Submitted:
20 September 2024
Posted:
20 September 2024
You are already at the latest version
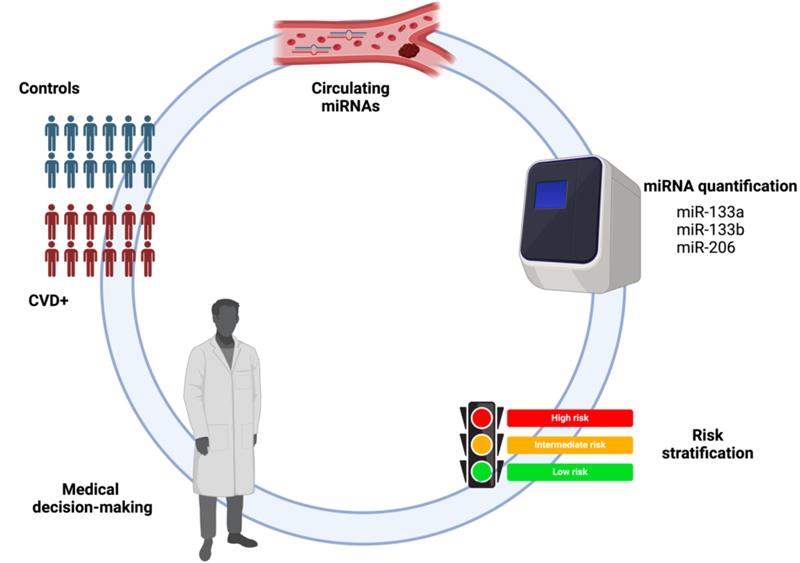
Abstract
Keywords:
1. Introduction
2. Results
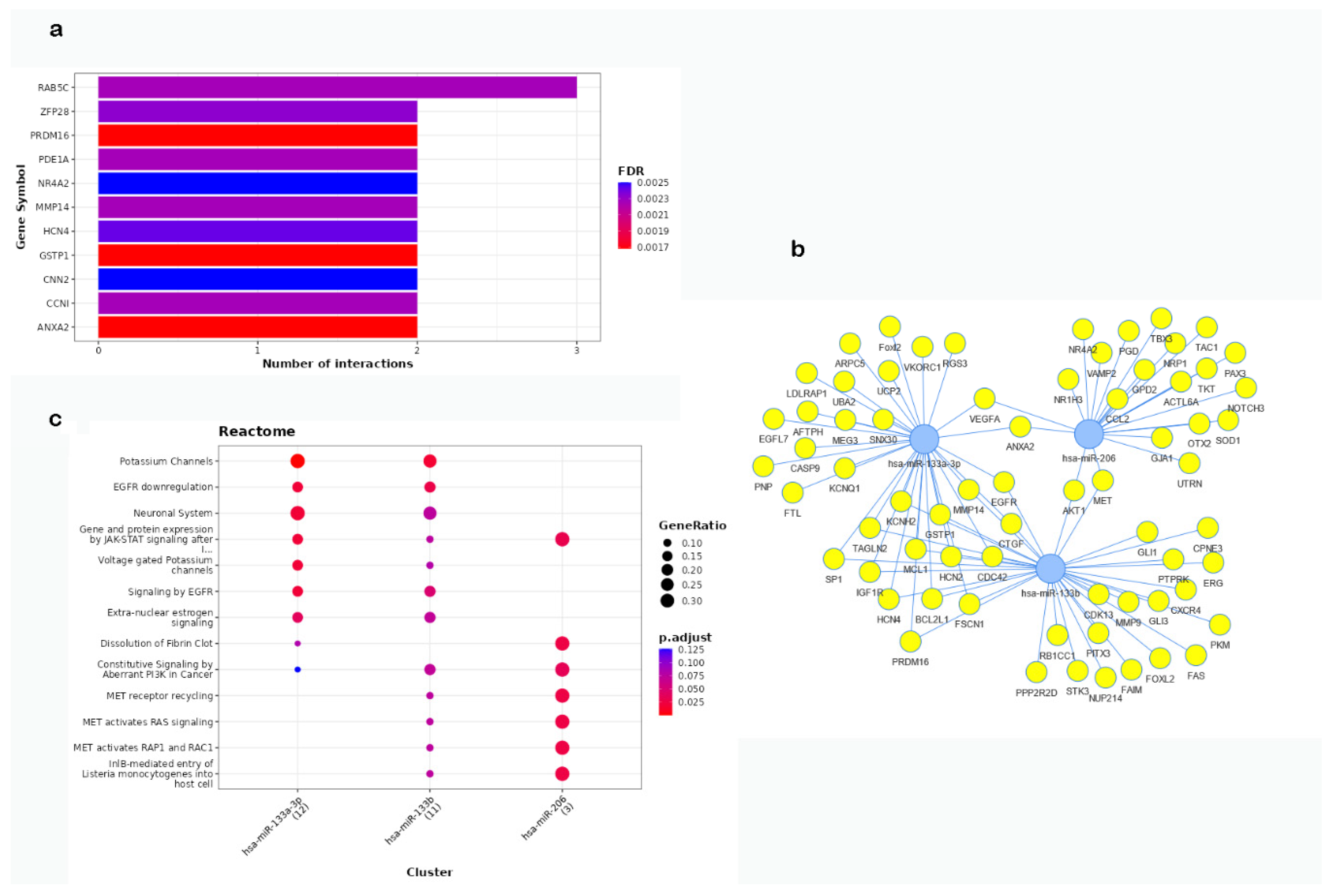
2.1. In Silico Functional Characterization
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Clinical and Laboratory Assessments
4.3. Plasma RNA Extraction
4.4. Quantitative Real-Time Polymerase Chain Reaction
4.5. Pathway Enrichment Analysis of the Predicted Targets
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z,; Benjamin, E.J.; Benziger, C.P.; et al. GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020, 76, 2982-3021.
- Vaduganathan, M.; Mensah, G.; Turco, J.V; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J Am Coll Cardiol. 2022, 80, 2361–2371.
- Filipowicz, W.; Bhattacharyya, S.N., Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nature reviews genetics, 2008; 9, 102-114.
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D., Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as biomarkers in disease: latest findings regarding their role in diagnosis and prognosis. Cells, 2020; 9, 276. [CrossRef]
