1. Introduction
Bening paroxysmal positional vertigo (BPPV) is one of the most common causes of dizziness. Peripheral vestibular disorders are a diverse group of diseases characterized by vestibular symptoms that may be acute, episodic or permanent [
1,
2]. The most common peripheral vestibular disorders are BPPV and Meniere's Disease, and the others are vestibular neuritis, bilateral vestibulopathy, vestibular paroxysm and superior canal dehiscence syndrome [
2,
3].
Due to the complexity of the etiopathogenesis and clinical presentation of peripheral vestibular diseases, their diagnosis and treatment can be challenging for the clinician, and prognosis can sometimes be difficult to predict [
4]. In general, these diseases are diagnosed by history, vestibular examination and tests, but there is no systemic biomarker specifically related to the inner ear [
5]. Otolin-1 is a glycoprotein whose mRNA expression is limited to the inner ear. It is specifically produced by marginal cells in the vestibular macula, semicircular canal crystal, organ of Corti and stria vascularis. Its main function is to interact with other inner ear-specific proteins such as otolith-associated protein otoconin-90 (OC90) and to form otoconia [
6,
7,
8]. Therefore, otolin-1 protein is a serologic biomarker recently defined in the literature as specific for the inner ear [
6]. Otolin-1 is specific for the inner ear, can be easily detected in serum as it crosses the blood-brain barrier and its level increases in correlation with pathology [
9]. In this sense, it may be used as an ideal biomarker in inner ear pathologies and specifically in peripheral vestibular diseases.
During otolith development, the organic matrix is formed before calcium carbonate deposition. Collagen OC90 is required for otolith formation [
10]. OC90 is the main matrix protein of the mammalian calcifying otolith: It retains the ability to bind calcium and is highly acidic so that calcium or CaCO3 can bind. In the absence of OC90, matrix-bound calcium on the surface of the vestibular macula is greatly reduced [
11].
However, to our knowledge, no study investigating the association of serum OC90 and otolin 1 levels with BPPV has been published yet. The aim of this study was to evaluate the systemic specific biomarkers that may help clinicians in BPPV, that may be included in the diagnostic, treatment and follow-up criteria, that may help explain the etiopathogenesis of peripheral vestibular disorders, that may help differentiate them from central vestibulopathies, and that may also be used for different inner ear pathologies in the light of the literature.
2. Material and Method
2.1. Ethical Approval
Permission was obtained from the Clinical Research Ethics Committee of Istanbul University Cerrahpasa, Cerrahpasa Medical Faculty (Date: 22 April 2024, Code: E-74555795-050.04-971548) before the study was started. This study was conducted according to the Declaration of Helsinki. All subjects gave their informed consent for inclusion before they participated in the study. All subjects were of Turkish descent.
2.2. Research Design
This is a cross-sectional study conducted in the outpatient clinics of the neurology and otorhinolaryngology Departments of Medicine Hospital, Medical Faculty, Istanbul Atlas University. A total of 80 participants aged 21–62 years were recruited for this study. Our study involved 50 consecutive BPPV patients (26 females and 24 males) at the Department of neurology and otorhinolaryngology, and 30 age and sex- matched controls (17 females and 13 males).
All participants underwent audiometric examination after routine neurotologic examination.
2.3. Inclusion Criteria
Patients diagnosed with BPPV were included in this study. The diagnosis of BPPV was confirmed by considering the history of dizziness and the character of nystagmus during Dix-Hallpike maneuver. Epley maneuver was performed in all patients after the affected side was determined by considering the channel indicated by the nystagmus. The patients were evaluated two or three days later and the maneuvers were repeated. The Dix-Hallpike maneuver is the gold standard for diagnosing BPPV of verticalsemicircular canals. The maneuvers were continued until no nystagmus was observed in the Dix-Hallpike test [
12].
In the study, 30 healthy individuals with no known chronic diseases, not taking any medication and aged between 18 and 62 years were recruited as the control group. The control group consisted of patients who presented to our clinic with complaints other than dizziness, imbalance or dizziness, who had serum vitamin D level measurements and who had not consulted a physician for vertigo, dizziness or imbalance in the last 1 year before presentation.
