1. Introduction
Polyethylene (PE) stands as the predominant plastic in today’s market, accounting for 70% of the global plastic commodity market share [
1,
2,
3,
4]. As one of the five major synthetic resins, it boasts the largest production capacity and highest import volume among such materials in China [
5,
6,
7]. Polyethylene serves as a widely utilized and cost-effective general-purpose plastic due to its exceptional malleability, low melting temperature, and excellent fluidity making it well-suited for a wide range of polymer material forming and processing techniques [
8,
9]. Presently, there exists a diverse array of PE resin options to choose from including low-density polyethylene (LDPE), linear low-density polyethylene (LLDPE), medium-density polyethylene (MDPE), high-density polyethylene (HDPE) and ultra-high molecular weight polyethylene (UHMW- PE) — all enjoying significant popularity.
XLPE is a polymer material with high molecular weight that has undergone cross-linking of its polyethylene structure through chemical or physical methods. Polyethylene, widely used in packaging, piping, and cabling across various industries, has limited stability in high-temperature, high-pressure, and chemical environments [
10]. To address these limitations, scientists have developed cross-linked polyethylene to significantly enhance the material’s heat resistance, strength, and chemical stability through the cross-linking process. As a result, cross-linked polyethylene has found extensive applications in construction for plumbing systems due to its improved resistance to hot water and chemicals; water supply systems for its flexibility and corrosion resistance; radiant floor heating systems (underfloor heating) for its durability under varying temperatures; cable insulation for telecommunications and power distribution networks due to enhanced electrical properties; as well as medical applications owing to its biological compatibility and sterilization ability [
11].
The research on XLPE is of significant scientific and societal importance, primarily manifested in the following seven aspects. Firstly, in terms of enhancing material performance, the process of cross-linking significantly improves the properties of original polyethylene, including high temperature resistance, high pressure resistance, chemical corrosion resistance, and aging resistance. These enhancements enable the application of XLPE in extreme environments and expand its utility across various fields. Secondly, with regard to industrial applications, the use of XLPE in sectors such as construction, hydraulic engineering, energy production, cable manufacturing [
12] and medical technology effectively enhances product safety standards while also improving reliability and service life. This provides a dependable material foundation for industrial development [
13,
14,
15,
16]. Thirdly, XLPE is noted for its favorable recyclability from an environmental sustainability perspective. Research efforts aimed at developing more environmentally friendly synthetic processes and recycling technologies can propel the plastics industry towards sustainable practices by optimizing material life cycle management to reduce environmental impact [
17,
18,
19,
20,
21]. The fourth is to meet market demand. With the acceleration of global urbanization and the improvement of living standards, the demand for high-performance durable materials is increasing. Research into XLPE can help meet this growing need by providing essential support for building modern societies. Fifthly is scientific research & technological innovation: The study of XLPE spans multiple disciplines including materials science, chemical engineering and physics. Through this research progress can be made within these related fields promoting new material development as well as technological innovation [
22,
23,
24,
25]. The fifth aspect pertains to economic development: the production and application chain linked to XLPE encompasses all stages of the process, from raw material supply through manufacturing to sales, thereby fostering economic growth and generating employment opportunities in related industries [
26,
27,
28]. Lastly, we have market competitiveness: In today’s global marketplace research & development focused on XLPE has potential to enhance a country or region’s competitive advantage within modern materials sector thereby driving exportation of related products [
29,
30,
31,
32].
In conclusion, the research on cross-linked polyethylene holds immense significance for the advancement of science and technology, while also playing a pivotal role in fostering economic and social development as well as environmental protection. Further research and development are anticipated to augment the potential value of this material in the future.
2. Classification and Properties of Polyethylene Resins
PE is one of the five major synthetic resins, and even is the most productive and imported variety of synthetic resins in China. PE is a widely used and inexpensive general-purpose plastic. Due to its high plasticity, low melting temperature and good fluidity, it is suitable for most forming and processing methods of high molecular polymer materials.
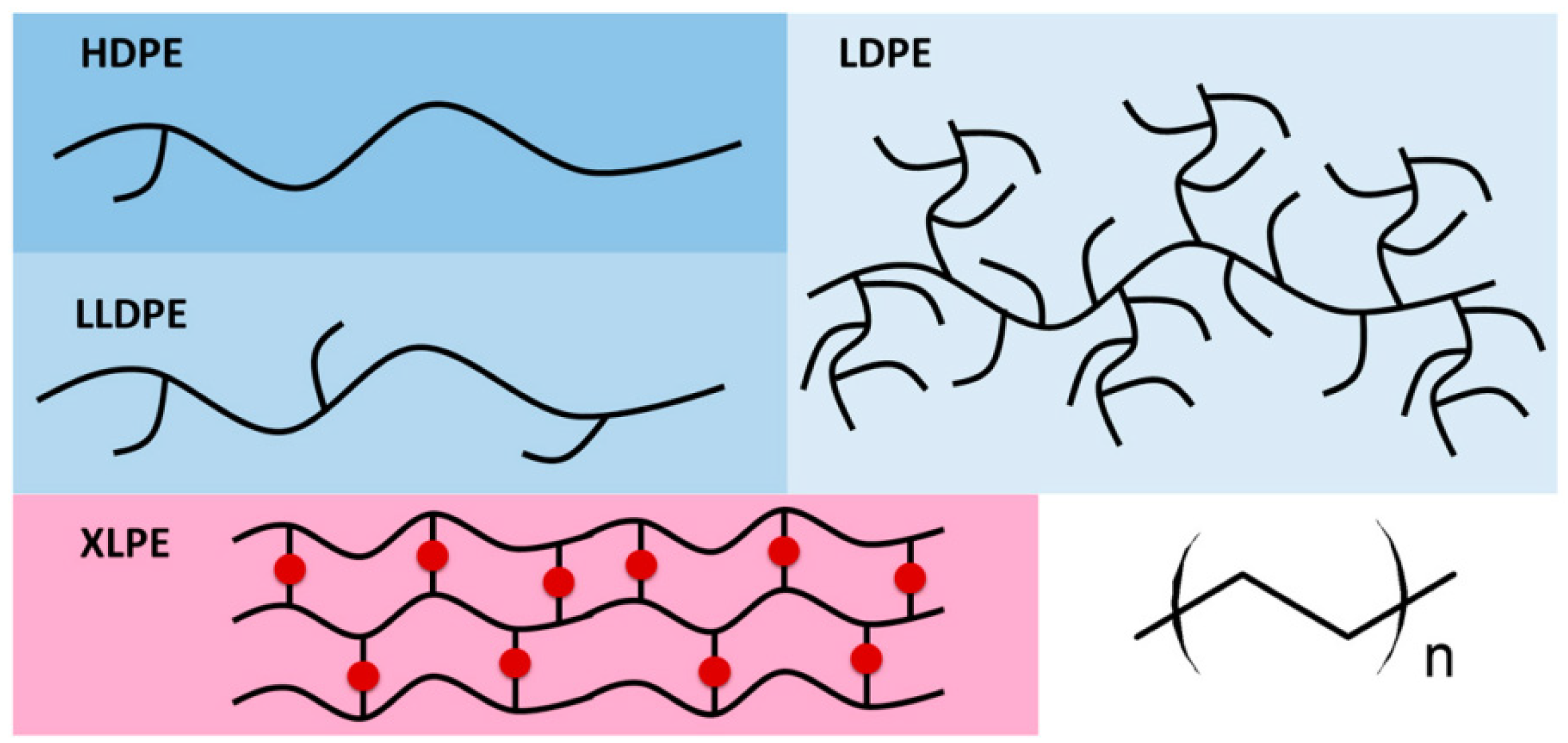
As illustrated in
Figure 1, polyethylene can be divided into HDPE, LDPE, and LLDPE, based on the method of polymerization, molecular weight, and chain structure [
33,
34]. I will introduce these three types of polyethylene from the perspectives of structure, features, and applications. Polyethylene is a high molecular weight compound synthesized through the polymerization of ethylene monomers, characterized by its molecular structure predominantly consisting of repeating units of “-CH
2-”. Various types of polyethylene exhibit distinct molecular architectures. HDPE features linear molecular chains with minimal branching, resulting in a tightly packed arrangement and elevated crystallization [
35]. In contrast, LDPE possesses elongated branches that lead to a less compact molecular configuration and consequently lower crystallization. LLDPE, while also exhibiting branching, has shorter and uniformly distributed branches, yielding an intermediate level of crystallization between HDPE and LDPE.
HDPE is known for its rigidity, hardness, and mechanical strength, as well as excellent heat and cold resistance [
36]. In contrast, LDPE offers remarkable flexibility, outstanding elongation properties, transparency, cold resistance, and great processability [
37,
38,
39]. LLDPE is recognized for its strong environmental stress crack resistance, high impact strength, and superb heat sealing capabilities [
40]. Ultra-high molecular weight polyethylene (UHMWPE) is distinguished by exceptional wear resistance, impact resilience, and chemical stability [
41].
Different types of polyethylene have widespread applications in many fields due to their unique physical and chemical properties. In the packaging industry, due to its good mechanical strength and chemical resistance, HDPE is commonly used to produce various containers, caps, bottles, etc. ; LDPE is commonly used to produce plastic bags, freshness bags, bubble wrap, etc. due to its good flexibility and transparency; and LLDPE is particularly suitable for producing high-strength, high-toughness film products, such as packaging films, due to its excellent resistance to environmental stress cracking and impact strength [
40]. In agriculture, HDPE film is valued for its mechanical strength and durability, allowing it to withstand significant external pressures and environmental conditions. This makes it ideal for crop cultivation areas requiring prolonged coverage. Conversely, LDPE offers flexibility and ductility, enabling it to closely adhere to the soil surface and effectively block water and wind infiltration, creating an optimal environment for plant growth [
42]. Additionally, LLDPE film resists environmental stress cracking well, maintaining structural integrity under challenging conditions while minimizing soil erosion and reducing crop damage [
43]. In the construction industry, HDPE pipes are commonly used for drainage, firefighting, and gas delivery due to their corrosion resistance, wear resistance, low temperature tolerance, and easy installation [
44]. LDPE is suitable for certain waterproof materials; its coating or film can be created by mixing it with specific additives. LLDPE exhibits exceptional resistance to environmental stress cracking, rendering it an optimal material for pipe and cable applications. Furthermore, LLDPE waterproofing membranes are employed in roofing and subterranean waterproofing projects due to their superior weather resistance, chemical stability, and flexibility [
33,
42,
45].
Cross-linking is a process in which linear or branched polymer chains are chemically or physically interconnected to form covalent bonds, resulting in the transformation of the original linear structure into a network structure and thereby significantly augmenting a spectrum of physical and chemical properties [
46,
47].
Figure 1.
Schematic representation of the structure of varying types of PEs [
48].
Figure 1.
Schematic representation of the structure of varying types of PEs [
48].
3. Advantages of Cross-linked Polyethylene Materials
XLPE is derived from linear or branched PE resins by treating with chemical or physical cross-linking modifications [
49]. A high molecular weight PE with a linear molecular structure experiences decreased VDM (Van der Waals’ force) between its linear molecules at a higher temperature (e.g., its temperature of critical deformation is approximately 55°C), leading to deformation of materials. In XLPE, cross-linking bonds are formed between single polymer chains to replace the original weak VDM, creating a stable network structure among molecules. This cross-linking process transforms thermoplastic polyethylene into thermosetting cross-linked polyethylene [
50], resulting in some enhanced properties for XLPE [
51,
52,
53].
XLPE exhibits an enhanced mechanical strength as well as a superior heat resistance, an improved resistance to chemicals, and even a better insulating property. Firstly, in regard to heat tolerance, the interconnected three-dimensional structure endows XLPE with an exceptional resilience, and its deterioration and charring occur only at temperatures above 300 °C. Its sustained operating temperature can reach 90 °C with a thermal longevity extending up to 40 years. Secondly, in terms of insulating performance, XLPE retains the favorable insulating traits of PE while augmenting its insulating capacity. The dielectric loss tangent remains minimal regardless of temperature fluctuations. Regarding mechanical attributes [
54], the formation of fresh chemical bonds among large molecules results in heightened hardness, rigidity, wear-resistance [
55], and impact-resistance within XLPE, countering PE’s susceptibility to cracking under environmental strain. Finally, in relation to chemical endurance, XLPE is insoluble in common solvents, bolstering PE solvent and chemical resilience while exhibiting robust immunity against acids, alkali and oils. Its combustion by-products primarily comprise water and carbon dioxide, minimizing environmental harm and in compliance with contemporary fire safety standards.
4. Cross-linking Methods of Polyethylene
Three primary cross-linking methods have been successfully developed to achieve industrial-scale cross-linked polyethylene: (1) chemical cross-linking using peroxides (e.g., DCP (Dicumyl peroxide) and DTBP (Di-tert-butyl hydrogen peroxide)); (2) chemical cross-linking employing silicone coupling agents (e.g., A171 (silane-ethenyltrimethoxy) and A151 (triethoxyvinylsilane)); and (3) physical cross-linking of polyethylene materials through irradiation with high-energy rays (e.g., electron beam and gamma ray of
60Co). Among these, irradiation cross-linked represents a form of physical cross-linking that enables polyethylene materials to undergo the process without the introduction of large quantities of low-molecular-weight chemicals, rendering it environmentally friendly and non-toxic. This characteristic is expected to significantly broaden the application scope of irradiation cross-linked polyethylene materials. Additionally, irradiation cross-linked offers advantages such as high efficiency, uniformity in cross-linked, and a high degree of cross-linked, all contributing to substantial enhancements in the performance stability of irradiation cross-linked polyethylene materials [
56,
57].
Table 1 describes the advantages, disadvantages and applications of four cross-linking methods: peroxide cross-linking, silica gel cross-linking, irradiation cross-linking and purple cross-linking.
4.1. Peroxide Cross-Linking
In contemporary industrial production, peroxide cross-linking is among the most prevalent methods for achieving cross-linked PE-based materials [
58,
59]. This process involves the introduction of a specific quantity of peroxide and antioxidant into the PE matrix, followed by treating under suitable temperature and pressure conditions to realize the transformation of linear or branched structure into cross-linked structure. Organic peroxide cross-linking agents represent a category of organic compounds characterized by the presence of an oxygen-oxygen (O-O) bond [
60,
61,
62,
63]. These compounds exhibit a tendency to decompose under external conditions such as elevated temperatures or exposure to light, resulting in the generation of free radicals. The high reactivity of these free radicals enables them to initiate cross-linking reactions among polymer molecules, thereby converting linear or slightly branched macro-molecular structures into three-dimensional network architectures and enhancing various properties of the material. The organic peroxides produced within the chemical industry primarily function as polymerization initiators and catalysts for synthetic resins [
64,
65]. In the field of polymer materials, it can serve as radical polymerization initiator, grafting reaction catalyst, cross-linking agent for rubber and plastics, curing agent for unsaturated polyesters, and regulator of molecular weight and distribution in the preparation of high-quality polypropylene suitable for spinning [
66,
67,
68].
Table 2 describes the advantages, disadvantages and applications of the three peroxide cross-linkers (i.e., DCP, DTBP and BIBP).
The peroxide cross-linking process exhibits some advantages over other methods due to the requirement for premixed polyethylene and cross-linking agents prior to extrusion through an extruding machine, resulting in significantly enhanced uniformity and depth of cross-linked products [
69,
70,
71]. In comparison with silicone cross-linking, the peroxide process extends product storage time and reduces production costs [
72]. Furthermore, the utilization of a plunger extruding machine in the peroxide cross-linking process eliminates the need for a post-heating device, leading to a more compact equipment structure and reduced production costs; however, this may limit production efficiency. When conducting peroxide cross-linking, it is crucial to maintain a controlled temperature to prevent carbonation of the cross-linked material due to exceeding the decomposition temperature of the peroxide [
73,
74]. Additionally, it is essential to regulate the extruding machine speed and utilize specialized extruding machine heads and high-pressure connection channels, which may pose limitations on application for medium-sized and small enterprises. Sun et al. developed a novel organic peroxide, 2,5-dimethyl-2,5-bis(tert-butylperoxy)hexane (Bi25) , which effectively initiates the cross-linking process of polyethylene (Bi25-XLPE) [
64]. Bi25-XLPE is characterized by the absence of by-product gases and an extended vulcanization duration. Its non-porous nature, coupled with a higher cross-linking density and crystallization, ensures that the physical and mechanical properties of Bi25-XLPE sheets comply with the insulation standards for 500 kV power cables. Furthermore, COMSOL has developed a 500 kV power cable model to validate the breakdown strength (reaching up to 61.66 kV/mm) and stability of Bi25-XLPE.
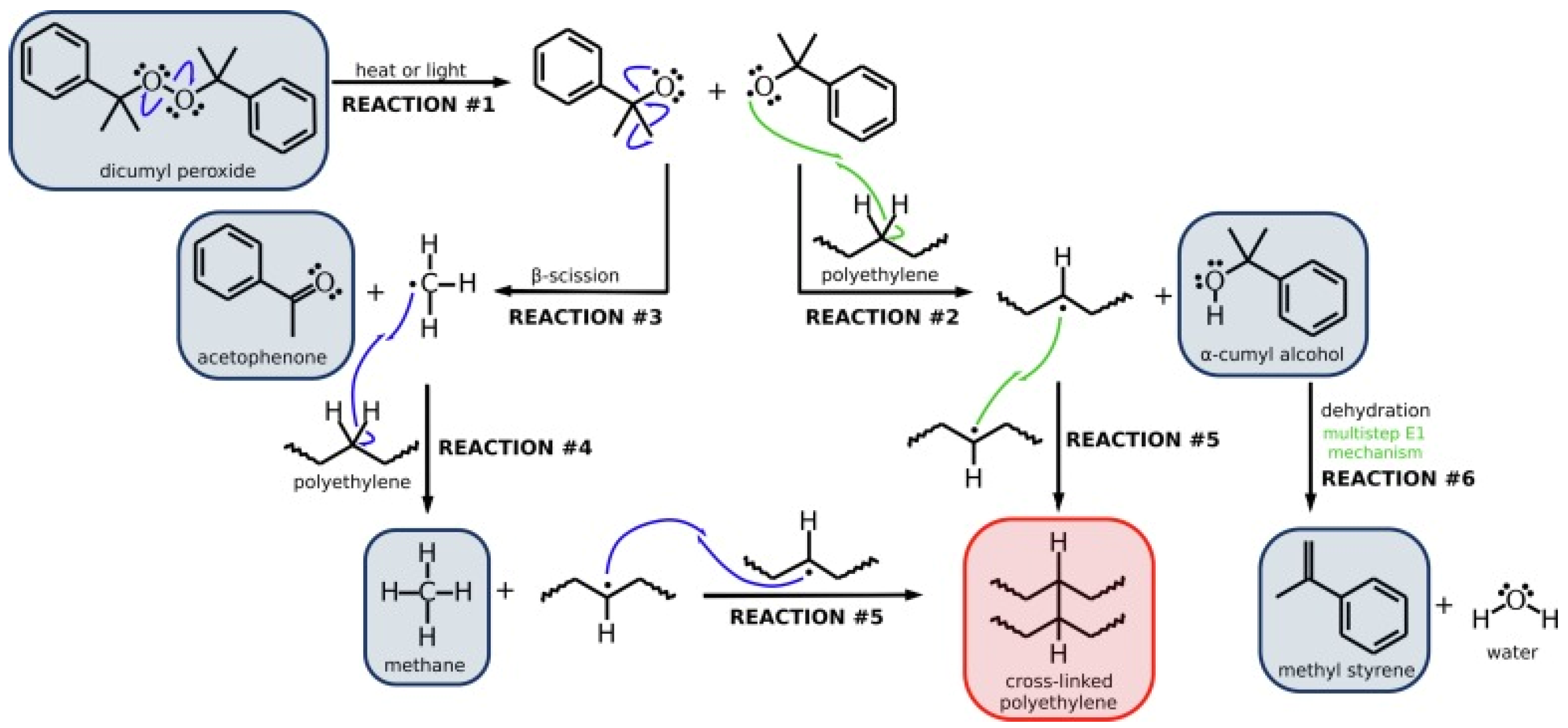
Figure 2 shows the reaction scheme and byproducts generated during DCP induced cross-linking procedure [
75,
76]. As
Figure 2 shows, the main steps in this process are: (1) Generation of free radicals: When PE and a peroxide cross-linking agent are mixed and melted under specific conditions, the peroxide decomposes at high temperatures to produce free radicals; (2) Hydrogen capture : The free radical attacks the PE macro-molecular chain, removing hydrogen atoms and generating a macro-molecular chain free radical; (3) Cross-linking: The highly reactive macro-molecular chain free radical of PE combines with another to form carbon-carbon bonds, resulting in a three-dimensional network structure that significantly enhances the mechanical properties and heat resistance of PE [
54,
77,
78,
79,
80]. Kang and Ha investigated the impact of various parameters, including temperature, shear rate, and cross-linking agent concentration, on the torque value and cross-linking reaction of HDPE that was cross-linked using 2,4-di-tert-butylperoxide [
44]. Their findings indicated that the effect of temperature was significantly more pronounced than that of shear rate. Elevated reaction temperatures facilitated the cross-linking process within the polymer matrix. Di et al. investigated the effects of varying concentrations of DCP on the cross-linking of LDPE. The findings indicated that as the concentration of the cross-linking agent increased, the degree of cross-linking in polyethylene initially rose before subsequently declining. Notably, when DCP content exceeded 1.0 wt%, a similar accumulation of polar space charges was observed. Furthermore, optimal rheological characteristics of processing properties for cross-linked polyethylene were noted within a DCP concentration range of 1.0-1.5 wt% [
81].
4.2. Silicone Cross-Linking
Silicone cross-linking is a chemical method used for the production of insulating materials. Silicone cross-linking is a chemical process that involves the interaction of silicone compounds with polymers under specific conditions to create a three-dimensional network structure material [
82]. This cross-linking technique significantly enhances the performance of materials, resulting in exceptional physical and chemical stability. Silicone cross-linked polyethylene usually exhibits outstanding physical properties, heat resistance, chemical resistance and excellent mechanical properties [
83,
84,
85,
86,
87,
88].
