1. Introduction
Lithium ions inhibit glycogen synthetase kinase-3β (GSK3β) and other target proteins thus simulating a rise in the levels of endogenous neurotrophic factors and neuroprotective effects of lithium salts. A complex experimental approbation of pharmacological properties of a lithium salt with an organic anion, lithium ascorbate (LiAsc), was previously reported on the pages of this journal [
1]. The neurocytological study indicated direct neuroprotective effects of LiAsc on cultured neurons under glutamate stress. The biodistribution studies in rats indicated accumulation of lithium ions in a sort of "depot" consisting of brain, aorta and femur. Studies of LiAsc (5, 10, 30 mg/kg) performed on a model of alcohol intoxication in rats shown the reduction of ischemical brain damage and the increase in preservation of the myelin sheaths of the neurons. Extremely low acute toxicity (LD50>5000 mg/kg) and even a moderate antitumor effect contribute favorably to the safety profile of LiAsc [
1].
The pharmacological action of the LiAsc is not likely to be restricted only to the above-mentioned effects. For example, chemoreactomic modeling of LiAsc, conducted using modern data mining technologies, allowed us to obtain estimates of the hemodynamic and anti-inflammatory properties of the substance. In particular, LiAsc might be characterized by anti-inflammatory action (modulation of prostaglandin metabolism, lipopolysaccharide-induced TNF-alpha production), demonstrate anticoagulant, antihyperlipidemic and antihyperglycemic effects whereas lithium carbonate (Li
2CO
3) was not predicted to exhibit any of the above. Chemoreactomic analysis indicated that ascorbate-anion in the LiAsc may be much more effective than nicotinate and oxybutyrate anions in the corresponding lithium salts for reducing glucose tolerance (activating the PPAR receptor, a known target protein for antidiabetic drugs) [
2]. The latter effect was, in fact, confirmed in a comparative study of the effects of LiAsc and Li
2CO
3 in the alloxan diabetes model: the use of LiAsc was statistically significantly associated with an increased animal survival and decreased hyperglycemia [
3].
Another study dealing with chemoreactomic modeling indicated possible benign influence of LiAsc on 38 commensal bacteria of human microbiota (including various types of bifido- and lactobacilli) and on the values of minimum inhibitory concentrations (MIC) for 120 pathogenic bacteria. On average over 38 representative of commensals LiAsc slightly more significantly supported the growth of all commensal bacteria (AUC=0.57±0.15) than lithium comenate (AUC=0.47±0.17), lithium nicotinate (AUC=0.45±0.22), lithium oxybutyrate (AUC=0.22±0.17), lithium aspartate (AUC=0.31± 0.14) and lithium orotate (AUC=0.50±0.21). In the case of pathogenic microorganisms, MIC values were significantly lower for LiAsc (4.50±3.69 μg/ml) than for comenate (6.31±5.58 μg/ml), nicotinate (10.98±9.37 μg/ml), oxybutyrate (7.45±4.73 μg/ ml) and aspartate (6.37±4.71 mcg/ml) anions of the lithium salts. The Li
2CO
3 , again, did not show any useful properties in this
in silico study [
4].
Thus, the results of mathematical modeling and of a few of experimental studies indicate a wider range of pharmacological properties of LiAsc than just neuroprotection. In this work we describe the hepatoprotective effects of LiAsc which we tested on a rat model of non-alcoholic fatty liver disease (NAFLD) with multiorgan pathology caused by combined intake of excess saturated fats, carbohydrates and inorganic iron salt.
NAFLD is a widespread disease that occurs as a result of dietary disorders (excess fats and simple carbohydrates, micronutrient deficiencies), toxic load on the liver, etc. A diet with excessive palm oil consumption contributed to the development of histologically confirmed steatohepatosis, characterized by fatty degeneration of hepatocytes localized predominantly in the periportal zone of the liver lobule [
5]. Combined with other factors (for instance, with iron overload due to iatrogenic or genetic causes), NAFLD develops faster and progresses to multiorgan pathology associated with damage to the liver, kidneys, heart, brain and other organs. Iron overload is one of the complications of NAFLD most difficult to treat: tissue iron overload initiates the formation of reactive oxygen species (ROS) and oxidative stress, insulin resistance, and proinflammatory reactions [
6,
7].