- Venneri, M.; Passantino, A. MiRNA: what clinicians need to know. European Journal of Internal Medicine, 2023, 113, 6-9.
- Wojciechowska, A.; Braniewska, A.; Kozar-Kamińska, K. MicroRNA in cardiovascular biology and disease. Advances in Clinical & Experimental Medicine 2017, 26, 5. [CrossRef]
- Varghese, L.N.; Schwenke, D.O.; Katare, R. Role of noncoding RNAs in cardiac ageing, Frontiers in Cardiovascular Medicine, 2023, 10, 1142575. [CrossRef]
- Poller, W.; Dimmeler, S.; Heymans, S., Zeller, T.; Haas, J.; Karakas, M.; Leistner, D.M.; Jakob, P.; Nakagawa, S.; Blankenberg, S.; et al. Non-coding RNAs in cardiovascular diseases: diagnostic and therapeutic perspectives. European heart journal, 2018, 39, 2704-2716. [CrossRef]
- Zhou; S.S.; Jin, J.P.; Wang, J.Q.; Zhang, Z.G.; Freedman, J.H.; Zheng, Y., Cai, L. miRNAS in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol Sin. 2018, 39, 1073–1084. [CrossRef]
- Çakmak, H.A., Demir, M. MicroRNA and Cardiovascular Diseases. Balkan Med J. 2020, 37, 60-71.