4.2.1. Sioplas Method
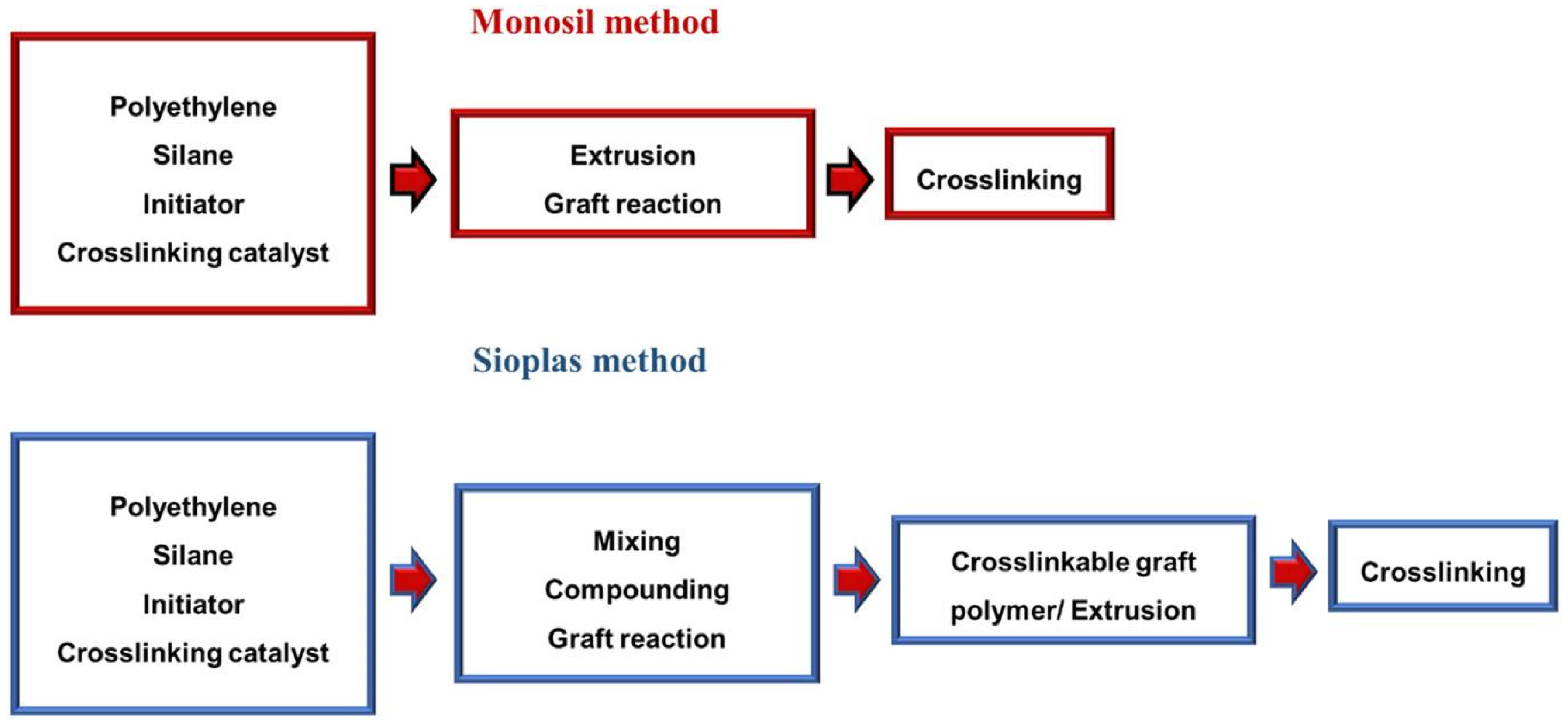
The two-step approach, as implied by its name, comprises of a pair of sequential processes. In the first step, peroxide serves as an initiator when PE molecules are exposed to silicone in a molten state. Under high temperature conditions, the peroxide acts as an initiator and decomposes into small molecular free radicals [
89]. These free radicals then combine with hydrogen atoms on the PE molecule to generate large molecular free radicals, which subsequently react with chemical bonds in the silicone. Subsequently, in the second step following this reaction, the linear structure of silicone transforms into a network structure through cross-linking reaction. During this process, water molecules replace silicone bonded to polymer chains via gaps within polymer molecules and undergo reactions with silicone bonded to polymer chains to form Si-O-Si cross-links.
The two-step approach is widely favored in low-voltage cable and small-scale production due to its ability to tailor products according to customer-specific requirements, minimal equipment demands, high insulation material grafting and cross-linked degree, simple process and low investment. However, this method has several drawbacks during the production process, such as susceptibility to impurity contamination, short product shelf life, premature cross-linking with air exposure, and they ultimately limit its applicability. Chen et al. conducted a comprehensive investigation into the thermal and mechanical properties of silicone-water cross-linked LLDPE in hot water, alongside an analysis of surfactant effects [
90]. Their findings indicated a reduction in crystallization, which was attributed to the increased concentration of silicone and the establishment of a silicone cross-linking network. Furthermore, variations in the silicone component’s content were found to significantly influence both tensile strength and elongation at break values.
4.2.2. Monosil Method
The one-step process has rapidly developed on the basis of the two-step process. Its basic principle is simple: to directly input basic materials into an extruding machine and produce electric wires and cables in a single step.
Figure 3 illustrates the procedural steps involved in one-step and two-step silane cross-linking. Compared to the two-step process, the one-step process offers numerous advantages, such as lower impurity levels and suitability for both low-voltage and medium-voltage cables. However, with advantages come disadvantages; this method demands high technical requirements and significant investment.
For example, BP (a British company) utilizes a hydrolysis type catalyst with high activity to yield an excellent performance of resulting cross-linked products. The quality of the produced goods depends on a key technology of the one-step process—grafting. A higher grafting rate leads to better product quality, while a lower rate results in inferior product quality [
91]. Yussuf et al. investigated the effects of varying silicone concentrations and the incorporation of nanoclay fillers on the degree of cross-linking, as well as the mechanical, thermal, rheological, and morphological properties [
85]. The findings indicated that an increase in silane concentration corresponded to a rise in gel content and degree of cross-linking. Notably, the gel content of XLPE containing MMT nanoclay reached an impressive 84%. Regarding mechanical properties, elevating the silicone concentration from zero to 2.0 phr resulted in an approximate increase of 2 MPa in tensile strength for native LLDPE; furthermore, incorporating MMT nanoclay into XLPE enhanced its tensile strength by approximately 3 MPa compared to pure LLDPE.
4.2.3. Precipitation Polymerization Approach
The precipitation polymerization method was developed by leveraging the advantages of both the two-step and one-step methods [
92]. In the 1980s, international researchers aimed to produce cross-linked polyethylene by directly introducing vinyl silicone into a high-pressure reactor. This approach entails a random precipitation polymerization reaction of ethylene and silicone units within the high-pressure reactor, without the use of peroxides during experimentation to prevent bubble formation, as no volatile substances are generated during the reaction [
93]. Similar to both the two-step and one-step methods, this precipitation polymerization technique utilizes silicone as its monomer. However, it diverges in process technology by integrating silicone directly into the monomer polymerization process instead of grafting it onto a silicone chain. Consequently, the resulting co-polymer is facile to process and exhibits wet-curing properties [
94].
The cross-linked polyethylene produced using this advanced and unique precipitation polymerization method offers numerous benefits such as high stability, prolonged durability, ease of shaping and shortened production time. The cross-linked material ensures high cleanliness while mitigating pollution issues stemming from peroxide residues during grafting [
95]. Notably, with this approach, injecting silicone co-polymer monomers once achieves uniform distribution of cross-linked crystals with lower required silicone content compared to that in silicone grafting compounds—resulting in improved product performance especially in terms of electrical properties [
96]. It can be utilized as an insulating material with a voltage rating of up to 33kV, and possesses inherent flame-retardant properties, rendering it suitable for use as a flame-retardant sheath [
97]. Camalov et al. investigated the breakdown strength of XLPE samples incorporating peroxides and silicone, revealing that at elevated voltage rise rates, both sample types exhibited enhanced breakdown strength values [
98]. Notably, the maximum increases in breakdown strength for the two XLPE samples were 17.96% and 23.80%, respectively. Additionally, the findings indicated that the silicone cross-linked sample consistently demonstrated superior breakdown strength across all tested conditions.
4.3. Irradiation Cross-Linking
Irradiation cross-linking, as a commonly used method of cross-linking, was first proposed by the Dole team in experiments with heavy water reactors. Subsequently, with further research, irradiation cross-linking technology of polyethylene has been primarily applied in the production of electric wires and cables, pipes, foam materials, films and gel materials [
99]. Cassidy et al. investigated the effects of gamma irradiation on HDPE [
100]. They examined the increase in intensity, noting that the material becomes brittle at a radiation dose of 60 kGy. Additionally, they analyzed the enhancement in ultimate strength, reduction in elongation, and increase in Rockwell hardness. Alsabbagh et al. conducted a comprehensive investigation into the effects of gamma irradiation on the mechanical and structural properties of polylactic acid (PLA) and HDPE [
101]. The samples were subjected to irradiation using a
60Co gamma-irradiation facility under ambient conditions, within a dose range of 0 to 175 kGy. The experimental findings revealed that gamma irradiation significantly influenced polymer properties through mechanisms such as cross-linking, chain scission, and oxidative degradation.
During the process of irradiation cross-linking, free radicals in polymers are generated through the irradiation of high-energy rays such as electron beams and neutron beams. In laboratory experiments, the rays are typically produced by a 60Co isotope irradiation source [
43]. In industrial settings, polymer cross-linking is commonly achieved using electron beams generated by large-scale electron accelerators [
102]. The principle of irradiation cross-linking [
103] is as follows: (1) High-energy irradiation acts on the bond energy of PE, breaking the C-H bonds and C-C bonds in PE to form free radicals and hydrogen atoms; (2) Hydrogen atoms are energized during the irradiation process and react with other C-H bonds and C-C bonds to generate secondary free radicals. In this process, hydrogen atoms undergo transformations and exist in a different state; (3) When the free radicals generated by irradiation encounter the secondary free radicals, they undergo cross-linked reactions. The issue of sensitization irradiation in polyethylene is currently a primary focus of research in the production of cross-linked polyethylene using the irradiation cross-linking method. Addressing this concern typically involves incorporating sensitizing agent or altering the irradiation atmosphere within the polyethylene [
104,
105]. Commonly used sensitizing agent include 2, 4-dimethyl-2,4-pentanediol acetate and trimethylol propane tris-(3-hydroxypropyl) acid ester while common sensitizing agent options include silicon tetrachloride, carbon tetrachloride, sodium fluoride and carbon black.
The use of the irradiation cross-linking method for cross-linked polyethylene offers several advantages [
106]: separate processes for cross-linking and extrusion allow for easy control over product quality, high production efficiency and low waste rates; no additional free radical initiator is required during the cross-linking process, maintaining material cleanliness and enhancing electrical properties [
107]; it is particularly suitable for producing small-sectioned, thin-walled insulated cables that are challenging to cross-link using chemical methods. This method avoids high-temperature peroxide-based cross-linking by utilizing shaping processing on a standard extruding machine followed by water-boiling cross-linking in warm water. As a result, degree of crystallization and density remain essentially unchanged [
108]. However, irradiation cross-linking also presents some drawbacks such as the requirement of an increased electron beam acceleration voltage for thick materials, the requirement of rotation or employing multiple electron beams for circular objects like electrical wires and cables to achieve uniform irradiation, substantial initial investment costs, complex operational and maintenance technology requirements and stringent safety protection measures during operation.
4.4. Ultraviolet Cross-Linking
The UV irradiation cross-linking technology represents a newly developed technique, and it provides an effective technical pathway for upgrading and replacing traditional products in the cable industry. Its application prospects are extensive, particularly in the manufacturing of various high-voltage cross-linked polyethylene insulated cable products, where UV cross-linking technology offers irreplaceable advantages [
109]. Zhang et al. synthesized functionalized SiO
2/XLPE nanocomposites by chemically grafting an auxiliary crosslinking agent onto the surface of nanoscale silica [
110]. Trihydroxymethyltrimethylolpropane triacrylate (TMPTA) served as an effective auxiliary crosslinking agent for polyethylene, which was successfully grafted onto the surface of nanoscale silica through a chemical reaction involving the thiol group of the coupling agent between sulfur silane and 3-mercaptopropyl trimethoxysilane (MPTMS).The findings indicate that the polar groups present on the surface of modified nanoscale silica inhibit the proliferation of electrical trees, while simultaneously introducing deep traps that hinder charge injection, thereby significantly enhancing both resistance and dielectric breakdown strength in electrical trees. This study proposes a strategy for developing high-resistance cross-linked polyethylene materials through the integration of surface functionalization and nanodielectric technology. Zhao et al. synthesized XLPE grafted with maleic anhydride (MAH) (denoted as XLPE-g-MAH) utilizing a photocatalyst under ultraviolet irradiation, and conducted a systematic investigation of its dielectric properties in comparison to pure XLPE [
111]. The fundamental mechanism underlying space charge suppression was elucidated through the analysis of the trapping energy level distribution within XLPE-g-MAH, which accounted for the observed enhancement in electrical breakdown performance.
Previously, the utilization of photosensitive chemical cross-linking techniques entailed the activation of a photo-sensitizer by ultraviolet light to induce cross-linking reaction in polyethylene materials [
112]. The UV cross-linkable photo-initiator system comprises two key components: photo-initiators and cross-linking agents [
113,
114,
115]. Photo-initiators are compounds that selectively absorb UV light energy to generate free radicals. They can be categorized into cleavage-type photo-initiators and hydrogen abstraction-type photo-initiators. The role of the cross-linking agent is primarily manifested in three aspects: enhancing the quantum efficiency of the photo-initiator, accelerating the rate of cross-linking reaction, and improving the uniformity of cross-linking [
116]. cross-linking agents typically contain two or more unsaturated structures and mainly participate in UV cross-linking reactions by forming allylic free radicals [
117,
118]. In contrast, monomers or homo-polymer forms of auxiliary cross-linking agents demonstrate a greater capacity to participate in the formation of polyethylene cross-linked nodes, thereby increasing the likelihood of cross-linking and enhancing photon-induced cross-linking efficiency [
118]. Ye Lei et al. examined the thermodynamic properties of flame-retardant materials based on UV cross-linked polyethylene vinyl acetate (EVA), demonstrating enhancements in thermal stability and mechanical strength in these samples [
119].
The lack of progress in UV cross-linking is primarily due to the utilization of low-energy UV radiation as the irradiation source and the inherent technical limitations [
120]. The constraints of UV cross-linking technology can be observed from two perspectives. Firstly, with regard to penetration power, UV light has limited the depth of penetration and is only suitable for thin and low-voltage samples; it cannot uniformly cross-link thicker samples. Secondly, in terms of speed, the cross-linking process with UV light is slow and often fails to meet industrial production requirements [
121,
122].
Cross-linked polyethylene insulated cables have a wide range of potential applications in the high-voltage and extra-high-voltage fields. The ultraviolet (UV) light-induced cross-linking technology for cross-linked polyethylene shows promise in addressing the limitations of peroxide cross-linking technology in high-voltage applications, enabling the production of long-length, high-voltage grade insulated cables. The UV light-induced cross-linking technology offers several advantages for manufacturing high-voltage cross-linked polyethylene cables: (1) Rapid production speed without causing insulation defects [
111,
123]: By absorbing specific wavelengths of UV light through a photo-initiator, polymerization reactions can occur within an extremely short time frame [
124]. Additionally, the polyethylene-based material containing a photo-initiator system undergoes cross-linking reactions only under UV light irradiation. As a result, materials trapped in mold dead corners during cable insulation extrusion do not form any cross-linking defects; (2) Reduced material cost and lower production energy consumption; (3) Minimal infrastructure investment: The mixing process of the photo-initiator system (non-thermally sensitive materials) can be synchronized with the melt filtration process used to produce high-purity cable raw materials for high-pressure cables, thereby eliminating the need to introduce DCP as a separate peroxide chemical-cross-linking agent via liquid-phase diffusion [
121]. Therefore, UV light-induced cross-linking technology for cross-linked polyethylene is anticipated to meet the demand for long-length submarine cables at higher voltage levels while reducing equipment investment and production costs associated with these types of cables [
109].
In practical experiments and in the production of UV-cross-linked polyethylene, a fluid mixture comprising LDPE, photo-initiator, and auxiliary cross-linking agent is directly exposed to ultraviolet light above the melting point temperature [
125,
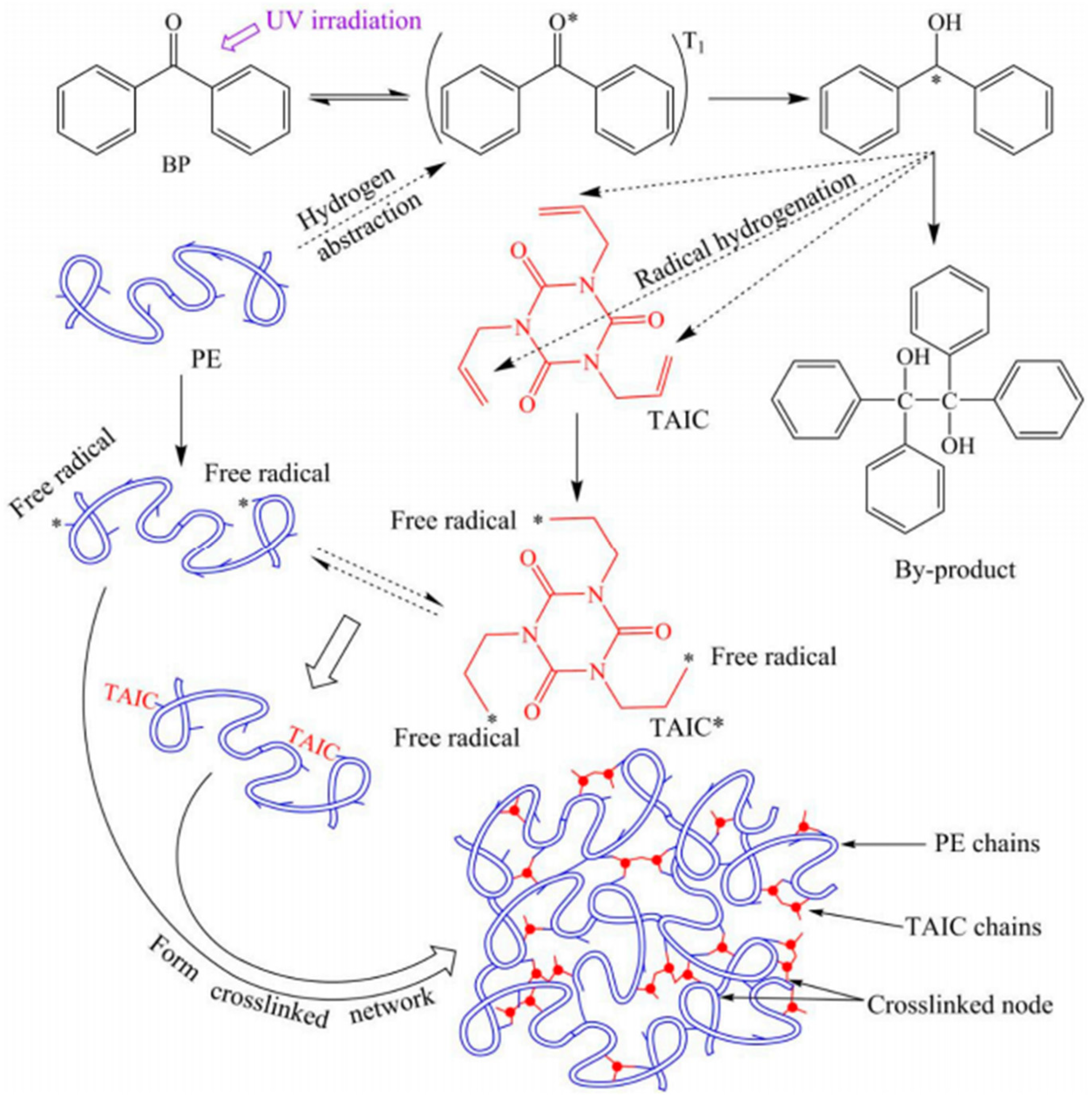
126]. This process facilitates the ultraviolet-induced molecular cross-linking of polyethylene. The cross-linking mechanism (
Figure 4) can be delineated into three steps [
127,
128,
129]. Initially, during the chain initiation stage, the photo-initiator absorbs ultraviolet light and transitions from the ground state to the singlet excited state. It then undergoes inter-system crossing to form a triplet excited state and abstracts hydrogen atoms from both polyethylene and cross-linked agents to generate corresponding alkyl free radicals P· and T·. Subsequently, in the chain growth stage, molecules with chain free radicals P· and T· respectively bond with cross-linking agent molecules. Finally, during the chain termination stage, the longer chain free radicals are formed. Polyethylene molecules containing these chain free radicals undergo reactions such as bi-molecular coupling or bi-molecular disproportion termination to form various cross-linked structures by bonding together long macro-molecular chains of polyethylene. Without an auxiliary cross-linking agent, polyethylene macro-molecules can only form cross-linked nodes through the chemical bonds of their own free radicals.
4.5. Other Cross-Linking Methods for PE-Based Materials
4.5.1. Salt Cross-Linking/Ion Cross-Linking
Salt cross-linking, also known as ion cross-linking, has similar principles to silicone cross-linking, but it solves the problem of resin recycling compared to silicone cross-linking [
130]. This establishes a foundation for the future advancement of resin production technology. The fundamental principle of salt cross-linking involves grafting reactive functional groups onto the chemical bonds of the resin monomer PE, such as -COOH or -SO
3H. Zinc hydroxide is employed for neutralization, leading to the formation of ionic salt bridges along the molecular chain, such as -COOZn
2+OOC- or -SO
3HZn
2+O
3S-. This results in a transition from a linear to a network structure.