2.4. Exlusion Criteria
Subjects with pathology on neurootologic examination, unexpected hearing loss on audiometric examination, history of inner ear disease disease (Meniere's disease, autoimmune inner ear disease, vestibular neuritis, acoustic neurinoma, sudden hearing loss, temporal bone fracture, drug-induced ototoxicity, etc.), history of head trauma, history of peripheral vestibular disease, history of otologic surgery, chronic systemic disease (such as hypertension, hyperlipidemia, hypothyroidism, hyperparathyroidism, diabetes mellitus or cardiac diseases) pregnancy, vitamin D replacement therapy for osteoporosis or osteopenia were excluded.
2.5. Sample collection and measurements
Blood samples were obtained from patients by venipuncture after an overnight fast (≥8 h). Serum samples were obtained after at least 30 min of clotting by centrifugation at 2500× g for 15 min and stored at −80 °C until OC90 and otolin-1 are studied. Routine biochemical parameters and 25-hydroxyvitamin D3 (25(OH)D3, Vit D3) were measured on the day of blood collection.
Complete blood count (CBC) was recorded with an automatic hematology analyzer (Sysmeks XN-1000, Norderstedt, Germany). The biochemical parameters were measured using the spectrophotometric method with an automated biochemistry analyzer (Architect i2000, Abbott Park, IL, USA). The serum CRP levels were measured using the nephelometric method (Immage 800 Beckman Coulter, CA 92821, USA). CRP analysis was performed at the time of admission. The vitamin D levels were assessed using an immunoassay based on electrochemiluminescence (ECL) technology.
2.6. Measurement of serum otoconin 90 (OC90) levels
Serum OC90 levels were measured according to the manufacturer’s instructions (Human Otoconin 90 ELISA Kit, Cat.No.: E2535Hu, BT LAB, Zhejiang, China). The coefficients of intra- and interassay variation were <8% (n = 20) and <10% (n = 20), respectively.
2.7. Measurement of serum otolin-1 levels
Serum otolin-1 levels were measured according to the manufacturer’s instructions (Human Otolin 1 ELISA Kit, Cat.No.: E3947Hu, BT LAB, Zhejiang, China). The coefficients of intra- and interassay variation were <8% (n = 20) and <10% (n = 20), respectively.
2.8. Statistical Analysis
The Statistical Package for the Social Sciences version 21.0 software package for Windows (IBM Corp., Armonk, NY, USA) and Jamovi 2.3.18 were used for data evaluation and analysis. Categorical variables are presented as frequencies (n) and percentages (%), and numerical variables are presented as mean ± standard deviation or medians (25th percentile- 75th percentile). Whether the data were normally distributed was analyzed through visual (histograms and Q‒Q plots) and descriptive (coefficient of variation, skewness, and kurtosis) techniques and analytical methods (Kolmogorov–Smirnov test). The independent samples t test or Mann‒Whitney U test was used to compare continuous variables between two independent groups. Spearman correlation analyze was used to evaluate the relationships between the numerical variables. ROC analysis was applied to evaluate the cutoff values, sensitivity and specificity of the parameters. A value of p < 0.05 was considered to indicate statistical significance.
3. Results
In the present study, 54.17% of the cases and 65.38% of the controls were female; the gender distributions of the case and control groups were similar (p=0.350). The mean age of the case group was 42.46±11.4 years, while the mean age of the control group was 38.69±11.2 years, and there was no significant difference between them (p=0.176). All the patients had dizziness, while 29.17% of the patients had tinnitus (35.71%-right; 64.29%-left). Symptoms were present within the last week in 29.17%, 1-3 weeks in 54.17%, and more than 4 weeks in 16.67% of the patients. Etiologically, 50% of the cases were idiopathic, 40% were after upper respiratory tract infection (URTI), 4% (n:2) were traumatic, and 4% (n:2) had a pressure difference. None of the patients had hearing loss on audiological examination. Nystagmus was present in 39.58% of the patients (
Table 1).
No statistically significant difference was observed between the case and control groups in terms of calcium, parathormone (PTH), IG%, WBC, RBC, HGB, HCT, MCV, MCH, MCHC, PDW, PCT, RDW-SD, lymphocytes, lymphocytes%, monocytes, monocytes%, monocytes, eosinophils, eosinophils%, or the SII.
