Submitted:
18 September 2024
Posted:
20 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Ocular Administration Routes
3. Advancements in Ocular Drug Delivery Systems
4. Liquid Crystals for Drug Delivery
5. Classification of Lyotropic Liquid Crystals
5.1. Bulk Forming Liquid Crystals for Ocular Drug Delivery
5.2. Liquid Crystal-Based Drug Nanoparticles (LCNP) for Ocular Drug Delivery
6. Beyond Traditional Lyotropic Techniques
6.1. Predictive Modeling and Molecular Ordering
6.2. Enhanced Biosensing Capabilities
6.3. Automated and Real-Time Analysis
6.4. Designing Light-Responsive Systems
6.5. Exploring Nonlinear Optical Properties
7. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Redd, T.K.; Al-Khaled, T.; Chan, R.M.P.; Campbell, J.P. Technology and Innovation in Global Ophthalmology: The Past, the Potential, and a Path Forward. Int. Ophthalmol. Clin. 2023, 63, 25–32. [CrossRef]
- Suri, R.; Beg, S.; Kohli, K. Target strategies for drug delivery bypassing ocular barriers. J. Drug Deliv. Sci. Technol. 2020, 55, 101389. [CrossRef]
- Acharya, A.; Goudanavar, P.; Joshi, V. Development and characterization of prolonged release Timolol maleate cubosomal gel for ocular drug delivery. Adv. Pharm. J. 2019, 4, 1–14. [CrossRef]
- Chen, Y.-Z.; Chen, Z.-Y.; Tang, Y.-J.; Tsai, C.-H.; Chuang, Y.-L.; Hsieh, E.-H.; Tucker, L.; Lin, I.-C.; Tseng, C.-L. Development of Lutein-Containing Eye Drops for the Treatment of Dry Eye Syndrome. Pharmaceutics 2021, 13, 1801. [CrossRef]
- Wang, F.; Song, Y.; Huang, J.; Wu, B.; Wang, Y.; Pang, Y.; Zhang, W.; Zhu, Z.; Ma, F.; Wang, X.; et al. Lollipop-Inspired Multilayered Drug Delivery Hydrogel for Dual Effective, Long-Term, and NIR-Defined Glaucoma Treatment. Macromol. Biosci. 2021, 21, 2100202. [CrossRef]
- Liu, Y.-C.; Lin, M.T.-Y.; Ng, A.H.C.; Wong, T.T.; Mehta, J.S. Nanotechnology for the Treatment of Allergic Conjunctival Diseases. Pharmaceuticals 2020, 13, 351. [CrossRef]
- Haghjou, N.; Soheilian, M.; Abdekhodaie, M.J. Sustained Release Intraocular Drug Delivery Devices for Treatment of Uveitis. Journal of Ophthalmic & Vision Research 2011, 6, 317–329.
- Korneeva, A.V.; Strakhova, S.S.; Loskutov, I.A. Clinical features of dry eye syndrome and evaluation of the effectiveness of artificial tear therapy in patients with anxiety and depression after cataract and keratorefractive surgery. Med Counc. 2023, 220–228. [CrossRef]
- Wong, C.W.; Wong, T.T. Posterior segment drug delivery for the treatment of exudative age-related macular degeneration and diabetic macular oedema. Br. J. Ophthalmol. 2019, 103, 1356–1360. [CrossRef]
- Silva, M.; Peng, T.; Zhao, X.; Li, S.; Farhan, M.; Zheng, W. Recent trends in drug-delivery systems for the treatment of diabetic retinopathy and associated fibrosis. Adv. Drug Deliv. Rev. 2021, 173, 439–460. [CrossRef]
- Wang, F.; He, S.; Chen, B. Retinoic acid-loaded alginate microspheres as a slow release drug delivery carrier for intravitreal treatment. Biomed. Pharmacother. 2018, 97, 722–728. [CrossRef]
- Wei, D.; Pu, N.; Li, S.-Y.; Wang, Y.-G.; Tao, Y. Application of iontophoresis in ophthalmic practice: an innovative strategy to deliver drugs into the eye. Drug Deliv. 2023, 30, 2165736. [CrossRef]
- Yamada N, Olsen TW. Routes for Drug Delivery to the Retina: Topical, Transscleral, Suprachoroidal and Intravitreal Gas Phase Delivery. Retinal Pharmacotherapeutics. 55. S.Karger AG; 2015:0.
- Waheed, A.; Aqil, M. Lyotropic liquid crystalline nanoparticles: Scaffolds for delivery of myriad therapeutics and diagnostics. J. Mol. Liq. 2021, 338, 116919. [CrossRef]
- Phan, S.; Fong, W.-K.; Kirby, N.; Hanley, T.; Boyd, B.J. Evaluating the link between self-assembled mesophase structure and drug release. Int. J. Pharm. 2011, 421, 176–182. [CrossRef]
- Wu W, Cao W, Cai Y, Zhang M, Chen T, Zhang N et al. In Situ Liquid Crystal Gel as a New Ophthalmic Drug Delivery System for Pilocarpine Nitrate: Improving Preocular Retention and Ocular Bioavailability. 2021.
- Le, T.C.; Tran, N. Using Machine Learning To Predict the Self-Assembled Nanostructures of Monoolein and Phytantriol as a Function of Temperature and Fatty Acid Additives for Effective Lipid-Based Delivery Systems. ACS Appl. Nano Mater. 2019, 2, 1637–1647. [CrossRef]
- Inokuchi, T.; Okamoto, R.; Arai, N. Predicting molecular ordering in a binary liquid crystal using machine learning. Liq. Cryst. 2020, 47, 438–448. [CrossRef]
- Zhang, Y.; Xu, S.; Zhang, R.; Deng, Z.; Liu, Y.; Tian, J.; Yu, L.; Hu, Q.; Ye, Q. Automated Calculation of Liquid Crystal Sensing Images Based on Deep Learning. Anal. Chem. 2022, 94, 12781–12787. [CrossRef]
- Cao, Y.; Yu, H.; Abbott, N.L.; Zavala, V.M. Machine Learning Algorithms for Liquid Crystal-Based Sensors. ACS Sensors 2018, 3, 2237–2245. [CrossRef]
- Wang, R.; Gao, Y.; Liu, A.; Zhai, G. A review of nanocarrier-mediated drug delivery systems for posterior segment eye disease: challenges analysis and recent advances. J. Drug Target. 2021, 29, 687–702. [CrossRef]
- Wan, K.H.; Chen, L.J.; Young, A.L. Efficacy and Safety of Topical 0.05% Cyclosporine Eye Drops in the Treatment of Dry Eye Syndrome: A Systematic Review and Meta-analysis. Ocul. Surf. 2015, 13, 213–225. [CrossRef]
- Gupta, M.P.; Koenig, L.R.; Doubrovina, E.; Hasan, A.; Dahi, P.B.; O’reilly, R.J.; Koehne, G.; Orlin, A.; Chan, R.V.P.; D’amico, D.J.; et al. Ocular Outcomes after Treatment of Cytomegalovirus Retinitis Using Adoptive Immunotherapy with Cytomegalovirus-Specific Cytotoxic T Lymphocytes. Ophthalmol. Retin. 2021, 5, 838–849. [CrossRef]
- Kim, S.J. Intravitreal Injections and Endophthalmitis. Int. Ophthalmol. Clin. 2015, 55, 1–10. [CrossRef]
- Sen HN, Vitale S, Gangaputra SS, Nussenblatt RB, Liesegang TL, Levy-Clarke GA et al. Periocular corticosteroid injections in uveitis: effects and complications. Ophthalmology 2014; 121 (11): 2275-2286.