It is well known that NAFLD and liver iron overload are aggravated by high fructose consumption [
8]. Excess dietary fructose stimulates rapid accumulation of intrahepatic fat, oxidative stress, mitochondrial dysfunction of hepatocytes [
9] and development of insulin resistance [
10]. It is known that patients with NAFLD and/or obesity consume almost 2 times more soft drinks compared to healthy controls [
11,
12,
13,
14,
15]. Therefore, the use of a fructose-overloaded diet is also an important direction for the development of NAFLD models.
To our knowledge, a NAFLD model in rats with iron overload, induced by a diet rich in palm oil and fructose, was not earlier described. The first objective of this work was to develop a biochemically and histologically confirmed model of NAFLD with iron overload against the background of excessive consumption of palm oil and fructose.
The second objective of the study - to compare the effectiveness of the hepatoprotective properties of LiAsc in comparison with a substance with known hepatoprotective properties - myoinositol (MI). Inositol-dependent proteins are involved in supporting the activity of the cardiovascular system, kidneys, liver, nervous tissue, immunity and sugar metabolism (primarily, the insulin signaling cascade) and pharmacological properties of MI include hepato- and nephroprotection [
16,
17].
3. Discussion
The results of chemoreactomic modeling of hepatoprotection of several lithium salts and of MI indicated, at the very least, a possibility that LiAsc might show some hepatoprotective properties (protection against D-GalN-induced cytotoxicity, D-galactosamine-induced cytotoxicity, modeling in Zucker diabetic rats), that are comparable to a substance with known hepatoprotective properties (MI). At the same time, no such properties were predicted for lithium carbonate nor lithium comenate (data were not shown).
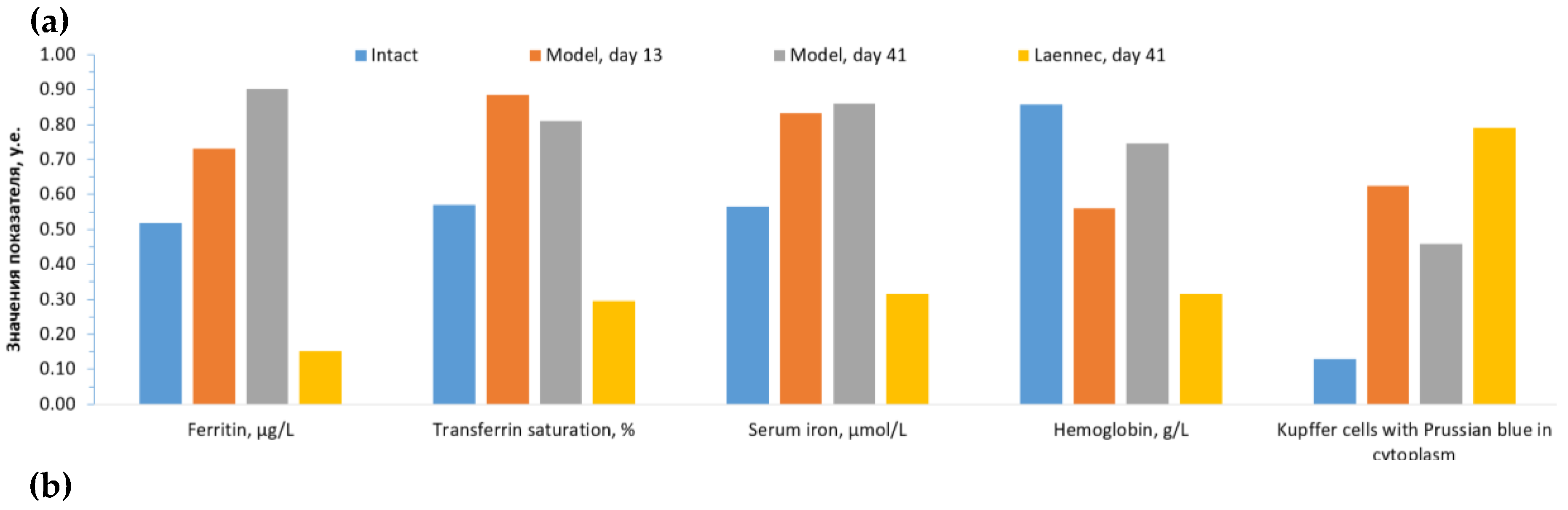
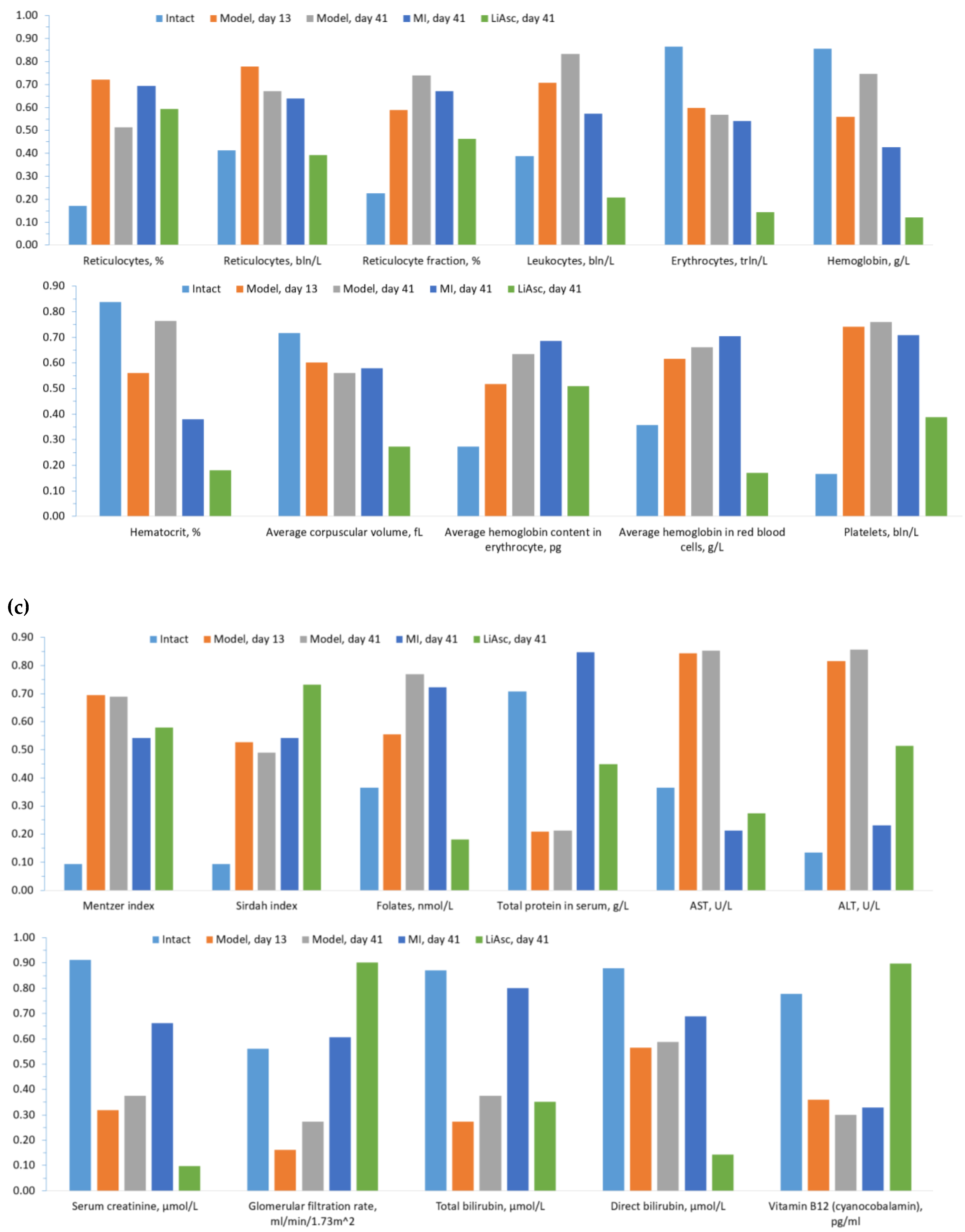
Analysis of the normalized profiles of biochemical parameters allowed us to obtain a more comprehensive picture of the differences in the effects of the studied approaches to iron overload therapy than using any individual parameters (AST/ALT, blood iron levels, etc.). We divided components of these profiles into three segments: iron metabolism parameters, general blood test parameters, and biochemical parameters of multiple organ dysfunction.
Among the indicators of iron metabolism both treatment with LiAsc and MI by day 41 resulted in a significant decrease in ferritin (MI: 173±52 μg/L, LiAsc: 203±4 μg/L), transferrin saturation (MI: 36±9%, LiAsc: 16±8%, all P<0.00005) and of serum iron (MI: 23±5 μmol/L, LiAsc: 13±4). Morphometrically determined number of Kupffer cells stained with Prussian blue was unaffected by MI and significantly reduced by LiAsc (18.8±2.5, P=0.1 when compared with intact group). This allows us to assume that LiAsc is quite efficient in reducing the iron overload of the hepatic tissue. This assumption was, actually, supported by the histological data.
