1. Introduction
Ash (Fraxinus excelsior L.) is a significant component of forest ecosystems, fulfilling both economic and ecological functions. It is a key species in riparian forests and contributes to global biodiversity (1). Economically, ash is not considered a priority tree species (2); however, its economic importance may increase due to dynamic climate change (3). Studies by Dyderski et al. (4) confirm a significant and positive impact of climate change on the potential distribution range of ash. However, climate change models do not account for the impact of the fungus Hymenoscyphus fraxineus (T. Kowalski) Baral, Queloz & Hosoya 2014 (anamorph Chalara fraxinea) (5-7).
One factor limiting the occurrence of ash in forests, and potentially leading to its complete elimination from some forest stands (8), is H. fraxineus, which causes ash dieback (ADB). H. fraxineus was first recorded in northeastern Poland in 1992 (7; 9) and has since spread across the continent. Infested stands decline rapidly (10), making sustainable forest management in ecosystems dependent on ash nearly impossible. The mortality rate of ash trees in forest stands is estimated at 69%, while in ash seed orchards, mortality can exceed 85% (11). Infection by H. fraxineus primarily occurs in summer when mature apothecia, formed on petioles of leaves from the previous growing season, release wind-dispersed ascospores (9). Characteristic symptoms of the disease include wilting of leaves, extensive shoot necrosis, and dieback of entire crowns, ultimately leading to tree death. In Poland, due to the high infection potential of H. fraxineus, the main administrator of the country's forests (State Forests) has ceased using ash trees in artificial forest regeneration efforts.
Scientific forecasts predict no decrease in the occurrence of H. fraxineus in Central Europe, and ash dieback is listed on the warning list of the European and Mediterranean Plant Protection Organisation (EPPO) (12-13). Due to the ongoing infection pressure and the ecological significance of F. excelsior, attempts are being made to reintroduce the species into forested areas. Initial results of reintroduction efforts under various site conditions have shown that the lowest ash mortality occurs in mesotrophic and dry habitats (13;14). Grosdidier et al. (15) demonstrated that lower ash mortality is observed in forests with lower ash density per unit area and in park or backyard settings where ash occurs as solitary trees. In southern and western Europe, the spread of H. fraxineus is strongly declining due to a climate that is unfavourable to the pathogen. The dynamics of ash dieback are favoured by heavy summer rainfall, high humidity, and low air temperatures, with optimal growth of H. fraxineus mycelium occurring at 20-25°C (16). Additionally, the decreasing density of ash trees and increasing fragmentation of forest stands are factors that limit the spread of the disease (13). A decrease in mortality and improved health of ash trees in a seed orchard where artificial removal of winter-fallen leaves was performed has also been demonstrated (17).
Research focused on identifying factors that contribute to lower susceptibility of ash to dieback has identified spring phenology as one of the key phenotypic markers associated with resistance. The start of the growing season is one of the most important factors determining the potential for growth under local environmental conditions (18). Smintina (19) and Kleinschmit et al. (20) demonstrated significant differences in phenology between ash provenances and families, and emphasised the importance of phenology in the potential selection of species for adaptive traits. Studies conducted under natural growing conditions in forest ecosystems have shown that ash trees that develop buds early in spring (21) or show early autumn leaf colouration (8) are less susceptible to crown dieback and exhibit higher resistance.
The main objective of the present study was to analyse the relationship between the degree of H. fraxineus infestation in F. excelsior genotypes and phenological observations of spring budburst. The study was conducted in a clonal seed orchard located in northeastern Poland. We hypothesised that: (a) lower susceptibility to H. fraxineus depends on the bioclimatic region, and (b) ash clones that develop buds early in spring show lower susceptibility to damage by H. fraxineus. Analyses were performed on 31 plus clones growing in a seed orchard infected with H. fraxineus. The pressure of H. fraxineus in the studied seed orchard systematically decreased over the observation years due to the removal of winter-fallen leaves. Therefore, the observations made in the context of forest management aimed to answer the question of whether breeding ash trees for resistant traits can be useful in identifying individuals that are relatively resistant to H. fraxineus.
2. Results
2.1. Bioclimatic Analyses
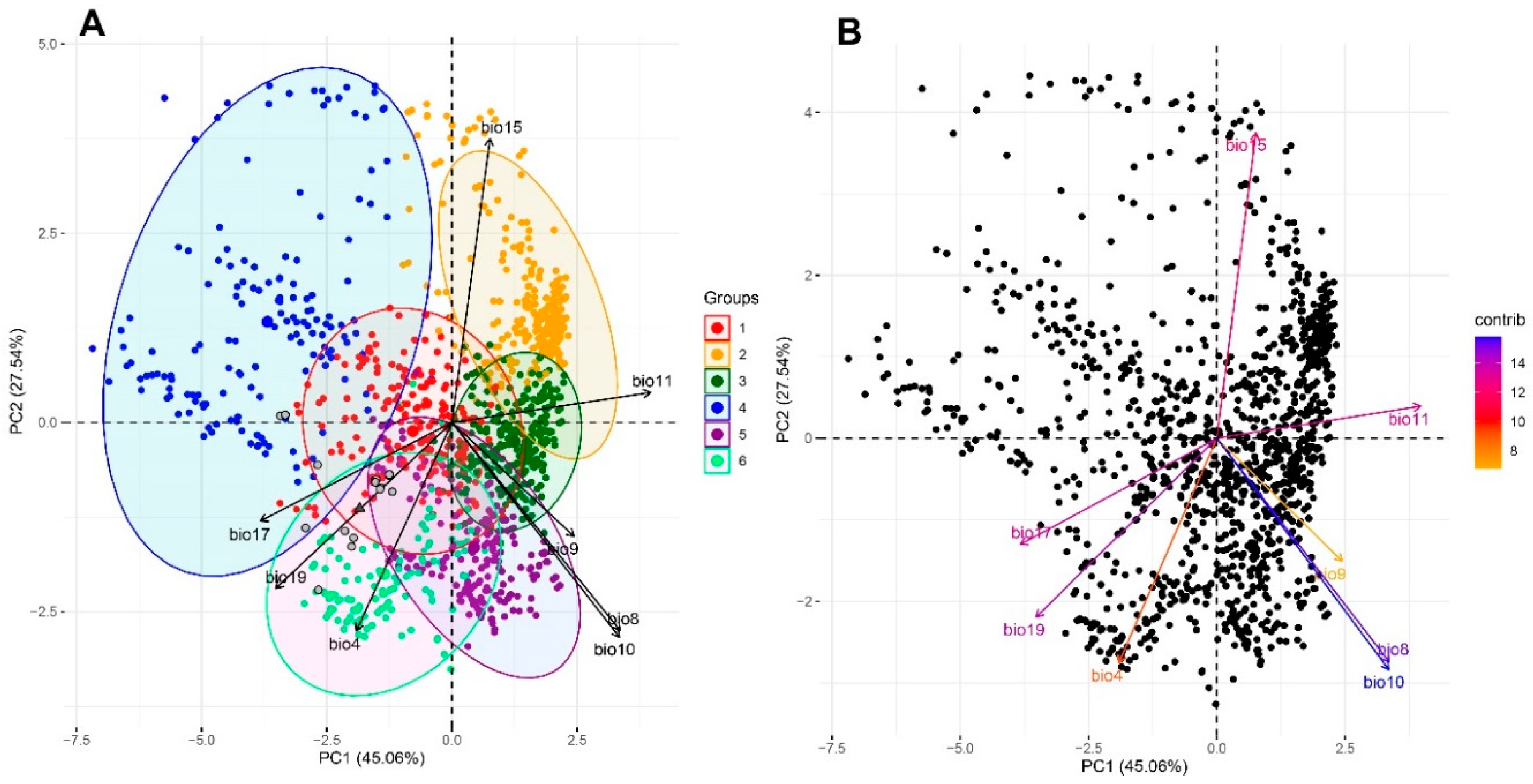
The 1450 European ash stands used for the PCA were sorted by eight components (bioclimatic variables), of which the first two were statistically significant and accounted for 72.60% of the total variance (
Figure 1 PCA Panel A).
