Submitted:
20 September 2024
Posted:
20 September 2024
You are already at the latest version
Abstract
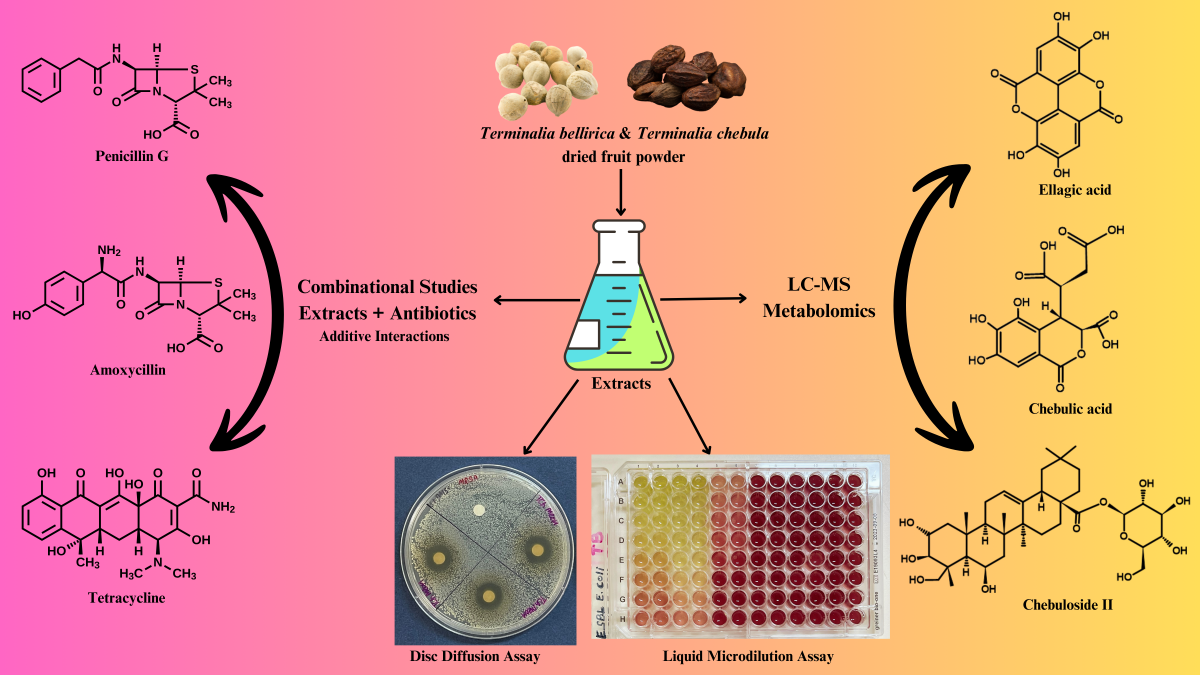
Keywords:
1. Introduction
2. Results
2.1. Antimicrobial Susceptibility Assays
2.2. Combination Assays: Sum of Fractional Inhibitory Concentration (ƩFIC) Determinations
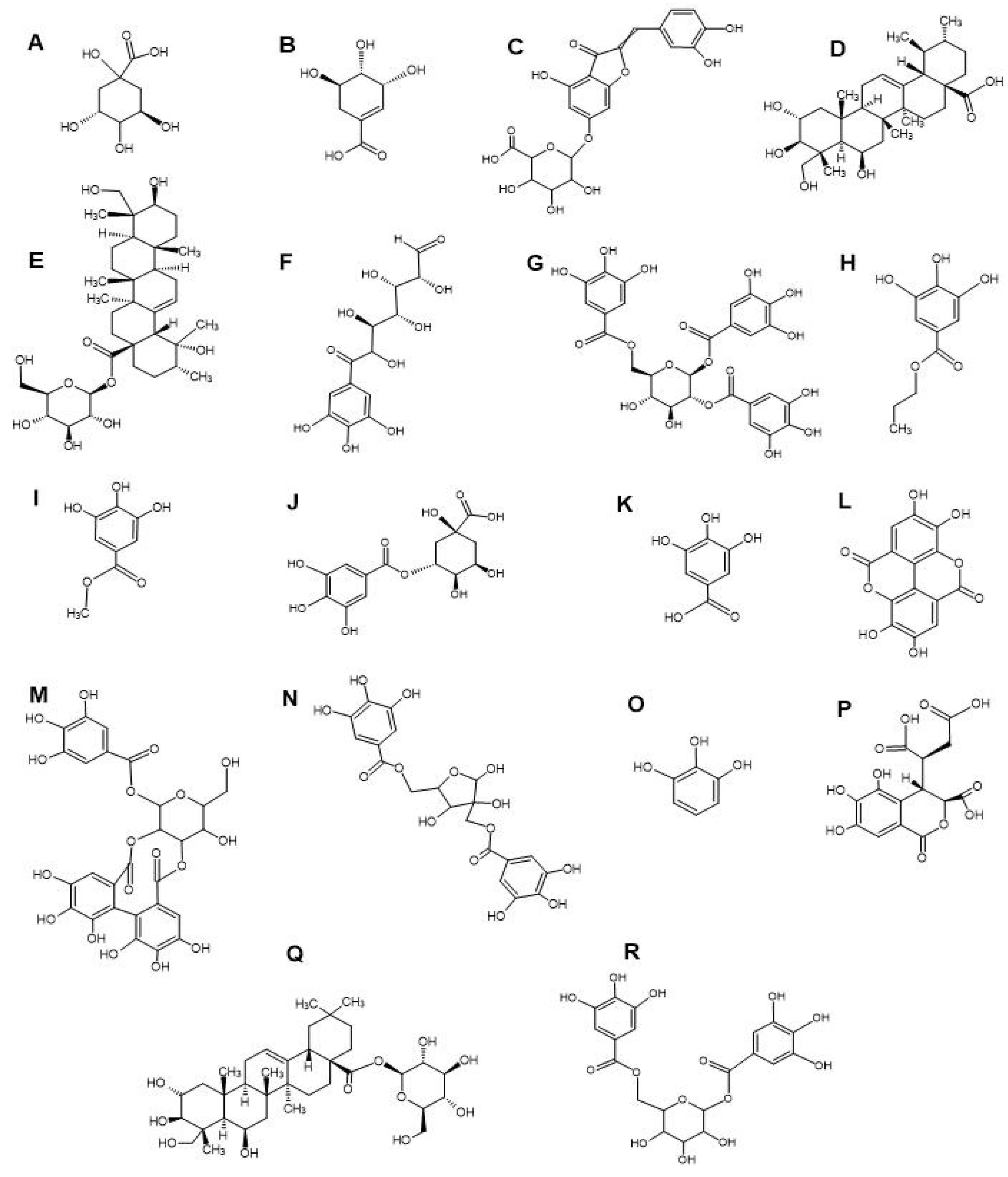
2.3. Compound Identification by LC-MS Metabolomics Fingerprinting
2.4. Toxicity Quantification
3. Discussion
4. Materials and Methods
4.1. Plant Origins
4.2. Extract Preparation
4.3. Antibiotics and Bacterial Strains
4.4. Antibacterial Susceptibility Screening
4.5. Minimum Inhibitory Concentration Determinations
4.6. Fractional Inhibitory Concentration Evaluation
4.7. Toxicity Assays
4.8. Non-Targeted Headspace LC-MS for Quantitative Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Antimicrobial resistance. World Health Organization. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 26 July 2024).
