1. Introduction
Urinary tract infections (UTIs) are a significant global health concern, affecting millions worldwide. These infections are caused by various bacterial species invading the urinary tract, potentially leading to functional or morphological disorders [
1]. Recurrent UTIs (rUTIs) profoundly impact the quality of life, influencing mental health, social interactions, work productivity, and sexual well-being [
2,
3]. This condition presents a substantial clinical challenge, particularly for women, who are disproportionately affected. The limitations of traditional management strategies, which heavily depend on antibiotics, have become increasingly evident. Growing concerns about antibiotic resistance and the adverse effects of long-term antibiotic use have intensified interest in alternative therapies [
4].
Uromune
®, a bacterial lysate vaccine, is a promising alternative in the fight against rUTIs. The vaccine is administered sublingually, stimulating the mucosal immune system, especially the gut-associated lymphoid tissue, which plays a crucial role in the body's immune defense [
5,
6,
7]. By exposing the immune system to these lysates, Uromune
® aims to promote the production of specific antibodies and bolster the overall immune response, thereby preventing the colonization and infection of the urinary tract.
Since its initial report in 2013 [
8], several studies have demonstrated the efficacy of Uromune
® in treating rUTIs, with good tolerance and minimal side effects. Importantly, these studies have shown the vaccine's effectiveness across diverse patient populations, including varying ages, sexes, and underlying conditions [
5,
6,
7,
9,
10,
11,
12]. Currently, this formulation is in the pre-licensing phase 3 of clinical development and is available under named patient programs in 26 countries [
7]. Developed by Immunotek S.L. (Alcalá de Henares, Spain) and commercialized by Q Pharma S.L. (Alicante, Spain), Uromune
® was approved by the Spanish Agency for Medicines and Health Products in October 2010 to prevent rUTIs. In March 2023, the European Association of Urology Guidelines on Urological Infections recognized Uromune
® as a beneficial immunoprophylactic agent [
13]. These guidelines are expected to facilitate the broader and more consistent use of this vaccine globally.
Uromune
® offers a hopeful outlook for managing rUTIs with minimal side effects despite current limitations in its clinical application. The vaccine was available in two formulations: (1) a generic formulation, termed MV140, consisting of a mixture of equal amounts of selected strains of
Escherichia coli,
Klebsiella pneumoniae,
Proteus vulgaris, and
Enterococcus faecalis, and (2) an autovaccine, composed of cultures derived directly from the patient's urine. Since January 2018, regulatory changes in Spain have mandated that Uromune
® be manufactured solely as an autovaccine [
5]. Despite its potential, data on the efficacy of this autovaccine remains limited.
This study exclusively investigated the efficacy of the Uromune® autovaccine. We did not study the MV140 formulation. Our aim was to expand global knowledge regarding the autovaccine's effectiveness and to explore the impact of comorbidities and other patient-specific factors on treatment outcomes. The findings of our study further support the incorporation of this autovaccine into routine clinical practice, emphasizing its beneficial effects.
2. Materials and Methods
We conducted a retrospective study of all patients treated with Uromune® for rUTIs in Hospital Universitari de Sant Joan de Reus between January 21, 2018, and August 31, 2022. This hospital, a member of the Hospital Network for Public Use in the Autonomous Community of Catalonia, Spain, has 367 inpatient beds and a 20-bed Intensive Care Unit. Serving a population of over 175,000, the hospital provides care to primary facilities and elderly residences in the region. Additionally, it serves as a referral center for Oncology and Radiotherapy for the entire Tarragona province, with a population of approximately 550,000.
UTI was defined as the presence of 100,000 or more colony-forming units of bacteria in the urinary system, accompanied by symptoms [
1]. Recurrent UTI was diagnosed in patients with three or more episodes of UTI within one year or two episodes within six months [
2]. At baseline, all patients underwent a comprehensive assessment, including anamnesis, abdominal and genital physical examinations, urine cultures, blood tests, and ultrasounds of the urinary tract with postvoid volume measurement. Additional diagnostic procedures such as CT scans, urethra-cystoscopy, urine cytology, or urodynamics were performed for patients showing signs of complicated UTI. Clinical, microbiological, and demographic data were meticulously gathered from patients' electronic medical records by the research staff, who individually reviewed each record to ensure thoroughness and accuracy. The McCabe score was calculated to assess clinical prognosis [
14], and the Charlson index was used to categorize patient comorbidities [
15].