The first component (PC1), explaining 45.06% of the variance (
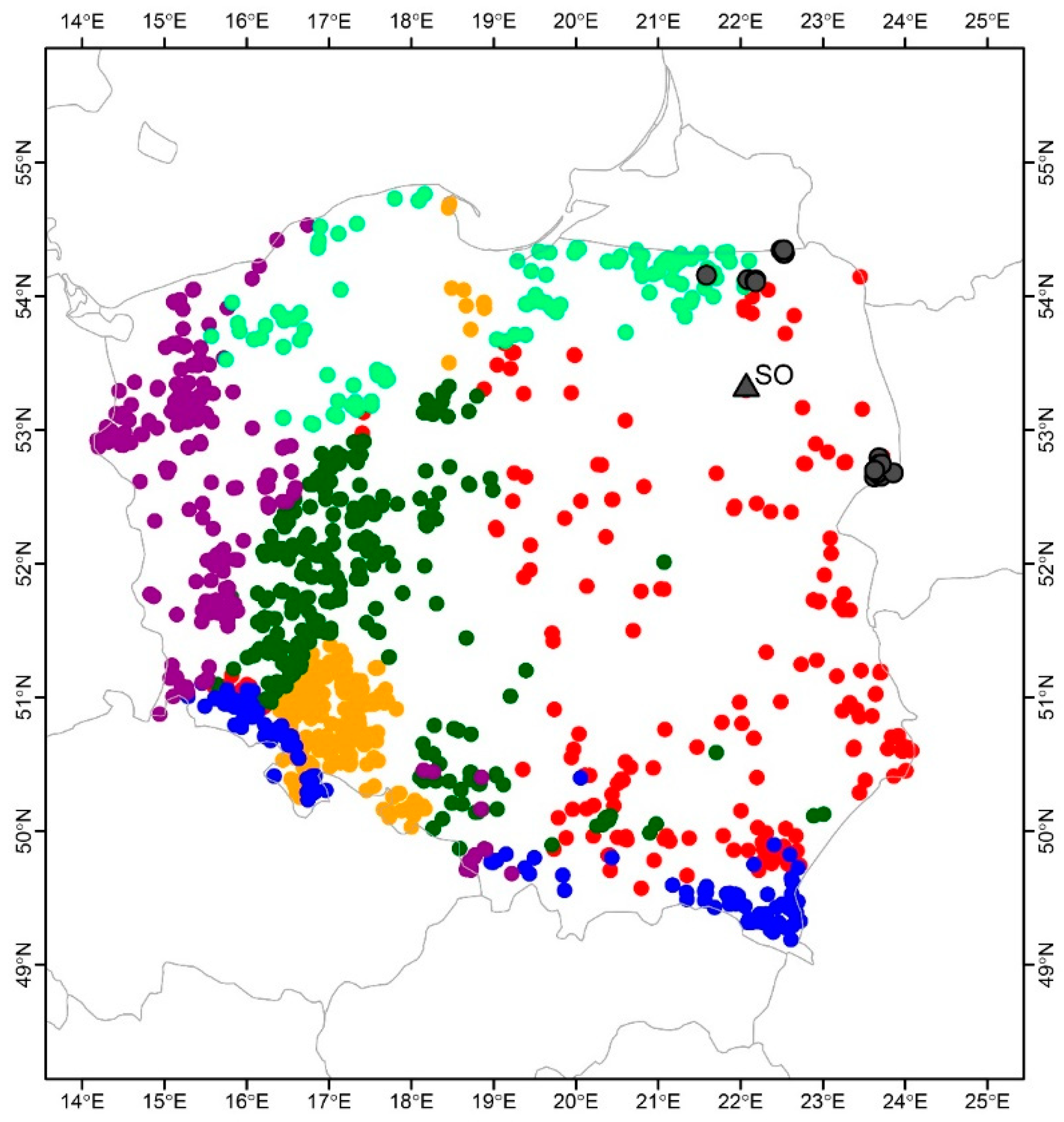
Table 1), was strongly correlated (|r| > 0.70) with the mean temperature of the coldest, wettest and warmest quarters (bio11, bio8 and bio10, respectively) and the precipitation of the driest (bio17) and coldest quarters (bio19). The second component (PC2) explained 27.54% of the variance and was strongly correlated with precipitation seasonality(|r| > 0.70) (bio15) and temperature seasonality (|0.50|<|r|<0.70) (bio4) and the mean temperature of the wettest (bio8) and warmest quarters (bio10). European ash populations have been assigned to two different ellipses determined at a 95% confidence level for the variability of climatic conditions in northeastern and eastern Poland (
Figure 2).
Implementation of the partitioning around medoids clustering algorithm led to the determination of six different bioclimatic regions in Poland for the European ash population (
Figure 2). Both the northeastern and eastern clusters are mainly determined by temperature seasonality and precipitation of the driest and coldest quarter conditions (bio4, bio17, and bio19, respectively;
Figure 1 PCA Panel B). However, the main bioclimatic variables differentiating European ash populations are the mean temperatures of the wettest and warmest quarters (bio8 and bio10, respectively).
Phenology and Defoliation Analyses
The grafts began bud burst between the end of April and the first week of May, with the entire bud development process lasting approximately 14 days. The most intensive period of growth and differentiation of the grafts occurred between the 5th and 7th day after the initial phenological observations. After this period, 80% of the grafts could be unequivocally assigned to a specific phenological phase.
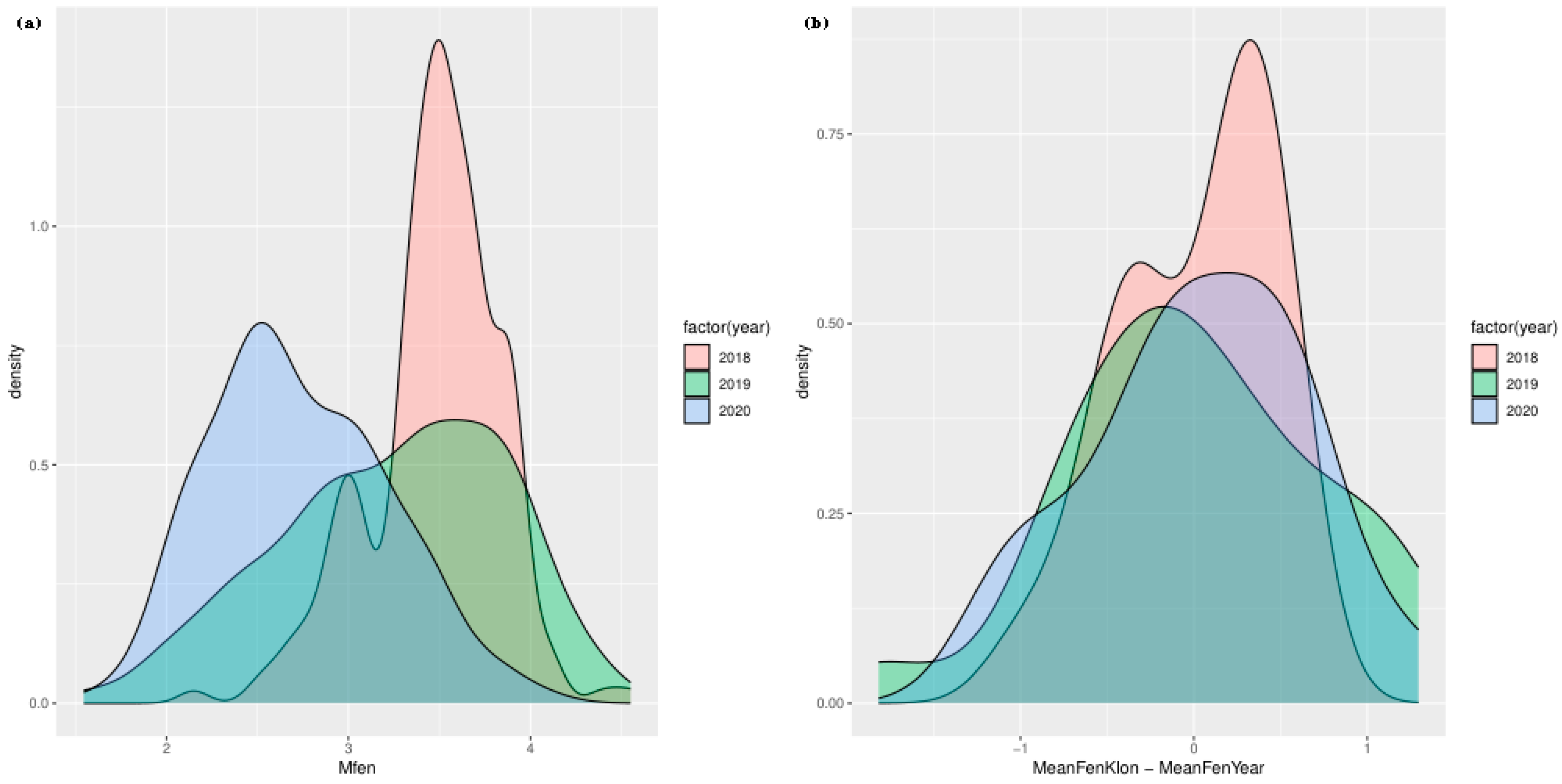
Figure 3 illustrates the distribution of the phenology of ash bud burst in different years. In panel (a), changing patterns of distribution are observed across the different years. The Mfen values from 2018 are characterised by a high concentration around a single peak, indicating lower phenological variability in that year. In contrast, the distributions for 2019 and 2020 show greater dispersion of values with several local peaks, indicating increased complexity and variability in the bud burst process during these years. Panel (b) of the figure presents the density distributions of the differences in phenology of clones relative to the annual mean (MeanFenKlon - MeanFenYear). In 2018, these differences are concentrated near zero, suggesting that the phenological values of the clones were close to the annual mean. In 2019 and 2020, the distributions of differences become more dispersed, and their breadth increases, reflecting greater deviations from the annual mean in the phenology of clones.
In 2019, the phenology of spring bud burst was not significantly different from 2018, indicating no substantial differences in the phenological process between individual genotypes in these years. In contrast, in 2020, significant differences from 2018 were observed in bud burst phenology. The estimated value for 2020 was -0.37, with a confidence interval ranging from -0.61 to -0.13, which was statistically significant at p = 0.003 (
Table 2). The Intraclass Correlation Coefficient (ICC) for the clones was 0.57, suggesting that 57% of the variability in bud burst phenology can be attributed to differences among clones, highlighting the importance of genotypic differences within the studied population. The standard deviation for random effects associated with clones was 0.22, indicating variability due to random factors within the clones (
Table 2). The total number of observations was 66, encompassing 22 clones, providing a robust basis for statistical analysis. The marginal R² was 0.058, while the conditional R² was 0.596, reflecting the contribution of random and fixed variables in explaining data variability and suggesting that the model accounted for a significant portion of the variability in the phenology of spring bud burst (
Table 2).
