Submitted:
19 September 2024
Posted:
23 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
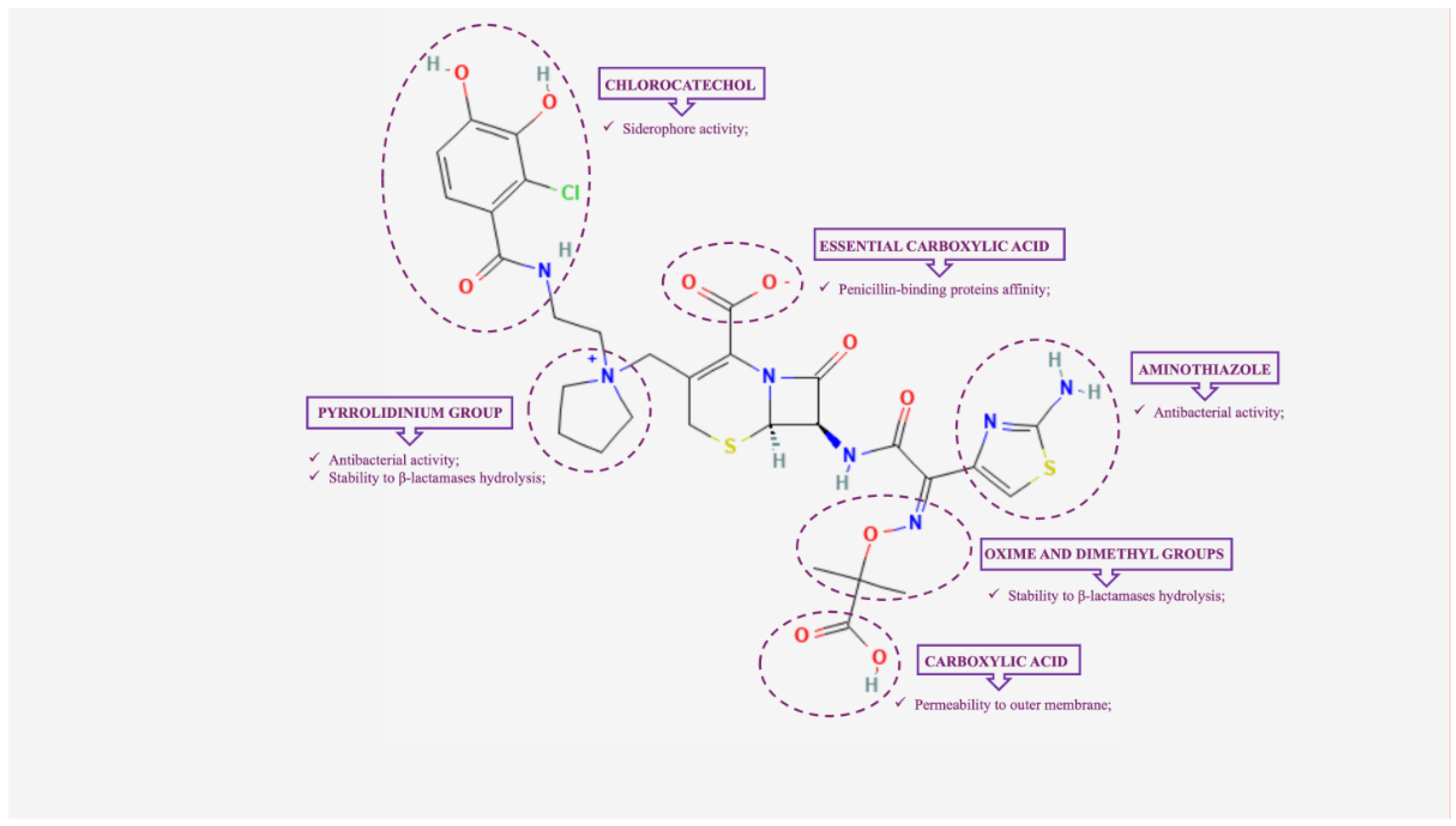
2. Cefiderocol: Mechanism of Action and In Vitro Activity
3. Mechanisms of Cefiderocol Resistance
3.1. Mutations in Genes Related to Iron Transfer Systems
3.2. Expression of β-Lactamases
3.2.1. Metallo β-Lactamases
3.2.2. KPC Variants
3.2.3. OXA-Type β-Lactamases
3.2.4. AmpC β-Lactamases
3.2.5. Other β-Lactamases
3.3. Mutations in Penicillin Binding Proteins
3.4. Porin Loss or Efflux Pump Overexpression
4. Pharmacokinetic/Pharmacodynamic Features
5. Clinical Usage
5.1. Carbapenem Resistant Pseudomonas aeruginosa
5.2. Stenotrophomonas maltophilia
5.3. Carbapenem Resistant Enterobacterales
5.4. Carbapenem Resistant Acinetobacter baumannii
6. Prospectives and Open Questions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bloom DE, Black S, Salisbury D, Rappuoli R. Antimicrobial resistance and the role of vaccines. Proc Natl Acad Sci U S A. 2018; 115(51):12868-12871. [CrossRef] [PubMed] [PubMed Central]
- Shi Z, Zhang J, Tian L, Xin L, Liang C, Ren X, Li M. A Comprehensive Overview of the Antibiotics Approved in the Last Two Decades: Retrospects and Prospects. Molecules. 2023; 28(4):1762. [CrossRef] [PubMed] [PubMed Central]
- Bassetti M, Kanj SS, Kiratisin P, Rodrigues C, Van Duin D, Villegas MV, Yu Y. Early appropriate diagnostics and treatment of MDR Gram-negative infections. JAC Antimicrob Resist. 2022; 4(5):dlac089. [CrossRef] [PubMed] [PubMed Central]
- Parmanik A, Das S, Kar B, Bose A, Dwivedi GR, Pandey MM. Current Treatment Strategies Against Multidrug-Resistant Bacteria: A Review. Curr Microbiol. 2022; 79(12):388. [CrossRef] [PubMed] [PubMed Central]
- Boyd SE, Livermore DM, Hooper DC, Hope WW. Metallo-β-Lactamases: Structure, Function, Epidemiology, Treatment Options, and the Development Pipeline. Antimicrob Agents Chemother. 2020; 64(10):e00397-20. [CrossRef] [PubMed] [PubMed Central]
- Echols R, Ariyasu M, Nagata TD. Pathogen-focused Clinical Development to Address Unmet Medical Need: Cefiderocol Targeting Carbapenem Resistance. Clin Infect Dis. 2019; 69(Suppl 7):S559-S564. [CrossRef] [PubMed] [PubMed Central]
- Heil EL, Tamma PD. Cefiderocol: the Trojan horse has arrived but will Troy fall? Lancet Infect Dis. 2021; 21(2):153-155. Reference for challenges with MBL-producing Enterobacterales. [CrossRef] [PubMed]
- Abdul-Mutakabbir JC, Alosaimy S, Morrisette T, Kebriaei R, Rybak MJ. Cefiderocol: A Novel Siderophore Cephalosporin against Multidrug-Resistant Gram-Negative Pathogens. Pharmacotherapy. 2020; 40(12):1228-1247. [CrossRef] [PubMed]
- Karakonstantis S, Rousaki M, Kritsotakis EI. Cefiderocol: Systematic Review of Mechanisms of Resistance, Heteroresistance and In Vivo Emergence of Resistance. Antibiotics (Basel). 2022; 11(6):723. Reference for cefiderocol's affinity for PBP2 in Klebsiella pneumoniae. [CrossRef] [PubMed] [PubMed Central]
- Ong'uti S, Czech M, Robilotti E, Holubar M. Cefiderocol: A New Cephalosporin Stratagem Against Multidrug-Resistant Gram-Negative Bacteria. Clin Infect Dis. 2022; 74(7):1303-1312. Reference for cefiderocol's stability against ESBLs and MBLs. [CrossRef] [PubMed] [PubMed Central]
- Karlowsky, J.A.; Hackel, M.A.; Takemura, M.; Yamano, Y.; Echols, R.; Sahm, D.F. In Vitro Susceptibility of Gram-Negative Pathogens to Cefiderocol in Five Consecutive Annual Multinational SIDERO-WT Surveillance Studies, 2014 to 2019. Antimicrob. Agents Chemother. 2022, 66, e0199021.
