1. Introduction
Among the various types of ores containing noble metals, those in which gold and silver are in close association with sulfide minerals occupy a special place. These metals not extracted by cyanidation even after ultrafine grinding of such an ore. According to Ref. [
1], the share of refractory ores is more than 30% of the total reserves of noble metals in the world.
This is due to the extremely fine dispersion of gold and silver in sulfide minerals and the presence of noble metals in the crystal lattice of sulfides [
2,
3,
4,
5,
6,
7].
The most reliable and universal methods in the world are those based on the use of pressure oxidation and neutral leaching for the quantitative oxidation of gold and silver containing sulfides (pyrite, arsenopyrite, and chalcopyrite) and subsequent cyanidation of the resulting cakes [
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18]. Along with high gold content, some sulfide concentrates contain significant amounts of silver as well. The extraction of silver from pressure oxidation cakes by cyanidation is difficult due to the formation of argentoyarosite by reaction 1 [
19,
20]:
Argentoyarosite is inert towards cyanide ion, therefore, silver extraction during cyanidation of the pressure oxidation residue usually does not exceed 4–20%. To increase silver extraction, it was proposed [
21] to treat pressure oxidation residues with a lime t 90 °C. The disadvantage of this method is the high consumption of lime (up to 100 kg per ton). Another method [
22,
23] is that pressure oxidation is carried out with the addition of limestone or lime. Binding of sulfate ion into gypsum inhibits the formation of argentoyarosite. However, this method also requires a large consumption of lime and promotes the formation of various deposits, reducing the efficiency of the equipment and affecting its heat balance. There is a known method of steaming pressure oxidation cakes with lime (1.5 times excess wrt stoichiometry, 370 K, L:S=5:1, duration 5–10 h) upon reaching pH = 10, argentoyarosite was destroyed according to the following reaction:
In sulfide concentrates processed in pressure oxidation plants, a significant amount of gold and silver is associated with arsenopyrite, where they are in the form of nano-inclusions of native metal, or in the form of single atoms. A gold atom may take the place of another atom in the structure of arsenopyrite, e.g., replace an atom of iron, arsenic or sulfur. In another case, gold atoms may be embedded in the voids of the crystal lattice of the mineral, and also occupy “defects” therein (various types of vacancies and dislocations). In the process of pressure oxidation oxidative leaching of arsenopyrite, iron arsenates are formed: FeAsO
4·2H
2O, FeAsO
4·0.68-0.77H
2O, Fe(AsO
4)
x(SO
4)
y(OH)
z·wH
2O, where 0.36≤x≤0.69, 0.19≤y≤0.5, 0.55≤z≤0.8 and 0.2≤w≤0.45 [
24,
25,
26,
27,
28]. During the conditioning of such cakes, necessary to increase gold recovery during subsequent cyanidation, solutions containing Fe (III) and As (V) are formed. They are neutralized with lime to obtain iron arsenates . Rapid neutralization of solutions with lime is one of the simplest methods for the precipitation of As (III) and As (V) as arsenites and arsenates of iron and calcium at pH = 3–4 [
29,
30]. The process of lime precipitation is a relatively economical way to immobilize arsenic, however, the precipitates thus obtained have poor long-term stability [
31,
32]. Co-precipitation of Fe (III) and As (V) under these conditions occurs in the form of ferrihydride, amorphous iron arsenates of poorly crystallized scorodite. During long-term storage and exposure to the environment these compounds are converted to alpha-goethite (α-FeOOH), which leads to the transition of arsenic to the liquid phase [
33,
34,
35,
36].
According to Ref. [
37], at temperatures below 100 °C, precipitation occurs with the formation of an amorphous solid phase which consists of gel-like iron hydroxide containing adsorbed arsenate ions. With increasing temperature, the crystalline structure of the precipitate improves, and adsorbed arsenate ions, interacting with iron (III), form the scorodite structure. This interaction occurs at temperatures of 150–200 °C with a Fe:As ratio of 1.5 and higher. Iron arsenates formed under pressure oxidation conditions are more stable in the long term, have a crystalline structure, and are less soluble compared to precipitates obtained under atmospheric conditions.
As a result of hydrothermal interaction, FeAsO4*2H2O, Fe3(AsO4)2SO4OH, FeSO4OH, Fe2(HAsO4)3*xH2O can be formed at temperatures of 150 °C and above. The precipitate obtained after 5 min of precipitation at 190 °C already contains crystalline scorodite. It has been established that acidity greatly affects the degree of arsenic precipitation: as it increases, arsenic extraction from the solution decreases. E.g., at 190 °C and 20 g/dm3 of free acid, 80% of arsenic precipitated from the solution.
The purpose of this work was to study the effect of a gypsum additive during pressure oxidation leaching of sulfide concentrate containing arsenopyrite on the extraction of gold, silver and the formation of secondary iron- and arsenic-containing phases.
2. Materials and Methods
2.1. Analysis
Chemical analysis of the starting minerals and the resulting solid dissolution products was carried out using an ARL Advant’X 4200 wavelength dispersive spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). Phase analysis was performed on an XRD 7000 Maxima diffractometer (Shimadzu Corp., Tokyo, Japan).
Particle size analysis was carried out by laser diffractometry using an Analysette 22 Nanotec Plus instrument (FRITSCH GmbH, Idar-Oberstein, Germany).
Chemical analysis of the resulting solutions was carried out by inductively coupled plasma mass spectrometry (ICP-MS) on an Elan 9000 instrument (PerkinElmer Inc., Waltham, MA, USA).
Scanning electron microscopy (SEM) was performed using a JSM-6390LV microscope (JEOL Ltd., Tokyo, Japan) equipped with a module for energy-dispersive X-ray spectroscopy analysis (EDX).
2.2. Materials and Reagents
The main raw materials used were sulfide gold- and silver-containing concentrate of the following composition, %: 30.7 Fe; 10.8 As; 32.3 S
total; 31.9 S
sulfide; 3.7 Si; 25.8 g/t Au; 52.05 g/t Ag.
