Submitted:
19 September 2024
Posted:
20 September 2024
You are already at the latest version
Abstract
Keywords:
Introduction
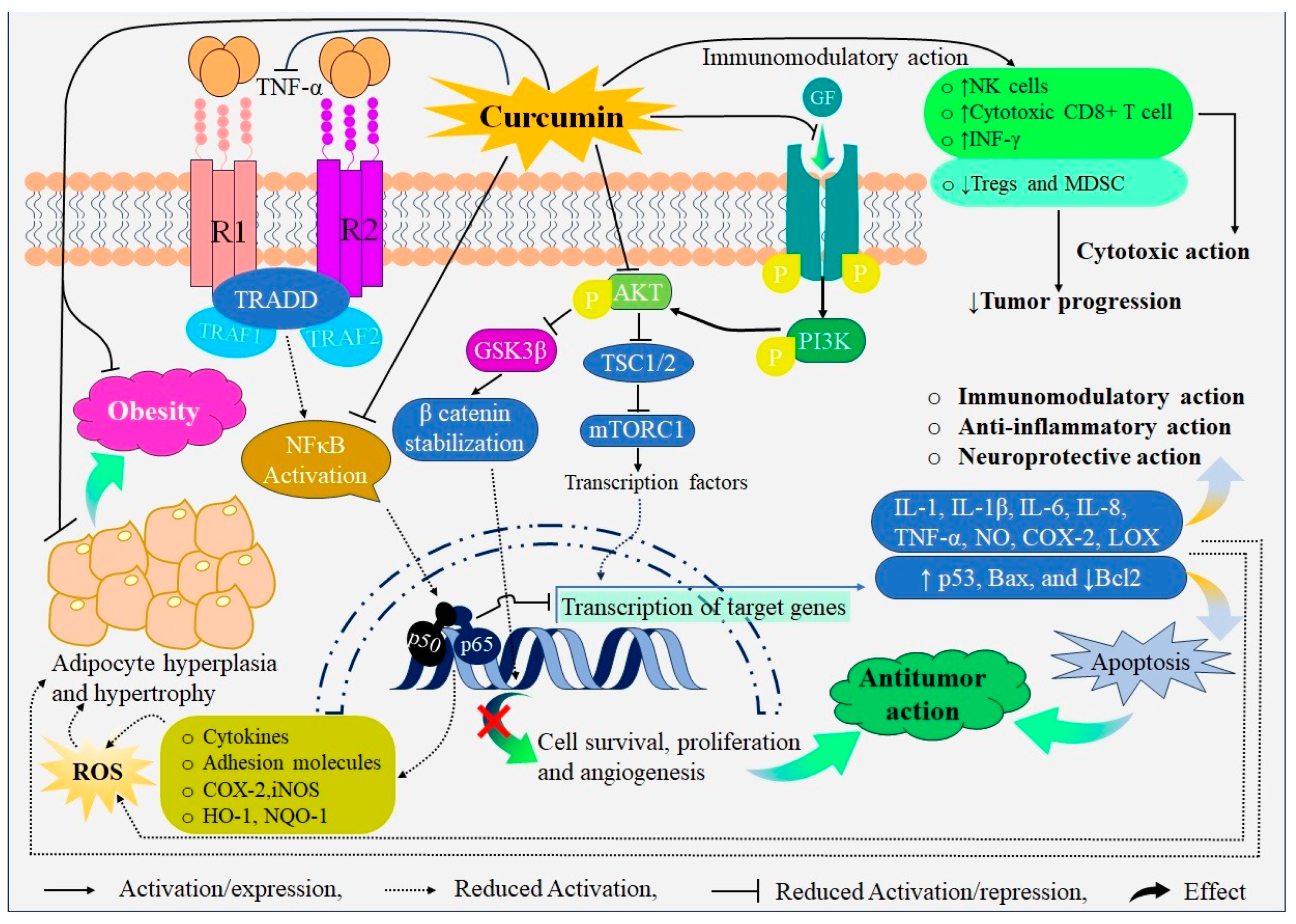
Probable Mechanisms of Action of Curcumin
Preclinical and Clinical Evidences for Therapeutic Applications of Curcumin
Therapy for Cancer
Colorectal Cancer
Inflammatory Bowel Disease
Osteoarthritis
Atherosclerosis
Peptic Ulcer
COVID-19
Psoriasis
Vitiligo
Depression
Safety Profile of Curcumin
Summary of Key Findings in Studies of Curcumin as a Therapeutic Agent
Conclusions
Funding Sources
Conflict of Interest Statement
References
- El-Saadony, M.T.; Yang, T.; Korma, S.A.; Sitohy, M.; Abd El-Mageed, T.A.; Selim, S.; Al Jaouni, S.K.; Salem, H.M.; Mahmmod, Y.; Soliman, S.M.; et al. Impacts of Turmeric and Its Principal Bioactive Curcumin on Human Health: Pharmaceutical, Medicinal, and Food Applications: A Comprehensive Review. Front. Nutr. 2022, 9, 1040259. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R. A Comprehensive Review on the Therapeutic Potential of Curcuma Longa Linn. in Relation to Its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Biswas, K.; Bandyopadhyay, U.; Banerjee, R.K. Turmeric and Curcumin: Biological Actions and Medicinal Applications. Curr. Sci. 2004, 44–53. [Google Scholar]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N. Biological Activities of Curcumin and Its Analogues (Congeners) Made by Man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential Therapeutic Effects of Curcumin, the Anti-Inflammatory Agent, against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Witkin, J.M.; Li, X. Curcumin, an Active Constiuent of the Ancient Medicinal Herb Curcuma Longa L.: Some Uses and the Establishment and Biological Basis of Medical Efficacy. CNS Neurol. Disord. Targets (Formerly Curr. Drug Targets-CNS Neurol. Disord. 2013, 12, 487–497. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sung, B. Pharmacological Basis for the Role of Curcumin in Chronic Diseases: An Age-Old Spice with Modern Targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef]
- Sharma, R.A.; McLelland, H.R.; Hill, K.A.; Ireson, C.R.; Euden, S.A.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J.; Gescher, A.J.; Steward, W.P. Pharmacodynamic and Pharmacokinetic Study of Oral Curcuma Extract in Patients with Colorectal Cancer. Clin. Cancer Res. 2001, 7, 1894–1900. [Google Scholar]
- DiSilvestro, R.A.; Joseph, E.; Zhao, S.; Bomser, J. Diverse Effects of a Low Dose Supplement of Lipidated Curcumin in Healthy Middle Aged People. Nutr. J. 2012, 11, 1–8. [Google Scholar] [CrossRef]
- Ide, H.; Tokiwa, S.; Sakamaki, K.; Nishio, K.; Isotani, S.; Muto, S.; Hama, T.; Masuda, H.; Horie, S. Combined Inhibitory Effects of Soy Isoflavones and Curcumin on the Production of Prostate-specific Antigen. Prostate 2010, 70, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M. Phase I Clinical Trial of Oral Curcumin: Biomarkers of Systemic Activity and Compliance. Clin. cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II Trial of Curcumin in Patients with Advanced Pancreatic Cancer. Clin. cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Golombick, T.; Diamond, T.H.; Badmaev, V.; Manoharan, A.; Ramakrishna, R. The Potential Role of Curcumin in Patients with Monoclonal Gammopathy of Undefined Significance—Its Effect on Paraproteinemia and the Urinary N-Telopeptide of Type I Collagen Bone Turnover Marker. Clin. Cancer Res. 2009, 15, 5917–5922. [Google Scholar] [CrossRef]
- He, Z.-Y.; Shi, C.-B.; Wen, H.; Li, F.-L.; Wang, B.-L.; Wang, J. Upregulation of P53 Expression in Patients with Colorectal Cancer by Administration of Curcumin. Cancer Invest. 2011, 29, 208–213. [Google Scholar] [CrossRef]
- Cruz–Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination Treatment with Curcumin and Quercetin of Adenomas in Familial Adenomatous Polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y. A Phase I/II Study of Gemcitabine-Based Chemotherapy plus Curcumin for Patients with Gemcitabine-Resistant Pancreatic Cancer. Cancer Chemother. Pharmacol. 2011, 68, 157–164. [Google Scholar] [CrossRef]
- Gupta, S.C.; Sung, B.; Kim, J.H.; Prasad, S.; Li, S.; Aggarwal, B.B. Multitargeting by Turmeric, the Golden Spice: From Kitchen to Clinic. Mol. Nutr. Food Res. 2013, 57, 1510–1528. [Google Scholar] [CrossRef]
- Bayet-Robert, M.; Kwiatowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.-A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I Dose Escalation Trial of Docetaxel plus Curcumin in Patients with Advanced and Metastatic Breast Cancer. Cancer Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef]
- Sathyabhama, M.; Priya Dharshini, L.C.; Karthikeyan, A.; Kalaiselvi, S.; Min, T. The Credible Role of Curcumin in Oxidative Stress-Mediated Mitochondrial Dysfunction in Mammals. Biomolecules 2022, 12, 1405. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Vinqvist, M.R.; Mukai, K.; Goto, H.; Hashimoto, Y.; Tokunaga, A.; Uno, H. On the Antioxidant Mechanism of Curcumin: Classical Methods Are Needed to Determine Antioxidant Mechanism and Activity. Org. Lett. 2000, 2, 2841–2843. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Paulraj, F.; Abas, F.; H. Lajis, N.; Othman, I.; Naidu, R. Molecular Pathways Modulated by Curcumin Analogue, Diarylpentanoids in Cancer. Biomolecules 2019, 9, 270. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Zhou, H.; S Beevers, C.; Huang, S. The Targets of Curcumin. Curr. Drug Targets 2011, 12, 332–347. [Google Scholar] [CrossRef]
- Zhang, F.; Altorki, N.K.; Mestre, J.R.; Subbaramaiah, K.; Dannenberg, A.J. Curcumin Inhibits Cyclooxygenase-2 Transcription in Bile Acid-and Phorbol Ester-Treated Human Gastrointestinal Epithelial Cells. Carcinogenesis 1999, 20, 445–451. [Google Scholar] [CrossRef]
- Setyono, J.; Harini, I.M.; Sarmoko, S.; Rujito, L. Supplementation of Curcuma Domestica Extract Reduces Cox-2 and Inos Expression on Raw 264.7 Cells. J. Phys. Conf. Ser. 2019, 1246, 12059. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Cho, S.-S.; Li, Y.; Bae, C.-S.; Park, K.M.; Park, D.-H. Anti-Inflammatory Effect of Curcuma Longa and Allium Hookeri Co-Treatment via NF-ΚB and COX-2 Pathways. Sci. Rep. 2020, 10, 5718. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Bradley, J. TNF-mediated Inflammatory Disease. J. Pathol. A J. Pathol. Soc. Gt. Britain Irel. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Anwar, M.J.; Alenezi, S.K.; Alhowail, A.H. Molecular Insights into the Pathogenic Impact of Vitamin D Deficiency in Neurological Disorders. Biomed. Pharmacother. 2023, 162, 114718. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Díez, J.J.; Hernanz, A.; Medina, S.; Bayón, C.; Iglesias, P. Serum Concentrations of Tumour Necrosis Factor-alpha (TNF-α) and Soluble TNF-α Receptor P55 in Patients with Hypothyroidism and Hyperthyroidism before and after Normalization of Thyroid Function. Clin. Endocrinol. (Oxf). 2002, 57, 515–521. [Google Scholar] [CrossRef]
- Tzanavari, T.; Giannogonas, P.; Karalis, K.P. TNF-α and Obesity. TNF Pathophysiol. 2010, 11, 145–156. [Google Scholar]
- Olivera, A.; Moore, T.W.; Hu, F.; Brown, A.P.; Sun, A.; Liotta, D.C.; Snyder, J.P.; Yoon, Y.; Shim, H.; Marcus, A.I. Inhibition of the NF-ΚB Signaling Pathway by the Curcumin Analog, 3, 5-Bis (2-Pyridinylmethylidene)-4-Piperidone (EF31): Anti-Inflammatory and Anti-Cancer Properties. Int. Immunopharmacol. 2012, 12, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Wang ShaoLing, W.S.; Li Ying, L.Y.; Wen Ying, W.Y.; Chen YanFeng, C.Y.; Na LiXin, N.L.; Li SongTao, L.S.; Sun ChangHao, S.C. Curcumin, a Potential Inhibitor of up-Regulation of TNF-Alpha and IL-6 Induced by Palmitate in 3T3-L1 Adipocytes through NF-KappaB and JNK Pathway. 2009. [Google Scholar]
- Ben, P.; Liu, J.; Lu, C.; Xu, Y.; Xin, Y.; Fu, J.; Huang, H.; Zhang, Z.; Gao, Y.; Luo, L. Curcumin Promotes Degradation of Inducible Nitric Oxide Synthase and Suppresses Its Enzyme Activity in RAW 264.7 Cells. Int. Immunopharmacol. 2011, 11, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Shoorei, H.; Bahroudi, Z.; Hussen, B.M.; Talebi, S.F.; Taheri, M.; Ayatollahi, S.A. Nrf2-Related Therapeutic Effects of Curcumin in Different Disorders. Biomolecules 2022, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Atiaa, A.; Abdullah, A. NQO1 Enzyme and Its Role in Cellular Protection; an Insight. Iberoam. J. Med. 2020, 2, 306–313. [Google Scholar] [CrossRef]
- Battino, M.; Giampieri, F.; Pistollato, F.; Sureda, A.; de Oliveira, M.R.; Pittalà, V.; Fallarino, F.; Nabavi, S.F.; Atanasov, A.G.; Nabavi, S.M. Nrf2 as Regulator of Innate Immunity: A Molecular Swiss Army Knife! Biotechnol. Adv. 2018, 36, 358–370. [Google Scholar] [CrossRef]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; Von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Nam, H.; Morrison, R.F. Curcumin Inhibits 3T3-L1 Preadipocyte Proliferation by Mechanisms Involving Post-Transcriptional P27 Regulation. Biochem. Biophys. reports 2016, 5, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yang, B.; Najafi, M. Targeting of Cancer Cell Death Mechanisms by Curcumin: Implications to Cancer Therapy. Basic Clin. Pharmacol. Toxicol. 2021, 129, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Hang, M.; Shi, Y. Curcumin Boosts Natural Killer Cell-Based Immunotherapy in Impeding Progression of Hepatocellular Carcinoma Through Androgen Receptor/UL16 Binding Protein-2 Signal. Sci. Adv. Mater. 2022, 14, 188–195. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Md Sakib Hossain, D.; Mohanty, S.; Sankar Sen, G.; Chattopadhyay, S.; Banerjee, S.; Chakraborty, J.; Das, K.; Sarkar, D.; Das, T.; et al. Curcumin Reverses T Cell-Mediated Adaptive Immune Dysfunctions in Tumor-Bearing Hosts. Cell. Mol. Immunol. 2010, 7, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Alinejad, S.; Khademvatan, S.; Amani, S.; Asadi, N.; Tappeh, K.H.; Yousefi, E.; Miandoabi, T. The Effect of Curcumin on the Expression of INFγ, TNF-α, and INOS Genes in PBMCs Infected with Leishmania Major [MRHO/IR/75/ER]. Infect. Disord. Drug Targets 2022, 22, e040422203031. [Google Scholar] [CrossRef]