- Candel, F.J.; Henriksen, A.S.; Longshaw, C.; Yamano, Y.; Oliver, A. In vitro activity of the novel siderophore cephalosporin, cefiderocol, in Gram-negative pathogens in Europe by site of infection. Clin. Microbiol. Infect. 2022, 28, 447.e1–447.e6.
- Golden, A.R.; Adam, H.J.; Baxter, M.; Walkty, A.; Lagacé-Wiens, P.; Karlowsky, J.A.; Zhanel, G.G. In Vitro Activity of Cefiderocol, a Novel Siderophore Cephalosporin, against Gram-Negative Bacilli Isolated from Patients in Canadian Intensive Care Units. Diagn. Microbiol. Infect. Dis. 2020, 97, 115012.
- Morris, C.P.; Bergman, Y.; Tekle, T.; Fissel, J.A.; Tamma, P.D.; Simner, P.J. Cefiderocol Antimicrobial Susceptibility Testing against Multidrug-Resistant Gram-Negative Bacilli: A Comparison of Disk Diffusion to Broth Microdilution. J. Clin. Microbiol. 2020, 59, e01649-20.
- Mushtaq, S.; Sadouki, Z.; Vickers, A.; Livermore, D.M.; Woodford, N. In Vitro Activity of Cefiderocol, a Siderophore Cephalosporin, against Multidrug-Resistant Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2020, 64, 12.
- E Choby, J.; Ozturk, T.; Satola, S.W.; Jacob, J.T.; Weiss, D.S. Widespread cefiderocol heteroresistance in carbapenem-resistant Gram-negative pathogens. Lancet Infect. Dis. 2021, 21, 597–598.
- Takemura, M.; Yamano, Y.; Matsunaga, Y.; Ariyasu, M.; Echols, R.; Nagata, T.D. 1266. Characterization of Shifts in Minimum Inhibitory Concentrations During Treatment with Cefiderocol or Comparators in the Phase 3 CREDIBLE-CR and APEKS-NP Studies. Open Forum Infect. Dis. 2020, 7, S649–S650.
- Wunderink, R.G.; Matsunaga, Y.; Ariyasu, M.; Clevenbergh, P.; Echols, R.; Kaye, K.S.; Kollef, M.; Menon, A.; Pogue, J.M.; Shorr, A.F.; et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): A randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2020, 21, 213–225. [CrossRef]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021, 21, 226–240.
- Klein, S.; Boutin, S.; Kocer, K.; O Fiedler, M.; Störzinger, D.; A Weigand, M.; Tan, B.; Richter, D.; Rupp, C.; Mieth, M.; et al. Rapid Development of Cefiderocol Resistance in Carbapenem-resistant Enterobacter cloacae During Therapy Is Associated With Heterogeneous Mutations in the Catecholate Siderophore Receptor cirA. Clin. Infect. Dis. 2021, 74, 905–908.
- Aoki T, Yoshizawa H, Yamawaki K, Yokoo K, Sato J, Hisakawa S, et al. Cefiderocol (S-649266), A new siderophore cephalosporin exhibiting potent activities against Pseudomonas aeruginosa and other gram-negative pathogens including multi-drug resistant bacteria: Structure activity relationship. Eur J Med Chem 2018; 155:847-68. [CrossRef]
- Sato T, Yamawaki K. Cefiderocol: Discovery, Chemistry, and In Vivo Profiles of a Novel Siderophore Cephalosporin. Clin Infect Dis 2019; 69:S538-S543. [CrossRef]
- Karakonstantis S, Rousaki M, Vassilopoulou L, Kritsotakis EI. Global prevalence of cefiderocol non-susceptibility in Enterobacterales, Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia: a systematic review and meta-analysis. Clin Microbiol Infect 2024; 30:178-88. [CrossRef]
- Borde K, Kareem MA, Sharma RM, Dass SM, Ravi V, Mathai D. In vitro activity of cefiderocol against comparators (ceftazidime-avibactam, ceftazidime-avibactam/ aztreonam combination, and colistin) against clinical isolates of meropenem-resistant Klebsiella pneumoniae from India. Microbiol Spectr 2023; 11:e0084723. [CrossRef]
- Gijón D, García-Castillo J, Fernández-López MC, Bou G, Siller M, Calvo-Montes J, et al. In vitro activity of cefiderocol and other newly approved antimicrobials against multi-drug resistant Gram-negative pathogens recovered in intensive care units in Spain and Portugal. Rev Esp Quimioter 2024; 37:69-77. [CrossRef]
- Daoud L, Al-Marzooq F, Ghazawi A, Anes F, Collyns T. High efficacy and enhanced synergistic activity of the novel siderophore-cephalosporin cefiderocol against multidrug-resistant and extensively drug-resistant Klebsiella pneumoniae from inpatients attending a single hospital in the United Arab Emirates. J Infect Public Health 2023; 16 Suppl 1:33-44. [CrossRef]
- Zhao J, Pu D, Li Z, Liu X, Zhang Y, Wu Yet al. In vitro activity of cefiderocol, a siderophore cephalosporin, against carbapenem-resistant hypervirulent Klebsiella pneumoniae in China. Antimicrob Agents Chemother 2023; 67:e0073523. [CrossRef]
- Malisova L, Vrbova I, Pomorska K, Jakubu V, Zemlickova H. In Vitro Activity of Cefiderocol Against Carbapenem-Resistant Enterobacterales and Pseudomonas aeruginosa. Microb Drug Resist 2023; 29:485-91. [CrossRef]
- Khanchandani H, Chaudhury M, Rao MS, Ramakrishna N, Venkataramana B, Chaudhury A. In vitro activity of the newly approved antimicrobial agent Cefiderocol against Carbapenem resistant Gram negative clinical isolates. Indian J Med Microbiol 2024; 48:100556. [CrossRef]
- Saxena S, Aggarwal P, Mitra S, Singh S, Kaim M, Sharma A. In vitro assessment of newer colistin-sparing antimicrobial agents for clinical isolates of carbapenem-resistant organisms. J Infect Chemother 2024: S1341-321X(24)00149-1. [CrossRef]
- Shields RK, Kline EG, Squires KM, Van Tyne D, Doi Y. In vitro activity of cefiderocol against Pseudomonas aeruginosa demonstrating evolved resistance to novel β-lactam/β-lactamase inhibitors. JAC Antimicrob Resist 2023; 5:dlad107. [CrossRef]
- Riccobene T, Ai C, Yu KC, Gregory S, Kim B, Debabov D, et al. Real-world in vitro activity of newer antibiotics against Enterobacterales and Pseudomonas aeruginosa, including carbapenem-non-susceptible and multidrug-resistant isolates: a multicenter analysis. Microbiol Spectr 2023; 11:e0312923. [CrossRef]
- Monogue ML, Desai D, Pybus CA, Sanders JM, Clark AE, Greenberg DE. In vitro activity of cefiderocol against Pseudomonas aeruginosa isolated from cystic fibrosis patients. Microbiol Spectr 2023; 11:e0304723. [CrossRef]
- Gill CM, Santini D, Nicolau DP. In vitro activity of cefiderocol against a global collection of carbapenem-resistant Pseudomonas aeruginosa with a high level of carbapenemase diversity. J Antimicrob Chemother 2024; 79:412-6. [CrossRef]