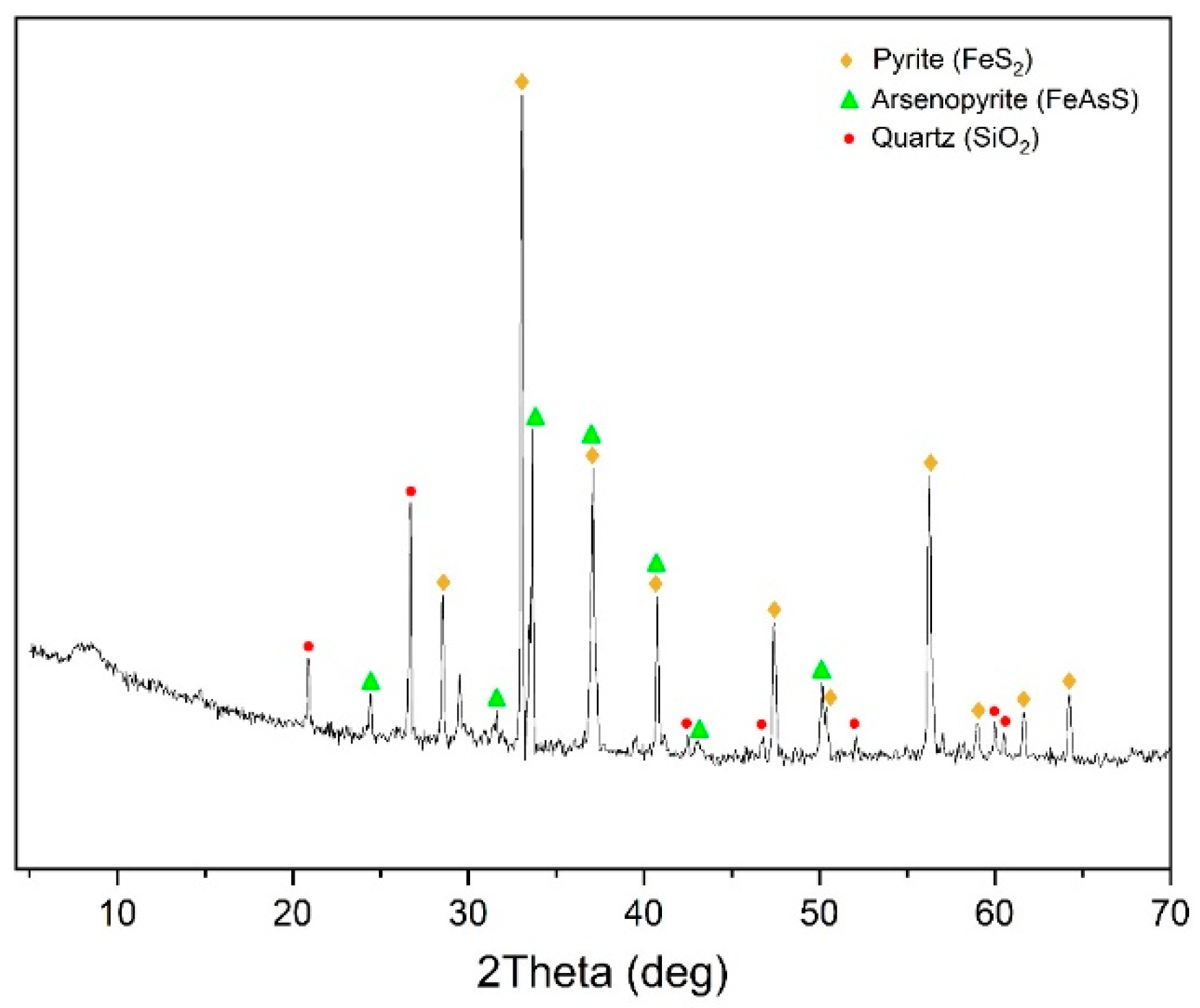
Figure 1 presents its X-ray pattern. According to the data presented, the main minerals in the concentrate are pyrite and arsenopyrite.
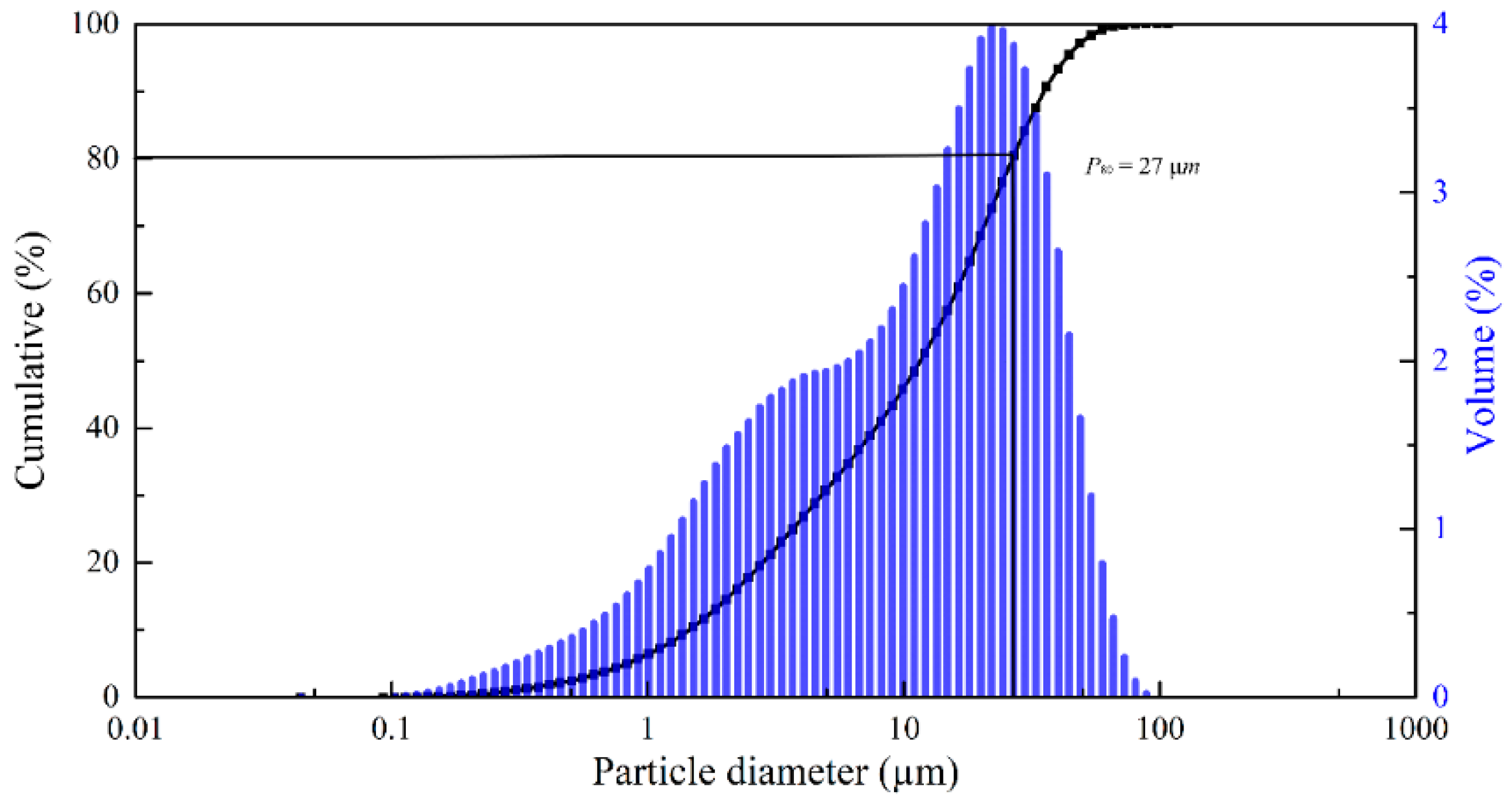
The concentrate was sieved on laboratory sieves and analyzed. Experiments were carried out with a fraction of 90% particle size class 20–40 μm, the granulometric composition is presented in
Figure 2. All other reagents used were of analytical grade.