In this study, only the Uromune® autovaccine was administered to patients. This autovaccine was personalized and tailored to each patient based on cultures derived from their own urine. The midstream portion of the patient's first-morning urine was collected in a sterile container under strict hygienic conditions. This method was chosen to ensure that the vaccine was specifically designed to target the bacteria present in the patient's urinary tract, thereby enhancing its effectiveness. Patients were given a sample transport kit, including a cotton swab, a plastic tube with a culture medium, and a padded envelope. The swab was used to inoculate the culture tube, which was then securely sealed and placed in the padded envelope for delivery to a pharmacy and subsequent shipment to Q Pharma/Immunotek Laboratories in Alicante, Spain. Based on the culture results, the laboratory prepared an individualized autovaccine, which was then returned to the pharmacy and subsequently delivered to the patient, ensuring safe and secure transportation.
The vaccine was administered as two sublingual puffs daily on an empty stomach for three months. To enhance absorption, patients were instructed to avoid swallowing saliva for one minute after application and to refrain from eating, drinking, or brushing their teeth for 30 minutes afterward. Treatment was interrupted only in the presence of fever, regardless of origin, to ensure patient safety and to prevent potential complications. Once the fever resolved, treatment was resumed. Each puff of approximately 100 µL contained 108 heat-inactivated whole bacteria. The formulation included 100% of the bacteria isolated from the patient's urine culture, and if two bacteria were present, the formulation contained 50% of each.
Unless otherwise specified, results are presented as numbers and percentages or as means and standard deviations. The χ² test was used to identify differences in categorical data, while the Student's t-test was applied to compare means between two groups. ANOVA was employed to examine the relationship between a continuous outcome and multiple predictor variables. Statistical significance was defined as p < 0.05. All analyses were conducted using SPSS 25.0 statistical software (SPSS Inc., Chicago, IL, USA), and graphs with GraphPrism version 9.