- Santerre Henriksen A, Jeannot K, Oliver A, Perry JD, Pletz MW, Stefani S, et al. In vitro activity of cefiderocol against European Pseudomonas aeruginosa and Acinetobacter spp., including isolates resistant to meropenem and recent β-lactam/β-lactamase inhibitor combinations. Microbiol Spectr 2024; 12:e0383623. [CrossRef]
- Maruri-Aransolo A, López-Causapé C, Hernández-García M, García-Castillo M, Caballero-Pérez JD, Oliver A, et al. In vitro activity of cefiderocol in Pseudomonas aeruginosa isolates from people with cystic fibrosis recovered during three multicentre studies in Spain. J Antimicrob Chemother 2024; 79:1432-40. [CrossRef]
- Valzano F, La Bella G, Lopizzo T, Curci A, Lupo L, Morelli E, et al. Resistance to ceftazidime-avibactam and other new β-lactams in Pseudomonas aeruginosa clinical isolates: a multi-center surveillance study. Microbiol Spectr 2024: e0426623. [CrossRef]
- Huang YS, Chuang YC, Chen PY, Chou PC, Wang JT. In vitro activity of cefiderocol and comparator antibiotics against multidrug-resistant non-fermenting Gram-negative bacilli. JAC Antimicrob Resist 2024; 6:dlae006. [CrossRef]
- Tunney MM, Elborn JS, McLaughlin CS, Longshaw CM. In vitro activity of cefiderocol against Gram-negative pathogens isolated from people with cystic fibrosis and bronchiectasis. J Glob Antimicrob Resist 2024; 36:407-10. [CrossRef]
- Bianco G, Boattini M, Comini S, Iannaccone M, Casale R, Allizond V, et al. Activity of ceftolozane-tazobactam, ceftazidime-avibactam, meropenem-vaborbactam, cefiderocol and comparators against Gram-negative organisms causing bloodstream infections in Northern Italy (2019-2021): emergence of complex resistance phenotypes. J Chemother 2022; 34:302-10. [CrossRef]
- Méndez-Sotelo BJ, Delgado-Beltrán M, Hernández-Durán M, Colín-Castro CA, Esquivel-Bautista J, Ortega-Oliva SA, et al. In vitro activity of ceftazidime/avibactam, cefiderocol, meropenem/vaborbactam and imipenem/relebactam against clinical strains of the Stenotrophomonas maltophilia complex. PLoS One 2024; 19:e0298577. [CrossRef]
- Takemura M, Nakamura R, Ota M, Nakai R, Sahm DF, Hackel MA, et al. In vitro and in vivo activity of cefiderocol against Achromobacter spp. and Burkholderia cepacia complex, including carbapenem-non-susceptible isolates. Antimicrob Agents Chemother 2023; 67:e0034623. [CrossRef]
- Jean-Pierre V, Sorlin P, Pantel A, Chiron R, Lavigne JP, Jeannot K, et al. Cefiderocol susceptibility of Achromobacter spp.: study of an accurately identified collection of 230 strains. Ann Clin Microbiol Antimicrob 2024; 23:54. [CrossRef]
- Jena J, Behera B, Nayak G, Mohanty S, Mahapatra A, Purushotham P, et al. In Vitro Susceptibility of Burkholderia pseudomallei Isolates to Cefiderocol and Ceftazidime/Avibactam from Odisha, India. J Lab Physicians 2023; 15:573-7. [CrossRef]
- Findlay J, Raro OHF, Poirel L, Nordmann P. Molecular analysis of metallo-beta-lactamase-producing Pseudomonas aeruginosa in Switzerland 2022-2023. Eur J Clin Microbiol Infect Dis 2024; 43:551-7. [CrossRef]
- Uskudar-Guclu A, Danyildiz S, Mirza HC, Akcil Ok M, Basustaoglu A. In vitro activity of cefiderocol against carbapenem-resistant Acinetobacter baumannii carrying various β-lactamase encoding genes. Eur J Clin Microbiol Infect Dis 2024; 43:1171-9. [CrossRef]
- Bulens SN, Campbell D, McKay SL, Vlachos N, Burgin A, Burroughs M, et al. Carbapenem-resistant Acinetobacter baumannii complex in the United States-An epidemiological and molecular description of isolates collected through the Emerging Infections Program, 2019. Am J Infect Control 2024: S0196-6553(24)00458-9. [CrossRef]
- Kayama S, Kawakami S, Kondo K, Kitamura N, Yu L, Hayashi W, et al. In vitro activity of cefiderocol against carbapenemase-producing and meropenem-non-susceptible Gram-negative bacteria collected in the Japan Antimicrobial Resistant Bacterial Surveillance. J Glob Antimicrob Resist 2024; 38:12-20. [CrossRef]
- Dahdouh E, Gómez-Marcos L, Cañada-García JE, de Arellano ER, Sánchez-García A, Sánchez-Romero I, et al. Characterizing carbapenemase-producing Escherichia coli isolates from Spain: high genetic heterogeneity and wide geographical spread. Front Cell Infect Microbiol 2024; 14:1390966. [CrossRef]
- to A, Sato T, Ota M, Takemura M, Nishikawa T, Toba S, Kohira N, Miyagawa S, Ishibashi N, Matsumoto S, Nakamura R, Tsuji M, Yamano Y. In Vitro Antibacterial Properties of Cefiderocol, a Novel Siderophore Cephalosporin, against Gram-Negative Bacteria. Antimicrob Agents Chemother. 2017; 62(1):e01454-17. [CrossRef]
- Luscher A, Moynié L, Auguste PS, Bumann D, Mazza L, Pletzer D, Naismith JH, Köhler T. TonB-Dependent Receptor Repertoire of Pseudomonas aeruginosa for Uptake of Siderophore-Drug Conjugates. Antimicrob Agents Chemother. 2018 ;62(6):e00097-18. [CrossRef]
- Gomis-Font MA, Clari MA, López-Causapé C, Navarro D, Oliver A. Emergence of cefiderocol resistance during ceftazidime/avibactam treatment caused by a large genomic deletion, including ampD and piuCD genes, in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2024; 68(1):e0119223. [CrossRef]
- Streling, A.P.; Al Obaidi, M.M.; Lainhart,W.D.; Zangeneh, T.; Khan, A.; Dinh, A.Q.; Hanson, B.; A Arias, C.; Miller,W.R. Evolution of Cefiderocol Non-Susceptibility in Pseudomonas aeruginosa in a Patient Without Previous Exposure to the Antibiotic. Clin. Infect. Dis. 2021, 73, 4472–4474. [CrossRef]
- Malik S, Kaminski M, Landman D, Quale J. Cefiderocol Resistance in Acinetobacter baumannii: Roles of β-Lactamases, Siderophore Receptors, and Penicillin Binding Protein 3. Antimicrob Agents Chemother. 2020; 64(11):e01221-20. [CrossRef]
- Yamano, Y.; Ishibashi, N.; Kuroiwa, M.; Takemura, M.; Sheng, W.-H.; Hsueh, P.-R. Characterisation of cefiderocol-non-susceptible Acinetobacter baumannii isolates from Taiwan. J. Glob. Antimicrob. Resist. 2021, 28, 120–124.