- Sessa, F.; Salerno, M.; Esposito, M.; Cocimano, G.; Pomara, C. miRNA dysregulation in cardiovascular diseases: current opinion and future perspectives. International Journal of Molecular Sciences, 2023, 24, 5192. [CrossRef]
- Searles, C.D. MicroRNAs and Cardiovascular Disease Risk. Curr Cardiol Rep 2024, 26, 51–60. [CrossRef]
- Lozano-Velasco, E.; Inácio, J.M.; Sousa, I.; Guimarães, A.R.; Franco, D.; Moura, G.; Belo, J.A. miRNAs in Heart Development and Disease. International Journal of Molecular Sciences, 2024, 25, 1673. [CrossRef]
- Li, X.; Wang, J.; Jia, Z.; Cui, Q.; Zhang, C.; Wang, W.; Chen, P.; Ma, K.; Zhou, C. MiR499 regulates cell proliferation and apoptosis during late-stage cardiac differentiation via Sox6 and cyclin D1. PLoS ONE. 2013, 8, e74504. [CrossRef]
- Zhao, X.; Wang, Y.; Sun, X. The functions of microRNA-208 in the heart. Diabetes Res Clin Pract. 2020;160: 108004. [CrossRef]
- Huang; X.H., Li, J.L., Li, X.Y., Wang, S.X., Jiao, Z.H., Li, S.Q., Liu, J; Ding, J. miR-208a in cardiac hypertrophy and remodeling. Frontiers in Cardiovascular Medicine, 2021, 8, 773314. [CrossRef]
- Zhu, Y.F.; Wang, R.; Chen, W.; Cao, Y.D.; Li, L.P.; Chen, X. miR-133a attenuates cardiomyocyte hypertrophy through inhibiting pyroptosis activation by targeting IKKε. Acta Histochemica, 2021, 123, 151653. [CrossRef]
- Ouyang, Z; Ke, W. miRNA in cardiac development and regeneration. Cell Regeneration 2021, 10.1, 14. [CrossRef]
- Song, Z.; Gao, R.; Yan, B. Potential roles of microRNA-1 and microRNA-133 in cardiovascular disease. Reviews in Cardiovascular Medicine 2020, 21(1), 57-64. [CrossRef]
- Abkhooie, L.; Sarabi, M.M.; Kahroba, H.; Eyvazi, S.; Montazersaheb, S.; Tarhriz, V.; Hejazi, M.S. Potential Roles of MyomiRs in Cardiac Development and Related Diseases. Curr Cardiol Rev. 2021, 17, e010621188335. [CrossRef]
- Care, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.L.; Segnalini, P.; Gu,Y.; Dalton, N.D.; et al. MicroRNA-133 controls cardiac hypertrophy. Nature medicine, 2007, 13, 613-618. [CrossRef]
- Boštjančič, E.; Zidar, N.; Štajer, D.; Glavač, D. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology, 2010, 115, 163-169. [CrossRef]
- Han, Z., Zhang, L.; Yuan, L.; Liu, X.; Chen, X.; Ye, X.; Yang, C., Yan, Z. Change of plasma microRNA-208 level in acute myocardial infarction patients and its clinical significance. Ann Transl Med. 2015, 3, 307. [CrossRef]
- Zhang, L.; Chen, X.; Su, T.; Li, H.; Huang, Q.; Wu, D.; Yang, C.; Han, Z. Circulating miR-499 are novel and sensitive biomarker of acute myocardial infarction. J Thorac Dis 2015, 7:303-308. [CrossRef]
- Kumar, D.; Narang, R.; Sreenivas, V.; Rastogi, V.; Bhatia, J.; Saluja, D.; Srivastava, K. Circulatory miR-133b and miR-21 as Novel Biomarkers in Early Prediction and Diagnosis of Coronary Artery Disease. Genes (Basel). 2020, 11, 164. [CrossRef]
- Yu, Y.; Zheng, Y.; Yu, T.; Bai, J.; Jiang, T. The relationship between serum rhoa and mir-133b in acute ischemic stroke Int. J. of Adv. Res. 2020, 8, 358-363.