- Draghiciu, O.; Lubbers, J.; Nijman, H.W.; Daemen, T. Myeloid Derived Suppressor Cells-An Overview of Combat Strategies to Increase Immunotherapy Efficacy. Oncoimmunology 2015, 4, e954829. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Jiang, H.; Ni, M.; Zou, Y.; Chen, Y.; Wu, T.; Ding, D.; Xu, H.; Li, X. Targeted Regulation of Tumor Microenvironment through the Inhibition of MDSCs by Curcumin Loaded Self-Assembled Nano-Filaments. Mater. today. Bio 2022, 15, 100304. [Google Scholar] [CrossRef]
- Ahmad, I.; Hoque, M.; Alam, S.S.M.; Zughaibi, T.A.; Tabrez, S. Curcumin and Plumbagin Synergistically Target the PI3K/Akt/MTOR Pathway: A Prospective Role in Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 6651. [Google Scholar] [CrossRef]
- Song, J.; Choi, B.; Jin, E.-J.; Yoon, Y.; Choi, K.-H. Curcumin Suppresses Streptococcus Mutans Adherence to Human Tooth Surfaces and Extracellular Matrix Proteins. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1347–1352. [Google Scholar] [CrossRef]
- Betts, J.W.; Wareham, D.W. In Vitro Activity of Curcumin in Combination with Epigallocatechin Gallate (EGCG) versus Multidrug-Resistant Acinetobacter Baumannii. BMC Microbiol. 2014, 14, 1–5. [Google Scholar] [CrossRef]
- Mun, S.-H.; Joung, D.-K.; Kim, Y.-S.; Kang, O.-H.; Kim, S.-B.; Seo, Y.-S.; Kim, Y.-C.; Lee, D.-S.; Shin, D.-W.; Kweon, K.-T. Synergistic Antibacterial Effect of Curcumin against Methicillin-Resistant Staphylococcus Aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, K.M.; Iranshahi, M.; Yazdi, M.C.; Shahverdi, A.R. The Combination Effect of Curcumin with Different Antibiotics against Staphylococcus Aureus. Int. J. Green Pharm. 2009, 3. [Google Scholar]
- Rudrappa, T.; Bais, H.P. Curcumin, a Known Phenolic from Curcuma Longa, Attenuates the Virulence of Pseudomonas Aeruginosa PAO1 in Whole Plant and Animal Pathogenicity Models. J. Agric. Food Chem. 2008, 56, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Lin, J.; Li, H.; Shen, Z.; Wang, Y.; Velkov, T.; Shen, J. The Natural Product Curcumin as an Antibacterial Agent: Current Achievements and Problems. Antioxidants 2022, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Rauf, A.; Akash, S.; Trisha, S.I.; Nasim, A.H.; Akter, M.; Dhar, P.S.; Ogaly, H.A.; Hemeg, H.A.; Wilairatana, P. Targeted Therapies of Curcumin Focus on Its Therapeutic Benefits in Cancers and Human Health: Molecular Signaling Pathway-Based Approaches and Future Perspectives. Biomed. Pharmacother. 2024, 170, 116034. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.-O. Curcumin in Cancer and Inflammation: An In-Depth Exploration of Molecular Interactions, Therapeutic Potentials, and the Role in Disease Management. Int. J. Mol. Sci. 2024, 25, 2911. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Rane, G.; Kanchi, M.M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Tan, B.K.H.; Kumar, A.P.; Sethi, G. The Multifaceted Role of Curcumin in Cancer Prevention and Treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef]
- Sivani, B.M.; Azzeh, M.; Patnaik, R.; Pantea Stoian, A.; Rizzo, M.; Banerjee, Y. Reconnoitering the Therapeutic Role of Curcumin in Disease Prevention and Treatment: Lessons Learnt and Future Directions. Metabolites 2022, 12. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Paciello, F.; Mezzogori, D.; Rolesi, R.; Eramo, S.L.M.; Paludetti, G.; Troiani, D. Molecular Targets for Anticancer Redox Chemotherapy and Cisplatin-Induced Ototoxicity: The Role of Curcumin on PSTAT3 and Nrf-2 Signalling. Br. J. Cancer 2015, 113, 1434–1444. [Google Scholar] [CrossRef]
- Zoi, V.; Galani, V.; Lianos, G.D.; Voulgaris, S.; Kyritsis, A.P.; Alexiou, G.A. The Role of Curcumin in Cancer Treatment. Biomedicines 2021, 9, 1086. [Google Scholar] [CrossRef]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical Effects of Curcumin in Enhancing Cancer Therapy: A Systematic Review. BMC Cancer 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer Potential of Curcumin: Preclinical and Clinical Studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar] [PubMed]
- Screening, P.D.Q.; Board, P.E. Colorectal Cancer Screening (PDQ®). In PDQ Cancer Information Summaries [Internet]; National Cancer Institute (US), 2023. [Google Scholar]
- Sharma, N.; Alam, M.S.; Sharma, A.; Garg, S.; Maity, M.K. Colorectal Cancer In Young Adults: Epidemiology, Risk Factors, Development, Symptoms, Traditional Herbal Therapy And Prevention. J. Pharm. Negat. Results 2022, 1370–1382. [Google Scholar]
- Garcea, G.; Berry, D.P.; Jones, D.J.L.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the Putative Chemopreventive Agent Curcumin by Cancer Patients: Assessment of Curcumin Levels in the Colorectum and Their Pharmacodynamic Consequences. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.E.; Benya, R. V; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens Jr, F.L. Phase IIa Clinical Trial of Curcumin for the Prevention of Colorectal Neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhuang, Z.; Lu, Y.; Tao, T.; Zhou, Y.; Liu, G.; Wang, H.; Zhang, D.; Wu, L.; Dai, H. Curcumin Mitigates Neuro-Inflammation by Modulating Microglia Polarization through Inhibiting TLR4 Axis Signaling Pathway Following Experimental Subarachnoid Hemorrhage. Front. Neurosci. 2019, 13, 496029. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin Inhibits LPS-Induced Neuroinflammation by Promoting Microglial M2 Polarization via TREM2/TLR4/NF-ΚB Pathways in BV2 Cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef]
- Holt, P.R.; Katz, S.; Kirshoff, R. Curcumin Therapy in Inflammatory Bowel Disease: A Pilot Study. Dig. Dis. Sci. 2005, 50, 2191–2193. [Google Scholar] [CrossRef]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Mitsuyama, K.; Sata, M. Curcumin Maintenance Therapy for Ulcerative Colitis: Randomized, Multicenter, Double-Blind, Placebo-Controlled Trial. Clin. Gastroenterol. Hepatol. 2006, 4, 1502–1506. [Google Scholar] [CrossRef]
- Lahiff, C.; Moss, A.C. Curcumin for Clinical and Endoscopic Remission in Ulcerative Colitis. Inflamm. Bowel Dis. 2011, 17, E66. [Google Scholar] [CrossRef]
- Sadeghi, N.; Mansoori, A.; Shayesteh, A.; Hashemi, S.J. The Effect of Curcumin Supplementation on Clinical Outcomes and Inflammatory Markers in Patients with Ulcerative Colitis. Phyther. Res. 2020, 34, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.; Docena, G.; MacDonald, T.T.; Sanderson, I.R. Curcumin Suppresses P38 Mitogen-Activated Protein Kinase Activation, Reduces IL-1β and Matrix Metalloproteinase-3 and Enhances IL-10 in the Mucosa of Children and Adults with Inflammatory Bowel Disease. Br. J. Nutr. 2010, 103, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Young, K.N.; Moniruzzaman, M.; Beyene, A.M.; Do, K.; Kalaiselvi, S.; Min, T. Curcumin and Its Modified Formulations on Inflammatory Bowel Disease (IBD): The Story so Far and Future Outlook. Pharmaceutics 2021, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Kaitha, S.; Bashir, M.; Ali, T. Iron Deficiency Anemia in Inflammatory Bowel Disease. World J. Gastrointest. Pathophysiol. 2015, 6, 62–72. [Google Scholar] [CrossRef]
- Jiao, Y.; Wilkinson IV, J.; Di, X.; Wang, W.; Hatcher, H.; Kock, N.D.; D’Agostino Jr, R.; Knovich, M.A.; Torti, F.M.; Torti, S. V Curcumin, a Cancer Chemopreventive and Chemotherapeutic Agent, Is a Biologically Active Iron Chelator. Blood, J. Am. Soc. Hematol. 2009, 113, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; He, B.; Guo, J.; Li, S.; Wang, J. Involvement of TLR4 in the Protective Effect of Intra-Articular Administration of Curcumin on Rat Experimental Osteoarthritis. Acta Cirúrgica Bras. 2019, 34, e201900604. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.; Zhang, H.; Li, H.; Liu, J.; Zhang, F.; Jiang, T.; Jiang, S. Curcumin Prevents Osteoarthritis by Inhibiting the Activation of Inflammasome NLRP3. J. Interf. Cytokine Res. 2017, 37, 449–455. [Google Scholar] [CrossRef]
- Guo, C.; Fu, R.; Wang, S.; Huang, Y.; Li, X.; Zhou, M.; Zhao, J.; Yang, N. NLRP3 Inflammasome Activation Contributes to the Pathogenesis of Rheumatoid Arthritis. Clin. Exp. Immunol. 2018, 194, 231–243. [Google Scholar] [CrossRef]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-Associated Uric Acid Crystals Activate the NALP3 Inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Nicoliche, T.; Maldonado, D.C.; Faber, J.; Silva, M.C.P. da Evaluation of the Articular Cartilage in the Knees of Rats with Induced Arthritis Treated with Curcumin. PLoS One 2020, 15, e0230228. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Er, B.; Durmus, A.S.; Ozercan, I.H.; Sahin, N.; Padigaru, M.; Morde, A.; Rai, D. Protective Effect of a Novel Highly Bioavailable Formulation of Curcumin in Experimentally Induced Osteoarthritis Rat Model. Curr. Dev. Nutr. 2020, 4, 1765. [Google Scholar] [CrossRef]