The vitamin D level was significantly lower in the case group than in the control group (14.3 (8.45-17.5)) vs 26.25 (21-32); p<0.001). CRP levels were significantly greater in the case group than in the control group (3.3 (2-5.35) vs 1.18 (0.68-1.7); p<0.001). The NLR was significantly greater in the case group than in the control group (1.66±0.52 vs 1.29±0.37; p<0.001). In addition, the neutrophil and neutrophil percentage were greater in the case group than in the control group. The PLR was significantly lower in the case group than in the control group (78.04 (62-94.74) vs 98 (85.6-117.96); p=0.003). In addition, the platelet count was lower in the case group than in the control group (
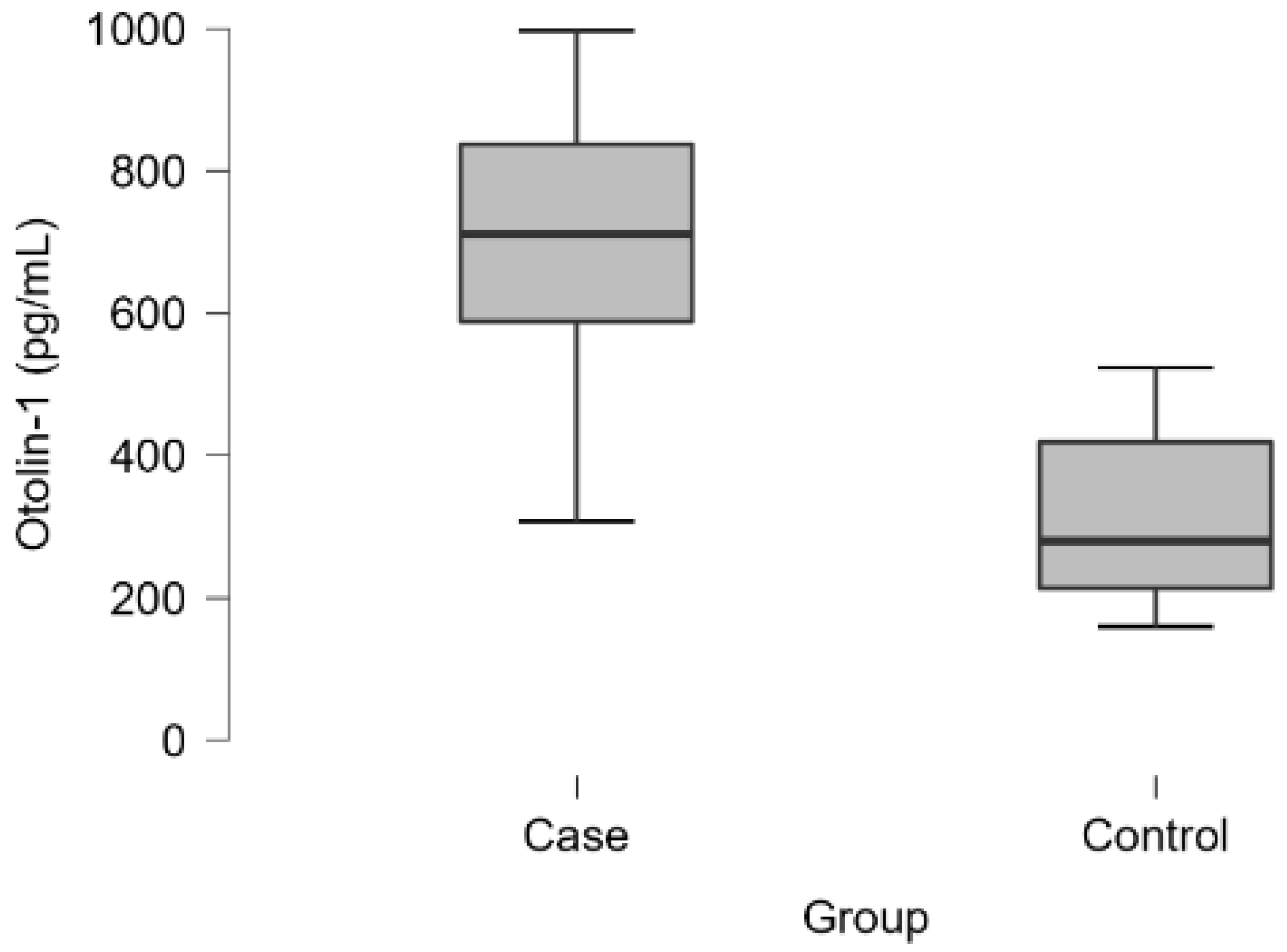
Table 2). Otolin-1 levels were significantly greater in the case group than in the control group (710.44 (584.35-837.39) vs 280.45 (212.7-419.61); p<0.001) (
Table 2-3,
Figure 1). No statistical significance was found although OC90 levels were greater in the case group than in the control group (
Table 2).