- Centers for Disease Control and Prevention (CDC). 2019 Antibiotic Resistance Threats Report. Available online: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/ (accessed on 26 July 2024).
- World Bank. Drug-Resistant Infections: A Threat to Our Economic Future. Available online: https://www.worldbank.org/en/topic/health/publication/drug-resistant-infections-a-threat-to-our-economic-future (accessed on 6 June 2024).
- Ajulo, S.; Awosile, B. Global antimicrobial resistance and use surveillance system (GLASS 2022): Investigating the relationship between antimicrobial resistance and antimicrobial consumption data across the participating countries. PLoS ONE 2024, 19, e0297921. [Google Scholar] [CrossRef] [PubMed]
- Renwick, M.J.; Brogan, D.M.; Mossialos, E. A systematic review and critical assessment of incentive strategies for discovery and development of novel antibiotics. J. Antibiot. (Tokyo) 2016, 69, 73–88. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO releases report on state of development of antibacterials. 2024. Available online: https://www.who.int/news/item/14-06-2024-who-releases-report-on-state-of-development-of-antibacterials (accessed on 18 June 2024).
- Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H.G. Back to nature: medicinal plants as promising sources for antibacterial drugs in the post-antibiotic era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef] [PubMed]
- Tiwana, G.; Cock, I.E.; Cheesman, M.J. A Review of Ayurvedic principles and the use of Ayurvedic plants to control diarrhoea and gastrointestinal infections. Pharmacogn. Commun. 2023, 13, 152–162. [Google Scholar] [CrossRef]
- Cheesman, M.; Ilanko, A.; Blonk, B.; Cock, I. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 2017, 11, 57. [Google Scholar] [CrossRef]
- Belmehdi, O.; Bouyahya, A.; Jekő, J.; et al. Synergistic interaction between propolis extract, essential oils, and antibiotics against Staphylococcus epidermidis and methicillin-resistant Staphylococcus aureus. Int. J. Sec. Metab. 2021, 8, 195–213. [Google Scholar] [CrossRef]
- Tiwana, G.; Cock, I.E.; White, A.; Cheesman, M.J. Use of specific combinations of the triphala plant component extracts to potentiate the inhibition of gastrointestinal bacterial growth. J. Ethnopharmacol. 2020, 260, 112937. [Google Scholar] [CrossRef]
- Thakur, M.; Khushboo; Yadav, A. ; et al. Antimicrobial activity against antibiotic-resistant pathogens and antioxidant activity and lcms/ms phytochemical content analysis of selected medicinal plants. J. Pure Appl. Microbiol. 2024, 18, 722–738. [Google Scholar] [CrossRef]
- Badoni, H.; Sharma, P.; Mohsin Waheed, S.; Singh, S. Phytochemical analyses and evaluation of antioxidant, antibacterial, and toxic properties of Emblica officinalis and terminalia bellirica fruit extracts. Asian J. Pharm. Clin. Res. 2016, 9. [Google Scholar] [CrossRef]
- Mani, P.; Devi, P.N.; Kaleeswari, S.; Poonkothai, M. Antimicrobial activity and phytochemical analysis of fruit extracts of Terminalia bellirica. Int. J. Pharm. Pharm. Sci. 2014, 43, 364. [Google Scholar]
- Sharma, R.; Raizada, S.; Gautam, A.; Bhatia, A.K. Phytochemical and antibacterial analysis of Terminalia chebula and Terminalia bellirica. In Green Chemistry in Environmental Sustainability and Chemical Education; Springer, 2018; pp. 131–137. [CrossRef]
- Parekh, J.; Chanda, S. Evaluation of antimicrobial activity of Terminalia chebula Retz. fruit in different solvents. J. Herbs Spices Med. Plants 2008, 13, 107–116. [Google Scholar] [CrossRef]
- Bag, A.; Kumar Bhattacharyya, S.; Bharati, P.; et al. Evaluation of antibacterial properties of Chebulic myrobalan (fruit of Terminalia chebula Retz.) extracts against methicillin-resistant Staphylococcus aureus and trimethoprim-sulphamethoxazole resistant uropathogenic Escherichia coli. Afr. J. Plant Sci. 2009, 3, 25–29. [Google Scholar]
- Zearah, S.A. Antifungal and antibacterial activity of flavonoid extract from Terminalia chebula retz. fruits. Journal of Basrah Researches (Sciences) 2014, 40, 122–131. [Google Scholar]
- Cajka, T.; Hricko, J.; Rudl Kulhava, L.; et al. Optimization of mobile phase modifiers for fast LC-MS-based untargeted metabolomics and lipidomics. Int. J. Mol. Sci. 2023, 24, 1987. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; et al. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Lin, S. Solubility and dissolution enhancement strategies: Current understanding and recent trends. Drug Dev. Ind. Pharm. 2015, 41, 875–887. [Google Scholar] [CrossRef]