- Hamdalla, H.M.; Ahmed, R.R.; Galaly, S.R.; Naguib, I.A.; Alghamdi, B.S.; Ahmed, O.M.; Farghali, A.; Abdul-Hamid, M. Ameliorative Effect of Curcumin Nanoparticles against Monosodium Iodoacetate-Induced Knee Osteoarthritis in Rats. Mediators Inflamm. 2022, 2022, 8353472. [Google Scholar] [CrossRef]
- Saber, M.M.; Mahmoud, M.M.; Amin, H.M.; Essam, R.M. Therapeutic Effects of Combining Curcumin and Swimming in Osteoarthritis Using a Rat Model. Biomed. Pharmacother. 2023, 166, 115309. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Product-Evaluation Registry of Meriva®, a Curcumin-Phosphatidylcholine Complex, for the Complementary Management of Osteoarthritis. Panminerva Med 2010, 52, 55–62. [Google Scholar] [PubMed]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Efficacy and Safety of Meriva®, a Curcumin-Phosphatidylcholine Complex, during Extended Administration in Osteoarthritis Patients. Altern Med Rev 2010, 15, 337–344. [Google Scholar] [PubMed]
- Chandran, B.; Goel, A. A Randomized, Pilot Study to Assess the Efficacy and Safety of Curcumin in Patients with Active Rheumatoid Arthritis. Phyther. Res. 2012, 26, 1719–1725. [Google Scholar] [CrossRef]
- Paultre, K.; Cade, W.; Hernandez, D.; Reynolds, J.; Greif, D.; Best, T.M. Therapeutic Effects of Turmeric or Curcumin Extract on Pain and Function for Individuals with Knee Osteoarthritis: A Systematic Review. BMJ open Sport Exerc. Med. 2021, 7, e000935. [Google Scholar] [CrossRef]
- Singh, L.; Sharma, S.; Xu, S.; Tewari, D.; Fang, J. Curcumin as a Natural Remedy for Atherosclerosis: A Pharmacological Review. Molecules 2021, 26. [Google Scholar] [CrossRef]
- Chen, Y.; Chai, Y.; Xie, K.; Yu, F.; Wang, C.; Lin, S.; Yang, Y.; Xu, F. Curcumin Promotes the Expression of IL-35 by Regulating Regulatory T Cell Differentiation and Restrains Uncontrolled Inflammation and Lung Injury in Mice. Inflammation 2020, 43, 1913–1924. [Google Scholar] [CrossRef]
- Cox, F.F.; Misiou, A.; Vierkant, A.; Ale-Agha, N.; Grandoch, M.; Haendeler, J.; Altschmied, J. Protective Effects of Curcumin in Cardiovascular Diseases—Impact on Oxidative Stress and Mitochondria. Cells 2022, 11, 342. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Hu, G.; Xu, C.; Jiang, H. Curcumin Attenuates Hydrogen Peroxide-Induced Premature Senescence via the Activation of SIRT1 in Human Umbilical Vein Endothelial Cells. Biol. Pharm. Bull. 2015, 38, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, I. Antioxidant and Radical Scavenging Properties of Curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S. V; Steenken, S.; Boone, C.W.; Simic, M.G. H-Atom Transfer Is a Preferred Antioxidant Mechanism of Curcumin. J. Am. Chem. Soc. 1999, 121, 9677–9681. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and Anti-Inflammatory Properties of Curcumin. Mol. targets Ther. uses curcumin Heal. Dis. 2007, 105–125. [Google Scholar]
- Faten, R.A.; Ibrahim, A.E.; Khaled, A.E. Protective and Modulatory Effects of Curcumin and L-Carnitine against Methotrexate-Induced Oxidative Stress in Albino Rats. Res. J. Pharm. Biol. Chem. Sci 2013, 4, 744–754. [Google Scholar]
- Gao, S.; Zhang, W.; Zhao, Q.; Zhou, J.; Wu, Y.; Liu, Y.; Yuan, Z.; Wang, L. Curcumin Ameliorates Atherosclerosis in Apolipoprotein E Deficient Asthmatic Mice by Regulating the Balance of Th2/Treg Cells. Phytomedicine 2019, 52, 129–135. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Abdollahi, E.; Nikfar, B.; Chaichian, S.; Ekhlasi-Hundrieser, M. Curcumin as a Potential Modulator of M1 and M2 Macrophages: New Insights in Atherosclerosis Therapy. Heart Fail. Rev. 2019, 24, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zou, J.; Li, P.; Zheng, X.; Feng, D. Curcumin Protects against Atherosclerosis in Apolipoprotein E-Knockout Mice by Inhibiting Toll-like Receptor 4 Expression. J. Agric. Food Chem. 2018, 66, 449–456. [Google Scholar] [CrossRef]
- Han, Y.; Sun, H.-J.; Tong, Y.; Chen, Y.-Z.; Ye, C.; Qiu, Y.; Zhang, F.; Chen, A.-D.; Qi, X.-H.; Chen, Q. Curcumin Attenuates Migration of Vascular Smooth Muscle Cells via Inhibiting NFκB-Mediated NLRP3 Expression in Spontaneously Hypertensive Rats. J. Nutr. Biochem. 2019, 72, 108212. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Pirro, M.; Gotto Jr, A.M.; Banach, M.; Atkin, S.L.; Majeed, M.; Sahebkar, A. Lipid-Modifying Activity of Curcuminoids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 1178–1187. [Google Scholar] [CrossRef]
- Sahebkar, A. A Systematic Review and Meta-Analysis of Randomized Controlled Trials Investigating the Effects of Curcumin on Blood Lipid Levels. Clin. Nutr. 2014, 33, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Alwi, I.; Santoso, T.; Suyono, S.; Sutrisna, B.; Suyatna, F.D.; Kresno, S.B.; Ernie, S. The Effect of Curcumin on Lipid Level in Patients with Acute Coronary Syndrome. Acta Med. Indones. 2008, 40, 201–210. [Google Scholar] [PubMed]
- Usharani, P.; Mateen, A.A.; Naidu, M.U.R.; Raju, Y.S.N.; Chandra, N. Effect of NCB-02, Atorvastatin and Placebo on Endothelial Function, Oxidative Stress and Inflammatory Markers in Patients with Type 2 Diabetes Mellitus: A Randomized, Parallel-Group, Placebo-Controlled, 8-Week Study. Drugs R. D. 2008, 9, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-S.; Su, Y.-F.; Yang, H.-W.; Lee, Y.-H.; Chou, J.I.; Ueng, K.-C. Lipid-Lowering Effects of Curcumin in Patients with Metabolic Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Phytother. Res. 2014, 28, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Mirzabeigi, P.; Mohammadpour, A.H.; Salarifar, M.; Gholami, K.; Mojtahedzadeh, M.; Javadi, M.R. The Effect of Curcumin on Some of Traditional and Non-Traditional Cardiovascular Risk Factors: A Pilot Randomized, Double-Blind, Placebo-Controlled Trial. Iran. J. Pharm. Res. IJPR 2015, 14, 479–486. [Google Scholar] [PubMed]
- Funamoto, M.; Sunagawa, Y.; Katanasaka, Y.; Miyazaki, Y.; Imaizumi, A.; Kakeya, H.; Yamakage, H.; Satoh-Asahara, N.; Komiyama, M.; Wada, H.; et al. Highly Absorptive Curcumin Reduces Serum Atherosclerotic Low-Density Lipoprotein Levels in Patients with Mild COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Czekaj, R.; Majka, J.; Magierowska, K.; Sliwowski, Z.; Magierowski, M.; Pajdo, R.; Ptak-Belowska, A.; Surmiak, M.; Kwiecien, S.; Brzozowski, T. Mechanisms of Curcumin-Induced Gastroprotection against Ethanol-Induced Gastric Mucosal Lesions. J. Gastroenterol. 2018, 53, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Xu, D.; Xu, S.; Zheng, Y.; Xu, S. Novel Role of Zn (II)–Curcumin in Enhancing Cell Proliferation and Adjusting Proinflammatory Cytokine-Mediated Oxidative Damage of Ethanol-Induced Acute Gastric Ulcers. Chem. Biol. Interact. 2012, 197, 31–39. [Google Scholar] [CrossRef]
- Mahattanadul, S.; Nakamura, T.; Panichayupakaranant, P.; Phdoongsombut, N.; Tungsinmunkong, K.; Bouking, P. Comparative Antiulcer Effect of Bisdemethoxycurcumin and Curcumin in a Gastric Ulcer Model System. Phytomedicine 2009, 16, 342–351. [Google Scholar] [CrossRef]
- Tuorkey, M.; Karolin, K. Anti-Ulcer Activity of Curcumin on Experimental Gastric Ulcer in Rats and Its Effect on Oxidative Stress/Antioxidant, IL-6 and Enzyme Activities. Biomed. Environ. Sci. 2009, 22, 488–495. [Google Scholar] [CrossRef]
- Mahattanadul, S.; Mustafa, M.W.; Kuadkaew, S.; Pattharachayakul, S.; Ungphaiboon, S.; Sawanyawisuth, K. Oral Ulcer Healing and Anti-Candida Efficacy of an Alcohol-Free Chitosan-Curcumin Mouthwash. Eur Rev Med Pharmacol Sci 2018, 22, 7020–7023. [Google Scholar] [PubMed]
- Kuadkaew, S.; Ungphaiboon, S.; Phdoongsombut, N.; Kaewsuwan, S.; Mahattanadul, S. Efficacy of a Chitosan-Curcumin Mixture in Treating Indomethacininduced Acute Gastric Ulcer in Rats. Curr. Pharm. Biotechnol. 2021, 22, 1919–1931. [Google Scholar] [CrossRef] [PubMed]
- Heikal, E.J.; Kaoud, R.M.; Gad, S.; Mokhtar, H.I.; Aldahish, A.A.; Alzlaiq, W.A.; Zaitone, S.A.; Moustafa, Y.M.; Hammady, T.M. Design and Optimization of Omeprazole-Curcumin-Loaded Hydrogel Beads Coated with Chitosan for Treating Peptic Ulcers. Pharmaceuticals (Basel). 2023, 16. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J.; Fernandez-Botran, R. Effects of Curcumin on Helicobacter Pylori Infection. Ann. Transl. Med. 2016, 4, 479. [Google Scholar] [CrossRef] [PubMed]
- Kositchaiwat, C.; Kositchaiwat, S.; Havanondha, J. Curcuma Longa Linn. in the Treatment of Gastric Ulcer Comparison to Liquid Antacid: A Controlled Clinical Trial. J. Med. Assoc. Thailand= Chotmaihet thangphaet 1993, 76, 601–605. [Google Scholar]
- Prucksunand, C.; Indrasukhsri, B.; Leethochawalit, M.; Hungspreugs, K. Phase II Clinical Trial on Effect of the Long Turmeric (Curcuma Longa Linn.) on Healing of Peptic Ulcer. Southeast Asian J. Trop. Med. Public Health 2001, 32, 208–215. [Google Scholar]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Jiang, H.; He, H.; Chen, Y.; Huang, W.; Cheng, J.; Ye, J.; Wang, A.; Tao, J.; Wang, C.; Liu, Q. Identification of a Selective and Direct NLRP3 Inhibitor to Treat Inflammatory Disorders. J. Exp. Med. 2017, 214, 3219. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, M.; Ceballos-Olvera, I.; del Barrio, L.; Re, F. Role of the Inflammasome, IL-1beta, and IL-18 in Bacterial Infections. ScientificWorld-Journal 2011, 11, 2037–2050. [Google Scholar] [CrossRef] [PubMed]
- Marín-Palma, D.; Tabares-Guevara, J.H.; Zapata-Cardona, M.I.; Flórez-Álvarez, L.; Yepes, L.M.; Rugeles, M.T.; Zapata-Builes, W.; Hernandez, J.C.; Taborda, N.A. Curcumin Inhibits in Vitro SARS-CoV-2 Infection in Vero E6 Cells through Multiple Antiviral Mechanisms. Molecules 2021, 26, 6900. [Google Scholar] [CrossRef]
- Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.; Bahadoram, M.; Alghasi, A. COVID-19: A Case for Inhibiting NLRP3 Inflammasome, Suppression of Inflammation with Curcumin? Basic Clin. Pharmacol. Toxicol. 2021, 128, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Rattis, B.A.C.; Ramos, S.G.; Celes, M. Curcumin as a Potential Treatment for COVID-19. Front. Pharmacol. 2021, 12, 675287. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, S.; Saeed, B.Q.; Temirgalieva, E.; Yumashev, A.V.; El-Esawi, M.A.; Navashenaq, J.G.; Valizadeh, H.; Sadeghi, A.; Aslani, S.; Yousefi, M. Nanocurcumin Improves Treg Cell Responses in Patients with Mild and Severe SARS-CoV2. Life Sci. 2021, 276, 119437. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, S.; El-Esawi, M.A.; Mahmoud, Z.H.; Timoshin, A.; Valizadeh, H.; Roshangar, L.; Varshoch, M.; Vaez, A.; Aslani, S.; Navashenaq, J.G. Immunomodulatory Effects of Nanocurcumin on Th17 Cell Responses in Mild and Severe COVID-19 Patients. J. Cell. Physiol. 2021, 236, 5325–5338. [Google Scholar] [CrossRef]
- Li, X.; Xu, D.; Sun, D.; Zhang, T.; He, X.; Xiao, D. Curcumin Ameliorates Monosodium Urate-induced Gouty Arthritis through Nod-like Receptor 3 Inflammasome Mediation via Inhibiting Nuclear Factor-kappa B Signaling. J. Cell. Biochem. 2019, 120, 6718–6728. [Google Scholar] [CrossRef]
- Saber-Moghaddam, N.; Salari, S.; Hejazi, S.; Amini, M.; Taherzadeh, Z.; Eslami, S.; Rezayat, S.M.; Jaafari, M.R.; Elyasi, S. Oral Nano-curcumin Formulation Efficacy in Management of Mild to Moderate Hospitalized Coronavirus Disease-19 Patients: An Open Label Nonrandomized Clinical Trial. Phyther. Res. 2021, 35, 2616–2623. [Google Scholar] [CrossRef]
- Kahkhaie, K.R.; Mirhosseini, A.; Aliabadi, A.; Mohammadi, A.; Mousavi, M.J.; Haftcheshmeh, S.M.; Sathyapalan, T.; Sahebkar, A. Curcumin: A Modulator of Inflammatory Signaling Pathways in the Immune System. Inflammopharmacology 2019, 27, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Fessler, S.N.; Chang, Y.; Liu, L.; Johnston, C.S. Curcumin Confers Anti-Inflammatory Effects in Adults Who Recovered from COVID-19 and Were Subsequently Vaccinated: A Randomized Controlled Trial. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Nicoliche, T.; Bartolomeo, C.S.; Lemes, R.M.R.; Pereira, G.C.; Nunes, T.A.; Oliveira, R.B.; Nicastro, A.L.M.; Soares, É.N.; da Cunha Lima, B.F.; Rodrigues, B.M. Antiviral, Anti-Inflammatory and Antioxidant Effects of Curcumin and Curcuminoids in SH-SY5Y Cells Infected by SARS-CoV-2. Sci. Rep. 2024, 14, 10696. [Google Scholar] [CrossRef]
- Golpour-Hamedani, S.; Pourmasoumi, M.; Askari, G.; Bagherniya, M.; Majeed, M.; Guest, P.C.; Sahebkar, A. Antiviral Mechanisms of Curcumin and Its Derivatives in Prevention and Treatment of COVID-19: A Review. Adv. Exp. Med. Biol. 2023, 1412, 397–411. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Niziński, P.; Hawrył, A.; Gancarz, M.; Hawrył, D.; Oliwa, W.; Pałka, M.; Markowska, J.; Oniszczuk, A. Potential of Curcumin in the Management of Skin Diseases. Int. J. Mol. Sci. 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Bahraini, P.; Rajabi, M.; Mansouri, P.; Sarafian, G.; Chalangari, R.; Azizian, Z. Turmeric Tonic as a Treatment in Scalp Psoriasis: A Randomized Placebo-Control Clinical Trial. J. Cosmet. Dermatol. 2018, 17, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Liu, L.; Sun, X.; Zhou, Y.; Chen, S.; Lu, Y.; Cai, X.; Hu, M.; Yan, G.; et al. Efficacy and Safety of Curcumin in Psoriasis: Preclinical and Clinical Evidence and Possible Mechanisms. Front. Pharmacol. 2022, 13, 903160. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Doppalapudi, S.; Domb, A.J.; Khan, W. Tacrolimus and Curcumin Co-Loaded Liposphere Gel: Synergistic Combination towards Management of Psoriasis. J. Control. release 2016, 243, 132–145. [Google Scholar] [CrossRef]
- Mao, K.-L.; Fan, Z.-L.; Yuan, J.-D.; Chen, P.-P.; Yang, J.-J.; Xu, J.; ZhuGe, D.-L.; Jin, B.-H.; Zhu, Q.-Y.; Shen, B.-X.; et al. Skin-Penetrating Polymeric Nanoparticles Incorporated in Silk Fibroin Hydrogel for Topical Delivery of Curcumin to Improve Its Therapeutic Effect on Psoriasis Mouse Model. Colloids Surf. B. Biointerfaces 2017, 160, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, M.R.; Jeon, S.H.; Jang, Y.; Yang, Y.D. Pathophysiological Roles of Ion Channels in Epidermal Cells, Immune Cells, and Sensory Neurons in Psoriasis. Int. J. Mol. Sci. 2024, 25, 2756. [Google Scholar] [CrossRef]
- Tabeshpour, J.; Banaeeyeh, S.; Eisvand, F.; Sathyapalan, T.; Hashemzaei, M.; Sahebkar, A. Effects of Curcumin on Ion Channels and Pumps: A Review. IUBMB Life 2019, 71, 812–820. [Google Scholar] [CrossRef]
- Kang, D.; Li, B.; Luo, L.; Jiang, W.; Lu, Q.; Rong, M.; Lai, R. Curcumin Shows Excellent Therapeutic Effect on Psoriasis in Mouse Model. Biochimie 2016, 123, 73–80. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Zhu, W.; Kuang, Y.; Liu, T.; Zhang, W.; Chen, X.; Peng, C. Skin and Gut Microbiome in Psoriasis: Gaining Insight Into the Pathophysiology of It and Finding Novel Therapeutic Strategies. Front. Microbiol. 2020, 11, 589726. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, W.; Zhang, Y.; Zeng, Y. Curcumin Alleviates Imiquimod-Induced Psoriasis-like Inflammation and Regulates Gut Microbiota of Mice. Immunity, Inflamm. Dis. 2023, 11, e967. [Google Scholar] [CrossRef]
- Li, L.; Liu, C.; Fu, J.; Wang, Y.; Yang, D.; Peng, B.; Liu, X.; Han, X.; Meng, Y.; Feng, F.; et al. CD44 Targeted Indirubin Nanocrystal-Loaded Hyaluronic Acid Hydrogel for the Treatment of Psoriasis. Int. J. Biol. Macromol. 2023, 243, 125239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, Q.; Li, Y.; He, Z.; Li, Z.; Guo, T.; Wu, Z.; Feng, N. CD44 Assists the Topical Anti-Psoriatic Efficacy of Curcumin-Loaded Hyaluronan-Modified Ethosomes: A New Strategy for Clustering Drug in Inflammatory Skin. Theranostics 2019, 9, 48–64. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Z.; Wang, L.; Cun, D.; Tong, H.H.Y.; Yan, R.; Chen, X.; Wang, R.; Zheng, Y. Enhanced Topical Penetration, System Exposure and Anti-Psoriasis Activity of Two Particle-Sized, Curcumin-Loaded PLGA Nanoparticles in Hydrogel. J. Control. release Off. J. Control. Release Soc. 2017, 254, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Lin, J.; Yang, C.; Wu, C.; He, J.; Chen, Z.; Yang, Q.; Chen, J.; Zheng, G.; Lv, L.; et al. Enhanced Penetration and Anti-Psoriatic Efficacy of Curcumin by Improved SmartPearls Technology with the Addition of Glycyrrhizic Acid. Int. J. Pharm. 2020, 578, 119101. [Google Scholar] [CrossRef]
- Reddy, S.; Aggarwal, B.B. Curcumin Is a Non-Competitive and Selective Inhibitor of Phosphorylase Kinase. FEBS Lett. 1994, 341, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Heng, M.C.Y.; Song, M.K.; Harker, J.; Heng, M.K. Drug-induced Suppression of Phosphorylase Kinase Activity Correlates with Resolution of Psoriasis as Assessed by Clinical, Histological and Immunohistochemical Parameters. Br. J. Dermatol. 2000, 143, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Kurd, S.K.; Smith, N.; VanVoorhees, A.; Troxel, A.B.; Badmaev, V.; Seykora, J.T.; Gelfand, J.M. Oral Curcumin in the Treatment of Moderate to Severe Psoriasis Vulgaris: A Prospective Clinical Trial. J. Am. Acad. Dermatol. 2008, 58, 625–631. [Google Scholar] [CrossRef]
- Asawanonda, P.; Klahan, S.-O. Tetrahydrocurcuminoid Cream plus Targeted Narrowband UVB Phototherapy for Vitiligo: A Preliminary Randomized Controlled Study. Photomed. Laser Surg. 2010, 28, 679–684. [Google Scholar] [CrossRef]
- Vaccaro, M.; Bagnato, G.; Cristani, M.; Borgia, F.; Spatari, G.; Tigano, V.; Saja, A.; Guarneri, F.; Cannavò, S.P.; Gangemi, S. Oxidation Products Are Increased in Patients Affected by Non-Segmental Generalized Vitiligo. Arch. Dermatol. Res. 2017, 309, 485–490. [Google Scholar] [CrossRef]
- Sun, Y.P.; Gu, J.F.; Tan, X. Bin; Wang, C.F.; Jia, X. Bin; Feng, L.; Liu, J.P. Curcumin Inhibits Advanced Glycation End Product-Induced Oxidative Stress and Inflammatory Responses in Endothelial Cell Damage via Trapping Methylglyoxal. Mol. Med. Rep. 2016, 13, 1475–1486. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection against Oxidative Injury. Curr. Mol. Med. 2020, 20, 116–133. [Google Scholar] [PubMed]
- Jalalmanesh, S.; Mansouri, P.; Rajabi, M.; Monji, F. Therapeutic Effects of Turmeric Topical Cream in Vitiligo: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J. Cosmet. Dermatol. 2022, 21, 4454–4461. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, A.A.; Bharti, R.K. Spontaneous Reversal of Vitiligo, a Rare Phenomenon Reported in a Case in Saudi Arabia with an Insight into Metabolic Biochemical Derangements. Medicina (Kaunas). 2023, 59. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The Role of Inflammation in Depression: From Evolutionary Imperative to Modern Treatment Target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 Signaling Pathway by Polyphenols: A Novel Therapeutic Strategy for Neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Song, Q.; Wang, P.; Li, Y.; Yang, M.; Liu, B.; Yu, S.Y. Curcumin Protects against Chronic Stress-Induced Dysregulation of Neuroplasticity and Depression-like Behaviors via Suppressing IL-1β Pathway in Rats. Neuroscience 2018, 392, 92–106. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.; Han, W.; Yang, M.; Wen, L.; Wang, K.; Jiang, P. Curcumin Relieves Depressive-like Behaviors via Inhibition of the NLRP3 Inflammasome and Kynurenine Pathway in Rats Suffering from Chronic Unpredictable Mild Stress. Int. Immunopharmacol. 2019, 67, 138–144. [Google Scholar] [CrossRef]