- Melega MV, Alves M, Lira RPC, da Silva IC, Ferreira BG, Filho HLGA et al. Safety and efficacy of intracameral moxifloxacin for prevention of post-cataract endophthalmitis: Randomized controlled clinical trial. J Cataract Refract Surg 2019; 45 (3).
- Lee, T.W.-Y.; Robinson, J.R. Drug Delivery to the Posterior Segment of the Eye: Some Insights on the Penetration Pathways after Subconjunctival Injection. J. Ocul. Pharmacol. Ther. 2001, 17, 565–572. [CrossRef]
- Alhassan, M.B.; Kyari, F.; Ejere, H.O. Peribulbar versus retrobulbar anaesthesia for cataract surgery. Cochrane Database Syst. Rev. 2015, 2015, CD004083. [CrossRef]
- Lai MM, Lai JC, Lee W-H, Huang JJ, Patel S, Ying HS et al. Comparison of Retrobulbar and Sub–Tenon's Capsule Injection of Local Anesthetic in Vitreoretinal Surgery. Ophthalmology 2005; 112 (4): 574-579.
- Gote V, Sikder S, Sicotte J, Pal D. Ocular drug delivery: present innovations and future challenges. Journal of Pharmacology and Experimental Therapeutics 2019; 370 (3): 602-624.
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for drug delivery to the anterior segment of the eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [CrossRef]
- Cholkar, K.; Patel, A.; Vadlapudi, A.D.; Mitra, A.K. Novel Nanomicellar Formulation Approaches for Anterior and Posterior Segment Ocular Drug Delivery. Recent Patents Nanomedicinee 2012, 2, 82–95. [CrossRef]
- Nagarwal, R.C.; Kant, S.; Singh, P.N.; Maiti, P.; Pandit, J.K. Polymeric nanoparticulate system: A potential approach for ocular drug delivery. J. Control. Release 2009, 136, 2–13. [CrossRef]
- Gan, L.; Han, S.; Shen, J.; Zhu, J.; Zhu, C.; Zhang, X.; Gan, Y. Self-assembled liquid crystalline nanoparticles as a novel ophthalmic delivery system for dexamethasone: Improving preocular retention and ocular bioavailability. Int. J. Pharm. 2010, 396, 179–187. [CrossRef]
- Ameeduzzafar; Ali, J.; Bhatnagar, A.; Kumar, N.; Ali, A. Chitosan nanoparticles amplify the ocular hypotensive effect of cateolol in rabbits. Int. J. Biol. Macromol. 2014, 65, 479–491. [CrossRef]
- Swetledge, S.; Jung, J.P.; Carter, R.; Sabliov, C. Distribution of polymeric nanoparticles in the eye: implications in ocular disease therapy. J. Nanobiotechnology 2021, 19, 1–19. [CrossRef]
- Balguri S, Adelli GR, Majumdar S. Topical ophthalmic lipid nanoparticle formulations (SLN, NLC) of indomethacin for delivery to the posterior segment ocular tissues. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV 2016; 109: 224-235.
- Lee, J.; Goh, U.; Lee, H.-J.; Kim, J.; Jeong, M.; Park, J.-H. Effective Retinal Penetration of Lipophilic and Lipid-Conjugated Hydrophilic Agents Delivered by Engineered Liposomes. Mol. Pharm. 2017, 14, 423–430. [CrossRef]
- Preeti, R.; Sambhakar, S.; Malik, R.; Bhatia, S.; Al Harrasi, A.; Rani, C.; Saharan, R.; Kumar, S.; Geeta; Sehrawat, R. Nanoemulsion: An Emerging Novel Technology for Improving the Bioavailability of Drugs. Scientifica 2023, 2023, 1–25. [CrossRef]
- Vaishya R, Khurana V, Patel S, Mitra AK. Controlled ocular drug delivery with nanomicelles. Wiley interdisciplinary reviews Nanomedicine and nanobiotechnology 2014; 6 5: 422-437.
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Ezzati Nazhad Dolatabadi, J.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. [CrossRef]
- Radwan, S.E.-S.; El-Kamel, A.H.; I Zaki, E.; Burgalassi, S.; Zucchetti, E.; El-Moslemany, R.M. Hyaluronic-Coated Albumin Nanoparticles for the Non-Invasive Delivery of Apatinib in Diabetic Retinopathy. Int. J. Nanomed. 2021, 16, 4481–4494. [CrossRef]
- Carvalho, I.; Marques, C.; Oliveira, R.; Coelho, P.; Costa, P.; Ferreira, D. Sustained drug release by contact lenses for glaucoma treatment—A review. J. Control. Release 2015, 202, 76–82. [CrossRef]
- Ervin, A.-M.; Law, A.; Pucker, A.D. Punctal occlusion for dry eye syndrome. Cochrane Database Syst. Rev. 2017, 2017, CD006775. [CrossRef]
- Jaffe, G.J.; Ben-Nun, J.; Guo, H.; Dunn, J.P.; Ashton, P. Fluocinolone acetonide sustained drug delivery device to treat severe uveitis. Ophthalmology 2000, 107, 2024–2033. [CrossRef]
- Singh, R.R.T.; Tekko, I.; McAvoy, K.; McMILLIAN, H.; Jones, D.; Donnelly, R. Minimally invasive microneedles for ocular drug delivery. Expert Opin. Drug Deliv. 2017, 14, 525–537. [CrossRef]
- Shah, P.; Thakkar, V.; Anjana, V.; Christian, J.; Trivedi, R.; Patel, K.; Gohel, M.; Gandhi, T. Exploring of Taguchi Design in the Optimization of Brinzolamide and Timolol Maleate Ophthalmic in-situ Gel Used in Treatment of Glaucoma. Curr. Drug Ther. 2020, 15, 524–542. [CrossRef]
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Castilho, R.O.; Yoshida, M.I.; Cardoso, V.N.; Fernandes, S.O.; Cronemberger, S.; Nogueira, J.C.; et al. Chitosan/hydroxyethyl cellulose inserts for sustained-release of dorzolamide for glaucoma treatment: In vitro and in vivo evaluation. Int. J. Pharm. 2019, 570, 118662. [CrossRef]
- Liu, D.; Wu, Q.; Chen, W.; Lin, H.; Zhu, Y.; Liu, Y.; Liang, H.; Zhu, F. A novel FK506 loaded nanomicelles consisting of amino-terminated poly(ethylene glycol)-block-poly(D,L)-lactic acid and hydroxypropyl methylcellulose for ocular drug delivery. Int. J. Pharm. 2019, 562, 1–10. [CrossRef]
- Imperiale, J.C.; Acosta, G.B.; Sosnik, A. Polymer-based carriers for ophthalmic drug delivery. J. Control. Release 2018, 285, 106–141. [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Gupta, S.; Boddu, S.H.S.; Sreeharsha, N.; Joseph, A.; Shinu, P.; Morsy, M.A. Lipid Nanoparticles as a Promising Drug Delivery Carrier for Topical Ocular Therapy—An Overview on Recent Advances. Pharmaceutics 2022, 14, 533. [CrossRef]