- Flanagan, J.N.; Steck, T.R. The relationship between agar thickness and antimicrobial susceptibility testing. Indian J. Microbiol. 2017, 57, 503–506. [Google Scholar] [CrossRef]
- Bhattacharjee, M.K. Better visualization and photodocumentation of zone of inhibition by staining cells and background agar differently. J. Antibiot. 2015, 68, 657–659. [Google Scholar] [CrossRef]
- Livermore, D.M. Antibiotic resistance in staphylococci. Int. J. Antimicrob. Agents 2000, 16, 3–10. [Google Scholar] [CrossRef]
- Kim, C.; Mwangi, M.; Chung, M.; et al. The Mechanism of heterogeneous beta-lactam resistance in MRSA: Key role of the stringent stress response. PLoS ONE 2013, 8, e82814. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; et al. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Huttner, A.; Bielicki, J.; Clements, M.N.; et al. Oral amoxicillin and amoxicillin–clavulanic acid: properties, indications, and usage. Clin. Microbiol. Infect. 2020, 26, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Oelschlaeger, P. β-Lactamases: Sequence, structure, function, and inhibition. Biomolecules 2021, 11, 986. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Eskin, N.A.M.; Iranshahy, M.; Fazly Bazzaz, B.S. Phytochemicals: A promising weapon in the arsenal against antibiotic-resistant bacteria. Antibiotics 2021, 10, 1044. [Google Scholar] [CrossRef]
- Platon, V.M.; Dragoi, B.; Marin, L. Erythromycin formulations—A journey to advanced drug delivery. Pharmaceutics 2022, 14, 2180. [Google Scholar] [CrossRef]
- Kehrenberg, C.; Schwarz, S.; Jacobsen, L.; et al. A new mechanism for chloramphenicol, florfenicol, and clindamycin resistance: Methylation of 23S ribosomal RNA at A2503. Mol. Microbiol. 2005, 57, 1064–1073. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, V.K.; Pathania, R. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J. Med. Res. 2019, 149, 129. [Google Scholar] [CrossRef]
- Tiwana, G.; Cock, I.E.; Cheesman, M.J. Phyllanthus niruri Linn.: Antibacterial activity, phytochemistry, and enhanced antibiotic combinatorial strategies. Antibiotics 2024, 13, 654. [Google Scholar] [CrossRef]
- Gibbons, S. Phytochemicals for bacterial resistance--strengths, weaknesses, and opportunities. Planta Med. 2008, 74, 594–602. [Google Scholar] [CrossRef]
- Cai, X.; Javor, S.; Gan, B.H.; et al. The antibacterial activity of peptide dendrimers and polymyxin B increases sharply above pH 7.4. Chem. Commun. 2021, 57, 5654–5657. [Google Scholar] [CrossRef] [PubMed]
- Zavascki, A.P.; Goldani, L.Z.; Li, J.; Nation, R.L. Polymyxin B for the treatment of multidrug-resistant pathogens: A critical review. J. Antimicrob. Chemother. 2007, 60, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Cock, I.E. The medicinal properties and phytochemistry of plants of the genus Terminalia (Combretaceae). Inflammopharmacology 2015, 23, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; J Krishana, M.; Joshi, A.B.; et al. A pharmacognostic, phytochemical, and pharmacological review of Terminalia bellirica. J. Pharmacogn. Phytochem. 2017, 6, 368–376. [Google Scholar]
- Bag, A.; Bhattacharyya, S.K.; Chattopadhyay, R.R. The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pac. J. Trop. Biomed. 2013, 3, 244. [Google Scholar] [CrossRef]
- Embaby, M.A.; El-Raey, M.A.; Zaineldain, M.; et al. Synergistic effect and efflux pump inhibitory activity of Ficus nitida phenolic extract with tetracycline against some pathogenic bacteria. Toxin Rev. 2021, 40, 1187–1197. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Q.; Wang, Y.; et al. Exploiting the synergistic antibacterial activity of shikimic acid and ceftiofur against methicillin-resistant Staphylococcus aureus. World J. Microbiol. Biotechnol. 2024, 40, 1–11. [Google Scholar] [CrossRef]
- Yang, X.; Gao, X.; Ou, J.; et al. Antimicrobial activity and mechanism of anti-MRSA of phloroglucinol derivatives. DARU J. Pharm. Sci. 2024, 32, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Duan, S.; Hu, Y.; et al. Antibacterial activity and synergistic antibiotic mechanism of trialdehyde phloroglucinol against methicillin-resistant Staphylococcus aureus. Phytother. Res. 2023, 37, 490–504. [Google Scholar] [CrossRef]