In all groups, otolin-1 was weakly positively correlated with OC90 (r=0.393; p=0.001) and strongly negatively correlated with vitamin D (r=0.682; p<0.001). OC90 was moderately negatively correlated with vitamin D (r=0.404; p<0.001). Similarly, there was a significant correlation between all three parameters in the case group. There was a strong positive correlation between otolin-1 and OC90 (r=0.693; p<0.001), a moderate negative correlation between otolin-1 and vitamin D (r=0.686; p<0.001), and a strong negative correlation between OC90 and vitamin D (r=0.672; p<0.001). In contrast, there were no correlations between the 3 parameters in the control group (p>0.05).
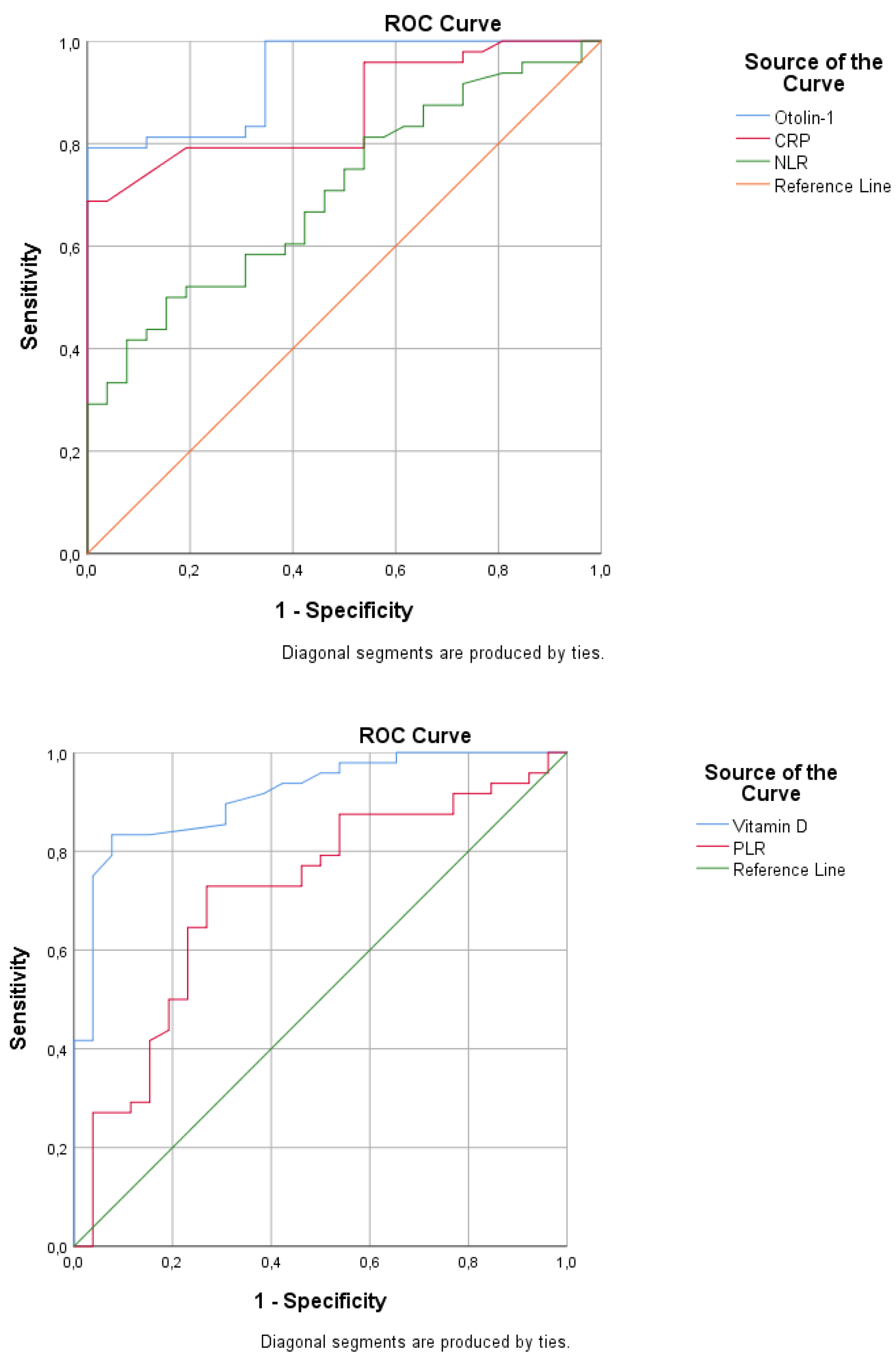
Table 4 and
Figure 2 present the ROC analysis results for BPPV. The AUC was 0.933 (95% CI: 0.881-0.986), with 79.2% sensitivity and 100% specificity, with a cutoff value greater than 525 for otolin-1. The AUCs were 0.867 (95% CI: 0.787-0.947) for CRP, 0.705 (0.587-0.823) for NLR, and 0.713 (0.588-0.838) for PLR. The AUC was 0.913 (0.848-0.979), with 83.3% sensitivity and 88.5% specificity, with a cutoff of less than 20 for vitamin D.