- Xin, H.; Li, Y.; Liu, Z.; Wang, X.; Shang, X.; Cui, Y.; Zhang, Z.G.; Chopp, M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013, 31, 2737–46. [CrossRef]
- Boštjančič, E.; Brandner, T.; Zidar, N.; Glavač, D.; Štajer, D. Down-regulation of miR-133a/b in patients with myocardial infarction correlates with the presence of ventricular fibrillation. Biomedicine & Pharmacotherapy. 2018, 99, 65-71. [CrossRef]
- D’Alessandra, Y.; Devanna, P.; Limana, F; Straino, S.; Di Carlo, A.; Brambilla, P.G.; Rubino, M.; Carena, M.C.; Spazzafumo, L.; De Simone, M.; et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J, 2010; 31, 2765-2773. [CrossRef]
- Sæther, J.C.; Vesterbekkmo, E.K.; Taraldsen, M.D.; Gigante, B-; Follestad, T.; Røsjø, H.R.; Omland, T.; Wiseth, R.; Madssen, E.; Bye, A. Associations between circulating microRNAs and lipid-rich coronary plaques measured with near-infrared spectroscopy. Sci Rep 2023, 13, 7580. [CrossRef]
- de Gonzalo-Calvo, D.; Martinez-Camblor, P.; Belmonte, T.; Barbé, F.; Duarte, K.; Cowie, M.R.; Angermann, C.E.; Korte, A.; Riedel, I.; Labus J; et al. Circulating miR-133a defines a low-risk subphenotype in patients with heart failure and central sleep apnea: a decision tree machine learning approach. Journal of Translational Medicine, 2023, 21, 742. [CrossRef]
- Modica, J.; Di Mauro, V.; Barandalla-Sobrados, M.; Chavez, S.E.P.; Carullo, P.; Nemska, S.; Anselmo, A.; Condorelli, G.; Iafisco, M.; Miragoli, M.; Catalucci D. Nano-miR-133a replacement therapy blunts pressure overload–induced heart failure. Circulation. 2021; 144, 1973-1976. [CrossRef]
- Sang, H.Q.; Jiang, Z.M.; Zhao, Q.P.; Xin, F. MicroRNA-133a improves the cardiac function and fibrosis through inhibiting Akt in heart failure rats. Biomed. Pharmacother. 2015, 71, 185–189. [CrossRef]
- Xiao, Y.; Zhao, J.; Tuazon, J.P.; Borlongan, C.V.; Yu, G. MicroRNA-133a and myocardial infarction. Cell transplantation 2019, 28, 831-838. [CrossRef]
- Yang, Y.; Del Re, D.P.; Nakano, N.; Sciarretta, S.; Zhai, P.; Park, J.; Sayed, D., Shirakabe, A.; Matsushima, S.; Park, Y:, et al. miR-206 Mediates YAP-Induced Cardiac Hypertrophy and Survival. Circ Res. 2015, 117, 891-904. [CrossRef]
- Caturano, A.; Vetrano, E.; Galiero, R.; Salvatore, T.; Docimo, G.; Epifani, R.; Alfano, M.; Sardu, C.; Marfella, R.; Rinaldi L, Sasso, F.C. Cardiac hypertrophy: from pathophysiological mechanisms to heart failure development. Reviews in Cardiovascular Medicine. 2022, 23, 165. [CrossRef]
- Yan, Y.; Dang, H.; Zhang, X.; Wang, X.; Liu, X. The protective role of MiR-206 in regulating cardiomyocytes apoptosis induced by ischemic injury by targeting PTP1B. Biosci Rep. 2020, 40, BSR20191000. [CrossRef]
- Shan, Z.X.; Lin, Q.X.; Fu, Y.H.; Deng, C.Y.; Zhou, Z.L.; Zhu, J.N.; Liu, X.Y.; Zhang, Y.Y.; Li, Y.; Lin, S.G.; Yu, X.Y. Upregulated expression of miR-1/miR-206 in a rat model of myocardial infarction. Biochemical and biophysical research communications. 2009, 381, 597-601. [CrossRef]
