Submitted:
19 September 2024
Posted:
19 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
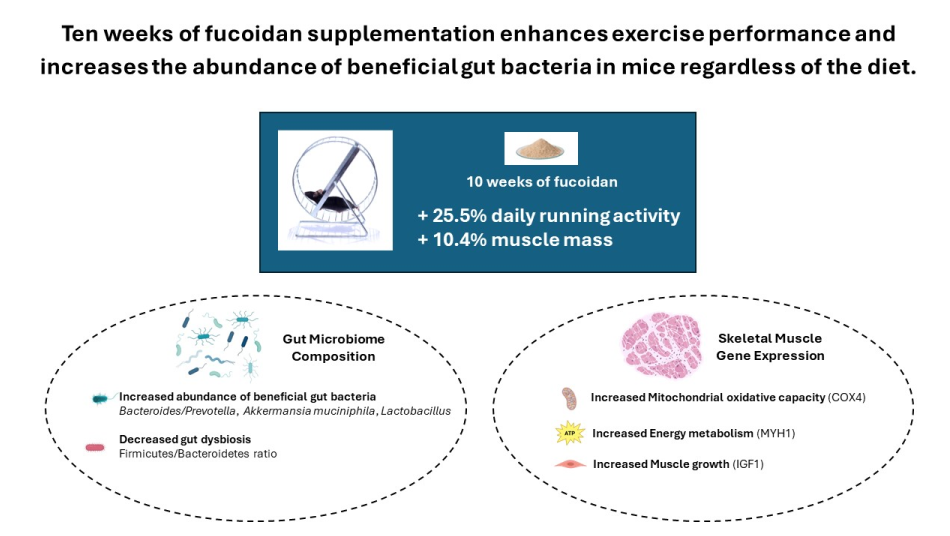
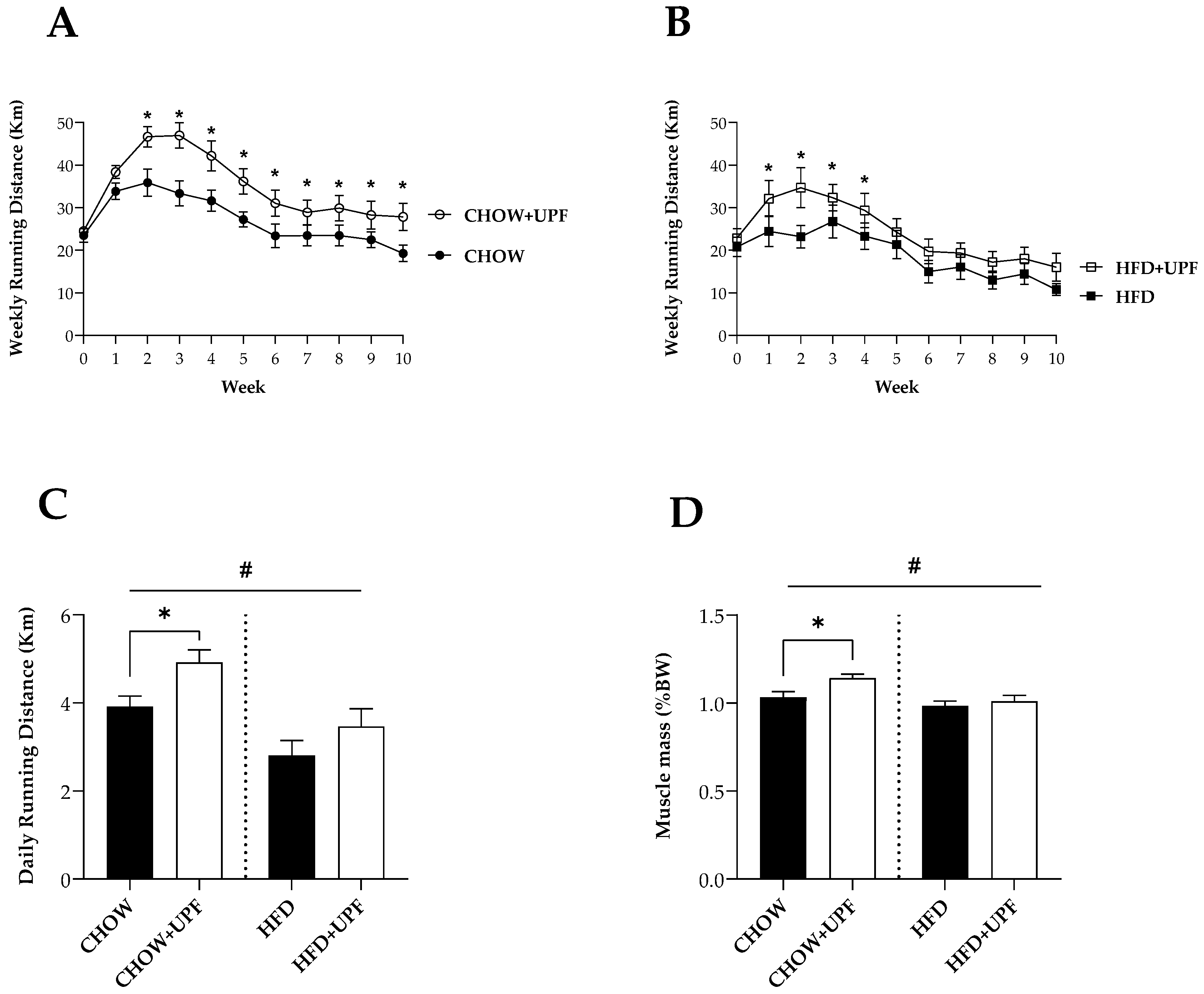
2.1. Effects of UPF on Running Activity and Muscle Mass
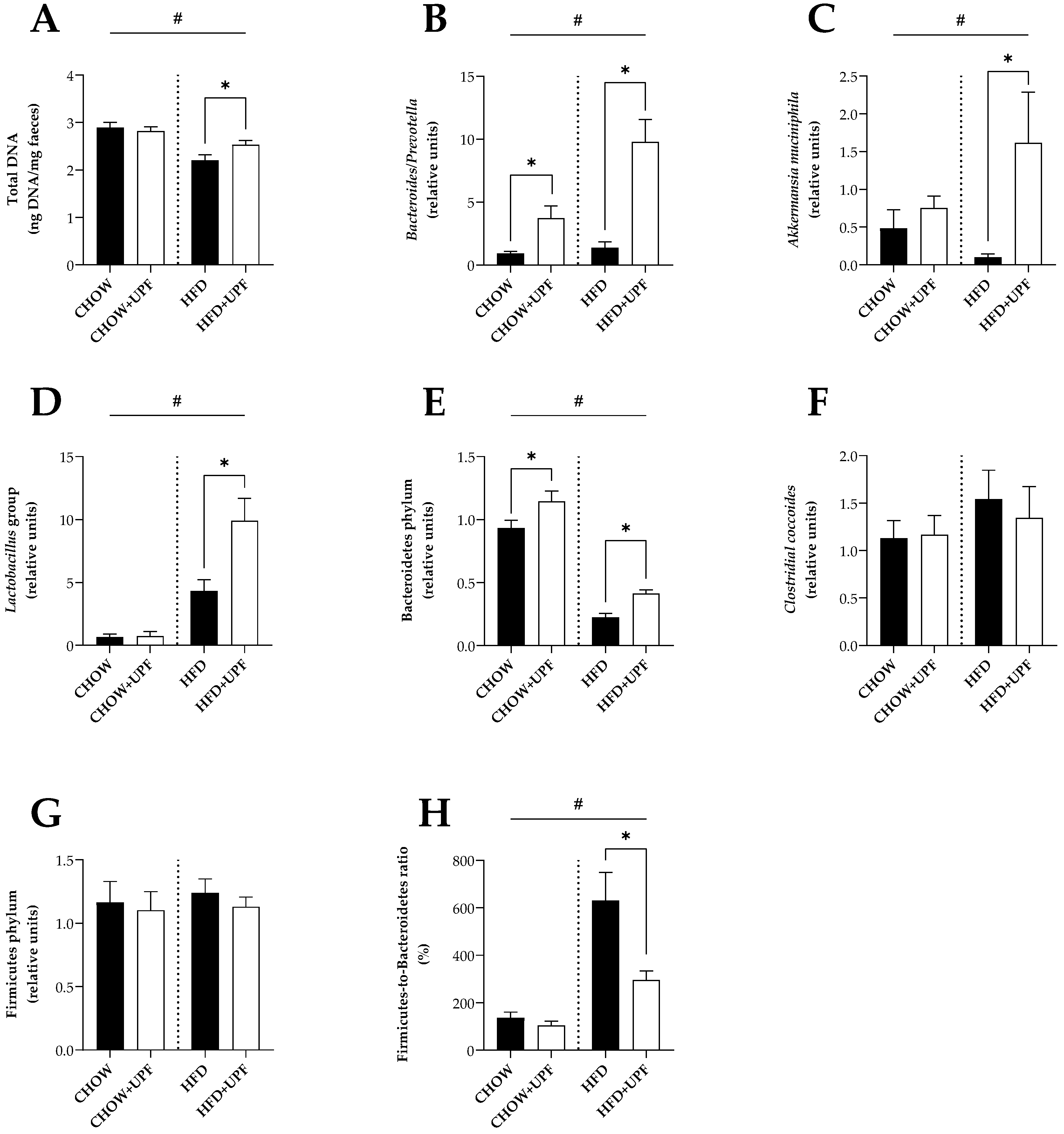
2.2. Effects of UPF on Total DNA Content in the Faeces
2.2.1. Effects of Diet and UPF Administration on Gut Microbial Profiling
2.3. Correlation of Gut Microbial Abundance with Mouse Body Weight, Visceral Fat, and Running Activity
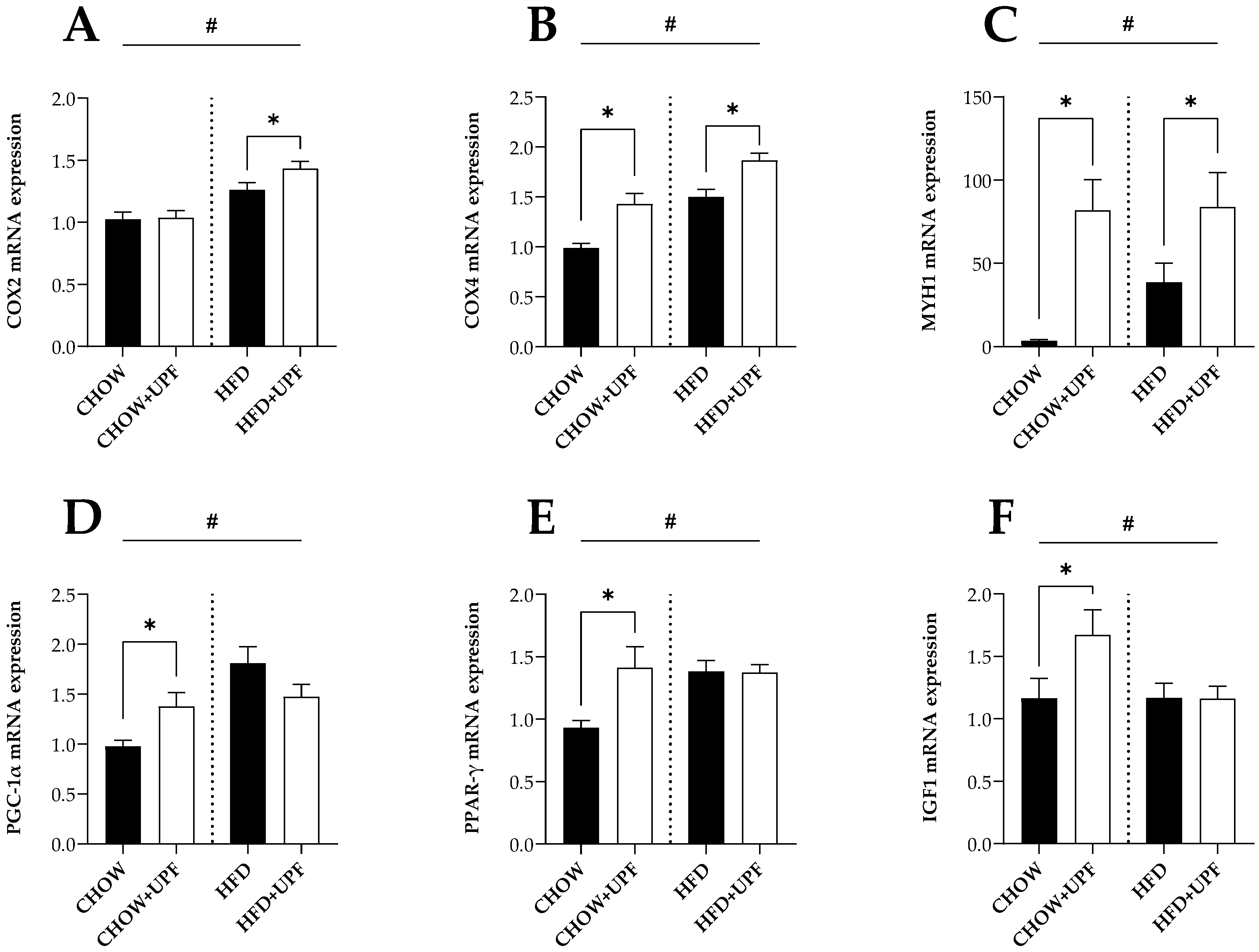
2.4. Effects of Diet and UPF on Muscle Gene Expression
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals and Diet
4.3. Experimental Groups
4.4. UPF Jelly Preparation
4.5. Training and Jelly Administration
4.6. Sample Collection
4.7. Gut Microbial Profile
4.8. Real-Time Quantitative PCR (RT-qPCR) Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li B, Lu F, Wei X, Zhao R. Fucoidan: Structure and Bioactivity. Molecules. 2008;13(8):1671-95. [CrossRef]
- Fitton HJ, Stringer DS, Park AY, Karpiniec SN. Therapies from Fucoidan: New Developments. Mar Drugs. 2019;17(10). [CrossRef]
- Ahmad T, Eapen MS, Ishaq M, Park AY, Karpiniec SS, Stringer DN, et al. Anti-Inflammatory Activity of Fucoidan Extracts In Vitro. Mar Drugs. 2021;19(12). [CrossRef]
- Mathew L, Burney M, Gaikwad A, Nyshadham P, Nugent EK, Gonzalez A, et al. Preclinical Evaluation of Safety of Fucoidan Extracts From Undaria pinnatifida and Fucus vesiculosus for Use in Cancer Treatment. Integr Cancer Ther. 2017;16(4):572-84. [CrossRef]
- Fitton J, Dell’Acqua G, Gardiner V-A, Karpiniec S, Stringer D, Davis E. Topical Benefits of Two Fucoidan-Rich Extracts from Marine Macroalgae. Cosmetics. 2015;2(2):66-81. [CrossRef]
