Submitted:
20 September 2024
Posted:
23 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
| Characteristic | Total (n=39) |
Study 1 (n-6) |
Study 2 (n=9) |
Study 3 (n=6) |
Study 4 (n=4) |
Study 5 (n=14) |
|---|---|---|---|---|---|---|
| Age, mean (SD) | 53.22 (16) | 38.7 (16.4) | 45.9 (13.2) | 63.3 (13.6) | 50.5 (4.8) | 49.9 (18.8) |
| Sex | ||||||
| Male | 13 | 4 | 1 | 2 | 1 | 5 |
| Female | 26 | 2 | 8 | 4 | 3 | 9 |
| Race | ||||||
| Caucasian | 33 | 5 | 7 | 6 | 4 | 11 |
| Asian/Pacific Islander | 3 | 1 | 1 | 0 | 0 | 1 |
| African American | 2 | 0 | 1 | 0 | 0 | 1 |
| American Indian/Alaska Native | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 1 | 0 | 0 | 0 | 0 | 1 |
| Number of Pain Conditions | ||||||
| 1 | 14 | 2 | 3 | 1 | 3 | 5 |
| 2 | 9 | 2 | 3 | 1 | 0 | 3 |
| 3+ | 16 | 2 | 3 | 4 | 1 | 6 |
| Duration of Pain | ||||||
| 6-12 months | 3 | 1 | 1 | 0 | 0 | 1 |
| 1-5 years | 15 | 2 | 4 | 1 | 2 | 6 |
| >5 years | 21 | 3 | 4 | 5 | 2 | 7 |
| Medications taken | ||||||
| Opioids | 3 | 0 | 0 | 2 | 0 | 1 |
| NSAID/Acetaminophen | 9 | 2 | 3 | 3 | 0 | 1 |
| Adjunctive Pain Medication | 3 | 1 | 0 | 0 | 0 | 2 |
| None/Did not specify | 24 | 1 | 5 | 0 | 2 | 6 |
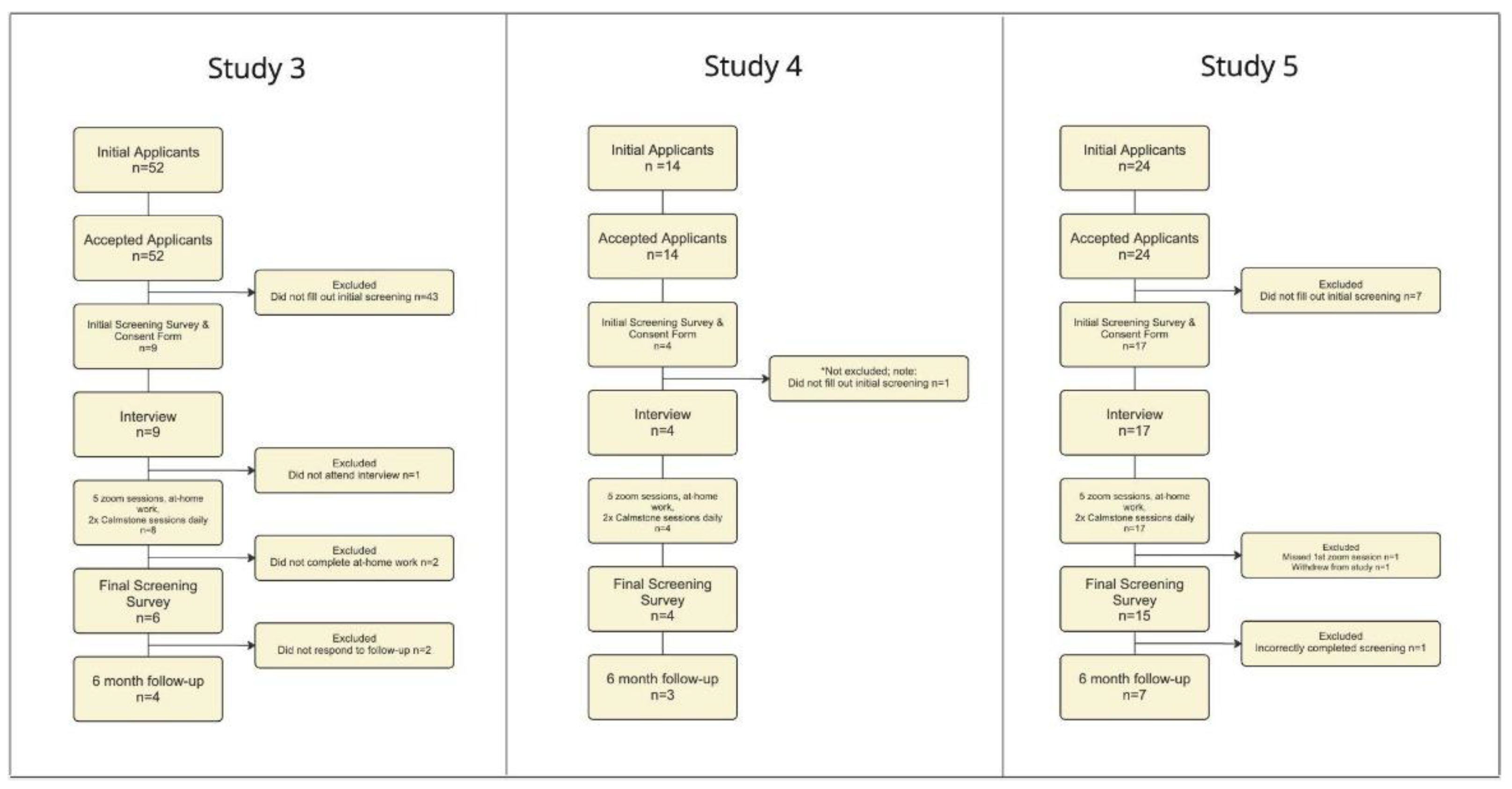
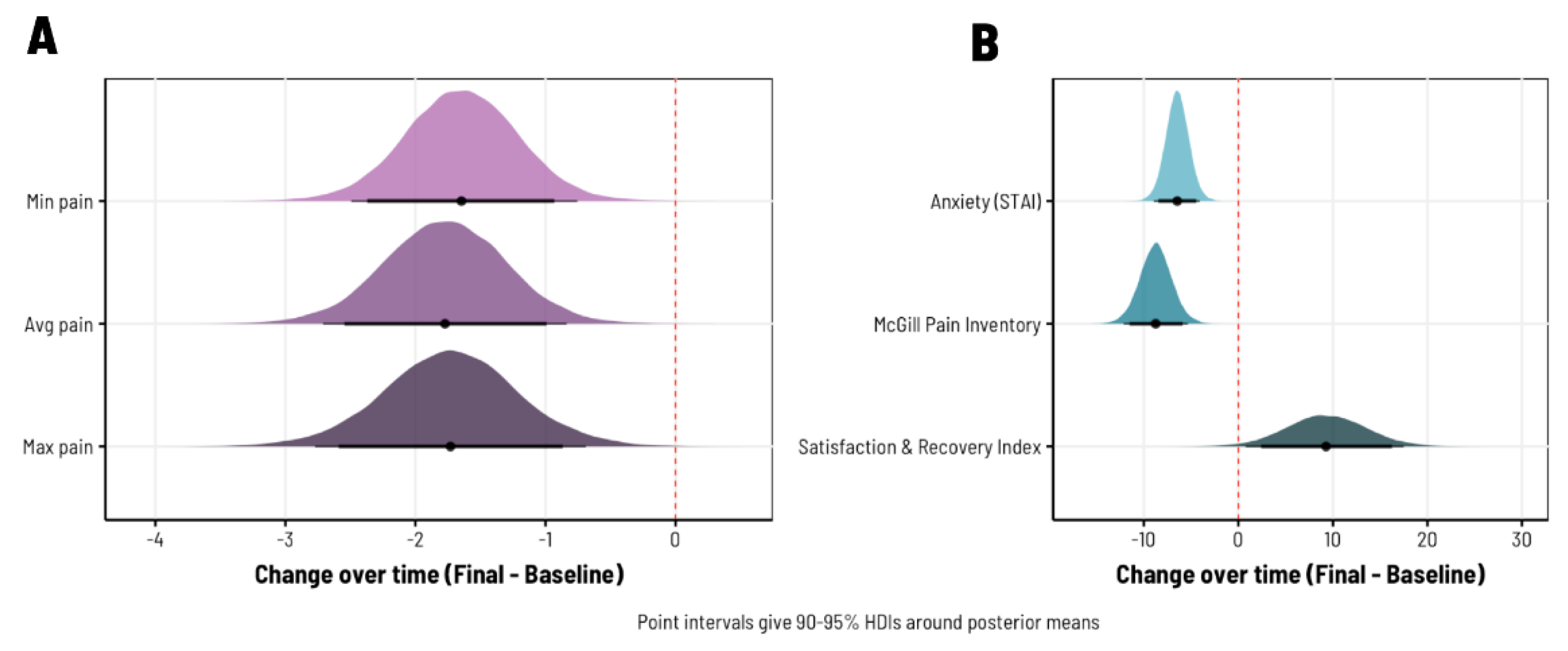
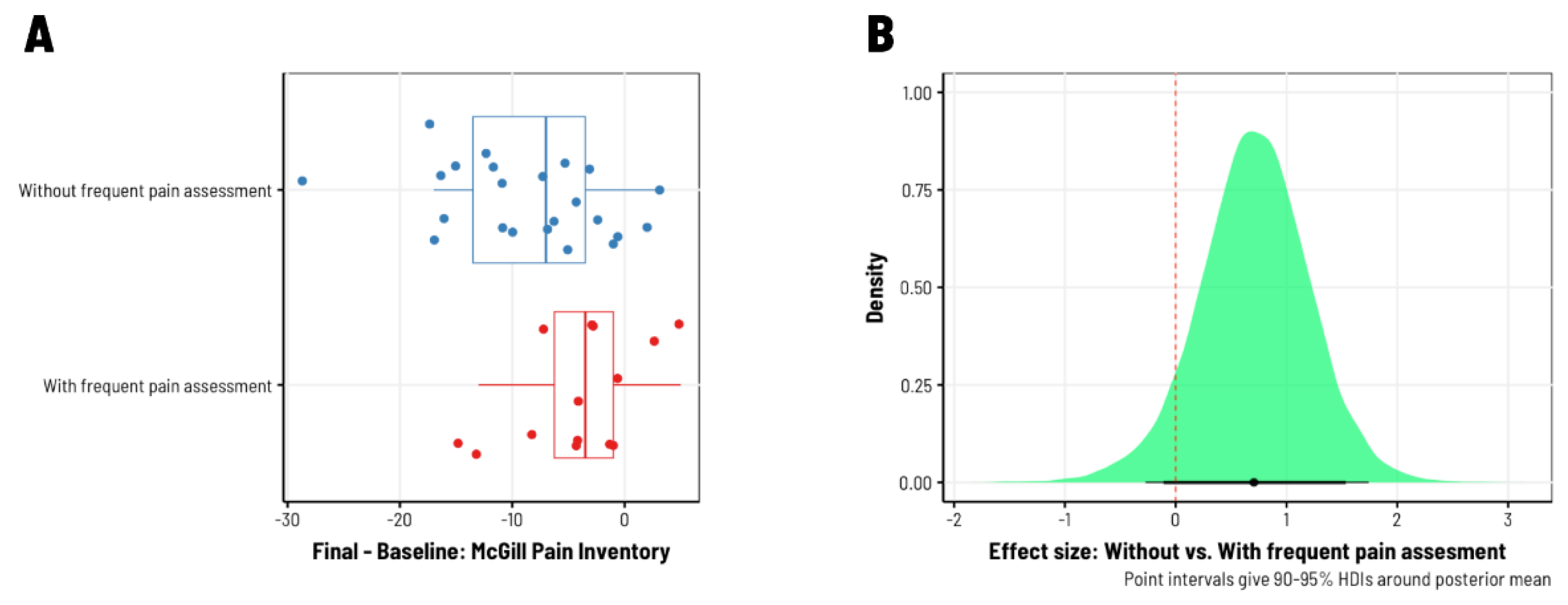
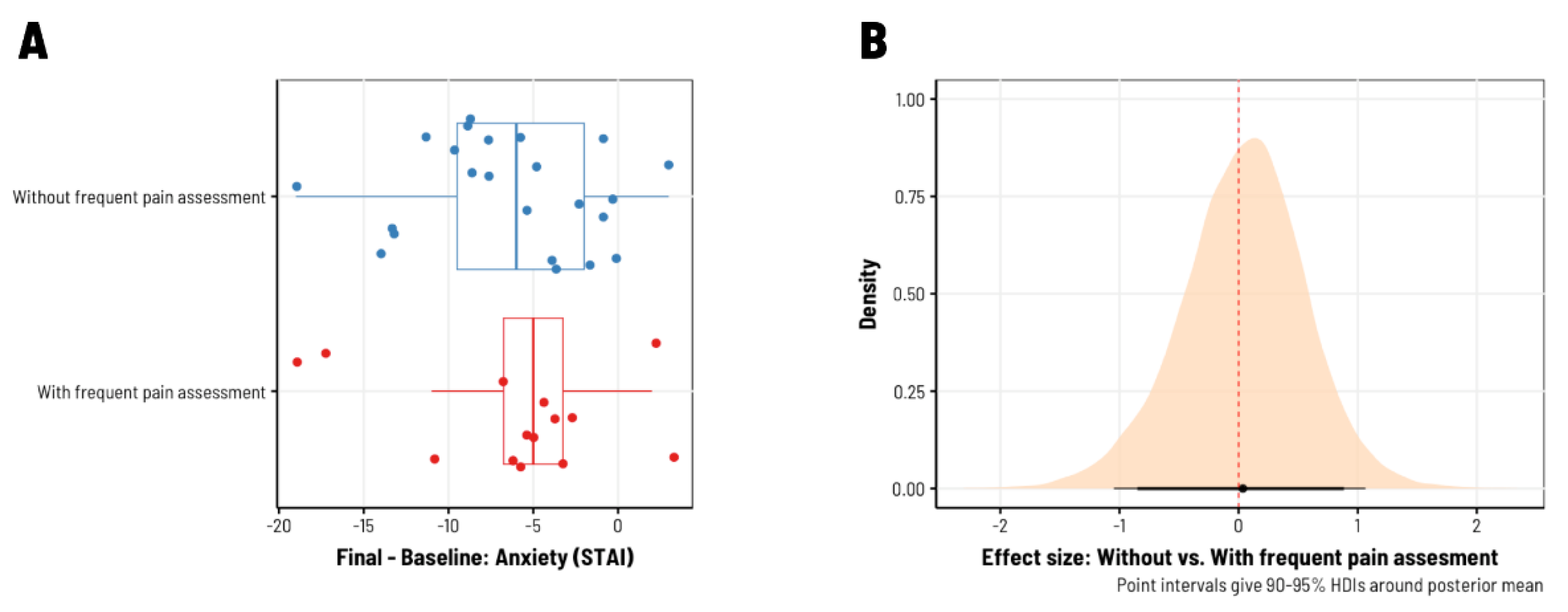
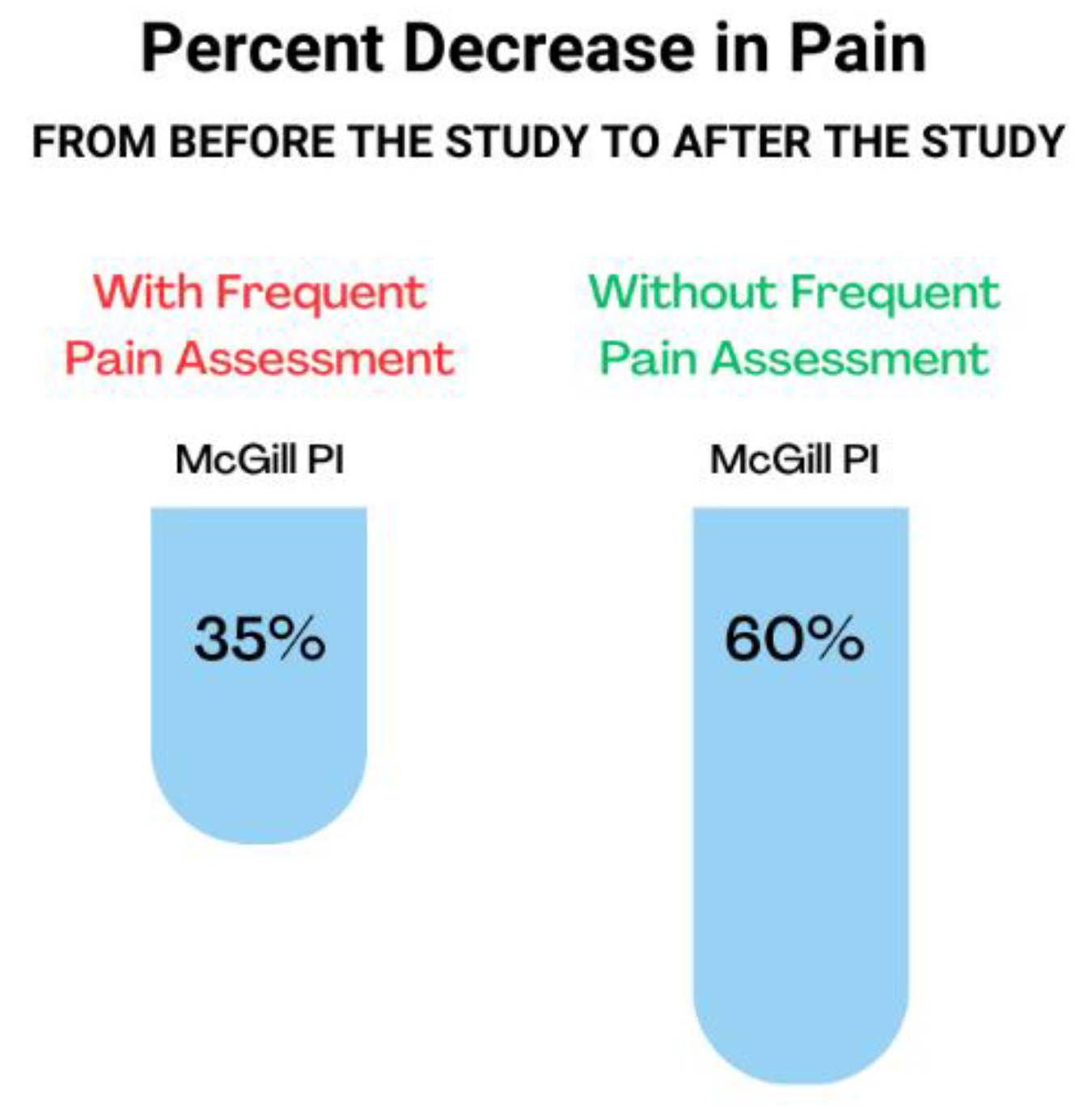