- Findlay J, Bianco G, Boattini M, Nordmann P. In vivo development of cefiderocol resistance in carbapenem-resistant Acinetobacter baumannii associated with the downregulation of a TonB-dependent siderophore receptor, PiuA. J Antimicrob Chemother. 2024; 79(4):928-930. [CrossRef]
- Huang E, Thompson RN, Moon SH, Keck JM, Lowry MS, Melero J, Jun S-R, Rosenbaum ER, Dare RK. Treatment-emergent cefiderocol resistance in carbapenem-resistant Acinetobacter baumannii is associated with insertion sequence ISAba36 in the siderophore receptor pirA. Antimicrob Agents Chemother. 2024: e0029024. [CrossRef]
- Nurjadi, D.; Kocer, K.; Chanthalangsy, Q.; Klein, S.; Heeg, K.; Boutin, S. New Delhi Metallo-Beta-Lactamase Facilitates the Emergence of Cefiderocol Resistance in Enterobacter cloacae. Antimicrob. Agents Chemother. 2022, 66, 2.
- McElheny, C.L.; Fowler, E.L.; Iovleva, A.; Shields, R.K.; Doi, Y. In Vitro Evolution of Cefiderocol Resistance in an NDM-Producing Klebsiella pneumoniae Due to Functional Loss of CirA. Microbiol. Spectr. 2021, 9, e0177921.
- Price, T.K.; Davar, K.; Contreras, D.;Ward, K.W.; Garner, O.B.; Simner, P.J.; Yang, S.; Chandrasekaran, S. Case Report and Genomic Analysis of Cefiderocol-Resistant Escherichia coli Clinical Isolates. Am. J. Clin. Pathol. 2021, 157, 257–265.
- Lan, P.; Lu, Y.; Chen, Z.;Wu, X.; Hua, X.; Jiang, Y.; Zhou, J.; Yu, Y. Emergence of High-Level Cefiderocol Resistance in Carbapenem-Resistant Klebsiella pneumoniae from Bloodstream Infections in Patients with Hematologic Malignancies in China. Microbiol. Spectr. 2022, 10, e0008422.
- Tascini C, Coppi M, Antonelli A, Niccolai C, Bartolini A, Pecori D, Sartor A, Giani T, Rossolini GM. In vivo evolution to high-level cefiderocol resistance of NDM-1-producing Klebsiella pneumoniae, followed by intra-hospital cross-transmission. Clin Microbiol Infect. 2024; 30(3):398-400. [CrossRef]
- Barker KR, Rebick GW, Fakharuddin K, MacDonald C, Mulvey MR, Mataseje LF. When the Trojan horse is unable to reach inside the city: investigation of the mechanism of resistance behind the first reported cefiderocol-resistant E. coli in Canada. Microbiol Spectr. 2024; 12(5):e0322323. [CrossRef]
- Arcari G, Cecilia F, Oliva A, Polani R, Raponi G, Sacco F, De Francesco A, Pugliese F, Carattoli A. Genotypic Evolution of Klebsiella pneumoniae Sequence Type 512 during Ceftazidime/Avibactam, Meropenem/Vaborbactam, and Cefiderocol Treatment, Italy. Emerg Infect Dis. 2023; 29(11):2266-2274. [CrossRef]
- Yamano, Y., Rio Nakamura, Miki Takemura, Roger Echols, Potential Mechanisms of Cefiderocol MIC Increase in Enterobacterales in In Vitro Resistance Acquisition Studies Open Forum Infectious Diseases Oxford Academic. 2020; 1455: URL https:// academic.oup.com/ofid/article/7/Supplement_1/S730/6057740.
- Padovani M, Bertelli A, Corbellini S, Piccinelli G, Gurrieri F, De Francesco MA. In Vitro Activity of Cefiderocol on Multiresistant Bacterial Strains and Genomic Analysis of Two Cefiderocol Resistant Strains. Antibiotics (Basel). 2023; 12:785. [CrossRef]
- Bao, J.; Xie, L.; Ma, Y.; An, R.; Gu, B.; Wang, C. Proteomic and Transcriptomic Analyses Indicate Reduced Biofilm-Forming Abilities in Cefiderocol-Resistant Klebsiella pneumoniae. Front. Microbiol. 2022, 12, 778190.
- Sato, T.; Ito, A.; Ishioka, Y.; Matsumoto, S.; Rokushima, M.; Kazmierczak, K.M.; Hackel, M.; Sahm, D.F.; Yamano, Y. Escherichia coli strains possessing a four amino acid YRIN insertion in PBP3 identified as part of the SIDERO-WT-2014 surveillance study. JAC-Antimicrob. Resist. 2020, 2, dlaa081.
- Zhang, Q.; Neidig, N.; Chu, T.-Y.; Divoky, C.; Carpenter, J.; Lee-Hsiao, C.; Threatt, H.; Sultana, R.; Bush, K. In vitro antibacterial activity of cefiderocol against recent multidrug-resistant carbapenem-nonsusceptible Enterobacterales isolates. Diagn. Microbiol. Infect. Dis. 2022, 103, 115651.
- Gill, C.M.; Abdelraouf, K.; Oota, M.; Nakamura, R.; Kuroiwa, M.; Gahara, Y.; Takemura, M.; Yamano, Y.; Nicolau, D.P. Discrepancy in sustained efficacy and resistance emergence under human-simulated exposure of cefiderocol against Stenotrophomonas maltophilia between in vitro chemostat and in vivo murine infection models. J. Antimicrob. Chemother. 2021, 76, 2615–2621.
- Werth, B.J.; Ashford, N.K.; Penewit, K.; Waalkes, A.; Holmes, E.A.; Bryan, A.; Salipante, S.J. Evolution of cefiderocol resistance in Stenotrophomonas maltophilia using in vitro serial passage techniques. JAC-Antimicrob. Resist. 2022, 4, dlac011.