4. Discussion
In present study, serum OC90, otolin-1 and vitamin D levels of BPPV patients and healthy subjects without vestibular complaints were compared. While serum otolin-1 levels were found to be high, vitamin D levels were found to be low in BPPV patients. No statistical significance was found although OC90, the main soluble matrix protein of otoconia were higher in BPPV. There was a strong positive correlation between otolin-1 and OC90, a moderate negative correlation between otolin-1 and vitamin D, and a strong negative correlation between OC90 and vitamin D in BPPV patient group. Vitamin D has high specificity and sensitivity while otolin-1 has high specificity and low sensitivity.
Although there is no conclusive evidence about the etiology, advanced age, head trauma, various diseases affecting the inner ear, female gender, hormonal factors, a viral or ischemic cause, hyperlipidemia, osteoporosis, vitamin D deficiency and familial predisposition are blamed [
13]. In present study, there was no difference between the groups in terms of age and gender. 52% of the cases were idiopathic, 40% after upper respiratory tract infection (URTI), 2% traumatic and 2% had pressure differential. The presence of a previous infection is evidence in favor of a viral etiology. It has been reported that more than 50% of patients with BBPV have idiopathic form [
14]. Our results are consistent with the literatüre.
Otoliths are crystalline structures composed of calcium carbonate and glycoproteins. The mechanism of BPPV is explained by otoliths as OC90 and otolin-1 that break away from the otolithic membrane and escape into the semi-circular canals [
15]. In present study, serum OC90 levels were greater in BPPV than in the control group. However, the difference in the serum OC90 values between the two groups was not found to be statistically significant. Bi et al. [
16] found that otoconin-90 could be detected in peripheral blood and the levels in patients with BPPV were significantly higher than those in healthy controls. Otoconin-90 blood levels showed a high positive correlation with age, reflecting the process of otoconia degradation with age. Zhao et al [
10] demonstrated that OC90-knockout mice do not effectively recruit Ca2+ to the macula from the bloodstream during development and have rod-like large calcitic aggregates that are susceptible to dissolution. In contrast to our study, Zhank et al. [
17] reported that patients with BPPV compared with in controls had notably lower plasma expressions levels of OC90. The reason for the lack of significant difference between the OC90 levels of the patient and control groups in our results is either inadequate study population or insufficient passage of the protein from the tissue to the systemic circulation. For example, otolith protein otolin-1 is specifically expressed in the inner ear, but can be detected in serum when it moves into systemic circulation via the blood-labyrinth barrier [
6,
18]. Further investigation is required to determine how the expression of inner ear-associated proteins such as OC90 changes in the systemic circulation.
Levels of otolin-1 in systemic circulation increase with age, while BPPV increases in prevalence with age [
19,
20,
21,
22,
23]. In present study, otolin-1 levels were significantly greater in the BPPV than in the control group. Parham et al. [
18] reported that even though the mean serum otolin-1 level was significantly higher in BPPV patients, absolute levels of serum otolin-1 were only higher than the control group in one-third of the postmenopausal BPPV patients. The authors explained this disparity possibly as a result of enrollment of the subjects up to two years from the BPPV episode. Wu et al. [
24] performed that serum levels of the otolin-1 protein were significantly higher in patients with BPPV than in healthy controls and may serve as a potential biomarker for BPPV episodes and be used to promote better management of BPPV clinically. Both parathyroid hormone (PTH) and total calcium levels affect otolin-1 levels, implying that the calcium dysregulation caused by primary hyperparathyroidism may contribute to the otoconia breakdown and may be associated with inner ear disorders such as BPPV [
25]. In a recent study the same way, the serum levels of otolin-1 in patients with BPPV are significantly higher compared with individuals without benign paroxysmal positional vertigo [
29]. Elevated serum levels of otolin-1 were also associated with an increased risk of recurrent BPPV [
9]. The meta-analysis indicated that there is a higher serum level of otolin-1 in patients with BPPV than in healthy controls. Therefore, otolin-1 may serve as a biomarker for the onset of BPPV [
27]. Contrary to the above findings, the serum levels of otolin-1 are not significantly different between the patients with Meniere disease (MD) in the interictal phase and the control group’s healthy ones [
28]. Our and these promising results suggested that serum otolin-1 levels may serve as a biomarker for BPPV episodes, not MD.