- Fattore, L.; Amchova, P.; Fadda, P.; Ruda-Kucerova, J. Olfactory Bulbectomy Model of Depression Lowers Responding for Food in Male and Female Rats: The Modulating Role of Caloric Restriction and Response Requirement. Biomedicines 2023, 11. [Google Scholar] [CrossRef]
- Xu, Y.; Ku, B.-S.; Yao, H.-Y.; Lin, Y.-H.; Ma, X.; Zhang, Y.-H.; Li, X.-J. Antidepressant Effects of Curcumin in the Forced Swim Test and Olfactory Bulbectomy Models of Depression in Rats. Pharmacol. Biochem. Behav. 2005, 82, 200–206. [Google Scholar] [CrossRef]
- Wang, R.; Xu, Y.; Wu, H.-L.; Li, Y.-B.; Li, Y.-H.; Guo, J.-B.; Li, X.-J. The Antidepressant Effects of Curcumin in the Forced Swimming Test Involve 5-HT1 and 5-HT2 Receptors. Eur. J. Pharmacol. 2008, 578, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The Role of BDNF on Neural Plasticity in Depression. Front. Cell. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Hurley, L.L.; Akinfiresoye, L.; Nwulia, E.; Kamiya, A.; Kulkarni, A.A.; Tizabi, Y. Antidepressant-like Effects of Curcumin in WKY Rat Model of Depression Is Associated with an Increase in Hippocampal BDNF. Behav. Brain Res. 2013, 239, 27–30. [Google Scholar] [CrossRef]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The Molecular Basis for the Pharmacokinetics and Pharmacodynamics of Curcumin and Its Metabolites in Relation to Cancer. Pharmacol. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.K.; Bishnoi, M.; Kulkarni, S.K. Anti-Depressant like Effect of Curcumin and Its Combination with Piperine in Unpredictable Chronic Stress-Induced Behavioral, Biochemical and Neurochemical Changes. Pharmacol. Biochem. Behav. 2009, 92, 39–43. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; Hosny, E.N.; Magdy, M.; Mohammed, H.S. Antidepressant Effects of Curcumin-Coated Iron Oxide Nanoparticles in a Rat Model of Depression. Eur. J. Pharmacol. 2021, 908, 174384. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, H.M.; Aboalasaad, F.A.; Mohamed, A.S.; Elhusseiny, F.A.; Khadrawy, Y.A.; Elmekawy, A. Evaluation of the Therapeutic Effect of Curcumin-Conjugated Zinc Oxide Nanoparticles on Reserpine-Induced Depression in Wistar Rats. Biol. Trace Elem. Res. 2024, 202, 2630–2644. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Y.; Li, N.; Pi, C.; Zhao, W.; Zhong, Y.; Li, W.; Shen, H.; Yang, Y.; Zheng, W. Preliminary Investigation Into the Antidepressant Effects of a Novel Curcumin Analogue (CACN136) In Vitro and In Vivo. Mol. Neurobiol. 2024, 1–24. [Google Scholar] [CrossRef]
- Ng, Q.X.; Koh, S.S.H.; Chan, H.W.; Ho, C.Y.X. Clinical Use of Curcumin in Depression: A Meta-Analysis. J. Am. Med. Dir. Assoc. 2017, 18, 503–508. [Google Scholar] [CrossRef]
- Kanchanatawan, B.; Tangwongchai, S.; Sughondhabhirom, A.; Suppapitiporn, S.; Hemrunrojn, S.; Carvalho, A.F.; Maes, M. Add-on Treatment with Curcumin Has Antidepressive Effects in Thai Patients with Major Depression: Results of a Randomized Double-Blind Placebo-Controlled Study. Neurotox. Res. 2018, 33, 621–633. [Google Scholar] [CrossRef]
- Zeng, L.; Yang, T.; Yang, K.; Yu, G.; Li, J.; Xiang, W.; Chen, H. Efficacy and Safety of Curcumin and Curcuma Longa Extract in the Treatment of Arthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Front. Immunol. 2022, 13, 891822. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Rayess, Y. El; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Zielińska, D.; Setzer, W.N. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 550909. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A. Are Curcuminoids Effective C-Reactive Protein-Lowering Agents in Clinical Practice? Evidence from a Meta-Analysis. Phytother. Res. 2014, 28, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Pelletier, J. Examen Chimique de La Racine de Curcuma. J. Pharm 1815, 1, 289–300. [Google Scholar]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of Curcumin, a Component of Golden Spice, and Its Miraculous Biological Activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Liju, V.B.; Jeena, K.; Kuttan, R. Acute and Subchronic Toxicity as Well as Mutagenic Evaluation of Essential Oil from Turmeric (Curcuma Longa L). Food Chem. Toxicol. an Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 53, 52–61. [Google Scholar] [CrossRef]
- Kim, D.-C.; Ku, S.-K.; Bae, J.-S. Anticoagulant Activities of Curcumin and Its Derivative. BMB Rep. 2012, 45, 221–226. [Google Scholar] [CrossRef]
- Chaudhari, S.P.; Tam, A.Y.; Barr, J.A. Curcumin: A Contact Allergen. J. Clin. Aesthet. Dermatol. 2015, 8, 43–48. [Google Scholar]
- Sreejayan, N.; Rao, M.N. Free Radical Scavenging Activity of Curcuminoids. Arzneimittelforschung. 1996, 46, 169–171. [Google Scholar]
- Sreejayan, X.X.; Rao, M.N.A. Nitric Oxide Scavenging by Curcuminoids. J. Pharm. Pharmacol. 1997, 49, 105–107. [Google Scholar] [CrossRef]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin Attenuates Oxidative Stress in RAW264. 7 Cells by Increasing the Activity of Antioxidant Enzymes and Activating the Nrf2-Keap1 Pathway. PLoS One 2019, 14, e0216711. [Google Scholar]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of Phenolic O-H and Methylene Hydrogen on the Free Radical Reactions and Antioxidant Activity of Curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Rao, L.J.; Sakariah, K.K. Antioxidant Activities of Curcumin, Demethoxycurcumin and Bisdemethoxycurcumin. Food Chem. 2006, 98, 720–724. [Google Scholar] [CrossRef]
- Carvalho, D.d.M.; Takeuchi, K.P.; Geraldine, R.M.; Moura, C.J.d.; Torres, M.C.L. Production, Solubility and Antioxidant Activity of Curcumin Nanosuspension. Food Sci. Technol. 2015, 35, 115–119. [Google Scholar] [CrossRef]
- Galli, G.M.; Griss, L.G.; Fortuoso, B.F.; Silva, A.D.; Fracasso, M.; Lopes, T.F.; Schetinger, M.R.S.; Gundel, S.; Ourique, A.F.; Carneiro, C. Feed Contaminated by Fumonisin (Fusarium Spp.) in Chicks Has a Negative Influence on Oxidative Stress and Performance, and the Inclusion of Curcumin-Loaded Nanocapsules Minimizes These Effects. Microb. Pathog. 2020, 148, 104496. [Google Scholar] [CrossRef]
- Nawab, A.; Li, G.; Liu, W.; Lan, R.; Wu, J.; Zhao, Y.; Kang, K.; Kieser, B.; Sun, C.; Tang, S. Effect of Dietary Curcumin on the Antioxidant Status of Laying Hens under High-Temperature Conditions. Brazilian J. Poult. Sci. 2019, 21, eRBCA–2018. [Google Scholar]
- Damiano, S.; Longobardi, C.; Andretta, E.; Prisco, F.; Piegari, G.; Squillacioti, C.; Montagnaro, S.; Pagnini, F.; Badino, P.; Florio, S. Antioxidative Effects of Curcumin on the Hepatotoxicity Induced by Ochratoxin a in Rats. Antioxidants 2021, 10, 125. [Google Scholar] [CrossRef]
- Huang, M.-T.; Lysz, T.; Ferraro, T.; Abidi, T.F.; Laskin, J.D.; Conney, A.H. Inhibitory Effects of Curcumin on in Vitro Lipoxygenase and Cyclooxygenase Activities in Mouse Epidermis. Cancer Res. 1991, 51, 813–819. [Google Scholar]
- Srimal, R.C.; Dhawan, B.N. Pharmacology of Diferuloyl Methane (Curcumin), a Non-steroidal Anti-inflammatory Agent. J. Pharm. Pharmacol. 1973, 25, 447–452. [Google Scholar] [CrossRef]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of Curcumin on Serum Cytokine Concentrations in Subjects with Metabolic Syndrome: A Post-Hoc Analysis of a Randomized Controlled Trial. Biomed. Pharmacother. 2016, 82, 578–582. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, Z.; Chen, G.; Wang, D.; Tang, S.; Deng, H.; Wang, J.; Li, S.; Lan, J.; Tong, J. Curcumin Attenuates Asthmatic Airway Inflammation and Mucus Hypersecretion Involving a PPARγ-Dependent NF-ΚB Signaling Pathway in Vivo and in Vitro. Mediators Inflamm. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, J.; Mohammadtursun, N.; Wu, J.; Dong, J.; Li, L. Curcumin Inhibits Cigarette Smoke-Induced Inflammation via Modulating the PPARγ-NF-ΚB Signaling Pathway. Food Funct. 2019, 10, 7983–7994. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.; Calvello, R.; Porro, C.; Trotta, T.; Salvatore, R.; Panaro, M.A. PI3k/Akt Signalling Pathway Plays a Crucial Role in the Anti-Inflammatory Effects of Curcumin in LPS-Activated Microglia. Int. Immunopharmacol. 2016, 36, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.L.; Luis, P.B.; Varuzza, P. V; Joseph, A.I.; Presley, S.H.; Chaturvedi, R.; Schneider, C. The Anti-Inflammatory Activity of Curcumin Is Mediated by Its Oxidative Metabolites. J. Biol. Chem. 2017, 292, 21243–21252. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Rahimnia, A.; Sharafi, M.; Alishiri, G.; Saburi, A.; Sahebkar, A. Curcuminoid Treatment for Knee Osteoarthritis: A Randomized Double-blind Placebo-controlled Trial. Phyther. Res. 2014, 28, 1625–1631. [Google Scholar] [CrossRef]
- Panahi, Y.; Saadat, A.; Beiraghdar, F.; Sahebkar, A. Adjuvant Therapy with Bioavailability-Boosted Curcuminoids Suppresses Systemic Inflammation and Improves Quality of Life in Patients with Solid Tumors: A Randomized Double-Blind Placebo-Controlled Trial. Phytother. Res. 2014, 28, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Machova Urdzikova, L.; Karova, K.; Ruzicka, J.; Kloudova, A.; Shannon, C.; Dubisova, J.; Murali, R.; Kubinova, S.; Sykova, E.; Jhanwar-Uniyal, M.; et al. The Anti-Inflammatory Compound Curcumin Enhances Locomotor and Sensory Recovery after Spinal Cord Injury in Rats by Immunomodulation. Int. J. Mol. Sci. 2015, 17. [Google Scholar] [CrossRef]