- Mittal, N.; Tesfu, H.H.; Hogan, A.M.; et al. Synthesis and antibiotic activity of novel acylated phloroglucinol compounds against methicillin-resistant Staphylococcus aureus. J. Antibiot. 2019, 72, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Cui, P.; Liu, X. Antibacterial activity and mechanism of madecassic acid against Staphylococcus aureus. Molecules 2023, 28, 1895. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, H.; Kataoka, S.; Okamoto, S.; et al. Antimicrobial triterpenes from Ilex integra and the mechanism of antifungal action. Phytother. Res. 1999, 13, 151–160. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Kim, S.H.; et al. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Sohn, M.J.; Lee, J.; Kim, W.G. Isolation and identification of pentagalloylglucose with broad-spectrum antibacterial activity from Rhus trichocarpa Miquel. Food Chem. 2010, 123, 501–506. [Google Scholar] [CrossRef]
- Masota, N.E.; Ohlsen, K.; Schollmayer, C.; et al. Isolation and Characterization of Galloylglucoses Effective against Multidrug-Resistant Strains of Escherichia coli and Klebsiella pneumoniae. Molecules 2022, 27, 5045. [Google Scholar] [CrossRef]
- Pfundstein, B.; El Desouky, S.K.; Hull, W.E.; et al. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar] [CrossRef]
- Avula, B.; Wang, Y.H.; Wang, M.; et al. Simultaneous determination and characterization of tannins and triterpene saponins from the fruits of various species of Terminalia and Phyllantus emblica using a UHPLC-UV-MS Method: Application to Triphala. Planta Med. 2013, 29, 181–188. [Google Scholar] [CrossRef]
- Manosroi, A.; Jantrawut, P.; Ogihara, E.; et al. Biological activities of phenolic compounds and triterpenoids from the Galls of Terminalia chebula. Chem. Biodivers. 2013, 10, 1448–1463. [Google Scholar] [CrossRef]
- Bag, A.; Chattopadhyay, R.R. Efflux-pump inhibitory activity of a gallotannin from Terminalia chebula fruit against multidrug-resistant uropathogenic Escherichia coli. Nat. Prod. Res. 2014, 28, 1280–1283. [Google Scholar] [CrossRef]
- Bag, A.; Chattopadhyay, R.R. Synergistic antibiofilm efficacy of a gallotannin 1,2,6-tri-O-galloyl-β-D-glucopyranose from Terminalia chebula fruit in combination with gentamicin and trimethoprim against multidrug resistant uropathogenic Escherichia coli biofilms. PLoS ONE 2017, 12, e0178712. [Google Scholar] [CrossRef] [PubMed]
- Hricovíniová, Z.; Mascaretti, Š.; Hricovíniová, J.; et al. New unnatural gallotannins: A way toward green antioxidants, antimicrobials and antibiofilm agents. Antioxidants 2021, 10, 1288. [Google Scholar] [CrossRef]
- Abbas, M.A.; Lee, E.B.; Boby, N.; et al. A pharmacodynamic investigation to assess the synergism of orbifloxacin and propyl gallate against Escherichia coli. Front. Pharmacol. 2022, 13, 989395. [Google Scholar] [CrossRef] [PubMed]
- Tamang, M.D.; Bae, J.; Park, M.; Jeon, B. Potentiation of β-Lactams against Methicillin-Resistant Staphylococcus aureus (MRSA) Using octyl gallate, a food-grade antioxidant. Antibiotics 2022, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Gesek, J.; Jakimiuk, K.; Atanasov, A.G.; Tomczyk, M. Sanguiins—Promising molecules with broad biological potential. Int. J. Mol. Sci. 2021, 22, 12972. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.J.; Fernández-López, S.; Cuñas-Figueroa, I.D.; et al. Sanguiin H-6 fractionated from cloudberry (Rubus chamaemorus) seeds can prevent the methicillin-resistant Staphylococcus aureus biofilm development during wound infection. Antibiotics 2021, 10, 1481. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.L.; Chen, G.; Chen, S.N.; et al. Characterization of polyphenolic constituents from Sanguisorba officinalis L. and its antibacterial activity. Eur. Food Res. Technol. 2019, 245, 1487–1498. [Google Scholar] [CrossRef]
- Hossain, M.A.; Park, H.C.; Park, S.W.; et al. Synergism of the combination of traditional antibiotics and novel phenolic compounds against Escherichia coli. Pathogens 2020, 9, 811. [Google Scholar] [CrossRef]
- Bassyouni, R.H.; Dwedar, R.A.; Farahat, M.G.; et al. Protective effect of hamamelitannin against biofilm production by methicillin-resistant Staphylococci Isolated from blood of patients at intensive Care Units. Microbiol. Res. J. Int. 2015, 10, 1–8. [Google Scholar] [CrossRef]
- Dhingra, A.K.; Chopra, B.; Grewal, A.S.; Guarve, K. Pharmacological properties of chebulinic acid and related ellagitannins from nature: An emerging contemporary bioactive entity. Pharmacol. Res. Mod. Chin. Med. 2022, 5, 100163. [Google Scholar] [CrossRef]