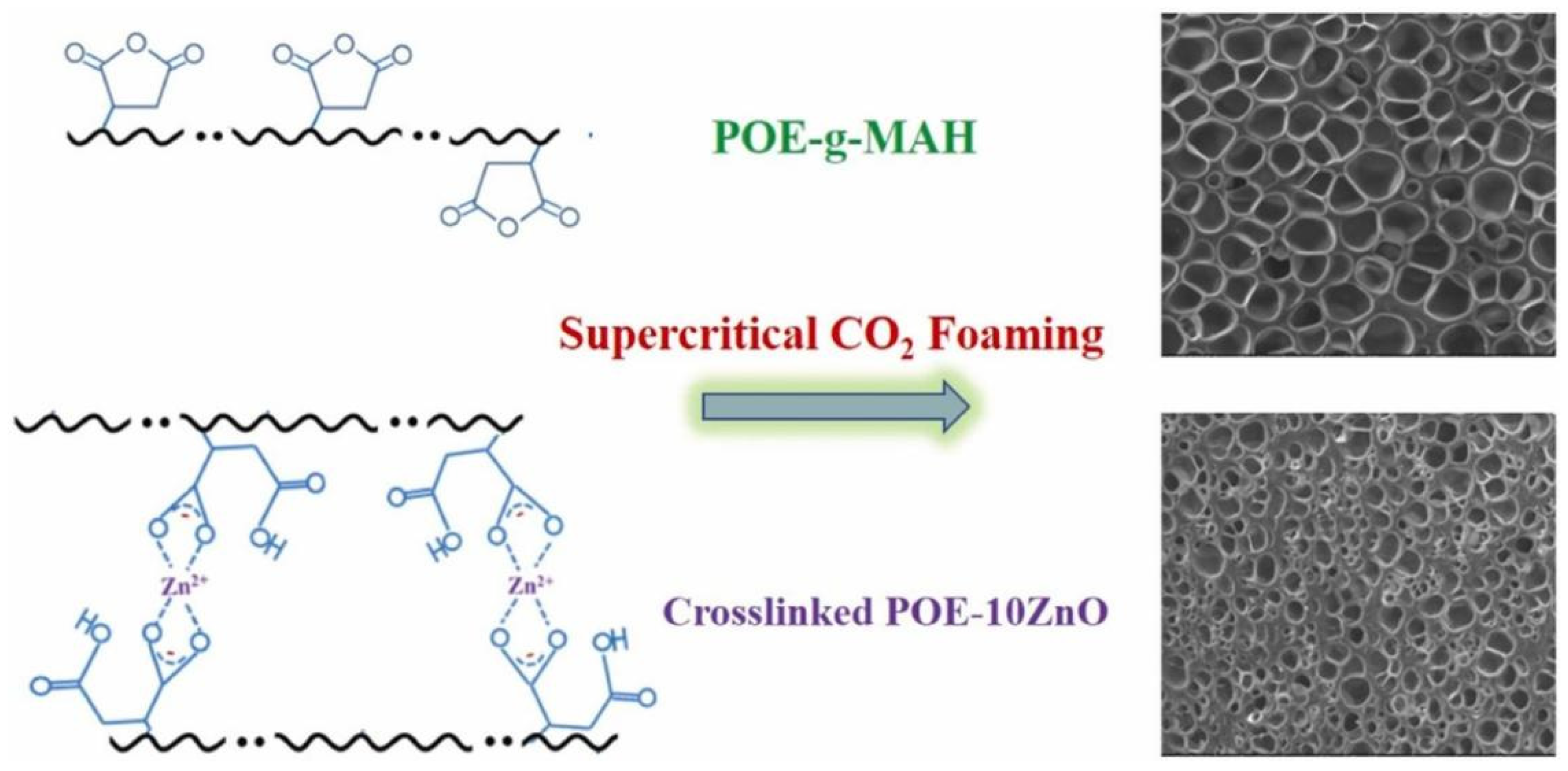
Figure 5 illustrates the successful synthesis of ionically cross-linked poly(ethylene oxide) (POE) via the incorporation of zinc oxide (ZnO) and stearic acid (StAc), resulting in a cross-linked POE that demonstrates improved tensile strength and enhanced anti-creep characteristics. Zhang et al. successfully grafted maleic anhydride (MAH) onto polyolefin elastomer (POE), resulting in POE-g-MAH, which was subsequently combined with ZnO and StAc to fabricate ionically cross-linked POE/ZnO composite materials through a straightforward melt mixing technique. The results showed that the POE-10ZnO foam exhibited good rebound resilience and high compressive strength [
131].
4.5.2. Azoic Cross-Linking
This method [
132]entails incorporating a azoic compound into PE and extruding it at a temperature below the decomposition point of the azoic compound. The extrusion objects then traverse through a high-temperature salt bath, where the azoic compound decomposes to generate free radicals, which in turn initiate cross-linking of PE. Azo compounds, such as aliphatic azo esters (2,2′-azobis [2-ethoxypropane]), are used as initiators for cross-linked PE. This technique is limited practical utility for plastics. Compounds featuring azobenzene motifs, frequently employed as initiators for free radical polymerization, exhibit a propensity for facile thermal degradation, photo-irradiation, or mechanical incorporation into polymers. Such derivatives containing azobenzene groups often function as macro-initiators in the synthesis of well-defined block co-polymers or the development of thermally degradable materials [
133].
5. Applications of Cross-Linked Polyethylene
Cross-linked polyethylene demonstrates superior mechanical properties, heat resistance, chemical resistance, and insulation compared to standard polyethylene. It is employed as an insulating material and coating for high-pressure and heat-resistant applications such as rockets, missiles, motors, transformers, among others. Furthermore, it is utilized in the production of heat-shrinkable tubing, various heat-resistant pipes and tubes, foam plastic products, corrosion-resistant chemical equipment components and containers, as well as flame-retardant building materials. The products of cross-linked polyethylene encompass pipes and cable materials. Currently, its most significant application areas lie within wires and cables, pipes and tubes, and foam plastics.
5.1. Wire and Cable Insulation Materials
In high-voltage cables, the electrical insulation performance is of paramount importance [
1,
13,
134,
135,
136]. The multiple layers of insulation surrounding typical high-voltage cables serve various crucial functions and possess important properties. The outer layer typically consists of polymers such as polyvinyl chloride or polyethylene [
137], providing excellent mechanical resistance at a low cost. As current is transmitted through high-voltage cables, the conductor temperature rises. Therefore, cross-linked polyethylene cables can transmit more power than non-cross-linked ones. Currently, XLPE, due to its simple extrusion manufacturing process and good electrical performance including thermal stability and mechanical properties, serves as the primary insulating material for HVAC power cables [
138].
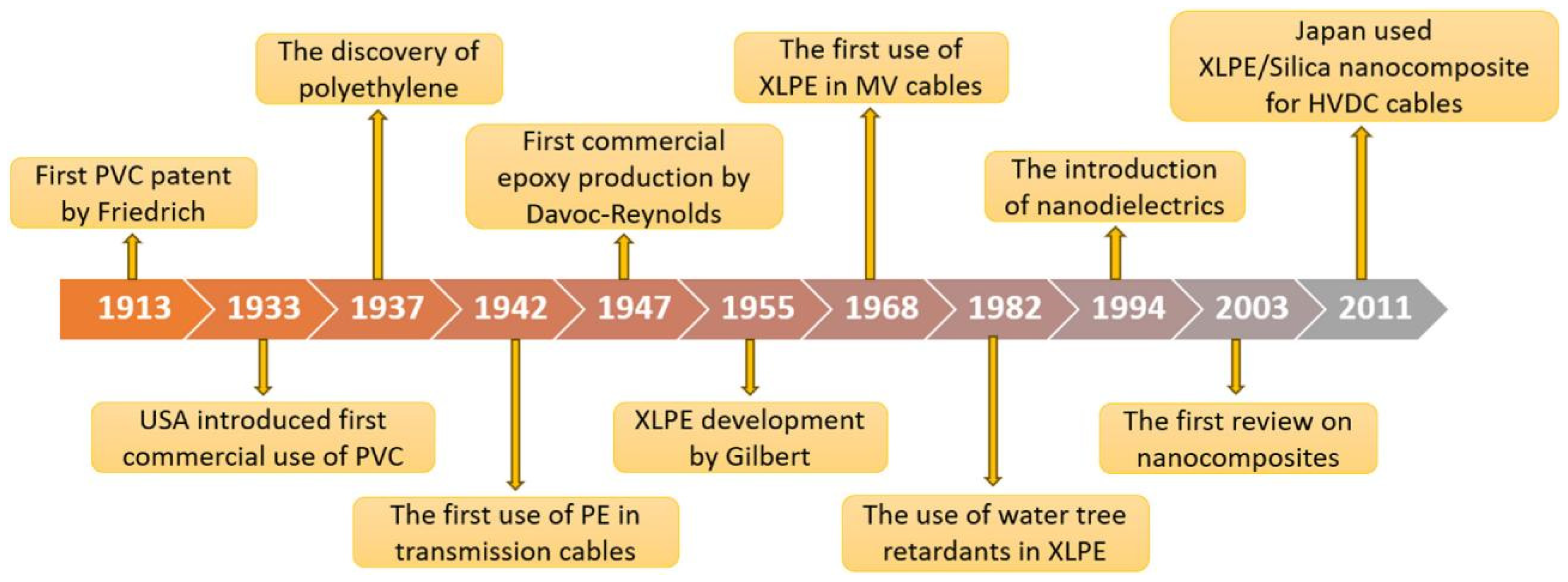
Figure 6 depicts the development history of insulated cables. It can be seen that the development of insulated cables has experienced a number of important milestones, which have not only promoted the progress of power, communications and other industries, but also greatly enriched people’s lives.
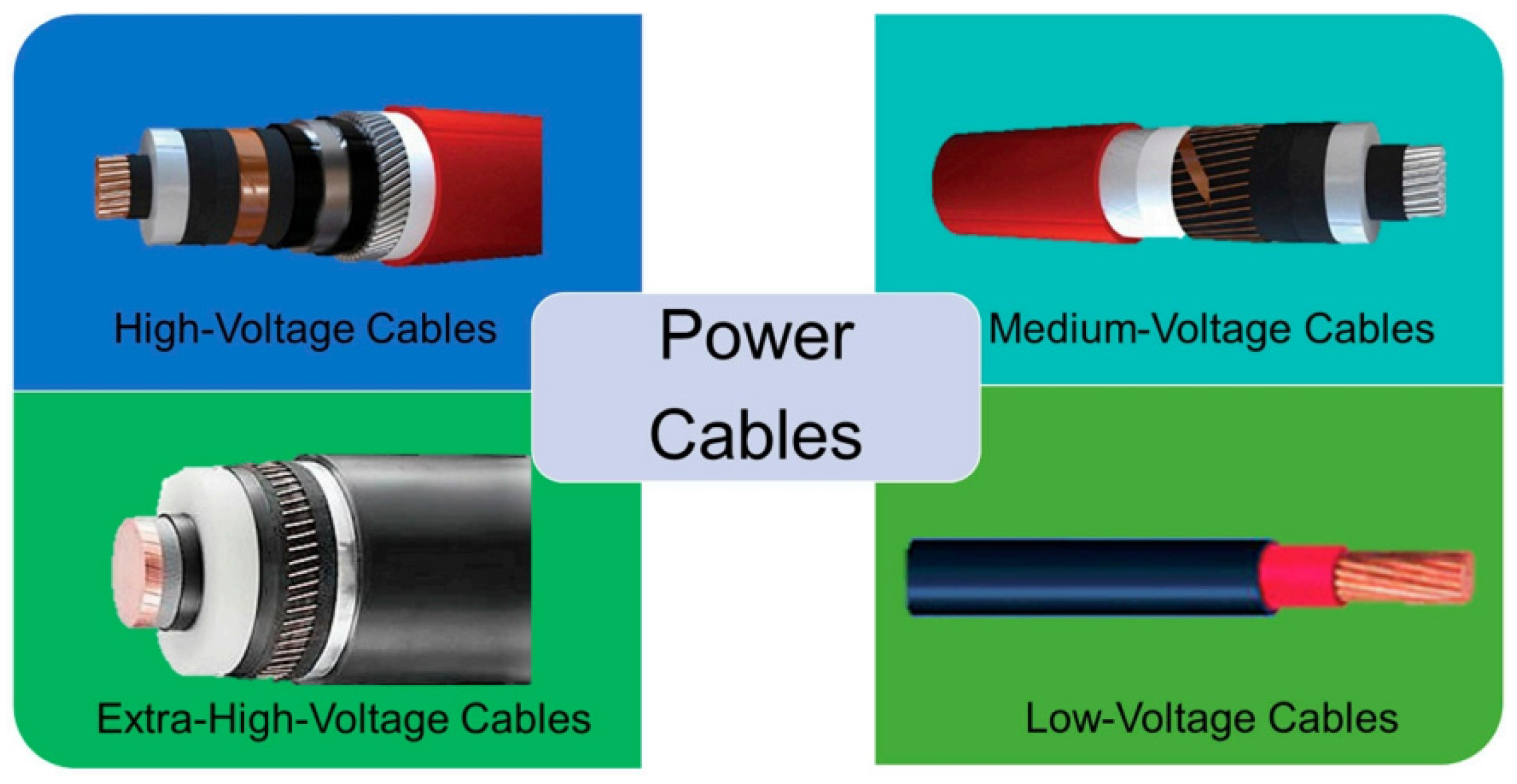
The cross-linked polyethylene cable material (
Figure 7) offers several advantages, including excellent wear resistance, environmental stress cracking resistance, high insulation resistance, and lightweight properties [
48]. It is primarily utilized as an overlay material [
140,
141] for electric wires and cables. The polyethylene cable sheath material serves as the protective sheath layer of cables, providing protection for optical cables, power cables, communication cables, etc. This material is available in LLDPE, HDPE, and MDPE variants-all of which are black to provide UV protection [
142]. Power cable sheaths can be made from either polyethylene or PVC materials [
143,
144]. The production process for polyethylene sheath materials does not require specialized equipment and can be achieved using twin-screw extruding machines and kneaders. Liu et al. developed a series of innovative halogen-free flame-retardant poly(vinyl chloride) composite insulating materials utilizing organic-inorganic flame-retardant blend agents [
145]. They incorporated CP-Allyl, a compound exhibiting both flame-retardant and radiation-sensitizing properties, into a matrix composed of low-density polyethylene and ethylene-vinyl acetate co-polymer through melt blending techniques. Furthermore, by subjecting the composite material to electron beam irradiation at doses ranging from 100 to 190 kGy, they facilitated cross-linking of the material and established correlations between the irradiation dose, degree of cross-linking, and resultant material properties. The findings indicate that the radiation cross-linking composite containing functional cyclotriphosphazene derivatives exhibits exceptional mechanical strength, flame resistance properties, and electrical insulation characteristics. Specifically, the mechanical strength exceeds 14.5 MPa, the limiting oxygen index ranges from 28.2% to 32.4%, and resistance surpasses 2.47×10
12 Ω, rendering it highly promising for applications in electrical wires and cables.
Cross-linked polyethylene forms a three-dimensional network structure, resulting in significantly enhanced heat resistance. Moreover, it exhibits shape memory functionality [
146,
147], enabling it to return to its pre-cross-linked state when heated. By harnessing these post-cross-linked characteristics, cross-linked PE products are widely utilized in applications such as heat shrink tubing, cables, heat-resistant pipes, and shrinkable films. The application of PE-based heat shrinkable materials in cable accessories encompasses indoor flame-retardant, outdoor, semi-conductive, stress control, and underground insulation heat shrink materials [
148,
149]. These materials have diverse requirements and formulations but all exhibit shape memory functionality. They are currently widely utilized and hold promising prospects for further development [
14].
5.2. Piping System
Compared to traditional plastic pipe materials, XLPE pipe materials offer numerous advantages, including extended service life, corrosion resistance, excellent heat resistance, strong impact performance, high creep strength, non-toxicity, and good pressure resistance [
150]. They are considered a new generation of environmentally friendly pipe materials [
151] and can be utilized for fluid transport pipelines in the petroleum industry and household water heater systems [
152,
153]. The raw materials used to produce peroxide-XLPE heat shrinkable tube products include PE, peroxide cross-linked agents, and antioxidants [
154]. XLPE polyethylene pipe materials demonstrate superior heat resistance and aging properties as well as exceptional corrosion resistance and mechanical performance compared to other cross-linked methods [
155,
156,
157,
158]. They are widely employed both domestically and internationally for the conveyance of gas, hot water, and other fluids with promising prospects for practical application.
XLPE, commonly used in many hot and cold water pipe systems, is typically produced from HDPE [
151,
159]. When utilizing various sizes of metal tubes, insert them into the heat shrink tubing and apply hot water or hot air to contract and secure it [
13]. This method is commonly employed for insulating and protecting internal wiring connections, wire terminals, and waterproofing communication cable joints. The use of shape memory heat-shrink materials in manufacturing cable accessories not only facilitates easy and quick installation but also ensures reliable performance at a low cost [
160,
161].
Due to the outstanding performance and complete non-toxic hygiene of cross-linked polyethylene pipe materials, they are regarded as a new generation of environmentally friendly pipes [
162]. They are primarily utilized in the following applications: 1) cold and hot water supply systems for buildings and potable water distribution systems [
163]; 2) air conditioning chilled water systems for buildings; 3) residential heating systems; 4) ground heating systems; 5) piping for domestic hot water heater systems [
10]; 6) fluid transport pipelines for beverages, alcoholic drinks, milk, etc., in the food industry; 7) fluid transport pipelines for chemical and petroleum industries; 8) pipelines for refrigeration systems and water treatment systems [
164]. Al-Malaika et al. examined the effectiveness of synthesized reactive antioxidants (r-AOs) with blocked phenolic and amine groups for grafting onto polyethylene, as well as their retention and stabilizing capabilities in peroxide-cross-linked polyethylene (PEX
a) pipes [
155]. The results revealed that adding graftable antioxidants did not affect the cross-linking density of PEX
a pipes, which consistently demonstrated a high level of cross-linking (>85%) and significant retention after solvent extraction from PEX
a materials. Additionally, the distribution of these antioxidants was notably uniform throughout both the length and thickness of the PEX
a pipes. The stabilizing performance of r-AOs was compared to that of conventional PEX
a materials stabilized with similar antioxidant functionalities produced through analogous methods. Across all testing conditions utilized in this research, the retention rates and long-term stabilization efficacy of graftable antioxidants within the pipes were markedly superior to those seen with traditional antioxidants.
5.3. Packing Material
Cross-linked technology has been demonstrated to be effective in the field of polyethylene materials foaming, as it functions to stabilize bubbles throughout the foaming process, enhance thermal resistance, and improve mechanical properties [
165,
166]. The performance of cross-linked polyethylene foam depends on both the cross-linked process (whether chemical or physical) and the extrusion process (also whether chemical or physical). Polyurethane foam demonstrates exceptional resistance to low temperatures, weathering, abrasion, chemicals, and thermal transfer. It also offers excellent insulation properties, buoyancy, and cushioning effects while remaining odorless and non-toxic [
167]. Moreover, it exhibits favorable secondary processing capabilities including cutting, thermoforming, embossing, vacuum-forming, and compatibility with other materials.
The closed-cell cross-linked polyethylene foam exhibits excellent mechanical properties, making it well-suited for a diverse range of applications such as providing insulation and protection for pipes, ducts, containers, and process equipment to prevent condensation, conserve energy, and block sound transmission [
168]. Additionally, the application of an aluminum sheet covering ensures both its mechanical and thermal properties are maintained, rendering it ideal for roof insulation. However, closed-cell foam is not suitable for applications requiring high gas or vapor permeability or selective permeability or absorption and damping of sound. In contrast, open-cell cross-linked polyethylene foam possesses these characteristics and may be more suitable for applications such as filters, separation membranes, diapers [
169].
Polyethylene materials thermal shrinkable films exhibit the unique ability to contract both longitudinally and transversely when exposed to heat [
170,
171,
172]. Once properly sized and applied to the surface of the product, they are passed through a hot air tunnel. Upon reaching an optimal temperature, the film undergoes significant contraction in both length and width, tightly enveloping the product’s surface to create a secure packaging layer. The resulting packages boast excellent sealing and moisture-proof properties, making them suitable for packaging metal parts and food items. The materials utilized in the production of thermal shrink film include PE [
173], polyvinyl chloride (PVC) [
174,
175,
176], polyester (PET) [
174,
177,
178], and polypropylene (PP) [
179,
180,
181] among others. Polyolefins foam (POE) [
182]is utilized in medical applications such as fillers for prosthetics and sports padding within plastic surgery. Additionally, it may serve as an alternative to EPDM foam material aviation, aerospace, and military contexts.
Cross-linked polyethylene foam offers diverse utility. In construction, it functions as both heat preservation and insulation and cushioning material for roof insulation, frost-resistant sheets, and related uses [
183]. In industry, it serves as thermal insulating material for cold storage and pipe installations [
184,
185,
186,
187]. Automotive aspects, it finds application in interior ceiling decoration, luggage compartments, floor mats, thermal insulation pads, sidewall panels, and waterproof curtains for car doors. Furthermore, in packaging, it acts as cushioning and protective materials within the realms of household appliances, medical equipment, glassware, and fruit. Additionally, these versatile materials employed as an insulating agent within agriculture. Xia et al. created two controllable cross-linked polyethylene foam systems by employing 2,5-dimethyl-2,5-bis(tert-butyl peroxy)hexane as the cross-linking agent, TEMPO to inhibit cross-linking, azodicarbonamide (AC) for foaming purposes, and citric acid as a promoter [
166]. They examined the density, expansion rate, cell structure, and mechanical properties of these two materials with controllable characteristics. The findings reveal that both systems display comparable performance trends: an increase in either the quantity of cross-linking inhibitor or foaming promoter results in larger pore sizes and higher expansion rates while simultaneously reducing density and mechanical strength. A comparative assessment indicates that the polyethylene foam system enhanced with citric acid as a foaming aid exhibits superior performance. Hong et al. chemically modified lignin using poly(ethylene-methacrylate) (PEGMA) to synthesize PEGMA-g-lignin (PEGMA-g-Lignin) [
188]. Both the original lignin and PEGMA-g-lignin were effectively integrated into the LDPE matrix. The analysis of thermal behavior and mechanical properties of the resulting biocomposite materials demonstrated that enhanced mechanical characteristics could be achieved without compromising the thermal properties of the LDPE matrix. Moreover, the foaming characteristics of these biocomposite materials, in conjunction with azodicarbonamide (a chemical blowing agent) and diaminodiphenylamine (a cross-linking agent), were investigated. The incorporation of 20 wt% PEGMA-g-lignin resulted in a high-quality lignin-reinforced LDPE biocomposite foam, which exhibited a uniform distribution of pore size frequency, comparable pore density, and an expansion rate akin to that observed in pure LDPE foam.