- Cox AJ, Cripps AW, Taylor PA, Fitton JH, West NP. Fucoidan Supplementation Restores Fecal Lysozyme Concentrations in High-Performance Athletes: A Pilot Study. Mar Drugs. 2020;18(8). [CrossRef]
- Irhimeh MR, Fitton JH, Lowenthal RM. Pilot clinical study to evaluate the anticoagulant activity of fucoidan. Blood Coagulation & Fibrinolysis. 2009;20(7):607-10. [CrossRef]
- Ahmad T, Ishaq M, Karpiniec S, Park A, Stringer D, Singh N, et al. Oral Macrocystis pyrifera Fucoidan Administration Exhibits Anti-Inflammatory and Antioxidant Properties and Improves DSS-Induced Colitis in C57BL/6J Mice. Pharmaceutics. 2022;14(11):2383. [CrossRef]
- Chen YM, Tsai YH, Tsai TY, Chiu YS, Wei L, Chen WC, et al. Fucoidan supplementation improves exercise performance and exhibits anti-fatigue action in mice. Nutrients. 2014;7(1):239-52. [CrossRef]
- McBean SE, Church JE, Thompson BK, Taylor CJ, Fitton JH, Stringer DN, et al. Oral fucoidan improves muscle size and strength in mice. Physiol Rep. 2021;9(3):e14730. [CrossRef]
- Ahn J, Ha TY, Ahn J, Jung CH, Seo HD, Kim MJ, et al. Undaria pinnatifida extract feeding increases exercise endurance and skeletal muscle mass by promoting oxidative muscle remodeling in mice. The FASEB Journal. 2020;34(6):8068-81. [CrossRef]
- McFadden BA, Vincenty CS, Chandler AJ, Cintineo HP, Lints BS, Mastrofini GF, et al. Effects of fucoidan supplementation on inflammatory and immune response after high-intensity exercise. J Int Soc Sports Nutr. 2023;20(1):2224751. [CrossRef]
- Antoni L, Nuding S, Weller D, Gersemann M, Ott G, Wehkamp J, et al. Human colonic mucus is a reservoir for antimicrobial peptides. J Crohns Colitis. 2013;7(12):e652-64. [CrossRef]
- Lopez-Santamarina A, Miranda JM, Mondragon ADC, Lamas A, Cardelle-Cobas A, Franco CM, et al. Potential Use of Marine Seaweeds as Prebiotics: A Review. Molecules. 2020;25(4). [CrossRef]
