1. Introduction
Keratins are structural fibrous proteins found in animal epithelial cells, e.g., feathers, claws, horns and hooves, highly cross-linked with disulfide and hydrogen bonds, containing approx. 50-60% hydrophobic amino acids. These properties result in the high mechanical strength of keratins but also their insolubility in most aqueous and organic solvents, making the isolation and processing of keratins challenging [
1,
2,
3]. The most abundant source of keratins is feathers, generated in approx. 8.5 billion tons annually by the poultry industry [
4]. Despite the high protein content, approx. 90%, the raw feathers are considered troublesome wastes due to their insolubility, indigestibility, strong resistance to natural proteolytic degradation and numerous surface microorganisms, including pathogenic ones. Therefore, the current methods for managing poultry feather waste include anaerobic digestion to produce biogas as well as pyrolysis and gasification to generate fuel [
5,
6]. However, beyond direct use, feathers can be processed into a more valuable product – soluble keratin with a wide range of applications [
7,
8].
To efficiently extract keratin, the waste feathers need to be purified. A typical feather pre-treatment approach, commonly used in laboratory methods, focuses on removing surface fat and includes mechanical processing such as shredding, to increase their surface area and enhance the penetration of chemical agents [
2,
7]. Despite its routine use, this method is time-consuming, energy-intensive, chemical-based, and poses scalability challenges. Moreover, the impact of feather pre-treatment on the extracted keratin's properties remains unclear, prompting the need for optimized processes that can be effectively scaled up. Recently, some reports have suggested that organic solvent degreasing, typically using hexane or petroleum ether, can be effectively replaced by washing feathers with detergents, such as 1% Triton X-100 or commercially available detergents (Ludwik® and Persil®), simplifying the process and reducing the use of hazardous chemicals [
9,
10].
Numerous methods of keratin extraction/solubilization have been developed so far, involving enzymatic, chemical (redox and hydrolysis reactions) and physical treatments, or their combination [
1]. Enzymatic treatment offers specificity but requires expensive enzymes and often results in a low keratin yield, typically below 50% [
11], while chemical reduction involves potentially hazardous chemicals. Thus, alkaline hydrolysis has emerged as a promising alternative [
8,
12,
13]. It not only provides a high keratin yield but also a straightforward process. Unlike many other chemical processes, this method requires only sodium hydroxide (for hydrolysis) and hydrochloric acid (for neutralization). However, the conditions of alkaline hydrolysis, including alkali concentration, reaction time, and temperature, strongly affect the keratin yield and physicochemical properties of soluble keratin obtained. It emphasizes the need to balance maximizing yield and maintaining high product quality. Although high alkali concentration reaction and temperature increase the probability of toxic by-product formation, these parameters facilitate reaching a higher keratin yield by increasing the degree of hydrolysis (DH) while simultaneously decreasing the protein's molecular weight [
12,
14,
15].
The soluble keratin has demonstrated great potential in the formulation of novel bioproducts. A large number of anionic groups, i.e., carboxyl, hydroxyl, and thiol groups in the polypeptides, facilitates the sorption of wastewater pollutants, including copper, aluminium, and lead [
16], making keratin-based materials useful for water treatment. Self-assembly, biocompatibility, ability to support cell proliferation, and lack of cytotoxicity enable the use of keratin in the formation of sponges, scaffolds, hydrogels, and wound dressing materials suitable for biomedical applications [
8]. Fractions with low DH and high molecular weight (HMW) display high structural similarity to synthetic polymers and allow the production of bioplastics useful as food packaging materials [
12]. HMW and middle molecular weight fractions show also antimicrobial activity [
17,
18], so keratin-based materials could extend the shelf life of food products. The high content of cysteine and hydrophobic amino acid residues grants keratin significant antioxidant activity [
19,
20,
21], with low molecular weight (LMW) fraction exhibiting stronger antioxidant properties than that of HMW or intact proteins [
22,
23,
24,
25]. LMW fractions also contain bioactive peptides and amino acids of high nutraceutical and cosmeceutical potential [
26,
27,
28]. Therefore, keratins can be used in food production as a valuable alternative source of protein in the diet and as a flavour enhancer, leavening agent for bakery, artificial meat flavour, and dietary supplements with particularly valuable antioxidant activity [
29,
30,
31].
Considering the growing interest in sustainable and biodegradable materials with added functional properties, beneficial in diverse applications ranging from food and biomedicine to environmental remediation, it is necessary to understand how biomass (feather) pre-treatment method and alkaline hydrolysis conditions affect the structural properties and biological activity of keratin. Although studies on keratin extraction from by-products of the food industry have been ongoing for many years [
8], the industrial use of poultry feathers remains insufficient. Currently employed laboratory methods for pre-treating and extracting soluble keratins, while effective, could be simplified and made more economically attractive for industrial applications due to their complexity and operational costs. According to Wang and Tong [
32], finding scalable methods is essential to expanding the applications of keratin.
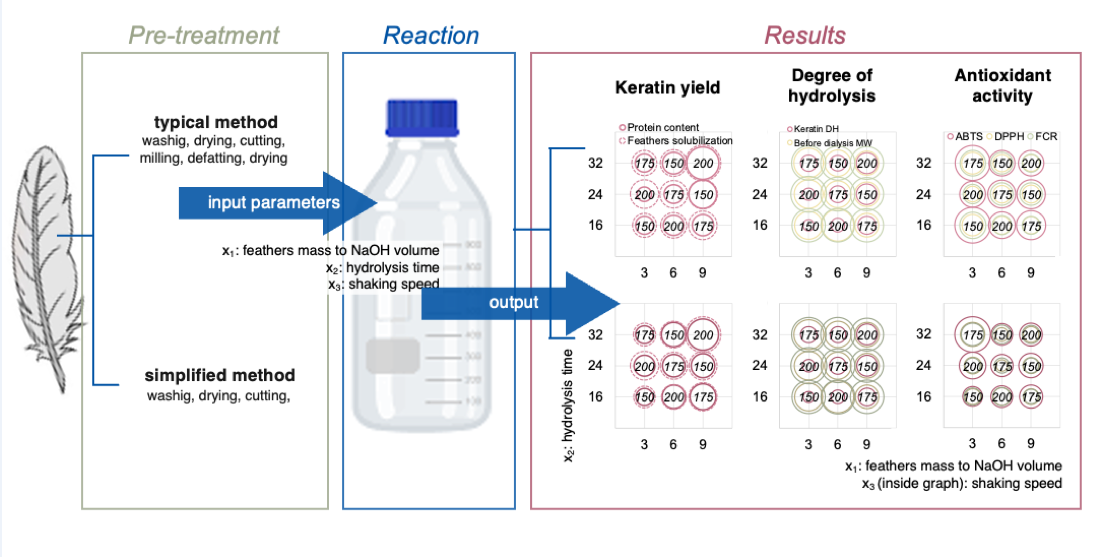
To address the challenges in keratin extraction, this study compares the standard feather pre-treatment method with a newly developed, significantly simplified method with higher scalability potential. The analysis focused on how the feather pre-treatment methods affect the efficiency of alkaline hydrolysis and the quality of keratin extracted, which is crucial for its functional and commercial applications. To compare the impact of pre-treatment methods on the obtained keratin, feathers pre-treated with typical or simplified methods were subjected to alkaline hydrolysis with three variable conditions, i.e. feather mass to NaOH volume ratio, time of hydrolysis and shaking speed. The more intensive hydrolysis conditions were expected to increase both the keratin yield and its antioxidant activity due to higher protein fragmentation, resulting in higher amounts of LMW peptides. To the best of our knowledge, this is the first study to systematically investigate the effects of typical vs simplified chicken feather pre-treatment methods and three different input variables (feather mass to NaOH volume ratio, time, and shaking speed) in the process of alkaline feather hydrolysis, examining the keratin yield, molecular weight, and antioxidant properties of the obtained keratin hydrolysates.