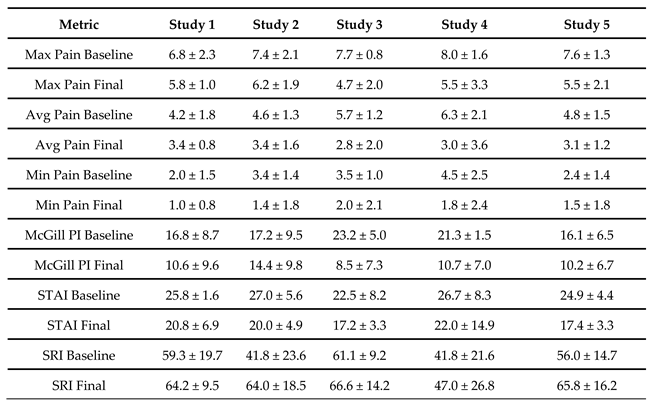
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Yong, R.J.; Mullins, P.M.; Bhattacharyya, N. Prevalence of chronic pain among adults in the United States. Pain 2022, 163, e328–e332. [Google Scholar] [CrossRef]
- Nahin, R.L.; Feinberg, T.; Kapos, F.P.; Terman, G.W. Estimated Rates of Incident and Persistent Chronic Pain Among US Adults, 2019-2020. JAMA Netw. Open 2023, 6, e2313563. [Google Scholar] [CrossRef]
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Fayaz, A.; Croft, P.; Langford, R.M.; Donaldson, L.J.; Jones, G.T. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open 2016, 6, e010364. [Google Scholar] [CrossRef]
- Crofford, L.J. Chronic Pain: Where the Body Meets the Brain. Trans. Am. Clin. Climatol. Assoc. 2015, 126, 167–183. [Google Scholar]
- Martinez-Calderon, J.; Jensen, M.P.; Morales-Asencio, J.M.; Luque-Suarez, A. Pain Catastrophizing and Function In Individuals With Chronic Musculoskeletal Pain. Clin. J. Pain 2019, 35, 279–293. [Google Scholar] [CrossRef]
- Wertli, M.M.; Eugster, R.; Held, U.; Steurer, J.; Kofmehl, R.; Weiser, S. Catastrophizing—a prognostic factor for outcome in patients with low back pain: a systematic review. Spine J. 2014, 14, 2639–2657. [Google Scholar] [CrossRef]
- Tegethoff, M.; Belardi, A.; Stalujanis, E.; Meinlschmidt, G. Comorbidity of Mental Disorders and Chronic Pain: Chronology of Onset in Adolescents of a National Representative Cohort. J. Pain 2015, 16, 1054–1064. [Google Scholar] [CrossRef]