- Mailing LJ, Allen JM, Buford TW, Fields CJ, Woods JA. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc Sport Sci Rev. 2019;47(2):75-85. [CrossRef]
- Clauss M, Gérard P, Mosca A, Leclerc M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front Nutr. 2021;8:637010. [CrossRef]
- Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, et al. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med Sci Sports Exerc. 2018;50(4):747-57. [CrossRef]
- Liu M, Ma L, Chen Q, Zhang P, Chen C, Jia L, et al. Fucoidan alleviates dyslipidemia and modulates gut microbiota in high-fat diet-induced mice. Journal of Functional Foods. 2018;48:220-7. [CrossRef]
- Huang J, Huang J, Li Y, Lv H, Yin T, Fan S, et al. Fucoidan Protects Against High-Fat Diet-Induced Obesity and Modulates Gut Microbiota in Institute of Cancer Research Mice. J Med Food. 2021;24(10):1058-67. [CrossRef]
- Monda V, Villano I, Messina A, Valenzano A, Esposito T, Moscatelli F, et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid Med Cell Longev. 2017;2017:3831972. [CrossRef]
- McLoughlin RF, Berthon BS, Jensen ME, Baines KJ, Wood LG. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: a systematic review and meta-analysis. The American Journal of Clinical Nutrition. 2017;106(3):930-45. [CrossRef]
- Meng D, Ai S, Spanos M, Shi X, Li G, Cretoiu D, et al. Exercise and microbiome: From big data to therapy. Comput Struct Biotechnol J. 2023;21:5434-45. [CrossRef]
- Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, et al. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients. 2020;12(5). [CrossRef]
