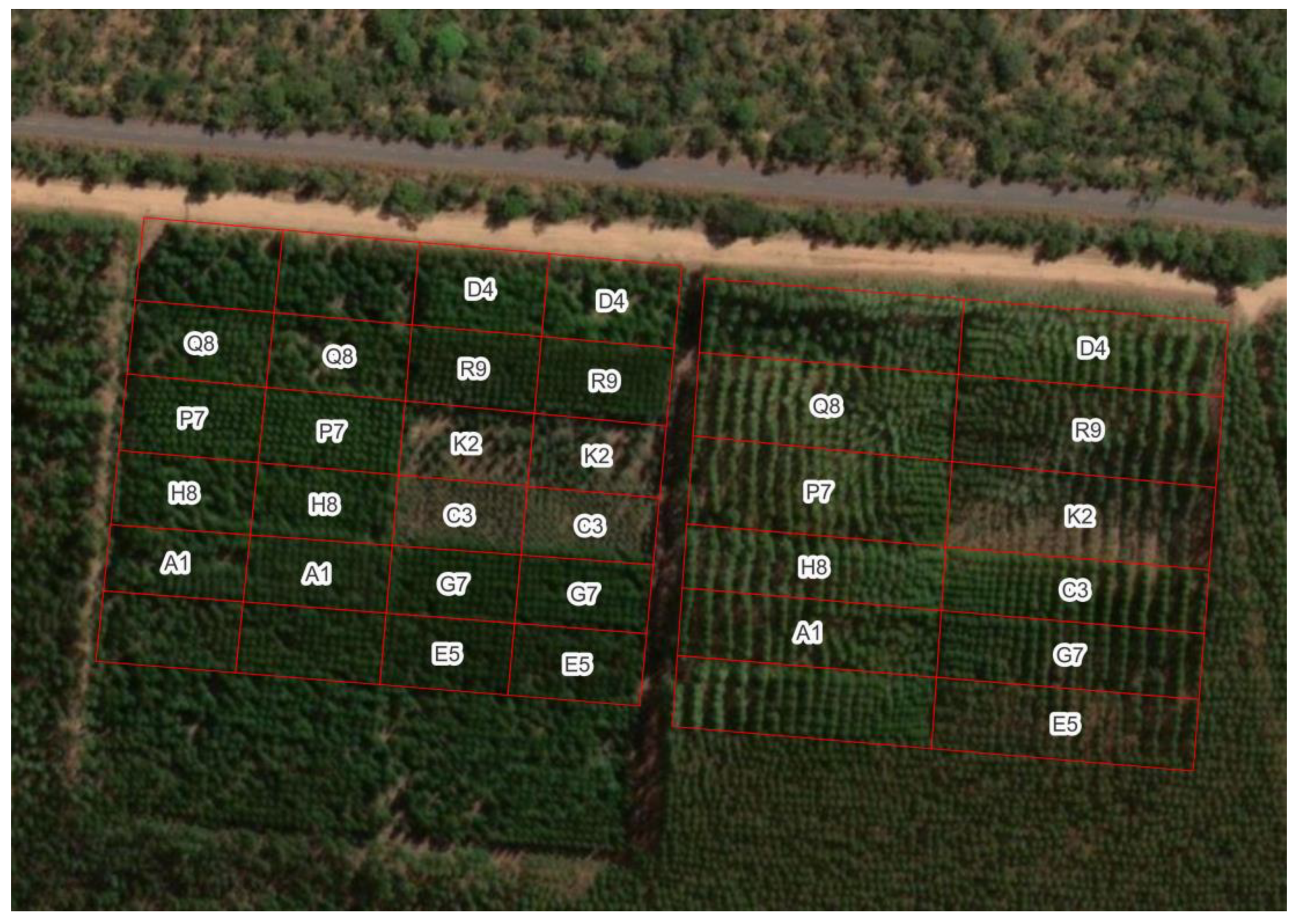
Figure 9.
Clonal test (left) and spacing test (right) at site 17, in Três Marias – Minas Gerais. Plots without the clone acronym have genotypes not evaluated in PCoppice.
Figure 9.