2. Materials and Methods
2.1. Materials
White chicken feathers were supplied by a local company (Drobful, Poland). Petroleum ether (POCH, Poland) was used for feather defatting, while sodium hydroxide (POCH, Poland) was used for hydrolysis. Sulfuric acid and Tashiro’s indicator (POCH, Poland), selenium catalyst (Chempur, Poland), boric acid and hydrochloric acid (Stanlab, Poland) were applied to determine the protein content by the Kjeldahl method. O-phthaldialdehyde (OPA), sodium tetraborate, sodium dodecyl sulphate (SDS) (Sigma Aldrich, USA), L-leucine (POCH, Poland) and dithiothreitol (DTT) (Chempur, Poland) were utilized for determination of keratin DH. A mobile phase of acetonitrile (POCH, Poland) and trifluoroacetic acid, along with molecular weight standards (Sigma Aldrich, USA) was used to determine molecular weight distribution. Investigation into the antioxidant activity of keratin hydrolysates involved 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS), 2,2’-diphenylo-1-picryl-hydrazyl (DPPH), Folin-Ciocâlteu reagent (FCR) (Sigma Aldrich, USA), as well as 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), sodium persulfate (Sigma Aldrich, USA) and methanol (POCH, Poland).
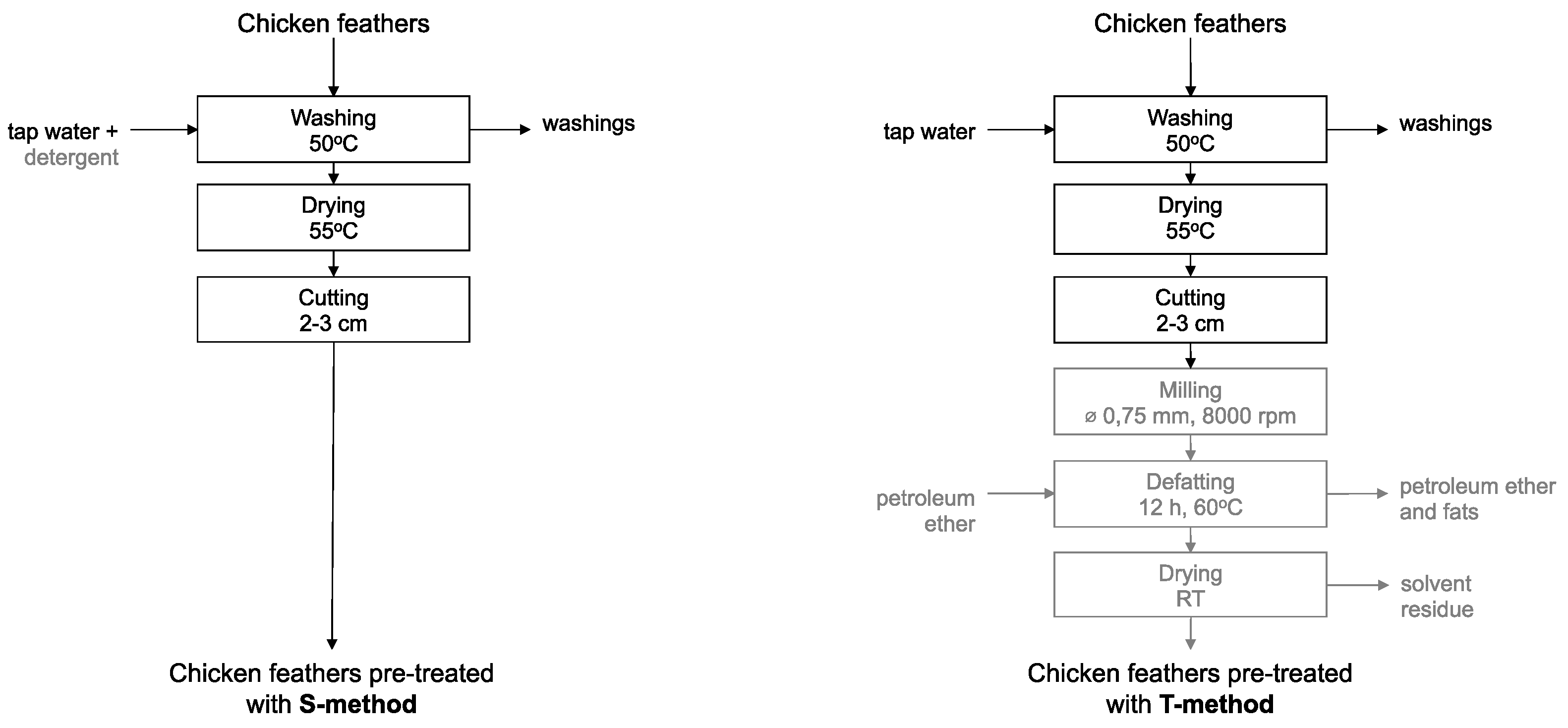
2.2. Feathers Pre-Treatment Methods
To achieve efficient extraction, feathers were pre-treated using the previously described methods: the typical pre-treatment (T-method) [
13] or the simplified pre-treatment (S-method) [
12].
In the T-method, the feathers were washed in tap water at 50°C, followed by rinse with distilled water, dried at 55°C for 12 h, cut into 20-30 mm long fragments, ground using an ultra-centrifugal mill (Retsch, Type ZM 200) with a mesh diameter of 0.75 mm at 8,000 rpm, and finally defatted with petroleum ether in a Soxhlet apparatus. In the S-method, the feathers were only washed with tap water and detergent at 50°C, followed by rinse in distilled water, dried at 55°C for 12 h, and then cut into 20-30 mm long fragments (
Figure 1).
2.3. Alkaline Hydrolysis
Keratin extraction was carried out at room temperature using the alkaline hydrolysis method. A Latin square experimental design (
Table 1) was employed to evaluate the effect of hydrolysis variables (x1 - feather mass to NaOH volume ratio, x2 - hydrolysis time, and x3 –shaking speed) at three levels of variation (p=3) and their interaction on the keratin extraction. This design resulted in 9 different experimental conditions for each pre-treatment method (T and S), totalling 18 experiments. The distribution of parameters in the Latin square method was based on the experimental design proposed by Dąbrowska et al. [
12].
Pre-treated feathers with the S- or T-method were shaken in DURAN® bottles with a 1:10 (m/v) ratio of distilled water at 150, 175, or 200 rpm for 1 h. Then, 1 M NaOH was added at ratios 1:3, 1:6, or 1:9 (w/v), the total solid-liquid ratio was completed with distilled water to 1:20 (m/v), and the shaking was continued for 16, 24, or 32 h. The resulting suspension, with insoluble feather residues and soluble keratin, was centrifuged at 5,000 rpm for 10 min, filtered, neutralized with HCl and dialyzed against water using Spectra/Por membranes of regenerated cellulose (molecular weight cut-off 3.5 kDa) for 72 h. Finally, hydrolysates were freeze-dried and stored at 4°C for further analysis.
Shaking speed values for the reaction mixture of 150, 175, and 200 (rpm) are included inside the Latin square.
2.4. Determination of Keratin Yield
The keratin yield was determined as the weight loss of the raw material (feathers solubilization) and the concentration of soluble protein after the reaction (soluble protein content).
2.4.1. Feather Solubilization
After filtering the soluble keratin, the insoluble feather residue was washed with distilled water until a neutral pH and dried to a constant weight at 105°C [
13]. The keratin yield was calculated as follows:
where: m
0 is the initial weight of feathers (g), m
dry is the dry weight of insoluble residue (g), and 0.903 is the dry weight content of pre-treated feathers.
2.4.2. Soluble Protein Content
The amount of nitrogen in the filtered soluble keratin hydrolysates was measured using the Kjeldahl method and then converted to total protein content using a conversion factor of 5.71 [
12,
33]. The keratin yield was calculated as follows:
where: H and F are the total protein content in hydrolysates and pre-treated feathers, respectively.