2.3. Apparatuses
POX experiments were performed in 1.2 dm3 titanium autoclave (Premex AG CH-2543 Lengnau, Switzerland), with the ability to supply and regulate oxygen flow using a flow meter (Bronkhorst EL-FLOW Prestige and Bronkhorst EL-PRESS Metal-Sealed pressure regulators), temperature control. Mixing was carried out using a top-drive mixer to ensure slurry homogeneity.
2.4. Stability Evaluation
The stability of arsenic (V) precipitation products was assessed by the Toxicity Characteristic Leaching Procedure (TCLP). To do this, 1 g of a solid sample was added to 20 ml of a buffer solution mixed with acetic acid and sodium acetate (pH ~ 4.9), and then shaken using a shaker at 22 °C for 18 h. The stability of the products was assessed by comparing the resulting arsenic concentration after leaching with the hazardous solid waste standard, which is 5 mg/l for arsenic [
30,
38].
2.5. Cyanidation of Pressure Oxidation Cakes
Pressure oxidation leaching cakes in experiments with gypsum additives were cyanidated without conditioning. Cyanidation was carried out for 24 h in a glass laboratory beaker at room temperature and mechanical stirring of the slurry. A sample of the material was mixed with distilled water to a solid content of 20% (wt.) and the pH of the slurry was adjusted to 10.5–11.0 using lime milk. After stabilizing this pH, potassium cyanide was added to obtain its concentration 2 g/l in the solution. If necessary, a concentrated KCN solution was added to the slurry, maintaining a given concentration. Upon completion of the cyanidation process, the slurry was filtered and the cake was washed. The wash water volume was equal to three times the volume of the liquid phase at the cyanidation stage. Next, the cake was dried and the residual content of gold and silver (cyanidation tailings) was analyzed.
3. Results and Discussion
3.1. Influence of Gypsum Addition on the Behavior of Iron and Arsenic during Pressure Oxidation Leaching and Silver Recovery during Subsequent Cyanidation of the Residue
A method is known of introducing lime and limestone with pressure oxidation leaching slurry. Binding of sulfate ion into gypsum inhibits the formation of argentoyarosite. However, this method also requires a large consumption of lime, the molar ratio S
2-:CO
3 =1:1. According to the data obtained, a positive effect on silver recovery at the stage of cyanidation of pressure oxidation leaching cakes is achieved even with a final acid concentration of 28 g/dm
3 when all the limestone turns into gypsum [
22].
Therefore, we studied the influence of the initial acid concentration, temperature and gypsum consumption on the behavior of iron, arsenic and sulfur during pressure oxidation leaching and the subsequent silver recovery during cyanidation. The oxidation degree of concentrate sulfides in all experiments was limited to 95–97%, controlling oxygen consumption, according to the industrial practice [
19,
20,
21,
22].
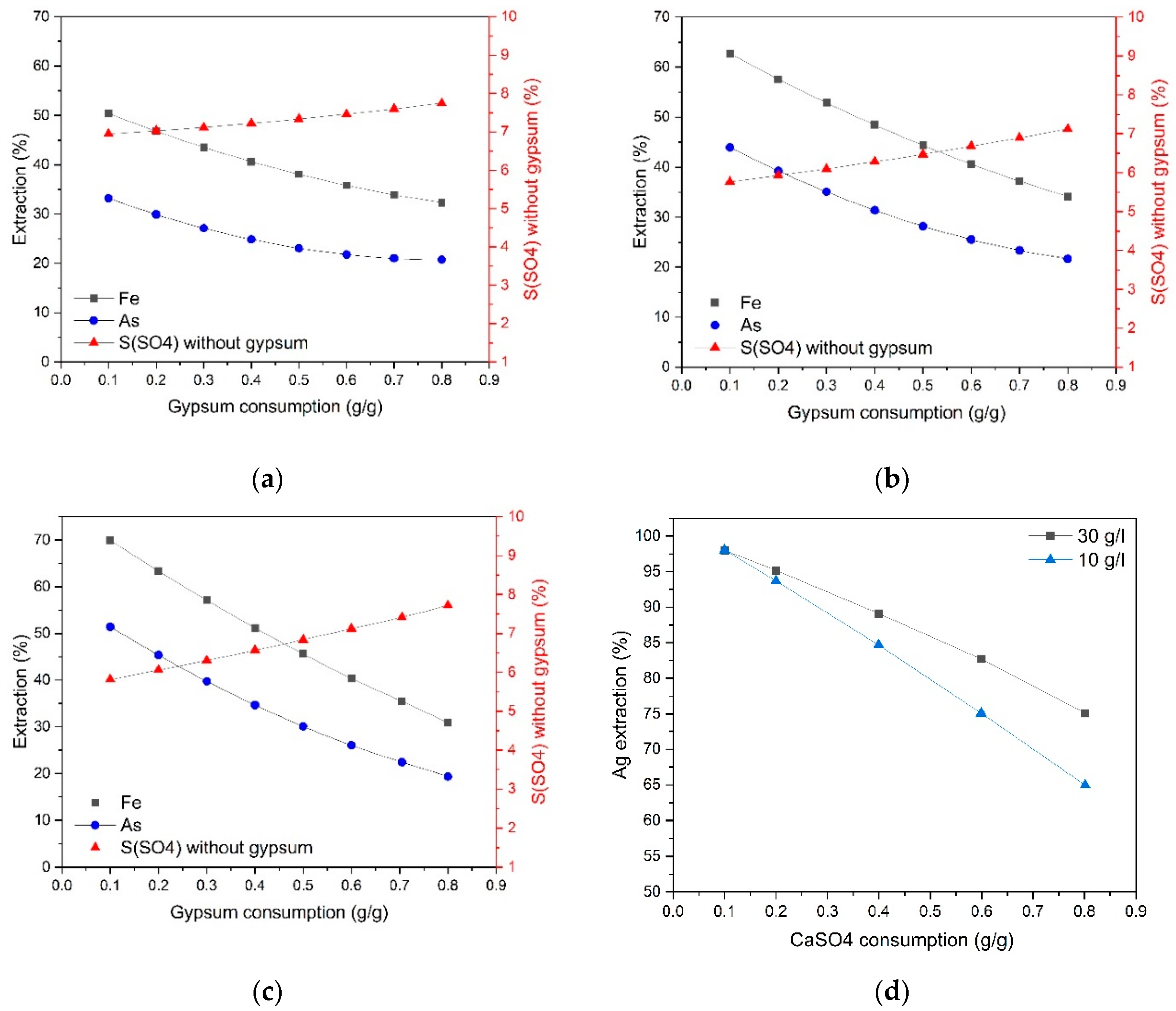
Figure 3 presents the dependence of the extraction of arsenic and iron into solution and the content of sulfate sulfur in the residue not associated with gypsum on gypsum consumption at 225 °C and various initial acid concentrations.