- Bianco G, Gaibani P, Comini S, Boattini M, Banche G, Costa C, Cavallo R, Nordmann P. Synergistic Effect of Clinically Available Beta-Lactamase Inhibitors Combined with Cefiderocol against Carbapenemase-Producing Gram-Negative Organisms. Antibiotics (Basel). 2022; 11:1681. [CrossRef]
- Kohira, N.; Hackel, M.A.; Ishioka, Y.; Kuroiwa, M.; Sahm, D.F.; Sato, T.; Maki, H.; Yamano, Y. Reduced susceptibility mechanism to cefiderocol, a siderophore cephalosporin, among clinical isolates from a global surveillance programme (SIDERO-WT-2014). J. Glob. Antimicrob. Resist. 2020, 22, 738–41.
- Poirel, L.; Sadek, M.; Nordmann, P. Contribution of PER-Type and NDM-Type _-Lactamases to Cefiderocol Resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, e0087721.
- Poirel, L.; de la Rosa, J.-M.O.; Sadek, M.; Nordmann, P. Impact of Acquired Broad-Spectrum _-Lactamases on Susceptibility to Cefiderocol and Newly Developed _-Lactam/_-Lactamase Inhibitor Combinations in Escherichia coli and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2022, 66, e0003922.
- Fröhlich C, Sørum V, Tokuriki N, Johnsen PJ, Samuelsen Ø. Evolution of β-lactamase-mediated cefiderocol resistance. J Antimicrob Chemother. 2022; 77:2429-36. [CrossRef]
- Mezcord V, Traglia GM, Pasteran F, Escalante J, Lopez C, Wong O, Rojas L, Marshall SH, Tolmasky ME, Bonomo RA, Ramirez MS. Characterisation of cefiderocol-resistant spontaneous mutant variants of Klebsiella pneumoniae-producing NDM-5 with a single mutation in cirA. Int J Antimicrob Agents. 2024; 63:107131. [CrossRef]
- Simner, P.J.; Mostafa, H.H.; Bergman, Y.; Ante, M.; Tekle, T.; Adebayo, A.; Beisken, S.; Dzintars, K.; Tamma, P.D. Progressive Development of Cefiderocol Resistance in Escherichia coli during Therapy is Associated With an Increase in blaNDM-5 Copy Number and Gene Expression. Clin. Infect. Dis. 2021, ciab888.
- Bianco G, Boattini M, Comini S, Iannaccone M, Bondi A, Cavallo R, Costa C. In vitro activity of cefiderocol against ceftazidime-avibactam susceptible and resistant KPC-producing Enterobacterales: cross-resistance and synergistic effects. Eur J Clin Microbiol Infect Dis. 2022; 41(1):63-70. [CrossRef]
- Hobson CA, Cointe A, Jacquier H, Choudhury A, Magnan M, Courroux C, Tenaillon O, Bonacorsi S, Birgy A. Cross-resistance to cefiderocol and ceftazidime-avibactam in KPC β-lactamase mutants and the inoculum effect. Clin Microbiol Infect. 2021 Aug;27(8):1172.e7-1172.e10. [CrossRef]
- Poirel L, Sadek M, Kusaksizoglu A, Nordmann P. Co-resistance to ceftazidime-avibactam and cefiderocol in clinical isolates producing KPC variants. Eur J Clin Microbiol Infect Dis. 2022; 41:677-80. [CrossRef]
- Gaibani P, Amadesi S, Lazzarotto T, Ambretti S. Genome characterization of a Klebsiella pneumoniae co-producing OXA-181 and KPC-121 resistant to ceftazidime/avibactam, meropenem/vaborbactam, imipenem/relebactam and cefiderocol isolated from a critically ill patient. J Glob Antimicrob Resist. 2022; 30:262-4. [CrossRef]
- Di Pilato V, Codda G, Niccolai C, Willison E, Wong JLC, Coppo E, Frankel G, Marchese A, Rossolini GM. Functional features of KPC-109, a novel 270-loop KPC-3 mutant mediating resistance to avibactam-based β-lactamase inhibitor combinations and cefiderocol. Int J Antimicrob Agents. 2024; 63:107030. [CrossRef]
- Amadesi S, Bianco G, Secci B, Fasciana T, Boattini M, Costa C, Gaibani P. Complete Genome Sequence of a Klebsiella pneumoniae Strain Carrying Novel Variant blaKPC-203, Cross-Resistant to Ceftazidime/Avibactam and Cefiderocol, but Susceptible to Carbapenems, Isolated in Italy, 2023. Pathogens. 2024; 13:507. [CrossRef]
- Giufrè M, Errico G, Del Grosso M, Pagnotta M, Palazzotti B, Ballardini M, Pantosti A, Meledandri M, Monaco M. Detection of KPC-216, a Novel KPC-3 Variant, in a Clinical Isolate of Klebsiella pneumoniae ST101 Co-Resistant to Ceftazidime-Avibactam and Cefiderocol. Antibiotics (Basel). 2024; 13:507. [CrossRef]
- Jacob AS, Chong GL, Lagrou K, Depypere M, Desmet S. No in vitro activity of cefiderocol against OXA-427-producing Enterobacterales. J Antimicrob Chemother. 2021; 76:3317-8. [CrossRef]
- Shields RK, Iovleva A, Kline EG, Kawai A, McElheny CL, Doi Y. Clinical Evolution of AmpC-Mediated Ceftazidime-Avibactam and Cefiderocol Resistance in Enterobacter cloacae Complex Following Exposure to Cefepime. Clin Infect Dis. 2020; 71:2713-6. [CrossRef]
- Kawai A, Shropshire WC, Suzuki M, Borjan J, Aitken SL, Bachman WC, McElheny CL, Bhatti MM, Shields RK, Shelburne SA, Doi Y. Structural insights into the molecular mechanism of high-level ceftazidime-avibactam resistance conferred by CMY-185. mBio. 2024; 15:e0287423. [CrossRef]
- Amadesi S, Gatti M, Rinaldi M, Pea F, Viale P, Gaibani P. Novel CMY-186 variant conferring cross-resistance to cefiderocol and ceftazidime/avibactam in Klebsiella pneumoniae from a critically ill patient during cefiderocol and ceftazidime/avibactam treatment. Int J Antimicrob Agents. 2024; 63(4):107107. [CrossRef]
- Simner, P.J.; Beisken, S.; Bergman, Y.; E Posch, A.; E Cosgrove, S.; Tamma, P.D. Cefiderocol Activity Against Clinical Pseudomonas aeruginosa Isolates Exhibiting Ceftolozane-Tazobactam Resistance. Open Forum Infect. Dis. 2021, 8, ofab311.
- Ballesté-Delpierre, C.; Ramírez, Á.; Muñoz, L.; Longshaw, C.; Roca, I.; Vila, J. Assessment of In Vitro Cefiderocol Susceptibility and Comparators against an Epidemiologically Diverse Collection of Acinetobacter baumannii Clinical Isolates. Antibiotics 2022, 11, 187.