2.5. Determination of Properties of Keratin Hydrolysates.
2.5.1. Degree of Keratin Hydrolysis
The DH of keratin was determined spectrophotometrically according to Bavaro et al. [
34], with a slight modification. Briefly, 10 µL of filtered hydrolysates were mixed with 200 µL derivatizing reagent consisting of 0.8 mg/mL OPA, 38.1 mg/mL sodium tetraborate, 1 mg/mL SDS, and 0.88 mg/mL DTT. The absorbance at 340 nm was measured using a multi-plate reader (Synergy HT, BIOKOM) with 15 minutes of reaction time. A standard curve was generated using 1 to 10 mM L-leucine solution. The results were expressed as mmol NH
2/g protein.
2.5.2. Molecular Weight Distribution
The molecular weight distribution of keratin hydrolysates was determined using an HPLC system (1200 series, Agilent Technologies, Wilmington, DE, USA) equipped with a DAD detector. The keratin hydrolysates, pre- and post-dialysis, were dissolved in a mobile phase composed of acetonitrile, water, and trifluoroacetic acid (30: 60.9: 0.1 v/v) to a final concentration of 0.5 mg/mL, and then centrifuged at 10,000 rpm for 10 min. Supernatants of 30 μL were injected into ReproSil 50 SEC (300 x 8 mm, 5 μm) column with a fractionation range from 0.5 to 10 kDa, eluted with the mobile phase at a flow rate of 0.2 mL/min and monitored at 205 nm [
35]. The column was calibrated with standards: aprotinin (6.5144 kDa), bovine insulin (5.7335 kDa), bovine insulin oxidized, chain B (3.4959 kDa), bacitracin A (1.423 kDa) and bradykinin (1.0602 kDa).
2.6. Determination of Antioxidant Activity of Keratin Hydrolysates
The antioxidant activity of keratin hydrolysates was determined using mixed tests based on both electron and hydrogen transfer (ABTS and DPPH tests, respectively) and one-electron transfer (FCR test). The calibration curves were prepared using the standard antioxidant Trolox. Antioxidant activity was expressed in mg of Trolox Equivalents (TE) per g of hydrolysate (mg TE/g).
The ABTS cation radical stock solution was prepared by dissolving ABTS in 2.45 mM Na2S2O8 to reach a concentration of 7 mM and leaving it in the dark for at least 24 h before measurements. Directly before measurements, the ABTS stock and DPPH were diluted until absorbance reached 0.7 at 734 nm with water and 1.0 at 515 nm with methanol, respectively, and commercial FCR indicator with water 1:9 (v/v).
In the case of ABTS and DPPH tests, 1 mL of diluted solution of the radical was mixed with 10 μL and 30 μL, respectively, of diluted keratin suspension or Trolox solution, incubated for 15 min in the dark and centrifuged at 18,000 rpm for 5 min. The absorbance was measured at 734 and 515 nm in the ABTS and DPPH tests, respectively. In the case of the FCR assay, 1 mL of diluted FCR was mixed with 100 μL of keratin suspension or Trolox solution and incubated for 8 minutes. Then, 0.9 mL of 10% sodium carbonate was added and incubated for 1 h, followed by centrifugation at 18,000 rpm for 5 min. Finally, the absorbance was measured at 750 nm. All absorbance measurements were performed using a multi-plate reader (Synergy HT, BIOKOM).
2.7. Statistical Analysis of the Results
The feathers pre-treatment was not considered a variable within the Latin square design but was analysed separately using one-way ANOVA. This separate analysis allowed us to distinctly evaluate the effects of the pre-treatment methods (T-method vs. S-method) without confounding them, with the three primary variables being optimized within each pre-treatment group. SigmaPlot 11.0 (SYSTAT Software, Germany) was used to perform statistical analysis of the experimental results using one-way ANOVA with a significance level of p < 0.05.
3. Results
3.1. Keratin Yield
3.1.1. Feather Solubilization
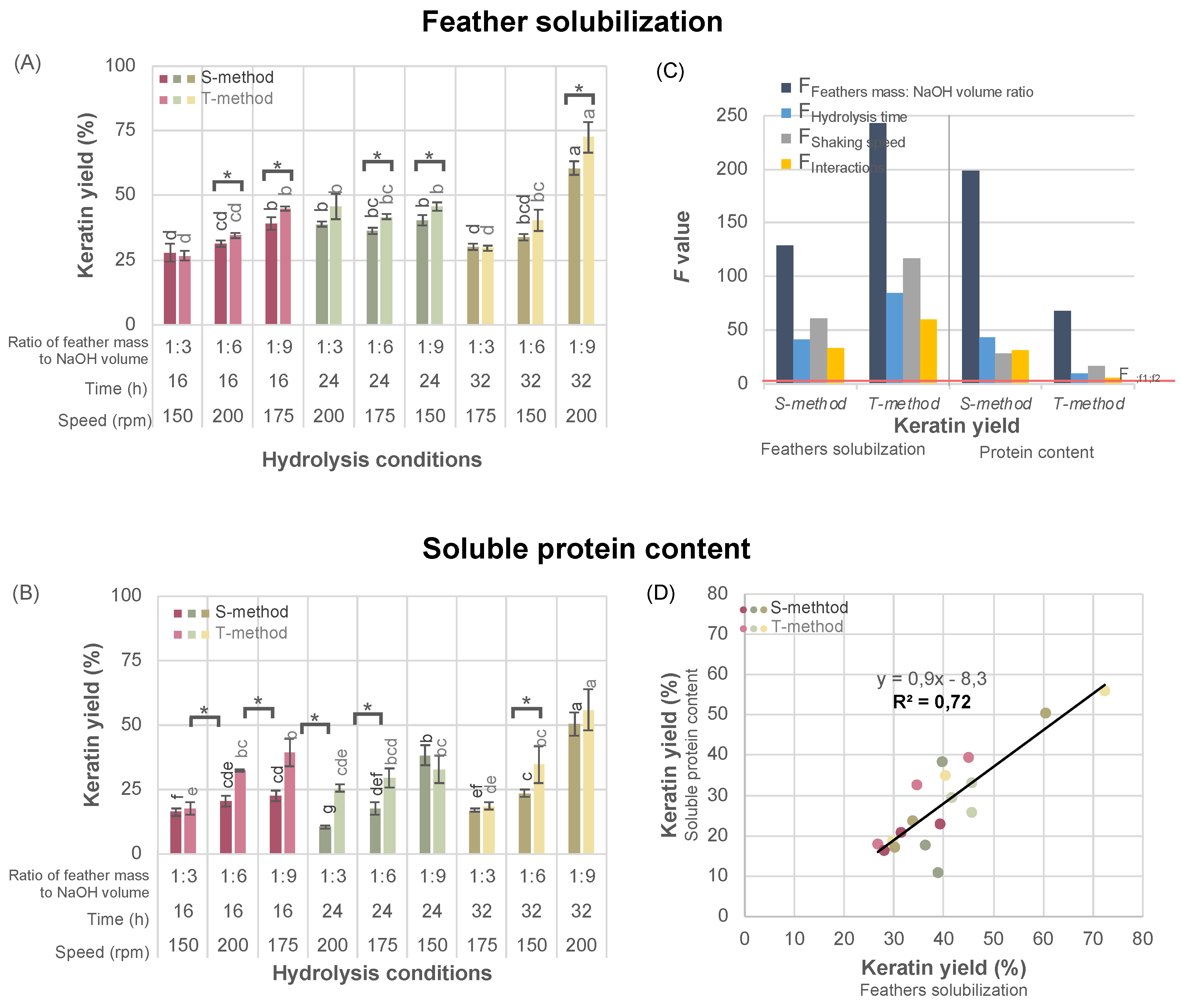
The keratin yield determined as feather solubilization ranged from 27-72%, depending on the pre-treatment method and hydrolysis conditions (
Figure 2A). The T-method of feather pretreatment resulted in approx. 10% higher keratin yield than the S-method, with statistical significance when using a feather mass to NaOH volume ratio of 1:6 or 1:9 (as denoted by an asterisk in
Figure 2A). When a 1:3 mass to volume ratio of NaOH was applied, the pre-treatment method did not significantly affect the yield (
Figure 2A), and under these conditions, the same keratin yield in both pre-treatment methods was achieved. This indicates that the steps in the T-method, including feather grinding and chemical defatting (
Figure 1), might be omitted when the lowest NaOH concentration is used.