3. Results
3.1. Patient Demographics and Clinical Characteristics
We included 49 patients. The mean age at the start of therapy was 61 years, ranging from 14 to 92 years. Twenty-nine (59.2%) of these patients were women. Eleven patients (22.4%) were current smokers, and six (12.2%) regularly consumed alcoholic beverages. Thirty-two patients (65.3%) were treated at the Urology Department, while 17 (34.7%) were treated at the Internal Medicine Department. The most common comorbidities were cardiovascular diseases, including arterial hypertension (67.3%) and dyslipidemia (40.8%). Cystitis was the most prevalent type of urinary infection (59.3%), with most infections caused by Gram-negative bacteria (81.6%).
3.2. Influence of Uromune® Administration
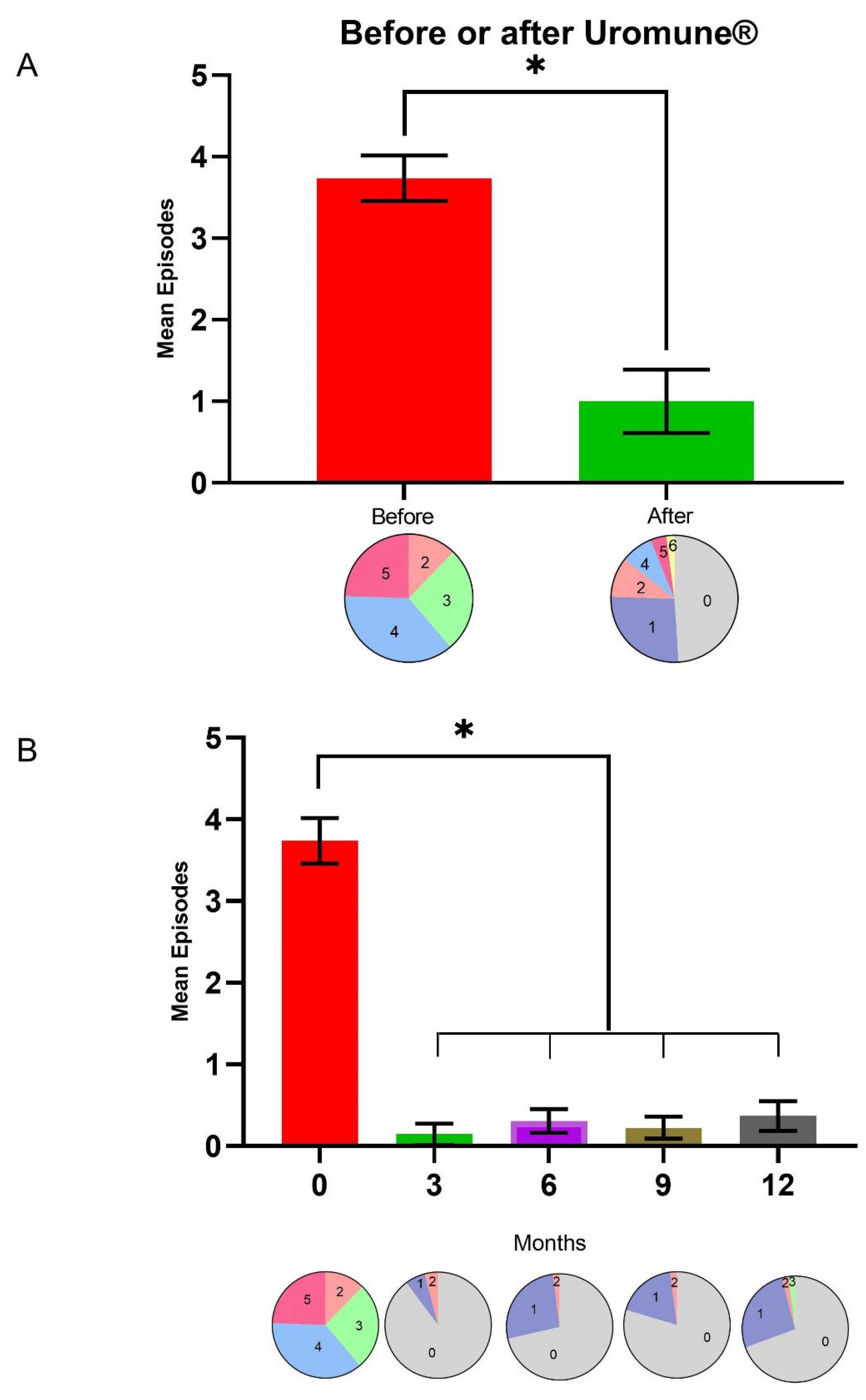
Uromune
® treatment led to a remarkable decrease in UTI episodes, from 3.73 ± 0.97 (mean ± SD) the year before to 0.98 ± 1.36 (
p < 0.001) the year after the autovaccine administration. Twenty-four patients did not report any episode of UTI during the first year post-Uromune
®. Similarly, the number of patients who suffered three or more episodes per year dropped from 43 (87.7%) before the intervention to 7 (14.3%) afterward (
Figure 1A). The maximum effectiveness of the autovaccine was observed three months post-administration, with 44 patients not experiencing any UTI episode, three patients experiencing one episode, and two patients having two episodes. While there was a slight increase in UTI during the remainder of the follow-up period, the incidence remained considerably lower than before treatment (
Figure 1B).
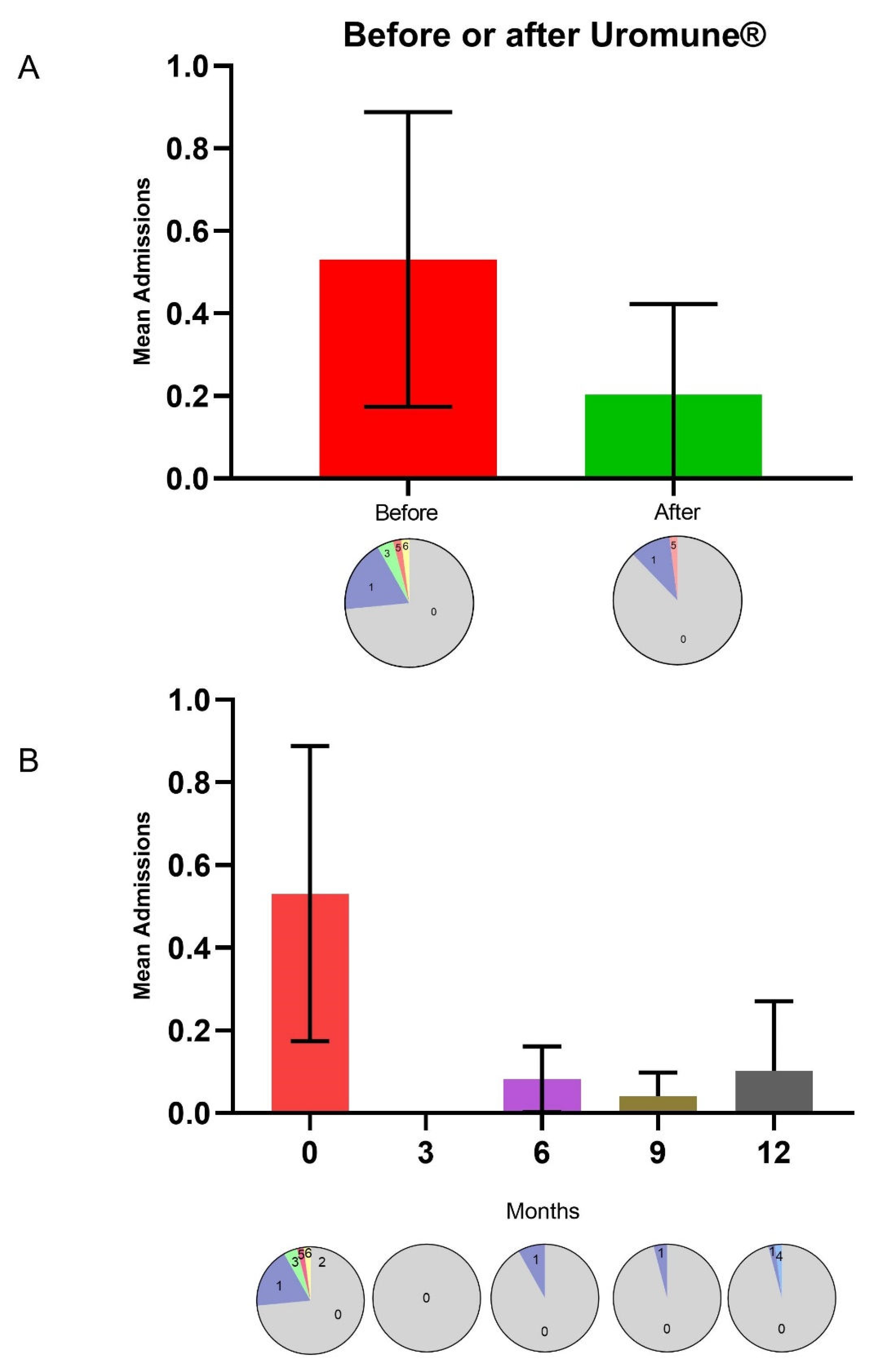
Similarly, the trend in hospital admissions followed that of UTI episodes, decreasing from 0.53 ± 1.24 before treatment to 0.20 ± 0.76 afterward, although differences did not reach statistical significance, probably due to the low number of hospitalized patients. Before treatment, 13 patients required hospitalization; one needed five hospitalizations, and one needed six. After treatment, only six patients required hospitalization, with just one needing five hospitalizations (