According to the data obtained, the extraction degree of arsenic and iron decreases with increasing gypsum consumption, and the slope of the curves is similar at various acid concentrations, which indicates their interaction to form joint compounds—iron arsenates. With an increase in the initial concentration of sulfuric acid from 10 to 30 g/dm
3, the dependence of the extraction of arsenic and iron on gypsum consumption rises. The content of sulfate sulfur, minus the gypsum added, increases with gypsum consumption, and with an increase in the initial concentration of sulfuric acid from 10 to 30 g/dm
3, this dependence becomes more pronounced. As can be seen, the content of sulfate sulfur decreases with increasing acid concentration at low gypsum consumption from 0.1 to 0.5 g/g; its further increase neutralizes the effect of acidity and contributes to obtaining a residue containing 6.6–7.4% sulfate sulfur. Similar things are observed in the process of pressure oxidation leaching at a temperature of 200 °C and an initial acid concentration of 20 g/dm
3 (
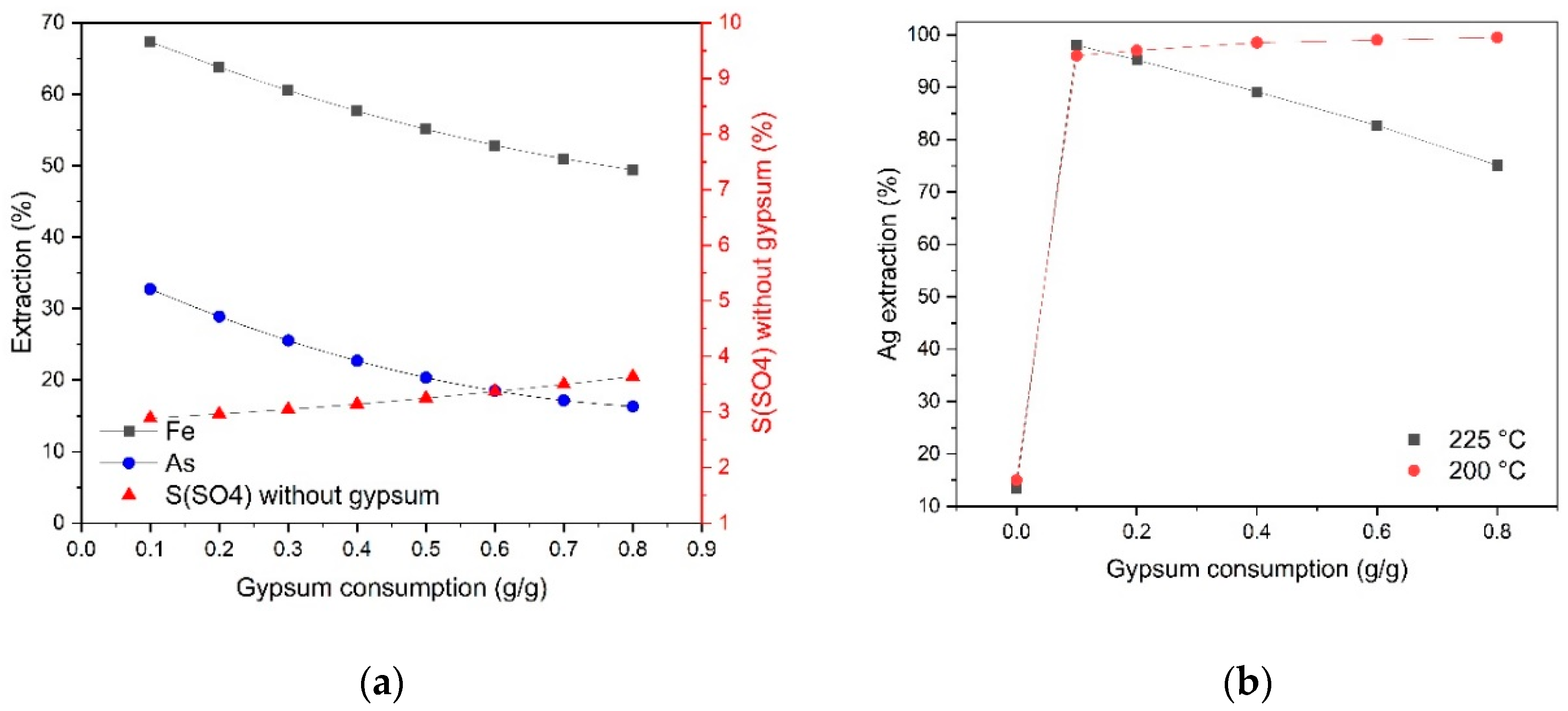
Figure 4). With an increase in gypsum consumption from 0.1 up to 0.8 g/g, the content of sulfate sulfur not associated with gypsum increases less noticeably, from 2.9 to 3.6%, while, in contrast to pressure oxidation leaching at 225 °C, silver extraction increases.
The results obtained are consistent with the data of previous researches, where it was shown that with an increase in the initial concentration of sulfuric acid, the amount of basic ferric sulfate in the cake (Fe(SO
4)OH BFS) decreases and the content of ferric arsenate sulfate increases. (BFAS (Fe
x[(SO
4),(AsO
4)]
∑1(OH)
y·nH
2O)) [
24,
25,
26,
27,
39].
Silver recovery increases with increasing initial sulfuric acid concentration and decreases at 225 °C and gypsum consumption above 0.1 g/g (
Figure 3 (d) and
Figure 4 (b)). Gypsum introduction when pressure oxidation leaching contributes to a sharp increase in the sulfate sulfur content in the cake from 2.4 to 5.8% at 225 °C and from 1.3 to 3.0% at 200 °C, respectively. In this case, the addition of gypsum 0.1 g/g of concentrate leads to an increase in silver extraction from 13.4 to 95–98% at the cyanidation stage, but a further increase in consumption above 0.1 g/g promotes to reduce silver transition into solution at 225 °C and has a positive effect at 200 °C. Gold recovery was 99%.