- Liu C, Yi J, Lu M, Yang P, Du C, Jiang F, Du P, Shen N. Dynamic within-host cefiderocol heteroresistance caused by blaSHV-12 amplification in pandrug-resistant and hypervirulent Klebsiella pneumoniae sequence type 11. Drug Resist Updat. 2024; 73:101038. [CrossRef]
- Nordmann, P.; Shields, R.K.; Doi, Y.; Takemura, M.; Echols, R.; Matsunaga, Y.; Yamano, Y. Mechanisms of Reduced Susceptibility to Cefiderocol Among Isolates from the CREDIBLE-CR and APEKS-NP Clinical Trials. Microb. Drug Resist. 2022, 28, 398–407.
- Rolston KVI, Gerges B, Shelburne S, Aitken SL, Raad I, Prince RA. Activity of Cefiderocol and Comparators against Isolates from Cancer Patients. Antimicrob Agents Chemother. 2020; 64:e01955-19. [CrossRef]
- Simner, P.J.; Beisken, S.; Bergman, Y.; Ante, M.; Posch, A.E.; Tamma, P.D. Defining Baseline Mechanisms of Cefiderocol Resistancein the Enterobacterales. Microb. Drug Resist. 2022, 28, 161–170.
- Ito, A.; Nishikawa, T.; Ishii, R.; Kuroiwa, M.; Ishioka, Y.; Kurihara, N.; Sakikawa, I.; Ota, T.; Rokushima, M.; Tsuji, M.; et al. 696. Mechanism of Cefiderocol high MIC mutants obtained in non-clinical FoR studies. Open Forum Infect. Dis. 2018, 5, S251.
- Magallon, A.; Amoureux, L.; Garrigos, T.; Sonois, M.; Varin, V.; Neuwirth, C.; Bador, J. Role of AxyABM overexpression in acquired resistance in Achromobacter xylosoxidans. J. Antimicrob. Chemother. 2022, 77, 926–929.
- Bilal M, El Tabei L, Büsker S, Krauss C, Fuhr U, Taubert M. Clinical Pharmacokinetics and Pharmacodynamics of Cefiderocol. Clin Pharmacokinet. 2021; 60(12):1495-1508. [CrossRef] [PubMed] [PubMed Central]
- Katsube T, Echols R, Wajima T. Pharmacokinetic and Pharmacodynamic Profiles of Cefiderocol, a Novel Siderophore Cephalosporin. Clin Infect Dis. 2019; 69(Suppl 7):S552-S558. [CrossRef] [PubMed] [PubMed Central]
- Chen IH, Kidd JM, Abdelraouf K, Nicolau DP. Comparative <i>In Vivo</i> Antibacterial Activity of Human-Simulated Exposures of Cefiderocol and Ceftazidime against <i>Stenotrophomonas maltophilia</i> in the Murine Thigh Model. Antimicrob Agents Chemother. 2019; 63(12):e01558-19. [CrossRef] [PubMed] [PubMed Central]
- Monogue ML, Tsuji M, Yamano Y, Echols R, Nicolau DP. Efficacy of Humanized Exposures of Cefiderocol (S-649266) against a Diverse Population of Gram-Negative Bacteria in a Murine Thigh Infection Model. Antimicrob Agents Chemother. 2017; 61(11):e01022-17. [CrossRef] [PubMed] [PubMed Central]
- Ghazi IM, Monogue ML, Tsuji M, Nicolau DP. Pharmacodynamics of cefiderocol, a novel siderophore cephalosporin, in a Pseudomonas aeruginosa neutropenic murine thigh model. Int J Antimicrob Agents. 2018; 51(2):206-212. [CrossRef] [PubMed]
- Stainton SM, Monogue ML, Tsuji M, Yamano Y, Echols R, Nicolau DP. Efficacy of Humanized Cefiderocol Exposures over 72 Hours against a Diverse Group of Gram-Negative Isolates in the Neutropenic Murine Thigh Infection Model. Antimicrob Agents Chemother. 2019; 63(2):e01040-18. [CrossRef] [PubMed] [PubMed Central]
- Nakamura R, Ito-Horiyama T, Takemura M, Toba S, Matsumoto S, Ikehara T, Tsuji M, Sato T, Yamano Y. <i>In Vivo</i> Pharmacodynamic Study of Cefiderocol, a Novel Parenteral Siderophore Cephalosporin, in Murine Thigh and Lung Infection Models. Antimicrob Agents Chemother. 2019; 63(9):e02031-18. [CrossRef] [PubMed] [PubMed Central]
- Sumi CD, Heffernan AJ, Lipman J, Roberts JA, Sime FB. What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review. Clin Pharmacokinet. 2019; 58(11):1407-1443. [CrossRef] [PubMed]
- Gatti M, Cojutti PG, Pea F. Impact of attaining aggressive vs. conservative PK/PD target on the clinical efficacy of beta-lactams for the treatment of Gram-negative infections in the critically ill patients: a systematic review and meta-analysis. Crit Care. 2024; 28(1):123. [CrossRef] [PubMed] [PubMed Central]
- Gatti M, Bartoletti M, Cojutti PG, Gaibani P, Conti M, Giannella M, Viale P, Pea F. A descriptive case series of pharmacokinetic/pharmacodynamic target attainment and microbiological outcome in critically ill patients with documented severe extensively drug-resistant Acinetobacter baumannii bloodstream infection and/or ventilator-associated pneumonia treated with cefiderocol. J Glob Antimicrob Resist. 2021; 27:294-298. [CrossRef] [PubMed]
- Mornese Pinna S, Corcione S, De Nicolò A, Montrucchio G, Scabini S, Vita D, De Benedetto I, Lupia T, Mula J, Di Perri G, D'Avolio A, De Rosa FG. Pharmacokinetic of Cefiderocol in Critically Ill Patients Receiving Renal Replacement Therapy: A Case Series. Antibiotics (Basel). 2022; 11(12):1830. [CrossRef] [PubMed] [PubMed Central]
- Gatti M, Giannella M, Rinaldi M, Gaibani P, Viale P, Pea F. Pharmacokinetic/Pharmacodynamic Analysis of Continuous-Infusion Fosfomycin in Combination with Extended-Infusion Cefiderocol or Continuous-Infusion Ceftazidime-Avibactam in a Case Series of Difficult-to-Treat Resistant <i>Pseudomonas aeruginosa</i> Bloodstream Infections and/or Hospital-Acquired Pneumonia. Antibiotics (Basel). 2022; 11(12):1739. [CrossRef] [PubMed] [PubMed Central]
- Gatti M, Rinaldi M, Tonetti T, Gaibani P, Siniscalchi A, Viale P, Pea F. Pharmacokinetics/pharmacodynamics of cefiderocol administered by continuous infusion in a case series of critically ill patients with carbapenem-resistant Acinetobacter baumannii infections undergoing continuous venovenous haemodiafiltration (CVVHDF). Int J Antimicrob Agents. 2023; 62(2):106852. [CrossRef] [PubMed]
- Pea F, Viale P. The antimicrobial therapy puzzle: could pharmacokinetic- pharmacodynamic relationships be helpful in addressing the issue of appropriate pneumonia treatment in critically ill patients? Clin Infect Dis. 2006; 42(12):1764-71. [CrossRef] [PubMed]
- Katsube T, Nicolau DP, Rodvold KA, Wunderink RG, Echols R, Matsunaga Y, Menon A, Portsmouth S, Wajima T. Intrapulmonary pharmacokinetic profile of cefiderocol in mechanically ventilated patients with pneumonia. J Antimicrob Chemother. 2021; 76(11):2902-2905. doi: 10.1093/jac/dkab280. Erratum in: J Antimicrob Chemother. 2021 ;76(11):3069. [CrossRef] [PubMed] [PubMed Central]
- Kawaguchi N, Katsube T, Echols R, Wajima T, Nicolau DP. Intrapulmonary Pharmacokinetic Modeling and Simulation of Cefiderocol, a Parenteral Siderophore Cephalosporin, in Patients With Pneumonia and Healthy Subjects. J Clin Pharmacol. 2022; 62(5):670-680. [CrossRef] [PubMed] [PubMed Central]
- From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO); Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, Eesa M, Fischer U, Hausegger K, Hirsch JA, Shazam Hussain M, Jansen O, Jayaraman MV, Khalessi AA, Kluck BW, Lavine S, Meyers PM, Ramee S, Rüfenacht DA, Schirmer CM, Vorwerk D. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int J Stroke. 2018; 13(6):612-632. [CrossRef] [PubMed]