5.4. Other Materials
Cross-linked polyethylene is widely used in various applications, such as electric wires and cables, pipes, automotive components, foam materials, sports equipment, and packaging materials. In conclusion, the study of cross-linked polyethylene holds significant importance for advancing science and technology while also making substantial contributions to economic and social development as well as environmental protection.
5.4.1. Auto Parts
Polyethylene materials elastomer (POE), a novel thermoplastic elastomer (TPE), finds extensive applications in the automotive sector [
13]. Stringent environmental regulations call for lightweight [
189,
190], halogen-free, and recyclable materials in automotive manufacturing. Consequently, cross-linked POE can serve as an alternative to PVC for producing decorative materials used on automotive surfaces or interiors. cross-linked POE demonstrates exceptional heat resistance and resistance to permanent compression set deformation, which can be further improved through compounding and incorporating fillers [
34]. In thermoplastic blends containing tire rubber, the interaction of free radicals generated by organic peroxides provides a possibility for molecular cross-linking between rubber and matrix phases. For example, Li et al. ‘s research shows that combining DCP with other organic compatibilizers in polyethylene-tire rubber composites can obtain excellent impact strength and elongation at break [
191]. However, the mechanical properties of polythylene-tire rubber composites are poor in terms of tensile strength and hardness [
192].
5.4.2. Sport Equipment
Modified polyethylene is distinguished by its exceptional wear resistance, low coefficient of friction, water resistance, excellent impact resistance, chemical corrosion resistance, and outstanding low-temperature and electrical insulation properties. It finds extensive application in the manufacturing of sports equipment. In the realm of sports equipment, a variety of modified polyethylene products are widely utilized in ball sports, water sports, and ice sports. The use of modified polyethylene can enhance the impact resistance [
193,
194], heat resistance [
195,
196], and shock absorption [
183,
197] of sports equipment. This not only enhances athletes’ comfort during exercise but also safeguards them while prolonging the service life of sports equipment and reducing costs. Modified polyethylene exhibits superior wear resistance which further improves with increasing relative molecular mass [
132,
198]. Compared to unmodified polyethylene, modified polyethylene has a lower coefficient of friction making it an ideal lubricating plastic. Additionally, it absorbs minimal water with one of the lowest rates among most engineering plastic products; even when soaked in rainwater it does not swell.
The modified polyethylene has specific applications in the sports equipment field as follows: 1) Applying modified polyethylene to the fireproof layer of artificial grass can render the grass fiber soft, enhance its wear and chemical resistance, thereby improving the safety of artificial turf. 2) Sports equipment crafted from modified polyethylene exhibits excellent flexibility [
199] and robust shock absorption [
200], offering protection for athletes and suiting high-intensity sports such as football and American football. 3) Modified polyethylene fibers are utilized to laminate the surface, core, and base of a snowboard sequentially [
201]. In certain high-performance ski boards, a surface material made of cross-linked polyethylene may be utilized to optimize glide performance and enhance durability. By integrating a variety of materials such as wood, carbon fiber, or alternative plastics, cross-linked polyethylene can enhance the structural integrity of ski boards and improve their ability to withstand impacts [
202]. Furthermore, its exceptional water resistance effectively protects against material deterioration in slippery conditions, ultimately extending the overall lifespan of the ski board [
203]. Tomin et al. conducted a comprehensive study on various densities of XLPE foam, which is widely recognized as one of the most prevalent foam types in the sports and packaging sectors due to its superior heat resistance and enhanced recovery from mechanical loads attributed to its relatively weak cross-linked structure [
193]. The research involved compression tests alongside two dynamic mechanical assessments performed on closed-cell XLPE foam with densities ranging from 30 to 70 kg/m³ and thicknesses between 10 and 60 mm. The findings indicate that the thickness of the foam significantly influences its impact damping performance, while the energy absorption capacity is primarily determined by both the structural characteristics and density of the material. Excessive compaction can lead to irreversible micro-structural deformation; consequently, repeated impacts may compromise both impact damping and energy absorption capabilities of the foam material. These insights have valuable implications across various industries, including sports equipment manufacturing and packaging solutions.
5.4.3. Food Packaging
The metal content in polyethylene materials elastomer (POE) is minimal, and it boasts a high level of purity, rendering it highly suitable for applications in medical equipment and food packaging [
204,
205,
206]. The majority of polyethylene is utilized for film products, with most being employed for packaging purposes. Additionally, a portion is used as agricultural film and building film. Polyethylene packaging film finds extensive use in the food industry for wrapping candies, vegetables, frozen foods, etc., while also serving as inner lining film, shrink packaging film, elastic packaging film, and heavy-duty packaging film for non-food items [
207]. Manukumar et al. developed a novel stable antibacterial packaging film by using a new photo-cross-linking technology to cross-link cinnamaldehyde (Cin-C) onto LDPE. This new approach is highly promising for developing food packaging containing natural antibacterial agents targeting cell membrane components [
208].
6. Challenges and Limitations
Cross-linked polyethylene is a commonly employed material, especially in the fields of pipe and cable insulation; however, it also faces significant challenges [
92]. The primary challenge for cross-linked polyethylene lies in the fact that the insulated cable is subjected to various stresses, including heat, electricity, and mechanical forces during operation. These factors interact with one another, resulting in space charge accumulation, accelerating the aging of the cable insulation, and posing a threat to the stability of cable operation. The aging process [
209,
210,
211] of cross-linked polyethylene materials typically takes place under various environmental factors such as electric fields, thermal conditions, oxygen exposure, moisture ingress, mechanical stress, light exposure, chemical substances, and microbial presence encountered during use. These factors can cause irreversible damage to the insulation material and even lead to power accidents, presenting incipient fault to the power grid’s operation. Similar to pipelines, cable materials are poor conductors of heat and electricity, yet due to the existence of micro-cavities, gas particles, and conductive particles, water-tree formation occurs. Water trees do not necessarily result in medium failure, but in certain cases, it can directly lead to electric tree development. The incorporation of hydrophilic clusters can effectively inhibit the formation of water trees, but improper addition may compromise the dielectric strength. Therefore, it is imperative to develop strategies for mitigating water tree formation without compromising dielectric performance. Cross-linked polyethylene cables and pipes encounter substantial challenges in resisting moisture and corrosive chemicals due to their inherent nature. Introducing self-healing properties has been necessitated by difficulties in detecting and rectifying damages sustained by these cables and pipes [
212].
An additional obstacle confronting both cross-linked polyethylene itself as well as its products pertains to waste disposal and recycling issues. The complexities associated with recycling this material contribute significantly towards environmental pollution [
213,
214]. Moreover, when compared to traditional materials, the initial cost of cross-linked polyethylene may be higher, especially when assessing its cost-effectiveness on a large scale of deployment. Considering these limitations, what trajectory can be anticipated for cross-linked polyethylene? Let’s observe and analyze.
7. Research Trend
The development trend of cross-linked polyethylene primarily focuses on three areas: nanocomposites, biodegradability, and recycling. These research directions not only enhance the performance and application range of cross-linked polyethylene but also stimulate the creation of new products and market expansion. As science and technology advance, the cross-linked polyethylene industry will continue to innovate, leading to broader prospects for development.
7.1. Nanocomposite Material
Materials of polyethylene nanocomposite with enhanced high-voltage direct current (HVDC) performance are crucial for the research and development of new insulating materials [
48]. This approach holds great promise for expanding applications in high-voltage direct current transmission cables. The incorporation of small amounts of inorganic nanoparticles [
215] into insulating materials can effectively enhance their electrical properties, including improving dielectric strength, volume resistivity, and modifying spatial charge behavior [
216,
217,
218,
219].When nanoparticles are functional modification with polyethylene materials, the resulting material demonstrates significant enhancements in terms of strength, toughness, and elongation. Various types of nano reinforcements have been documented so far, including grapheme [
220], layered silicates [
221], nanoscale silica, carbon nanotubes (CNTs), and polyhedral oligomeric silsesquioxane [
160,
222] (POSS). The primary techniques for fabricating polyethylene materials nanocomposites [
223] involve blending, embed [
224], and in-situ polymerization.
The research into enhancing the electrical insulation performance of polyethylene insulating materials through the incorporation of grapheme oxide (GO) has emerged as a burgeoning area of study. Research indicates that the introduction of GO into the LDPE matrix can create numerous deep traps within the matrix, impeding the movement of charge carriers and thereby restraining the injection and accumulation of space charges. The grapheme nanoparticles establish multiple interaction regions in the nanocomposite material, elevating the trapping energy level of cross-linked polyethylene [
225]. Grapheme nanoparticles create numerous interconnected zones within nanocomposite materials, resulting in a significant increase in breakdown strength, minimal accumulation of space charge, and improved electrical conductivity in cross-linked polyethylene/GO nanocomposites [
226]. Han et al. found that adding nano-graphene oxide to the XLPE could effectively suppress the space charge accumulation and increase its breakdown strength [
227].
Boron nitride (BN) filler not only has excellent thermal conductivity and insulation properties but also demonstrates thermal stability, strong mechanical attributes, and resistance to both corrosion and oxidation. Li et al. utilized a melt blending method to create XLPE/BN nanocomposites with different concentrations of fillers, incorporating boron nitride nanoparticles (BNNP) and boron nitride nanoflakes (BNNS) as the nanofillers, while XLPE, designed for high-voltage direct current (HVDC) cable applications, acted as the matrix material. The introduction of BNNS and BNNP enhances the dielectric constant of the composite materials [
228]. The peak relative permittivity is observed at a concentration of 1 wt%, measuring 2.35 for XLPE/BNNSs and 2.32 for XLPE/BNNP—an increase of approximately 11% and 9%, respectively, compared to that of the pure XLPE matrix. For both BNNSs and BNNP composites, the breakdown strength initially rises before declining as BN concentration increases; it reaches its highest value at a concentration of 0.5 wt%. Specifically, these values are recorded at 403.8 kV/mm for XLPE/BNNSs and 349.2 kV/mm for XLPE/BNNP—representing enhancements of about 33% and 13% over that of the unmodified XLPE matrix.
Carbon nanotubes (CNTs) exhibit good dispersion and compatibility within the HDPE matrix; their incorporation enhances the yield strength and tensile modulus of the composite material, while also improving processing performance [
229].
Cross-linked polyethylene composite silica nanomaterials have a wide range of applications. In the construction industry, these materials can be used for thermal insulation of building walls, roofs, floors, and other components, effectively reducing energy consumption and enhancing the energy-saving performance of buildings. In industrial equipment applications, this material is suitable for thermal insulation in various industrial equipment such as petrochemical facilities, boilers, pipelines, etc., thereby improving equipment insulation and reducing energy loss. For automotive manufacturing purposes, this material can be employed for soundproofing, heat preservation, and sound absorption to enhance in-car comfort and reduce noise levels [
230]. In cold chain logistics scenarios, it is applicable to the cold chain logistics industry by providing effective thermal insulation in cold storage and freezing environments to maintain product quality and freshness [
231]. Cross-linked polyethylene composite silica nanomaterials demonstrate exceptional thermal insulation, high strength, temperature resistance, corrosion resistance, and aging resistance, as well as environmental sustainability [
110]. As a result, they have extensive applications in these fields. Additionally, the material also possesses environmentally sustainable characteristics that align with current requirements for environmental protection and sustainable development [
232]. In summary, cross-linked polyethylene composite silica nanomaterials hold promising prospects for broad application in construction, industry, automobiles, etc [
233]. Liu et al. developed composite materials (PE-Al-Si) exhibiting enhanced thermal conductivity and mechanical properties by melt blending LDPE, aluminum oxide (Al
2O
3), and nano-silica (SiO
2). The resulting material was subsequently modified through electron beam irradiation. When the mass fraction of nano-SiO
2 was 1% and the electron beam irradiation dose reached 120 kGy, the thermal conductivity of PE-Al-Si attained 0.759 W/(m·K), representing a 22% increase compared to the composite material (PE-Al) devoid of SiO
2. Furthermore, the tensile strength of PE-Al-Si improved from 21.6 MPa to 26.2 MPa, marking a 17% enhancement relative to PE-Al. This demonstrates that SiO
2 not only augments the mechanical properties of the composite but also enhances its radiation efficiency and thermal conductivity [
234].
In order to enhance the electrical insulation properties of polyethylene materials insulating materials, two main technologies are primarily utilized: ultrapure technology and composite material technology. However, ultrapure technology is associated with high industrial costs and is constrained by the maximum direct current breakdown strength of polyethylene materials insulating materials. On the other hand, composite material technology (
Figure 8) is predominantly restricted to blending polyethylene materials with polymers and doping polyethylene materials with nanoparticles. Moreover, it is challenging for modified nanoparticles to effectively interact with non-polar polyethylene materials. Consequently, novel technologies are urgently required for enhancing the electrical insulation properties of polyethylene materials insulating materials in high-voltage direct current power transmission systems and reducing China’s long-term reliance on imported insulating materials for high-voltage direct current cables [
216].
7.2. Restoration
XLPE has undergone significant enhancements in its physical and chemical properties due to cross-linking modifications. XLPE cables are extensively utilized in urban and industrial power supply system owing to their advantages of flexible spatial configuration, excellent electrical and mechanical properties, and high reliability in power delivery. However, during practical applications, the material is inevitably subjected to various external factors such as mechanical stress, chemical corrosion, and temperature fluctuations, which can lead to a deterioration in performance or even structural damage. Through advanced repair technologies, it is possible to restore the original performance of cross-linked polyethylene, thereby extending its service life and reducing both replacement frequency and associated costs.
Currently, repair systems are classified into two categories: endogenous and exogenous [
235,
236,
237]. Endogenous repair primarily leverages the material’s inherent properties for restoration, utilizing reversible valence bond alterations and chemical reactions to modify the intrinsic chemical structure of the material, thereby facilitating damage repair. However, the application of intrinsic repair systems to insulating materials poses challenges due to valence bond reactions that can alter the substrate’s chemical structure and significantly impair its fundamental properties. Exogenous repair materials are composite substances composed of foreign elements and a matrix, aimed at restoring the integrity of the matrix. Exogenous repair systems primarily encompass micro-capsules, bio-mimetic blood vessels, and hollow fibers [
238].
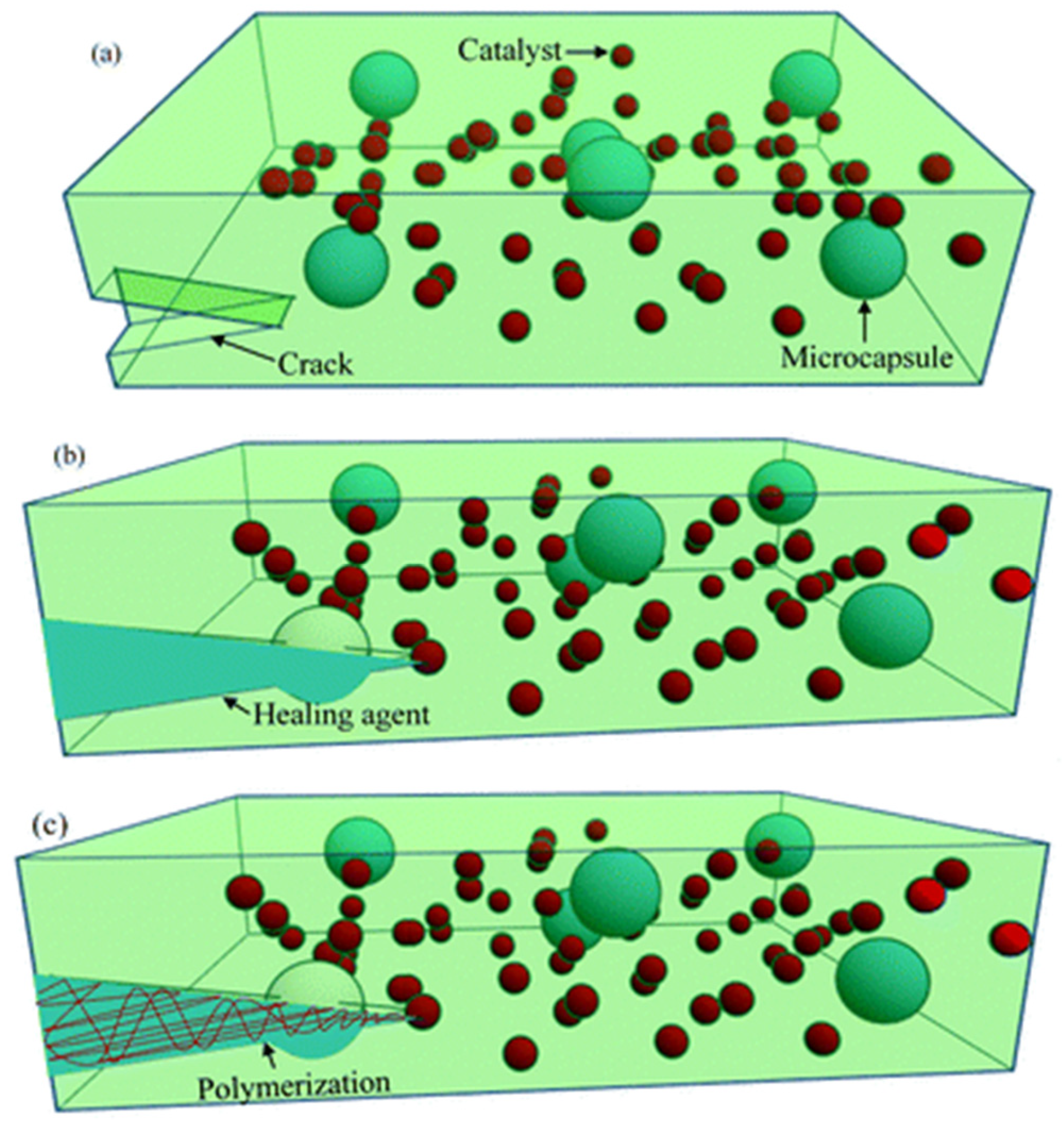
The micro-capsule repair system not only exhibits excellent stability and a high repair efficiency but also exerts minimal influence on the properties of the matrix [
239]. This innovative micro-capsule repair technology was initially introduced by White et al. [
240], involving encapsulating the repair material within specialized micro-capsules that are integrated into the matrix material. Upon damage to the matrix, stress concentration at the affected site induces rupture of the micro-capsule. In response to external stimuli, the internal reparative agent rapidly solidifies and fills in the compromised region, thereby facilitating the material’s restoration process.
Figure 9 illustrates the mechanism underlying micro-capsule repair. Zhu et al. proposed a micro-capsule-based XLPE repair material to address the degradation of insulation performance caused by water tree aging in XLPE. UF@SiO
2, modified with nano-silica dioxide (SiO
2), was utilized as the wall material, while octadecyl trimethoxysilane (DTMS) and linear alkylbenzenesulfonic acid (LABSA) were selected as core materials [
241]. The three-layer shell-core structure micro-capsules were synthesized through interfacial polymerization. The preparation method for the three-layer shell microcapsule/XLPE composite material was investigated. Results indicate that an increased doping concentration of the three-layer shell-core micro-capsules leads to a significant decline in the electrical performance of the composite compared to XLPE without microcapsules. At a doping concentration of 1.0 wt%, there is no substantial change in breakdown strength or dielectric loss factor; however, spatial charge density decreases significantly, resulting in markedly improved space charge performance. Furthermore, during water tree aging tests, the composite demonstrated effective inhibition of water tree growth and notable repair capabilities post-aging due to the presence of micro-capsules. Additionally, nanoscale SiO
2 on the surface of these micro-capsules enhances their adhesion to the matrix, preventing common issues related to capsule aggregation when incorporated into composites and thereby improving repair efficiency. Zhou et al. investigated the impact of a novel repair solution on the restoration of aging cables affected by water tree phenomena [
242]. The repair solution incorporated long-chain siloxanes and voltage stabilizers, and the long-term anti-aging performance against water tree degradation in the repaired cables was evaluated through a secondary water tree aging test. Concurrently, a comparative analysis was conducted to assess the effects of both repaired and unrepaired conditions on the initiation and growth of electrical trees, elucidating the operational mechanisms of the repair solution as well as examining the degradation trends associated with voltage stabilizers.