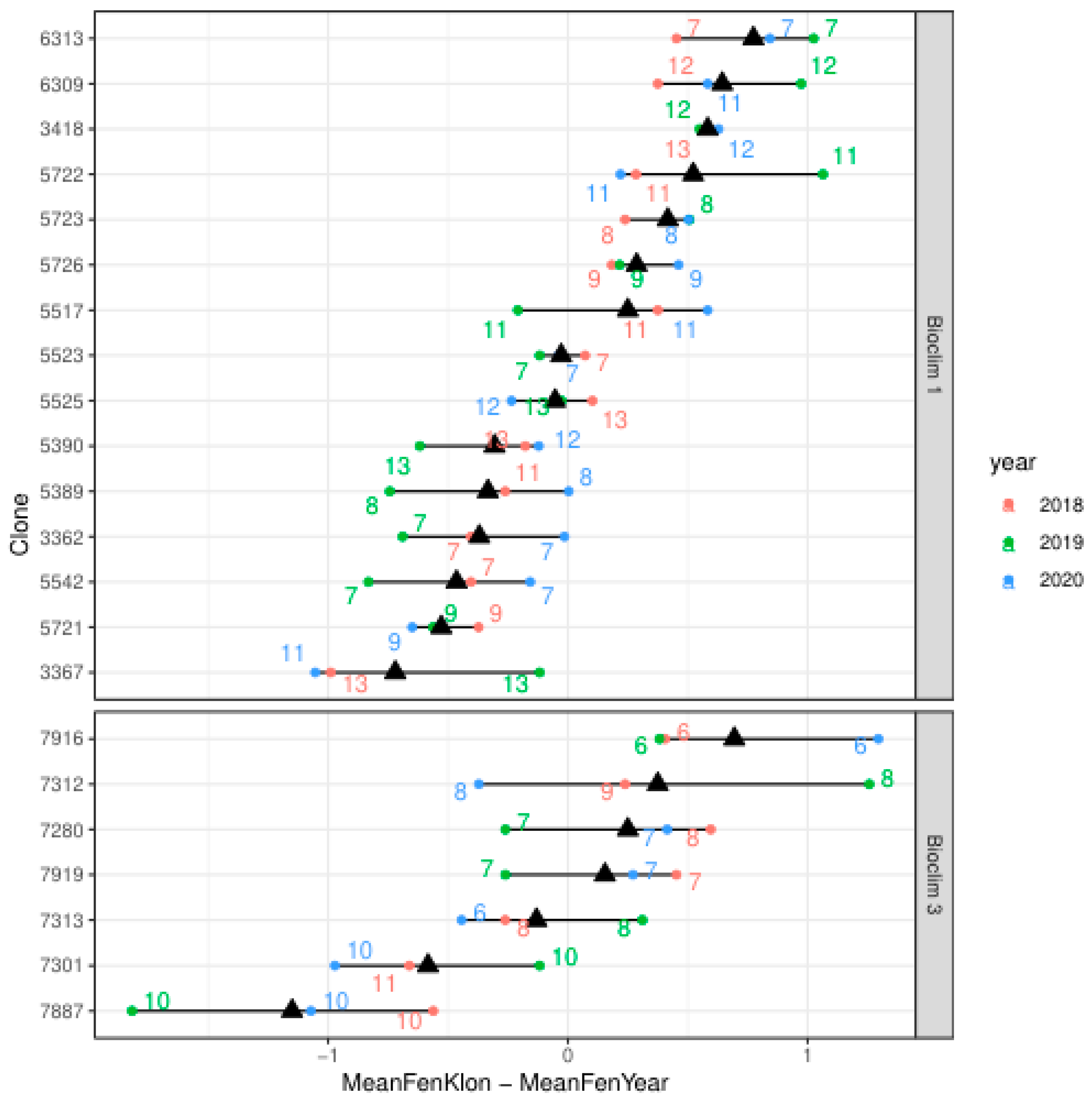
The
Figure 4 illustrates the phenological variability of ash clones in two bioclimatic regions from 2018 to 2020. The distinct differences in phenological timing, particularly in clones 6313 and 7887, indicate substantial phenological diversity within the regions. The number of grafts allows for the assessment of clone health, which can be useful in analyzing their viability. An overall decline in the number of grafts was observed in most clones across both bioclimatic regions, which may suggest ongoing issues with infection by the fungus
Hymenoscyphus fraxineus. While the trends are similar, some clones exhibit greater resistance or susceptibility to conditions affecting their viability, potentially due to their specific genetic traits.
Based on the analysis of available data, the Bioclim 1 region (
Figure 4) demonstrates greater adaptive capacity. This is due to the greater stability in the number of grafts and favorable phenological deviations, which suggest better adaptation to environmental conditions. Clone 6313, with a constant number of grafts over three years, deserves special attention. Its positive phenological deviations suggest that it is better adapted to earlier phenological onset, which may be advantageous under certain stress conditions. Clone 5723 shows smaller declines in the number of grafts compared to other clones in the Bioclim 1 region. On the other hand, it should be noted that in Bioclim 3, clone 7301 maintains a relatively stable number of grafts compared to other clones, despite some declines (
Figure 4).
The phenological distribution of the clones shows that Bioclim 1 has more clones with early phenological onset, while Bioclim 3 is characterized by a greater number of late clones. This differentiation may indicate the adaptation of clones to specific bioclimatic conditions in these regions. It should be noted that clones 6313 from Bioclim 1 and 7301 from Bioclim 3 show early phenological onset, which is consistent with their high survival rate.
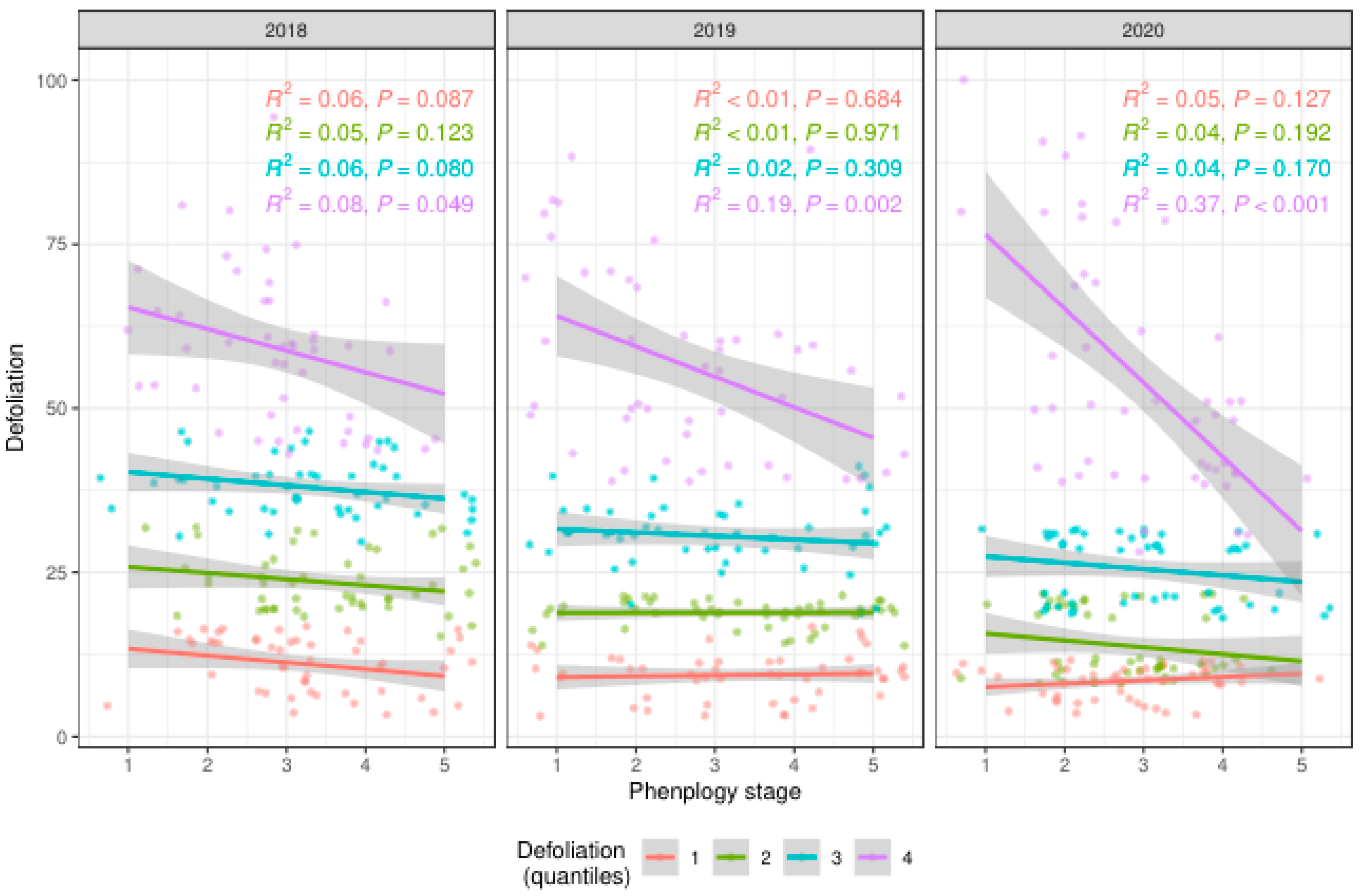
Figure 5 illustrates the degree of defoliation in a group of ash clones over three years (2018-2020), based on their phenological stages. Each year is presented in a separate panel, highlighting the relationship between phenological stages and the degree of defoliation.