- Jin, Y.; Zhou, T.Y.; Cao, J.N.; Feng, Q.T.; Fu, Y.J.; Xu, X.; Yang, C.J. MicroRNA-206 Downregulates Connexin43 in Cardiomyocytes to Induce Cardiac Arrhythmias in a Transgenic Mouse Model. Heart Lung Circ. 2019, 28, 1755–1761. [CrossRef]
- Wang, D.Z.; Chang, P.S.; Wang, Z.; Sutherland, L.; Richardson, J.A.; Small, E.; Krieg, P.A.; Olson, E.N. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001. 105, 851-862. [CrossRef]
- Cesana, M.; Cacchiarelli, D.; Legnini, I.; Santini, T.; Sthandier, O.; Chinappi, M.; Tramontano, A.; Bozzoni, I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011, 147, 358-369. [CrossRef]
- Cui, S.; Li, L.; Mubarokah, S.N.; Meech, R. Wnt/β-catenin signaling induces the myomiRs miR-133b and miR-206 to suppress Pax7 and induce the myogenic differentiation program. Journal of cellular biochemistry. 2019, 120, 12740-12751. [CrossRef]
- Ni, B.; Sun, M.; Zhao, J.; Wang, J.; Cao, Z. The role of β-catenin in cardiac diseases. Frontiers in Pharmacology, 2023, 14, 1157043. [CrossRef]
- Balatskyi, V.V.; Sowka, A.; Dobrzyn, P.; Piven, O.O. WNT/β-catenin pathway is a key regulator of cardiac function and energetic metabolism. Acta Physiol (Oxf). 2023, 237, e13912. [CrossRef]
- Fu, W.B.; Wang, W.E.; Zeng, C.Y. Wnt signaling pathways in myocardial infarction and the therapeutic effects of Wnt pathway inhibitors. Acta Pharm. Sin. 2019, 40, 9-12. [CrossRef]
- Zhang, Q.; Wang, L.; Wang, S.; Cheng, H.; Xu, L.; Pei, G.; Wang, Y.; Fu, C.; Jiang, Y.; He, C.; Wei, Q. Signaling pathways and targeted therapy for myocardial infarction. Sig Transduct Target Ther 2022, 7, 78 (2022). [CrossRef]
- Cibi, D.M.; Bi-Lin, K.W.; Shekeran, S.G.; Sandireddy, R.; Tee, N.; Singh, A.; Wu, Y, Srinivasan, D.K.; Kovalik, J.P.; Ghosh, S.; Seale, P.; Singh, M.K. Prdm16 deficiency leads to age-dependent cardiac hypertrophy, adverse remodeling, mitochondrial dysfunction, and heart failure. Cell reports. 2020, 33, 108288. [CrossRef]
- Hennis, K.; Biel, M.; Fenske, S.; Wahl-Schott, C. Paradigm shift: new concepts for HCN4 function in cardiac pacemaking. Pflugers Arch. 2022, 474, 649-663. [CrossRef]
- Andrukhova, O.; Salama, M.; Rosenhek, R.; Gmeiner, M.; Perkmann, T.; Steindl, J.; Aharinejad, S. Serum Glutathione S-Transferase P1 1 in Prediction of Cardiac Function. J. Card. Fail. 2012, 18, 253-261. [CrossRef]
- Simeunovic, D.; Odanovic, N.; Pljesa-Ercegovac, M.; Radic, T.; Radovanovic, S.; Coric, V.; Milinkovic, I.; Matic, M.; Djukic, T.; Ristic, A.; et al. Glutathione Transferase P1 Polymorphism Might Be a Risk Determinant in Heart Failure. Dis. Markers 2019, 2019, 6984845. [CrossRef]
- Méndez-Barbero, N.; San Sebastian-Jaraba, I.; Blázquez-Serra, R., Martín-Ventura, J.L.; Blanco-Colio, L.M. Annexins and cardiovascular diseases: Beyond membrane trafficking and repair. Frontiers in cell and developmental biology, 2002; 10, 1000760. [CrossRef]
- Kayejo, V. G.; Fellner, H.; Thapa, R.; Keyel, P.A. Translational implications of targeting annexin A2: From membrane repair to muscular dystrophy, cardiovascular disease and cancer. Clinical and Translational Discovery, 2023, 3, e240. [CrossRef]