7.3. Reutilization
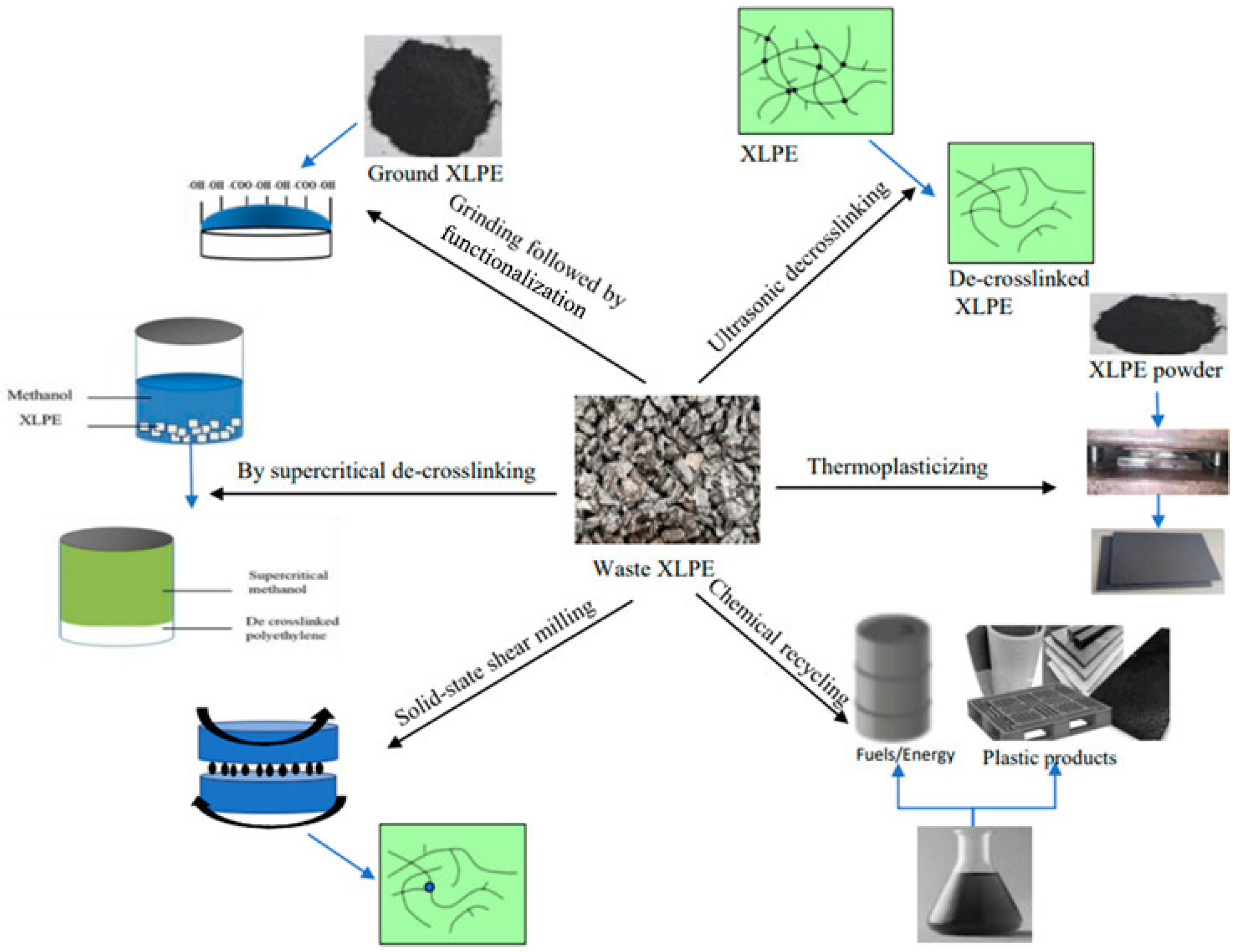
The significance of plastic waste recycling cannot be overstated in addressing global environmental issues (
Figure 10) [
244]. By processing discarded waste and utilizing it as a new energy source, recycled products are created. Cross-linked polyethylene is widely utilized as a thermosetting plastic due to its cost-effectiveness, lightweight nature, and exceptional insulation properties against electricity and heat [
244]. It is commonly used as an insulating material for wires and cables. As a thermosetting plastic, cross-linked polyethylene exhibits limited fluidity and poor plasticity. Additionally, chemical bonds exist between its molecular chains, causing the molecular structure to transition from linear to three-dimensional, resulting in a thermosetting material that does not melt upon heating and is difficult to dissolve in most solvents [
245]. Consequently, recycling cross-linked polyethylene presents significant challenges but holds great importance. The irreversible covalent cross-linking in cross-linked polyethylene makes its recycling a highly challenging task. An energy-efficient, economical, and environmentally friendly recycling process is essential to protect our ecosystem.
Disposal of cross-linked polyethylene through landfill dumping poses environmental concerns due to the material’s stable properties that prevent decay over extended periods. This not only occupies limited land resources but also contributes to soil and water pollution. Incineration releases toxic and harmful gases that can adversely impact the ecological environment. Therefore, research into scientific and effective technological methods for the recycling and reuse of waste cross-linked polyethylene and other thermosetting plastics holds significant implications for resource regeneration and environmental protection [
159,
246]. Recycling methods for cross-linked polyethylene include grinding [
247], thermal-assisted shearing, supercritical fluid processing, ultrasonic-assisted single-screw extrusion, as well as solid-state mechanical-chemical grinding.
7.3.1. Powdered Filler Recovery Method
This approach primarily entails employing basic recycling techniques to recover discarded cross-linked polyethylene edge trim through sorting, cleaning, and crushing it into powdered fillers [
248]. Subsequently these fillers undergo direct processing via blending with other thermoplastic polymers [
249]. The recycled powdered filler regeneration technology offers a streamlined process with low costs and minimal investment requirements; however product quality cannot be assured as they fail to match those derived from pure materials in terms of strength, elasticity, toughness, durability or any other aspect, resulting in significant limitations on their applications [
250]. Hence, it is anticipated that leveraging more efficient grinding equipment and compatibility technology will enhance the performance characteristics of this blended system. White et al. systematically investigated the viability of utilizing recycled XLPE cable material as a powder filler through three perspectives: separation techniques, granule refinement methods, and molding processes [
67].
7.3.2. Hot Shear Plasticizing Recovery Technology
The core of the hot shear plastic recycling process lies in the thermal oxidation and mechanical action that result in the breakdown of the cross-linked three-dimensional structure. Specifically, at certain temperatures and under mechanical shear force, some carbon-carbon bonds and cross-linked carbon-carbon bonds within the main chain of cross-linked polyethylene molecules are partially disrupted, leading to a shift away from a three-dimensional molecular structure and an increase in material thermal plasticity [
251]. Xie et al. examined how roll temperature and the duration of plasticization affect the degradation of waste XLPE scrap using a twin-roll calender, maintaining constant roll speed and gap settings [
252,
253]. Their results indicated that as plasticizing time increased at 170°C, the gel content in XLPE significantly diminished, suggesting an improved level of degradation. Additionally, the recycled XLPE was mixed with a specific ratio of PE-LD prior to molding and foaming processes. The resulting foam material had a density between 518 and 610 kg/m³, making it appropriate for uses such as foam-type cable filler strips and packaging solutions.
7.3.3. Supercritical Fluid Treatment Recovery Method
Waste HDPE is commonly incinerated due to its thermosetting nature, making it non-recyclable. Therefore, there is an urgent need for the development of innovative technologies aimed at recycling cross-linked HDPE to reduce environmental pollution [
254]. Numerous experiments involving cross-linked HDPE have been conducted under various sub-critical and supercritical conditions [
255], with methanol being used as the supercritical solvent [
213,
256,
257]. The DCXLPE foam sheets were fabricated using the supercritical methanol technique. With an increase in the concentration of the cross-linking agent, there was a corresponding elevation in both gel fraction and hardness of the DCXLPE foam sheets, while the expansion rate and cell size decreased. The resulting foam products exhibit analogous structures and properties to traditional low-density polyethylene foam, and also demonstrate potential for exploring new application areas for regenerated cross-linked polyolefins through supercritical de-crosslinked reactions [
258]. Supercritical fluid refers to a liquid that is above its critical temperature and pressure, such as water. In the supercritical state, water demonstrates the following characteristics: (1) It exhibits more vigorous movement than at normal temperatures, with low viscosity and fast diffusion speed; (2) Its dielectric constant is similar to that of polar solvents, enabling potentially insoluble substances in water at normal temperature and pressure to dissolve in supercritical water. Consequently, supercritical water can facilitate the decomposition of plastics containing polar groups like ester, amide, and ether on molecular chains, thereby creating new pathways for recycling waste high-molecular-weight materials [
259]. Lee et al. investigated the de-crosslinking reaction of cross-linked polypropylene (XLPP) and XLPE under supercritical methanol conditions. Baek et al. indicates that the extrusion method can function as a continuous reactor for the de-crosslinking and recycling of XLPE using supercritical methanol [
260]. As both the reaction temperature and methanol ratio increase, the gel content in XLPE progressively diminishes. Despite the fact that XLPE’s retention time in a continuous supercritical extruder with methanol is under 2 minutes, complete de-crosslinking of XLPE can be achieved when temperatures surpass 390°C and the methanol feed rate reaches 7 ml/min.
7.3.4. Ultrasonic Assisted Extrusion Recycling Technology
Ultrasonic fields have the capability to induce high-frequency tensile stresses in various media, while high-amplitude oscillating waves can lead to solid fracture and liquid cavitation [
261,
262]. The phenomenon of acoustic cavitation enables the concentration of energy in local molecular bonds, selectively breaking the lower-energy cross-linked bonds within the three-dimensional network structure while preserving the main chains of macromolecules, thus achieving depolymerization and regeneration [
263]. During extrusion of cross-linked polyethylene, apart from undergoing ultrasonic action, it also experiences significant thermal shear forces, resulting in damage to its cross-linked bonds and potential breakage of the main chains of macromolecules. The characteristic feature of ultrasonic-assisted extrusion recycling technology is a favorable regeneration effect but with low production efficiency [
18,
261]. Exploring methods to economically and efficiently subject materials to ultrasonic action during extrusion for maximal damage to cross-linked bonds while retaining the integrity of macro-molecular chains represents a key research direction for future studies. Lee et al. developed a kinetic model explaining reaction kinetics related to depolymerization of cross-linked low-density polyethylene based on experimental findings; this model illustrated that reaction rates are directly proportional to gel concentration while exhibiting exponential relationships with temperature changes [
264]. The observed process conformed well with first-order reaction kinetics characterized by a kinetic constant of 0.867 cm³/(mg·min) along with an activation energy requirement of 578 kJ/mol under optimal conditions.
7.3.5. Solid Phase Shear Milling Method
The solid-phase shearing milling method is an innovative technique for processing high molecular weight materials using the force chemistry reactor in the form of a grinding disc [
265,
266]. Operating at room temperature in an air medium without chemical initiators or solvents, this method achieves solid-phase force chemistry depolymerization of cross-linked polyethylene to endow it with thermoplastic process-ability while maintaining good mechanical properties of the regenerated material. The solid-phase shearing milling method effectively addresses the challenge of reprocessing cross-linked waste high molecular weight materials, offering a more efficient, economical, and environmentally friendly approach for their recycling and utilization [
246,
267]. Further research should focus on mechanistic elucidation and feasibility analysis for scaled-up production. Wu et al. successfully accomplished the solid-phase force chemistry depolymerization and regeneration of waste XLPE utilizing solid-phase shearing milling technology [
268]. The study revealed that following the solid-phase shearing milling process, the gel content of XLPE was markedly diminished while the relative molecular mass of the soluble fraction exhibited minimal variation, thereby confirming the occurrence of solid-phase depolymerization. Furthermore, the thermoplastic processability of XLPE was enhanced, and the regenerated sheet produced via compression molding demonstrated commendable mechanical properties, with peak tensile strength and elongation at break recorded at 16.4 MPa and 450%, respectively. Sun et al. studied the de-crosslinking mechanism and products of Si-XLPE material in the solid phase shear milling method process by controlling the powdering cycle [
265]. The results indicated that the cross-linked structures were destroyed due to the fracture of Si–O–Si chemical bonds in the solid phase shear milling method process, and the molecular structures of the resulting products prepared by the solid phase shear milling method technology were close to that of silane-grafted PE.
8. Conclusions
This article presents an overview of the classification and related properties of polyethylene, the preparation methods, structural features, and performance advantages of cross-linked polyethylene. The primary cross-linking methods include peroxide cross-linking, silicone cross-linking, radiation cross-linking, and ultraviolet light cross-linking. Each method has distinct advantages and disadvantages that impact the structure and properties of the resulting cross-linked polyethylene materials. Furthermore, the thermal and mechanical properties of these materials are significantly improved through this process which expands their applicability in high-temperature pressure environments.
The article also discusses examples of applications for cross-linked polyethylene different fields including building water supply systems, floor heating systems, and cable materials while analyzing their performance characteristics. Despite possessing high market competitiveness, cross-linked polyethylene still faces challenges such as environmental friend lines sand recycling. Additionally, the article explores future trends for cross-linked polyethylene such as research on bio-based alternatives, green production processes, and further enhancement of material properties. These research directions will help promote broader application and development of cross-linked polyethylene materials.
In summary, this review introduces six aspects: the classification and performance of cross-linked polyethylene, the advantages of cross-linked polymers, cross-linking methods and relevant mechanisms, applications of cross-linked polymers, challenges and limitations faced by cross-linked polymers, and research trends in this field.
Author Contributions
Conceptualization, J.Z., J.L. and B.L.; literature review and resources, J.Z. and Y.L.; methodology, J.L., H.L., M.Y., Y.Q. and Y.X.; investigation, Z.J., J.L and Y.L.; data curation, H.L. and M.Y.; writing—original draft preparation, J.Z. and Y.L.; writing—review and editing, W.H. and B.J.; visualization, J.Z., Y.L., and Y.Q.; supervision, Y.X., W.H. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for this project was provided by the Science and Technology Development Plan of Jilin Province, China (No. 20220201126GX) and the National Natural Science Foundation of China (Nos.: 52073118 and 52073044).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pourrahimi, A.M.; Mauri, M.; D’Auria, S.; Pinalli, R.; Müller, C. Alternative Concepts for Extruded Power Cable Insulation: from Thermosets to Thermoplastics. Advanced Materials 2024. [Google Scholar] [CrossRef] [PubMed]
- Aouinet, M.; Aissat, S. Climatic Ageing of Low Density Polyethylene in Agricultural Greenhouses. Advances in Materials Science 2024, 24, 127–138. [Google Scholar] [CrossRef]
- Cecon, V.S.; Da Silva, P.F.; Vorst, K.L.; Curtzwiler, G.W. The effect of post-consumer recycled polyethylene (PCRPE) on the properties of polyethylene blends of different densities. Polymer Degradation and Stability 2021, 190. [Google Scholar] [CrossRef]
- Jian, X.; Wang, P.; Sun, N.; Xu, W.; Liu, L.; Ma, Y.; Chen, W.-Q. Material flow analysis of China’s five commodity plastics urges radical waste infrastructure improvement. Environmental Research: Infrastructure and Sustainability 2022, 2. [Google Scholar] [CrossRef]
- Galchun, A.; Korab, N.; Kondratenko, V.; Demchenko, V.; Shadrin, A.; Anistratenko, V.; Iurzhenko, M. Nanostructurization and thermal properties of polyethylenes’ welds. Nanoscale Research Letters 2015, 10. [Google Scholar] [CrossRef]
- Karaki, A.; Hammoud, A.; Masad, E.; Khraisheh, M.; Abdala, A.; Ouederni, M. A review on material extrusion (MEX) of polyethylene- Challenges, opportunities, and future prospects. Polymer 2024, 307. [Google Scholar] [CrossRef]
- Gerassimidou, S.; Geueke, B.; Groh, K.J.; Muncke, J.; Hahladakis, J.N.; Martin, O.V.; Iacovidou, E. Unpacking the complexity of the polyethylene food contact articles value chain: A chemicals perspective. Journal of Hazardous Materials 2023, 454. [Google Scholar] [CrossRef]
- Ahmad, H.; Rodrigue, D. Crosslinked polyethylene: A review on the crosslinking techniques, manufacturing methods, applications, and recycling. Polymer Engineering & Science 2022, 62, 2376–2401. [Google Scholar]
- Ding, M.-Q.; Ding, J.; Zhang, Z.-R.; Li, M.-X.; Cui, C.-H.; Pang, J.-W.; Xing, D.-F.; Ren, N.-Q.; Wu, W.-M.; Yang, S.-S. Biodegradation of various grades of polyethylene microplastics by Tenebrio molitor and Tenebrio obscurus larvae: Effects on their physiology. Journal of Environmental Management 2024, 358. [Google Scholar] [CrossRef]
- Schramm, D.; Asme. PE-RT, a new class of polyethylene for industrial pipes6. In Proceedings of the 25th International Conference on Offshore Mechanics and Arctic Engineering (OMAE 2006), Hamburg, GERMANY, 2006 Jun 04-09. [Google Scholar]
- Below, H.; Quilitz, G.; Schumann, W. Electron beam crosslinking of large diameter thick-waited polyethylene pipes. Plastics Rubber and Composites 2005, 34, 34–39. [Google Scholar] [CrossRef]
- Fazal, A.; Hao, M.; Vaughan, A.S.; Chen, G.; Cao, J.; Wang, H.; Ieee. The effect of composition and processing on electric characteristics of XLPE in HVDC cable applications. In Proceedings of the IEEE Electrical Insulation Conference (EIC), Montreal, CANADA, 2016 Jun 19-22. [Google Scholar]
- Rouif, S. Radiation cross-linked plastics: a versatile material solution for packaging, automotive, Electrotechnic and Electronics. Radiation Physics and Chemistry 2004, 71, 527–530. [Google Scholar] [CrossRef]
- Qu, B.J. Recent developments in photoinitiated crosslinking of polyethylene and its industrial applications. Chinese Journal of Polymer Science 2001, 19, 189–207. [Google Scholar]
- Qu, B.; Bao, W.; Wu, Q.; Shi, W.; Ma, S.; Jia, Z.; Ieee. Recent Devolpments on Photoinitiated Crosslinking of Polyethylene and Its Applications for Manufacturing Insulated Wire and Cable. In Proceedings of the 9th International Conference on Properties and Applications of Dielectric Materials, Harbin Univ Sci & Technol, Harbin, PEOPLES R CHINA, 2009 Jul 19-23. [Google Scholar]
- de Sousa Barros, A.B.; Pereira Barros, J.J.; Corte Real Coutinho, S.V.; Calvacanti Albuquerque, A.K.; Siqueira, D.D.; Ries, A.; Ramos Wellen, R.M. Thermal degradation kinetics of industrial batch crosslinked polyethylene. Journal of Applied Polymer Science 2022, 139. [Google Scholar] [CrossRef]
- Kocak, B.; Paksoy, H. Sensible Thermal Energy Storage in Packed Bed for Industrial Solar Applications. In Proceedings of the 12th International Conference on Solar Energy for Buildings and Industry (ISES EuroSun), Fachhochschule Ostschweiz, Hochschule Technik Rapperswil, Rapperswil, SWITZERLAND, 2018; pp. 1130–1136. [Google Scholar]
- Huang, K.; Isayev, A.I. Comparison between decrosslinking of crosslinked high and low density polyethylenes via ultrasonically aided extrusion. Polymer 2015, 70, 290–306. [Google Scholar] [CrossRef]
- Wu, H.; Liang, M.; Lu, C. Morphological and Structural Development of Recycled Crosslinked Polyethylene During Solid-State Mechanochemical Milling. Journal of Applied Polymer Science 2011, 122, 257–264. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, T.; Song, H.; Zhang, H. Investigation of the Rheological Properties and Storage Stability of Waste Polyethylene/Ethylene-Vinyl Acetate-Modified Asphalt with Crosslinking and a Silicone Coupling Agent. Materials 2023, 16. [Google Scholar] [CrossRef]
- Isayev, A.I.; Huang, K. Comparison between Decrosslinking of Crosslinked High and Low Density Polyethylenes via Ultrasonically Aided Extrusion. In Proceedings of the 31st International Conference of the Polymer-Processing-Society (PPS), South Korea, 2016 Jun 07-11. [Google Scholar]
- Gappert, G. Crystallization of swollen, crosslinked polymers; 2004.
- Yamamoto, K.; Tateiwa, T.; Takahashi, Y. Vitamin E-stabilized highly crosslinked polyethylenes: The role and effectiveness in total hip arthroplasty. Journal of Orthopaedic Science 2017, 22, 384–390. [Google Scholar] [CrossRef]
- Salehi, M. Exploring Coupled Physical, Biological and Chemical Processes that Control Lead Fate and Transport through Plastic Plumbing Materials. 2020.
- Forster, A.L. Long term stability and implications for performance of high strength fibers used in body armor; 2012.
- Asadi, S.; Babaizadeh, H.; Foster, N.; Broun, R. Environmental and economic life cycle assessment of PEX and copper plumbing systems: A case study. Journal of Cleaner Production 2016, 137, 1228–1236. [Google Scholar] [CrossRef]
- Hammons, T.J. Power cables in the twenty-first century. Electric Power Components and Systems 2003, 31, 967–994. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, Y.; Chen, J.; Zheng, H. Wireless Transmission Based on GPRS Used in Cable On-line Monitoring System. In Proceedings of the International Conference on Electrical, Electronics and Mechatronics (ICEEM), Thailand, 2016 Dec 20-21. [Google Scholar]
- Birajdar, R.S.; Chikkali, S.H. Insertion copolymerization of functional olefins: Quo Vadis? European Polymer Journal 2021, 143. [Google Scholar] [CrossRef]
- Kaiser, K.M.A. Recycling of multilayer packaging using a reversible cross-linking adhesive. Journal of Applied Polymer Science 2020, 137. [Google Scholar] [CrossRef]
- Wang, D.M. Synthesis study on one kind of crosslinked hdpe special resin. 2011.