Consideration of changes in the general blood test parameters allowed us to evaluate hematopoietic effects of the reproduction of the NAFLD model and of the pharmacotherapeutic agents applied. One of the main parameters of iron metabolism and hematopoiesis is the level of hemoglobin in the blood. When reproducing the model, an observable decrease in the total number of red blood cells was noted (intact 8.7±0.8 trln cells/l, model day 13 7.4±1.2 trln/l). This may be due to the fact that iron/fat overload stimulates destruction of erythrocytes and/or shortens the red blood cell life cycle.
The acceleration of the erythrocyte life cycle (which implies, in particular, activation of hematopoiesis processes) under conditions of iron overload is also indicated by a sharp increase in the reticulocyte levels during the model reproduction. Recall that reticulocytes are young erythrocytes formed in the bone marrow, precursor cells of erythrocytes. The reticulocyte content in the blood reflects the state of the hematopoietic system (primarily erythropoiesis). All three indicators characterizing various aspects of the reticulocyte content in the blood (% of reticulocytes, reticulocyte content, reticulocyte fraction) were significantly increased during the model reproduction. MI did not have a significant effect on all the three parameters of reticulocytes (P>0.1) but LiAsc stimulated a decrease in total reticulocyte count (291±45 bln/L, P=0.0052) and reticulocyte fraction (51±6%, P=0.0275) towards the normal range on day 41 thus, probably, normalizing hemopoiesis towards the state of intact animals.
Biochemical indicators of multiple organ dysfunction characterized inflammation (white blood cells), thrombus formation (platelets) and the degree of cytolysis of the tissues (AST, ALT etc). One of the main signs of multiple organ dysfunction is an increase in proinflammatory and prothrombotic reactions of the body. In the present study, this corresponded to a significant increase in the levels of leukocytes (inflammation) and platelets (coagulation/aggregation of blood) when reproducing the NAFLD model with iron overload. Therapeutic interventions, on the contrary, contributed to a decrease in both leukocytes and platelets.
The state of the liver function is often characterized by levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total serum protein, bilirubin (total, direct), vitamins B9 (folates) and vitamin B12 (cyanocobalamin). With damage to parenchyma of the liver, the levels of AST/ALT, bilirubin, vitamin B12 and folates, as a rule, increase (due to cytolysis of hepatocytes with the release of cell contents) while the levels of total protein - fall (due to deterioration of the biosynthetic function of the liver).
When reproducing the NAFLD model, the levels of AST, ALT, folates did increase, while the levels of total protein decreased. However, we observed an unusual dynamics of bilirubin levels (a decrease in total and direct bilirubin) and a decrease in vitamin B12 levels. In general, both LiAsc and MI contributed to the restoration of the values of these parameters towards the range of values characteristic for intact animals.
The enzymes AST and ALT are present in the highest concentrations in hepatocytes (also in cardiomyocytes and muscles). Normally, the levels/activity of AST/ALT in the blood are relatively low which prompts researchers and doctors to use AST and ALT as “liver function tests”. An increase in AST/ALT is indeed a characteristic feature of NAFLD, and a decrease in AST/ALT towards the reference range characterizes the effectiveness of an NAFLD therapy.
Reproducing the model resulted in a drastic increase in AST from 114.9±27.3 to 301.3±30.3 U/L (P<0.000001) on day 41. MI and LiAsc contributed to achieving AST levels similar to those in intact animals on day 41 (MI 94±35 U/L, P<0.000001; LiAsc 121.9±2.0 U/L, P=0.000013). Almost entirely similar picture was observed with ALT levels (see Results section). Changes in AST/ALT levels in groups 2–4 were consistent with changes in serum total protein levels (which represent a biomarker of biosynthetic function of liver). Both MI and LiAsc had a positive effect on liver biosynthetic function, promoting recovery of total protein levels by day 41 (MI 60.7±3.7 g/L, P=0.0001; LiAsc 52.5±1.0 g/L, P=0.01).