Regardless of the pre-treatment method, an increase in feather mass to NaOH volume ratio, reaction time, and shaking speed led to a higher keratin yield (left side of
Figure 2B). The Latin square analysis revealed that these three hydrolysis parameters significantly affect the keratin yield, as the calculated Fisher-Snedecor statistics value (F value for feather mass to NaOH volume ratio, reaction time, and shaking speed, assigned as a bar in the dark blue, blue and grey at
Figure 2B, respectively) exceeds the critical threshold (F
α;f1;f2=3.55, a red line at
Figure 2B). The F values for these parameters were higher than the F value for their interaction (F
Inferactions, assigned as a yellow bar at
Figure 2B), suggesting that each of the variables individually had a more substantial impact on the keratin yield than their mutual interaction.
3.1.2. Soluble Protein Content
The keratin yield, described as a soluble protein content (determined by the Kjeldahl method), ranged from 11-56%, and like that defined as feather solubilization, depended on the pre-treatment method and hydrolysis conditions (
Figure 2C). Additionally, both methods showed statistically significant higher yield when the T-method was used. However, the effect of feather pre-treatment on keratin yield defined as soluble protein content, unlike that defined as feather solubilization, could not be easily related to the ratio of feather mass to NaOH volume or other reaction conditions.
The statistical analysis based on the Latin square confirmed also that increased feather mass to NaOH volume ratio, hydrolysis time, and shaking speed increased the keratin yield defined as soluble protein content. Regardless of the applied pre-treatment method, the calculated F values (the right side of
Figure 2B) significantly exceed the critical threshold (F
α;f
1;f
2). The interactions between parameters were also significant, indicating interactions between the feather mass to NaOH volume ratio, hydrolysis time, and shaking speed. These results proved that optimizing reaction conditions requires considering both hydrolysis parameters and their interactions, emphasizing the importance of a holistic approach to achieve optimal results.
There was a linear relationship between the keratin yield, defined as the feather solubilization and the soluble protein content (
Figure 2D, R
2 > 0.7). Additionally, conditions of the alkaline hydrolysis used led to the extraction of protein with a comparable efficiency to that documented in our previous work [
12].
3.2. Degree of Keratin Hydrolysis
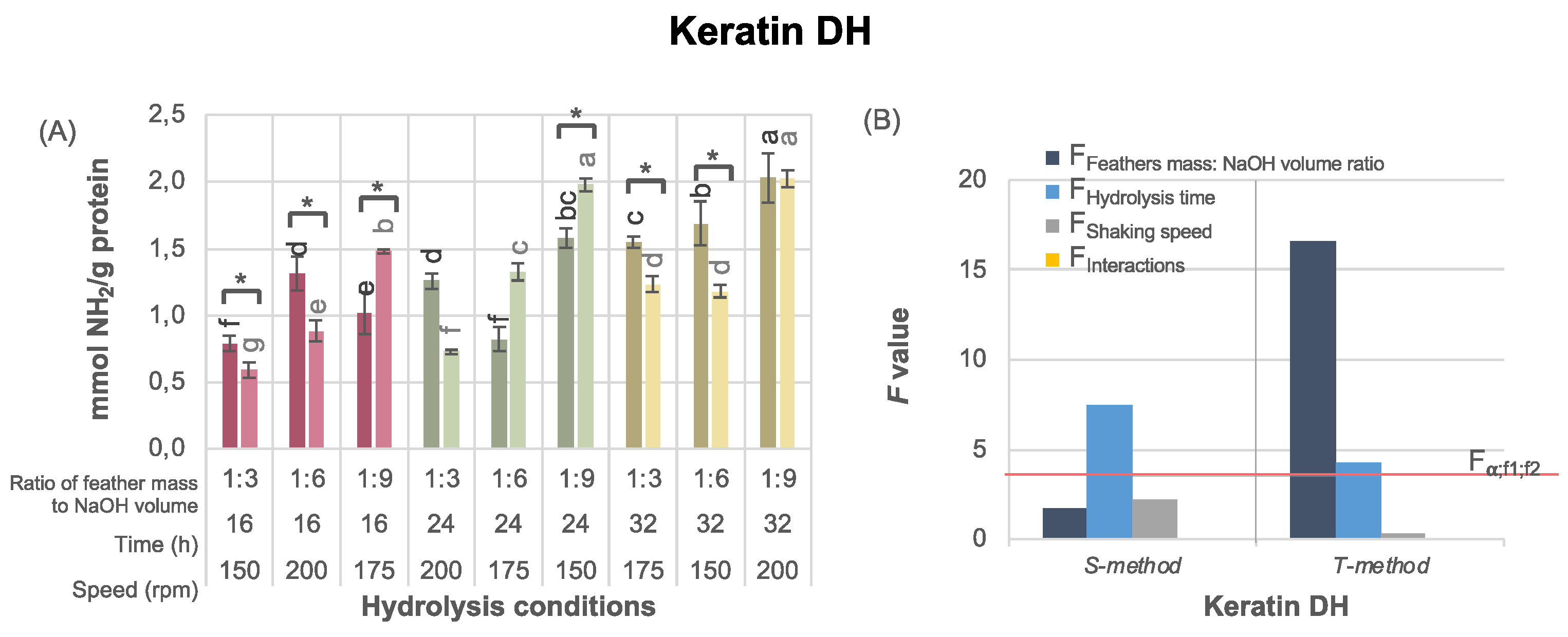
The results of the OPA assay confirmed that peptide bonds were broken by feather alkaline hydrolysis with significant differences in the DH of keratin depending on both the pre-treatment method and the hydrolysis conditions (
Figure 3). No clear pattern regarding the effect of pre-treatment methods on the DH of keratin was recognized; in some cases with the same reaction parameters, the DH was higher when the T-method was used, while in others when the S-method was applied (
Figure 3A). As expected, the lowest DH (0.59 mmol NH
2/g protein) was noted for the keratin obtained under the least intensive hydrolysis conditions, i.e., with 1:3 feather mass to NaOH volume ratio, 16 h hydrolysis time and at 150 rpm, when feathers were pre-treated with the T-method. The highest DH (2.03 mmol NH
2/g protein) was shown by keratin hydrolysates processed under the most intensive hydrolysis conditions, i.e. 1:9 feather mass to NaOH ratio, 32 hours, and 200 rpm shaking speed, regardless of the pre-treatment method (
Figure 3A).
As revealed by the statistical analysis based on the Latin square, the feather mass to NaOH volume ratio and hydrolysis time had a statistically significant effect on keratin DH in the case of the feathers pre-treated with the T-method, while only the hydrolysis time had a significant effect when feathers were pre-treated with the S-method (
Figure 3B). Although shaking speed affected keratin yield expressed as feather solubilization and soluble protein (
Figure 2B), no effect of this parameters was observed on DH (
Figure 3B). This indicates that keratin yield does not solely depend simply on the DH.