- Mustafa, E.; Adam, T.Z.; Afia, R.S.A.; Asipuela, A. Thermal Degradation and Condition Monitoring of Low Voltage Power Cables in Nuclear Power Industry. In Proceedings of the 10th IFIP WG 5.5/SOCOLNET Advanced Doctoral Conference on Computing, Electrical and Industrial Systems (DoCEIS), Costa de Caparica, PORTUGAL, 2019 May 08-10. [Google Scholar]
- Azizi, H.; Morshedian, J.; Barikani, M. Effect of EVA copolymer on properties of different polyethylenes in silane crosslinking process. Plastics Rubber and Composites 2010, 39, 357–363. [Google Scholar] [CrossRef]
- Omar, M.F.; Akil, H.M.; Ahmad, Z.A. Effect of molecular structures on dynamic compression properties of polyethylene. Materials Science and Engineering a-Structural Materials Properties Microstructure and Processing 2012, 538, 125–134. [Google Scholar] [CrossRef]
- Lee, J.-G.; Jeong, J.-O.; Jeong, S.-I.; Park, J.-S. Radiation-Based Crosslinking Technique for Enhanced Thermal and Mechanical Properties of HDPE/EVA/PU Blends. Polymers 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, O.; Stewart, H.E.; O’Rourke, T.D. Thermal and mechanical properties of polyethylene pipes. Journal of Materials in Civil Engineering 2007, 19, 1043–1052. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ikeda, M.; Tanaka, Y. Electrical properties and morphology of polyethylene produced with a novel catalyst. Ieee Transactions on Dielectrics and Electrical Insulation 2004, 11, 881–890. [Google Scholar] [CrossRef]
- Ono, H.; Araoka, M.; Hirai, N.; Ohki, Y. Factors influencing the dielectric properties of linear low-density polyethylene. Electrical Engineering in Japan 2002, 140, 1–7. [Google Scholar] [CrossRef]
- Dadbin, S.; Frounchi, M.; Saeid, M.H.; Gangi, F. Molecular structure and physical properties of E-beam crosslinked low-density polyethylene for wire and cable insulation applications. Journal of Applied Polymer Science 2002, 86, 1959–1969. [Google Scholar] [CrossRef]
- Andreev, M.; Nicholson, D.A.; Kotula, A.; Moore, J.D.; den Doelder, J.; Rutledge, G.C. Rheology of crystallizing LLDPE. Journal of Rheology 2020, 64, 1379–1389. [Google Scholar] [CrossRef]
- Cardoso, P.S.M.; Ueki, M.M.; Barbosa, J.D.V.; Garcia Filho, F.C.; Lazarus, B.S.; Azevedo, J.B. The Effect of Dialkyl Peroxide Crosslinking on the Properties of LLDPE and UHMWPE. Polymers 2021, 13. [Google Scholar] [CrossRef]
- Gulmine, J.V.; Janissek, P.R.; Heise, H.M.; Akcelrud, L. Degradation profile of polyethylene after artificial accelerated weathering. Polymer Degradation and Stability 2003, 79, 385–397. [Google Scholar] [CrossRef]
- Perera, R.; Albano, C.; Sanchez, Y.; Karam, A.; Silva, P.; Pastor, J.M. Changes in structural characteristics of LLDPE functionalized with DEM using gamma-irradiation. Journal of Applied Polymer Science 2012, 124, 1106–1116. [Google Scholar] [CrossRef]
- Kang, T.K.; Ha, C.S. Effect of processing variables on the crosslinking of HDPE by peroxide. Polymer Testing 2000, 19, 773–783. [Google Scholar] [CrossRef]
- Liang, R. Processing flow behavior and modeling of polyethylene melts. Journal of Central South University of Technology 2007, 14, 178–182. [Google Scholar] [CrossRef]
- Liu, Y.; Long, Y.; Wang, Q.; Hao, C.; Lei, Q.; Guo, X. Effect of the cross-linking agent on the cross-linking degree and electrical properties of cross-linked polyethylene. Materials Research Express 2023, 10. [Google Scholar]
- Montoya Ospina, M.C. Processing and Characterization of Polyethylene Vitrimers; 2023.
- Pleşa, I.; Noţingher, P.V.; Stancu, C.; Wiesbrock, F.; Schlögl, S. Polyethylene Nanocomposites for Power Cable Insulations. Polymers 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Meola, C.; Nele, L.; Giuliani, M.; Suriano, P. Chemical and Irradiation Cross-Linking of Polyethylene. Technological Performance over Costs. Polymer-Plastics Technology and Engineering 2004, 43, 631–648. [Google Scholar] [CrossRef]
- Neidhart, E.K.; Hua, M.; Peng, Z.; Kearney, L.T.; Bhat, V.; Vashahi, F.; Alexanian, E.J.; Sheiko, S.S.; Wang, C.; Helms, B.A.; et al. C-H Functionalization of Polyolefins to Access Reprocessable Polyolefin Thermosets. Journal of the American Chemical Society 2023, 145, 27450–27458. [Google Scholar] [CrossRef]
- Montoya-Ospina, M.C.; Verhoogt, H.; Ordner, M.; Tan, X.; Osswald, T.A. Effect of cross-linking on the mechanical properties, degree of crystallinity and thermal stability of polyethylene vitrimers. Polymer Engineering and Science 2022, 62, 4203–4213. [Google Scholar] [CrossRef]
- Bergmann, F.; Halmen, N.; Scalfi-Happ, C.; Reitzle, D.; Kienle, A.; Mittelberg, L.; Baudrit, B.; Hochrein, T.; Bastian, M. Investigation of the degree of cross-linking of polyethylene and thermosets using absolute optical spectroscopy and Raman microscopy. Journal of Sensors and Sensor Systems 2023, 12, 175–185. [Google Scholar] [CrossRef]
- Simis, K.S.; Bistolfi, A.; Bellare, A.; Pruitt, L.A. The combined effects of crosslinking and high crystallinity on the microstructural and mechanical properties of ultra high molecular weight polyethylene. Biomaterials 2006, 27, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-h.; Yang, H.-m.; Song, Y.-h.; Zheng, Q. Influence of crosslinking on physical properties of low density polyethylene. Chinese Journal of Polymer Science 2012, 30, 837–844. [Google Scholar] [CrossRef]
- Wu, J.-h.; Dong, P.; Wang, Z.-r.; Wang, K.; Zhang, Q.; Fu, Q. Improving Wear Resistance of Ultrahigh Molecular Weight Polyethylene: Cross-linking versus Crystallinity. Acta Polymerica Sinica 2023, 54, 622–630. [Google Scholar]
- Krupa, I.; Luyt, A.S. Mechanical properties of uncrosslinked and crosslinked linear low-density polyethylene/wax blends. Journal of Applied Polymer Science 2001, 81, 973–980. [Google Scholar] [CrossRef]
- Huseynova, Z.N. MECHANO-CHEMICAL SYNTHESIS OF THE CROSS-LINKED COMPOSITE MATERIALS ON THE BASIS OF LOW DENSITY POLYETHYLENE. Processes of Petrochemistry and Oil Refining 2020, 21, 118–123. [Google Scholar]
- Backens, S.; Ofe, S.; Schmidt, S.; Glueck, N.; Fluegge, W. Influence of peroxide cross-linking temperature and time on mechanical, physical and thermal properties of polyethylene. Materials Testing 2022, 64, 186–194. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, H.; Zhang, J. The effect of peroxide cross-linking on the thermal conductivity and crystallinity of low-density polyethylene. Materials Today Communications 2022, 31. [Google Scholar] [CrossRef]
- Anbarasan, R.; Babot, O.; Maillard, B. Crosslinking of high-density polyethylene in the presence of organic peroxides. Journal of Applied Polymer Science 2004, 93, 75–81. [Google Scholar] [CrossRef]
- Poh, B.T.; Cheong, S.K. Adhesion behavior of natural rubber-based adhesives crosslinked by benzoyl peroxide. Journal of Applied Polymer Science 2012, 124, 1031–1035. [Google Scholar] [CrossRef]
- Gul, R.M. Comparison of peroxides used for cross-linking ultra high molecular weight polyethylene. European Polymer Journal 1999, 35, 2001–2005. [Google Scholar] [CrossRef]
- Choudhury, A.; Adhikari, B. Dynamic vulcanization of recycled milk pouches (LDPE-LLDPE) and EPDM blends using dicumyl peroxide. Polymer International 2007, 56, 1213–1223. [Google Scholar] [CrossRef]
- Sun, W.-J.; Liu, X.-J.; Yuan, L.-J.; Xiao, H.; Lu, J.-M. An efficient strategy triggered crosslinking of polyethylene and its application in degassing-free ultrahigh voltage power cables. Journal of Applied Polymer Science 2023, 140. [Google Scholar] [CrossRef]
- Rossiello, L.; Buttafava, A.; Martinotto, L.; Peruzzotti, F.; Ghisoni, G.M.; Faucitano, A. The mechanism of action of crosslinking additives in polyethylene vulcanisation: an EPR investigation. Research on Chemical Intermediates 2002, 28, 89–100. [Google Scholar] [CrossRef]
- Molloy, B.M.; Hyslop, D.K.; Parent, J.S. Comparative Analysis of Delayed-Onset Peroxide Crosslinking Formulations. Polymer Engineering and Science 2014, 54, 2645–2653. [Google Scholar] [CrossRef]
- Bhowmick, A.K.; Heslop, J.; White, J.R. Effect of stabilizers in photodegradation of thermoplastic elastomeric rubber-polyethylene blends—a preliminary study. Polymer Degradation and Stability 2001, 74, 513–521. [Google Scholar] [CrossRef]
- Aigbodion, A.I.; Ressia, J.A.; Ciolino, A.E.; Failla, M.D.; Valles, E.M. Effect of the Vinyl Concentration on the Structural and Rheological Characteristics of Peroxide-Modified High-Density Polyethylenes. Journal of Applied Polymer Science 2010, 115, 1942–1951. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, H.; Xu, Z.; Zhang, C.; Yang, J.; Zheng, C.; Lei, J. Accelerated water tree aging of crosslinked polyethylene with different degrees of crosslinking. Polymer Testing 2016, 56, 83–90. [Google Scholar] [CrossRef]
- Lee, J.R.; Lee, D.G.; Hong, S.M.; Kang, H.J. Crosslinking characteristics of high density polyethylene by reactive melt processing. Polymer-Korea 2005, 29, 385–391. [Google Scholar]
- Ahmad, H.; Rostami-Tapeh-Esmaeil, E.; Rodrigue, D. The effect of chemical crosslinking on the properties of Rotomolded high density polyethylene. Journal of Applied Polymer Science 2024, 141. [Google Scholar] [CrossRef]
- Madani, M.; Sharifi-Sanjani, N.; Rezaei-Zare, E.; Faridi-Majidi, R. Preparation of granular crosslinkable medium-density polyethylene. Journal of Applied Polymer Science 2007, 104, 1873–1879. [Google Scholar] [CrossRef]
- Akbarian, D.; Hamedi, H.; Damirchi, B.; Yilmaz, D.E.; Penrod, K.; Woodward, W.H.H.; Moore, J.; Lanagan, M.T.; van Duin, A.C.T. Atomistic-scale insights into the crosslinking of polyethylene induced by peroxides. Polymer 2019, 183. [Google Scholar] [CrossRef]
- Li, J.; Shang, K.; Wang, S.; Feng, Y.; Chen, G.; Li, S. Temperature-dependent synergistic antioxidants for high-efficiency modulation of anti-scorch performance and crosslinking degree in peroxide-initiated low-density polyethylene. Materials Letters 2024, 362. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, Y.; Li, M.; Zhao, H.; Wang, X.; Han, B. Theoretical study on the radical reaction mechanism in the cross-linking process of polyethylene. RSC Advances 2015, 5, 90343–90353. [Google Scholar] [CrossRef]
- Sahyoun, J.; Crepet, A.; Gouanve, F.; Keromnes, L.; Espuche, E. Diffusion mechanism of byproducts resulting from the peroxide crosslinking of polyethylene. Journal of Applied Polymer Science 2017, 134. [Google Scholar] [CrossRef]
- Molloy, B.M.; Johnson, K.-a.; Ross, R.J.; Parent, J.S. Functional group tolerance of AOTEMPO-mediated peroxide cure chemistry. Polymer 2016, 99, 598–604. [Google Scholar] [CrossRef]
- Khonakdar, H.A.; Jafari, S.H.; Haessler, R. Glass-transition-temperature depression in chemically crosslinked low-density polyethylene and high-density polyethylene and their blends with ethylene vinyl acetate copolymer. Journal of Applied Polymer Science 2007, 104, 1654–1660. [Google Scholar] [CrossRef]
- Saci, H.; Bouhelal, S.; Bouzarafa, B.; Lopez, D.; Fernandez-Garcia, M. Reversible crosslinked low density polyethylenes: structure and thermal properties. Journal of Polymer Research 2016, 23, 1–9. [Google Scholar] [CrossRef]
- Likhitlert, S.; Wongchaleo, C.; Kositchaiyong, A.; Wimolmala, E.; Mitrprasertporn, S.; Sombatsompop, N. Thermal Characteristics and Temperature Profile Changes of Structurally Different Polyethylenes With Peroxide Modifications. Journal of Vinyl & Additive Technology 2014, 20, 80–90. [Google Scholar]
- Di, L.; Qin, C.; Wang, W.; Huang, A.; Wei, F.; Xu, H.; Yang, S. Influence of Crosslink Density on Electrical Performance and Rheological Properties of Crosslinked Polyethylene. Polymers 2024, 16. [Google Scholar] [CrossRef]
- de Melo, R.P.; Aguiar, V.d.O.; Vieira Marques, M.d.F. Silane Crosslinked Polyethylene from Different Commercial PE’s: Influence of Comonomer, Catalyst Type and Evaluation of HLPB as Crosslinking Coagent. Materials Research-Ibero-American Journal of Materials 2015, 18, 313–319. [Google Scholar]
- Lee, G.J.; Han, M.G.; Chung, S.C.; Suh, K.D.; Im, S.S. Effect of crosslinking on the positive temperature coefficient stability of carbon black-filled HDPE/ethylene-ethylacrylate copolymer blend system. Polymer Engineering and Science 2002, 42, 1740–1747. [Google Scholar] [CrossRef]
- Sirisinha, K.; Boonkongkaew, M.; Kositchaiyong, S. The effect of silane carriers on silane grafting of high-density polyethylene and properties of crosslinked products. Polymer Testing 2010, 29, 958–965. [Google Scholar] [CrossRef]
- Yussuf, A.A.; Al-Saleh, M.A.; Al-Enezi, S.T.; Abraham, G. Effect of silane concentration on the properties of crosslinked linear low density polyethylene-montmorillonite nanocomposite. Polymer Composites 2021, 42, 2268–2281. [Google Scholar] [CrossRef]
- Tang, C.Y.; Xie, X.L.; Wu, X.C.; Li, R.K.Y.; Mai, Y.W. Enhanced wear performance of ultra high molecular weight polyethylene crosslinked by organosilane. Journal of Materials Science-Materials in Medicine 2002, 13, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.L.; Costa, M.F. Optimization of process conditions, characterization and mechanical properties of silane crosslinked high-density polyethylene. Materials Science and Engineering a-Structural Materials Properties Microstructure and Processing 2010, 527, 4593–4599. [Google Scholar] [CrossRef]
- Bengtsson, M.; Oksman, K.; Stark, N.M. Profile extrusion and mechanical properties of crosslinked wood-thermoplastic composites. Polymer Composites 2006, 27, 184–194. [Google Scholar] [CrossRef]
- Grubbstrom, G.; Holmgren, A.; Oksman, K. Silane-crosslinking of recycled low-density polyethylene/wood composites. Composites Part a-Applied Science and Manufacturing 2010, 41, 678–683. [Google Scholar] [CrossRef]
- Chen, T.; Wang, J.; Shi, P.; Li, Q.; Wu, C. Effect of Hot Water and Water-carrying Agent on the Properties of Si lane-water Cross linked Linear Low Density Polyethylen. International Polymer Processing 2013, 28, 180–187. [Google Scholar] [CrossRef]
- Pessanha, A.B.; Rocha, M.C.G.; da Silva, A.H.M.F.T. Silane Grafting and Mositure Crosslinking of LLDPE by a Reactive Extrusion Process: Effect of Processing Conditions and Reaction System. Polimeros-Ciencia E Tecnologia 2011, 21, 53–58. [Google Scholar] [CrossRef]
- Gahleitner, M.; Pham, T.; Machl, D. Polyolefin Blends with Selectively Crosslinked Disperse Phase Based on Silane-Modified Polyethylene. Polymers 2023, 15. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, J.; Fan, H. The preparation of cross-linkable polyethylene via coordination copolymerization of ethylene with alkene-derived chlorosilane. European Polymer Journal 2022, 163. [Google Scholar] [CrossRef]
- Pape, P.G. Moisture crosslinking process for foamed polymers. Journal of Vinyl & Additive Technology 2000, 6, 49–52. [Google Scholar]
- Mauri, M.; Hofmann, A.I.; Gomez-Heincke, D.; Kumara, S.; Pourrahimi, A.M.; Ouyang, Y.; Hagstrand, P.-O.; Gkourmpis, T.; Xu, X.; Prieto, O.; et al. Click chemistry-type crosslinking of a low-conductivity polyethylene copolymer ternary blend for power cable insulation. Polymer International 2020, 69, 404–412. [Google Scholar] [CrossRef]
- Montanari, G.C. Bringing an Insulation to Failure: the Role of Space Charge. Ieee Transactions on Dielectrics and Electrical Insulation 2011, 18, 339–364. [Google Scholar] [CrossRef]
- Dubois, S.M. Effect of silane cross-linking on polyolefin syntactic foam extrudate for cable jacketing; 2006.