- Evans CC, LePard KJ, Kwak JW, Stancukas MC, Laskowski S, Dougherty J, et al. Exercise Prevents Weight Gain and Alters the Gut Microbiota in a Mouse Model of High Fat Diet-Induced Obesity. PLOS ONE. 2014;9(3):e92193. [CrossRef]
- Watson SA, McStay GP. Functions of Cytochrome c Oxidase Assembly Factors. International Journal of Molecular Sciences. 2020;21(19):7254. [CrossRef]
- Allen JM, Miller MEB, Pence BD, Whitlock K, Nehra V, Gaskins HR, et al. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. Journal of Applied Physiology. 2015;118(8):1059-66. [CrossRef]
- Aykut MN, Erdoğan EN, Çelik MN, Gürbüz M. An Updated View of the Effect of Probiotic Supplement on Sports Performance: A Detailed Review. Current Nutrition Reports. 2024. [CrossRef]
- Alvehus M, Boman N, Söderlund K, Svensson MB, Burén J. Metabolic adaptations in skeletal muscle, adipose tissue, and whole-body oxidative capacity in response to resistance training. European Journal of Applied Physiology. 2014;114(7):1463-71. [CrossRef]
- Gómez-Pérez Y, Capllonch-Amer G, Gianotti M, Lladó I, Proenza AM. Long-term high-fat-diet feeding induces skeletal muscle mitochondrial biogenesis in rats in a sex-dependent and muscle-type specific manner. Nutrition & Metabolism. 2012;9(1):15. [CrossRef]
- Čunátová K, Reguera DP, Houštěk J, Mráček T, Pecina P. Role of cytochrome c oxidase nuclear-encoded subunits in health and disease. Physiol Res. 2020;69(6):947-65. [CrossRef]
- Kadenbach, B. Complex IV—The regulatory center of mitochondrial oxidative phosphorylation. Mitochondrion. 2021;58:296-302. [CrossRef]
- Pajuelo Reguera D, Čunátová K, Vrbacký M, Pecinová A, Houštěk J, Mráček T, et al. Cytochrome c Oxidase Subunit 4 Isoform Exchange Results in Modulation of Oxygen Affinity. Cells. 2020;9(2):443. [CrossRef]