Figure 2A). The most substantial reduction in hospitalizations, similar to the decrease in UTI episodes, was observed three months after starting treatment, although the differences did not reach statistical significance (
Figure 2B).
3.3. Factors Influencing UTI recurrence after Uromune® Administration
We did not find any significant differences in demographic characteristics, department of care, associated diseases, or comorbidities between patients who experienced recurrent infections after autovaccine administration and those who did not (
Table 1). However, ANOVA including demographic variables, the most relevant urological complications and comorbidities (those with a frequency >10%), and the Charlson and McCabe indices, revealed that having undergone a urostomy, having chronic kidney disease or being immunosuppressed were significantly and independently associated with an increased number of recurrences following autovaccine administration (
Table 2).
3.4. Adverse Effects
We did not find any adverse effects associated with Uromune® autovaccine treatment.
4. Discussion
A systematic review reported the potential of Uromune
® in treating rUTIs, presenting it as a promising alternative to antibiotics [
5]. However, despite the encouraging findings, the application of this vaccine to clinical practice remains limited. The recent inclusion of Uromune
® in the European Association of Urology's Guidelines on Urological Infections [
13] has opened up a significant opportunity for widespread adoption. This development presents a hopeful prospect for the management of rUTIs. Most studies to date have focused on MV140 generic formulation, and little data have been reported on the efficacy of the autovaccine. This new endorsement has inspired us to share the experience of our institution with this therapy, further fueling our hope for the future of rUTIs treatment.