Vitamin B9 (folates) is a water-soluble vitamin required for the synthesis and methylation of DNA, the amino acids glycine and methionine. Measuring folate levels in the blood is commonly used to diagnose folate deficiency, which occurs due to (1) insufficient dietary intake (including consumption of thermally processed food), (2) decreased intestinal absorption of folates, (3) intake of drugs that impair absorption/metabolism of folates (estrogens, NSAIDs, cytostatic drugs, etc.). Causes of increased folate levels include intake of special folate-containing drugs or hepatocyte cytolysis. Also, an increase in folates during model reproduction may be compensatory in nature: folates are important for cell division, including restoration of the pool of hepatocytes and erythrocytes. When reproducing the model, blood folate levels showed an upward trend (67.6±3.1 nmol/L, P=0.043). While MI had no significant effects on folates. LiAsc contributed to a decrease to the normal range (49.6±2.9 nmol/L, P<0.000005 when compared with model at day 41, P>0.1 when compared with intact) which is yet another indication of the hepatoprotective properties of LiAsc (lower cytolysis).
Folate level usually correlate with vitamin B12 levels (since folate and vitamin B12 deficiencies often coexist). However, when reproducing the present model, the above-mentioned increase in folate levels was combined with a decrease in blood vitamin B12 levels (intact 264.5±58.1 pg/mL, model, day 13 190.5±7.1 pg/mL, P=0.013) which indicated the development of a deficiency. MI did not affect B12 levels (P=0.096) but LiAsc promoted normalization of vitamin B12 levels, to levels significantly higher than in intact animals (day 41: 325.3±23.1 pg/mL, P<0.00001 compared with model on day 41, P<0.001 compared with intact).
The main reasons for a decrease in vitamin B12 levels in the blood are insufficient intake, inflammatory and other bowel diseases, absorption disorders, including against the background of liver and kidney diseases or taking a number of drugs (colchicine, anticonvulsants, antibiotics reduce the concentration of B12). Since renal dysfunction was indeed observed in the model (see above), this is the most likely factor determining the decrease in vitamin B12 levels.
A statistically significant restoration of the concentration of vitamin B12 in the blood to the values of intact animals represent rather unexpected effect of lithium ascorbate. In the pharmacology of micronutrients, the so-called "Thérèse Turuan phenomenon" is known, in which the intake of one or another vitamin helps to improve the supply of other vitamins to the body. This effect may be due to the direct effects of the lithium ion on target proteins of the proteome [
18] or might be an indirect consequence of the hepatoprotective effect of LiAsc.
Despite the fact that hepatocyte cytolysis (reflected by an increase in AST, ALT, vitamin B9, bilirubin and a decrease in total protein) is characteristic of liver pathology, when reproducing the present model, total bilirubin levels significantly decreased (intact 5.9±0.6 μmol/L, model, day 13 1.5±1.1 μmol/L, P=0.000016), with an insignificant increase by day 41 (2.1±1.4 μmol/L). Recall that bilirubin and its fractions (total, direct, indirect) are metabolites of heme breakdown (a component of the hemoglobin protein). Therefore, the levels of various fractions of bilirubin increase with activation of red blood cell destruction, dysfunction of the liver and biliary tract, accompanying a wide variety of pathologies (hemolytic anemia, myocardial infarction, sepsis, viral hepatitis, biliary atresia, alcoholic liver disease, NAFLD, etc.).
Causes of decreased total/direct bilirubin are much rarer and include renal failure, acute leukemia, tuberculosis, anemias of various origins, including those resulting from taking various drugs (barbiturates, valproates, caffeine, corticosteroids, penicillin, sulfonamides, etc.). In our view, the most likely explanation for the decrease in bilirubin levels when reproducing the NAFLD model with iron overload was renal dysfunction (as indicated by decrease in creatinine and GFR levels). Only MI contributed to a significant restoration of total bilirubin levels on day 41 (5.3±1.2 μmol/L, P=0.0011), with no significant effect for lithium ascorbate (1.9±0.7 μmol/L).
Histological confirmations of the model and of the effects of the substances indicated progression from normality (as evidenced by histology of the intact group) to iron/lipid overload after reproducing the NAFLD model. Liver samples indicated progression of fatty degeneration of hepatocytes, inflammatory cell infiltrate (T-lymphocytes, eosinophils, intracellular and extracellular deposition of Prussian blue granules on the periphery of the liver lobule. Nephrocytes of the distal tubules began to actively accumulate "iron grains" in the cytoplasm; a focal accumulation of iron-containing substances was noticed in the perivascular space of neural tissue. In general, the developed complex model of secondary hemochromatosis and liver steatosis had reliable morphological confirmation.