- Zhang L, Zhou Y, Wu W, Hou L, Chen H, Zuo B, et al. Skeletal Muscle-Specific Overexpression of PGC-1α Induces Fiber-Type Conversion through Enhanced Mitochondrial Respiration and Fatty Acid Oxidation in Mice and Pigs. International Journal of Biological Sciences. 2017;13(9):1152-62. [CrossRef]
- Sellers RS, Mahmood SR, Perumal GS, Macaluso FP, Kurland IJ. Phenotypic Modulation of Skeletal Muscle Fibers in LPIN1-Deficient Lipodystrophic (fld) Mice. Veterinary Pathology. 2019;56(2):322-31. [CrossRef]
- Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, et al. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci U S A. 2008;105(22):7815-20. [CrossRef]
- Yoshida T, Delafontaine P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells. 2020;9(9):1970. [CrossRef]
- Wegierska AE, Charitos IA, Topi S, Potenza MA, Montagnani M, Santacroce L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sports Med. 2022;52(10):2355-69. [CrossRef]
- Cerdá B, Pérez M, Pérez-Santiago JD, Tornero-Aguilera JF, González-Soltero R, Larrosa M. Gut Microbiota Modification: Another Piece in the Puzzle of the Benefits of Physical Exercise in Health? Frontiers in Physiology. 2016;7. [CrossRef]
- Koutouratsas T, Philippou A, Kolios G, Koutsilieris M, Gazouli M. Role of exercise in preventing and restoring gut dysbiosis in patients with inflammatory bowel diseases: A review. World J Gastroenterol. 2021;27(30):5037-46. [CrossRef]
- Abenavoli L, Scarpellini E, Colica C, Boccuto L, Salehi B, Sharifi-Rad J, et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients. 2019;11(11). [CrossRef]
- Belzer C, de Vos WM. Microbes inside--from diversity to function: the case of Akkermansia. Isme j. 2012;6(8):1449-58. [CrossRef]
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066-71. [CrossRef]