- Kuch, K. Psychological Factors and the Development of Chronic Pain. Clin. J. Pain 2001, 17, S33–S38. [Google Scholar] [CrossRef]
- Sator-Katzenschlager, S. Pain and neuroplasticity. Rev. Medica Clin. Las Condes 2014, 25, 699–706. [Google Scholar] [CrossRef]
- Melzack, R.; Coderre, T.J.; Katz, J.; Vaccarino, A.L. Central Neuroplasticity and Pathological Pain. Ann. New York Acad. Sci. 2001, 933, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Deitos, A.M.; Dussán-Sarria, J.A.; de Souza, A.M.; Medeiros, L.M.; Tarragô, M.d.G.; Sehn, F.M.; Chassot, M.M.; Zanette, S.; Schwertner, A.; Fregni, F.; et al. Clinical Value of Serum Neuroplasticity Mediators in Identifying the Central Sensitivity Syndrome in Patients With Chronic Pain With and Without Structural Pathology. Clin. J. Pain 2015, 31, 959–967. [Google Scholar] [CrossRef]
- Hiraga, S.-I.; Itokazu, T.; Nishibe, M.; Yamashita, T. Neuroplasticity related to chronic pain and its modulation by microglia. Inflamm. Regen. 2022, 42, 15. [Google Scholar] [CrossRef] [PubMed]
- McCracken, L.M. “Attention” to pain in persons with chronic pain: A behavioral approach. Behav. Ther. 1997, 28, 271–284. [Google Scholar] [CrossRef]