- Möhlmann JE, van Luin M, Uijtendaal EV, Zahr N, Sikma MA. Continuous infusion of cefiderocol in a critically ill patient with continuous venovenous haemofiltration. Br J Clin Pharmacol. 2023; 89(12):3753-3757. [CrossRef] [PubMed]
- Gatti M, Pea F. Jumping into the future: overcoming pharmacokinetic/pharmacodynamic hurdles to optimize the treatment of severe difficult to treat-Gram-negative infections with novel beta-lactams. Expert Rev Anti Infect Ther. 2023; 21(2):149-166. [CrossRef] [PubMed]
- 117 Naseer S, Weinstein EA, Rubin DB, Suvarna K, Wei X, Higgins K, Goodwin A, Jang SH, Iarikov D, Farley J, Nambiar S. US Food and Drug Administration (FDA): Benefit-Risk Considerations for Cefiderocol (Fetroja®). Clin Infect Dis. 2021; 72(12):e1103-e1111. [CrossRef] [PubMed]
- Bassetti M, Echols R, Matsunaga Y et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen focused, descriptive, phase 3 trial. Lancet Infect Dis 2021; 21: 226–40. [CrossRef]
- Portsmouth S., van Veenhuyzen D., Echols R., et al 2017. Cefiderocol compared with imipenem/cirastatin in the treatment of adults with complicated urinary tract infections with or without pyelonephritis or acute uncomplicated pyelonephritis: results from a multicenter, double-blind, randomized study, abstr OS0250D. Abstr 27th Eur Congr Clin Microbiol Infect Dis.
- EMA, 2020. Fetcroja. European Medicines Agency. URL https://www.ema.europa.eu/en/medicines/human/EPAR/fetcroja.
- World Health Organization Regional Office for Europe, European Centre for Disease Prevention and Control. 2022. Antimicrobial resistance surveillance Europe. https://www.ecdc.europa.eu/sites/default/files/documents/Joint-WHO-ECDC-AMR-report-2022.pdf.
- Satlin MJ, Simner PJ, Slover CM, et al Cefiderocol Treatment for Patients with Multidrug- and Carbapenem-Resistant Pseudomonas aeruginosa Infections in the Compassionate Use Program. Antimicrob Agents Chemother. 2023; 67(7):e0019423. [CrossRef] [PubMed]
- de la Fuente C, Rodríguez M, Merino N, et al. Real-life use of cefiderocol for salvage therapy of severe infections due to carbapenem-resistant Gram-negative bacteria, International Journal of Antimicrobial Agents, 2023; 62(1): 106818, ISSN 0924-8579. [CrossRef]
- Gavaghan V, Miller JL, Dela-Pena J. Case series of cefiderocol for salvage therapy in carbapenem-resistant Gram-negative infections. Infection. 2023; 51(2):475-482. [CrossRef] [PubMed] [PubMed Central]
- Vacheron, CH., Kaas, A., Rasigade, JP. et al. Correction: Cefiderocol in Difficult-to-Treat Nf-GNB in ICU Settings. Ann. Intensive Care 2024; 14, 81 . [CrossRef]
- Ramirez P et al. Real-world effectiveness and safety of cefiderocol in patients with Gram-negative bacterial infections in the early access programme in Spain: results of the PERSEUS study. Abstract. ECCMID 2024.
- Paez JG, Costa S. Risk factors associated with mortality of infections caused by Stenotrophomonas maltophilia: a systematic review. J Hosp Infect. 2008; 70(2):101–108. [CrossRef]
- Cai B, Nguyen ST, Copeland JD, Song HJ, Slover CM. 2751. Cefiderocol Use in Treating Patients with Confirmed Stenotrophomonas maltophilia Infections in US Hospitals During January 2020 - June 2022. Open Forum Infect Dis. 2023; 10(Suppl 2):ofad500.2362. [CrossRef]
- Timsit JF, Paul M, Shields RK, et al. Cefiderocol for the Treatment of Infections Due to Metallo-B-lactamase-Producing Pathogens in the CREDIBLE-CR and APEKS-NP Phase 3 Randomized Studies. Clin Infect Dis. 2022; 75(6):1081-1084. [CrossRef]
- Falcone M, Tiseo G. Cefiderocol for the Treatment of Metallo-β-Lactamases Producing Gram-Negative Bacilli: Lights and Shadows From the Literature. Clin Infect Dis. 2022; 75(6):1085-1087. [CrossRef]
- Isler, B., Vatansever, C., Özer, B., et al Higher rates of cefiderocol resistance among NDM producing Klebsiella bloodstream isolates applying EUCAST over CLSI breakpoints. Infectious Diseases, 2023; 55(9), 607–613. [CrossRef]
- Bavaro, D.F.; Belati, A.; Diella, L.; et al. Cefiderocol- Based Combination Therapy for “Difficult-to-Treat” Gram-Negative Severe Infections: Real-Life Case Series and Future Perspectives. Antibiotics 2021, 10, 652. [CrossRef]
- Marco Falcone, Giusy Tiseo, Manuela Nicastro, et al Cefiderocol as Rescue Therapy for Acinetobacter baumannii and Other Carbapenem-resistant Gram-negative Infections in Intensive Care Unit Patients, Clinical Infectious Diseases, 2021; 72(11): 2021–2024. [CrossRef]
- Bavaro, D.F., Papagni, R., Belati, A. et al. Cefiderocol Versus Colistin for the Treatment of Carbapenem-Resistant Acinetobacter baumannii Complex Bloodstream Infections: A Retrospective, Propensity-Score Adjusted, Monocentric Cohort Study. Infect Dis Ther 2023; 12, 2147–2163 . [CrossRef]
- Dalfino, L.; Stufano, M.; Bavaro, D.F.; et al. Effectiveness of First-Line Therapy with Old and Novel Antibiotics in Ventilator-Associated Pneumonia Caused by Carbapenem-Resistant Acinetobacter baumannii: A Real Life, Prospective, Observational, Single-Center Study. Antibiotics 2023, 12, 1048. [CrossRef]
- Emanuele Rando, Salvatore Lucio Cutuli, Flavio Sangiorgi, et al, Cefiderocol-containing regimens for the treatment of carbapenem-resistant A. baumannii ventilator-associated pneumonia: a propensity-weighted cohort study, JAC-Antimicrobial Resistance, 2023; 5(4) dlad085. [CrossRef]
- Russo A, Bruni A, Gullì S, Bet al. Efficacy of cefiderocol- vs colistin-containing regimen for treatment of bacteraemic ventilator-associated pneumonia caused by carbapenem-resistant Acinetobacter baumannii in patients with COVID-19. Int J Antimicrob Agents. 2023; 62(1):106825. [CrossRef]
- Oliva A, Liguori L, Covino S, et al. Clinical effectiveness of cefiderocol for the treatment of bloodstream infections due to carbapenem-resistant Acinetobacter baumannii during the COVID-19 era: a single center, observational study. Eur J Clin Microbiol Infect Dis. 2024; 43(6):1149-1160. [CrossRef]
- Renato Pascale, Zeno Pasquini, Michele Bartoletti, et al. Cefiderocol treatment for carbapenem-resistant Acinetobacter baumannii infection in the ICU during the COVID-19 pandemic: a multicentre cohort study, JAC-Antimicrobial Resistance, 2021; 3(4): dlab174. [CrossRef]
- Maddalena Giannella, Stefano Verardi, Andreas Karas, et al , Carbapenem-Resistant Acinetobacter spp Infection in Critically Ill Patients With Limited Treatment Options: A Descriptive Study of Cefiderocol Therapy During the COVID-19 Pandemic, Open Forum Infectious Diseases, 2023; 10(7): ofad329. [CrossRef]
- Pranita D. Tamma, Emily L. Heil, Julie Ann Justo et al Infectious Diseases Society of America Antimicrobial-Resistant Treatment Guidance: Gram-Negative Bacterial Infections. Infectious Diseases Society of America 2024; Version 4.0. Available at https://www.idsociety.org/practice-guideline/amr-guidance/].