When reproducing the model, structural changes in the liver, kidneys, brain and myocardium at the microscopic level were established, characteristic of multiple organ pathology. In the liver, accumulation of iron-containing products (hemosiderin) in the cytoplasm of hepatocytes and in the Disse space of the periportal zone of the liver lobule was observed. Macrophage activity of stellate reticuloendotheliocytes (Kupffer cells) was revealed with a tendency to an increase in the level of phagocytosis of iron-containing products by the 41st day of the experiment. Fatty degeneration of hepatocytes (as the main morphological criterion of steatosis) was diffuse-focal in nature with localization in the centers of liver lobules and was expressed by small-droplet obesity of hepatocytes on the 13th day of the experiment, with large-droplet obesity by the 41st day. The presence of inflammatory cell infiltrate with eosinophils in the stroma of portal tracts indicates the development of hepatitis.
After the model was reproduced (day 13), infiltration of iron-containing products into the cytoplasm of nephrocytes along the entire length of the tubules was established in the kidneys, without significant blood flow disorders and inflammatory reaction. By the 41st day of the experiment, excretion and reabsorption of iron was noted, which led to an overload of iron-containing products in the nephrocytes of the proximal and distal convoluted tubules.
In the brain and myocardium, the content of "iron grains" was minimal and was observed as focal areas of accumulation of small lumps of Prussian blue in the absence of morphological signs of any toxic effect on the parenchymatous elements of these organs. Possible reasons for the minimal effect on the brain include short periods after the reproduction of the model and the filtering role of the blood-brain barrier. In the case of the myocardium, the minimal amount of iron deposits is most likely associated with the constant motor activity of this organ and with the high number of mitochondria in cardiomyocytes (which accelerates the cell detoxification cycle).
Histological studies also confirmed the results of biochemical tests in the sense that LiAsc apparently had “anti-hemosiderin” properties as evidenced by morphometrics with Kuppfer cells and by quite a number of positive effects on the state of tissues of liver, brain and kidneys, including lowering of inflammation, lipid hyperaccumulation and iron overload of the tissues.
It also seems proper to discuss the
putative molecular mechanisms of hepatoprotective action of the LiAsc which are related to the molecular-biological properties of the ascorbate anion and of the lithium ion. The hepatoprotective properties of
ascorbic acid, ascorbates and of a few of the ascorbic acid derivatives [
19,
20] are known, especially when used in combinations with other substances of hepatoprotective quality: alpha-lipoic acid, polyphenol silymarin [
21], anti-diabetic drug metformin [
22], vitamin PP (nicotinamide) [
23]
etc.
Possible mechanisms of the hepatoprotective action of ascorbate might included the obvious antioxidant effect [
24] (which includes restoration of the levels of glutathione GSH and of antioxidant enzymes SOD-1/2, CAT [
25]), inhibition of sulfatase-2 and reduced expression of pro-inflammatory protein factor NFkB, CRP, TNF-a, IL-1b, IL-6 [
26], reduction of expression of pro-apoptotic caspase-3, of pro-fibrotic TGF-beta and collagen-I genes [
27]. Ascorbic acid supplementation in such cases is done in quite high doses. For example, 1.5 mg/ml in the drinking water to mice is roughly equivalent to 225 mg/kg/day does which, in turn, corresponds to intake of 1000-2000 mg/day of vitamin C by an adult human [
28].
On the contrary, studies of
lithium carbonate (done in high doses, corresponding to intake of 1-10 g/day of Li
2CO
3 by human patients) usually indicate hepatic injury as a typical consequence. Rather, the effects of various substances against Li
2CO
3-induced hepatotoxicity are investigated [
29,
30]. However, results of at least one clinical study indicate that more discrete usage even of relatively toxic Li
2CO
3 can, actually, reduce hepatic injury or leukopenia in patients with Graves' disease [
31].
Potential mechanism of the
hepatoprotective action of lithium ion involves, most likely, the inhibition of the glycogen synthase kinase-3 beta (GSK-3β) target protein. It is common knowledge that lithium ion can specifically bind to and inhibit GSK-3β. The experimental evidence available allows us to state that inhibition of GSK-3β can favorably influence hepatic function. For example, one of the histologically confirmed toxic effects of lead acetate includes significantly reduced phosphorylation of the liver GSK-3β. On the contrary, supplementation of rats with hepatoprotective L-carnitine resulted in an increase in phosphorylation of the liver GSK-3β. It is well known that phosphorylation of serine-9 in GSK-3β (serine-21 in GSK-3α) results in inhibition of GSK-3 kinases [
32], thus inhibition of GSK-3β would have a benign influence on the liver.