Figure 8 reveals a clear association between phenological stages and the severity of defoliation in ash clones. Similar to 2018, in 2019, the correlation between phenological stages and defoliation is generally weak, except for the highly defoliated clones (purple). The correlation for this group is somewhat more pronounced, with P<0.005. In 2020, the correlation is more evident for the highly defoliated clones (purple), showing R²=0.37 and P<0.001. This strong correlation indicates that clones in the latest phenological stages are more susceptible to severe defoliation. The phenological stage appears to be a key factor influencing the severity of defoliation caused by H. fraxineus infection, with late-developing clones being more vulnerable to damage.
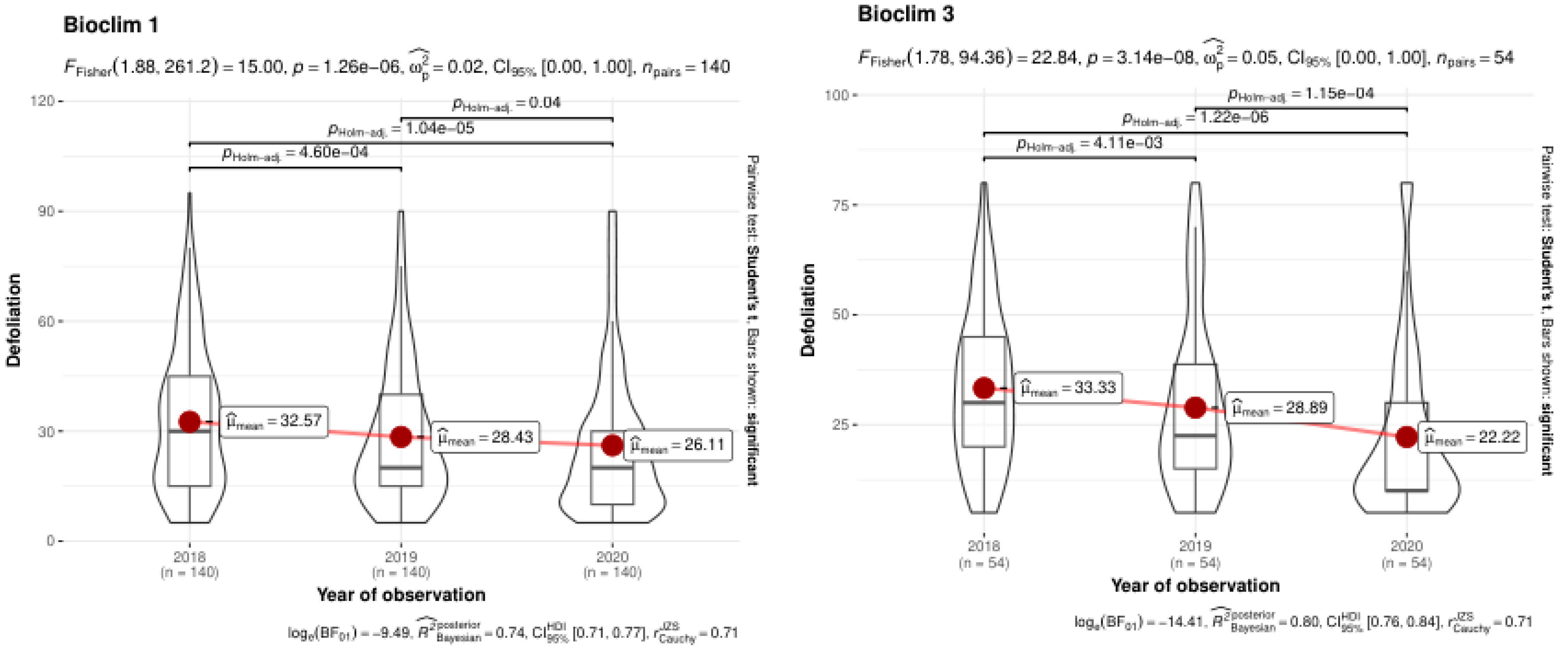
Figure 6 illustrates the changes in defoliation levels from 2018 to 2020 in two bioclimatic regions: Bioclim 1 and Bioclim 3, highlighting differences in the rate of defoliation reduction between these regions. In Bioclim 1, there is a notable decrease in the mean defoliation levels over the observed years, with mean defoliation values (Dmean ) of 32.57 in 2018, 28.43 in 2019, and 26.11 in 2020. Similarly, Bioclim 3 also shows a decrease in defoliation levels over the years. The better vitality of trees is more pronounced in Bioclim 3, suggesting a more effective response to defoliation factors. Fisher's statistic (
F=22.84) indicates highly significant differences in defoliation levels across the years in Bioclim 3, and Bayesian analysis confirms a significant reduction trend during the observed years. The variance components (σG
2=0.05 and σE
2=0.05) underscore the role of genetic factors in shaping the dynamics of defoliation.
3. Discussion
Ash dieback caused by the fungal pathogen Hymenoscyphus fraxineus is a serious ecological problem in Europe. It is estimated that in some regions of Europe, even 70-90% of ash stands have been severely affected, with many trees dying as a result of the infection. Depending on the region, the intensity of ash dieback can vary; however, in the most severely affected areas, losses can reach up to 95% of ash trees (10). The estimated economic losses due to ash dieback are projected to reach £15 billion in the UK alone over the next 100 years (22). Currently, there are no effective methods to protect ash trees from infection with H. fraxineus (13). Ongoing scientific research focused on mitigating the ecological consequences of the epidemic is investigating evolutionary mechanisms of resistance in ash trees by selecting genotypes that are less susceptible to dieback.
In the present study, we evaluate the natural evolutionary mechanism of spring bud burst timing and its impact on the dynamics of infection. We confirmed the well-known relationship between phenology timing and provenance, and also demonstrated higher survival and vitality in clones with earlier bud burst times. These findings may have practical implications, providing guidelines for selecting genotypes to be used as parent generations for generative and vegetative reproduction.
The obtained results are a logical consequence of the nature of the epidemic, whose gradual expansion, combined with the high intensity of the disease, suggests that
H. fraxineus is an invasive alien organism (23). In its native range (Japanese populations), the fungus acts as a leaf endophyte, and it is possible that its invasiveness in Europe would be negligible with limited inoculum. However, the currently observed high inoculum density allows the fungus to challenge host defense responses (24), leading to an epidemic state. The dynamic nature of ash dieback was confirmed in the present study, where 30% of plants died within the first few years of growth; now, the dynamics of dieback are decreasing (
Figure 5). The significant reduction in defoliation levels was a result of removing fallen ash leaves during winter, which are a source of reinfection by the pathogen in the spring—a treatment and its effectiveness described by (17). Observations of the relationship between leaf shedding and the level of secondary infections by
H. fraxineus—along with the spring bud burst phenology—are natural defense mechanisms of ash trees against pathogen infection. The present study demonstrated a significant difference in clones regarding the timing of spring bud burst (
Figure 7), which had a significant impact (P<0.001) on the health of ash trees most affected by
H. fraxineus (
Figure 5). The level of ash tree infection is influenced by the evolutionary adaptations discussed in this study, genetic factors (26), and environmental interactions (13).
On the other hand, the lack of population structure of H. fraxineus in Europe (6 and 26) and the much lower allelic richness compared to native populations (27) prompt the search for effective methods to naturally reduce epidemics. In addition, field observations indicate that only a small proportion of foliar infections result in shoot infections (6). This is possible because in many cases, the infected leaves are shed before the pathogen reaches the host's phloem or xylem tissue (28). For these reasons, intensive research is underway to select genotypes that are less susceptible to the disease. One of the most important phenotypic traits influencing the degree of infestation of ash trees by H. fraxineus is the spring bud burst phenology examined in the present study (29 and 30).
Significant genetic correlations between phenological traits and crown damage caused by H. fraxineus infection have been found in Danish and Swedish studies (8 and 30). The genetic correlation between spring phenology (bud burst) and clonal health (percentage of crown defoliation) was significant. Additionally, the same studies found a strong correlation between ash infestation levels and leaf senescence (measured as autumn leaf color), with fewer infested ash clones exhibiting earlier yellowing. The results confirm that leaves, as one of the primary symptoms of infection, play a key role in the dynamics of disease recognition in ash trees. Similar results were obtained by Baliuckas and Pliura (29) in experiments with Lithuanian and Western European populations, in which populations with earlier spring phenology had significantly (r = 0.81) better health. The aforementioned analysis by Baliuckas and Pliura (29) clearly demonstrates the relationship between health status and spring frost occurrence, as well as differences among populations. Baliuckas and Pliura (29) showed that populations from higher latitudes and greater longitudes were less damaged. These findings demonstrate that the spring development of ash trees is an epigenetic trait inherited by offspring. Our study confirms the results obtained in previous studies (8, 29 and 30). The present study also confirms the genetic dependence of phenological timing on the provenance of the clones. Although the northern clones grew under different climatic conditions, they retained the genetic pattern of later spring phenology. This phenomenon likely has ecological consequences related to the evolutionary escape of northern ash trees from late frosts (31).
The analysis of climatic data related to the occurrence of ash in Poland confirmed the bioclimatic regionalization of ash (
Figure 1 and
Figure 2). Eight bioclimatic variables responsible for the occurrence of the species were identified (
Table 1). The studied ash trees, distinguished by their regions of origin, were grouped into two basic provenances (bioclimatic region 1 and bioclimatic region 3), which differed from each other in terms of the timing of phenological events and health status. Trees from northern Poland (bioclimatic region 3) were significantly characterized by later timing of phenological appearance.