- Abdel Aziz, M.T.; El-Asmar, M.F.; Rezq, A.M.; Mahfouz, S.M.; Wassef, M.A.; Fouad, H.H.; Ahmed, H.H.; Taha, F.M. The Effect of a Novel Curcumin Derivative on Pancreatic Islet Regeneration in Experimental Type-1 Diabetes in Rats (Long Term Study). Diabetol. Metab. Syndr. 2013, 5, 1–14. [Google Scholar] [CrossRef]
- El-Bahr, S.M. Curcumin Regulates Gene Expression of Insulin like Growth Factor, B-Cell CLL/Lymphoma 2 and Antioxidant Enzymes in Streptozotocin Induced Diabetic Rats. BMC Complement. Altern. Med. 2013, 13, 1–11. [Google Scholar] [CrossRef]
- Ezati, M.; Ghavamipour, F.; Khosravi, N.; Sajedi, R.H.; Chalabi, M.; Farokhi, A.; Adibi, H.; Khodarahmi, R. Synthesis and Potential Antidiabetic Properties of Curcumin-Based Derivatives: An In Vitro and In Silico Study of α-Glucosidase and α-Amylase Inhibition. Med. Chem. 2022, 19, 99–117. [Google Scholar] [CrossRef]
- Sayeli, V.K.; Shenoy, A.K. Antidiabetic Effect of Bio-Enhanced Preparation of Turmeric in Streptozotocin-Nicotinamide Induced Type 2 Diabetic Wistar Rats. J. Ayurveda Integr. Med. 2021, 12, 474–479. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, S.; Chen, Y.; Li, K.; Chen, W.; Liu, Y. Curcumin Anti-Diabetic Effect Mainly Correlates with Its Anti-Apoptotic Actions and PI3K/Akt Signal Pathway Regulation in the Liver. Food Chem. Toxicol. 2020, 146, 111803. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, M.; Ullah, H.; Iqbal, R.; Shaheen, G.; Albalawi, M.A.; Alatawi, F.S.; Omran, A.; Alareefy, A.; Abdulsalam, N.M.; Khateeb, N.A. Antidiabetic Effect of Black Pepper, Turmeric, and Ajwa Date Pulp, Seed, and Their Mixtures as Antioxidants in Alloxan Diabetic Rats. J. Anim. Feed Sci. 2023, 33, 28–46. [Google Scholar] [CrossRef]
- Rai, D.; Singh, J.K.; Roy, N.; Panda, D. Curcumin Inhibits FtsZ Assembly: An Attractive Mechanism for Its Antibacterial Activity. Biochem. J. 2008, 410, 147–155. [Google Scholar] [CrossRef]
- De, R.; Kundu, P.; Swarnakar, S.; Ramamurthy, T.; Chowdhury, A.; Nair, G.B.; Mukhopadhyay, A.K. Antimicrobial Activity of Curcumin against Helicobacter Pylori Isolates from India and during Infections in Mice. Antimicrob. Agents Chemother. 2009, 53, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Zandi, K.; Ramedani, E.; Mohammadi, K.; Tajbakhsh, S.; Deilami, I.; Rastian, Z.; Fouladvand, M.; Yousefi, F.; Farshadpour, F. Evaluation of Antiviral Activities of Curcumin Derivatives against HSV-1 in Vero Cell Line. Nat. Prod. Commun. 2010, 5, 1934578 × 1000501220. [Google Scholar] [CrossRef]
- Teow, S.-Y.; Ali, S.A. Synergistic Antibacterial Activity of Curcumin with Antibiotics against Staphylococcus Aureus. Pak. J. Pharm. Sci 2015, 28, 2109–2114. [Google Scholar]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal Activity of Curcumin I Is Associated with Damaging of Bacterial Membrane. PLoS One 2015, 10, e0121313. [Google Scholar] [CrossRef]
- Hettiarachchi, S.S.; Perera, Y.; Dunuweera, S.P.; Dunuweera, A.N.; Rajapakse, S.; Rajapakse, R.M.G. Comparison of Antibacterial Activity of Nanocurcumin with Bulk Curcumin. ACS omega 2022, 7, 46494–46500. [Google Scholar] [CrossRef]
- Milano, F.; Mari, L.; van de Luijtgaarden, W.; Parikh, K.; Calpe, S.; Krishnadath, K.K. Nano-Curcumin Inhibits Proliferation of Esophageal Adenocarcinoma Cells and Enhances the T Cell Mediated Immune Response. Front. Oncol. 2013, 3, 137. [Google Scholar] [CrossRef]
- Gholipour, F.; Amini, M.; Baradaran, B.; Mokhtarzadeh, A.; Eskandani, M. Anticancer Properties of Curcumin-Treated Lactobacillus Plantarum against the HT-29 Colorectal Adenocarcinoma Cells. Sci. Rep. 2023, 13, 2860. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Xia, J.; Chen, Z.; Zhang, S.; Ahmad, A.; Miele, L.; Sarkar, F.H.; Wang, Z. Inhibitory Effect of Curcumin on Oral Carcinoma CAL-27 Cells via Suppression of Notch-1 and NF-κB Signaling Pathways. J. Cell. Biochem. 2011, 112, 1055–1065. [Google Scholar] [CrossRef]
- Liu, H.-T.; Ho, Y.-S. Anticancer Effect of Curcumin on Breast Cancer and Stem Cells. Food Sci. Hum. wellness 2018, 7, 134–137. [Google Scholar] [CrossRef]
- Wang, J.; Qi, L.; Zheng, S.; Wu, T. Curcumin Induces Apoptosis through the Mitochondria-Mediated Apoptotic Pathway in HT-29 Cells. J. Zhejiang Univ. Sci. B 2009, 10, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Kasinathan, A.; Ganesan, R.; Balasubramanian, A.; Bhaskaran, J.; Suresh, S.; Srinivasan, R.; Aravind, K.B.; Sivalingam, N. Curcumin Induces Apoptosis and Cell Cycle Arrest via the Activation of Reactive Oxygen Species-Independent Mitochondrial Apoptotic Pathway in Smad4 and P53 Mutated Colon Adenocarcinoma HT29 Cells. Nutr. Res. 2018, 51, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.C.; Vatsala, P.G.; Keshamouni, V.G.; Padmanaban, G.; Rangarajan, P.N. Curcumin for Malaria Therapy. Biochem. Biophys. Res. Commun. 2005, 326, 472–474. [Google Scholar] [CrossRef]
- Cui, L.; Miao, J.; Cui, L. Cytotoxic Effect of Curcumin on Malaria Parasite Plasmodium Falciparum: Inhibition of Histone Acetylation and Generation of Reactive Oxygen Species. Antimicrob. Agents Chemother. 2007, 51, 488–494. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Rawat, P.S.; Cooke, B.M.; Coppel, R.L.; Patankar, S. Cellular Effects of Curcumin on Plasmodium Falciparum Include Disruption of Microtubules. PLoS One 2013, 8, e57302. [Google Scholar] [CrossRef]
- Ali, A.H.; Sudi, S.; Basir, R.; Embi, N.; Sidek, H.M. The Antimalarial Effect of Curcumin Is Mediated by the Inhibition of Glycogen Synthase Kinase-3β. J. Med. Food 2017, 20, 152–161. [Google Scholar] [CrossRef]
- Busari, Z.A.; Dauda, K.A.; Morenikeji, O.A.; Afolayan, F.; Oyeyemi, O.T.; Meena, J.; Sahu, D.; Panda, A.K. Antiplasmodial Activity and Toxicological Assessment of Curcumin PLGA-Encapsulated Nanoparticles. Front. Pharmacol. 2017, 8, 622. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, O.-K.; Yoon, H.-G.; Park, J.; You, Y.; Kim, K.; Lee, Y.-H.; Choi, K.-C.; Lee, J.; Jun, W. Anti-Obesity Effect of Extract from Fermented Curcuma Longa L. through Regulation of Adipogenesis and Lipolysis Pathway in High-Fat Diet-Induced Obese Rats. Food Nutr. Res. 2016, 60, 30428. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.; Hamayel, A.; Assaassa, A.; Hammad, F.; Mosa, A.; Nafaa, F.; Ghanim, M.; Dwikat, M.; AlQub, M.; Rahim, A.A. Hexane Extract of Curcuma Longa L. Inhibits the Activities of Key Enzymes and pro-Inflammatory Adipokines Linked to Obesity. Eur. J. Integr. Med. 2021, 48, 101400. [Google Scholar]
- Hassan, M.H.; Awadalla, E.A.; Abd El-Kader, A.E.-K.M.; Seifeldin, E.A.; Mahmoud, M.A.; Muddathir, A.R.M.; Abdelsadik, A. Antitoxic Effects of Curcumin against Obesity-Induced Multi-Organs’ Biochemical and Histopathological Abnormalities in an Animal Model. Evid. Based. Complement. Alternat. Med. 2022, 2022, 9707278. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Kim, K.-W.; Jin, B.-R.; Lee, D.-S.; Ko, W.; Kim, H.-M.; Yang, C.Y.; Yoon, D.; Kim, G.-S.; An, H.-J. The Ethanolic Extract of Korean Curcuma Longa Rhizome Inhibits Adipogenesis in 3T3-L1 Adipocytes and High-Fat Diet-Induced Obese Mice via Activating AMPK Signaling Pathway. J. Funct. Foods 2023, 110, 105854. [Google Scholar] [CrossRef]
- Islam, T.; Scoggin, S.; Gong, X.; Zabet-Moghaddam, M.; Kalupahana, N.S.; Moustaid-Moussa, N. Anti-Inflammatory Mechanisms of Curcumin and Its Metabolites in White Adipose Tissue and Cultured Adipocytes. Nutrients 2023, 16. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Song, Q.; Wang, P.; Li, Y.; Yang, M.; Yu, S.Y. Neuroprotective Effects of Curcumin on IL-1β-Induced Neuronal Apoptosis and Depression-like Behaviors Caused by Chronic Stress in Rats. Front. Cell. Neurosci. 2019, 12, 516. [Google Scholar] [CrossRef]
- Qi, X.-J.; Liu, X.-Y.; Tang, L.-M.-Y.; Li, P.-F.; Qiu, F.; Yang, A.-H. Anti-Depressant Effect of Curcumin-Loaded Guanidine-Chitosan Thermo-Sensitive Hydrogel by Nasal Delivery. Pharm. Dev. Technol. 2020, 25, 316–325. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, Y. Curcumin Protects against Intracellular Amyloid Toxicity in Rat Primary Neurons. Int. J. Clin. Exp. Med. 2012, 5, 44–49. [Google Scholar]
- Wu, J.; Li, Q.; Wang, X.; Yu, S.; Li, L.; Wu, X.; Chen, Y.; Zhao, J.; Zhao, Y. Neuroprotection by Curcumin in Ischemic Brain Injury Involves the Akt/Nrf2 Pathway. PLoS One 2013, 8, e59843. [Google Scholar] [CrossRef]
- Song, S.; Nie, Q.; Li, Z.; Du, G. Curcumin Improves Neurofunctions of 6-OHDA-Induced Parkinsonian Rats. Pathol. Res. Pract. 2016, 212, 247–251. [Google Scholar] [CrossRef]
- Teter, B.; Morihara, T.; Lim, G.P.; Chu, T.; Jones, M.R.; Zuo, X.; Paul, R.M.; Frautschy, S.A.; Cole, G.M. Curcumin Restores Innate Immune Alzheimer’s Disease Risk Gene Expression to Ameliorate Alzheimer Pathogenesis. Neurobiol. Dis. 2019, 127, 432–448. [Google Scholar] [CrossRef]
- Yang, J.; Song, S.; Li, J.; Liang, T. Neuroprotective Effect of Curcumin on Hippocampal Injury in 6-OHDA-Induced Parkinson’s Disease Rat. Pathol. Res. Pract. 2014, 210, 357–362. [Google Scholar] [CrossRef]