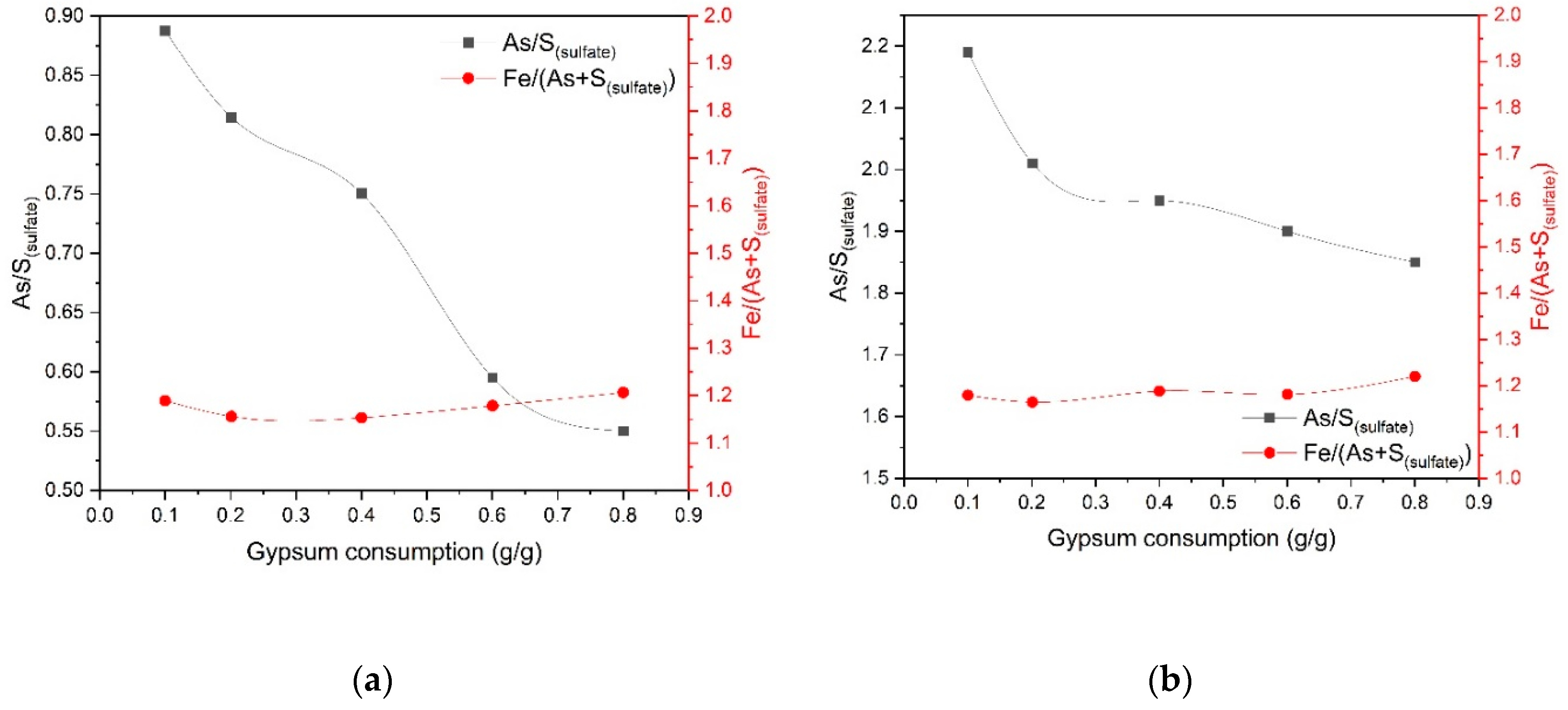
Figure 5 shows the effect of gypsum consumption on the molar ratio of arsenic to sulfate sulfur not associated with gypsum As/S
(sulfate) and the molar ratio of iron to the sum of arsenic and sulfate sulfur Fe/(As+ S
(sulfate)) in the cake.
An increase in gypsum consumption from 0.1 up to 0.8 g/g during pressure oxidation leaching at 225 °C helps to reduce silver extraction from 98 to 75.1% and the molar ratio of arsenic to sulfate sulfur not associated with gypsum As/S(sulfate) in the cake from 0.88 down to 0.55. At 200 °C, an increase in gypsum consumption affects the As/S(sulfate) ratio in the cake to a lesser extent and helps to increase silver extraction from 96 up to 99.5%, while the Fe/(As+ S(sulfate)) molar ratio remains almost unchanged and is 1.15–1.21 over the entire range of temperatures, acid concentrations and gypsum consumption. These data show that with an increase in gypsum consumption in cakes, the content of sulfur-associated iron compounds increases, and with an increase in temperature from 200 up to 225 °C, the degree of precipitation of these compounds increases as well. Gold recovery in all experiments was 97–98%.
3.2. Analysis of the Resulting Precipitation
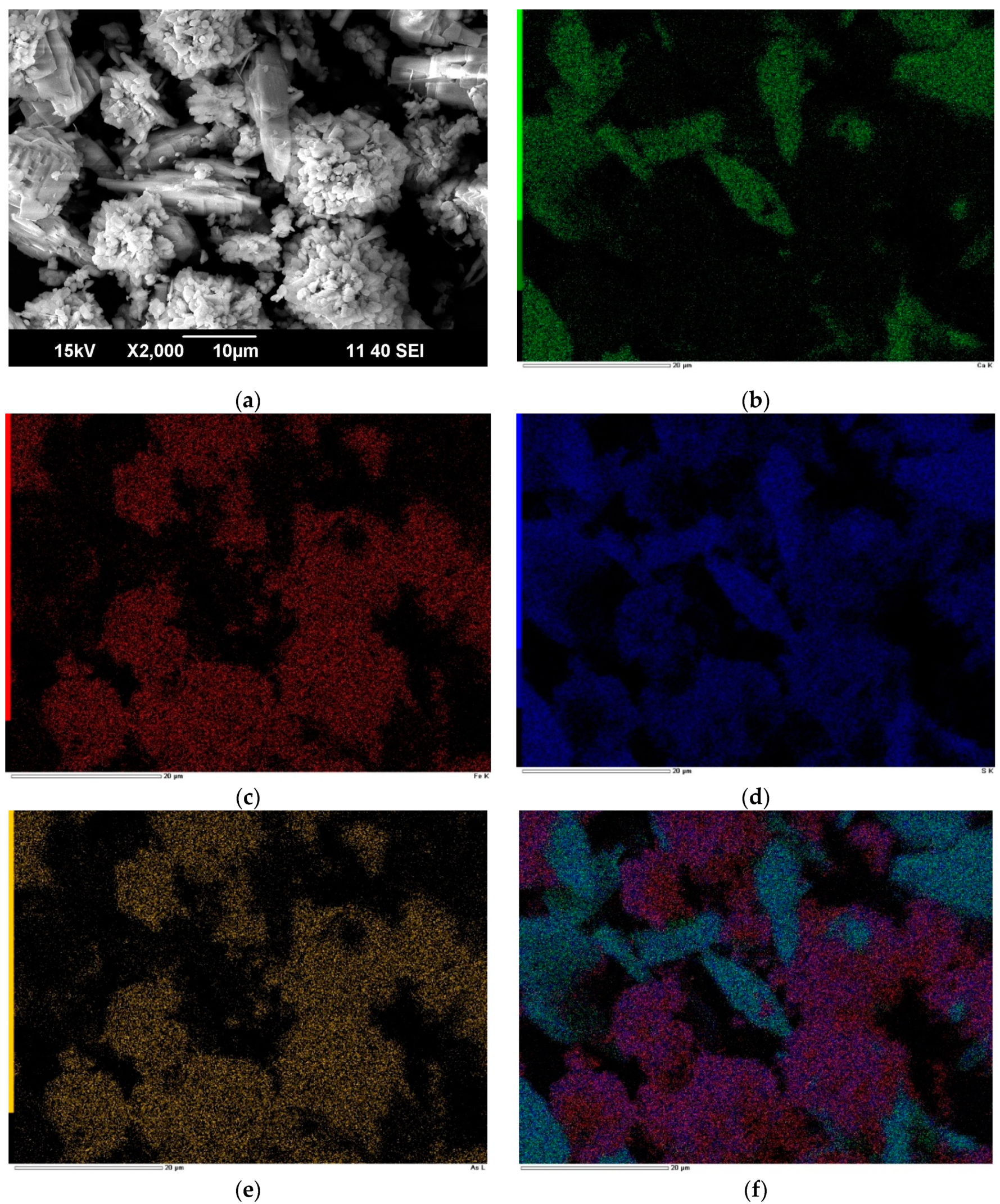
Figure 6 presents microphotographs of the resulting precipitate at 225 °C, gypsum consumption of 0.4 g/g and its EDS mapping. The resulting cake contains, %: 9.1 As; 20.1 Fe; 8.6 Ca; 12.4 S
(sulfate) (As/S
(sulfate) = 0.7).