Our study evaluated 49 patients with a diverse age range, reflecting a broad spectrum of the population affected by rUTIs. The gender distribution was slightly skewed, with women constituting most of the cohort, aligning with the known higher prevalence of UTIs in females. Lifestyle factors included relevant percentages of current smokers and patients regularly consuming alcohol, both of which can impact immune function and potentially affect susceptibility to infections [
16]. The average age in our study is consistent with previous reports [
2,
5,
6,
12] and reflects the typical aged profile of patients with UTIs who need to be seen in a hospital consultation. Most patients received treatment in the Urology Department, with the remainder treated in Internal Medicine, indicating that these infections often necessitate specialized care. The high prevalence of urological disorders and urinary incontinence underscores the significant burden of these conditions in our population. Additionally, many patients were using urinary catheters, a known risk factor for UTI. The comorbidity profile revealed a high prevalence of cardiovascular diseases and dyslipidemia, which is expected in older individuals. These comorbid conditions might contribute to the complexity of managing UTI in these patients. The predominance of cystitis as the type of urinary infection and the high rate of Gram-negative bacterial infections align with common etiological patterns observed in patients with UTI [
17].
The administration of Uromune
® autovaccine was associated with a substantial reduction in UTI episodes. Twenty-four patients did not report any episodes in the first year post-administration, and the proportion of patients experiencing three or more episodes per year decreased dramatically. This marked improvement was most pronounced three months after initiating the treatment, where 44 patients remained episode-free, indicating the peak effectiveness of the autovaccine. The autovaccine reduced the frequency of UTIs, the severity of symptoms, and the need for antibiotic use. Although there was a slight uptick in the incidence of UTIs over the subsequent months, the overall rates stayed much lower than pre-treatment levels. This slight increase could be due to factors such as the natural progression of the disease or a certain development of resistance to the autovaccine. Our data are consistent with those previously reported with the generic preparation MV-140 [
2,
5,
6,
10,
12,
18,
19,
20]. A recent randomized placebo-controlled efficacy study demonstrated that treatment with MV140 was associated with reducing the burden of UTI, reducing symptoms and antibiotic use, and significantly improving patients' quality of life. There is very little data on the efficacy of autovaccines, but two reports suggested that, as in our case, they are also highly effective for treating rUTI. [
19,
21]. Hospital admissions also decreased notably in the year following the Uromune
® administration. Before treatment, 13 patients required hospitalization, with several experiencing multiple hospitalizations. Post-treatment, only six patients needed hospitalization, suggesting a substantial reduction in severe episodes necessitating hospital care. However, while the trend in reduced hospitalizations was clear, it did not achieve statistical significance, potentially due to the limited sample size or variability in patient responses.
Despite the general effectiveness of Uromune®, some patients continued to experience recurrent infections. Our analysis found no significant differences in demographic characteristics, department of care, associated diseases, comorbidities, or clinical indices (Charlson and McCabe) between those who did and did not experience recurrences. This finding suggests that these factors alone do not predict the likelihood of recurrence after autovaccine administration. However, multiple regression analysis identified that having a urostomy, a chronic kidney disease, or being immunosuppressed were independent predictors of UTI recurrence post-Uromune® treatment. This finding highlights the need for targeted strategies to manage UTIs in these high-risk groups. These targeted strategies could include frequent monitoring, personalized treatment plans, or additional preventive measures.
While providing valuable insights into the efficacy of the Uromune® autovaccine for rUTI, this study has several limitations that should be acknowledged: The retrospective nature of the study inherently limits the ability to establish causal relationships. Relying on historical data from electronic medical records may lead to incomplete or biased information. Since the study was conducted at a single hospital and the sample size was small, the patient population may not represent the broader population with rUTIs. Moreover, the absence of a control group or a comparison with patients receiving alternative treatments or placebo limits the ability to definitively attribute observed outcomes to the autovaccine.
5. Conclusions
This study suggests that the administration of Uromune® autovaccine can significantly reduce the frequency of rUTIs and associated hospitalizations, particularly within the first few months of treatment. While autovaccine is broadly effective, patients with urological or renal diseases or immunosuppression may require additional therapeutic strategies to prevent recurrences. The findings of this study underscore the urgent need for further prospective, randomized trials with larger cohorts and extended follow-up periods to confirm these findings and optimize treatment protocols for different patient subgroups. The urgency and importance of this research cannot be overstated, as it has the potential to improve the management of rUTIs significantly.