- Dai, W.; Wang, H.; Fang, J.; Zhu, Y.; Zhou, J.; Wang, X.; Zhou, Y.; Zhou, M. Curcumin Provides Neuroprotection in Model of Traumatic Brain Injury via the Nrf2-ARE Signaling Pathway. Brain Res. Bull. 2018, 140, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Yang, Y.; Xu, Z.; Zhou, P.; Gong, W.; Li, Y.; Fan, J.; He, F. Downregulation of B Lymphocyte Stimulator Expression by Curcumin in B Lymphocyte via Suppressing Nuclear Translocation of NF-ΚB. Eur. J. Pharmacol. 2011, 650, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Atabaki, M.; Shariati-Sarabi, Z.; Tavakkol-Afshari, J.; Mohammadi, M. Significant Immunomodulatory Properties of Curcumin in Patients with Osteoarthritis; a Successful Clinical Trial in Iran. Int. Immunopharmacol. 2020, 85, 106607. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.S.; Mishra, K.P.; Singh, D.P.; Mehrotra, S.; Singh, V.K. Immunomodulatory Effects of Curcumin. Immunopharmacol. Immunotoxicol. 2005, 27, 485–497. [Google Scholar] [CrossRef]
- Antony, S.; Kuttan, R.; Kuttan, G. Immunomodulatory Activity of Curcumin. Immunol. Invest. 1999, 28, 291–303. [Google Scholar] [CrossRef]
- Park, Y.-G.; Cho, J.-H.; Choi, J.; Ju, E.-M.; Adam, G.O.; Hwang, D.-I.; Lee, J.-H.; An, S.-Y.; Choi, H.-K.; Park, C.-B. Immunomodulatory Effects of Curcuma Longa L. and Carthamus Tinctorius L. on RAW 264.7 Macrophages and Cyclophosphamide-Induced Immunosuppression C57BL/6 Mouse Models. J. Funct. Foods 2022, 91, 105000. [Google Scholar] [CrossRef]
| Activity | Study type | Subjects/Methods | Main findings | References |
|---|---|---|---|---|
| Anti-oxidant | In vitro | DPPH scavenging method | Curcumin exhibited more potency in scavenging of superoxide free radicals followed by demethoxycurcumin and bisdemethoxycurcumin. | [181,182] |
| In vitro | Styrene oxidation method | Curcumin showed phenolic chain-breaking antioxidant activity | [21] | |
| In vitro | RAW264.7 cells | Curcumin demonstrated resistance to oxidising agents by activating the Nrf2-Keap1 pathway and boosting the activity of antioxidant enzymes. | [183] | |
|
In vitro, In vivo & In silico |
DPPH scavenging method Radiation-induced peroxidation of lipid in liver microsomes of rats DFT studies |
Curcumin inhibited lipid peroxidation by 82% and dimethoxy curcumin by 24%. In curcumin, the hydrogen of -OH is more labile for separation than the hydrogen of -CH(2). |
[184] | |
| In vitro | Laser flash photolysis and pulse radiolysis | The donation of H-atom by curcumin is the preferred antioxidant mechanism over electron donation to free radicals. | [95] | |
| In vitro | DPPH scavenging method ABTS radical scavenging activity DMPD radical scavenging activity Total antioxidant activity |
Curcumin exhibited free radical scavenging activity against DPPH, ABTS, DMPD, superoxide anion free radical, and H2O2, as well as for ferrous (Fe2+) ion chelation and ferric ion (Fe3+) reduction. |
[94] | |
| In vitro | Phosphomolybdenum peroxidation method Linoleic acid peroxidation methods |
Curcumin showed maximum anti-oxidant activity followed by demethoxycurcumin and then bisdemethoxycurcumin | [185] | |
| In vitro | DPPH scavenging method | Curcumin nanosuspension samples exhibited similar anti-oxidant activity to simple curcumin mixture. | [186] | |
| In vivo | Fumonisin-induced oxidative stress in birds | Compared to curcumin, nanocurcumin (10 mg/kg) exhibits stronger antioxidant effects, as demonstrated by the reduction of thiobarbituric acid reactive substance (TBARS), ALT, AST, and ROS levels, as well as an increase in SOD and CAT concentrations. | [187] | |
| In vivo | Stress-induced oxidative in hens | Curcumin supplementation produces reversal of heat-induced increase in lipid peroxidation, and decrease in antioxidant profile. | [188] | |
| In vivo | Ochratoxin A-induced hepatotoxicity in rats | Curcumin restored the ochratoxin A-induced reduction in SOD, CAT, and GPx levels in liver tissues. | [189] | |
| Anti-inflammatory |
In vitro& In vivo |
Arachidonic acid metabolism 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced epidermal inflammation and tumor progression in mice |
Addition of curcumin (5-10 microM) to epidermal microsomes produces inhibition of arachidonic acid metabolism into PGE2, PGF2α, and PGD2. Topically applied curcumin results in inhibition of the activity of LOX and COX in epidermal inflammation. |
[190] |
| In vivo | Carrageenan-induced oedema in rats, mouse, and cats. | Curcumin is less ulcerogenic than phenylbutazone and exerts anti-inflammatory activity that is comparable to that of phenylbutazone. It also impeded the increased levels of SGOT and SGPT that were induced by inflammation. | [191] | |
| In vivo | Patients with metabolic syndrome | In patients with metabolic syndrome, the administration of curcumin (1 g/day) once daily results in a substantial decrease in serum levels of inflammatory cytokines. | [192] | |
|
In vitro& In vivo |
BEAS-2B cells Ovalbumin (OVA) to induce chronic asthma in mice |
In vitro and in vivo models, curcumin suppresses PPAR activation, inhibits NF-κB p65 translocation, and improves the increased expression of MCP-1 and MUC5AC induced by OVA and IL-4. | [193] | |
| In vivo | Chronic unpredictable mild stress-induced inflammation in rats | Curcumin demonstrated an antidepressant effect by inhibiting the activation of NF-κB and reducing the expression of pro-inflammatory cytokines. | [160] | |
| In vitro | BV2 cells | Curcumin suppresses inflammation induced by LPS by controlling microglia polarization (M1/M2), balancing TREM2/TLR4, and inhibiting NF-κB activity. | [69] | |
|
In vitro& In vivo |
Beas-2B cells cigarette smoke (CS)-induced COPD in rats |
Curcumin prevents cigarette smoke-induced inflammation in both vivo and in vitro, possibly by regulating the PPARγ/NF-κB signaling pathway. | [194] | |
| In vivo | Ulcerative colitis patients | Combining drug therapy with curcumin supplementation significantly reduced serum high-sensitivity CRP and ESR levels in ulcerative colitis patients. | [73] | |
| In vitro | BV-2 microglia | Curcumin reduces LPS-induced NO and pro-inflammatory cytokine production in microglial cells. | [195] | |
| In vitro | HeLa and RAW264.7 cells | Curcumin’s oxidative intermediates blocked IKKβ, an activating kinase upstream of NF-κB. | [196] | |
| In vivo | Patients of knee osteoarthritis | Curcuminoids produced significant anti-inflammatory effect. | [197] | |
| In vivo | Patients with solid tumor | Curcuminoid preparation (180 mg/day) produces significant reduction in TNFα, IL-6, substance P, hs-CRP, CGRP and TGF-β as compared to placebo control. | [198] | |
| In vivo | Spinal cord injury-induced inflammation in rats | Curcumin inhibited the formation of glial scars by preventing the production of MIP1α, IL-2, and CCL5 and by reducing NF-κB activation. | [199] | |
| Antidiabetic | In vivo | Streptozotocin (STZ) model of diabetes in rats | The microstructural alterations of pancreatic tissue were restored, and plasma glucose, insulin, and C-peptide levels were significantly reduced in diabetic rats after 40 days of treatment with a novel curcumin derivative (NCD). | [200] |
| In vivo | STZ model of diabetes in rats | Curcumin restored the levels of TBARS and GSH in diabetic rats, thereby normalizing blood glucose and hepatic oxidative stress. Additionally, it upregulates the expression of the IGF-1, Bcl2, SOD, and GST genes in hepatic tissues. | [201] | |
|
In vitro & In Silico |
α-Glucosidase and α-Amylase Inhibition methods | Curcumin-based benzaldehyde derivatives (L8, L11, and L13) reduce the glycemic index and inhibit the primary pathways that generate reactive oxygen species (ROS). | [202] | |
| In vitro | Streptozotocin-Nicotinamide (STZ-NA) model in rats | In comparison to the diabetic control, turmeric extracts (bioenhanced turmeric extract, BTE; regular turmeric extract, RTE) resulted in a decrease in blood glucose and an increase in oral glucose tolerance. Additionally, it demonstrated improvements in pancreatic β cell function and insulin sensitivity, as well as a decrease in insulin resistance. | [203] | |
| In vivo | High fat diet and low dose STZ model of diabetes in rats | Curcumin (at a higher dose) resulted in a substantial decrease in the levels of fasting blood glucose, total cholesterol, TGs, LDL-C, HDL-C, ALT, and AST, as well as liver coefficient and MDA, and BCL2-associated X expression in rats with type 2 diabetes mellitus. | [204] | |
| In vivo | Alloxan-induced diabetes in rats | Turmeric, either alone or in combination with Ajwa date seed and black pepper, produced antihyperlipidemic and weight-stabilizing effects in alloxan-induced diabetic mice. | [205] | |
| Antimicrobial | In vitro | B. subtilis | The formation of the cytokinetic Z-ring in B. subtilis was significantly inhibited by curcumin. Additionally, impeded the assembly of FtsZ protofilaments and enhanced the GTPase activity of FtsZ. | [206] |
|
In vitro& In vivo |
Clinical isolates of H. pylori H. pylori infection in mice |
Curcumin showed promising anti H. pylori action against clinical isolates, with MIC ranging between 5 and 50 μg/ml. It exhibited high effectiveness in eradication of H. pylori from infected mice as well as in restoration of H. pylori-induced gastric damage. | [207] | |
| In vitro | HSV-1 in cell culture | Curcumin and its novel compounds exhibit considerable antiviral activity against HSV-1 in cell culture. | [208] | |
| In vitro | Broth microdilution method Checkerboard dilution test, and Time-kill assay |
The combination of oxacillin, ampicillin, ciprofloxacin, and norfloxacin with curcumin demonstrated synergistic activity against MRSA. | [52,209] | |