- Marino, A.; Stracquadanio, S.; Campanella, E.; et al Intravenous Fosfomycin: A Potential Good Partner for Cefiderocol. Clinical Experience and Considerations. Antibiotics 2023, 12,49. [CrossRef]
- Paranos P, Vourli S, Pournaras S, Meletiadis J. Assessing Clinical Potential of Old Antibiotics against Severe Infections by Multi-Drug-Resistant Gram-Negative Bacteria Using In Silico Modelling. Pharmaceuticals (Basel). 2022; 15(12):1501. [CrossRef] [PubMed] [PubMed Central]
- Simner PJ, Beisken S, Bergman Y, Ante M, Posch AE, Tamma PD. Defining Baseline Mechanisms of Cefiderocol Resistance in the Enterobacterales. Microb Drug Resist. 2022; 28(2):161-170. [CrossRef] [PubMed] [PubMed Central]
- Mezcord, V.; Escalante, J.; Nishimura, B.; et al. Induced Heteroresistance inCarbapenem-Resistant Acinetobacter baumannii (CRAB) via Exposure to Human Pleural Fluid (HPF) and Its Impact on Cefiderocol Susceptibility. Int. J. Mol. Sci. 2023, 24, 11752. [CrossRef]
- Amadesi S, Amedeo A, Rinaldi M, Palombo M, Giannella M, Gaibani P. In vivo emergence of cefiderocol and ceftazidime/avibactam cross-resistance in KPC- producing Klebsiella pneumoniae following ceftazidime/avibactam -based therapies. Diagn Microbiol Infect Dis. 2024; 110(1):116372. [CrossRef]
- Bovo F, Amadesi S, Palombo M, Lazzarotto T, Ambretti S, Gaibani P. Clonal dissemination of Klebsiella pneumoniae resistant to cefiderocol, ceftazidime/avibactam, meropenem/vaborbactam and imipenem/relebactam co- producing KPC and OXA-181 carbapenemase. JAC Antimicrob Resist. 2023; 5(4):dlad099. [CrossRef]
- Lewis RE, Palombo M, Diani E, Secci B, Gibellini D, Gaibani P. Synergistic Activity of Cefiderocol in Combination with Avibactam, Sulbactam or Tazobactam against Carbapenem-Resistant Gram-Negative Bacteria. Cells. 2024; 13(16):1315. [CrossRef]

| Cefiderocol susceptibility % | Study remarks | Country | Reference | |||||||||||||
| Carbapenemase-producing strains | Carbapenem-non-susceptible strains | Overall | ||||||||||||||
| BURK | BURK | |||||||||||||||
| ACB | PA | EB | ACHR | ACB | PA | EB | ACHR | STEMA | ACB | PA | EB | |||||
| 55.3-59.1 | 77.1-98.2 | 61.2-75.1 | 86.8 | 96.5 | 87.6 | 99.6 | 91.2 | 98.6 | 97 | Meta-analysis | Worldwide | [3] | ||||
| 80.6 | 82.3 | K. pneumoniae | India | [4]* | ||||||||||||
| 100 | 98.4 | 100 | 99.5 | Spain, Portugal | [5]* | |||||||||||
| 93.3 | 97.9 | K. pneumoniae | UAE | [6]* | ||||||||||||
| 86-90 | 90.3 | 95.6 | K. pneumoniae | China | [7]* | |||||||||||
| 84 | 82 | Czech Republic | [8] | |||||||||||||
| 100 | 95.2 | 93.3 | India | [9]* | ||||||||||||
| 93.4 | 100 | 97.2 | India | [10]* | ||||||||||||
| 93.8 | USA | [11]* | ||||||||||||||
| 93.3 | 95.6 | USA | [12]* | |||||||||||||
| 63 | 80.7 | CFP | USA | [13]* | ||||||||||||
| 69-100 | 95 | Worldwide | [14] | |||||||||||||
| 85 | 97.8 | 92.4 | 98.9 | Europe | [15] | |||||||||||
| 70.6 | 91.5 | CFP | Spain | [16] | ||||||||||||
| 95.8 | Italy | [17] | ||||||||||||||
| 100 | 100 | 97.9 | 94.4 | Taiwan | [18]* | |||||||||||
| 70.7 | 87.8 | 86.2 | CFP | UK | [19] | |||||||||||
| 83.4-87.5 | 97.2 | 96 | 100 | Italy | [20] | |||||||||||
| 95.1 | Mexico | [21]* | ||||||||||||||
| 92.9 | 88.5 | 94.8 | 96.7 | Worldwide | [22] | |||||||||||
| 96.3 | 99.1 | CFP | France, Denmark | [23] | ||||||||||||
| 100 | India | [24]* | ||||||||||||||
| 79.5 | MBL | Switzerland | [25] | |||||||||||||
| 59.6 | Turkey | [26] | ||||||||||||||
| 97.9 | 96.9 | USA | [27]* | |||||||||||||
| 91.2 | 100 | 83.3-100 | Japan | [28]* | ||||||||||||
| 92.2 | E. coli | Spain | [29] | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