- Yang, Z.N.; Su, B.J.; Wang, Y.Q.; et al. Isolation, absolute configuration, and biological activities of chebulic acid and brevifolincarboxylic acid Derivatives from Euphorbia hirta. J. Nat. Prod. 2020, 83, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lin, Y.; Li, B.; et al. Antibacterial constituents of Fructus Chebulae Immaturus and their mechanisms of action. BMC Complement. Altern. Med. 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tasduq, S.A.; Singh, K.; Satti, N.K.; et al. Terminalia chebula (fruit) prevents liver toxicity caused by sub-chronic administration of rifampicin, isoniazid and pyrazinamide in combination. Hum. Exp. Toxicol. 2006, 25, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; et al. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Jenic, D.; Waller, H.; Collins, H.; Erridge, C. Reversal of tetracycline resistance by cepharanthine, cinchonidine, ellagic Acid and propyl gallate in a multi-drug resistant Escherichia coli. Nat. Prod. Bioprospect. 2021, 11, 345–355. [Google Scholar] [CrossRef]
- Quave, C.L.; Estévez-Carmona, M.; Compadre, C.M.; et al. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS ONE 2012, 7, e28737. [Google Scholar] [CrossRef]
- Anek, P.; Kumpangcum, S.; Roytrakul, S.; et al. Antibacterial activities of phenolic compounds in Miang extract: Growth inhibition and change in protein expression of extensively drug-Resistant Klebsiella pneumoniae. Antibiotics 2024, 13, 536. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Alcorn, S.R.; White, A.; Cock, I.E. Hamamelis virginiana L. leaf extracts inhibit the growth of antibiotic-Resistant Gram-positive and Gram-negative bacteria. Antibiotics 2023, 12, 1195. [Google Scholar] [CrossRef]
- Vitko, N.P.; Richardson, A.R. Laboratory maintenance of methicillin-resistant Staphylococcus aureus (MRSA). Curr. Protoc. Microbiol. 2013, 28, 9C.2.1–9C.2.14. [Google Scholar] [CrossRef]
- Doern, C.D. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar] [CrossRef]
- Ruebhart, D.R.; Wickramasinghe, W.; Cock, I.E. Protective efficacy of the antioxidant’s vitamin E and Trolox against Microcystis aeruginosa and microcystin-LR in Artemia franciscana Nauplii. J. Toxicol. Environ. Health A 2009, 72, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
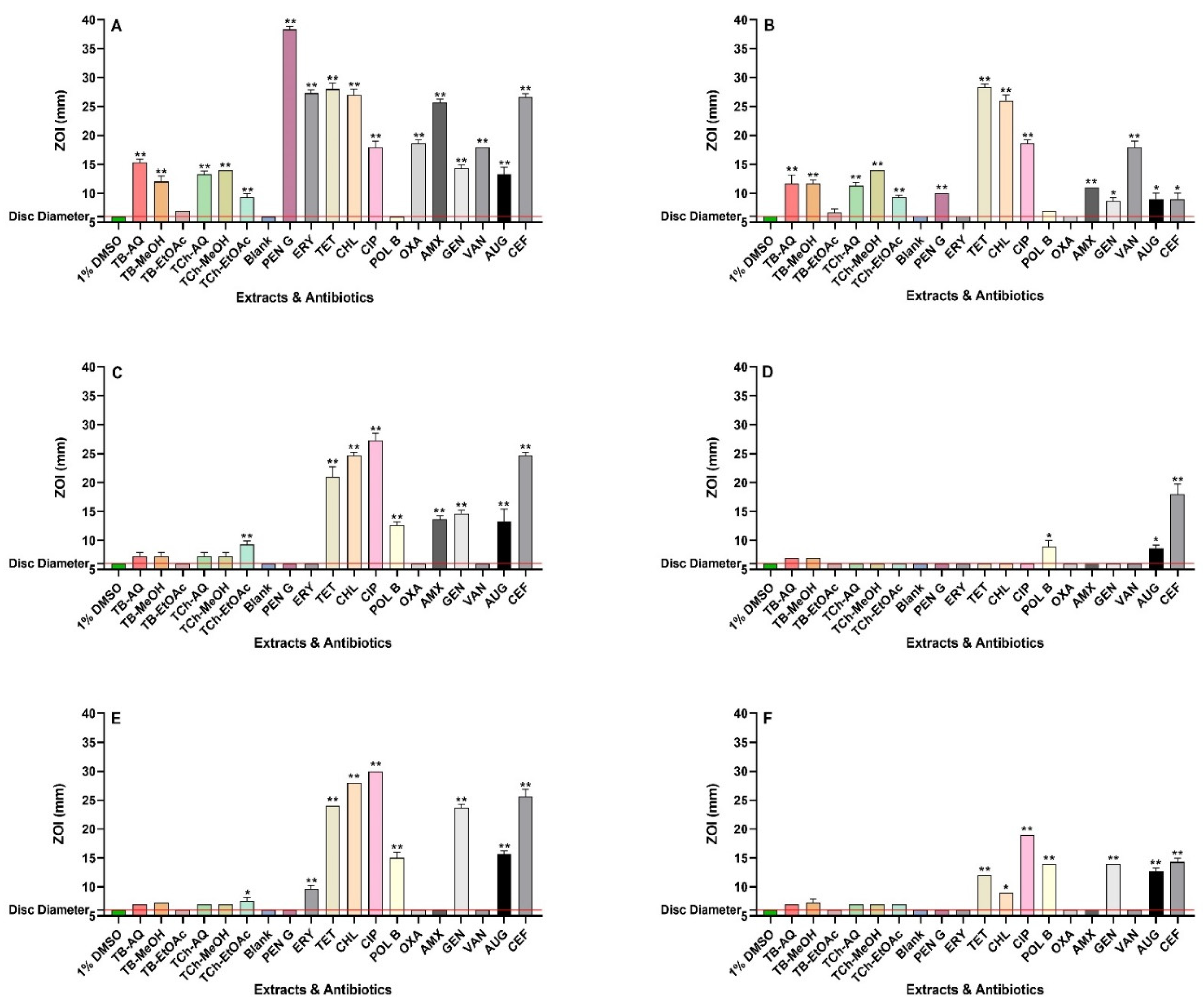
| Extract Type or Antibiotic | Bacterial species & MIC (µg/mL) | |||||
|---|---|---|---|---|---|---|
| S. aureus | MRSA | E. coli | ESBL E. coli | K. pneumoniae | ESBL K. pneumoniae | |
| TB-AQ | 392 | 196 | 3138 | 1569 | 784 | 3138 |
| TB-MeOH | 94 | 189 | 755 | 755 | 1509 | 755 |
| TB-EtOAc | 900 | 900 | Inactive | Inactive | Inactive | Inactive |
| TCh-AQ | 139 | 139 | 2225 | 2225 | 556 | 2225 |
| TCh-MeOH | 189 | 189 | 3019 | 1509 | 1509 | 1509 |
| TCh-EtOAc | 306 | 306 | Inactive | Inactive | 1225 | Inactive |
| PENG | 1.25 | >2.5 | >2.5 | >2.5 | >2.5 | >2.5 |
| ERY | 0.31 | >2.5 | >2.5 | >2.5 | >2.5 | >2.5 |
| TET | 0.16 | 0.04 | 0.31 | >2.5 | 0.625 | >2.5 |
| CHL | >2.5 | >2.5 | 2.5 | >2.5 | 2.5 | >2.5 |
| CIP | 0.16 | 0.625 | 0.02 | >2.5 | 0.02 | 0.16 |
| POLB | >2.5 | >2.5 | 0.02 | 0.02 | 0.02 | 0.04 |
| OXA | 0.16 | >2.5 | >2.5 | >2.5 | >2.5 | >2.5 |
| AMX | 0.625 | >2.5 | >2.5 | >2.5 | >2.5 | >2.5 |
| GEN | >2.5 | >2.5 | 0.625 | >2.5 | 0.625 | >2.5 |
| Bacteria | Extracts | PENG | ERY | TET | CHL | CIP | POLB | OXA | AMX | GEN | VAN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | TB-AQ | 0.53 | 1.26 | 1.50 | - | 1.50 | - | 1.25 | 0.56 | - | 1.06 |
| TB-MeOH | 1.02 | 2.13 | 1.13 | - | 2.25 | - | 1.25 | 1.03 | - | 2.03 | |
| TB-EtOAc | 0.75 | 1.13 | 1.06 | - | 2.06 | - | 2.13 | 1.25 | - | 1.50 | |
| TChAQ | 1.06 | 1.25 | 0.75 | - | 1.50 | - | 1.50 | 1.13 | - | 1.06 | |
| TCh-MeOH | 1.03 | 1.13 | 1.25 | - | 1.25 | - | 1.25 | 1.06 | - | 1.03 | |
| TCh-EtOH | 0.75 | 0.75 | 1.25 | - | 2.50 | - | 2.50 | 1.00 | - | 1.50 | |
| MRSA | TB-AQ | - | - | 1.00 | - | 1.06 | - | - | - | - | 1.03 |
| TB-MeOH | - | - | 1.50 | - | 1.03 | - | - | - | - | 1.02 | |
| TB-EtOAc | - | - | 1.02 | - | 0.63 | - | - | - | - | 1.50 | |
| TCh-AQ | - | - | 1.00 | - | 2.13 | - | - | - | - | 2.06 | |
| TCh-MeOH | - | - | 1.00 | - | 1.06 | - | - | - | - | 1.03 | |
| TCh-EtOAc | - | - | 1.06 | - | 1.00 | - | - | - | - | 1.50 | |
| E. coli | TB-AQ | - | - | 1.50 | 1.25 | 2.25 | 64.50 | - | - | 2.00 | - |
| TB-MeOH | - | - | 1.52 | 1.06 | 4.00 | 17.50 | - | - | 1.25 | - | |
| TCh-AQ | - | - | 1.50 | 1.25 | 2.25 | 64.50 | - | - | 2.00 | - | |
| TCh-MeOH | - | - | 0.76 | 1.25 | 2.25 | 32.25 | - | - | 2.00 | - | |
| ESBL E. coli | TB-AQ | - | - | - | - | - | 16.50 | - | - | - | - |
| TB-MeOH | - | - | - | - | - | 5.00 | - | - | - | - | |