Calcium channel proteins involved in calcium metabolism in vestibular end organs have been shown to be vitamin D dependent. Studies on this subject have shown that rats lacking vitamin D receptors in vestibular cells develop vestibular dysfunction [
29,
30]. In present study, the vitamin D level was significantly lower in the BPPV patients than in the control group. There was a strong positive correlation between otolin-1 and OC90, a moderate negative correlation between otolin-1 and vitamin D, and a strong negative correlation between OC90 and vitamin D in the BPPV patients. Jeong et al. [
31] found that serum 25-OH vit D levels were lower in 100 patients with BPPV compared to the control group (n=192) and that vitamin D deficiency and osteoporosis were risk factors that could significantly affect BPPV recurrence in these patients. Büki et al. [
32] reported that serum vitamin D levels were lower in patients with BPPV recurrence compared to patients without recurrence and no recurrence was reported when vitamin D replacement was performed in these patients. Talaat et al. [
33] reported that vitamin D replacement therapy had positive effects on BPPV recurrence. Similarly, it has been reported that in patients with recurrent BPPV who are under rehabilitation therapy, raising serum 25-OHD to normal value reduces recurrent rate of BPPV significantly [
34]. In a study involving 232 patients diagnosed with BPPV, vitamin D deficiency was found to be a risk factor for the development of BPPV independent of age, gender and type of BPPV [
35]. On the other hand, Karatas et al. [
36] reported that there was no association between BPPV and vitamin D deficiency and that such a situation could only be a coincidence. Vitamin D deficiency are also not risk factors for BPPV [
36]. The results of studies on vitamin D in patients with BPPV are controversial [
35,
36,
37,
38,
39,
40,
41]. Therefore, more large-scale prospective studies are needed.
The present study reveals that the serum levels of OC90 are not significantly different between the patients with BPPV patients and the healthy controls. High serum levels of otolin-1 were associated with an increased risk of BPPV. Serum levels of otolin-1 can potentially be used as a biomarker for acute onset of inner ear disorders due to its significant increase in patients with BPPV. Vitamin D level was significantly lower in the BPPV patients and has high specificity and sensitivity in the patients with BPPV patients. It also provides evidence that BPPV patients with vitamin D deficiency may improve their complaints with replacement therapy. There was a significant correlation between all three parameters in the BPPV patients. The otoconia organic matrix is composed of a variety of proteins, the chief constituents of which are otoconin-90 and otolin-1, but additional work is needed to establish their value and clarify the exact mechanisms. Evaluation of serum biomarkers of CO90 and otolin-1, including vitamin D, in a larger cohort of patients with a clinical diagnosis of BPPV may help to further elucidate the role of otolithic biomechanical disruption. The evaluation of serum biomarkers, an evolving area of research with the potential to reveal the pathophysiological mechanisms underlying various inner ear pathologies, will be included in our future projects.
Author Contributions
DA: SD, MNE, MSA, and HU conceptualized and designed this study. DA performed data acquisition. SD performed the statistical analyses. DA, SD, MNE, MSA, and HU drafted the manuscript. All authors finalized the manuscript. All authors supervised the entire process. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was received for conducting this study.
Institutional Review Board Statement
Permission was obtained from the Clinical Research Ethics Committee of Istanbul University Cerrahpasa, Cerrahpasa Medical Faculty (Date: 22 April 2024, Code: E-74555795-050.04-971548) before the study was started.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data underlying this article are available in the article. If needed, please contact the corresponding author. The email address is huzun59@hotmail.com.
Conflicts of Interest
The authors declare no competing interests.