According to
Figure 6, the precipitate is represented by needle-shaped particles with a smooth surface and spherical particles with a rough surface. There is a heterogeneous distribution of iron with arsenic and calcium with sulfur (
Figure 6), which is caused by the presence of anhydrite (CaSO
4) and iron arsenates (
Figure 6 (f)).
The EDS mapping results show that arsenic is distributed with iron in one area and is almost absent in the calcium area (
Figure 6 (b), (c), (e)). According to the microimages obtained, one can notice the presence of two minerals which may contain Ca, S, Fe, As, the first being gypsum, and the second being sulfur-containing iron arsenate. This confirms the formation of iron arsenates other than scorodite. The distribution of sulfur and arsenic over iron-containing particles is uniform, which indicates the absence of basic ferric sulfate particles in the precipitate.
According to previous researches, the peaks on the diffraction patterns of basic ferric sulfate (Fe(SO
4)(OH)) and basic ferric arsenate sulfate (Fe
x[(SO
4),(AsO
4)]
∑1(OH)
y·nH
2O) have minor differences [
24,
25,
26,
27,
39].
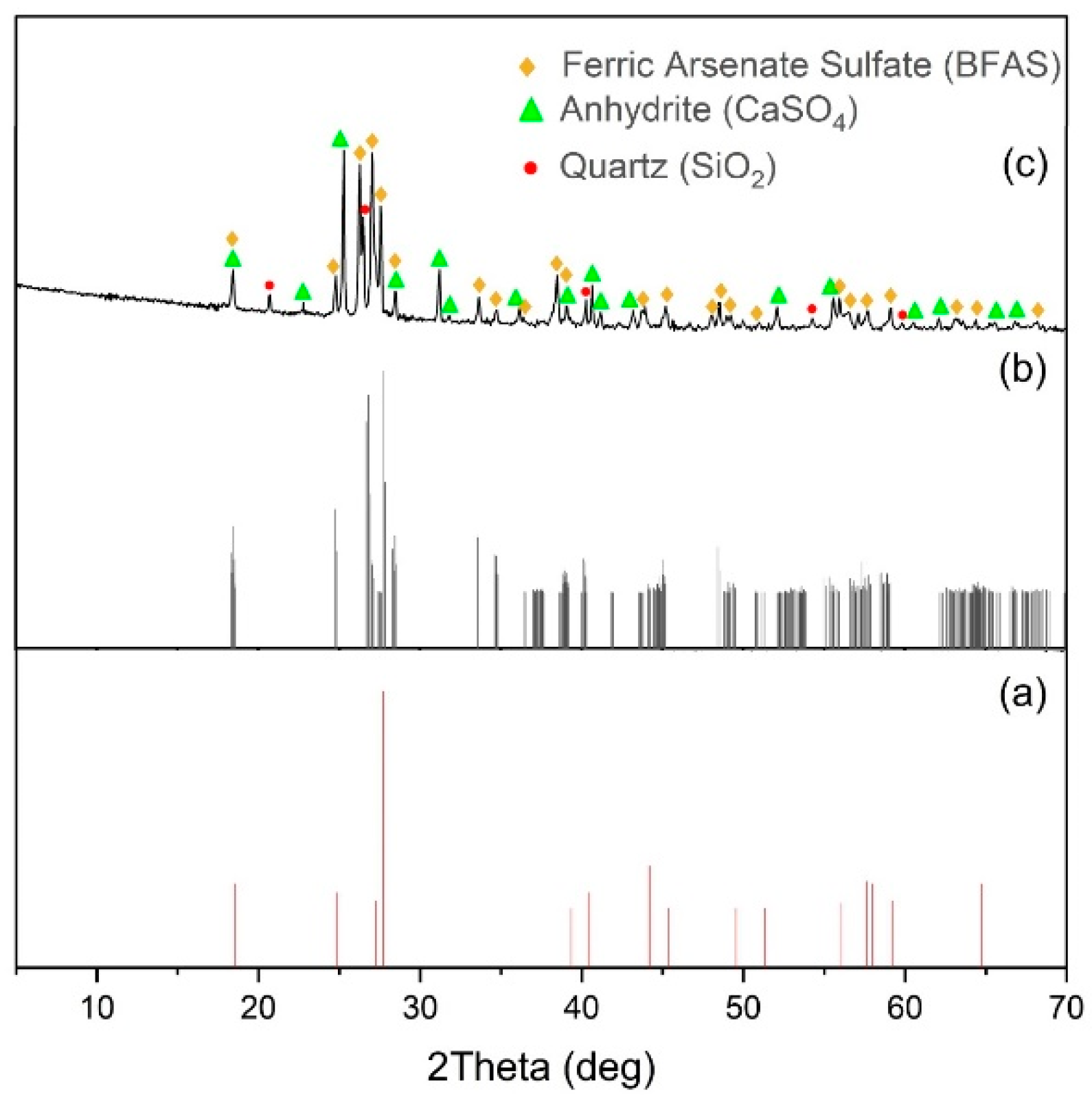
Figure 7 shows the diffraction pattern of the cake after pressure oxidation leaching.
As shown in
Figure 7, BFAS is present in the precipitate since its characteristic peaks with angles 2θ = 28.2 and 34.5 were detected. The peaks of BFS coincide with those of BFAS, making it difficult to detect by X-ray diffraction [
24,
25,
26,
27,
39]. Gypsum recrystallizes into its anhydrite (CaSO
4).
According to the data obtained, the addition of gypsum contributes to a change in the phase composition of the cake after pressure oxidation leaching. An increase in gypsum consumption promotes the formation of BFAS with an increased content of sulfate sulfur, and a decrease in the As/S
(sulfate) molar ratio in the cake from 3.7 to 0.88 contributes to an increase in silver extraction from 13.4 to 98%, and its further decrease from 0.88 to 0.58 leads to a gradual decrease of silver transfer into solution from 98 to 75.1% at the cyanidation stage. This is possibly due to the formation of more crystalline BFAS with increased sulfate sulfur content with increasing gypsum consumption. The positive effect of acidity on silver recovery and the decrease in sulfate sulfur content minus gypsum could also be explained by a decrease in the formation of BFAS with an increased content of sulfate sulfur, since according to EDS mapping the distribution of arsenic and sulfur over iron-containing particles is uniform [
24,
25,
26,
27].