- Garland, E.L.; Howard, M.O. Mindfulness-Oriented Recovery Enhancement Reduces Pain Attentional Bias in Chronic Pain Patients. Psychother. Psychosom. 2013, 82, 311–318. [Google Scholar] [CrossRef]
- Schütze, R.; Rees, C.; Smith, A.; Slater, H.; O’sullivan, P. Metacognition, perseverative thinking, and pain catastrophizing: A moderated-mediation analysis. Eur. J. Pain 2020, 24, 223–233. [Google Scholar] [CrossRef]
- Semeru, G.M.; Halim, M.S. Acceptance versus catastrophizing in predicting quality of life in patients with chronic low back pain. Korean J. Pain 2019, 32, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Darnall, B.; Sturgeon, J.; Kao, M.-C.; Hah, J.; Mackey, S. From Catastrophizing to Recovery: a pilot study of a single-session treatment for pain catastrophizing. J. Pain Res. 2014, 7, 219–226. [Google Scholar] [CrossRef]
- Ly, F.S.; Santander, T.; Pavlov, S.; Zhao, J.; Zhang, M.; Arroyo, D.; Sokolovskiy, S.; Iyer, A.; Yankauskas, Y.; Chen, J.; et al. Home-Use and Portable Biofeedback Lowers Anxiety and Pain in Chronic Pain Subjects. Am. J. Lifestyle Med. 2023, 15598276231221112. [Google Scholar] [CrossRef]
- Thong, I.S.K.; Jensen, M.P.; Miró, J.; Tan, G. The validity of pain intensity measures: what do the NRS, VAS, VRS, and FPS-R measure? Scand. J. Pain 2018, 18, 99–107. [Google Scholar] [CrossRef]
- Price, D.D.; McGrath, P.A.; Rafii, A.; Buckingham, B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 1983, 17, 45–56. [Google Scholar] [CrossRef]