Author Contributions
Conceptualization, Simona Iftimie and Ana F. López-Azcona; Data curation, Simona Iftimie and Jordi Camps; Formal analysis, Jordi Camps; Funding acquisition, Simona Iftimie; Investigation, Simona Iftimie, Ana F. López-Azcona, Laia Pujol-Galarza, Antoni Pont-Salvadó and Mercè Pascual-Queralt; Methodology, Simona Iftimie and Paula Ladero-Palacio; Project administration, Jordi Camps; Resources, Jorge Joven, Antoni Castro and Mercè Pascual-Queralt; Software, Paula Ladero-Palacio and Xavier Gabaldó-Barrios; Supervision, Jorge Joven and Antoni Castro; Validation, Simona Iftimie; Visualization, Simona Iftimie and Jordi Camps; Writing – original draft, Jordi Camps; Writing – review & editing, Simona Iftimie, Jordi Camps and Mercè Pascual-Queralt.
Funding
The APC was funded by Q PHARMA S.L. (Alicante, Spain).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of HOSPITAL UNIVERSITARI DE SANT JOAN (protocol code 10-12-23/12proj December 12, 2010).
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269−284. [CrossRef]
- Carrión-López, P.; Martínez-Ruiz, J.; Librán-García, L.; Giménez-Bachs, J.M.; Pastor-Navarro, H.; Salinas-Sánchez, A.S. Analysis of the efficacy of a sublingual bacterial vaccine in the prophylaxis of recurrent urinary tract infection. Urol. Int. 2020, 104, 293−300. [CrossRef]
- Newlands, A.F.; Roberts, L.; Maxwell, K.; Kramer, M.; Price, J.L.; Finlay, K.A. Development and psychometric validation of a patient-reported outcome measure of recurrent urinary tract infection impact: the Recurrent UTI Impact Questionnaire. Qual. Life Res. 2023, 32, 1745−1758. [CrossRef]
- Geerlings, S.E.; Beerepoot, M.A.; Prins, J.M. Prevention of recurrent urinary tract infections in women: antimicrobial and nonantimicrobial strategies. Infect. Dis. Clin. North Am. 2014, 28, 135−147. [CrossRef]
- Ramírez Sevilla, C.; Gómez Lanza, E.; Puyol Pallàs, M. Immunoactive prophylaxis protocol of uncomplicated recurrent urinary tract infections in a cohort of 1104 women treated with Uromune® vaccine. Life (Basel) 2024, 14, 464. [CrossRef]
- Ramírez Sevilla, C.; Gómez Lanza, E.; Manzanera, J.L.; Martín, J.A.R.; Sanz, M.Á.B. Active immunoprophyilaxis with Uromune® decreases the recurrence of urinary tract infections at three and six months after treatment without relevant secondary effects. BMC Infect. Dis. 2019, 19, 901. [CrossRef]
- Nickel, J.C.; Saz-Leal, P.; Doiron, R.C. Could sublingual vaccination be a viable option for the prevention of recurrent urinary tract infection in Canada? A systematic review of the current literature and plans for the future. Can. Urol. Assoc. J. 2020, 14, 281−287. [CrossRef]
- Lorenzo-Gómez, M.F.; Padilla-Fernández, B.; García-Criado, F.J.; Mirón-Canelo, J.A.; Gil-Vicente, A.; Nieto-Huertos, A.; Silva-Abuin, J.M. Evaluation of a therapeutic vaccine for the prevention of recurrent urinary tract infections versus prophylactic treatment with antibiotics. Int. Urogynecol. J. 2013, 24, 127−134. [CrossRef]
- Lorenzo-Gómez, M.F.; Foley, S.; Nickel, J.C.; García-Cenador, M.B.; Padilla-Fernández, B.Y.; González-Casado, I.; Martínez-Huélamo, M.; Yang, B.; Blick, C.; Ferreira, F.; Caballero, R.; Saz-Leal, P.; Casanovas, M. Sublingual MV140 for prevention of recurrent urinary tract infections. NEJM Evid. 2022, 1, EVIDoa2100018. [CrossRef]