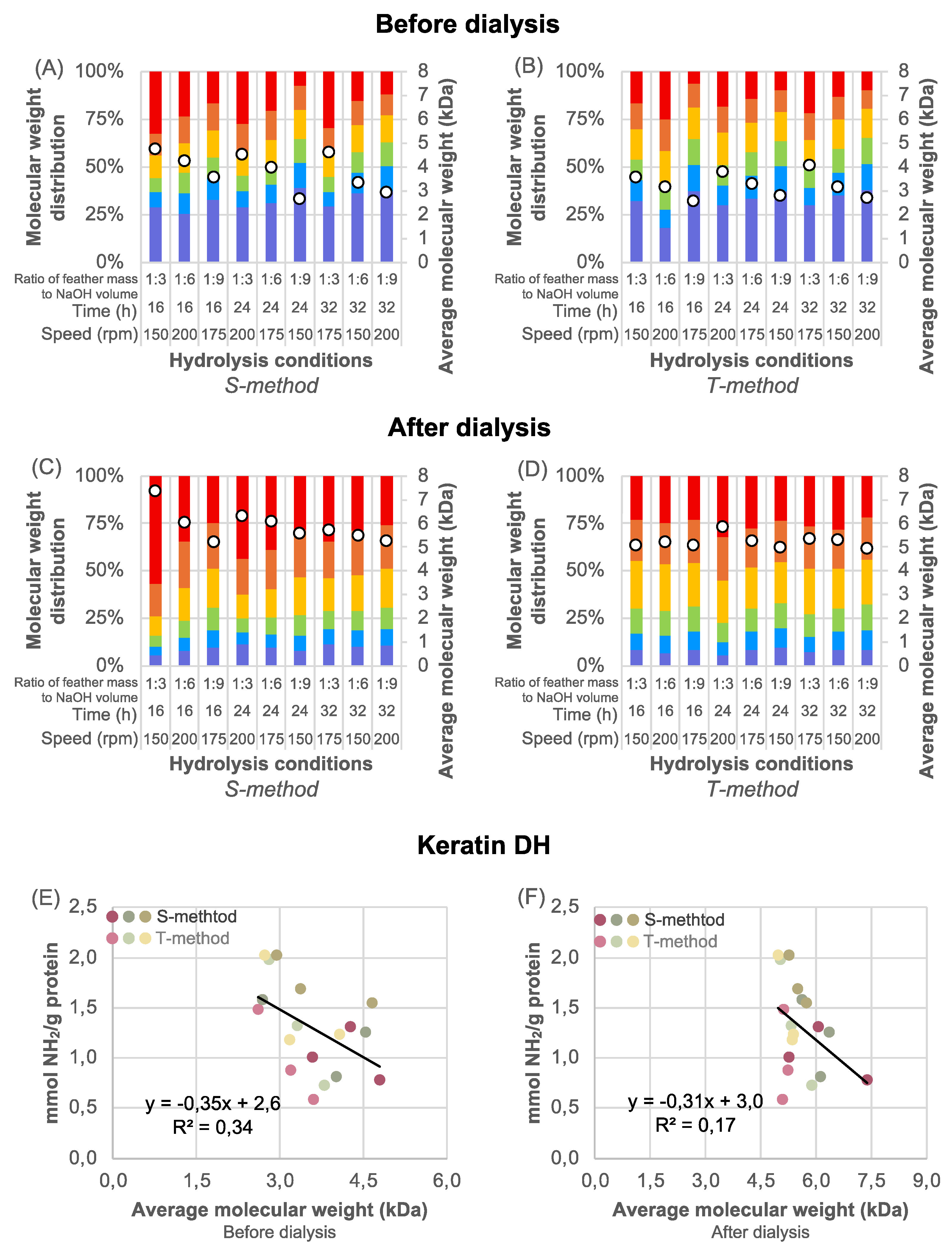
3.3. Molecular Weight Distribution
Regardless of the pre-treatment method and hydrolysis conditions, the most evident differences in the percentage content of the HMW (>9.9 kDa) and LMW (<0.5 kDa) fractions were observed as the effect of dialysis. These two fractions were the significant determinants of the average molecular weight of the hydrolysates (assigned as a circle on individual bars in
Figure 4A–D). The feathers pre-treated with the T-method resulted in similar average molecular weight of the post-dialysis peptides, ranging from 5.0 to 5.9 kDa (
Figure 4D); however, this was approx.15% lower than that obtained using the S-method, which was more diversified, ranging from 5.3 to 7.4 kDa (
Figure 4C).
Although the reaction time and shaking speed had a low impact on the molecular weight distribution of hydrolysates before dialysis (
Figure 4A,B), the use of feather mass and NaOH volume in a 1:9 ratio resulted in a decrease in HMW, an increase in LMW fraction percentage content, and a reduction in average molecular weight. In contrast, for feathers pre-treated with the T-method, the hydrolysis conditions did not affect the content of the HMW- and LMW-fractions, nor the average molecular weight of the post-dialysis keratin hydrolysates (
Figure 4D). As expected, the average molecular weight of all samples significantly increased from 5.1-5.9 kDa to 6.8-7.4 kDa because of dialysis due to the loss of most LMW peptides that easily passed through the dialysis membrane pores (with a molecular weight cut-off of 3.5 kDa).
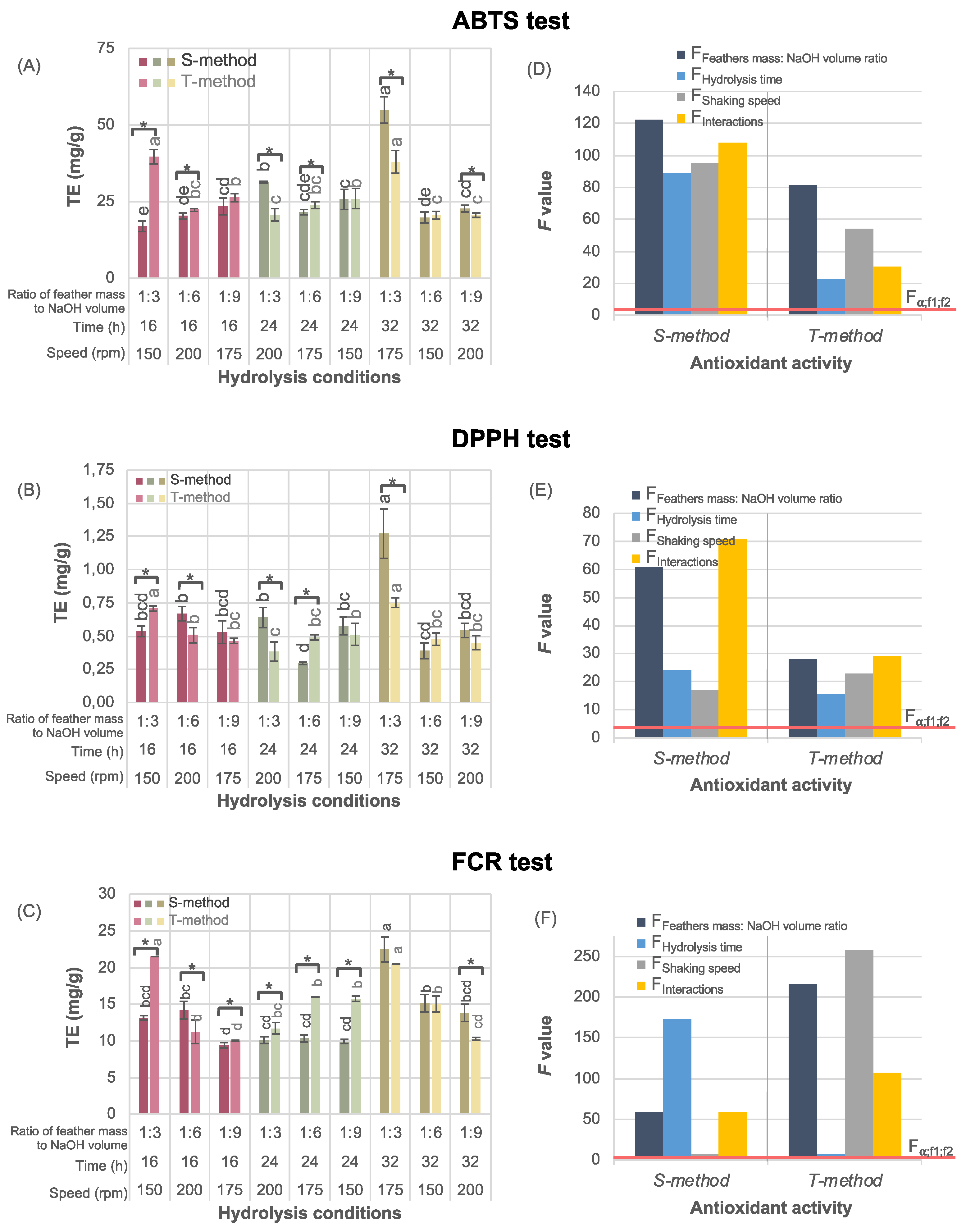
3.4. Antioxidant Activity
Antioxidant activity of the keratin hydrolysates obtained varied depending on the method of its determination (
Figure 5). The antioxidant activity tests used, i.e., those based on the organic radical scavenging ABTS and DPPH test and that based on reducing the FCR with phenolic compounds in an alkaline state, showed that the ABTS test was more efficient than those of DPPH and FCR. However, these methods differ regarding reaction mechanisms, reagents, and reaction conditions [
36,
37].