- Huang, J.; Peng, T.; Li, Y.; Zhan, Z.; Zeng, Y.; Huang, Y.; Pan, X.; Wu, C.-Y.; Wu, C. Ocular Cubosome Drug Delivery System for Timolol Maleate: Preparation, Characterization, Cytotoxicity, Ex Vivo, and In Vivo Evaluation. Aaps Pharmscitech 2017, 18, 2919–2926. [CrossRef]
- Marano, R.J.; Toth, I.; Wimmer, N.; Brankov, M.; Rakoczy, P.E. Dendrimer delivery of an anti-VEGF oligonucleotide into the eye: a long-term study into inhibition of laser-induced CNV, distribution, uptake and toxicity. Gene Ther. 2005, 12, 1544–1550. [CrossRef]
- Marcano, D.C.; Shin, C.S.; Lee, B.; Isenhart, L.C.; Liu, X.; Li, F.; Jester, J.V.; Pflugfelder, S.C.; Simpson, J.; Acharya, G. Synergistic Cysteamine Delivery Nanowafer as an Efficacious Treatment Modality for Corneal Cystinosis. Mol. Pharm. 2016, 13, 3468–3477. [CrossRef]
- Rajabalaya, R.; Musa, M.N.; Kifli, N.; David, S.R. Oral and transdermal drug delivery systems: role of lipid-based lyotropic liquid crystals. Drug Des. Dev. Ther. 2017, 11, 393–406. [CrossRef]
- Marriott, M.; Lupi, L.; Kumar, A.; Molinero, V. Following the nucleation pathway from disordered liquid to gyroid mesophase. J. Chem. Phys. 2019, 150, 164902. [CrossRef]
- Rapalli, V.K.; Waghule, T.; Hans, N.; Mahmood, A.; Gorantla, S.; Dubey, S.K.; Singhvi, G. Insights of lyotropic liquid crystals in topical drug delivery for targeting various skin disorders. J. Mol. Liq. 2020, 315, 113771. [CrossRef]
- Tschierske, C.; Nürnberger, C.; Ebert, H.; Glettner, B.; Prehm, M.; Liu, F.; Zeng, X.-B.; Ungar, G. Complex tiling patterns in liquid crystals. Interface Focus 2011, 2, 669–680. [CrossRef]
- Silvestrini, A.V.P.; Caron, A.L.; Viegas, J.; Praça, F.G.; Bentley, M.V.L.B. Advances in lyotropic liquid crystal systems for skin drug delivery. Expert Opin. Drug Deliv. 2020, 17, 1781–1805. [CrossRef]
- Liu, K.; Chen, D.; Marcozzi, A.; Zheng, L.; Su, J.; Pesce, D.; Zajaczkowski, W.; Kolbe, A.; Pisula, W.; Müllen, K.; et al. Thermotropic liquid crystals from biomacromolecules. Proc. Natl. Acad. Sci. 2014, 111, 18596–18600. [CrossRef]
- Shoaib, A.; Mangla, B.; Javed, S.; Sultan, M.H.; Alqahtani, S.S.; Shakeel, F. Vicissitudes of liquid crystals for solubility enhancement of poorly soluble drugs. J. Mol. Liq. 2021, 321, 114924. [CrossRef]
- Li, M.; Yu, X.; Zhu, L.; Jin, Y.; Wu, Z. Ocular lamellar crystalline gels for sustained release and enhanced permeation of resveratrol against corneal neovascularization. Drug Deliv. 2021, 28, 206–217. [CrossRef]
- Blanco-Fernández, G.; Blanco-Fernandez, B.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Lipidic lyotropic liquid crystals: Insights on biomedical applications. Adv. Colloid Interface Sci. 2023, 313, 102867. [CrossRef]
- Milak, S.; Chemelli, A.; Glatter, O.; Zimmer, A. Vancomycin ocular delivery systems based on glycerol monooleate reversed hexagonal and reversed cubic liquid crystalline phases. Eur. J. Pharm. Biopharm. 2019, 139, 279–290. [CrossRef]
- Libster, D.; Aserin, A.; Garti, N. Interactions of biomacromolecules with reverse hexagonal liquid crystals: Drug delivery and crystallization applications. J. Colloid Interface Sci. 2011, 356, 375–386. [CrossRef]
- Guo, C.; Wang, J.; Cao, F.; Lee, R.J.; Zhai, G. Lyotropic liquid crystal systems in drug delivery. Drug Discov. Today 2010, 15, 1032–1040. [CrossRef]
- Xingqi, W.; Yong, Z.; Xing, L.; Yang, W.; Jie, H.; Rongfeng, H.; Shuangying, G.; Xiaoqin, C. Cubic and hexagonal liquid crystal gels for ocular delivery with enhanced effect of pilocarpine nitrate on anti-glaucoma treatment. Drug Deliv. 2019, 26, 952–964. [CrossRef]
- Rodríguez-Abreu, C.; Shrestha, L.K.; Varade, D.; Aramaki, K.; Maestro, A.; Quintela, A.L.; Solans, C. Formation and Properties of Reverse Micellar Cubic Liquid Crystals and Derived Emulsions. Langmuir 2007, 23, 11007–11014. [CrossRef]
- Dieterich, S.; Sottmann, T.; Giesselmann, F. Gelation of Lyotropic Liquid-Crystal Phases—The Interplay between Liquid Crystalline Order and Physical Gel Formation. Langmuir 2019, 35, 16793–16802. [CrossRef]
- Alam, M.M.; Shrestha, L.K.; Aramaki, K. Glycerol effects on the formation and rheology of cubic phase and related gel emulsion. J. Colloid Interface Sci. 2009, 329, 366–371. [CrossRef]
- de Souza JF, Pontes KdS, Alves TFR, Amaral VA, Rebelo MdA, Hausen MdA et al. Spotlight on Biomimetic Systems Based on Lyotropic Liquid Crystal. Molecules : A Journal of Synthetic Chemistry and Natural Product Chemistry 2017; 22.
- Martiel, I.; Baumann, N.; Vallooran, J.J.; Bergfreund, J.; Sagalowicz, L.; Mezzenga, R. Oil and drug control the release rate from lyotropic liquid crystals. J. Control. Release 2015, 204, 78–84. [CrossRef]
- Gupta V. AN OVERVIEW ON CUBOSOMES AS REMARKABLE NANOCARRIER FOR DRUG DELIVERY. Malaysian Journal of Pharmaceutical Sciences 2023.
- Gagliardi, A.; Cosco, D.; Udongo, B.P.; Dini, L.; Viglietto, G.; Paolino, D. Design and Characterization of Glyceryl Monooleate-Nanostructures Containing Doxorubicin Hydrochloride. Pharmaceutics 2020, 12, 1017. [CrossRef]
- Chen, Y.; Lu, Y.; Zhong, Y.; Wang, Q.; Wu, W.; Gao, S. Ocular delivery of cyclosporine A based on glyceryl monooleate/poloxamer 407 liquid crystalline nanoparticles: preparation, characterization,in vitrocorneal penetration and ocular irritation. J. Drug Target. 2012, 20, 856–863. [CrossRef]
- Milak, S.; Chemelli, A.; Glatter, O.; Zimmer, A. Vancomycin Loaded Glycerol Monooleate Liquid Crystalline Phases Modified with Surfactants. Pharmaceutics 2020, 12, 521. [CrossRef]
- Wang X, Zhang Y, Huang J, Tian C, Xia M, Liu L et al. A novel phytantriol-based lyotropic liquid crystalline gel for efficient ophthalmic delivery of pilocarpine nitrate. AAPS PharmSciTech 2019; 20: 1-14.