References
- Strupp, M., Mandalà, M., López-Escámez, J. A. Peripheral vestibular disorders. Current Opinion in Neurology 2019, 32, 165–173. [CrossRef]
- Yang TH, Xirasagar S, Cheng YF, Chen CS, Lin HC. Increased prevalence of peripheral vestibular disorder among patients with Fabry disease. Orphanet J Rare Dis. 2024;19:97. [CrossRef]
- Welgampola MS, Akdal G, Halmagyi GM. Neuro-otology- some recent clinical advances [published correction appears in J Neurol. 2017 Jan;264(1):204. doi: 10.1007/s00415-016-8320-z]. J Neurol. 2017;264:188-203. [CrossRef]
- Maudoux A, Vitry S, El-Amraoui A. Vestibular Deficits in Deafness: Clinical Presentation, Animal Modeling, and Treatment Solutions. Front Neurol. 2022;13:816534. [CrossRef]
- Gomaa NA, Jimoh Z, Campbell S, Zenke JK, Szczepek AJ. Biomarkers for Inner Ear Disorders: Scoping Review on the Role of Biomarkers in Hearing and Balance Disorders. Diagnostics (Basel). 2020;11:42. [CrossRef]
- Deans MR, Peterson JM, Wong GW. Mammalian Otolin: a multimeric glycoprotein specific to the inner ear that interacts with otoconial matrix protein Otoconin-90 and Cerebellin-1. PLoS One. 2010;5:e12765. [CrossRef]
- Yang H, Zhao X, Xu Y, Wang L, He Q, Lundberg YW. Matrix recruitment and calcium sequestration for spatial specific otoconia development. PLoS One. 2011;6(5):e20498. [CrossRef]
- Andrade LR, Lins U, Farina M, Kachar B, Thalmann R. Immunogold TEM of otoconin 90 and otolin - relevance to mineralization of otoconia, and pathogenesis of benign positional vertigo. Hear Res. 2012;292(1-2):14-25. [CrossRef]
- Fan Z, Hu Z, Han W, et al. High Serum Levels of Otolin-1 in Patients With Benign Paroxysmal Positional Vertigo Predict Recurrence. Front Neurol. 2022;13:841677. [CrossRef]
- Zhao X, Yang H, Yamoah EN, Lundberg YW. Gene targeting reveals the role of Oc90 as the essential organizer of the otoconial organic matrix. Dev Biol. 2007;304(2):508-524. [CrossRef]
- Huang S, Qian S. Advances in otolith-related protein research. Front Neurosci. 2022; 16:956200. [CrossRef]
- Epley JM. Human experience with canalith repositioning maneuvers. Ann N Y Acad Sci 2001; 942: 179–191. [CrossRef]
- Nuti D, Zee DS, Mandalà M. Benign Paroxysmal Positional Vertigo: What We Do and Do Not Know. Semin Neurol. 2020;40(1):49-58. [CrossRef]
- Guerra J, Devesa J. Causes and treatment of idiopathic benign paroxysmal positional vertigo based on endocrinological and other metabolic factors. J Otol. 2020;15(4):155-160. [CrossRef]
- Lundberg YW, Xu Y, Thiessen KD, Kramer KL. Mechanisms of otoconia and otolith development. Dev Dyn. 2015;244(3):239-253. [CrossRef]
- Bi J, Liu B, Zhang Y, Zhou Q. Study on the Bone Metabolism Indices and Otoconin-90 in Benign Paroxysmal Positional Vertigo. Otol Neurotol. 2021;42(6):e744-e749. [CrossRef]
- Zhang S, Xing J, Gong Y, Li P, Wang B, Xu L. Downregulation of VDR in benign paroxysmal positional vertigo patients inhibits otolith-associated protein expression levels. Mol Med Rep. 2021;24(2):591. [CrossRef]
- Parham K, Sacks D, Bixby C, Fall P. Inner ear protein as a biomarker in circulation?. Otolaryngol Head Neck Surg. 2014;151(6):1038-1040. [CrossRef]
- Tabtabai R, Haynes L, Kuchel GA, Parham K. Age-Related Increase in Blood Levels of Otolin-1 in Humans. Otol Neurotol. 2017;38(6):865-869. [CrossRef]
- Feng MY, Zhuang JH, Gu HH, Tian Q, Zhang ZH. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019;33(12):1138-1147.