Regardless of the antioxidant activity test, feathers pre-treated with the T-method resulted in the high antioxidant activity under both the lowest feather mass to NaOH volume ratio (1:3), the longest hydrolysis time (32 h) at the medium shaking speed (175 rpm), and under the same feather mass to NaOH ratio (1:3) but with the shorter hydrolysis time (16 h) and at lower shaking speed (150 rpm). However, the feathers pre-treated with the S-method led to even higher antioxidant activity under the same hydrolysis conditions. Further, no clear trends in the effect of hydrolysis conditions on antioxidant activity were seen. The statistical analysis based on the Latin square methodology revealed that the effect of hydrolysis parameters on antioxidant activity differed for each determination method (
Figure 5D–F). In particular, in the ABTS test, the feather mass to NaOH volume ratio had the greatest F value; in the DPPH test, the interactions between parameters were most significant; and for the FCR test, the shaking speed had the highest statistical significance.
Generally, the radical scavenging activity was enhanced when 1:3 and 1:6 feather mass to NaOH volume ratios were used (
Figure 5A,B). Moreover, the improved antioxidant activity of feathers pre-treated with the T-method was affected by a reduction in the shaking speed from 200 to 150 rpm, while in the S-method, improved antioxidant activity was achieved with an extended reaction time from 16 to 32 h (
Figure 5D,E).
Although the trends in keratin yield and antioxidant activity depending on the hydrolysis conditions remained very similar in all tests applied (the outcome depends mainly on feather mass to NaOH volume ratio), the FCR assay is the only method in which shaking speed caused the highest Fisher-Snedecor statistic (the grey bar at
Figure 5F). For feathers pre-treated with the T-method, the shaking speed played a crucial role in antioxidant activity determined with FCR method (
Figure 5F on the right), while with the S-method, the reaction time primarily determined the result (
Figure 5F on the left).
4. Discussion
More intensive hydrolysis conditions result in higher extraction yield but lower peptide molecular weight. In particular, the highest keratin yield of 72% was achieved with a 1:9 feather mass to NaOH volume ratio, 32 hours of hydrolysis, and a 200 rpm of shaking speed. In contrast, the lowest yield of 27% was obtained under milder conditions, with a 1:3 feather mass to NaOH volume ratio, 16 hours of hydrolysis, and a 150 rpm of shaking speed. These findings align with those of Ossai et al. [
7], who reported that alkaline hydrolysis can yield up to 90%, making it one of the most effective keratin extraction methods, second only to hydrothermal extraction. However, such high efficiency is typically achieved at elevated, higher-than-room temperatures [
7,
13,
16], while in the presented study, the reactions were conducted at room temperature. Although the temperature is crucial for enhancing keratin yield, excessively high temperatures can lead to the degradation or racemization of certain amino acids, thereby diminishing the biological value of the resultant protein [
14]. The room temperature of the reaction supports not only the preservation of the biological value of protein but also energy-saving goals, making the process more scalable for industrial applications due to reduced energy consumption and associated costs [
38]. This emphasizes the need for optimize reaction conditions to maximize keratin yield while preventing unnecessary protein degradation or chemical reagent consumption. Ossai et al. [
7] argue that alkaline hydrolysis is not industrially viable due to the high costs of using acids or alkalis. However, the results obtained suggest that it should be considered due to its simplicity, reagent availability, and eco-friendliness compared to more commonly used redox methods, which require more complex and often toxic extraction reagents.
The feather pretreatment with the T-method resulted in a 9-16% higher keratin yield (determined both as feather solubilization and protein content) compared to the S-method. This could be attributed to several factors. First, the feathers differed in size: in the S-method, the fragments were about 20-30 mm, whereas in the T-method, they were about 0.75 mm. The smaller particle size can enhance the reaction yield by increasing the surface area available for interaction with reagents, facilitating more efficient penetration of reagents and a higher yield of the reaction. Second, defatting feathers with petroleum ether was used only in the T-method, ensuring that all NaOH was employed in the keratin extraction procedure. Inaccurate defatting can lead to some NaOH being involved in a competing saponification reaction, reducing the amount of NaOH available for keratin hydrolysis. As could be seen in
Figure 2A,C, NaOH concentration is crucial for high keratin yield.
During alkaline feather hydrolysis, disulfide bonds undergo redox reactions, leading to the breakage of disulfide bridges, and peptide bonds are cleaved through acyl nucleophilic substitution. Consequently, the hydrolysate obtained is composed of peptides, soluble salts, amino acids and sulphides [
7,
39]. According to Mokrejs et al. [
40], alkaline hydrolysis of keratin results in free amino acids and LMW peptides with short chains devoid of crosslinks and soluble in water. The presented study demonstrated that the higher the keratin yield, the more amino groups were released, but there was no correlation between these values (
Figure 4E,F). This indicates that the reaction with NaOH contributes not only to breaking peptide bonds but also to various other bonds. This breaking of bonds causes the formation of smaller peptides from keratin – the more intensive the reaction conditions, the smaller the MW of the peptides.
Chicken feathers consist of proteins with sizes of 10-70 kDa, with a fraction of 9.9 kDa representing β-keratin being the most abundant [
12,
22,
41]. As reported by Endo et al. [
42], the molecular weight of peptides in keratin hydrolysates obtained from poultry feathers via alkaline hydrolysis ranged between 1.2-5.7 kDa, with the highest peptide concentration observed in the 4.5-5 kDa fraction. Dąbrowska et al. [
12] found that alkaline hydrolysates of feather keratin primarily consisted of peptides with molecular weights <10 kDa.
According to Zhang et al. [
43], the presence of HMW peptides (>10 kDa) suggests that protein extraction involved not only hydrolysis of peptide bonds but also the disruption of disulfide and non-covalent bonds. The results presented confirm this hypothesis, as evidenced by the lack of correlation between molecular weight and DH of keratin (
Figure 4E,F, R
2 < 0.7). If hydrolysis was limited to peptide bonds, a strong linear correlation between molecular weight and the number of free NH
2 groups (higher DH) would be expected.
According to Ferraro et al. [
22], Liu et al. [
44] and Zou et al. [
25], the strongest antioxidant activity is usually exhibited by the LMW hydrolysate fractions. However, during dialysis, many of these smallest peptides were lost. Therefore, it can be suspected that dialysis may worsen the antioxidant activity of keratin hydrolysates. Nevertheless, all hydrolysates exhibited antioxidant activity, but no correlation was observed between molecular weight and antioxidant activity.