- Wu, W.; Cao, W.; Chen, J.; Cai, Y.; Dong, B.; Chu, X. In Situ Liquid Crystal Gel as a Promising Strategy for Improving Ocular Administration of Dexamethasone: Preparation, Characterization, and Evaluation. Aaps Pharmscitech 2021, 23, 1–13. [CrossRef]
- Tarsitano, M.; Mancuso, A.; Cristiano, M.C.; Urbanek, K.; Torella, D.; Paolino, D.; Fresta, M. Perspective use of bio-adhesive liquid crystals as ophthalmic drug delivery systems. Sci. Rep. 2023, 13, 1–12. [CrossRef]
- Li, J.; Wu, L.; Wu, W.; Wang, B.; Wang, Z.; Xin, H.; Xu, Q. A potential carrier based on liquid crystal nanoparticles for ophthalmic delivery of pilocarpine nitrate. Int. J. Pharm. 2013, 455, 75–84. [CrossRef]
- Achouri, D.; Hornebecq, V.; Piccerelle, P.; Andrieu, V.; Sergent, M. Self-assembled liquid crystalline nanoparticles as an ophthalmic drug delivery system. Part I: influence of process parameters on their preparation studied by experimental design. Drug Dev. Ind. Pharm. 2015, 41, 109–115. [CrossRef]
- E Hartnett, T.; O’connor, A.J.; Ladewig, K. Cubosomes and other potential ocular drug delivery vehicles for macromolecular therapeutics. Expert Opin. Drug Deliv. 2015, 12, 1513–1526. [CrossRef]
- Liu, R.; Wang, S.; Fang, S.; Wang, J.; Chen, J.; Huang, X.; He, X.; Liu, C. Liquid Crystalline Nanoparticles as an Ophthalmic Delivery System for Tetrandrine: Development, Characterization, and In Vitro and In Vivo Evaluation. Nanoscale Res. Lett. 2016, 11, 1–12. [CrossRef]
- Verma, P.; Ahuja, M. Cubic liquid crystalline nanoparticles: optimization and evaluation for ocular delivery of tropicamide. Drug Deliv. 2016, 23, 3043–3054. [CrossRef]
- Ali, Z.; Sharma, P.K.; Warsi, M.H. Fabrication and Evaluation of Ketorolac Loaded Cubosome for Ocular Drug Delivery. J. Appl. Pharm. Sci. 2016, 6, 204–208. [CrossRef]
- Younes, N.F.; Abdel-Halim, S.A.; Elassasy, A.I. Corneal targeted Sertaconazole nitrate loaded cubosomes: Preparation, statistical optimization, in vitro characterization, ex vivo permeation and in vivo studies. Int. J. Pharm. 2018, 553, 386–397. [CrossRef]
- Silva RO, da Costa BL, da Silva FR, da Silva CN, De Paiva MB, Dourado LFN et al. Treatment for chemical burning using liquid crystalline nanoparticles as an ophthalmic delivery system for pirfenidone. Int J Pharm 2019; 568: 118466.
- Eldeeb, A.E.; Salah, S.; Ghorab, M. Formulation and evaluation of cubosomes drug delivery system for treatment of glaucoma: Ex-vivo permeation and in-vivo pharmacodynamic study. J. Drug Deliv. Sci. Technol. 2019, 52, 236–247. [CrossRef]
- El-Gendy, M.A.; Mansour, M.; El-Assal, M.I.; Ishak, R.A.; Mortada, N.D. Delineating penetration enhancer-enriched liquid crystalline nanostructures as novel platforms for improved ophthalmic delivery. Int. J. Pharm. 2020, 582, 119313. [CrossRef]
- Kaul S, Nagaich U, Verma N. Investigating nanostructured liquid crystalline particles as prospective ocular delivery vehicle for tobramycin sulfate: Ex vivo: and: in vivo: studies. J Adv Pharm Technol Res 2021; 12 (4): 356-361.
- Kaul, S.; Nagaich, U.; Verma, N. Preclinical assessment of nanostructured liquid crystalline particles for the management of bacterial keratitis: in vivo and pharmacokinetics study. Drug Deliv. Transl. Res. 2022, 12, 1719–1737. [CrossRef]
- Bessone, C.D.V.; Akhlaghi, S.P.; Tártara, L.I.; Quinteros, D.A.; Loh, W.; Allemandi, D.A. Latanoprost-loaded phytantriol cubosomes for the treatment of glaucoma. Eur. J. Pharm. Sci. 2021, 160, 105748. [CrossRef]
- Elfaky, M.A.; Sirwi, A.; Tolba, H.H.; Shaik, R.A.; Selmi, N.M.; Alattas, A.H.; Albreki, R.S.; Alshreef, N.M.; Gad, H.A. Development, Optimization, and Antifungal Assessment of Ocular Gel Loaded With Ketoconazole Cubic Liquid Crystalline Nanoparticles. J. Pharm. Sci. 2021, 110, 2210–2220. [CrossRef]
- Shoman, N.A.; Gebreel, R.M.; El-Nabarawi, M.A.; Attia, A. Optimization of hyaluronan-enriched cubosomes for bromfenac delivery enhancing corneal permeation: characterization, ex vivo, and in vivo evaluation. Drug Deliv. 2023, 30, 2162162. [CrossRef]
- Nasr, M.; Teiama, M.; Ismail, A.; Ebada, A.; Saber, S. In vitro and in vivo evaluation of cubosomal nanoparticles as an ocular delivery system for fluconazole in treatment of keratomycosis. Drug Deliv. Transl. Res. 2020, 10, 1841–1852. [CrossRef]
- El-Gendy, M.A.; Mansour, M.; El-Assal, M.I.A.; Ishak, R.A.H.; Mortada, N.D. Travoprost Liquid Nanocrystals: An Innovative Armamentarium for Effective Glaucoma Therapy. Pharmaceutics 2023, 15, 954. [CrossRef]
- Priya, S.; Srividya, G.; Chandra, N.S.; Singh, P.P.; Saha, R.N.; Sathe, P.; Nirmal, J.; Singhvi, G. Loteprednol etabonate loaded lyotropic liquid crystalline nanoparticles in-situ ophthalmic gel: Qbd driven optimization and in-vitro, ex-vivo evidence of sustained precorneal residence time. J. Drug Deliv. Sci. Technol. 2023, 89. [CrossRef]
- Malaekeh-Nikouei B, Vafaei F, Karimi M, Nosrati R, Kamali H. Preparation and in-vitro evaluation of fluorometholone cubosomes for ocular delivery. Nanomedicine Journal 2023; 10 (4).
- Sharadha, M.; Gupta, N.V.; Rahamathulla, M.; Ahmed, M.M.; Farhana, S.A.; Osmani, R.A.M.; Veeranna, B.; Koteshwara, K. Subconjunctival therapy by cubic liquid crystalline nanoparticles to deliver Triamcinolone acetonide for the management of diabetic Retinopathy: In vivo evidences. Int. J. Pharm. 2023, 646, 123443. [CrossRef]
- Omran, S.; Elnaggar, Y.S.; Abdallah, O.Y. Controlled release, chitosan-tethered luteolin phytocubosomes; Formulation optimization to in-vivo antiglaucoma and anti-inflammatory ocular evaluation. Int. J. Biol. Macromol. 2024, 254, 127930. [CrossRef]
- Iyer, B.A.N.; Vasantha, P.V. Development and Characterization of Moxifloxacin Hydrochloride Cubogel for Enhanced Ocular Drug Delivery. Int. J. Pharm. Investig. 2024, 14, 472–481. [CrossRef]
- Chakorkar, S.S.; Mulla, J.A.S.G. Cubosome-based Corticosteroidal Drug Delivery System for Sustained Ocular Delivery: A Pharmacokinetic Investigation. Indian J. Pharm. Educ. Res. 2024, 58, s502–s514. [CrossRef]
- Aher S, Nimase B. Cubosomes; An Approach to Sustain and Improve the Ocular Delivery for Glaucoma Treatment: Box Benhken Optimization, Formulation, In Vitro Characterization and Ex Vivo Permeation Study. Journal for Research in Applied Sciences and Biotechnology 2024; 3 (3): 209-222.