3.3. Study of the Solubility and Toxicity of the resulting Iron Arsenate Precipitates
According to Ref. [
28], the “Fe(III) oxyhydride – ferrihydrite” phase could sorb AsO
43- ions. Sorption occurs through the following reactions:
When these precipitates are stored for a long time in atmospheric conditions, reactions (3, 4) may proceed in the opposite direction, thereby creating a danger of environmental contamination with toxic, water-soluble arsenic compounds. Therefore the solubility of arsenic from precipitates is one of the most important indicators for organizing their long-term storage.
The toxicity of the precipitate was tested on pressure oxidation leaching cake after cyanidation, obtained at a temperature of 225 °C, oxygen pressure 0.5 MPa, gypsum consumption 0.4 g/g, the following composition, %: 9.1 As; 20.0 Fe; 15 S; 8.6 Ca. The cake was washed in distilled water and kept for 24 h at temperatures of 24–26 °C with periodic stirring. After washing and measuring the arsenic concentration in the liquid phase, 1 g of the solid sample was added to 20 cm
3 of a buffer solution mixed with acetic acid and sodium acetate (pH ~ 4.9) and stirred for 18 h according to the TCLP method [
30,
39].
According to TCLP analysis, the cake obtained after pressure oxidation leaching and cyanidation is stable and suitable for disposal, since the final concentration of arsenic in the solution was 0.45 mg/dm3, while the threshold concentration for this method is 5 mg/dm3.
4. Conclusions
In this work, studies were carried out on the extraction of silver and gold from cakes of pressure oxidation leaching of sulfide concentrate containing pyrite and arsenopyrite. The addition of gypsum at a consumption of 0.1 g/g of concentrate helps to increase silver extraction from 13.4 to 95–98% at the cyanidation stage, with no conditioning operation. Gold recovery was 99%.
An increase in gypsum consumption contributes to the formation of BFAS with an increased content of sulfate sulfur, and a decrease in the As/S(sulfate) molar ratio in the cake from 3.7 down to 0.88 contributes to an increase in silver extraction at the cyanidation stage up to 98%, and its further decrease from 0.88 down to 0.58 leads to a gradual reducing silver transfer into solution from 98 down to 75.1% at the cyanidation stage. This is possibly due to the formation of more crystalline BFAS with increased sulfate sulfur content with increasing gypsum consumption. The positive effect of acidity on silver recovery and the reduction of sulphate sulfur minus gypsum may also be explained by the reduction in the formation of BFAS with increased sulphate sulfur content. Basic ferric sulfate (BFS) is not formed in this case, since according to EDS mapping the distribution of arsenic and sulfur over iron-containing particles is uniform.
According to TCLP analysis, the cake obtained after pressure oxidation leaching with the addition of gypsum and cyanidation is stable and suitable for disposal, since the final concentration of arsenic in the solution was 0.45 mg/dm3.
Author Contributions
Conceptualization, K.K. and I.F.; methodology, O.D. and M.T.; validation, A.Z.; formal analysis, M.T. and O.D.; investigation, K.K. and I.F.; resources, D.R.; data curation, K.K., D.R. and I.F.; writing—original draft preparation, K.K. and M.T.; writing—review and editing, K.K., D.R. and I.F.; visualization A.Z.; supervision, K.K.; project administration, I.F.; funding acquisition, D.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Russian Science Foundation Project No. 22-79-10290. Analytical studies were carried out with the financial support of the State Task of the Russian Federation under Grant № 075-03-2024-009/1 (FEUZ-2024-0010)
Conflicts of Interest
The authors declare no conflict of interest
References
- Jiafeng, L., Hongying, Y., Rongxin, Z., Linlin, T., Qiao, C. Mineralogical characteristics and recovery process optimization analysis of a refractory gold ore with gold particles mainly encapsulated in pyrite and Arsenopyrite. Geochemistry 2023, 83, 125941. [CrossRef]
- Yan, Z., Mingyao, C., Jianguo, W., Xiaoliang, L., Xianjun, L. A review of gold extraction using alternatives to cyanide: focus on current status and future prospects of the novel eco-friendly synthetic gold lixiviants. Minerals Engineering 2022, 176, 107336. [CrossRef]
- Abdelnasser, A., Kumral, M. The nature of gold-bearing fluids in Atud gold deposit. central eastern desert, Egypt. International Geology Review 2017, 59, 1845-1860. [CrossRef]
- Xin-yuan, N., Xin, C., Jun, K. Pretreatment process on refractory gold ores with As. ISIJ International 2014, 54, 543-547. [CrossRef]
- Hong, Q., Xueyi, G., Qinghua, T., Dawei, Y., Lei, Z. Recovery of gold from sulfide refractory gold ore: oxidation roasting pretreatment and gold extraction. Minerals Engineering 2021, 164, 106822. [CrossRef]
- Chryssoulis, S.L., McMullen, J. Mineralogical investigation of gold ores. Developments in Mineral Processing, 2005, 15, 21–72. [CrossRef]
- Rogozhnikov, D.A., Mamyachenkov, S.V., Karelov, S.V., Anisimova, O.S. Nitric acid leaching of polymetallic middlings of concentration. Russian Journal of Non-Ferrous Metals 2013, 54(6), 440–442. [CrossRef]
- Rogozhnikov, D.A., Rusalev, R.E., Dizer, O.A., Naboychenko, S.S. Nitric acid loosening of rebellious sulphide concentrates containing precious metals. Tsvetnye Metally 2018, 16, 38–44. [CrossRef]
- Karimov, K., Shoppert, A., Rogozhnikov, D., Kuzas, E., Zakhar’yan, S., Naboichenko, S. Effect of preliminary alkali desilication on ammonia pressure leaching of low-grade copper–silver concentrate. Metals 2020, 10(6), 1–17, 812. [CrossRef]
- McMullen, J., Thomas, G. Gold roasting, autoclaving or bio-oxidation process selection based on benchscale and pilot plant test work and costs. Mineral Processing Plant Design, Practice, and Control at Vancouver 2002, 211–250.