| TCh-AQ | - | - | - | - | - | 32.50 | - | - | - | - | |
| TCh-MeOH | - | - | - | - | - | 16.50 | - | - | - | - | |
| K. pneumoniae | TB-AQ | - | - | 1.25 | 1.06 | 2.00 | 9.00 | - | - | 1.25 | - |
| TB-MeOH | - | - | 1.50 | 1.12 | 3.00 | 16.50 | - | - | 1.50 | - | |
| TB-EtOAc | - | - | - | - | - | - | - | - | - | - | |
| TCh-AQ | - | - | 1.25 | 1.06 | 4.00 | 9.00 | - | - | 1.25 | - | |
| TCh-MeOH | - | - | 0.75 | 0.56 | 3.00 | 16.50 | - | - | 1.50 | - | |
| TCh-EtOAc | - | - | 0.63 | 1.00 | 2.12 | 63.00 | - | - | 4.00 | - | |
| ESBL K. pneumoniae | TB-AQ | - | - | - | - | 1.25 | 8.25 | - | - | - | - |
| TB-MeOH | - | - | - | - | 1.00 | 2.50 | - | - | - | - | |
| TCh-AQ | - | - | - | - | 2.50 | 16.60 | - | - | - | - | |
| TCh-MeOH | - | - | - | - | 1.50 | 8.75 | - | - | - | - |
| Retention Time (min) | Molecular Weight | Empirical Formula | Putative Compounds | % Relative Abundance (TB) | % Relative Abundance (TCh) | ||||
|---|---|---|---|---|---|---|---|---|---|
| AQ | MeOH | EtOAc | AQ | MeOH | EtOAc | ||||
| 1.496 | 192.06305 | C7H12O6 | Quinic acid | 3.13% | 0.33% | 0.19% | 0.39% | - | 1.53% |
| 1.573 | 344.07409 | C14H16O10 | Theogallin | - | 3.47% | - | - | 0.03% | 0.02% |
| 1.784 | 302.06328 | C12H14O9 | Pyrogallol-2-O-glucuronide | - | 0.21% | - | 0.07% | - | - |
| 1.793 | 244.0581 | C10H12O7 | 1-O-Galloylglycerol | - | - | 0.02% | - | - | - |
| 1.905 | 236.0684 | C12H12O5 | Dillapional | 0.01% | - | - | - | T | 0.01% |
| 1.923 | 316.0793 | C13H16O9 | Ginnalin B | 0.01% | - | - | - | - | - |
| 1.975 | 149.08397 | C9H11NO | Venoterpine | - | - | 0.05% | 0.04% | - | - |
| 2.045 | 294.03749 | C13H10O8 | Banksiamarin B | - | - | 0.15% | 0.17% | 0.05% | 0.13% |
| 2.058 | 174.05268 | C7H10O5 | Shikimic acid | - | 0.41% | 0.67% | 2.64% | 3.14% | 4.80% |
| 2.237 | 154.02646 | C7H6O4 | Gentisic acid | 0.02% | 0.05% | - | 0.01% | - | 0.09% |
| 2.296 | 448.15769 | C19H28O12 | 8-O-Acetyl shanzhiside methyl ester | - | - | - | 0.19% | 0.18% | - |
| 2.356 | 332.07411 | C13H16O10 | 6-Galloylglucose | 6.06% | 3.97% | 3.39% | 3.74% | 0.68% | - |
| 2.444 | 448.06386 | C20H16O12 | Ellagic acid 2-rhamnoside | 0.11% | 1.44% | 0.69% | 0.01% | 0.21% | 0.33% |
| 2.51 | 292.02177 | C13H8O8 | Brevifolincarboxylic acid | 0.09% | - | - | 0.11% | 0.03% | 0.61% |
| 2.56 | 288.0844 | C12H16O8 | Phlorin | - | 0.02% | - | 0.03% | 0.02% | - |
| 3.11 | 484.0848 | C20H20O14 | Gallic acid 3-O-(6-galloylglucoside) | 0.74% | - | - | - | - | - |
| 3.158 | 212.06824 | C10H12O5 | Propyl gallate | - | - | - | 0.30% | 0.35% | 0.12% |
| 4.4 | 277.05821 | C13H11NO6 | Salfredin C1 | - | - | - | 0.14% | - | 0.03% |
| 4.847 | 184.03703 | C8H8O5 | Methyl gallate | 0.25% | 11.39% | - | 0.04% | 1.05% | - |
| 5.638 | 444.19969 | C21H32O10 | Cynaroside A | - | - | - | 0.02% | 0.02% | - |
| 8.848 | 636.09627 | C27H24O18 | 1,3,4-Trigalloyl-β-D-glucopyranose | - | - | - | - | 0.07% | 0.25% |
| 8.44 | 484.0855 | C20H20O14 | 1,6-Bis-O-(3,4,5-trihydroxybenzoyl) hexopyranose | 5.13% | 5.19% | 2.52% | 6.52% | 2.16% | 3.78% |
| 8.961 | 478.07436 | C21H18O13 | Quercetin 7-glucuronide | - | - | - | - | - | 0.49% |
| 8.985 | 478.0743 | C21H18O13 | Quercetin 3-O-glucuronide | - | 0.08% | - | - | - | - |
| 9.316 | 470.01176 | C21H10O13 | Sanguisorbic acid dilactone | - | 0.08% | - | 0.56% | 0.61% | 0.19% |
| 9.567 | 634.08082 | C27H22O18 | Sanguiin H4 | 0.84% | - | 0.07% | - | 5.63% | 0.54% |
| 9.811 | 478.07452 | C21H18O13 | Miquelianin | 0.04% | 0.42% | - | 0.37% | 0.60% | 0.39% |
| 9.902 | 152.04703 | C8H8O3 | Vanillin | - | - | - | 0.16% | 0.17% | 0.17% |
| 9.921 | 126.03146 | C6H6O3 | Phloroglucinol | 0.10% | 1.93% | - | 0.07% | 0.24% | 0.27% |
| 10.043 | 636.09614 | C27H24O18 | 1,2,6-Trigalloyl-β-D-glucopyranose | 0.09% | 5.44% | 2.85% | 0.11% | 1.42% | 2.22% |
| 10.158 | 634.08037 | C27H22O18 | Corilagin | - | - | - | - | 0.15% | - |
| 10.357 | 524.1533 | C24H28O13 | Barbatoflavan | 0.01% | - | - | - | - | - |
| 10.419 | 484.08522 | C20H20O14 | Hamamelitannin | 2.10% | 0.02% | 0.62% | 0.02% | 0.11% | - |
| 10.421 | 126.0316 | C6H6O3 | Pyrogallol | 6.28% | 5.28% | 2.19% | 6.29% | 3.82% | 4.87% |
| 10.422 | 296.05248 | C13H12O8 | cis-Coutaric acid | 0.33% | 0.86% | 0.19% | 0.31% | 0.93% | 1.05% |
| 10.476 | 636.09612 | C27H24O18 | 1,4,6-Trigalloyl-β-D-glucopyranose | - | - | - | - | - | 0.63% |
| 10.55 | 601.99658 | C28H10O16 | Diellagilactone | - | - | - | 1.13% | 0.60% | - |
| 10.703 | 372.1055 | C16H20O10 | Veranisatin C | 0.02% | 0.04% | - | - | - | - |