It is necessary for the selection and breeding of ash to determine whether the reduced damage is due to adaptation to growing conditions or to reduced susceptibility to
H. fraxineus. In this context, data showing the degree of defoliation of ramets as a function of their origin and phenology are important. It should be noted that the spore pressure of
H. fraxineus in the present study decreases in years when fallen leaves, which serve as a source of infection the following spring, are removed (
Figure 6). Our results revealed that the ramets from different bioclimatic regions had the greatest differences in damage levels in 2018, while the difference between ramets in 2020 was not significant (
Figure 6). Therefore, it can be hypothesized that the cause of damage to ash crowns is
H. fraxineus, which in 2018 significantly damaged ash crowns at a later spring bud break due to high spore pressure. On the other hand, with low spore pressure from
H. fraxineus, variations in the extent of damage to ash trees were negligible. This conclusion confirms the general knowledge about the development of
H. fraxineus. The pathogen forms its apothecia mainly in petioles and rachitic leaves lying on the ground, releasing ascospores that infect ash trees (23). The number of airborne ascospores is highest in the early morning and depends on the humidity protecting the ascospores from drying out (32). Thus, despite the release of spores over a long period (33), ecological conditions for the infection of ash trees by the pathogen are most favorable for a few days in early spring; later, high temperatures reduce the effectiveness of infection.
4. Materials and Methods
Study Site
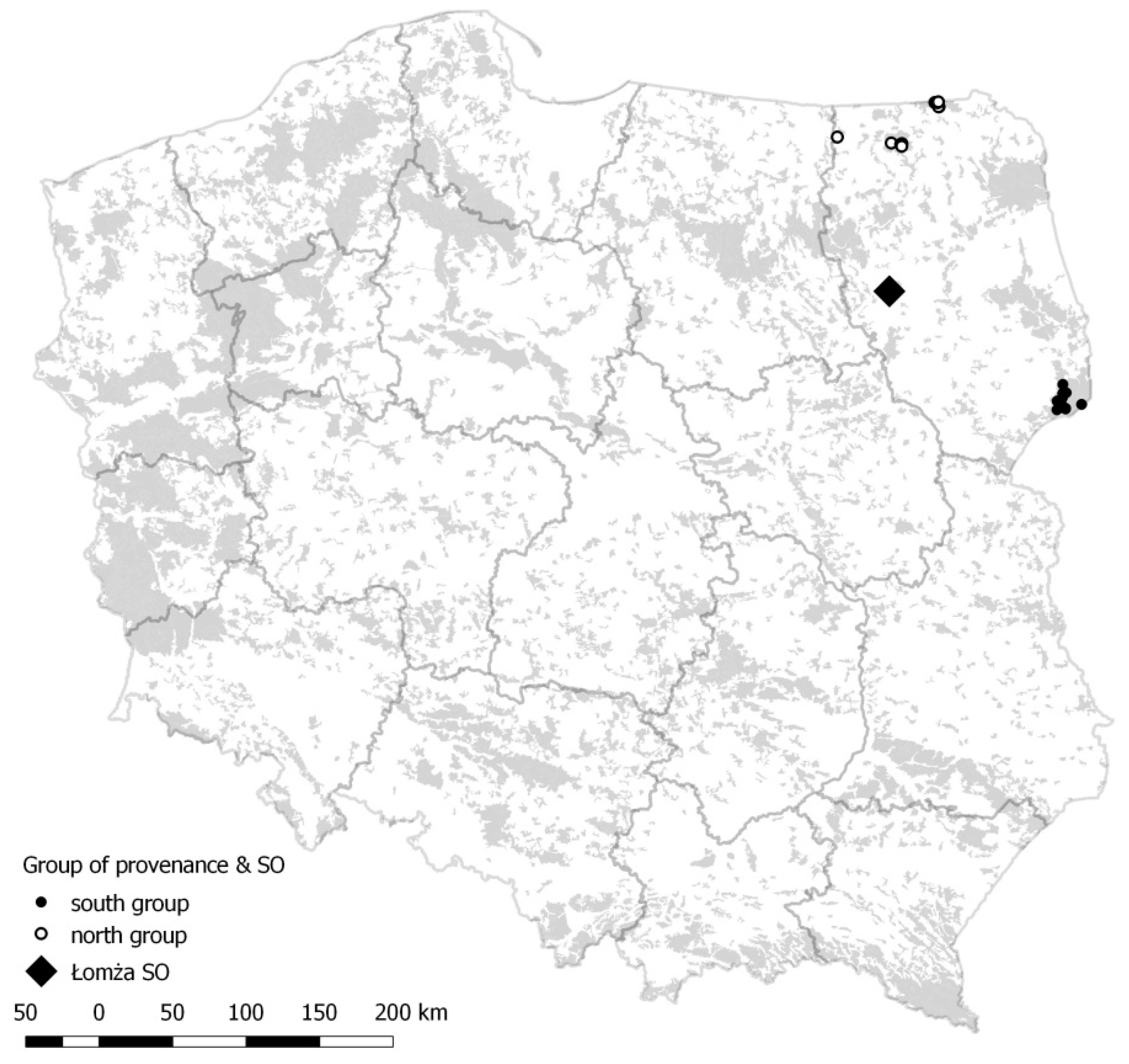
This study was performed in a clonal seed orchard located in the Łomża Forest District (N 22.06, E 53.31) in northeastern Poland. The orchard was planted on a moist forest site in spring 2002 on an area of 6.22 ha and divided into 6 plots (blocks) where 1544 one-year-old ash trees were planted in a single tree plot design with a spacing of 6 × 6 m. The grafts were grown from 31 clones of plus trees originating from 6 populations: Białowieża, Borki, Browsk, Czerwony Dwór, Gołdap, and Hajnówka (
Figure 1). For this study plot 2 with the highest survival rate (64.0%) was selected. The mean annual temperature in the years of observations ranged from 9.6 °C to 10.1 °C, while the relative humidity ranged from 74.8 to 77.3% (
Table 3). For detailed data on forest habitat and topography of the analysed seed orchards, see Przybylski et al. (2020). Individual clones were represented by different numbers of grafts (from 2 to 13 individuals). Ramets whose abundance per seed orchard did not exceed 4 were removed from the statistical analyses (
Table 4).
Climate of Seed Orgin and Study Area
Plus trees whose genotype was analysed in the present study grew in two Polish climatic zones, i.e., south and north (
Figure 7 and
Table 4). The southern zone is warmer, with an average annual air temperature of approximately 7.5 °C and a growing season approximately 200 days long. The northern zone is colder, with an average annual temperature that does not reach 7.0 °C and a growing season length that does not exceed 180 days. In both, the total annual precipitation is 550-600 mm. The analysed seed orchard was located at the border between the isotherms and isohyets of the two areas.
Table 4.
Classification of the studied provenances into climatic zones; the single clone number was assigned to the climatic zone together with the individual number of grafts (Forest Research Institute of Poland database) in the seed orchard. Number of grafts in plot 2 in the seed orchard and number of grafts that survived in plot 2 in 2020. Grafts whose numbers were less than four were not analysed.
Table 4.
Classification of the studied provenances into climatic zones; the single clone number was assigned to the climatic zone together with the individual number of grafts (Forest Research Institute of Poland database) in the seed orchard. Number of grafts in plot 2 in the seed orchard and number of grafts that survived in plot 2 in 2020. Grafts whose numbers were less than four were not analysed.
| |
Group of climatic zone |
Provenance name |
Individual N° of Plus trees |
Numbers of grafts in plot 2 of seed orchard |
Ramets survival, year 2020 [%] |
Selected for statistical analysis |
| 1 |
south |
Browsk |
3362 |
7 |
87.5 |
+ |
| 2 |
Browsk |
3367 |
13 |
73.3 |
+ |
| 3 |
Białowieża |
3385 |
3 |
50.0 |
- |
| 4 |
Hajnówka |
3418 |
13 |
85.71 |
+ |
| 5 |
Hajnówka |
5389 |
11 |
57.14 |
+ |
| 6 |
Hajnówka |
5390 |
13 |
75.0 |
+ |
| 7 |
Hajnówka |
5517 |
11 |
84.62 |
+ |
| 8 |
Hajnówka |
5523 |
7 |
43.75 |
+ |
| 9 |
Hajnówka |
5524 |
2 |
33.33 |
- |
| 10 |
Hajnówka |
5525 |
13 |
75 |
+ |
| 11 |
Białowieża |
5542 |
7 |
58.33 |
+ |
| 12 |
Hajnówka |
5721 |
9 |
69.23 |
+ |
| 13 |
Hajnówka |
5722 |
11 |
78.57 |
+ |
| 14 |
Hajnówka |
5723 |
8 |
72.73 |
+ |
| 15 |
Hajnówka |
5726 |
10 |
64.29 |
+ |
| 16 |
Browsk |
6309 |
12 |
78.57 |
+ |
| 17 |
Browsk |
6313 |
7 |
50 |
+ |
| 18 |
Hajnówka |
6318 |
2 |
100 |
- |
| 19 |
north |
Borki |
7246 |
3 |
75 |
- |
| 20 |
Gołdap |
7268 |
3 |
50 |
+ |
| 21 |
Gołdap |
7280 |
8 |
70 |
+ |
| 22 |
Borki |
7301 |
10 |
76.92 |
+ |
| 23 |
Czerwony Dwór |
7312 |
9 |
72.73 |
+ |
| 24 |
Czerwony Dwór |
7313 |
8 |
60 |
+ |
| 25 |
Czerwony Dwór |
7875 |
4 |
75 |
- |
| 26 |
Gołdap |
7882 |
3 |
16.67 |
+ |
| 27 |
Gołdap |
7887 |
11 |
76.92 |
+ |
| 28 |
Gołdap |
7888 |
4 |
66.67 |
+ |
| 29 |
Gołdap |
7916 |
6 |
100 |
+ |
| 30 |
Gołdap |
7918 |
3 |
37.5 |
+ |
| 31 |
Gołdap |
7919 |
7 |
77.78 |
+ |
Figure 7.