- Camalov, M.; Orucov, A.; Hashimov, A.; Arikan, O.; Akin, F. Breakdown strength analysis of XLPE insulation types: A comparative study for multi-layer structure and voltage rise rate. Electric Power Systems Research 2023, 223. [Google Scholar] [CrossRef]
- Basfar, A.A.; Mosnacek, J.; Shukri, T.M.; Bahattab, M.A.; Noireaux, P.; Courdreuse, A. Mechanical and thermal properties of blends of low-density polyethylene and ethylene vinyl acetate crosslinked by both dicumyl peroxide and ionizing radiation for wire and cable applications. Journal of Applied Polymer Science 2008, 107, 642–649. [Google Scholar] [CrossRef]
- Cassidy, J.; Nesaei, S.; McTaggart, R.; Delfanian, F. Mechanical response of high density polyethylene to gamma radiation from a Cobalt-60 irradiator. Polymer Testing 2016, 52, 111–116. [Google Scholar] [CrossRef]
- Alsabbagh, A.; Abu Saleem, R.; Almasri, R.; Aljarrah, S.; Awad, S. Effects of gamma irradiation on 3D-printed polylactic acid (PLA) and HDPE. Polymer Bulletin 2021, 78, 4931–4945. [Google Scholar] [CrossRef]
- Tamada, M.; Seko, N.; Yoshii, F. Application of radiation-graft material for metal adsorbent and crosslinked natural polymer for healthcare product. Radiation Physics and Chemistry 2004, 71, 223–227. [Google Scholar] [CrossRef]
- Singh, A. Irradiation of polyethylene: Some aspects of crosslinking and oxidative degradation. Radiation Physics and Chemistry 1999, 56, 375–380. [Google Scholar] [CrossRef]
- Lotfy, S.; Basfar, A.A. Influence of gamma irradiation on acrylate-based shape memory polymers in the presence of various crosslinkers. Journal of Applied Polymer Science 2023, 140. [Google Scholar] [CrossRef]
- Dadbin, S.; Frounchi, M.; Sabet, M. Studies on the properties and structure of electron-beam crosslinked low-density polyethylene/poly ethylene-co-(vinyl acetate) blends. Polymer International 2005, 54, 686–691. [Google Scholar] [CrossRef]
- Sethi, M.; Gupta, N.K.; Srivastava, A.K. Dynamic mechanical analysis of polyethylene and ethylene vinylacetate copolymer blends irradiated by electron beam. Journal of Applied Polymer Science 2002, 86, 2429–2434. [Google Scholar] [CrossRef]
- Khan, F.; Kwek, D.; Kronfli, E.; Ahmad, S.R. Laser-induced crosslinking of ultra-low-and high-density polyethylene. Macromolecular Rapid Communications 2007, 28, 158–163. [Google Scholar] [CrossRef]
- Milicevic, D.; Trifunovic, S.; Popovic, M.; Milic, T.V.; Suljovrujic, E. The influence of orientation on the radiation-induced crosslinking/oxidative behavior of different PEs. Nuclear Instruments & Methods in Physics Research Section B-Beam Interactions with Materials and Atoms 2007, 260, 603–612. [Google Scholar]
- Li, C.; Zhang, C.; Zhao, H.; Zhang, H.; Wang, X.; Han, B. Grafted UV absorber as voltage stabilizer against electrical degradation and breakdown in cross-linked polyethylene for high voltage cable insulation. Polymer Degradation and Stability 2021, 185. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Yu, P.-L.; Sun, W.-F.; Wang, X. Ameliorated Electrical-Tree Resistant Characteristics of UV-Initiated Cross-Linked Polyethylene Nanocomposites with Surface-Functionalized Nanosilica. Processes 2021, 9. [Google Scholar] [CrossRef]
- Zhao, H.; Xi, C.; Zhao, X.-D.; Sun, W.-F. Elevated-Temperature Space Charge Characteristics and Trapping Mechanism of Cross-Linked Polyethylene Modified by UV-Initiated Grafting MAH. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Grause, G.; Chien, M.-F.; Inoue, C. Changes during the weathering of polyolefins. Polymer Degradation and Stability 2020, 181. [Google Scholar] [CrossRef]
- Uehara, H.; Sekii, Y.; Iwata, S.; Takada, T.; Cao, Y.; Ieee. Suppression of Electrical Tree Initiation by Antioxidant and Ultraviolet Absorber, Using A Density-Functional Study. In Proceedings of the IEEE Conference on Electrical Insulation and Dielectric Phenomenon (CEIDP), Fort Worth, TX, 2017 Oct 22-25. [Google Scholar]
- Behnke, S.; Ulbricht, M. Thin-film composite membranes for organophilic nanofiltration based on photo-cross-linkable polyimide. Reactive & Functional Polymers 2015, 86, 233–242. [Google Scholar]
- Kim, H.J.; Skinner, M.; Yu, H.; Oh, J.H.; Briseno, A.L.; Emrick, T.; Kim, B.J.; Hayward, R.C. Water Processable Polythiophene Nanowires by Photo-Cross-Linking and Click-Functionalization. Nano Letters 2015, 15, 5689–5695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shang, Y.; Zhao, H.; Wang, X.; Han, B.; Li, Z. Further discussion on the reaction behaviour of triallyl isocyanurate in the UV radiation cross-linking process of polyethylene: a theoretical study. Royal Society Open Science 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-W.; Sun, W.-F.; Wang, X. UV-Initiated Crosslinking Reaction Mechanism and Electrical Breakdown Performance of Crosslinked Polyethylene. Polymers 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, J.; Zhang, H.; Shang, Y.; Wang, X.; Han, B.; Li, Z. Theoretical study on the reaction of triallyl isocyanurate in the UV radiation cross-linking of polyethylene. Rsc Advances 2017, 7, 37095–37104. [Google Scholar] [CrossRef]
- Ye, L.; Wu, Q.; Qu, B. Photocrosslinking and related properties of intumescent flame-retardant LLDPE/EVA/IFR blends. Polymers for Advanced Technologies 2012, 23, 858–865. [Google Scholar] [CrossRef]
- Rodriguez, A.K.; Mansoor, B.; Ayoub, G.; Colin, X.; Benzerga, A.A. Effect of UV-aging on the mechanical and fracture behavior of low density polyethylene. Polymer Degradation and Stability 2020, 180. [Google Scholar] [CrossRef]
- Mills, D.H.; Lewin, P.L.; Chen, G.; Ieee. Ageing of High Voltage Cable Insulation. In Proceedings of the 30th Electrical Insulation Conference (EIC), Annapolis, MD, 2011 Jun 05-08. [Google Scholar]
- Tireau, J.; Van Schoors, L.; Benzarti, K.; Colin, X. Consequences of thermo- and photo-oxidation on end-use properties of pure PE. In Proceedings of the 5th International Conference on Times of Polymers (TOP) and Composites, Ischia, ITALY, 2010 Jun 20-23. [Google Scholar]
- Aarya, S.; Kumar, P.; Bhatia, M.; Kumar, S.; Sharma, J.; Siddhartha. Gamma Rays Induced Modification in Ultrahigh Molecular Weight Polyethylene (UHMWPE). Advances in Polymer Technology 2021, 2021. [Google Scholar] [CrossRef]
- Temmel, S.; Kern, W.; Luxbacher, T. Zeta potential of photochemically modified polymer surfaces. In Proceedings of the 10th Dresden Polymer Meeting, Dresden, GERMANY, 2006 Apr 10-13. [Google Scholar]
- Lim, C.-M.; Hur, J.; Jang, H.; Seo, J.-H. Developing a thermal grafting process for zwitterionic polymers on cross-linked polyethylene with geometry-independent grafting thickness. Acta Biomaterialia 2019, 85, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Basfar, A.A.; Ali, K.M.I.; Mofti, S.M. UV stability and radiation-crosslinking of linear low density polyethylene and low density polyethylene for greenhouse applications. Polymer Degradation and Stability 2003, 82, 229–234. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, Y.; Li, M.; Zhao, H.; Wang, X.; Han, B. Theoretical study on the reaction mechanism in the UV radiation cross-linking process of polyethylene. RSC Advances 2016, 6, 110831–110839. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Zhao, H.; An, T.; Du, X.; Lu, Y.; Chen, Z. Theoretical study on the grafting reaction of maleimide and its derivatives to polyethylene in the UV radiation cross-linking process. Structural Chemistry 2019, 30, 1033–1039. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, C.; Shang, Y.; Zhao, H.; Wang, X.; Han, B.; Li, Z. Theoretical study on the hydrogen addition reactions to bismaleimide in the ultra-violet radiation cross-linking process of polyethylene. Journal of Molecular Graphics & Modelling 2020, 100. [Google Scholar]
- Li, C.; Han, B.; Zhang, H.; Zhao, H.; Zhang, C.; Ieee. Effect of Acetophenone on the Breakdown Strength of Polyethylene. In Proceedings of the 2015 IEEE 11th International Conference on the Properties and Applications of Dielectric Materials (ICPADM), Sydney, AUSTRALIA, 2015 Jul 19-22. [Google Scholar]
- Zhang, F.; Zhang, X.; Yu, K.; Li, K.; Hou, J.; Yang, Y.; Shen, C.; Chen, J.; Park, C.B. Preparation and microcellular foaming of crosslinked polyethylene-octene elastomer by ionic modification. Journal of Supercritical Fluids 2023, 202. [Google Scholar] [CrossRef]
- Yang, S.; Yi, S.; Yun, J.; Li, N.; Jiang, Y.; Huang, Z.; Xu, C.; He, C.; Pan, X. Carbene-Mediated Polymer Cross-Linking with Diazo Compounds by C-H Activation and Insertion. Macromolecules 2022, 55, 3423–3429. [Google Scholar] [CrossRef]
- Ayer, M.A.; Simon, Y.C.; Weder, C. Azo-Containing Polymers with Degradation On-Demand Feature. Macromolecules 2016, 49, 2917–2927. [Google Scholar] [CrossRef]
- Severengiz, M.; Sprenger, T.; Seliger, G. Challenges and Approaches for a Continuous Cable Production. In Proceedings of the 13th Global Conference on Sustainable Manufacturing—Decoupling Growth from Resource Use, Binh Dong New City, VIETNAM, 2016 Sep 16-18. [Google Scholar]
- Mead, J.L.; Tao, Z.; Liu, H.S. Insulation materials for wire and cable applications. Rubber Chemistry and Technology 2002, 75, 701–712. [Google Scholar] [CrossRef]
- Miceli, M.; Carvelli, V.; Drissi-Habti, M. Modelling Electro-Mechanical Behaviour of an XLPE Insulation Layer for Hi-Voltage Composite Power Cables: Effect of Voids on Onset of Coalescence. Energies 2023, 16. [Google Scholar] [CrossRef]
- Hairaldin, S.Z.; Baharin, R.; Mansur, S.A.; Yusoff, M.S.J.; Zainal, M.Z.M.; Ali, A. Determination of WUBHS speed range for electron beam irradiation of wire and cable. In Proceedings of the 8th International Nuclear Science, Technology and Engineering Conference (iNuSTEC), Univ Kebangsaan Malaysia, Bangi, MALAYSIA, 2020 Oct 29-31. [Google Scholar]
- Sun, S.-Q.; Peng, L.; Wang, G.-X.; Wu, Y.-H.; Zhou, J.; Bing, H.-J.; Yu, D.; Luo, J. An improved open-top chamber warming system for global change research. Silva Fennica 2013, 47. [Google Scholar] [CrossRef]
- Nazrin, A.; Kuan, T.M.; Mansour, D.-E.A.; Farade, R.A.; Ariffin, A.M.; Abd Rahman, M.S.; Wahab, N.I.B.A. Innovative approaches for augmenting dielectric properties in XLPE: A review. Heliyon 2024, 10. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, P.; Hui, B.; Cheng, Y.; Zhu, W.; Fu, M.; Li, X. Broadband impedance spectrum detection of buffer layer defects in 110 kV crosslinked polyethylene cable with corrugated aluminium sheath. High Voltage 2023, 8, 967–976. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J. Design of Smooth Aluminum Bonded Sheaths in HV XLPE Cables Based on the Bending Performance Research. Ieee Access 2020, 8, 102493–102501. [Google Scholar] [CrossRef]
- Islam, M.; Rahaman, M.; Aldalbahi, A.; Paikaray, B.; Moharana, J.; Mondal, S.; Das, N.C.; Gupta, P.; Giri, R. High density polyethylene and metal oxides based nanocomposites for high voltage cable application. Journal of Applied Polymer Science 2021, 139. [Google Scholar] [CrossRef]
- Kiger, C.J.; Sexton, C.D.; Hashemian, H.M.; O’Hagan, R.D.; Dormann, L.; Wasfy, W. Implementation of New Cable Condition-Monitoring Technology at Oyster Creek Nuclear Generating Station. Nuclear Technology 2017, 200, 93–105. [Google Scholar] [CrossRef]
- Gillen, K.T.; Assink, R.; Bernstein, R.; Celina, M. Condition monitoring methods applied to degradation of chlorosulfonated polyethylene cable jacketing materials. Polymer Degradation and Stability 2006, 91, 1273–1288. [Google Scholar] [CrossRef]
- Leng, B.; Yang, J.; Zhu, C.; Wang, Z.; Shi, C.; Liu, Y.; Zhang, H.; Xu, W.; Liu, B. Synthesis of a Cyclophosphazene Derivative Containing Multiple Cyano Groups for Electron-Beam Irradiated Flame-Retardant Materials. Polymers 2021, 13. [Google Scholar] [CrossRef]
- Han, Q.; Liu, W.; Xu, Y.; Cao, Z.; Chen, Y. Fabrication of EPDM/LDPE shape memory composites: the effect of vulcanization and crystals. Polymer Bulletin 2024, 81, 2179–2196. [Google Scholar] [CrossRef]
- Chen, T.; Li, Q.; Fu, Z.; Sun, L.; Guo, W.; Wu, C. The shape memory effect of crosslinked ultra-high-molecular-weight polyethylene prepared by silane-induced crosslinking method. Polymer Bulletin 2018, 75, 2181–2196. [Google Scholar] [CrossRef]
- Kurup, S.N.; Ellingford, C.; Wan, C. Shape memory properties of polyethylene/ethylene vinyl acetate/carbon nanotube composites. Polymer Testing 2020, 81. [Google Scholar] [CrossRef]
- Mishra, J.K.; Chang, Y.-W.; Lee, B.C.; Ryu, S.H. Mechanical properties and heat shrinkability of electron beam crosslinked polyethylene-octene copolymer. Radiation Physics and Chemistry 2008, 77, 675–679. [Google Scholar] [CrossRef]
- Yayla, P.; Bilgin, Y. Squeeze-off of polyethylene pressure pipes: Experimental analysis. Polymer Testing 2007, 26, 132–141. [Google Scholar] [CrossRef]
- Kelley, K.M.; Stenson, A.C.; Dey, R.; Whelton, A.J. Release of drinking water contaminants and odor impacts caused by green building cross-linked polyethylene (PEX) plumbing systems. Water Research 2014, 67, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Hadiuzzaman, M.; Ladner, D.A.A.; Salehi, M. Impact of the surface aging of potable water plastic pipes on their lead deposition characteristics. Environmental Science-Water Research & Technology 2023, 9, 2501–2514. [Google Scholar]
- Rzeznik, I. Investigation of temperature drop in domestic hot water circuit. In Proceedings of the 10th Conference on Interdisciplinary Problems in Environmental Protection and Engineering (EKO-DOK), Polanica Zdroj, POLAND, 2018 Apr 16-18. [Google Scholar]
- de Melo, R.P.; Marques, M.F.V. PEX Synthesized via Peroxide for Oil Pipes, Starting from Different Commercial Polyethylenes: Influence of Comonomer and Catalyst Type. In Proceedings of the 10th Brazilian Polymer Congress (CBPol), Foz do Iguacu, BRAZIL, 2011 Oct 13-17. [Google Scholar]
- Al-Malaika, S.; Riasat, S.; Lewucha, C. Reactive antioxidants for peroxide crosslinked polyethylene. Polymer Degradation and Stability 2017, 145, 11–24. [Google Scholar] [CrossRef]
- Ghoochani, S.; Hadiuzzaman, M.; Mirza, N.; Brown, S.P.; Salehi, M. Effects of water chemistry and flow on lead release from plastic pipes versus copper pipes, implications for plumbing decontamination. Environmental Pollution 2023, 337. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wu, B.; Zhao, X.; Ye, L. A novel strategy for construction of multiple dynamic hybrid crosslinking network for polyethylene pipes and self-healing behavior via thermal stimulation. Composite Structures 2023, 319. [Google Scholar] [CrossRef]
- Chang, N.Y.; Yoo, S.H.; Kim, Y.; Kang, W.-G.; Jung, S.-t.; Lee, J.; Lee, D.-M.; Kim, J. Processing Technology of Crosslinked Polyethylene (PE-X) by Electron Beam. Journal of Radiation Industry 2019, 13, 295–303. [Google Scholar]
- Sirisinha, K.; Chuaythong, P. Reprocessable silane-crosslinked polyethylene: property and utilization as toughness enhancer for high-density polyethylene. Journal of Materials Science 2014, 49, 5182–5189. [Google Scholar] [CrossRef]
- Pruksawan, S.; Lim, J.W.R.; Lee, Y.L.; Lin, Z.; Chee, H.L.; Chong, Y.T.; Chi, H.; Wang, F. Enhancing hydrogel toughness by uniform cross-linking using modified polyhedral oligomeric silsesquioxane. Communications Materials 2023, 4. [Google Scholar] [CrossRef]
- Waller, H. Systematical research on welded joints of crosslinked polyethylene. Materialwissenschaft Und Werkstofftechnik 2000, 31, 38–47. [Google Scholar] [CrossRef]
- Whelton, A. Towards a Safer and Greener Indoor Environment: Chemical Liberation from Polyethylene Plumbing Pipes. 2012.
- Colin, X.; Audouin, L.; Verdu, J. Towards a Non Empirical Kinetic Model for the Lifetime Prediction of Polyethylene Pipes Transporting Drinking Water. In Proceedings of the 4th International Conference on Times of Polymers TOP and Composites, Ischia, ITALY, 2009 Sep 21-24. [Google Scholar]
- Holder, S.L.; Hedenqvist, M.S.; Nilsson, F. Understanding and modelling the diffusion process of low molecular weight substances in polyethylene pipes. Water Research 2019, 157, 301–309. [Google Scholar] [CrossRef]
- Xia, B.; Dong, W. Study on Cellular Structure and Mechanical Property of Foaming/Cross-linking Polyethylene System. In Proceedings of the International Conference on Energy, Environment and Bioengineering (ICEEB), Electr Network, 2020 Aug 07-09. [Google Scholar]
- Xia, B.; Dong, W.; Zhang, X.; Wang, Y.; Jiang, J.; Li, T.; Ma, P.; Chen, M. Preparation of Controllable Cross-Linking Polyethylene Foaming Materials and Their Properties. Journal of Wuhan University of Technology-Materials Science Edition 2022, 37, 1014–1019. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Wang, C.; Wang, S.; Wen, S.; Phule, A.D. A lightweight, thermal insulation and excellent weatherability foam crosslinked by electron beam irradiation. Radiation Physics and Chemistry 2020, 173. [Google Scholar] [CrossRef]
- Bawareth, M.; Xu, W.; Ravichandran, D.; Zhu, Y.; Jambhulkar, S.; Fonseca, N.; Miquelard-Garnier, G.; Camille, V.; Matthew, L.; Campbell, W.; et al. Crosslinked Polyethylene (XLPE) Recycling via Foams. Polymers 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Wang, J.; Park, C.B. Extrusion of microcellular open-cell LDPE-based sheet foams. Journal of Applied Polymer Science 2006, 102, 3376–3384. [Google Scholar] [CrossRef]
- da Veiga, L.P.; Jeanguenat, C.; Lisco, F.; Li, H.-Y.; Nicolay, S.; Ballif, C.; Ingenito, A.; Leon, J.J.D. Ultrathin ALD Aluminum Oxide Thin Films Suppress the Thermal Shrinkage of Battery Separator Membranes. Acs Omega 2022, 7, 45582–45589. [Google Scholar] [CrossRef]
- Jacomino, A.P.; Kluge, R.A.; Sarantópoulos, C.I.G.; Sigrist, J.M.M. Evaluation of plastic packages for Guava refrigerated preservation. Packaging Technology and Science 2001, 14, 11–19. [Google Scholar] [CrossRef]
- LaChance, A.M.; Hou, Z.; Farooqui, M.M.; Carr, S.A.; Serrano, J.M.; Odendahl, C.E.; Hurley, M.E.; Morrison, T.E.; Kubachka, J.L.; Samuels, N.T.; et al. Polyolefin films with outstanding barrier properties based on one-step coassembled nanocoatings. Advanced Composites and Hybrid Materials 2022, 5, 1067–1077. [Google Scholar] [CrossRef]
- Bobovitch, A.L.; Sagron, A.; Unigovski, Y.; Jarashneli, A.; Gutman, E.M. Stress relaxation in low-shrink-force polyolefin films. Polymer Engineering and Science 2004, 44, 1716–1720. [Google Scholar] [CrossRef]
- Nishitsuji, S.; Shinozaki, C.; Miyata, K.; Yamada, S.; Yoshida, J. Study of the interfacial adhesive strength of a heat-shrinkable multilayer film. Polymer Engineering and Science 2021, 61, 836–842. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, X.; Jia, H.; Zhou, Y.; Ma, J.; Liu, X.; Jiang, L.; Chen, S. Superhydrophobic Polytetrafluoroethylene/Heat-Shrinkable Polyvinyl Chloride Composite Film with Super Anti-Icing Property. Polymers 2019, 11. [Google Scholar] [CrossRef]
- Hong, C.K.; Maeng, H.; Song, K.; Kaang, S. Thermal Behaviors of Heat Shrinkable Poly(vinyl chloride) Film. Journal of Applied Polymer Science 2009, 112, 886–895. [Google Scholar] [CrossRef]
- Kim, K.C.; Jang, S.S. nnnEffects of thermal shrinkage temperatures and comonomers on thermal shrinkage of uniaxially-stretched PET copolymer films: a molecular dynamics simulation approach. New Journal of Chemistry 2018, 42, 4991–4997. [Google Scholar] [CrossRef]
- Kim, K.C.; Jang, S.S. Molecular dynamics simulation study on the structural properties of poly (ethylene terephthalate) under uniaxial extension and thermal shrinkage processes. Current Applied Physics 2018, 18, 19–26. [Google Scholar] [CrossRef]
- Diaz, J.A.; Youngblood, J.P. Multivariable dependency of thermal shrinkage in highly aligned polypropylene tapes for self-reinforced polymer composites. Composites Part a-Applied Science and Manufacturing 2016, 90, 771–777. [Google Scholar] [CrossRef]
- Yarysheva, A.Y.; Tunyan, A.A.; Bol’shakova, A.V.; Strel’tsov, D.R.; Malakhov, S.N.; Yarysheva, L.M.; Chvalun, S.N.; Volynskii, A.L. Creation of a Microrelief by Thermally Stimulated Shrinkage of Metal-Coated Polypropylene Films. Doklady Physical Chemistry 2017, 477, 219–221. [Google Scholar] [CrossRef]
- Wu, L.-P.; Kang, W.-L.; Chen, Y.; Zhang, X.; Lin, X.-H.; Chen, L.-Y.; Gai, J.-G. Structures and properties of low-shrinkage polypropylene composites. Journal of Applied Polymer Science 2017, 134. [Google Scholar] [CrossRef]
- Li, H.; Wang, T.; Cui, C.; Mu, Y.; Niu, K. Low-Density and High-Performance Fiber-Reinforced PP/POE Composite Foam via Irradiation Crosslinking. Polymers 2024, 16. [Google Scholar] [CrossRef]
- McGee, S.D.; Batt, G.S.; Gibert, J.M.; Darby, D.O. Predicting the Effect of Temperature on the Shock Absorption Properties of Polyethylene Foam. Packaging Technology and Science 2017, 30, 477–494. [Google Scholar] [CrossRef]
- Liu, S.; Veysey, S.W.; Fifield, L.S.; Bowler, N. Quantitative analysis of changes in antioxidant in crosslinked polyethylene (XLPE) cable insulation material exposed to heat and gamma radiation. Polymer Degradation and Stability 2018, 156, 252–258. [Google Scholar] [CrossRef]