- Sánchez-Ramón, S.; Fernández-Paredes, L.; Saz-Leal, P.; Diez-Rivero, C.M.; Ochoa-Grullón, J.; Morado, C.; Macarrón, P.; Martínez, C.; Villaverde, V.; de la Peña, A.R.; Conejero, L.; Hernández-Llano, K.; Cordero, G.; Fernández-Arquero, M.; Gutierrez, B.F.; Candelas, G. Sublingual bacterial vaccination reduces recurrent infections in patients with autoimmune diseases under immunosuppressant treatment. Front. Immunol. 2021, 12, 675735. [CrossRef]
- Ciudin, A.; Padulles, B.; Popescu, R.; Manasia, P. Autovaccine-based immunotherapy: A promising approach for male recurrent urinary tract infections. Life (Basel) 2024, 14, 111. [CrossRef]
- Yang, B.; Foley, S. First experience in the UK of treating women with recurrent urinary tract infections with the bacterial vaccine Uromune®. BJU Int. 2018, 121, 289−292. [CrossRef]
- Bonkat, G.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S.E.; Köves, B.; Kranz, J.; Schubert, S.; Pilatz, A.; Veeratterapillay, R.; Wagenlehner, F. EAU Guidelines on urological infections. https://uroweb.org/guidelines/urological-infections/chapter/the-guideline (accessed 25 June 2024).
- McCabe, W.R.; Jackson, G.G. Gram-negative bacteremia. I. Etiology and ecology. Arch. Intern. Med. 1962, 110, 847–864. [CrossRef]
- Berkman, L.F.; Leo-Summers, L.; Horwitz, R.I. Emotional support and survival after myocardial infarction. A prospective, population-based study of the elderly. Ann. Intern. Med. 1992, 117, 1003−1009. [CrossRef]
- Roseman, C.; Truedsson, L.; Kapetanovic, M.C. The effect of smoking and alcohol consumption on markers of systemic inflammation, immunoglobulin levels and immune response following pneumococcal vaccination in patients with arthritis. Arthritis Res. Ther. 2012, 14, R170. [CrossRef]
- Jacobsen, S.M.; Stickler, D.J.; Mobley, H.L.; Shirtliff, M.E. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 2008, 21, 26−59. [CrossRef]
- Shabaka, A.; López de la Manzanara, V.; Zapata, N.; Santiago, J.L.; Pérez-Flores, I.; Moreno de la Higuera, M.A.; Sánchez-Ramón, S.; Sánchez-Fructuoso, A.I. Clinical and immunological response to sublingual vaccination for the prevention of recurrent urinary tract infections in kidney transplant patients results after 1 year of follow-up. Transplantation 2018, 10, S320. [CrossRef]
- Lorenzo-Gómez, M.F.; Padilla-Fernández, B.; Flores-Fraile, J.; Valverde-Martínez, S.; González-Casado, I.; Hernández, J.D.; Sánchez-Escudero, A.; Vicente Arroyo, M.J.; Martínez-Huélamo, M.; Criado, F.H.; Blanco-Tarrío, E.; Márquez-Sánchez, M.; Flores-Fraile, M.C.; Saz-Leal, P.; Mirón-Canelo, J.A.; García-Perdomo, H.A.; García-Cenador, M.B. Impact of whole-cell bacterial immunoprophylaxis in the management of recurrent urinary tract infections in the frail elderly. Vaccine 2021, 39, 6308−6314. [CrossRef]
- Curtis Nickel, J.; Foley, S.; Yang, B.; Casanovas, M.; Caballero, R.; Diez-Rivero, C.M.; Lorenzo-Gómez, M.F. Reducing recurrent urinary tract infections in women with MV140 impacts personal burden of disease: Secondary analyses of a randomized placebo-controlled efficacy Study. Eur. Urol. Open Sci. 2024, 63, 96−103. [CrossRef]
- Esteve-Palau, E.; Martinez Franco, E.M.; Gonzalez-Cuevas, A.; Capella, A.; Moreno, E.; Alvarez, M.C.; Garro, M.C.; Carreras, M.; Cespedes, M.; Cuadras, D.; Diaz-Brito Fernandez, V. Individualised autovaccination is a promising strategy for managing recurrent urinary tract infections in women. Proceedings of the 30 European Society of Clinical Microbiology and Infectious Diseases Congress, Paris, France, 18 April 2020, abstract 954, p. 506.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).