- Sacks D, Parham K. Preliminary Report on the Investigation of the Association Between BPPV and Osteoporosis Using Biomarkers. Otol Neurotol. 2015;36(9):1532-1536. [CrossRef]
- Parham K, Kuchel GA. A Geriatric Perspective on Benign Paroxysmal Positional Vertigo. J Am Geriatr Soc. 2016;64(2):378-385. [CrossRef]
- Qian S, Zhang X, Wang Y. Serum Estradiol Correlates With Benign Paroxysmal Positional Vertigo in Postmenopausal Women. Endocr Pract. 2022;28(7):673-677. [CrossRef]
- Wu Y, Han W, Yan W, Lu X, Zhou M, Li L, Guan Q, Fan Z. Increased Otolin-1 in Serum as a Potential Biomarker for Idiopathic Benign Paroxysmal Positional Vertigo Episodes. Front Neurol. 2020;11:367. [CrossRef]
- McKenna K, Rahman K, Parham K. Otoconia degeneration as a consequence of primary hyperparathyroidism. Med Hypotheses. 2020;144:109982. [CrossRef]
- Irugu DVK, Singh A, Yadav H, et al. Serum otolin-1 as a biomarker for benign paroxysmal positional vertigo: a case-control study. J Laryngol Otol. 2021;135(7):589-592. [CrossRef]
- Liu X, Han K, Zhou M, Wu Y. Association between otolin-1 and benign paroxysmal positional vertigo: A meta-analysis. Front Neurol. 2022;13:950023. [CrossRef]
- Singh A, Yadav H, Verma H, Sikka K, Abraham RA, Irugu DVK. Normal Serum Levels of Otolin-1 in Patients with Meniere Disease in Remission. Int Arch Otorhinolaryngol. 2023;27(3):e440-e444. [CrossRef]
- Hoenderop JG, Nilius B, Bindels RJ. Calcium absorption across epithelia. Physiological reviews 2005;85:373-422. [CrossRef]
- Yamauchi D, Raveendran NN, Pondugula SR, Kampalli SB, Sanneman JD, Harbidge DG, Marcus DC. Vitamin D upregulates expression of ECaC1 mRNA in semicircular canal. Biochem Biophys Res Commun 2005; 331:1353-1357. [CrossRef]
- Jeong SH, Kim JS, Shin JW, et al. Decreased serum vitamin D in idiopathic benign paroxysmal positional vertigo. J Neurol. 2013;260(3):832-838. [CrossRef]
- Büki B, Ecker M, Jünger H, Lundberg YW. Vitamin D deficiency and benign paroxysmal positioning vertigo. Med Hypotheses. 2013;80(2):201-204. [CrossRef]
- Talaat HS, Abuhadied G, Talaat AS, Abdelaal MS. Low bone mineral density and vitamin D deficiency in patients with benign positional paroxysmal vertigo. Eur Arch Otorhinolaryngol. 2015;272(9):2249-2253. [CrossRef]
- Sheikhzadeh M, Lotfi Y, Mousavi A, Heidari B, Bakhshi E. The effect of serum vitamin D normalization in preventing recurrences of benign paroxysmal positional vertigo: A case-control study. Caspian J Intern Med. 2016;7(3):173-177.
- Rhim GI. Serum vitamin D and recurrent benign paroxysmal positional vertigo. Laryngoscope Investig Otolaryngol. 2016;1(6):150-153. [CrossRef]
- Karataş A, Acar Yüceant G, Yüce T, Hacı C, Cebi IT, Salviz M. Association of Benign Paroxysmal Positional Vertigo with Osteoporosis and Vitamin D Deficiency: A Case Controlled Study. J Int Adv Otol. 2017;13(2):259-265. [CrossRef]
- Ren YY, Wang YJ, Li JL, Liu M, Xia F. Low vitamin D and uric acid status in patients with benign paroxysmal positional vertigo. Sci Prog. 2023;106(4):368504231205397. [CrossRef]
- Rhim G, Kim MJ. Vitamin D Supplementation and Recurrence of Benign Paroxysmal Positional Vertigo. Nutrients. 2024;16(5):689. [CrossRef]
- Jeong SH, Kim JS, Kim HJ, et al. Prevention of benign paroxysmal positional vertigo with vitamin D supplementation: A randomized trial. Neurology. 2020;95(9):e1117-e1125. [CrossRef]
- Abdelmaksoud AA, Fahim DFM, Bazeed SES, Alemam MF, Aref ZF. Relation between vitamin D deficiency and benign paroxysmal positional vertigo. Sci Rep. 2021;11(1):16855. [CrossRef]
- Sharma K, Ojha T, Dabaria R, Chhabra B, Trivedi BB, Bansal M. Relation Between Posterior Canal Benign Paroxysmal Positional Vertigo and Vitamin D Deficiency. Indian J Otolaryngol Head Neck Surg. 2022;74(Suppl 3):4405-4408. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).