- Lovejoy, T.I.; Turk, D.C.; Morasco, B.J. Evaluation of the Psychometric Properties of the Revised Short-Form McGill Pain Questionnaire. J. Pain 2012, 13, 1250–1257. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Turk, D.C.; Trudeau, J.J.; Benson, C.; Biondi, D.M.; Katz, N.P.; Kim, M. Validation of the Short-Form McGill Pain Questionnaire-2 (SF-MPQ-2) in Acute Low Back Pain. J. Pain 2015, 16, 357–366. [Google Scholar] [CrossRef]
- Jumbo, S.U.M.; MacDermid, J.C.; Kalu, M.E.M.; Packham, T.L.; Athwal, G.S.; Faber, K.J. Measurement Properties of the Brief Pain Inventory-Short Form (BPI-SF) and Revised Short McGill Pain Questionnaire Version-2 (SF-MPQ-2) in Pain-related Musculoskeletal Conditions: A Systematic Review. Clin. J. Pain 2021, 37, 454–474. [Google Scholar] [CrossRef]
- Marteau, T.M.; Bekker, H. The development of a six-item short-form of the state scale of the Spielberger State—Trait Anxiety Inventory (STAI). Br. J. Clin. Psychol. 1992, 31, 301–306. [Google Scholar] [CrossRef]
- Zsido, A.N.; Teleki, S.A.; Csokasi, K.; Rozsa, S.; Bandi, S.A. Development of the short version of the spielberger state—trait anxiety inventory. Psychiatry Res. 2020, 291, 113223. [Google Scholar] [CrossRef]
- Modarresi, S.; Walton, D.M. Reliability, discriminative accuracy, and an exploration of response shift as measured using the satisfaction and Recovery Index over 12 months from musculoskeletal trauma. Musculoskelet. Sci. Pr. 2020, 51, 102300. [Google Scholar] [CrossRef]