- Nemr, A.A.; Ahmed, S.; Adel, I.M. Limonene-enriched ultra-structural cubosomes to augment ocular delivery of a poorly water soluble anti-fungal drug: Fabrication, characterization, statistical optimization, in vivo corneal uptake and histopathological evaluation in rabbits. J. Drug Deliv. Sci. Technol. 2024, 98. [CrossRef]
- Bhageerathy, A.; Prasanth, V.V. Formulation and Evaluation of Moxifloxacin Hydrochloride Loaded Cubosomal Gel for Ocular Delivery. J. Drug Deliv. Ther. 2024, 14, 88–99. [CrossRef]
- Teba, H.E.; Khalil, I.A.; El Sorogy, H.M. Novel cubosome based system for ocular delivery of acetazolamide. Drug Deliv. 2021, 28, 2177–2186. [CrossRef]
- Han S, Shen J-q, Gan Y, Geng H-m, Zhang X-x, Zhu C-l et al. Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability. Acta Pharmacol Sin 2010; 31 (8): 990-998.
- Gaballa, S.A.; El Garhy, O.H.; Moharram, H.; Abdelkader, H. Preparation and Evaluation of Cubosomes/Cubosomal Gels for Ocular Delivery of Beclomethasone Dipropionate for Management of Uveitis. Pharm. Res. 2020, 37, 1–23. [CrossRef]
- Ding, Y.; Chow, S.H.; Chen, J.; Le Brun, A.P.; Wu, C.-M.; Duff, A.P.; Wang, Y.; Song, J.; Wang, J.-H.; Wong, V.H.; et al. Targeted delivery of LM22A-4 by cubosomes protects retinal ganglion cells in an experimental glaucoma model. Acta Biomater. 2021, 126, 433–444. [CrossRef]
- El-Emam, G.A.; Motawea, A.; El Hady, W.E.A.; Saber, S.; Mourad, A.A.; Ramadan, H.A.; El-Baz, A.M. Salutary influence of gemifloxacin mesylate nanocubosomes based-in situ ocular gel as a novel approach for the management of experimental keratitis induced by MRSA. J. Drug Deliv. Sci. Technol. 2023, 89. [CrossRef]
- Le, T.C.; Tran, N. Using Machine Learning To Predict the Self-Assembled Nanostructures of Monoolein and Phytantriol as a Function of Temperature and Fatty Acid Additives for Effective Lipid-Based Delivery Systems. ACS Appl. Nano Mater. 2019, 2, 1637–1647. [CrossRef]
- Inokuchi, T.; Okamoto, R.; Arai, N. Predicting molecular ordering in a binary liquid crystal using machine learning. Liq. Cryst. 2020, 47, 438–448. [CrossRef]
- Sigaki, H.Y.D.; de Souza, R.T.; Zola, R.S.; Ribeiro, H.V. Estimating physical properties from liquid crystal textures via machine learning and complexity-entropy methods. Phys. Rev. E 2019, 99, 013311. [CrossRef]
- Cao, Y.; Yu, H.; Abbott, N.L.; Zavala, V.M. Machine Learning Algorithms for Liquid Crystal-Based Sensors. ACS Sensors 2018, 3, 2237–2245. [CrossRef]
- Otón, E.; Otón, J.M.; Caño-García, M.; Escolano, J.M.; Quintana, X.; Geday, M.A. Rapid detection of pathogens using lyotropic liquid crystals. Opt. Express 2019, 27, 10098–10107. [CrossRef]
- Wang, Z.; Xu, T.; Noel, A.; Chen, Y.-C.; Liu, T. Applications of liquid crystals in biosensing. Soft Matter 2021, 17, 4675–4702. [CrossRef]
- Zhang, Y.; Xu, S.; Zhang, R.; Deng, Z.; Liu, Y.; Tian, J.; Yu, L.; Hu, Q.; Ye, Q. Automated Calculation of Liquid Crystal Sensing Images Based on Deep Learning. Anal. Chem. 2022, 94, 12781–12787. [CrossRef]
- Rodriguez-Palomo, A.; Lutz-Bueno, V.; Cao, X.; Kádár, R.; Andersson, M.; Liebi, M. In Situ Visualization of the Structural Evolution and Alignment of Lyotropic Liquid Crystals in Confined Flow. Small 2021, 17, 2006229. [CrossRef]
- Aleandri, S.; Speziale, C.; Mezzenga, R.; Landau, E.M. Design of Light-Triggered Lyotropic Liquid Crystal Mesophases and Their Application as Molecular Switches in “On Demand” Release. Langmuir 2015, 31, 6981–6987. [CrossRef]
- Klimusheva GV, Garboskiy Y, Bugaychuk S, Tolochko AS, Melnik DA, Tokmenko II et al. Nonlinear Optics of Electrochromic and Photosensitive Cells of Ionic Liquid Crystals and Mesomorphic Glasses. Mol Cryst Liq Cryst 2011; 541: 142/[380] - 151/[389].
- Deniset-Besseau A, De Sa Peixoto P, Mosser G, Schanne-Klein M-C. Nonlinear optical imaging of lyotropic cholesteric liquid crystals. Opt Express 2010; 18 2: 1113-1121.
- Lucchetti, L.; Fraccia, T.P.; Nava, G.; Turiv, T.; Ciciulla, F.; Bethge, L.; Klussmann, S.; Lavrentovich, O.D.; Bellini, T. Elasticity and Viscosity of DNA Liquid Crystals. ACS Macro Lett. 2020, 9, 1034–1039. [CrossRef]
- Lucchetti, L.; Fraccia, T.P.; Ciciulla, F.; Simoni, F.; Bellini, T. Giant optical nonlinearity in DNA lyotropic liquid crystals. Opt. Express 2017, 25, 25951–25959. [CrossRef]