Geographical location in Poland of the study seed orchards, the square. Black dots indicate locations of plus trees from the southern region. White dots indicate locations of plus trees from the northern region. The grey background represents the forest areas of Poland.
Figure 7.
Geographical location in Poland of the study seed orchards, the square. Black dots indicate locations of plus trees from the southern region. White dots indicate locations of plus trees from the northern region. The grey background represents the forest areas of Poland.
Phyto-Pathological Evaluation
To confirm the presence of
H. fraxineus in the seed orchards, shoot samples of the side pedals were collected from trees showing symptoms of infestation (
Figure 8a). Samples were taken from seed orchards one ramet per clone (clones: 7301, 7888, 7268, 6309, 5542, 5525, 5723, 7919, and 5389). Six to seven samples from each ramet has been placed in media. If
H. fraxineus was detected in one sample from a clone, it was considered a confirmation of infection. Laboratory analyses and macroscopic identification of the obtained cultures were performed according to the methodology described by Kowalski and Bartnik (9) (
Figure 8b). The obtained isolates exhibited the characteristics of type B colonies with unevenly discoloured white to yellow‒orange mycelia (
Figure 8c). Morphological identification of selected
H. fraxineus isolates was supported by sequencing of the barcode region ITS according to the methodology described by Chandelier et al. (34). The barcode region sequences were deposited in the GenBank database [ncbi.nlm.nih.gov] under accession numbers MT053856 and MT053857.
Figure 8.
Macroscopic identification of the obtained cultures of the pathogen. a) Phenotypic signs of H. fraxineus on ash trees; b) methodological confirmations of H. fraxineus occurrences by Kowalski and Bartnik (35) (2010); c) type B colonies H. fraxineus.
Figure 8.
Macroscopic identification of the obtained cultures of the pathogen. a) Phenotypic signs of H. fraxineus on ash trees; b) methodological confirmations of H. fraxineus occurrences by Kowalski and Bartnik (35) (2010); c) type B colonies H. fraxineus.
Phenological Observations and Health Condition
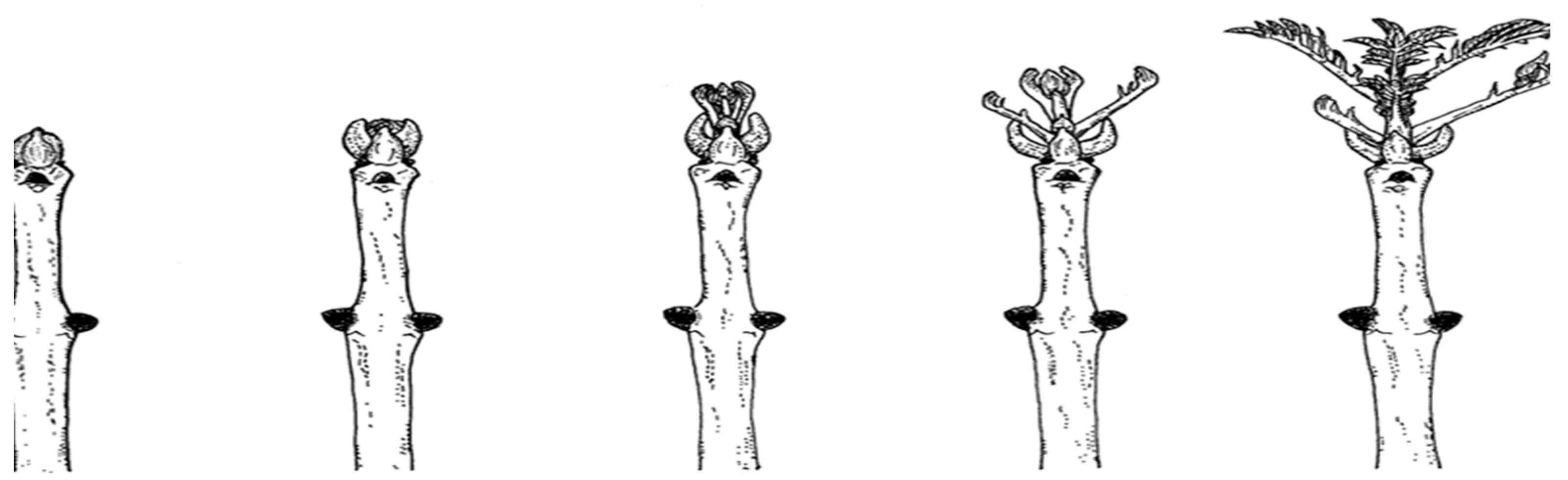
The phenology of ash budburst was assessed for all 238 ramets in block 2 using the methodology developed in the Trees4Future project (Trees4Future - Home). Phenological phase assessment assumes the identification of five spring developmental phases of buds (
Figure 9). The phenological phases were as follows:
dormant bud
swelling of bud, slight greening of bud scales
buds begin to burst, first green visible
bud burst, petioles of leaves visible, no lengthening of twig
bud burst, petioles of leaves visible, twig has started lengthening, leaves are fully expanded.
Bud development monitoring was conducted every 1-3 days from April to May (in 2018: 111-151 days of the year (DOY); in 2019: 108-146 DOY; and in 2020: 115-154 DOY) by observing the buds from dormancy to full leaf development. Numerical values assigned to individual stages of bud development were averaged (arithmetic mean) for all trees of a given clone (238 ramets).
Plant health was determined in August 2018-2020 by the degree of defoliation of the assimilation apparatus in the central part of the tree crown. Defoliation was assessed from the ground simultaneously by two people who observed the ramets from opposite sides of the crown. The degree of defoliation used in the analysis was the arithmetic mean of two values of crown defoliation measured on a scale from 0 to 100% (dead tree). The methodology described is consistent with international standards adopted by the ICP Forests and ICP-Focus projects (35).
Bioclimatic Analysis
To determine the position of ash populations in the Polish range of European ash, which is necessary for bioclimatic analysis, we used a dataset of sites compiled from the Polish State Forests IT System (SILP) (36). After cleaning the dataset for erroneous and doubtful locations, as well as locations outside the country, 1450 European ash stands were available.
Then, 19 bioclimatic parameters were extracted from BIOCLIM 2.0 bioclimatic maps (37) with a resolution of 0.5 arcmin using the R package 'raster' version 3.5-2. From this dataset, eight variables were selected (
Table 1), removing those that provided too general climatic description (annual temperatures and precipitation) and then excluding variables that were highly correlated with each other (R > 0.7). The climatic variables were used to characterise the climatic variability of European ash stands selected from the database.
The analysis was performed to define the climate-related clustering of European ash by means of the partitioning around medoids clustering algorithm, which is an extension of the k-means clustering algorithm (38). To determine the optimal number of clusters, we applied the within-sum-of-squares method (WSS), which minimises the distance between points in a cluster. Finally, we defined six clusters as optimal for grouping the European ash distribution dataset. The optimal number of clusters and the PAM clustering were calculated using the 'cluster' and 'factoextra' packages in R (38). To determine the climatic position of ash populations within the climatic gradient of European ash distribution in Poland We performed principal component analysis (PCA) of bioclimatic variables for all Polish occurrences of European ash based on a correlation matrix. A set of statistically significant axes was determined using a 1000-fold bootstrap analysis. Pearson's correlation coefficient was used to identify the climatic factors according to which the occurrences of European ash were ranked by principal components (
Table 1).
Statistical Analyses
The mean phenology of spring bud burst in ash clones in 2018, 2019 and 2020 was analysed using a linear mixed-effects model in R (R Core Team, 2020). The
lme4 package (39), which was developed for fitting fixed and random effects models, is therefore particularly suitable for data with hierarchical structures. A linear mixed-effects model was specified to investigate the influence of year on the timing of bud burst while accounting for the random variability associated with the clone effect. The model included year as a fixed effect to assess temporal trends, with 2018 serving as the reference year. Clone-specific variability was modelled as a random effect so that each clone has its own intercept value to capture clone-specific deviations from the overall average stage of bud burst. The model can be formally expressed as follows:
where:
represents the mean bud burst stage for clone i in year j;
is the intercept, reflecting the estimated mean bud burst stage in 2018;
and are the fixed effect coefficients for the years 2019 and 2020, respectively;
is the random effect associated with clone i, representing the deviation of each clone from the overall mean, assumed to follow a normal distribution with mean 0 and variance ;
is the residual error term, normally distributed with mean 0 and variance .
The model estimated the fixed effects of the year and the random effects of the individual clones. The fixed effects provided information on the annual variation in the timing of bud burst, while the random effects captured the variability between clones. Confidence intervals were calculated for the fixed effects to assess the precision of the estimates and the statistical significance of the differences from year to year. The random effects for each clone were extracted to understand how each clone differed from the mean of the whole population. This extraction was complemented by tools from the mixedup package, which provided a comprehensive summary of the random effects and facilitated the interpretation of clone-specific patterns.