- Walton, D.M.; MacDermid, J.C.; Pulickal, M.; Rollack, A.; Veitch, J. Development and Initial Validation of the Satisfaction and Recovery Index (SRI) for Measurement of Recovery from Musculoskeletal Trauma. Open Orthop. J. 2014, 8, 316–325. [Google Scholar] [CrossRef]
- Modarresi, S.; Farzad, M.; Shafiee, E.; Modarresi, G.; Maleki, M.; Bakhshi, E.; Hosseini, S.A.; Walton, D.M. Cross-cultural Adaptation and Psychometric Evaluation of the Persian Version of the Satisfaction and Recovery Index (SRI): Structural Validity, Construct Validity, Internal Consistency, and Test-retest Reliability. Arch. Bone Jt. Surg. 2023, 11, 53–63. [Google Scholar] [CrossRef]
- Bürkner, P.-C. brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef]
- Westfall, J.; Kenny, D.A.; Judd, C.M. Statistical power and optimal design in experiments in which samples of participants respond to samples of stimuli. J. Exp. Psychol. Gen. 2014, 143, 2020–2045. [Google Scholar] [CrossRef]
- Delgado-Gallén, S.; Soler, M.D.; Cabello-Toscano, M.; Abellaneda-Pérez, K.; Solana-Sánchez, J.; España-Irla, G.; Roca-Ventura, A.; Bartrés-Faz, D.; Tormos, J.M.; Pascual-Leone, A.; et al. Brain system segregation and pain catastrophizing in chronic pain progression. Front. Neurosci. 2023, 17, 1148176. [Google Scholar] [CrossRef] [PubMed]