4. Materials and Methods
The complex of studies reported here included an in silico chemoreactomic modeling of several lithium salts, synthesis of the lithium salt(s), development and histological confirmation of the in vivo rat model of NAFLD with iron overload and approbation of the effects of LiAsc and of MI on the NFALD model.
Chemoreactomic modeling of hepatoprotective effects of substances. As in our previous paper [
1] before trying any actual experimental studies we modeled putative hepatoprotective properties of LiAsc and of several other lithium salts using the chemoreactomic approach to the analysis of the "structure-property" problem of organic molecules. The approach represents a relatively new direction of application of artificial intelligence (machine learning) systems in the field of post-genomic pharmacology. The rigorous mathematical methodology based on the theory of topological data analysis [
33] and the algorithms that were realized within an original proprietary software developed by the authors that was previously described [
34] and extensively tested [
35]. The training of the algorithms was carried out on the basis of the data on structure and properties of the molecules presented in the PubChem database for which hepatoprotective properties were found. The resulting train/test subset of PubChem included the data for 3423 molecules for which 486 hepatoprotective activities were determined experimentally (as documented in PubChem). The algorithms and software developed were realized within the framework of the topological and metrical theories of the analysis of complex data with highly heterogeneous features [
34,
35]; additional information about the algorithms and their testing is presented in the internet at web sites
www.chemoinformatics.ru and
www.pharmacoinformatics.ru.
Substances used. The synthesis of the organic salts was based on the classical reaction of neutralization carried out with the organic acids and the lithium carbonate as described in [
1]. The synthesis of LiAsc was carried out in accordance with the equation of the reaction of neutralization 2C
6H
8O
6 + Li
2CO
3=2C
6H
7O
6Li + H
2O + CO
2↑ from L-Ascorbic acid (C
6H
8O
6, Sigma-Aldrich, A5960, BioXtra, ≥99.0%) and lithium carbonate (Li₂CO₃, Merck, 1.05671.1000, certificates Ph Eur, BP, USP). Synthesis of sodium ascorbate (used in a preliminary comparative study) was performed similarly from sodium carbonate (Na₂CO₃, Merck, 1.06392.1000, grade ISO-1). Myoinositol was also of pharmaceutical grade (C
6 H
12O
6, Мerck, 1.04731.1000, certificates Ph Eur, FCC, NF).
Animal studies. During the animal studies, the animals were kept under standard conditions in accordance with Directive 2010/63/EU of the European Parliament and of the Council of the European Union of 22 September 2010 concerning the protection of animals used in scientific studies [
36]. Indoor air control in compliance with environmental parameters (temperature 18-26 °C, humidity 46-65%). The rats were kept in standard plastic cages with a bedding, the cages were covered with steel lattice covers with a stern recess. Floor area per animal met regulatory standards. The animals were fed in accordance with Directive 2010/63/EU. The animals were given water
ad libitum. The water was purified and normalized for organoleptic properties, in terms of pH, dry residue, reducing substances, carbon dioxide, nitrates and nitrites, ammonia, chlorides, sulfates, calcium and heavy metals in standard drinkers with steel spout lids. A 12-hour lighting cycle was maintained in the animal housing rooms. Environmental conditions were monitored using a Testo combined meter (manufactured by TestoAG, Germany) and recorded in a corresponding log. For acclimatization, laboratory animals were kept individually in cages for 5 days before the start of the study. During this period, the animals' clinical condition was monitored daily by visual inspection. Animals with deviations detected during the inspection were not included in the experimental groups.
The development of the iron overload NAFLD model was based on the NAFLD model induced by palm oil [
5]. The development of the NAFLD model with multiple organ pathology and iron
overload and testing of the effects of LiAsc therapy were carried out on 30 white male rats weighing 300-400 g. The animals were divided into 4 groups. At the beginning of the experiment, the animals were distributed into groups so that the individual body weight did not deviate from the average body weight for the group by more than 20%. Weighing was carried out on electronic scales for weighing rats/mice (manufactured by Cas Corporation, Russia).
The first group (n=6) was an intact control (on a normal diet and drinking pure drinking water). In the second (n=12), third, and fourth (n=6) groups of animals, the model of liver iron overload was reproduced under conditions of adding saturated fats and fructose to the diet. To reproduce the model, chemically pure divalent iron sulfate (manufactured by JSC LenReaktiv, passport No. 070051-81, Russia) was administered intraperitoneally to the animals at a dose of 50 mg/kg/day for 12 days. At the same time, a solid fraction of palm oil (CandleM , Indonesia) was added to the diet at a dose of 30 g/kg/day. A fructose solution (LLC Sladkiy Mir Company, TU 10.86.10-027-72315488-2019. Batch 210723, Russia) at a dose of 1 g/kg/day was used instead of drinking water for 12 days.