5. Conclusions
Ash dieback (Fraxinus excelsior), caused by the invasive fungal pathogen Hymenoscyphus fraxineus, poses a serious ecological and economic threat to European forests. Our study confirmed that phenological variation, particularly in the timing of early spring bud burst, plays a crucial role in reducing the susceptibility of ash trees to infection. Clones exhibiting earlier bud burst were characterised by higher survival rates and less crown damage, suggesting that phenological timing is a key factor in selecting genotypes resistant to H. fraxineus.
The observed relationship between phenology and the health of ash trees underscores the importance of further research into the genetic and ecological mechanisms that could support the development of resistant ash populations. These findings have significant practical implications, particularly in the context of selecting and breeding genotypes intended for natural regeneration and forest resource conservation. In the future, it will be crucial to understand how these phenotypic traits can be integrated into forest management strategies to minimise the impact of ash dieback on an ecosystem scale.
Further research is necessary to better understand the long-term consequences of phenology at the ecosystem level and its impact on the survival of the species under changing climatic conditions. Understanding these relationships may support the development of more effective forest management strategies that will not only protect ash resources but also preserve biodiversity and ecosystem stability in the face of global environmental changes.
Author Contributions
Conceptualization, PP. and KŚ.; methodology, PP. and KŚ.; software, PP..; validation, PP. and KŚ; formal analysis, PP..; investigation, PP; resources, PP and KŚ.; data curation, VM.; writing—original draft preparation, PP., KŚ., VM; writing—review and editing, PP.; visualization, PP. and VM.; supervision, PP.; project administration, PP.; funding acquisition, PP. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Forest Research Institute (grant number 90.02.51).
Data Availability Statement
The data are publicly available in annual reports held in the library of the Forest Research Institute in Poland.
Acknowledgments
Regional Directorate of State Forests of Bialystok and Marcin Klisz support with bioclimatic analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dobrowolska, D.; Hein, S.; Oosterbaan, A.; Wagner, S.; Clark, J.; Skovsgaard, J.P. A review of European ash (Fraxinus excelsior L.): Implications for silviculture. Forestry 84, (2011):133–148. [CrossRef]
- Hemery, G.E. Forest management and silvicultural responses to projected climate change impacts on European broadleaved trees and forests. Int For Rev 10, (2008):591–607. [CrossRef]
- Rawlik, K.; Jagodzinski, A.M. Ekologiczne znaczenie roslin runa lesnego. ACADEMIA-mag Pol. Akademii Nauk 3-4: (2019), 50–53.
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Global Change Biology, 24, (2018): 1150−1163. DOI: DOI: . [CrossRef]
- Bakys, R.; Vasaitis, R.; Barklund, P.; Ihrmark, K.; Stenlid, J. Investigations concerning the role of Chalara fraxinea in declining fraxinus excelsior. Plant Pathol 58, (2009):284–292. [CrossRef]
- Gross, A.; Holdenrieder, O.; Pautasso, M.; Queloz, V.; Sieber, T.N. Hymenoscyphus pseudoalbidus,; the causal agent of European ash dieback. Mol plant pathol 15, (2014):5–21. [CrossRef]
- Kowalski, T.; Holdenrieder, O. Pathogenicity of Chalara fraxinea. For Pathol 39, (2009):1–7.
- McKinney, L.V.; Nielsen, L.R.; Hansen, J.K.; Kjær, E.D. Presence of natural genetic resistance in fraxinus excelsior (Oleraceae) to Chalara fraxinea (Ascomycota): An emerging infectious disease. Heredity (Edinb) 106, (2011):788–797. [CrossRef]
- Kowalski, T.; Bartnik, C. Morphologial variation in colonies of Chalara fraxinea isolated from ash (Fraxinus excelsior L.) stems with symptoms of dieback and effects of temperature on colony growth and structure. Acta Agrobot 63. (2010) . [CrossRef]
- Pautasso, M.; Aas, G.; Queloz, V.; Holdenrieder, O. European ash (Fraxinus excelsior) dieback–A conservation biology challenge. Biol Conserv 158: (2013), 37–49.
- Coker, T.L.; Rozsypalek, J.; Edwards, A.; Harwood, T.P.; Butfoy, L.; Buggs, R.J. Estimating mortality rates of European ash (Fraxinus excelsior) under the ash dieback (Hymenoscyphus fraxineus) epidemic. Plants, People, Planet 1, (2019):48–58. [CrossRef]
- Musolin, D.L.; Selikhovkin, A.V.; Shabunin, D.A.; Zviagintsev, V.B.; Baranchikov, Y.N. Between ash dieback and emerald ash borer: Two Asian invaders in Russia and the future of ash in Europe. Baltic For 23: (2017), 316–333.
- Turczański, K.; Rutkowski, P.; Dyderski, M.K.; Wronska-Pilarek, D.; Nowiński, M. Soil pH and organic matter content affects European ash (Fraxinus excelsior l.) crown defoliation and its impact on understory vegetation. Forests 11: (2020).
- Davydenko, K.; Meshkova, V. The current situation concerning severity and causes of ash dieback in Ukraine caused by Hymenoscyphus fraxineus, (2017).
- Grosdidier, M.; Scordia, T.; Ioos, R.; Marçais, B. Landscape epidemiology of ash dieback. J Ecol 108:1789–1799. (2020) . [CrossRef]
- Wylder, B.; Biddle, M.; King, K.; Baden, R.; Webber, J. Evidence from mortality dating of Fraxinus excelsior indicates ash dieback (Hymenoscyphus fraxineus) was active in England in 2004 – 2005. (2018). Forestry. [CrossRef]
- Przybylski, P.; Sikora, K.; Mohytych, V.; Wlostowski, M. Wpływ zabiegu agrotechnicznego na stan zdrowotny klonalnej plantacji nasiennej jesionu wyniosłego (Fraxinus excelsior L.) w kontekście jej porażenia przez Hymenoscyphus fraxineus (T. Kowalski). sylwan 164: (2020) 404–413.
- Anderson, J.T.; Inouye, D.W.; McKinney, A.M.; Colautti, R.I.; Mitchell-Olds, T. Phenotypic plasticity and adaptive evolution contribute to advancing flowering phenology in response to climate change. Proc Biol Sci 279, (2012):3843–3852. [CrossRef]
- Smintina, I. Provenance trials of fraxinus excelsior. Results 10 years after planting. Revista Padurilor 108: (1993), 10–17.
- Kleinschmit, J.; Svolba, .J; Enescu, V.; Franke, A.; Rau, H.; Ruetz, W. First results of provenance trials of fraxinus excelsior established in 1982. Forstarchiv 67, (1996):114–122.
- Bakys, R.; Vasaitis, R.; Skovsgaard, J.P. Patterns and severity of crown dieback in young even-aged stands of european ash (Fraxinus excelsior L.) in relation to stand density; bud flushing phenotype; and season. Plant Protect Sci 49, (2013):120–126. [CrossRef]
- Hill, L.; Jones, G.; Atkinson, N.; Hector, A.; Hemery, G.; Brown, N. The £15 billion cost of ash dieback in Britain. Curr Biol. 2019 May 6;29(9):R315-R316. [CrossRef]
- Queloz, V.; Grünig, C.R.; Berndt, R.; Kowalski, T.; Sieber, T.N.; Holdenrieder, O. Cryptic speciation in Hymenoscyphus albidus. For Pathol 41: (2010), 133–142. [CrossRef]
- Drenkhan, R.; Riit, T.; Adamson, K.; Hanso, M. The earliest samples of Hymenoscyphus albidus vs. H. fraxineus in Estonian mycological herbaria. (2016).
- Meger, J.; Ulaszewski, B.; Pałucka, M.; Kozioł, C.; Burczyk, J. . Genomic prediction of resistance to Hymenoscyphus fraxineus in common ash (Fraxinus excelsior L.) populations. Evolutionary Applications, 17, (2024). [CrossRef]
- Burokiene, D.; Prospero, S.; Jung, E.; Marciulyniene, D.; Moosbrugger, K.; Norkute, G.; Rigling, D.; Lygis, V.; Schoebel, C.N. Genetic population structure of the invasive ash dieback pathogen Hymenoscyphus fraxineus in its expanding range. Biol Invasions 17, (2015):2743–2756. [CrossRef]
- Zhao, Y.J.; Hosoya, T.; Baral, H.O.; Hosaka, K.; Kakishima, M. Hymenoscyphus pseudoalbidus; the correct name for Lambertella albida reported from Japan. Mycotaxon -Ithaca Ny- 122: (2012). 25–41. [CrossRef]
- Kirisits, T.; Matlakova, M.; Mottinger-Kroupa, S.; Halmschlager, E.; Lakatos, F. Chalara fraxinea associated with dieback of narrow-leafed ash (Fraxinus angustifolia). Plant Pathol 59, (2010):411. [CrossRef]
- Baliuckas, V., Pliura, A. Development of dynamic multiple population breeding and conservation system of Quercus robur L. Proc Nordic Baltic Situ Symp.8, (1998):14.
- Stener, L.G. Clonal differences in susceptibility to the dieback of fraxinus excelsior in southern Sweden. Scand J For Res 28: (2013), 205–216. [CrossRef]
- Pliura, A.; Baliuckas, V. Genetic variation in adaptive traits of progenies of Lithuanian and western European populations of fraxinus excelsior L. Baltic For 13: (2007), 28–38.
- Timmermann, V.; Borja, I.; Hietala, A.M.; Kirisits, T.; Solheim, H. Ash dieback: Pathogen spread and diurnal patterns of ascospore dispersal; with special emphasis on Norway. EPPO Bull 41: (2011), 14–20. [CrossRef]
- Kirisits, T., Cech, T.L. Beobachtungen zum sexuellen stadium des eschentriebsterben-erregers Chalara fraxinea in osterreich. Forstsch Aktuell 48, (2009):21–25.