5. Conclusions
In the presented study, the success of two methods of feather pre-treatment and various alkaline hydrolysis conditions were compared. The T-method resulted in a higher keratin yield than the S-method, especially when 1:6 and 1:9 feather mass to NaOH volume ratios were used. This suggests that although the T-method is more labour-intensive, it may be more efficient for industrial applications seeking maximum yield. On the other hand, although the simplified pre-treatment S-method resulted in 1.26 times lower keratin yield than the T-method, it led to hydrolysates with a higher average molecular weight. Moreover, the S-method is less complex and less time-consuming, making it suitable for applications when scalability is prioritized.
The feather mass to NaOH volume ratio was the most critical factor affecting keratin yield, DH, and molecular weight distributions. Increase in hydrolysis parameters (feather mass to NaOH volume ratio, hydrolysis time and shaking speed) led to higher DH and peptide with lower MW, indicating extensive bond cleavage and higher yields of hydrolysed keratin.
Both pre-treatment methods and hydrolysis conditions influenced the antioxidant activity of keratin hydrolysates. The S-method with extended hydrolysis reaction time resulted in the products with higher antioxidant activity. However, the dialysis process, necessary for the purification of keratin hydrolysates after its neutralization, resulted in the loss of LMW peptides, primarily responsible for antioxidant properties. Therefore, further studies should focus on optimizing the purification process of keratin alkaline hydrolysates to preserve LMW peptides with higher antioxidant activity than HMW. Additionally, exploring the specific bond hydrolysis and amino acid sequences will help better understand the mechanisms behind the antioxidant properties of keratin hydrolysates.
This study provides a foundation for developing scalable and efficient keratin extraction methods from chicken feathers. These methods contribute to sustainable environmental management by converting waste into valuable products, emphasizing the need to balance yield, quality, and process simplicity for various industrial applications, including biomedicine, environmental remediation, and food packaging.
Author Contributions
Agata Sommer: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing - original draft. Antoni Taraszkiewicz: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing - original draft. Hanna Staroszczyk: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing - review & editing.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable
Acknowledgments
The authors would like to acknowledge with thanks Mrs. Paulina Świderska for professional laboratory assistance and Dr. Izabela Sinkiewicz for scientific discussions on keratin hydrolysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Donato, R. K.; Mija, A. Keratin Associations with Synthetic, Biosynthetic and Natural Polymers: An Extensive Review. Polymers (Basel) 2019, 12 (1), 32. [CrossRef]
- Rouse, J. G.; Van Dyke, M. E. A Review of Keratin-Based Biomaterials for Biomedical Applications. Materials 2010, 3 (2), 999–1014. [CrossRef]
- Sinkiewicz, I.; Staroszczyk, H.; Śliwińska, A. Solubilization of Keratins and Functional Properties of Their Isolates and Hydrolysates. J Food Biochem 2018, 42 (2), 1–11. [CrossRef]
- Jagadeesan, Y.; Meenakshisundaram, S.; Raja, K.; Balaiah, A. Sustainable and Efficient-Recycling Approach of Chicken Feather Waste into Liquid Protein Hydrolysate with Biostimulant Efficacy on Plant, Soil Fertility and Soil Microbial Consortium: A Perspective to Promote the Circular Economy. Process Saf Environ Prot 2023, 170, 573–583. [CrossRef]
- Zhang, L.; Ren, J.; Bai, W. A Review of Poultry Waste-to-Wealth: Technological Progress, Modeling and Simulation Studies, and Economic- Environmental and Social Sustainability. Sustainability 2023, 15 (7), 5620. [CrossRef]
- Kar, P.; Misra, M. Use of Keratin Fiber for Separation of Heavy Metals from Water. J Chem Technol Biotechnol 2004, 79 (11), 1313–1319. [CrossRef]
- Ossai, I. C.; Hamid, F. S.; Hassan, A. Valorisation of Keratinous Wastes: A Sustainable Approach towards a Circular Economy. Waste Manage 2022, 151, 81–104. [CrossRef]
- Feroz, S.; Muhammad, N.; Ranayake, J.; Dias, G. Keratin - Based Materials for Biomedical Applications. Bioact Mater 2020, 5 (3), 496–509. [CrossRef]
- Wrześniewska-Tosik, K.; Zajchowski, S.; Ryszkowska, J.; Tomaszewska, J.; Mirowski, J.; Szoła, K. Wpływ Sposobu Przygotowania Włókien Keratynowych z Piór Drobiowych Na Właściwości Kompozytów z Recyklatów Polietylenu Dużej Gęstości. Polimery/Polymers 2015, 60 (2), 109–117. [CrossRef]
- Eslahi, N.; Hemmatinejad, N.; Dadashian, F. From Feather Waste to Valuable Nanoparticles. Part Sci Technol 2014, 32 (3), 242–250. [CrossRef]
- Gupta, S.; Nadda, A. K.; Gupta, A.; Singh, J.; Mulla, S. I.; Sharma, S. Transforming Wastes into High Value-Added Products: An Introduction. In Biopolymers. Springer Series on Polymer and Composite Materials; Nadda, A. K., Sharma, S., Bhat, R., Eds.; Springer : Cham, 2022; pp 1–18.
- Dąbrowska, M.; Sommer, A.; Sinkiewicz, I.; Taraszkiewicz, A.; Staroszczyk, H. An Optimal Designed Experiment for the Alkaline Hydrolysis of Feather Keratin. Environ Sci Pollut Res 2022, 29 (16), 24145–24154. [CrossRef]
- Sinkiewicz, I.; Śliwińska, A.; Staroszczyk, H.; Kołodziejska, I. Alternative Methods of Preparation of Soluble Keratin from Chicken Feathers. Waste Biomass Valori 2017, 8 (4), 1043–1048. [CrossRef]
- Cai, G.; Moffitt, K.; Navone, L.; Zhang, Z.; Robins, K.; Speight, R. Valorisation of Keratin Waste: Controlled Pretreatment Enhances Enzymatic Production of Antioxidant Peptides. J Environ Manage 2022, 301, 113945. [CrossRef]
- Momen, S.; Alavi, F.; Aider, M. Alkali-Mediated Treatments for Extraction and Functional Modification of Proteins: Critical and Application Review. Trends Food Sci Technol 2021, 110, 778–797. [CrossRef]
- Reddy, C. C.; Khilji, I. A.; Gupta, A.; Bhuyar, P.; Mahmood, S.; Saeed AL-Japairai, K. A.; Chua, G. K. Valorization of Keratin Waste Biomass and Its Potential Applications. J Water Proc Eng 2021, 40, 101707. [CrossRef]
- Paul, T.; Mandal, A.; Mandal, S. M.; Ghosh, K.; Mandal, A. K.; Halder, S. K.; Das, A.; Maji, S. K.; Kati, A.; Mohapatra, P. K. D.; Pati, B. R.; Mondal, K. C. Enzymatic Hydrolyzed Feather Peptide, a Welcoming Drug for Multiple-Antibiotic-Resistant Staphylococcus Aureus: Structural Analysis and Characterization. Appl Biochem Biotechnol 2015, 175 (7), 3371–3386. [CrossRef]