| 10.754 | 636.09608 | C27H24O18 | 1,3,6-Tri-O-galloyl-β-D-glucose | - | 0.25% | 0.93% | 0.64% | - | - |
| 11.305 | 170.02138 | C7H6O5 | Phloroglucinic acid | 0.98% | 2.80% | 4.33% | 0.98% | 0.83% | 1.24% |
| 11.091 | 610.1535 | C27H30O16 | Rutin | - | 0.14% | 0.10% | - | 0.03% | 0.08% |
| 11.176 | 432.10539 | C21H20O10 | Vitexin | - | - | - | - | 0.05% | - |
| 11.257 | 304.0579 | C15H12O7 | Nigrescin | T | 0.02% | - | - | - | - |
| 11.272 | 610.1527 | C27H30O16 | Quercetin 3-O-rhamnoside-7-O-glucoside | 0.05% | - | - | - | - | - |
| 11.448 | 302.04213 | C15H10O7 | Quercetin | 0.11% | 0.33% | 0.17% | 0.02% | - | - |
| 11.473 | 464.0951 | C21H20O12 | Myricitrin | 0.05% | 0.13% | 0.19% | - | - | - |
| 11.529 | 422.0846 | C19H18O11 | 1,5,8-Trihydroxy-9-oxo-9H-xanthen-3-yl β-D-glucopyranoside | 0.02% | 0.05% | - | - | - | - |
| 11.589 | 170.02147 | C7H6O5 | Gallic acid | 26.23% | 8.64% | 2.60% | 23.90% | 1.90% | 1.56% |
| 11.756 | 216.0994 | C10H16O5 | (4S,5S,8S,10R)-4,5,8-trihydroxy-10-methyl-3,4,5,8,9,10-hexahydro-2H-oxecin-2-one | 0.05% | - | - | 0.06% | - | 1.23% |
| 11.822 | 462.07961 | C21H18O12 | Aureusidin 6-glucuronide | - | - | 2.52% | - | - | 0.04% |
| 11.905 | 286.04729 | C15H10O6 | Maritimetin | - | - | - | T | 0.01% | 0.01% |
| 11.906 | 594.15829 | C27H30O15 | Palasitrin | - | - | - | 0.01% | - | 0.03% |
| 11.938 | 176.04708 | C10H8O3 | 4-Methylumbelliferone hydrate | - | - | - | 0.03% | 0.03% | - |
| 11.953 | 310.10477 | C15H18O7 | (E)-1-O-Cinnamoyl-β-D-glucose | - | - | - | - | - | 1.67% |
| 12.141 | 584.1169 | C28H24O14 | 2”-O-Galloylisovitexin | - | - | - | - | - | 0.01% |
| 12.183 | 448.0996 | C21H20O11 | Maritimein | - | 0.01% | 0.02% | - | - | - |
| 12.185 | 302.00627 | C14H6O8 | Ellagic acid | 2.45% | 10.74% | 33.49% | 8.39% | 10.46% | 11.96% |
| 12.201 | 220.07336 | C12H12O4 | Eugenitin | - | - | - | - | - | 0.04% |
| 12.233 | 262.0476 | C13H10O6 | Maclurin | - | 0.02% | - | 0.04% | - | - |
| 12.254 | 148.05223 | C9H8O2 | trans-Cinnamic acid | - | - | - | 0.76% | 0.96% | 0.87% |
| 12.38 | 334.0325 | C15H10O9 | 3,5,6,7,2′,3′,4′-Heptahydroxyflavone | - | 0.20% | - | - | - | - |
| 12.503 | 192.07847 | C11H12O3 | (R)-Shinanolone | - | - | - | 0.04% | 0.03% | 0.05% |
| 12.504 | 310.10483 | C15H18O7 | (2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl) oxan-2-yl (2E)-3-phenylprop-2-enoate | - | - | - | 2.25% | - | - |
| 12.509 | 610.1894 | C28H34O15 | Neohesperidin | - | - | 0.16% | - | - | - |
| 12.584 | 498.1741 | C23H30O12 | Eucaglobulin | - | 0.02% | - | - | - | - |
| 12.593 | 436.13688 | C21H24O10 | Nothofagin | - | - | - | - | - | 0.12% |
| 12.721 | 680.37692 | C36H56O12 | Tenuifolin | - | - | - | - | 0.11% | 0.37% |
| 12.8 | 486.33397 | C30H46O5 | Bassic acid | - | - | - | - | - | 0.05% |
| 12.844 | 518.1785 | C26H30O11 | Phellodensin E | - | - | 0.05% | - | - | - |
| 12.889 | 346.1052 | C18H18O7 | Hamilcone | - | - | - | 0.02% | - | 0.01% |
| 12.914 | 190.13544 | C13H18O | β-Damascenone | - | - | - | - | 0.02% | 0.06% |
| 12.915 | 666.39739 | C36H58O11 | Chebuloside II | - | - | - | - | 3.85% | - |
| 12.924 | 302.1152 | C17H18O5 | Lusianin | 0.01% | - | - | - | - | - |
| 12.929 | 356.03763 | C14H12O11 | (+)-Chebulic acid | 0.54% | - | - | 3.98% | 1.40% | 2.88% |
| 12.967 | 330.14651 | C19H22O5 | Hericenone A | - | - | - | - | 0.03% | - |
| 12.969 | 504.34417 | C30H48O6 | Madecassic acid | - | - | - | - | - | 1.42% |
| 12.971 | 202.13522 | C14H18O | (±)-Anisoxide | - | - | - | - | - | 0.05% |
| 12.973 | 244.14583 | C16H20O2 | Lahorenoic acid C | - | - | - | - | - | 0.02% |
| 13.208 | 288.06318 | C15H12O6 | Eriodictyol | - | - | - | - | - | 0.27% |
| 13.354 | 314.1154 | C18H18O5 | Crotaoprostrin | - | - | - | - | - | 0.01% |
| 13.428 | 442.1992 | C25H30O7 | Exiguaflavanone M | - | - | 0.13% | - | - | - |
| 13.443 | 460.13716 | C23H24O10 | 7-Hydroxy-5,6-dimethoxyflavone 7-glucoside | - | - | - | - | 0.01% | - |
| 13.451 | 274.08371 | C15H14O5 | Phloretin | - | - | - | 0.01% | 0.02% | 0.01% |
| 13.536 | 462.11641 | C22H22O11 | Leptosin | - | 0.01% | - | 0.01% | 0.06% | - |
| 13.59 | 302.04255 | C15H10O7 | Bracteatin | - | - | - | 0.01% | 0.01% | - |
| 13.592 | 450.07961 | C20H18O12 | Quercetin 4′-galactoside | - | - | - | 0.01% | - | - |
| 13.593 | 502.1836 | C26H30O10 | Flavaprin | - | - | 0.07% | - | - | - |
| 13.642 | 514.18403 | C27H30O10 | Baohuoside 1 | - | - | - | T | 0.01% | - |
| 13.678 | 568.12178 | C28H24O13 | Isoorientin 2”-p-hydroxybenzoate | - | - | - | - | - | 0.04% |