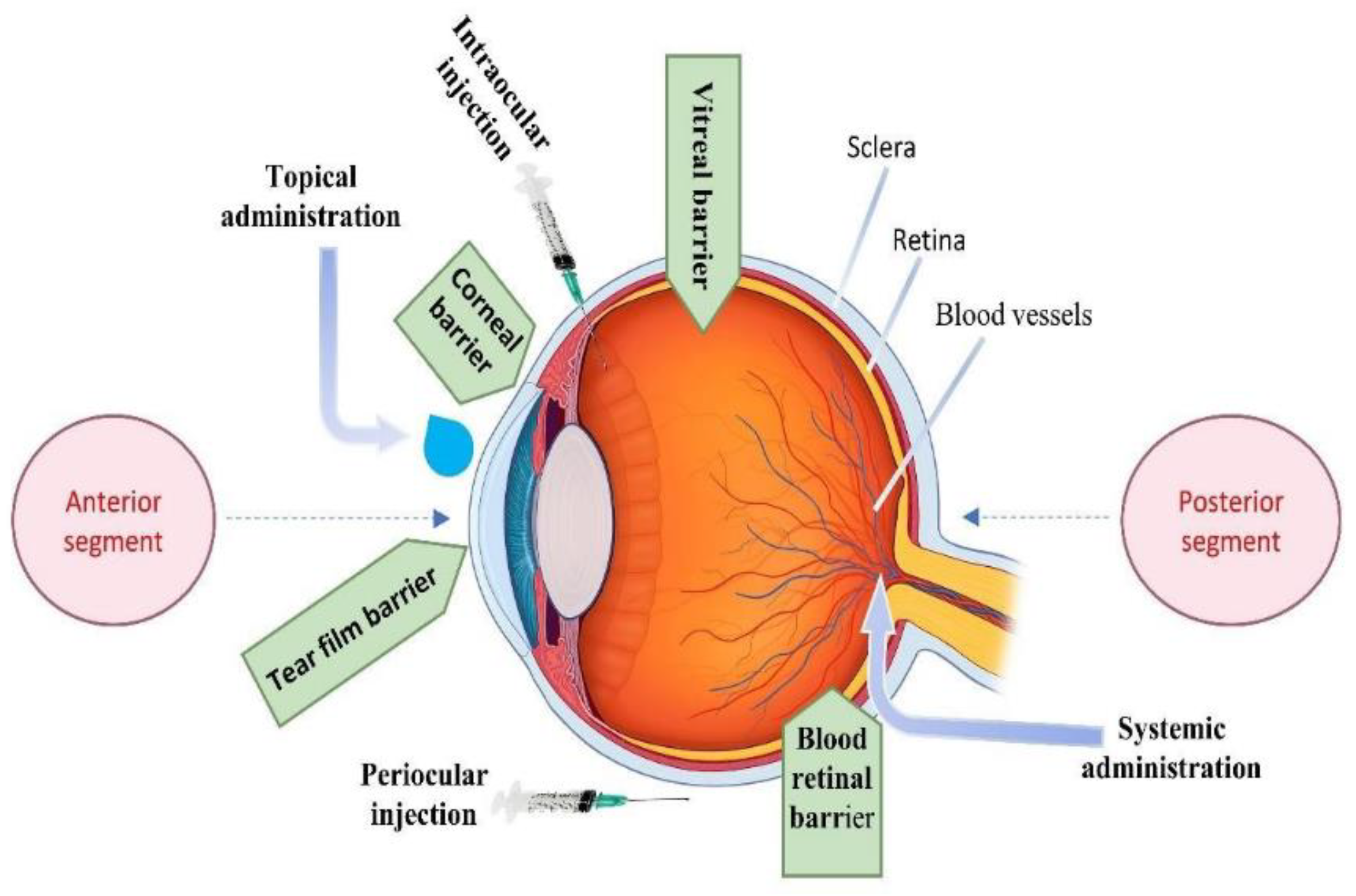
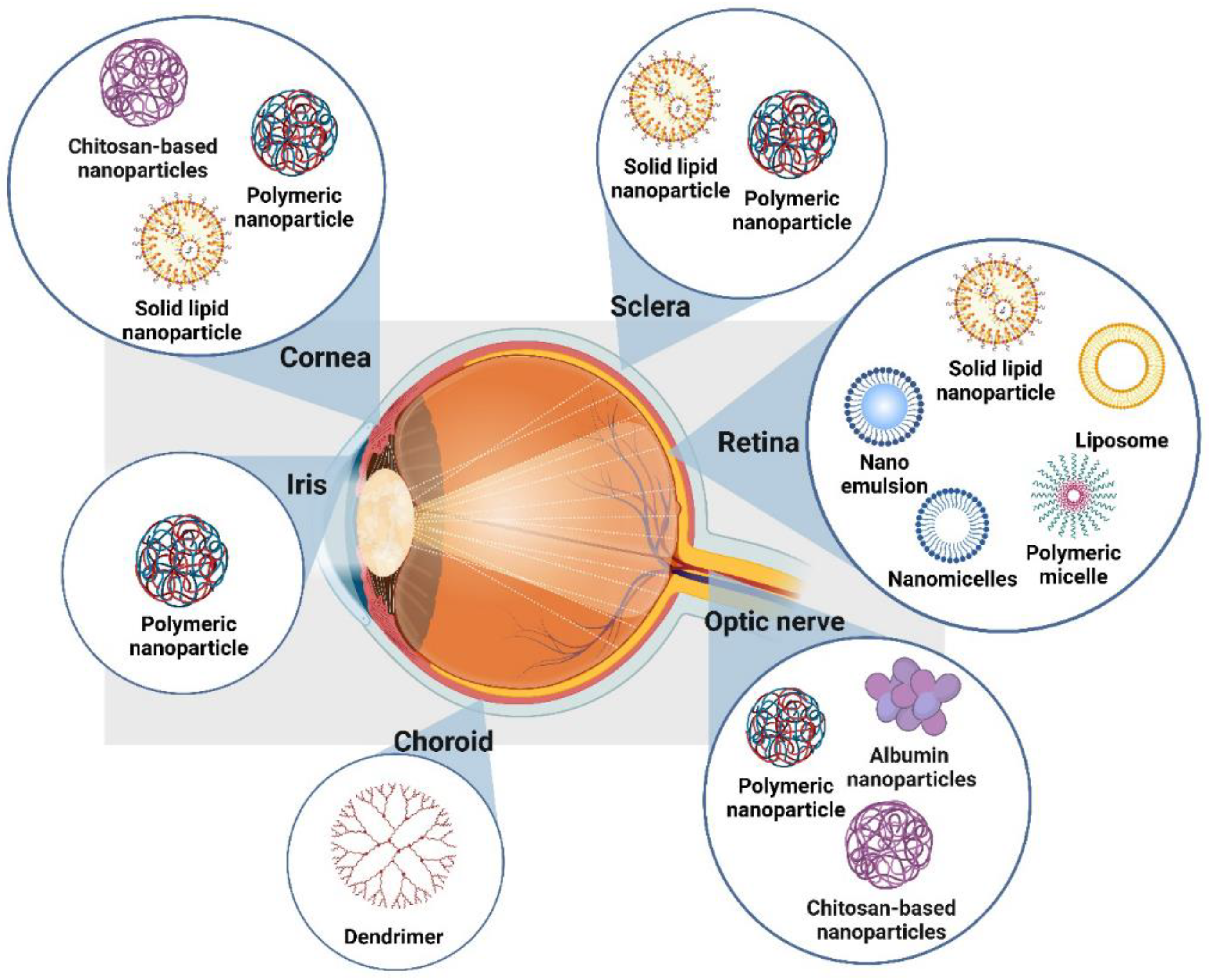
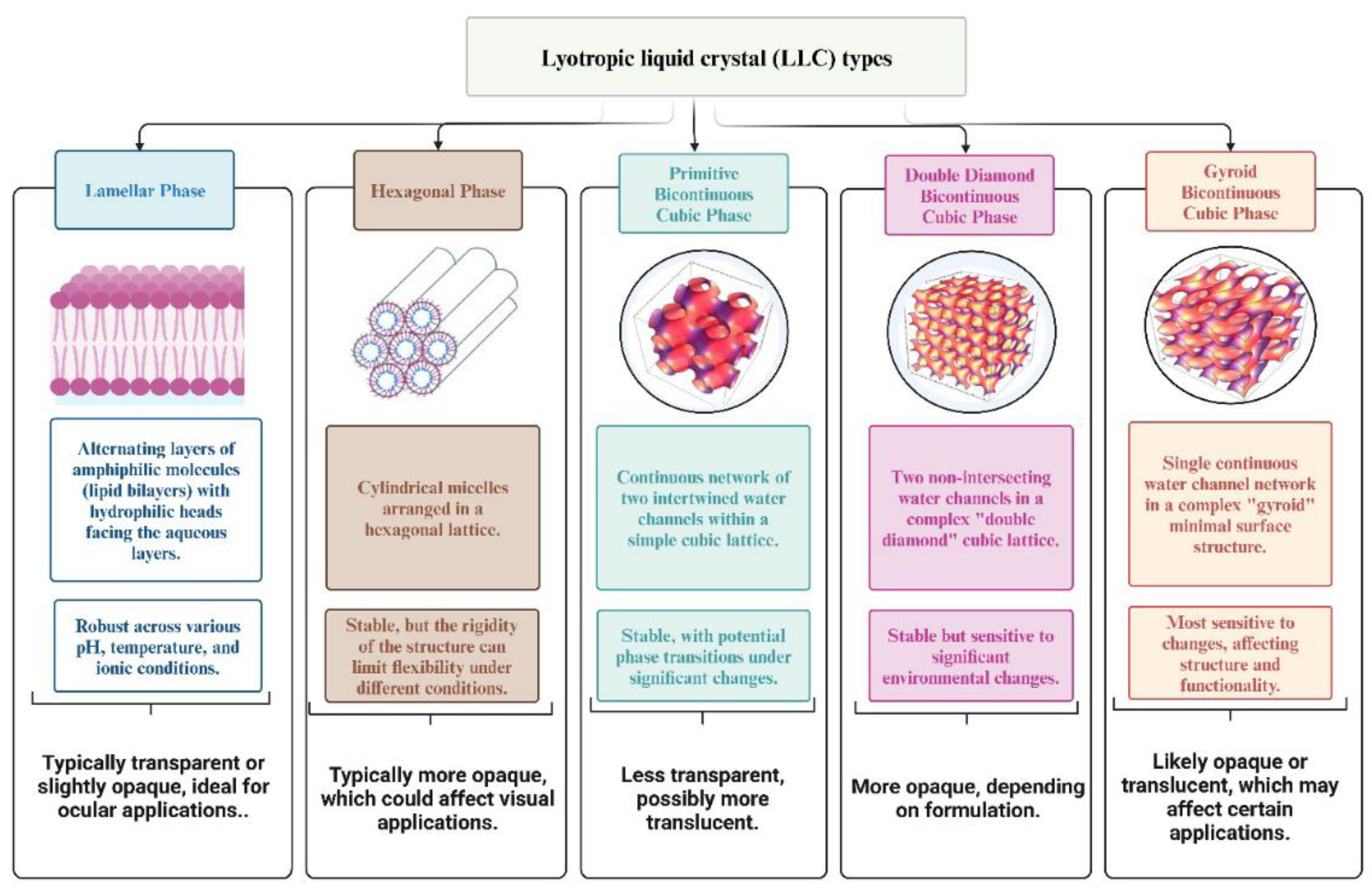
| Segment | Disease | Challenges in Drug Delivery | References |
|---|---|---|---|
| Anterior Segment | Dry Eye Syndrome | High tear turnover leads to rapid drug clearance and low corneal penetration. | [4] |
| Glaucoma | Need for sustained IOP control; low bioavailability of topical agents; frequent dosing required. | [5] | |
| Allergic Conjunctivitis | Requires rapid action with minimal systemic absorption; barriers like the conjunctival epithelium. | [6] | |
| Anterior Uveitis | High risk of systemic side effects with oral therapy; low penetration with topical steroids. | [7] | |
| Cataract | Requires precise drug delivery post-surgery to prevent infection and inflammation. | [8] | |
| Posterior Segment | Age-Related Macular Degeneration (AMD) | Poor penetration of drugs to the retina; risk of damage with repeated injections. | [9] |
| Diabetic Retinopathy (DR) | Sustained delivery needed to reduce frequent IVT injections; blood-retinal barrier limits drug access. | [10] | |
| Diabetic Macular Edema (DME) | Requires precise targeting to avoid damage to the retina; high systemic absorption risks with systemic therapy. | [9] | |
| Proliferative Vitreoretinopathy | High recurrence rate necessitates long-term therapy; limited options for effective drug penetration. | [11] | |
| Cytomegalovirus Retinitis | Often requires systemic treatment with significant side effects; local delivery challenging due to retinal barriers. | [12] | |
| Retinal Vein Occlusion | Requires sustained anti-VEGF therapy; risks associated with repeated intraocular injections. | [13] |
| Route | Application | Pros | Cons | References |
|---|---|---|---|---|
| Topical (Eye Drops) | Dry Eye Syndrome, Glaucoma, Conjunctivitis | Easy administration; high patient compliance; direct application to the eye. | Low bioavailability (<5%); frequent dosing required; low posterior segment delivery. | [22] |
| Systemic | Severe Infections, CMV Retinitis, Uveitis | Bypasses ocular barriers; effective for widespread or severe infections. | Low ocular bioavailability; significant systemic side effects; poor targeting. | [23] |
| Intraocular (IVT) | AMD, DR, Retinal Vein Occlusion, DME | Direct delivery to posterior segment; high drug concentration at target site. | Invasive; risks of endophthalmitis, retinal detachment, cataracts; repeated injections needed. | [24] |
| Periocular | Posterior Uveitis, DR, DME | Direct targeting of posterior segment; reduces systemic exposure. | Risk of local complications; requires technical expertise; may cause discomfort. | [25] |
| Intracameral (IC) | Post-Cataract Surgery | High local drug concentration; effective prevention of post-surgical infections. | Invasive; risks include increased IOP, corneal edema, or endothelial cell loss. | [26] |
| Subconjunctival (SC) | Posterior Uveitis, DME, DR | Enhanced drug penetration to the posterior segment; reduced dosing frequency. | Potential for local irritation or fibrosis; requires careful injection technique. | [27] |
| Retrobulbar (RB)/Peribulbar (PB) | Anesthesia for Cataract or Retinal Surgery | Effective anesthesia for surgical procedures; long-lasting anesthetic effects. | Risks include optic nerve injury, globe perforation, or hematoma formation. | [28] |
| Sub-Tenon (ST) | Uveitis, DME, Surgical Anesthesia | Safer than RB/PB injections; avoids sharp needles; effective for anti-inflammatory delivery. | Less effective for deep retinal conditions; potential for discomfort and patient anxiety. | [29] |
| System Type | Mechanism | Applications | Benefits | Limitations | Reference |
|---|---|---|---|---|---|
| Contact Lenses | Sustained drug release through the cornea | Glaucoma | Prolong contact with corneal tissues increases bioavailability and reduces dosing frequency. | Limited to certain drug types may require patient adaptation. | [43] |
| Punctum Plugs | Inhibit tear drainage; sustained drug release | Dry Eye Syndrome | Prolong drug retention on the ocular surface reduces the need for frequent dosing. | May cause discomfort; potential for blockage or infection. | [44] |