- Gracely, R.H.; Geisser, M.E.; Giesecke, T.; Grant, M.A.B.; Petzke, F.; Williams, D.A.; Clauw, D.J. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 2004, 127, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Linton, S.J.; Flink, I.K.; Vlaeyen, J.W.S. Understanding the Etiology of Chronic Pain From a Psychological Perspective. Phys. Ther. 2018, 98, 315–324. [Google Scholar] [CrossRef]
- Linton, S.J.; Shaw, W.S. Impact of Psychological Factors in the Experience of Pain. Phys. Ther. 2011, 91, 700–711. [Google Scholar] [CrossRef]
- Edwards, R.R.; Dworkin, R.H.; Sullivan, M.D.; Turk, D.C.; Wasan, A.D. The Role of Psychosocial Processes in the Development and Maintenance of Chronic Pain. J. Pain 2016, 17, T70–T92. [Google Scholar] [CrossRef] [PubMed]
- Nees, F.; Becker, S. Psychological Processes in Chronic Pain: Influences of Reward and Fear Learning as Key Mechanisms - Behavioral Evidence, Neural Circuits, and Maladaptive Changes. Neuroscience 2018, 387, 72–84. [Google Scholar] [CrossRef]
- Hölzl, R.; Kleinböhl, D.; Huse, E. Implicit operant learning of pain sensitization. Pain 2005, 115, 12–20. [Google Scholar] [CrossRef]
- van Wilgen, C.P.; Keizer, D. The Sensitization Model to Explain How Chronic Pain Exists Without Tissue Damage. Pain Manag. Nurs. 2012, 13, 60–65. [Google Scholar] [CrossRef]
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