- Liu, H., Liu, P., Shi, X., Yin, D., Zhao, J. NR4A2 protects cardiomyocytes against myocardial infarction injury by promoting autophagy. Cell Death Discov. 2018, 4, 27. [CrossRef]
- Ashraf, S.; Taegtmeyer, H.; Harmancey, R. Prolonged cardiac NR4A2 activation causes dilated cardiomyopathy in mice. Basic Res Cardiol. 2022, 117, 33. [CrossRef]
- Ye, P.; Zhu, Y.R.; Gu, Y.; Zhang, D.M.; Chen, S.L. Functional protection against cardiac diseases depends on ATP-sensitive potassium channels. Journal of cellular and molecular medicine. 2018, 22, 5801-5806. [CrossRef]
- Weisbrod, D. Small and intermediate calcium activated potassium channels in the heart: role and strategies in the treatment of cardiovascular diseases. Frontiers in Physiology 2020, 11, 590534. [CrossRef]
- Lei, M.; Salvage, S.C.; Jackson, A.P.; Huang, C.L.H. Cardiac arrhythmogenesis: roles of ion channels and their functional modification. Frontiers in Physiology, 2024, 15, 1342761. [CrossRef]
- Forrester, S.J.; Kawai, T.; O'Brien, S.; Thomas, W.; Harris, R.C.; Eguchi, S. Epidermal growth factor receptor transactivation: Mechanisms, pathophysiology, and potential therapies in the cardiovascular system. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 627-653. [CrossRef]
- AlShatnawi, M.N.; Shawashreh, R.A.; Sunoqrot, MA.; Yaghi, A.R. A systematic review of epidermal growth factor receptor tyrosine kinase inhibitor-induced heart failure and its management. The Egyptian Journal of Internal Medicine, 2022, 34, 85. [CrossRef]
- Algül, S.; Schuldt, M.; Manders, E.; Jansen, V.; Schlossarek, S.; de Goeij-de Haas, R.; Henneman, A.A.; Piersma, S.R.; Jimenez, C.R.; Michels, M; Carrier, L., et al.EGFR/IGF1R signaling modulates relaxation in hypertrophic cardiomyopathy. Circulation Research, 2023, 133, 387-399. [CrossRef]
- Bolli, R.; Dawn, B.; Xuan, Y.T. Role of the JAK–STAT pathway in protection against myocardial ischemia/reperfusion injury. Trends Cardiovasc. Med. 2003; 13:72–79. [CrossRef]
- Barry, S.P.; Townsend, P.A.; Latchman, D.S.; Stephanou, A. Role of the JAK–STAT pathway in myocardial injury. Trends Mol. Med. 2007; 13:82–89. [CrossRef]
- Gallo, S.; Sala, V.; Gatti, S.; Crepaldi, T. HGF/Met Axis in Heart Function and Cardioprotection. Biomedicines. 2014, 2, 247-262. [CrossRef]
- Gallo, S.; Sala, V.; Gatti, S.; Crepaldi, T. Cellular and molecular mechanisms of HGF/Met in the cardiovascular system. Clin Sci (Lond). 2015, 129, 1173-1193. [CrossRef]
- Ząbczyk, M.; Ariëns, R.A.; Undas, A. Fibrin clot properties in cardiovascular disease: from basic mechanisms to clinical practice. Cardiovascular research, 2023, 119, 94-111. [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3,1101–1108. [CrossRef]
- Licursi, V.; Conte, F.; Fiscon, G.; Paci, P. MIENTURNET: An interactive web tool for microNA-target enrichment and network-based analysis. BMC Bioinform. 2019; 20, 545. [CrossRef]
| Variables | CVD- (N=119) |
CVD + (N=90) |
P-value |
|---|---|---|---|
| Age (mean, SD) | 82 (7.8) | 85 (6.3) | <0.001 |
| Sex (men, %) | 33 (28%) | 28 (31%) | 0.705 |
| BMI, Kg/m2 (mean, SD) | 26 (6.1) | 25 (6.3) | 0.813 |
| SBP, mmHg (mean, SD) | 128 (11) | 126 (13) | 0.353 |