- Yan, H.-D.; Zhang, C.; Li, W.-K.; Zha, J.-W. Effect of trap level density on breakdown strength and space charge distribution of polypropylene/low-density polyethylene composites. Polymer Composites 2020, 41, 780–787. [Google Scholar] [CrossRef]
- Kaewunruen, S.; Ngamkhanong, C.; Papaelias, M.; Roberts, C. Wet/dry influence on behaviors of closed-cell polymeric cross-linked foams under static, dynamic and impact loads. Construction and Building Materials 2018, 187, 1092–1102. [Google Scholar] [CrossRef]
- Reynoso, L.E.; Carrizo Romero, A.B.; Viegas, G.M.; San Juan, G.A. Characterization of an alternative thermal insulation material using recycled expanded polystyrene. Construction and Building Materials 2021, 301. [Google Scholar] [CrossRef]
- Hong, S.-H.; Hwang, S.-H. Construction and foamability of lignin-reinforced low-density polyethylene biocomposites. Materials Today Communications 2021, 28. [Google Scholar] [CrossRef]
- He, Z.; Zhai, F.; Tan, C.; Chen, X.; Chen, T.; Ma, P. Trial Manufacture and Performance Research of Hydraulic Oil Tank for Three Kinds of Non-Metallic Materials. Machines 2024, 12. [Google Scholar] [CrossRef]
- Graziano, A.; Garcia, C.; Jaffer, S.; Tjong, J.; Yang, W.; Sain, M. Functionally tuned nanolayered graphene as reinforcement of polyethylene nanocomposites for lightweight transportation industry. Carbon 2020, 169, 99–110. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Zhang, Y.X. Mechanical properties of high-density polyethylene/scarp rubber powder composites modified with ethylene-propylene-diene terpolymer, dicumyl peroxide, and silicone oil. Journal of Applied Polymer Science 2003, 88, 2020–2027. [Google Scholar] [CrossRef]
- Soleimani, M.; Cree, D.; Olson, L.; Tabil, L.G.; Panigrahi, S. Fabrication of nonextruded recycled rubber-polyethylene composites. Journal of Applied Polymer Science 2020, 137. [Google Scholar] [CrossRef]
- Tomin, M.; Kmetty, A. Evaluating the cell structure-impact damping relation of cross-linked polyethylene foams by falling weight impact tests. Journal of Applied Polymer Science 2021, 138. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, X.; Chen, L.; Li, Y.; Sun, M.; Duan, X.; Liang, W. Structures and impact strength variation of chemically crosslinked high-density polyethylene: effect of crosslinking density. Rsc Advances 2021, 11, 6791–6797. [Google Scholar] [CrossRef]
- Lv, H.; Yang, K.; Xiao, X.; Kuang, G.; Yu, S.; Kang, H.; Jin, H.; Iop. Research on Thermal Endurance Properties of XLPE Aged in Different Media. In Proceedings of the 5th International Conference on Environmental Science and Material Application (ESMA), Xian, PEOPLES R CHINA, 2020 Dec 15-16. [Google Scholar]
- Dong, T.; Niu, F.; Qiang, Z.; Wang, X.; Guo, J.; Wang, Y.; He, Y. Thermal aging behavior and heat resistance mechanism of ultraviolet crosslinked ultra-high molecular weight polyethylene fiber. Journal of Applied Polymer Science 2024, 141. [Google Scholar] [CrossRef]
- Tomin, M.; Kmetty, A. Investigation of the energy absorption properties of cross linked polyethylene foams. In Proceedings of the 12th Hungarian Conference on Materials Science (HMSC), Balatonkenese, HUNGARY, 2020 Oct 13-15. [Google Scholar]
- Wang, Z.; Li, Q.; Chen, X.; Zhang, Q.; Wang, K. High crystallinity makes excellent wear resistance in crosslinked UHMWPE. Polymer 2023, 280. [Google Scholar] [CrossRef]
- Zhen, Q.; Zhang, H.; Sun, H.-W.; Zhang, Y.-F. Tailoring the softness performance of polyethylene/polypropylene micro-nanofibrous fabrics for skin contacts. Journal of Applied Polymer Science 2022, 139. [Google Scholar] [CrossRef]
- Wu, J.Z.; Pan, C.S.; Ronaghi, M.; Wimer, B.M.; Reischl, U. Application of polyethylene air-bubble cushions to improve the shock absorption performance of Type I construction helmets for repeated impacts. Bio-Medical Materials and Engineering 2021, 32, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rohm, S.; Knoflach, C.; Nachbauer, W.; Hasler, M.; Kaserer, L.; van Putten, J.; Unterberger, S.H.; Lackner, R. Effect of Different Bearing Ratios on the Friction between Ultrahigh Molecular Weight Polyethylene Ski Bases and Snow. Acs Applied Materials & Interfaces 2016, 8, 12552–12557. [Google Scholar]
- Sobajima, A.; Okihara, T.; Moriyama, S.; Nishimura, N.; Osawa, T.; Miyamae, K.; Haniu, H.; Aoki, K.; Tanaka, M.; Usui, Y.; et al. Multiwall Carbon Nanotube Composites as Artificial Joint Materials. Acs Biomaterials Science & Engineering 2020, 6, 7032–7040. [Google Scholar]
- Chen, J.-Q.; Wang, X.; Sun, W.-F.; Zhao, H. Water-Tree Resistability of UV-XLPE from Hydrophilicity of Auxiliary Crosslinkers. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Zibaei, R.; Ebrahimi, B.; Rouhi, M.; Hashami, Z.; Roshandel, Z.; Hasanvand, S.; Guimaraes, J.d.T.; Goharifar, M.; Mohammadi, R. Development of packaging based on PLA/POE/SeNPs nanocomposites by blown film extrusion method: Physicochemical, structural, morphological and antioxidant properties. Food Packaging and Shelf Life 2023, 38. [Google Scholar] [CrossRef]
- Mahand, S.N.; Yazdanbakhsh, A.; Tayouri, M.I.; Zarei, A.; Nouranian, S.; Ruckdaeschel, H.; Khonakdar, H.A. Theoretical and experimental investigation of selective gas permeability in polystyrene/polyolefin elastomer/nanoclay nanocomposite films. Polymer Testing 2023, 120. [Google Scholar] [CrossRef]
- Barikloo, H.; Ahmadi, E.; Ahmadi, S. Evaluation of PE/POE/PA6 blends containing silica and clay toward nano composite packaging film. Journal of Food Measurement and Characterization 2021, 15, 2297–2308. [Google Scholar] [CrossRef]
- Yang, Y.; Nair, A.K.N.; Sun, S. Adsorption and Diffusion of Methane and Carbon Dioxide in Amorphous Regions of Cross-Linked Polyethylene: A Molecular Simulation Study. Industrial & Engineering Chemistry Research 2019, 58, 8426–8436. [Google Scholar]
- Manukumar, H.M.; Umesha, S. Photocrosslinker technology: An antimicrobial efficacy of cinnamaldehyde cross-linked low-density polyethylene (Cin-C-LDPE) as a novel food wrapper. Food Research International 2017, 102, 144–155. [Google Scholar] [CrossRef]
- Lv, H.; Lu, T.; Xiong, L.; Zheng, X.; Huang, Y.; Ying, M.; Cai, J.; Li, Z. Assessment of thermally aged XLPE insulation material under extreme operating temperatures. Polymer Testing 2020, 88. [Google Scholar] [CrossRef]
- Wan, D.; Qi, F.; Zhou, Q.; Zhou, H.; Zhao, M.; Duan, X. Effect of Thermal Aging on Threshold Field Strength and Relative Permittivity of Cross-Linked Polyethylene with Different Cross-Linking Agent Contents. Journal of Electrical Engineering & Technology 2021, 16, 2885–2892. [Google Scholar]
- Wang, S.; Zhou, Q.; Li, J.; Liao, R.; Wu, N.; Li, Y.; Chen, M. Influence of Thermal Aging on Space Charge Accumulation and Dissipation of Cross-Linked Polyethylene With Different Cross-Linking Agent Contents. Ieee Access 2020, 8, 163541–163557. [Google Scholar] [CrossRef]
- Li, E.; Du, W.; Zhuang, R.; Ba, M.; Yuan, L.; Zhang, Q.; Zhang, Y. Preparation and Characterization of Electromagnetic-Induced Rupture Microcapsules for Self-Repairing Mortars. Materials 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-K.; Kim, H. Preparation of De-crosslinked Polyethylene from Waste Crosslinked High-Density Polyethylene Using Supercritical Twin-Screw Extrusion. Korean Journal of Chemical Engineering 2024, 41, 1767–1773. [Google Scholar] [CrossRef]
- Hong, Z.; Yan, K.; Ge, D.; Wang, M.; Li, G.; Li, H. Effect of styrene-butadiene-styrene (SBS) on laboratory properties of LDPE/ethylene-vinyl acetate (EVA) compound modified asphalt. Journal of Cleaner Production 2022, 338. [Google Scholar] [CrossRef]
- Helal, E.; Pottier, C.; David, E.; Fréchette, M.; Demarquette, N.R. Polyethylene/thermoplastic elastomer/Zinc Oxide nanocomposites for high voltage insulation applications: Dielectric, mechanical and rheological behavior. European Polymer Journal 2018, 100, 258–269. [Google Scholar] [CrossRef]
- Thomas, J.; Joseph, B.; Jose, J.P.; Maria, H.J.; Main, P.; Ali Rahman, A.; Francis, B.; Ahmad, Z.; Thomas, S. Recent Advances in Cross-linked Polyethylene-based Nanocomposites for High Voltage Engineering Applications: A Critical Review. Industrial & Engineering Chemistry Research 2019, 58, 20863–20879. [Google Scholar]
- Gao, J.-G.; Li, X.; Yang, W.-H.; Zhang, X.-H. Space Charge Characteristics and Electrical Properties of Micro-Nano ZnO/LDPE Composites. Crystals 2019, 9. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Xiao, K. Investigation of the electrical properties of XLPE/SiC nanocomposites. Polymer Testing 2016, 50, 145–151. [Google Scholar] [CrossRef]
- Hui, L.; Schadler, L.S.; Nelson, J.K. The Influence of Moisture on the Electrical Properties of Crosslinked Polyethylene/Silica Nanocomposites. Ieee Transactions on Dielectrics and Electrical Insulation 2013, 20, 641–653. [Google Scholar] [CrossRef]
- Yussuf, A.A.; Al-Saleh, M.A.; Samuel, J.; Ahmad, N.; Alshammar, T.; Al-Banna, A.; Abraham, G. Effect of magnesium hydroxide and graphene nanoplatelets on the properties of peroxide cross-linked linear low-density polyethylene nanocomposite. Polymer Engineering and Science 2023, 63, 4207–4219. [Google Scholar] [CrossRef]
- Gill, Y.Q.; Ehsan, H.; Mehmood, U.; Irfan, M.S.; Saeed, F. A novel two-step melt blending method to prepare nano-silanized-silica reinforced crosslinked polyethylene (XLPE) nanocomposites. Polymer Bulletin 2022, 79, 10077–10093. [Google Scholar] [CrossRef]
- Zhao, B.; Hang, G.; Li, L.; Zheng, S. Nanocomposites of polyethylene with polyhedral oligomeric silsesquioxane: from thermoplastics to vitrimers through silyl ether metathesis. Materials Today Chemistry 2022, 24. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Pypeć, K.; Irska, I.; Piesowicz, E. Functional Polymer Hybrid Nanocomposites Based on Polyolefins: A Review. Processes 2020, 8. [Google Scholar] [CrossRef]
- Jubinville, D.; Lee, H.-S.; Mekonnen, T. High-Biocontent Polymer Blends and Their Wood Plastic Composites: Blending, Compatibilization, and Their Recyclability. Applied Composite Materials 2024. [Google Scholar] [CrossRef]
- Cao, L.; Zhong, L.; Li, Y.; Li, W.; Gao, J.; Xu, L.; Chen, G. Crosslinking-modulated direct-current conductivity of XLPE-PS composite via charge trap characteristics. Applied Physics Letters 2022, 120. [Google Scholar] [CrossRef]
- Han, J.; Wu, M.; Duan, W. GO Enhanced, Irradiation Cross-linked UHMWPE/VE Nanocomposites with Increased Hardness and Scratch Resistance. Polymer-Korea 2020, 44, 589–595. [Google Scholar] [CrossRef]
- Han, C.; Du, B.X.; Li, J.; Li, Z.; Tanaka, T. Investigation of charge transport and breakdown properties in XLPE/GO nanocomposites part 1: The role of functionalized GO quantum wells. Ieee Transactions on Dielectrics and Electrical Insulation 2020, 27, 1204–1212. [Google Scholar] [CrossRef]
- Li, G.; Zhou, X.; Li, X.; Wei, Y.; Hao, C.; Li, S.; Lei, Q. DC breakdown characteristics of XLPE/BNNS nanocomposites considering BN nanosheet concentration, space charge and temperature. High Voltage 2020, 5, 280–286. [Google Scholar] [CrossRef]
- Shamsi, R.; Sadeghi, G.M.M. Novel polyester diol obtained from PET waste and its application in the synthesis of polyurethane and carbon nanotube-based composites: swelling behavior and characteristic properties. Rsc Advances 2016, 6, 38399–38415. [Google Scholar] [CrossRef]
- Bhanushali, S.; Srivats, D.S.; Mishra, P.; More, A.P. Silica/coconut shell charcoal/high-density polyethylene/linear low-density polyethylene composites. Iranian Polymer Journal 2023, 32, 571–584. [Google Scholar] [CrossRef]
- Yang, S.B.; Lee, J.; Yeasmin, S.; Park, J.M.; Han, M.D.; Kwon, D.-J.; Yeum, J.H. Blown Composite Films of Low-Density/Linear-Low-Density Polyethylene and Silica Aerogel for Transparent Heat Retention Films and Influence of Silica Aerogel on Biaxial Properties. Materials 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-t.; Kang, M.; Bae, J.Y. The Facile Synthesis and Application of Mesoporous Silica Nanoparticles with a Vinyl Functional Group for Plastic Recycling. International Journal of Molecular Sciences 2024, 25. [Google Scholar] [CrossRef]
- Rahaman, M.; Paikaray, B.; Islam, M.; Mondal, S.; Moharana, J.; Pandiaraj, S.; Periyasami, G.; Giri, R. Simultaneous Effect of EBR on LLDPE and PDMS Rubber Blends and Its Nanocomposites for Cable Applications. Acs Omega 2023, 9, 828–836. [Google Scholar] [CrossRef]
- Shi, C.; Yang, J.; Liu, Y.; Wang, Y.; Xu, W.; Xu, Y.; Hu, W.; Liu, B. Thermally Conductive Study of Polyethylene/Al<sub>2</sub>O<sub>3</sub>Composite Networks Cross-linked Pipes by Electron Beam Irradiation. Chemical Research in Chinese Universities 2020, 36, 940–945. [Google Scholar]
- Song, M.-M.; Wang, Y.-M.; Liang, X.-Y.; Zhang, X.-Q.; Zhang, S.; Li, B.-J. Functional materials with self-healing properties: a review. Soft Matter 2019, 15, 6615–6625. [Google Scholar] [CrossRef]
- Zhong, N.; Post, W. Self-repair of structural and functional composites with intrinsically self-healing polymer matrices: A review. Composites Part a-Applied Science and Manufacturing 2015, 69, 226–239. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Rong, M.Z.; Zhang, M.Q. Self-healing polymeric materials based on microencapsulated healing agents: From design to preparation. Progress in Polymer Science 2015, 49-50, 175–220. [Google Scholar] [CrossRef]
- Ekeocha, J.; Ellingford, C.; Pan, M.; Wemyss, A.M.; Bowen, C.; Wan, C. Challenges and Opportunities of Self-Healing Polymers and Devices for Extreme and Hostile Environments. Advanced Materials 2021, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Li, Y.; Zheng, R. Self-healing of electrical tree damage of polyethylene/microcapsules insulation composite material. Journal of Materials Research and Technology-Jmr&T 2022, 19, 1614–1626. [Google Scholar]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Tao, X.; Sun, H.; Zhu, Y.; He, S.; Han, X. Self-Healing Properties of Water Tree with Microcapsule/Cross-Linked Polyethylene Composite Material Based on Three-Layer Core-Shell Structure. Polymers 2024, 16. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.; Xu, Q.; Chen, Y.; Ren, X.; Zhou, K. Thermal aged XLPE cables restoration by injecting hydrolyzable voltage stabilizer. Journal of Polymer Research 2022, 29. [Google Scholar] [CrossRef]
- Islam, S.; Bhat, G. Progress and challenges in self-healing composite materials. Materials Advances 2021, 2, 1896–1926. [Google Scholar] [CrossRef]
- Selvin, M.; Shah, S.; Maria, H.J.; Thomas, S.; Tuladhar, R.; Jacob, M. Review on Recycling of Cross-Linked Polyethylene. Industrial & Engineering Chemistry Research 2024, 63, 1200–1214. [Google Scholar]
- Gall, M.; Freudenthaler, P.J.; Fischer, J.; Lang, R.W. Characterization of Composition and Structure-Property Relationships of Commercial Post-Consumer Polyethylene and Polypropylene Recyclates. Polymers 2021, 13. [Google Scholar] [CrossRef]
- Liao, Y.; Lai, S.; Yang, S.; Liu, J.; Kelly, A.L.; Bai, S. Using Asphalt as an Additive for Waste Cross-Linked Polyethylene Recycled Materials to Improve Thermoplastic Processing. Acs Omega 2022, 7, 19113–19121. [Google Scholar] [CrossRef]
- Manas, D.; Manas, M.; Mizera, A.; Stoklasek, P.; Navratil, J.; Sehnalek, S.; Drabek, P. The High Density Polyethylene Composite with Recycled Radiation Cross-Linked Filler of rHDPEx. Polymers 2018, 10. [Google Scholar] [CrossRef]
- Manas, D.; Manas, M.; Mizera, A.; Navratil, J.; Ovsik, M.; Tomanova, K.; Sehnalek, S. Use of Irradiated Polymers after Their Lifetime Period. Polymers 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Ryu, T.; Shin, J.; Lee, D.-H.; Ryu, J.; Park, I.; Hong, H.; Huh, Y.S.; Kim, B.-G.; Chung, K.-S. Development of multi-stage column for lithium recovery from an aqueous solution. Hydrometallurgy 2015, 157, 39–43. [Google Scholar] [CrossRef]
- Navratil, J.; Manas, M.; Mizera, A.; Bednarik, M.; Stanek, M.; Danek, M. Recycling of irradiated high-density polyethylene. Radiation Physics and Chemistry 2015, 106, 68–72. [Google Scholar] [CrossRef]
- Billotte, C.; Romana, L.; Flory, A.; Kaliaguine, S.; Ruiz, E. Recycled from waste tires carbon black/high-density polyethylene composite: Multi-scale mechanical properties and polymer aging. Polymer Composites 2024. [Google Scholar] [CrossRef]
- Li, L.; Zhang, K.; Zhong, L.; Chen, N.; Xu, M.; Xie, D.; Chen, G.; Ieee. Dielectric Behaviors of Recyclable Thermo-plastic Polyolefin Blends for Extruded Cables. In Proceedings of the 2015 IEEE 11th International Conference on the Properties and Applications of Dielectric Materials (ICPADM), Sydney, AUSTRALIA, 2015 Jul 19-22. [Google Scholar]
- Zhang, K.; Li, L.; Zhong, L.; Chen, N.; Xu, M.; Xie, D.; Chen, G.; Ieee. The Mechanical Properties of Recyclable Cable Insulation Materials Based on Thermo-plastic Polyolefin Blends. In Proceedings of the 2015 IEEE 11th International Conference on the Properties and Applications of Dielectric Materials (ICPADM), Sydney, AUSTRALIA, 2015 Jul 19-22. [Google Scholar]
- Cho, H.-K.; Hong, S.M.; Koo, C.M. Crystallization and the Mechanical and Rheological Properties of Decrosslinked-Crosslinked-High-Density Polyethylenes Using a Supercritical Fluid. Journal of Applied Polymer Science 2011, 120, 2090–2094. [Google Scholar] [CrossRef]
- Lee, H.-s.; Jeong, J.H.; Hong, G.; Cho, H.-K.; Baek, B.K.; Koo, C.M.; Hong, S.M.; Kim, J.; Lee, Y.-W. Effect of Solvents on De-Cross-Linking of Cross-Linked Polyethylene under Subcritical and Supercritical Conditions. Industrial & Engineering Chemistry Research 2013, 52, 6633–6638. [Google Scholar]
- Kwon, Y.J.; Hong, S.M.; Koo, C.M. Viscoelastic Properties of Decrosslinked Irradiation-Crosslinked Polyethylenes in Supercritical Methanol. Journal of Polymer Science Part B-Polymer Physics 2010, 48, 1265–1270. [Google Scholar] [CrossRef]
- Goto, T.; Yamazaki, T.; Sugeta, T.; Okajima, I.; Sako, T. Selective decomposition of the siloxane bond constituting the crosslinking element of silane-crosslinked polyethylene by supercritical alcohol. Journal of Applied Polymer Science 2008, 109, 144–151. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Park, W.Y.; Hong, S.M.; Koo, C.M. Foaming of recycled crosslinked polyethylenes via supercritical decrosslinking reaction. Journal of Applied Polymer Science 2012, 126, E21–E27. [Google Scholar] [CrossRef]
- Baek, B.K.; La, Y.H.; Lee, A.S.; Han, H.; Kim, S.H.; Hong, S.M.; Koo, C.M. Decrosslinking reaction kinetics of silane-crosslinked polyethylene in sub- and supercritical fluids. Polymer Degradation and Stability 2016, 130, 103–108. [Google Scholar] [CrossRef]
- Baek, B.K.; Shin, J.W.; Jung, J.Y.; Hong, S.M.; Nam, G.J.; Han, H.; Koo, C.M. Continuous Supercritical Decrosslinking Extrusion Process for Recycling of Crosslinked Polyethylene Waste. Journal of Applied Polymer Science 2015, 132. [Google Scholar] [CrossRef]
- Jia, R.-J.; Teng, K.-Y.; Huang, J.-Y.; Wei, X.; Qin, Z.-Y. Hydrogen Bonding Crosslinking of Starch-Polyvinyl Alcohol Films Reinforced by Ultrasound-Assisted and Cellulose Nanofibers Dispersed Cellulose Nanocrystals. Starch-Starke 2022, 74. [Google Scholar] [CrossRef]
- Isayev, A.I.; Huang, K. Decrosslinking of Crosslinked High-Density Polyethylene via Ultrasonically Aided Single-Screw Extrusion. Polymer Engineering and Science 2014, 54, 2715–2730. [Google Scholar] [CrossRef]
- Huang, K.; Isayev, A.I. Ultrasonic decrosslinking of crosslinked high-density polyethylene: effect of degree of crosslinking. Rsc Advances 2014, 4, 38877–38892. [Google Scholar] [CrossRef]
- Lee, H.-s.; Jeong, J.H.; Cho, H.-K.; Koo, C.M.; Hong, S.M.; Kim, H.; Lee, Y.-W. A kinetic study of the decross-linking of cross-linked polyethylene in supercritical methanol (vol 93, pg 2084, 2008). Polymer Degradation and Stability 2009, 94, 1598–1598. [Google Scholar] [CrossRef]
- Sun, F.; Yang, S.; Wang, Q. Selective Decomposition Process and Mechanism of Si-O-Si Cross-Linking Bonds in Silane Cross-Linked Polyethylene by Solid-State Shear Milling. Industrial & Engineering Chemistry Research 2020, 59. [Google Scholar]
- Yu, P.; Guo, X.-S.; Bao, R.-Y.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. Photo-Driven Self-Healing of Arbitrary Nondestructive Damage in Polyethylene-Based Nanocomposites. Acs Applied Materials & Interfaces 2020, 12, 1650–1657. [Google Scholar]
- Innes, J.R.; Shriky, B.; Allan, S.; Wang, X.; Hebda, M.; Coates, P.; Whiteside, B.; Benkreira, H.; Caton-Rose, P.; Lu, C.H.; et al. Effect of solid-state shear milled natural rubber particle size on the processing and dynamic vulcanization of recycled waste into thermoplastic vulcanizates. Sustainable Materials and Technologies 2022, 32. [Google Scholar] [CrossRef]
- Wu, H.; Liang, M.; Lu, C. Non-isothermal crystallization kinetics of peroxide-crosslinked polyethylene: Effect of solid state mechanochemical milling. Thermochimica Acta 2012, 545, 148–156. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).