| Ocular Implants | Long-term, controlled drug release at targeted sites | Uveitis | Provides consistent therapeutic levels; minimizes systemic exposure; long-lasting effects. | Invasive procedure; risk of local complications; potential for device migration. | [45] |
| Microneedles | Minimally invasive drug delivery through ocular barriers | AMD, Retinal Disorders | Direct drug delivery to the retina or vitreous reduces systemic exposure and is less invasive than IVT. | Requires skilled application; potential for local irritation or damage. | [46] |
| In Situ Gels | Liquid-to-gel transformation upon contact with ocular fluids | Glaucoma, Uveitis | Prolonged drug release; improved patient compliance; reduced dosing frequency. | Limited to specific formulations; potential for discomfort during gel formation. | [47] |
| Ocular Inserts | Controlled drug release from solid or semi-solid inserts | Glaucoma, Dry Eye Syndrome | Sustained drug release; improves patient adherence; reduces systemic side effects. | It may cause discomfort or foreign body sensation, and it has the potential for dislodgement. | [48] |
| Nanomicelles | Self-assembly of amphiphilic block copolymers in aqueous environments | Glaucoma, Uveitis | Enhanced solubility of hydrophobic drugs; improved corneal penetration; sustained release. | Limited size capacity for drug encapsulation; potential for polymer-related toxicity. | [49] |
| Polymeric Nanoparticles | Biodegradable polymer-based nano-carriers | AMD, Glaucoma, Ocular Infections | Prolonged drug retention; biocompatible and biodegradable; potential for targeted delivery. | Potential immunogenicity; complex manufacturing process; stability issues. | [50] |
| Lipid-Based Nanoparticles | Solid and liquid lipids | Post-Surgical Inflammation, Retinal Disorders | Improved drug loading, sustained release profiles, and better patient tolerance. | Risk of lipid crystallization affecting drug release; challenges in large-scale production. | [51] |
| Cubosomes | Nanostructured particles with cubic symmetry | Uveitis, Retinal Disorders | Efficient encapsulation of both hydrophilic and hydrophobic drugs; enhanced stability. | Opaque appearance might limit visual applications; complex formulation process. | [52] |
| Dendrimers | Hyperbranched polymer-based carriers | Anti-VEGF Therapy | High surface area for drug conjugation; controlled release. | Expensive to produce; potential toxicity due to surface charge; complex synthesis. | [53] |
| Nanowafers | Dissolvable wafer that releases drugs over time | Ocular Infections | Sustained release; enhances therapeutic efficacy; reduces systemic exposure. | Limited by the types of drugs that can be loaded; potential for irritation. | [54] |
| Drug | Carrier System | Amphiphilic Lipid | Significant Outcome | Reference |
|---|---|---|---|---|
| Vancomycin HCl | Bulk hexagonal and cubic phases | GMO | GMO-based liquid crystalline phases were able to increase the bioavailability and effectiveness of vancomycin in the eye. | [64] |
| Vancomycin HCl | Bulk LC phases modulated with tuning agents | GMO | Effectively delivering Vancomycin HCl in vivo intravitreally for 2880 minutes. | [76] |
| Pilocarpine Nitrate | Reversed bicontinuous cubic (QII) phase | PHY | Pilocarpine nitrate could maintain sustained release from the gels for 12 hours. | [77] |
| Pilocarpine Nitrate | Cubic (Q2) and hexagonal (H2) phases | PHY | LC gels exhibited sustained release behavior for pilocarpine nitrate and more cumulative drug penetration across the cornea. | [67] |
| Dexamethasone | ISLG | PHY | Significant enhancement of corneal penetration. | [78] |
| Resveratrol | ROLG | GMO | ROLGs demonstrated strong retention on the ocular surface and a high capacity for drug loading. | [62] |
| Acyclovir | The lamellar phase transitions into a cubic phase in situ | GMO or PHY | The enhanced bio-adhesion and extended residence time of the LC systems led to improved ocular drug bioavailability. | [79] |
| Drug | Carrier System | Amphiphilic Lipid | Stabilizer | Significant Outcome | Reference |
|---|---|---|---|---|---|