| DBP, mmHg (mean, SD) | 75 (7.7) | 71 (8.2) | 0.010 |
| Total Cholesterol, mg/dl (mean, SD) | 164 (41) | 149 (39) | 0.009 |
| HDL Cholesterol, mg/dl (mean, SD) | 49 (15) | 49 (12) | 0.958 |
| LDL Cholesterol, mg/dl (mean, SD) | 92 (32) | 78 (32) | 0.004 |
| Triglycerides, mg/dl (mean, SD) | 120 (69) | 118 (64) | 0.882 |
| Statin users (Yes, %) | 22 (18%) | 26 (29%) | 0.109 |
| Fasting plasma glucose, mg/dL (mean, SD) | 103 (48) | 98 (28) | 0.333 |
| Glycated Haemoglobin A1c, % (mean, SD) | 6.2 (1.6) | 7 (8.6) | 0.385 |
| Total protein, g/dL (mean, SD) | 6.5 (0.59) | 6.5 (0.64) | 0.556 |
| Albumin, g/dL (mean, SD) | 5.3 (7.6) | 5.3 (6.7) | 0.787 |
| C-reactive Protein, mg/L (mean, SD) | 14 (23) | 17 (35) | 0.498 |
| Urea, mg/dL (mean, SD) | 46 (17) | 54 (29) | 0.019 |
| Creatinine, mg/dL (mean, SD) | 1 (0.3) | 1.2 (0.58) | 0.029 |
| Uric acid, mg/dL (mean, SD) | 4.4 (1.2) | 6 (7.5) | 0.030 |
| Sodium, mM/L (mean, SD) | 141 (2.7) | 140 (2.8) | 0.016 |
| Potassium, mM/L (mean, SD) | 4.4 (0.5) | 4.5 (0.64) | 0.365 |
| Chloride, mM/L (mean, SD) | 105 (3.9) | 101 (15) | 0.014 |
| Calcium, mg/dL (mean, SD) | 9.1 (0.62) | 9 (0.65) | 0.612 |
| Phosphorus, mg/dL (mean, SD) | 3.5 (0.92) | 3.5 (0.64) | 0.962 |
| Magnesium, mg/dL (mean, SD) | 1.9 (0.33) | 1.9 (0.34) | 0.247 |
| Iron, μg/dL (mean, SD) | 56 (26) | 49 (24) | 0.087 |
| Ferritin, ng/mL (mean, SD) | 189 (249) | 160 (228) | 0.432 |
| Total bilirubin, mg/dL (mean, SD) | 0.72 (0.39) | 0.64 (0.31) | 0.112 |
| Hypertension (Yes, %) | 80 (67%) | 73 (81%) | 0.037 |
| Ischemic cardiomyopathy (Yes, %) | - | 47 (52%) | - |
| Atrial fibrillation (Yes, %) | - | 26 (29%) | - |
| Heart failure (Yes, %) | - | 16 (18%) | - |
| Stroke (Yes, %) | - | 28 (31%) | - |
| Deceased (Yes, %) | 28 (27%) | 40 (49%) | 0.004 |
| Predictors | Odds Ratios | CI | p | Odds Ratios | CI | p | Odds Ratios | CI | p |
|---|---|---|---|---|---|---|---|---|---|
| (Intercept) | 0.04 | 0.00 – 2.97 | 0.146 | 0.07 | 0.00 – 5.18 | 0.226 | 0.02 | 0.00 – 2.48 | 0.147 |
| Age | 1.05 | 1.00 – 1.10 | 0.044 | 1.04 | 0.99 – 1.09 | 0.100 | 1.06 | 1.01 – 1.11 | 0.028 |
| Sex | 0.97 | 0.45 – 2.00 | 0.932 | 0.92 | 0.42 – 1.94 | 0.835 | 0.90 | 0.41 – 1.87 | 0.776 |
| Statin | 1.51 | 0.66-3.76 | 0.353 | 1.37 | 0.59 - 3.44 | 0.475 | 1.60 | 0.68 – 4.13 | 0.301 |
| miR-133a | 0.71 | 0.42 – 1.14 | 0.170 | ||||||
| miR-133b | 0.66 | 0.44 – 0.97 | 0.038 | ||||||
| miR-206 | 0.57 | 0.36 – 0.85 | 0.009 |
| Ischemic cardiomyopathy |
Atrial fibrillation |
Heart failure |
Stroke | |||||
|---|---|---|---|---|---|---|---|---|
| miRNA | OR (95% CI)# | p* | OR (95% CI)# | p* | OR (95% CI)# | p* | OR (95% CI)# | p* |
| miR-133a | 1.02 (0.63-1.70) | 0.943 | 1.16 (0.64-2.22) | 0.647 | 0.47 (0.24-0.95) | 0.031 | 1.02 (0.58-1.89) | 0.935 |
| miR-133b | 0.84 (0.57-1.23) | 0.378 | 0.85 (0.52-1.38) | 0.517 | 0.82 (0.46-1.44) | 0.495 | 0.63 (0.39-0.99) | 0.048 |
| miR-206 | 1.28 (0.89-1.89) | 0.202 | 1.06 (0.68- 1.73) | 0.795 | 0.64 (0.38-1.08) | 0.090 | 0.82 (0.54-1.24) | 0.332 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