| In vitro | Human neutrophil peptide-1 (HNP-1) | Curcumin I demonstrated time and dose-dependent action against S. aureus and E. coli at concentrations as low as 25 μM, killing 50% of bacteria after 2 hours of incubation. The damage to the cell membrane may have contributed to the broad-spectrum antibacterial action. | [210] | |
| In vitro |
S. aureus E. coli |
Nanocurcumin cream demonstrated superior antibacterial efficacy against S. aureus and E. coli, with a broader zone of inhibition than curcumin cream. | [211] | |
| Antitumor and anticancer | In vitro | Esophageal adenocarcinoma (EAC) cells | Nanocurcumin therapy with T cells on EAC cells (OE19 and OE33) demonstrated potential by boosting T cell cytotoxicity. This could be because curcumin increases EAC sensitivity to T cells’ cytotoxic actions. | [212] |
| In vitro | HT-29 colorectal adenocarcinoma cells | Curcumin influences the metabolomics of probiotics in intestinal flora, with a particular emphasis on Lactobacillus plantarum. This, in turn, induces apoptosis, which may impact their anticancer properties. | [213] | |
| In vitro | Oral carcinoma CAL-27 cells | Curcumin therapy reduces cell viability by inducing apoptosis and down-regulating Notch-1 and NF-κB. | [214] | |
| In vitro | Breast cancer stem cells | Curcumin induces apoptosis and suppresses the proliferation of breast cancer stem cells (BCSCs) irrespective of the expression of hormone receptors. | [215] | |
| In vitro | HT-29 cells | Curcumin (10–80 mol/L) inhibits the proliferation of HT-29 cells and promotes apoptosis. In addition, it promotes the expression of Bax and Bad while simultaneously suppressing the expression of Bcl-2, Bcl-xL, and survivin. | [216] | |
| In vitro | HT-29 cells | Curcumin induces DNA fragmentation, chromatin condensation, nuclear shrinkage, and increased cellular death in HT-29 cells via producing ROS in a dose- and duration-dependent manner. | [217] | |
| Antimalarial |
In vitro & In vivo |
Plasmodium falciparum culture Plasmodium berghei-infected mice |
The growth of chloroquine-resistant Plasmodium falciparum in culture medium is suppressed in a dose-dependent manner by curcumin, with an IC50 of approximately 5 μM. Oral curcumin administration produced a reduction in blood parasitemia in Plasmodium berghei-infected mice. |
[218] |
| In vitro | P. falciparum culture | Curcumin treatment damages both mitochondrial and nuclear DNA, most likely due to an increase in intracellular ROS. It also inhibits PfGCN5 HAT activity by reducing histone H3 acetylation at K9 and K14. | [219] | |
|
In vitro & In silico |
P. falciparum culture Molecular docking by using homology modeling by SWISS-MODEL server |
Curcumin produces dose-dependent morphological changes and suppresses parasitic growth, as evidenced by changes in microtubule morphology compared to untreated. Both the diketo and enol forms of curcumin showed more than 250 binding positions, mostly at the alpha and beta subunit interfaces, which overlap with colchicine. |
[220] | |
| In vivo | Plasmodium berghei NK65-infected mice | Curcumin treatment (i.p.) inhibits the GSK3β, resulting in a dose-dependent reduction of parasitemia and levels of pro- and anti-inflammatory cytokines in the aftermath of P. berghei infection. | [221] | |
| In vitro & In vivo |
RAW 264.7 cell line Peter’s 4-day suppressive protocol in mice model |
Curcumin-loaded PLGA nanoparticles were more cytotoxic than free curcumin to the RAW 264.7 cell line. PLGA-encapsulated curcumin (5 and 10 mg/kg) effectively suppresses parasitic growth (56.8%) compared to free curcumin (40.5%), without causing significant alterations in serum markers of hematologic and liver toxicity. |
[222] | |
| Anti-obesity | In vivo | Animal model of obesity | The ethanolic extract of curcumin enhances lipid breakdown and β-oxidation by increasing the expression of lipases, including adipose triglyceride lipase, hormone-sensitive lipase, adiponectin, and AMP-activated protein kinase. | [223] |
| In vitro | Abdominal subcutaneous adipose tissue (ASAT) explants, and lLPS-induced-mononuclear cells (iMC) | The hexane extract of Curcuma longa, which contains a variety of curcuminoids, exhibits anti-obesity, significant inhibitory activity against lipase, α-amylase, and α-glucosidase. | [224] | |
| In vivo | High fat diet-induced obesity in rats | Curcumin demonstrated antitoxic, antioxidant, cytoprotective, and anti-obesity effects by reversing the effects of a high-fat diet on glucose, TAGs, and insulin, as well as DNA fragmentation, MPO, GSH, and SOD in hepatic tissue, and the expression of TLR4, IL-6, and TNF-α. | [225] | |
|
In vitro& In vivo |
3T3-L1 adipocytes High-fat diet (HFD)-induced obesity in mice |
The ethanolic extract of Curcuma longa (CLE) inhibited lipid accumulation and restored differentiation-induced alterations in adipogenesis and lipolysis-related proteins in 3T3-L1 cells by restoring AMPK phosphorylation. Furthermore, CLE reduced HFD-induced increases in body weight, AST, ALT, cholesterol, LDL, ACC, PPAR-g, SREBP1, FABP4, FAS, adiponectin, and leptin, as well as activation of AMPK. | [226] | |
|
In vitro& In vivo |
3T3-L1 adipocytes High-fat diet (HFD)-induced obesity in mice |
Curcumin upregulates the EIF2 and mTOR signaling pathways, thereby suppressing the LPS-induced increase in IL-6 in 3T3-L1 adipocytes. Furthermore, the administration of curcumin in HFD-induced obese mice led to the detection of metabolites such as tetrahydrocurcumin (THC) and curcumin-O-glucuronide (COG). | [227] | |
| Neuroprotective | In vivo | CUMS-induced depression in rats | The administration of chronic curcumin (40 mg/kg, i.p.) results in the suppression of neuronal apoptosis within neurons of the ventromedial prefrontal cortex (vmPFC), as well as the reduction of depression-like behaviors and the expression of interleukin-1β (IL-1β). | [228] |
| In vivo | FST and TST model of depression in mice | Curcumin-loaded thermos-sensitive hydrogel reduces immobility duration in FST and TST in mice and enhances neurotransmitters such as NE, DA, 5-HT, and their metabolites in the hippocampus and striatum. | [229] | |
| In vitro | Primary hippocampal neurons | Curcumin ameliorated the cellular oxidative stress in cultured primary hippocampal neurons of rats, thereby inhibiting the Aβ-mediated intracellular toxicity. | [230] | |
| In vivo | MCAO-induced ischemic brain injury in rats | Curcumin exhibited neuroprotective effects by inhibiting the intracellular transcription of NAD(P)H: quinone oxidoreductase1 (NQO1) and Akt phosphorylation, which in turn ensued in an increase in the binding of NRF2 with ARE. | [231] | |
| In vivo | 6-OHDA-induced Parkinson’s disease in rat | Curcumin has been observed to protect neurons from 6-OHDA-induced injury, as evidenced by improved memory function, which is achieved by reducing neuronal oxidative stress and increasing DA and ACh levels in the substantia nigra of rats. Additionally, subsequent to curcumin administration, there was a decrease in intercalatum heat shock protein 70 (HSP70) and an upsurge in the expression of basic fibroblast growth factor (bFGF), nerve growth factor (NGF), and receptor tyrosine kinase A (TrkA). | [232] | |
| In vivo | APPsw transgenic mice | Curcumin at a lower dose stimulated microglial migration to and phagocytosis of amyloid plaques, decreased miR-155-mediated neurodegenerative phenotype, and reduced amyloid stress in mouse brains. | [233] | |
| In vivo | 6-OHDA-induced Parkinson’s disease in rat | Curcumin reduced 6-OHDA-induced hippocampus damage by raising the expression of BDNF, TrkB, and PI3K, as well as elevating neurotransmitters like DA and NE in hippocampal neurons. | [234] | |
| In vivo | Traumatic brain injury in mice | Curcumin protects against TBI-induced secondary brain injury, as evidenced by reduced water content, reactive oxygen species (ROS), neurological impairment score, and cell death. This protection is accomplished by increasing Bcl-2 levels and translocating Nrf2, which prevents a decrease in antioxidant enzymes by increasing the expression of heme oxygenase 1 (HO1) and NAD(P)H: quinone oxidoreductase 1 (NQO1). | [235] | |
| Immunomodulatory | In vitro | Human B lymphocyte cell lines and HepG2 cell line | Curcumin inhibits the translocation of p65 into the nucleus, thereby inhibiting the activity of B lymphocytes, by diminishing the DNA binding to the promotor region of the B lymphocyte stimulator, thereby suppressing NF-κB signaling. |
[236] |
| In vivo | Patients with osteoarthritis | Osteoarthritis patients experience a decrease in the frequency of Visual Analog Score following administration of curcumin (80 mg). | [237] | |
| In vitro | RAW-264.7 cells of mouse | Curcumin suppresses PHA-induced T-cell growth, production of IL-2, NO generation, and LPS-induced NF-κB, while increasing NK cell cytotoxicity. | [238] | |
| In vivo | Balb/c mice | Administration of curcumin leads to elevated white blood cell count, circulating antibodies targeting sheep red blood cells, plaque-forming cells (PFC) in the spleen, and increased bone marrow cellularity in mice. | [239] | |
|
In vitro& In vivo |
RAW 264.7 macrophages CPA-induced immunosuppression in mice |
Curcumin treatment produces inhibition of NO production, reduced expression of iNOS and COX-2 by inhibiting ERK 1/2 and p38 activation in RAW 264.7 macrophages. Additionally, curcumin produces reversal of CPA-induced changes in body weight, immunoglobulins, and NK cell activity in mice. | [240] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