- Shang Q, Song G, Zhang M, Shi J, Xu C, Hao J, et al. Dietary fucoidan improves metabolic syndrome in association with increased Akkermansia population in the gut microbiota of high-fat diet-fed mice. Journal of Functional Foods. 2017;28:138-46. [CrossRef]
- Kim NY, Kim JM, Son JY, Ra CH. Synbiotic Fermentation of Undaria pinnatifida and Lactobacillus brevis to Produce Prebiotics and Probiotics. Appl Biochem Biotechnol. 2023;195(10):6321-33. [CrossRef]
- Li H, Dong T, Tao M, Zhao H, Lan T, Yan S, et al. Fucoidan enhances the anti-tumor effect of anti-PD-1 immunotherapy by regulating gut microbiota. Food & Function. 2024;15(7):3463-78. [CrossRef]
- Baenas I, Camacho-Barcia L, Miranda-Olivos R, Solé-Morata N, Misiolek A, Jiménez-Murcia S, et al. Probiotic and prebiotic interventions in eating disorders: A narrative review. European Eating Disorders Review. 2024. [CrossRef]
- Dempsey E, Corr SC. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front Immunol. 2022;13:840245. [CrossRef]
- Sonnenburg ED, Zheng H, Joglekar P, Higginbottom SK, Firbank SJ, Bolam DN, et al. Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell. 2010;141(7):1241-52. [CrossRef]
- Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano G, Gasbarrini A, et al. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019;7(1):14. [CrossRef]
- Takezawa K, Fujita K, Matsushita M, Motooka D, Hatano K, Banno E, et al. The Firmicutes/Bacteroidetes ratio of the human gut microbiota is associated with prostate enlargement. The Prostate. 2021;81(16):1287-93. [CrossRef]
- Stojanov S, Berlec A, Štrukelj B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms. 2020;8(11). [CrossRef]
- An J, Kwon H, Kim YJ. The Firmicutes/Bacteroidetes Ratio as a Risk Factor of Breast Cancer. J Clin Med. 2023;12(6). [CrossRef]
- Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070-5. [CrossRef]
- Mujico JR, Baccan GC, Gheorghe A, Díaz LE, Marcos A. Changes in gut microbiota due to supplemented fatty acids in diet-induced obese mice. Br J Nutr. 2013;110(4):711-20. [CrossRef]
- Fitton JH, Stringer DN, Karpiniec SS. Therapies from Fucoidan: An Update. Mar Drugs. 2015;13(9):5920-46. [CrossRef]
- Health N, Council MR. Australian code of practice for the care and use of animals for scientific purposes: Australian Government Publishing Service; 1997. [CrossRef]
- Zhang, L. Method for voluntary oral administration of drugs in mice. STAR Protoc. 2021;2(1):100330. [CrossRef]
- Chaplin A, Parra P, Serra F, Palou A. Conjugated Linoleic Acid Supplementation under a High-Fat Diet Modulates Stomach Protein Expression and Intestinal Microbiota in Adult Mice. PLoS One. 2015;10(4):e0125091. [CrossRef]
- Mujico JR, Baccan GC, Gheorghe A, Díaz LE, Marcos A. Changes in gut microbiota due to supplemented fatty acids in diet-induced obese mice. British Journal of Nutrition. 2013;110(4):711-20. [CrossRef]
- Pfaffl, MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. [CrossRef]
| Bacterial Groups/species | Body Weight | Visceral Fat | Running Distance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | ||||
| Bacteroidetes phylum | - | 0.708 ** | 0.0001 | - | 0.779 ** | 0.0001 | 0.598 ** | 0.0001 | |
| Firmicutes phylum | 0.136 | 0.4022 | 0.111 | 0.4950 | - | 0.261 | 0.1035 | ||
| Clostridial coccoides | 0.107 | 0.5362 | 0.217 | 0.2039 | - | 0.430 ** | 0.0089 | ||
| Akkermansia muciniphila | - | 0.399 * | 0.0354 | - | 0.140 | 0.4756 | 0.420 * | 0.0259 | |
| F/B ratio | 0.568 ** | 0.0001 | 0.593 ** | 0.0001 | - | 0.559 ** | 0.0002 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