- Qin, X.; Xu, X.; Guo, Y.; Shen, Q.; Liu, J.; Yang, C.; Scott, E.; Bitter, H.; Zhang, C. A Sustainable and Efficient Recycling Strategy of Feather Waste into Keratin Peptides with Antimicrobial Activity. Waste Manage 2022, 144, 421–430. [CrossRef]
- Acquah, C.; Stefano, E. Di; Udenigwe, C. C. Role of Hydrophobicity in Food Peptide Functionality and Bioactivity. J Food Bioact 2018, 4, 88–98. [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M. Bioinformatics and Bioactive Peptides from Foods: Do They Work Together? In Advances in Food and Nutrition Research; 2024; pp 35–111. [CrossRef]
- Prajapati, S.; Koirala, S.; Anal, A. K. Bioutilization of Chicken Feather Waste by Newly Isolated Keratinolytic Bacteria and Conversion into Protein Hydrolysates with Improved Functionalities. Appl Biochem Biotechnol 2021, 193 (8), 2497–2515. [CrossRef]
- Ferraro, V.; Anton, M.; Santé-Lhoutellier, V. The “Sisters” α-Helices of Collagen, Elastin and Keratin Recovered from Animal by-Products: Functionality, Bioactivity and Trends of Application. Trends Food Sci Technol 2016, 51, 65–75. [CrossRef]
- Lv, R.; Dong, Y.; Bao, Z.; Zhang, S.; Lin, S.; Sun, N. Advances in the Activity Evaluation and Cellular Regulation Pathways of Food-Derived Antioxidant Peptides. Trends Food Sci Technol 2022, 122, 171–186. [CrossRef]
- Nwachukwu, I. D.; Aluko, R. E. Structural and Functional Properties of Food Protein-derived Antioxidant Peptides. J Food Biochem 2019, 43 (1), 1–13. [CrossRef]
- Zou, T. Bin; He, T. P.; Li, H. Bin; Tang, H. W.; Xia, E. Q. The Structure-Activity Relationship of the Antioxidant Peptides from Natural Proteins. Molecules 2016, 21 (1). [CrossRef]
- Callegaro, K.; Brandelli, A.; Daroit, D. J. Beyond Plucking: Feathers Bioprocessing into Valuable Protein Hydrolysates. Waste Manage 2019, 95, 399–415. [CrossRef]
- Mokrejs, P.; Hutta, M.; Pavlackova, J.; Egner, P.; Benicek, L. The Cosmetic and Dermatological Potential of Keratin Hydrolysate. J Cosmet Dermatol 2017, 16 (4), e21–e27. [CrossRef]
- Taraszkiewicz, A.; Sinkiewicz, I.; Sommer, A.; Dąbrowska, M.; Staroszczyk, H. Prediction of Bioactive Peptides from Chicken Feather and Pig Hair Keratins Using in Silico Analysis Based on Fragmentomic Approach. Curr Pharm Des 2022, 28 (10), 841–851. [CrossRef]
- Dias, G. J.; Haththotuwa, T. N.; Rowlands, D. S.; Gram, M.; Bekhit, A. E.-D. A. Wool Keratin – A Novel Dietary Protein Source: Nutritional Value and Toxicological Assessment. Food Chem 2022, 383, 132436. [CrossRef]
- Houltham, S.; Starck, C.; Stannard, S. Two Week Keratin-Based Protein Supplementation Is Comparable in Gastrointestinal Handling to a Milk-Based Equivalent. J Hum Nutr Food Sci 2014, 2 (4), 1020.
- Lasekan, A. O.; Abu Bakar, F.; Hashim, D. Potential of Chicken By-Products as Sources of Useful Biological Resources. Waste Manage 2013, 33 (3), 552–565. [CrossRef]
- Wang, R.; Tong, H. Preparation Methods and Functional Characteristics of Regenerated Keratin-Based Biofilms. Polymers (Basel) 2022, 14 (21), 4723. [CrossRef]
- Grazziotin, A.; Pimentel, F. A.; De Jong, E. V.; Brandelli, A. Nutritional Improvement of Feather Protein by Treatment with Microbial Keratinase. Anim Feed Sci Technol 2006, 126 (1–2), 135–144. [CrossRef]
- Bavaro, S. L.; Mamone, G.; Picariello, G.; Callanan, M. J.; Chen, Y.; Brodkorb, A.; Giblin, L. Thermal or Membrane Processing for Infant Milk Formula: Effects on Protein Digestion and Integrity of the Intestinal Barrier. Food Chem 2021, 347, 129019. [CrossRef]
- Johns, P. W.; Jacobs, W. A.; Phillips, R. R.; McKenna, R. J.; O’Kane, K. A.; McEwen, J. W. Characterisation of Peptide Molecular Mass Distribution in Commercial Hydrolysates and Hydrolysate-Based Nutritional Products. Food Chem 2011, 125 (3), 1041–1050. [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of Methods to Determine Antioxidant Capacities. Food Anal Methods 2009, 2 (1), 41–60. [CrossRef]
- Munteanu, I. G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int J Mol Sci 2021, 22 (7), 3380. [CrossRef]
- Lee, J.-Y.; Chen, P.-Y. Optimization of Heat Recovery Networks for Energy Savings in Industrial Processes. Processes 2023, 11 (2), 321. [CrossRef]
- Holkar, C. R.; Jain, S. S.; Jadhav, A. J.; Pinjari, D. V. Valorization of Keratin Based Waste. Process Saf Environ Prot 2018, 115, 85–98. [CrossRef]
- Mokrejš, P.; Huťťa, M.; Pavlačková, J.; Egner, P. Preparation of Keratin Hydrolysate from Chicken Feathers and Its Application in Cosmetics. J Visualized Exp 2017, 2017 (129), 1–9. [CrossRef]
- Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E. H.; Britto, R.; Bye-A-Jee, H.; Cukura, A.; Denny, P.; Dogan, T.; Ebenezer, T.; Fan, J.; Garmiri, P.; da Costa Gonzales, L. J.; Hatton-Ellis, E.; Hussein, A.; Ignatchenko, A.; Insana, G.; Ishtiaq, R.; Joshi, V.; Jyothi, D.; Kandasaamy, S.; Lock, A.; Luciani, A.; Lugaric, M.; Luo, J.; Lussi, Y.; MacDougall, A.; Madeira, F.; Mahmoudy, M.; Mishra, A.; Moulang, K.; Nightingale, A.; Pundir, S.; Qi, G.; Raj, S.; Raposo, P.; Rice, D. L.; Saidi, R.; Santos, R.; Speretta, E.; Stephenson, J.; Totoo, P.; Turner, E.; Tyagi, N.; Vasudev, P.; Warner, K.; Watkins, X.; Zaru, R.; Zellner, H.; Bridge, A. J.; Aimo, L.; Argoud-Puy, G.; Auchincloss, A. H.; Axelsen, K. B.; Bansal, P.; Baratin, D.; Batista Neto, T. M.; Blatter, M.-C.; Bolleman, J. T.; Boutet, E.; Breuza, L.; Gil, B. C.; Casals-Casas, C.; Echioukh, K. C.; Coudert, E.; Cuche, B.; de Castro, E.; Estreicher, A.; Famiglietti, M. L.; Feuermann, M.; Gasteiger, E.; Gaudet, P.; Gehant, S.; Gerritsen, V.; Gos, A.; Gruaz, N.; Hulo, C.; Hyka-Nouspikel, N.; Jungo, F.; Kerhornou, A.; Le Mercier, P.; Lieberherr, D.; Masson, P.; Morgat, A.; Muthukrishnan, V.; Paesano, S.; Pedruzzi, I.; Pilbout, S.; Pourcel, L.; Poux, S.; Pozzato, M.; Pruess, M.; Redaschi, N.; Rivoire, C.; Sigrist, C. J. A.; Sonesson, K.; Sundaram, S.; Wu, C. H.; Arighi, C. N.; Arminski, L.; Chen, C.; Chen, Y.; Huang, H.; Laiho, K.; McGarvey, P.; Natale, D. A.; Ross, K.; Vinayaka, C. R.; Wang, Q.; Wang, Y.; Zhang, J. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res 2023, 51 (D1), D523–D531. [CrossRef]
- Endo, R.; Kamei, K.; Iida, I.; Kawahara, Y. Dimensional Stability of Waterlogged Wood Treated with Hydrolyzed Feather Keratin. J Archaeol Sci 2008, 35 (5), 1240–1246. [CrossRef]
- Zhang, Y.; Zhao, W.; Yang, R. Steam Flash Explosion Assisted Dissolution of Keratin from Feathers. ACS Sustain Chem Eng 2015, 3 (9), 2036–2042. [CrossRef]
- Liu, R.; Xing, L.; Fu, Q.; Zhou, G.; Zhang, W. A Review of Antioxidant Peptides Derived from Meat Muscle and By-Products. Antioxidants 2016, 5 (3), 32. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).