Clonal test (left) and spacing test (right) at site 17, in Três Marias – Minas Gerais. Plots without the clone acronym have genotypes not evaluated in PCoppice.
Figure 10.
Stump before pruning (left) and pruned stump (right) at site 8, in Inhambupe - Bahia.
Figure 10.
Stump before pruning (left) and pruned stump (right) at site 8, in Inhambupe - Bahia.
Table 1.
Pearson correlations between sprouting height at 6 months and stem biomass at 12 months with climatic variables of initial and coppice rotation.
Table 1.
Pearson correlations between sprouting height at 6 months and stem biomass at 12 months with climatic variables of initial and coppice rotation.
| Age (months) |
Variable |
Initial rotation |
Coppice rotation |
| Rainfall |
Average Temperature |
Water deficit |
Rainfall |
Average Temperature |
Water deficit |
| 6 |
Sprouting height (m) |
-0.80 |
0.78 |
0.89 |
-0.48 |
0.89 |
0.81 |
| 12 |
Stem biomass (Mg/ha) |
-0.87 |
0.81 |
0.77 |
0.29 |
0.75 |
0.14 |
Table 2.
The locations of the PCoppice sites spanned a gradient of 3000 km, 6.5 oC in mean annual temperature, and 600 mm of annual rainfall.
Table 2.
The locations of the PCoppice sites spanned a gradient of 3000 km, 6.5 oC in mean annual temperature, and 600 mm of annual rainfall.
| Site |
Lat
(degrees) |
Long
(degrees) |
Altitude
(m) |
Average annual ppt
(mm)1
|
Average temperature
(°C)1
|
Soil Type |
Water holding capacity
(mm)2
|
Average annual water deficit
(mm)1
|
| 6 |
-30.19 |
-51.62 |
150 |
1576.0 |
20.0 |
Ultisol |
145 |
124 |
| 8 |
-11.86 |
-38.37 |
218 |
1131.1 |
25.6 |
Ultisol |
89 |
538 |
| 13 |
-20.9 |
-51.9 |
361 |
1062.3 |
25.2 |
Oxisol |
87 |
558 |
| 17 |
-18.25 |
-45.1 |
806 |
914.3 |
24.1 |
Oxisol |
76 |
938 |
| 20 |
-22.35 |
-46.97 |
633 |
1199.0 |
22.2 |
Oxisol |
165 |
341 |
| 22 |
-24.23 |
-50.53 |
888 |
1458.5 |
20.8 |
Latosol |
214 |
39 |
| 33 |
-23.85 |
-48.7 |
695 |
1386.7 |
20.6 |
Latosol |
196 |
42 |
| 35 |
-5.90 |
-35.35 |
650 |
1047.4 |
26.5 |
Oxisol |
89 |
1020 |
Table 3.
Genotypes evaluated in PCoppice1.
Table 3.
Genotypes evaluated in PCoppice1.
| Code |
Genotype |
Group2
|
Climate of origin3
|
| A1 |
E. urophylla |
Tropical |
Cwa |
| C3 |
E. grandis x E. camaldulensis |
Tropical |
As |
| D4 |
E. grandis x E. urophylla |
Tropical |
Aw |
| E5 |
E. urophylla |
Tropical |
Cwa |
| F6 |
E. benthamii |
Subtropical |
Cfb |
| G7 |
E. urophylla |
Tropical |
Cwa |
| H8 |
E. grandis x E. urophylla |
Tropical |
Am |
| I9 |
E. dunnii |
Subtropical |
Cfb |
| J1 |
E. benthamii |
Subtropical |
Cfb |
| K2 |
E. saligna |
Subtropical |
Cfb |
| L3 |
E. urophylla x E. globulus |
Subtropical |
Cfb |
| M4 |
E. dunnii |
Subtropical |
Cfb |
| N5 |
E. dunnii |
Subtropical |
Cfb |
| O6 |
E. grandis |
Subtropical |
Cfb |
| P7 |
E. urophylla x E. brassiana |
Tropical |
As |
| Q8 |
E. grandis x E. urophylla |
Tropical |
Af |
| R9 |
E. urophylla |
Tropical |
Aw |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).