- Chandelier, A.; Andre, F.; Laurent, F. Detection of Chalara fraxinea in common ash (fraxinus excelsior) using real time PCR. Forest Pathol 40, (2009):87–95. [CrossRef]
- Lorenz. M. International co-operative programme on assessment and monitoring of air pollution effects on forests-ICP forests. Water, Air, Soil Pollut 85, (1995):1221–1226.
- Starzycka, J. Geographic information system in the state forests. In: Gajos M, Styblinska M (ed) Geoinformation challenges, Croatian Information Technology Association, (2008), Silesia, pp 259–267.
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315. (2017) . [CrossRef]
- Kaufman, L.; Rousseeuw P.J. Finding groups in data: An introduction to cluster analysis. John Wiley & Sons INC., Hoboken, New Jersey. (2008).
- Bates, D.; Mächler, M.; Bolker, B. & Walker, S.. Fitting Linear Mixed-Effects Models Using lme4. (2015).
Figure 1.
Climate-related variability among European ash sites in Poland (Panel A: red, yellow, dark green, blue, violet, and light green circles; Panel B: black circles) and ash populations (grey circles). For the input data extraction of 8 BIOCLIM indices (
http://worldclim.org/bioclim) for 1450 records. The colours of the circles in Panel A are consistent with the colour code used for bioclimatic clusters (
Figure 2). The acronyms of bioclimatic indices (bio4–19) are explained in
Table 1. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.) Panel B: Variable vectors coloured according to their contribution in total variance: orange – low, blue – high.
Figure 1.
Climate-related variability among European ash sites in Poland (Panel A: red, yellow, dark green, blue, violet, and light green circles; Panel B: black circles) and ash populations (grey circles). For the input data extraction of 8 BIOCLIM indices (
http://worldclim.org/bioclim) for 1450 records. The colours of the circles in Panel A are consistent with the colour code used for bioclimatic clusters (
Figure 2). The acronyms of bioclimatic indices (bio4–19) are explained in
Table 1. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.) Panel B: Variable vectors coloured according to their contribution in total variance: orange – low, blue – high.
Figure 2.
Climate-related European ash grouping in Poland. The partitioning around medoids clustering algorithm was used to determine six clusters of European ash current distribution (marked with colours: red, yellow, dark green, blue, violet, and light green circles). Grey triangle indicate the location of the ash seed orchard analysed in this study (SO), and grey circles mark the locations of stands where plus trees were selected for the SO.
Figure 2.
Climate-related European ash grouping in Poland. The partitioning around medoids clustering algorithm was used to determine six clusters of European ash current distribution (marked with colours: red, yellow, dark green, blue, violet, and light green circles). Grey triangle indicate the location of the ash seed orchard analysed in this study (SO), and grey circles mark the locations of stands where plus trees were selected for the SO.
Figure 3.
The density distributions related to the phenology of ash bud burst over the years 2018, 2019, and 2020. Panel (a) illustrates the density distribution of the phenology of bud burst (Mfen) across these three years. Panel (b) presents the density distributions of differences in phenology of clones relative to the annual mean (MeanFenKlon - MeanFenYear).
Figure 3.
The density distributions related to the phenology of ash bud burst over the years 2018, 2019, and 2020. Panel (a) illustrates the density distribution of the phenology of bud burst (Mfen) across these three years. Panel (b) presents the density distributions of differences in phenology of clones relative to the annual mean (MeanFenKlon - MeanFenYear).
Figure 4.
Phenological Adaptation and Graft Stability in Ash Clones Across Bioclimatic Regions. Annotations: X-Axis (MeanFenKlon – MeanFenYear): Represents the deviation from the annual mean phenological phase, with values ranging from -1 to 1. Red Circles: Indicate the phenological phase of clones in 2018, Green in 2019, Blue in 2020. Numbers Inside Symbols: Denote the number of grafts per clone for the given year, and triangles: represent the average phenological phase of each clone across the three years.
Figure 4.
Phenological Adaptation and Graft Stability in Ash Clones Across Bioclimatic Regions. Annotations: X-Axis (MeanFenKlon – MeanFenYear): Represents the deviation from the annual mean phenological phase, with values ranging from -1 to 1. Red Circles: Indicate the phenological phase of clones in 2018, Green in 2019, Blue in 2020. Numbers Inside Symbols: Denote the number of grafts per clone for the given year, and triangles: represent the average phenological phase of each clone across the three years.
Figure 5.
: Relationship Between Phenological Stages and Defoliation Levels in Ash Clones from 2018 to 2020. Color-Coded Symbols: Orange Dots: Low defoliation, up to 20% (quantile 1), Green Dots: Medium defoliation, up to 30% (quantile 2), Blue Dots: Moderate defoliation, up to 40% (quantile 3), Purple Dots: High defoliation, over 40% (quantile 4).
Figure 5.
: Relationship Between Phenological Stages and Defoliation Levels in Ash Clones from 2018 to 2020. Color-Coded Symbols: Orange Dots: Low defoliation, up to 20% (quantile 1), Green Dots: Medium defoliation, up to 30% (quantile 2), Blue Dots: Moderate defoliation, up to 40% (quantile 3), Purple Dots: High defoliation, over 40% (quantile 4).
Figure 6.
Analysis of Defoliation Dynamics in Ash Clones Across Bioclimatic Regions Over Three Years.
Figure 6.
Analysis of Defoliation Dynamics in Ash Clones Across Bioclimatic Regions Over Three Years.
Figure 9.
Diagram of ash bud development according to phenological phases 1-5 (Trees4Future - Home).
Figure 9.
Diagram of ash bud development according to phenological phases 1-5 (Trees4Future - Home).
Table 1.
Pearson’s correlation coefficients between the 8 bioclimatic variables proposed and the first two principal components (PC1 and PC2).
Table 1.
Pearson’s correlation coefficients between the 8 bioclimatic variables proposed and the first two principal components (PC1 and PC2).
| Bioclimatic variables |
Abbreviation |
Pearson’s correlation |
| |
|
PC1 |
PC2 |
| Temperature Seasonality (STD * 100) |
bio4 |
-0.42 |
-0.6 |
| Mean Temperature of Wettest Quarter |
bio8 |
0.73 |
-0.6 |
| Mean Temperature of Driest Quarter |
bio9 |
0.53 |
-0.33 |
| Mean Temperature of Warmest Quarter |
bio10 |
0.73 |
-0.62 |
| Mean Temperature of Coldest Quarter |
bio11 |
0.87 |
0.09 |
| Precipitation Seasonality (CV) |
bio15 |
0.17 |
0.82 |
| Precipitation of Driest Quarter |
bio17 |
-0.84 |
-0.28 |
| Precipitation of Coldest Quarter |
bio19 |
-0.77 |
-0.48 |
Table 2.
Mixed-Effects Model Analysis of Spring Bud Burst Phenology in Ash Clones for 2018, 2019 and 2020.
Table 2.
Mixed-Effects Model Analysis of Spring Bud Burst Phenology in Ash Clones for 2018, 2019 and 2020.
| |
MeanFenKlon |
| Predictors |
Estimates |
CI |
p |
| year [2018] (Intercept) |
3.27 |
3.01 – 3.54 |
<0.001 |
| year [2019] |
-0.16 |
-0.40 – 0.08 |
0.196 |
| year [2020] |
-0.37 |
-0.61 – -0.13 |
0.003 |
| Random Effects |
| σ2
|
0.16 |
| τ00 Klon
|
0.22 |
| ICC |
0.57 |
| N Klon
|
22 |
| Observations |
66 |
| Marginal R2 / Conditional R2
|
0.058 / 0.596 |
Table 3.
Average temperature and humidity in the study seed orchard analysed with monthly data accuracy for 2018-2020.
Table 3.
Average temperature and humidity in the study seed orchard analysed with monthly data accuracy for 2018-2020.
| |
Jan. |
Feb. |
March |
Apr. |
May |
June |
July |
Aug. |
Spt. |
Oct. |
Nov. |
Dec. |
Mean |
| Temp. 2018 |
-1.2 |
-4.2 |
-0.6 |
12.4 |
18.1 |
19.2 |
21.2 |
20.7 |
16.0 |
9.6 |
3.4 |
0.1 |
9.6 |
| Temp. 2019 |
-3.4 |
1.9 |
4.7 |
9.9 |
13.5 |
22.3 |
19.0 |
20.1 |
14.3 |
10.6 |
5.3 |
2.4 |
10.1 |
| Temp. 2020 |
1.8 |
2.5 |
4.0 |
8.2 |
11.3 |
19.5 |
19.0 |
20.2 |
15.5 |
10.3 |
4.9 |
0.0 |
9.8 |
| Air humidity 2018 |
90.8 |
85.4 |
73.5 |
65.6 |
57.2 |
58.1 |
69.4 |
66.8 |
69.7 |
77.5 |
90.2 |
95.0 |
74.9 |
| Air humidity 2019 |
92.8 |
83.4 |
75.1 |
51.4 |
69.9 |
61.0 |
64.8 |
64.9 |
71.3 |
82.4 |
90.1 |
90.3 |
74.8 |
| Air humidity 2020 |
92.2 |
83.6 |
68.5 |
55.3 |
69.2 |
71.4 |
67.1 |
67.3 |
76.0 |
87.8 |
93.4 |
95.5 |
77.3 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).