- Thomas, G. Pressure oxidation overview. Developments in Mineral Processing 2005, 15, 346–369. [CrossRef]
- Papangelakis, V., Demopoulos, G. Acid pressure oxidation of pyrite: reaction kinetics. Hydrometallurgy 1991, 26, 309–325. [CrossRef]
- Papangelakis, V., Demopoulos, G. Acid pressure oxidation of arsenopyrite. Scientific Survey 1991, 26. [CrossRef]
- Dizer, O., Rogozhnikov, D., Karimov, K., Kuzas, E., Suntsov, A. Nitric Acid Dissolution of Tennantite, Chalcopyrite and Sphalerite in the Presence of Fe (III) Ions and FeS2. Materials, 2022, 15(4), 1545. [CrossRef]
- Rogozhnikov, D.A., Karelov, S.V., Mamyachenkov, S.V., Anisimova, O.S. Technology for the hydrometallurgical processing of a complex multicomponent sulfide-based raw material. Metallurgist, 2013, 57(3-4), 247–250. [CrossRef]
- Collins, M., Buban, K., Faris, M., Masters, I., Antonio, M. Design of the AGA Brazil refractory gold pressure oxidation plant. Pressure Hydrometallurgy Laval, 2012, 3-14.
- Fleming, C., Geldart, J., Blatter, P., Cousin, P., Robitalille, J. Flowsheet development for Agnico Eagles refractory gold Kittila project in Finland. Hydrometallurgy 2008: Proceedings of the 6th International Symposium 2008, 404–413.
- Zavalyuev, A., Rogozhnikov, D., Fomenko, I., Lyakh, S. Conditioning of POX slurry and its effect on pressure oxidation performance of refractory gold sulphide ore. Tsvetnye Metally 2023, 1, 44–50. [CrossRef]
- Du Plessis, C.A., Lambert, H., Gärtner, R.S., Ingram, K., Slabbert, W., Eksteen, J.J. Lime use in gold processing – a review. Minerals Engineering 2021, 174, 107231. [CrossRef]
- Islas, H., Flores, M., Juárez, J., Reyes, M., Blanco, A., Gutiérrez, E., Aguilar-Carrillo, J., Nolasco, M., Rodríguez, I., Reyes, I. Silver leaching from jarosite-type compounds using cyanide and non-cyanide lixiviants: a kinetic approach. Minerals Engineering 2021, 174, 107250. [CrossRef]
- Chan, T., Collins, M., Dennett, J., Stiksma, J., Ji, J., Kalanchey, R., Berezowsky, R. Pilot plant pressure oxidation of refractory gold-silver concentrate from Eldorado Gold Corporation's Certej Project in Romania. Canadian Metallurgical Quarterly 2015, 54, 252-260. [CrossRef]
- Simmons, G.L., Gathje, J.C. High temperature POX of precious/base metal concentrates from Newmont`s Phoenix Project, using controlled precipitation of sulphate species to enhance silver recovery. Pressure Hydrometallurgy 2004. Proceedings of the International Conference on the Use of Pressure Vessels for Metal Extraction and Recovery. 34th Annual Hydrometalluigy Meeting of CIM. 2004. Banff, 735-750.
- Gunaratnam, A.A., Dreisinger, D.B., Choi, Y. Characterisation of solid phases in the iron–sulphate–water system where silver is present. Canadian Metallurgical Quarterly 2018, 57, 1-11. [CrossRef]
- Fleuriault, C.M., Anderson, C.G., Shuey, S. Iron phase control during pressure oxidation at elevated temperature. Minerals Engineering 2016, 98, 161-168. [CrossRef]
- Dutrizac, J.E., Jambor, J.L. Characterization of the iron arsenate-sulphate compounds precipitated at elevated temperatures. Hydrometallurgy 2007, 86, 147-163. [CrossRef]
- Swash, P.M., Monhemius, A.J. Hydrothermal precipitation from aqueous solutions containing iron(III), arsenate and sulphate. Hydrometallurgy 1994, 177-190. [CrossRef]
- Gomez, M.A., Becze, L., Cutler, J.N., Demopoulos, G.P. Hydrothermal reaction chemistry and characterization of ferric arsenate phases precipitated from Fe2(SO4)3–As2O5–H2SO4 solutions. Hydrometallurgy 2011, 107, 74-90. [CrossRef]
- Sung Ng, W., Liu, Y., Chen, M. The effect of curing on arsenic precipitation and kinetic study of pressure oxidation of pyrite and arsenopyrite. Minerals Engineering 2022, 185, 107675. [CrossRef]
- Jiang, X., Peng, C., Fu, D., Chen, Z., Shen, L., Li, Q., Ouyang, T., Wang, Y. Removal of arsenate by ferrihydrite via surface complexation and surface precipitation. Applied Surface Science 2015, 353, 1087-1094. [CrossRef]
- Riveros, P.A., Dutrizac, J.E., Spencer, P. Arsenic Disposal Practices in the Metallurgical Industry. Canadian Metallurgical Quarterly 2001, 40, 395-420. [CrossRef]
- Nazari, A.M., Radzinski, R., Ghahreman, A. Review of arsenic metallurgy: treatment of arsenical minerals and the immobilization of arsenic. Hydrometallurgy 2017, 174, 258–281. [CrossRef]
- Clancy, T.M., Snyder, K.V., Reddy, R., Lanzirotti, A., Amrose, S.E., Raskin, L., Hayes, K.F. Evaluating the cement stabilization of arsenic-bearing iron wastes from drinking water treatment. Journal of Hazardous Materials 2015, 300, 522–529. [CrossRef]
- Robins, R. Solubility and stability of scorodite, FeAsO4*2H2O: Discussion. American Mineralogist 1987, 72, 842–844.
- Filippou, D., Demopoulos, G.P. Arsenic immobilization by controlled scorodite precipitation. The Journal of The Minerals 1997, 49, 52–55. [CrossRef]
- Rios-Valenciana, E.E., Briones-Gallardo, R., Cházaro-Ruiz, L.F., Martínez-Villegas, N., Celis, L.B. Role of indigenous microbiota from heavily contaminated sediments in the bioprecipitation of arsenic. Journal of Hazardous Materials 2017, 339, 114–121. [CrossRef]
- Ahoranta, S.H., Kokko, M.E., Papirio, S., Özkaya, B., Puhakka, J.A. Arsenic removal from acidic solutions with biogenic ferric precipitates. Journal of Hazardous Materials 2016, 306, 124–132. [CrossRef]
- Monhemius, A.J., Swash P.M. Removing and stabilizing As from copper refining circuits by hydrothermal processing. The Journal of the Minerals 1999, 51, 30-33. [CrossRef]
- Fujita, T., Taguchi, R., Abumiya, M., Matsumoto, M., Shibata, E., Nakamura, T. Effects of zinc, copper and sodium ions on ferric arsenate precipitation in a novel atmospheric scorodite process. Hydrometallurgy 2008, 93, 30-38. [CrossRef]
- Gomez, M.A., Assaaoudi, H., Becze, L., Cutler, J.N., Demopoulos, G.P. Vibrational spectroscopy study of hydrothermally produced scorodite (FeAsO4·2H2O), ferric arsenate sub-hydrate (FAsH; FeAsO4·0.75H2O) and basic ferric arsenate sulfate (BFAS; Fe[(AsO4)1−x(SO4)x(OH)x]·wH2O). Journal of Raman Spectroscopy 2009, 41, 212-221. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).