| Dexamethasone | Cubosome | GMO | Poloxamer 407 | Significant improvement in dexamethasone ocular bioavailability | [14] |
| Pilocarpine nitrate | Cubosome | GMO | Poloxamer 407 | Improved bioavailability superior to commercial eye drops | [80] |
| Pirfenidone | Cubosome | GMO | Poloxamer 407 | Sustained release profile compared to drug solution | [87] |
| Cyclosporine A | Cubosome | GMO | Poloxamer 407 | Enhanced penetration and retention compared to oil solution | [75] |
| Tropicamide | Cubosome | GMO | Poloxamer 407 | Faster onset and higher intensity of mydriatic action than conventional ophthalmic solution | [84] |
| Brinzolamide | Cubosome | GMO | Poloxamer 407 | Prolonged drug release compared to commercial product | [78] |
| Riboflavin | Cubosome | Peceol® | Poloxamer 407 | Improved preocular retention and ocular bioavailability | [81] |
| D-Mannitol | Cubosome | GMO | Poloxamer 407 | Effective as potential carriers for improved ocular delivery | [89] |
| Tetrandrine | Cubosome | GMO | Poloxamer 407, Gelucire 44/14 | Prolonged release profile compared to drug solution | [83] |
| Tobramycin | Cubosome | GMO | Poloxamer 407 | Improved effectiveness over marketed tobramycin eye drops | [90] |
| Bromfenac | Cubosome | GMO | Poloxamer 407 | Longer duration of action and higher bioavailability than drug solution | [94] |
| Acetazolamide | Cubosome | GMO | Poloxamer 407, Transcutol P | Greater therapeutic efficacy than commercial products | [106] |
| Brimonidine | Cubosome | GMO | Poloxamer 407 | Sustained IOP-lowering effect for 17.6 hours, compared to 1.9 hours with Alphagan®P | [88] |
| Ketorolac | Cubosome | Peceol® | Poloxamer 407 | Significantly increased transcorneal penetration | [85] |
| Flurpiprofen | Cubosome | GMO | Poloxamer 407 | Enhanced transcorneal permeation | [107] |
| Beclomethasone | Cubosome | GMO | Poloxamer 407 | Increased bioavailability and improved ocular permeability | [108] |
| Vancomycin | Cubosome | GMO | Poloxamer 407 | Considerable decrease in severity of keratitis | [90] |
| LM22A-4 | Cubosome | PHY | Pluronic 127 | Successfully targeted posterior retina and optic nerve head in vivo | [109] |
| Latanoprost | Cubosome | PHY | Poloxamer 407 | Persisted IOP reduction for at least 9 days, compared to 24h with commercial formulation | [92] |
| Sertaconazole | Cubosome | GMO | Poloxamer 407, Poloxamer 188 | Excellent in vivo corneal absorption and tolerability | [86] |
| Fluconazole | Cubosome | GMO | Poloxamer 407 | More effective and safer for treating keratomycosis than aqueous drug solution | [95] |
| Travoprost | Cubosome | GMO | Poloxamer 407, Tween®80 | Decrease in intraocular pressure lasting 48-72 hours compared to commercial formulation | [96] |
| Ketoconazole | Cubosome | GMO | Poloxamer 407 | Boosted antifungal activity in rabbit-induced fungal keratitis | [93] |
| Loteprednol Etabonate | Cubosome | Lipoid S 75 | Poloxamer 407, Poloxamer 338, Transcutol P | Improved ocular retention, efficacy, and patient compliance | [97] |
| Fluorometholone | Cubosome | GMO | Poloxamer 407 | Sustained release and increased permeability | [98] |
| Triamcinolone | Cubosome | GMO | Poloxamer 407 | Superior drug delivery and therapeutic outcomes | [99] |
| Luteolin | Cubosome | GMO | Poloxamer 407 | Remarkable efficacy in reducing intraocular pressure and inflammation | [100] |
| Gemifloxacin mesylate | Cubosome | GMO | Poloxamer 407 | Greater potency, significant reductions in corneal opacity and inflammation | [110] |
| Moxifloxacin Hydrochloride | Cubosome | GMO | Poloxamer 407 | Sustained drug release and increased bioavailability | [101] |
| Fluorometholone | Cubosome | GMO | Poloxamer 407 | Improved ocular bioavailability and drug release | [102] |
| Acetazolamide | Cubosome | GMO | Poloxamer 407, Polyvinyl alcohol | Increased corneal penetration and extended drug release | [103] |
| Fenticonazole Nitrate | Cubosome | GMO | Poloxamer 188, Poloxamer 407 | Enhanced corneal absorption and permeation | [104] |
| Moxifloxacin Hydrochloride | Cubosome | GMO | Poloxamer 407 | Increased permeability and sustained drug release | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