| 13.703 | 280.13067 | C15H20O5 | Artabsinolide A | - | - | - | - | - | 0.02% |
| 13.725 | 330.0374 | C16H10O8 | Blighinone | 0.12% | - | - | - | - | - |
| 13.805 | 444.10499 | C22H20O10 | 3′-O-Methylderhamnosylmaysin | - | - | - | 0.04% | 0.12% | 0.08% |
| 13.805 | 292.09447 | C15H16O6 | (S)-Angelicain | - | - | - | 0.05% | - | - |
| 13.806 | 462.11629 | C22H22O11 | 6-O-[(2E)-3-Phenyl-2-propenoyl]-1-O-(3,4,5-trihydroxybenzoyl)-β-D-glucopyranose | - | - | - | 0.08% | 1.02% | 0.18% |
| 14.024 | 500.1674 | C26H28O10 | Ikarisoside A | - | - | 0.03% | - | - | - |
| 14.078 | 534.28285 | C29H42O9 | Corchoroside A | - | - | - | - | - | 0.02% |
| 14.358 | 272.06832 | C15H12O5 | Naringenin | - | - | - | - | - | 0.18% |
| 14.367 | 470.33907 | C30H46O4 | Glycyrrhetinic acid | - | - | - | - | 0.08% | 0.01% |
| 14.561 | 226.1203 | C12H18O4 | Allixin | - | - | 0.03% | - | - | - |
| 14.783 | 234.16167 | C15H22O2 | Valerenic acid | - | - | - | - | - | 0.13% |
| 14.928 | 202.17175 | C15H22 | Rulepidadiene B | - | - | - | - | - | 0.08% |
| 14.93 | 222.16153 | C14H22O2 | Rishitin | - | - | - | - | - | 0.04% |
| 15.046 | 426.09477 | C22H18O9 | Epiafzelechin 3-O-gallate | - | - | - | - | - | 0.01% |
| 15.454 | 650.4026 | C36H58O10 | Pedunculoside | - | - | - | - | 1.18% | 2.61% |
| 16.257 | 738.41926 | C39H62O13 | Isonuatigenin 3-[rhamnosyl-(1->2)-glucoside] | - | - | - | - | 0.04% | 0.12% |
| 16.301 | 470.1941 | C26H30O8 | Limonin | - | - | 0.14% | - | - | - |
| 16.478 | 540.1663 | C25H32O11S | Sumalarin B | - | - | 0.02% | - | - | - |
| 16.532 | 316.1308 | C18H20O5 | Methylodoratol | - | - | 0.02% | - | - | - |
| 16.787 | 472.2097 | C26H32O8 | Kushenol H | - | - | 0.07% | - | - | - |
| 16.844 | 344.0532 | C17H12O8 | 3,4,3′-Tri-O-methylellagic acid | - | - | 0.01% | - | - | - |
| 16.876 | 252.20856 | C16H28O2 | Isoambrettolide | - | - | - | - | - | 0.32% |
| 16.889 | 300.1361 | C18H20O4 | Angoletin | - | - | T | - | - | - |
| 17.064 | 440.1828 | C25H28O7 | Lonchocarpol E | - | - | 0.15% | - | - | - |
| 17.209 | 544.2668 | C30H40O9 | Physagulin F | - | - | 0.10% | - | - | - |
| 17.275 | 342.14638 | C20H22O5 | Brosimacutin C | - | - | - | - | T | - |
| 17.276 | 180.11471 | C11H16O2 | Jasmolone | - | 0.61% | 1.48% | 0.05% | - | - |
| 17.31 | 504.34409 | C30H48O6 | Protobassic acid | - | - | - | 1.26% | 0.02% | 0.01% |
| 17.33 | 468.32326 | C30H44O4 | Glabrolide | - | - | - | 0.87% | - | 3.19% |
| 17.33 | 502.32964 | C30H46O6 | Medicagenic acid | - | - | - | - | T | 0.07% |
| 17.331 | 200.15605 | C15H20 | (S)-gamma-Calacorene | - | - | - | 0.02% | - | 0.29% |
| 17.363 | 696.40877 | C37H60O12 | Momordicoside E | - | - | - | - | 0.01% | 0.04% |
| 17.626 | 286.08357 | C16H14O5 | Homobutein | - | T | 0.01% | - | - | 0.01% |
| 17.635 | 282.12541 | C18H18O3 | Ohobanin | 0.01% | T | 0.09% | T | - | 0.06% |
| 17.671 | 652.27302 | C32H44O14 | Dicrocin | - | - | - | - | - | 0.03% |
| 17.902 | 424.1881 | C25H28O6 | Paratocarpin G | T | - | - | - | - | - |
| 17.949 | 456.2144 | C26H32O7 | Antiarone J | - | 0.54% | - | - | - | - |
| 18.124 | 372.2509 | C20H36O6 | Sterebin Q4 | - | - | 0.01% | - | - | - |
| 18.164 | 328.1309 | C19H20O5 | 2′,3′,4′,6′-Tetrameth oxychalcone | - | - | 0.05% | - | - | - |
| 18.237 | 428.1831 | C24H28O7 | Heteroflavanone B | - | - | 0.05% | - | - | - |
| 18.237 | 312.13615 | C19H20O4 | Desmosdumotin C | T | - | 0.02% | T | - | 0.02% |
| 18.292 | 546.35535 | C32H50O7 | Hovenidulcigenin B | - | - | - | 0.04% | - | - |
| 18.339 | 236.1776 | C15H24O2 | Capsidiol | - | - | - | - | - | 0.02% |
| 18.346 | 268.13069 | C14H20O5 | Kamahine C | 0.02% | 0.01% | - | - | 0.01% | 0.15% |
| 18.363 | 336.0994 | C20H16O5 | Ciliatin A | - | - | - | - | 0.01% | - |
| 18.47 | 466.1989 | C27H30O7 | Eriotriochin | - | 0.50% | - | - | - | - |
| 18.498 | 340.09447 | C19H16O6 | Ambanol | - | - | - | - | 0.01% | - |
| 18.507 | 484.31803 | C30H44O5 | Liquoric acid | - | - | - | T | 0.01% | 0.04% |
| 18.623 | 488.35017 | C30H48O5 | Pitheduloside I | - | - | - | T | 0.06% | 0.11% |
| 19.641 | 226.09903 | C15H14O2 | 7-Hydroxyflavan | - | 0.84% | - | 0.03% | - | 0.05% |
| 18.748 | 208.1096 | C12H16O3 | Isoelemicin | 0.01% | - | 0.19% | - | - | - |
| 18.767 | 526.2565 | C30H38O8 | Kosamol A | - | 0.52% | - | - | - | - |
| 18.824 | 300.0997 | C17H16O5 | 2′,4′-Dihydroxy-3,4-dimethoxychalcone | - | - | 0.07% | - | - | - |
| 18.987 | 372.1208 | C20H20O7 | Tangeretin | - | - | 0.03% | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