On the 13th day of the study, animals of the first and of the second groups were anesthetized, blood was collected for biochemical testing and autopsy material (liver, kidneys, brain, heart) was collected for pathohistological testing. Animals were withdrawn from the experiment by achieving anesthetic death using the drug Zoletil.
Exploring different approaches to therapy was carried out from the 13th day of the study. The third group (n=6) received LiAsc (manufactured by Normofarm LLC, Russia) at a dose of 30 mg/kg/day per os for 4 weeks. The fourth group received myoinositol (400 mg/kg/day per os) for 4 weeks. On the 41st day of the study, the animals of the second, third, and fourth groups (n=6) were anesthetized, blood was collected for biochemical analysis, and autopsy material (liver, kidneys, brain, heart) was collected for histopathological examination.
The animals were observed daily; their general condition, appetite, behavioral characteristics, intensity and nature of motor activity, frequency and depth of respiratory movements, condition of hair and skin, tail position, amount and consistency of feces were recorded. The values of more than 20 biochemical parameters were determined in the blood (
Table 2) using the methods described in
Table 3.
Histopathohistological studies. Material for histological examination was obtained during autopsy of experimental animals. The brain was removed entirely by craniotomy and fixed in 10% neutral formalin solution; after 24 hours, the precentral gyrus zone of the forebrain, cerebellum, and brainstem were isolated using frontal incisions. After evisceration, the liver, heart, and kidneys were fixed in 10% neutral formalin solution. After 24 hours, the organs were dissected, fragments of the left ventricular myocardium, right and left lobes of the liver, and cortical sections of the right and left kidneys were isolated and re-fixed. After secondary fixation and washing of the material, dehydration of the brain, liver, and kidney tissues was performed using 99% isopropyl alcohol. The pieces were then embedded in paraffin and histological sections 5-6 μm thick prepared on sliding microtome “Microm” and were stained with hematoxylin and eosin. Duplicates of the sections were stained according to Perls using a set of reagents from the Biovitrum (Russia) to detect trivalent iron in the tissues. In the case of the presence of an excess trivalent ionic iron in tissues the result of the Perls’ reaction should be the formation of the blue-colored salt – the Prussian blue (according to the equation of the chemical reaction FeIIICl3 + K4[FeII(CN)6] → KFeIII[FeII(CN)6] + 3KCl).
The qualitative assessment of pathological changes in the tissues took into account the degree of circulatory disorder, the presence and localization of Prussian blue, the characteristics of the inflammatory response, and structural changes in parenchymatous elements. Micrographs were obtained using a research microscope "Micros" MS-200 with a digital ocular camera DCM 900 (UK). Quantitative assessment of the iron overload through morphometric analysis was performed on the liver samples (item “Kupffer cells” in
Table 1) by counting the average number of Kupffer cells containing Prussian blue in their cytoplasm per field of view.
Statistical analysis. The results were processed using the Excel 2013 and Statistica 10.0 (USA) software packages. The significance of differences between groups was determined by a nonparametric U-test, the Wilcoxon – Mann – Whitney test.
To construct profile diagrams of the biochemical parameters listed in
Table 1, the cumulative empirical distribution functions (CDF) of each of the studied parameters were first calculated. Recall that the CDF F(X) of the values of parameter X is a function in the range of values [0…1], on the graph of which the values of parameter X (for example, ferritin levels) in all experiments are plotted along the X axis, and the probability that the value of X is less than a given value is plotted along the Y axis. For example, if the maximum value of ferritin in the blood collected during the experiments was 500 μg/L, then the probability that an arbitrary value ferritin less than 500 equals to 1 (i.e. all observed values were less than 500 μg/L).
Then, the values of each X parameter in their generally accepted units of measurement (e.g., μg/L for ferritin) were replaced by the values of the CDF F(X) in dimensionless units in the range of values [0...1]. Using the CDF values instead of the original values of the parameters allows for a clear comparison of the effects of various therapeutic interventions on a single scale of values in the range [0...1]. At the same time, the absolute values of the parameters studied may differ by 1-2 orders of magnitude. For example, the values of ferritin lie in the range of 72-277 μg/L, and total bilirubin - in the range of 0.3-6.7 μmol/L. Obviously, a diagram with such significant differences in the orders of magnitude of the parameter values will not be informative nor visually acceptable.
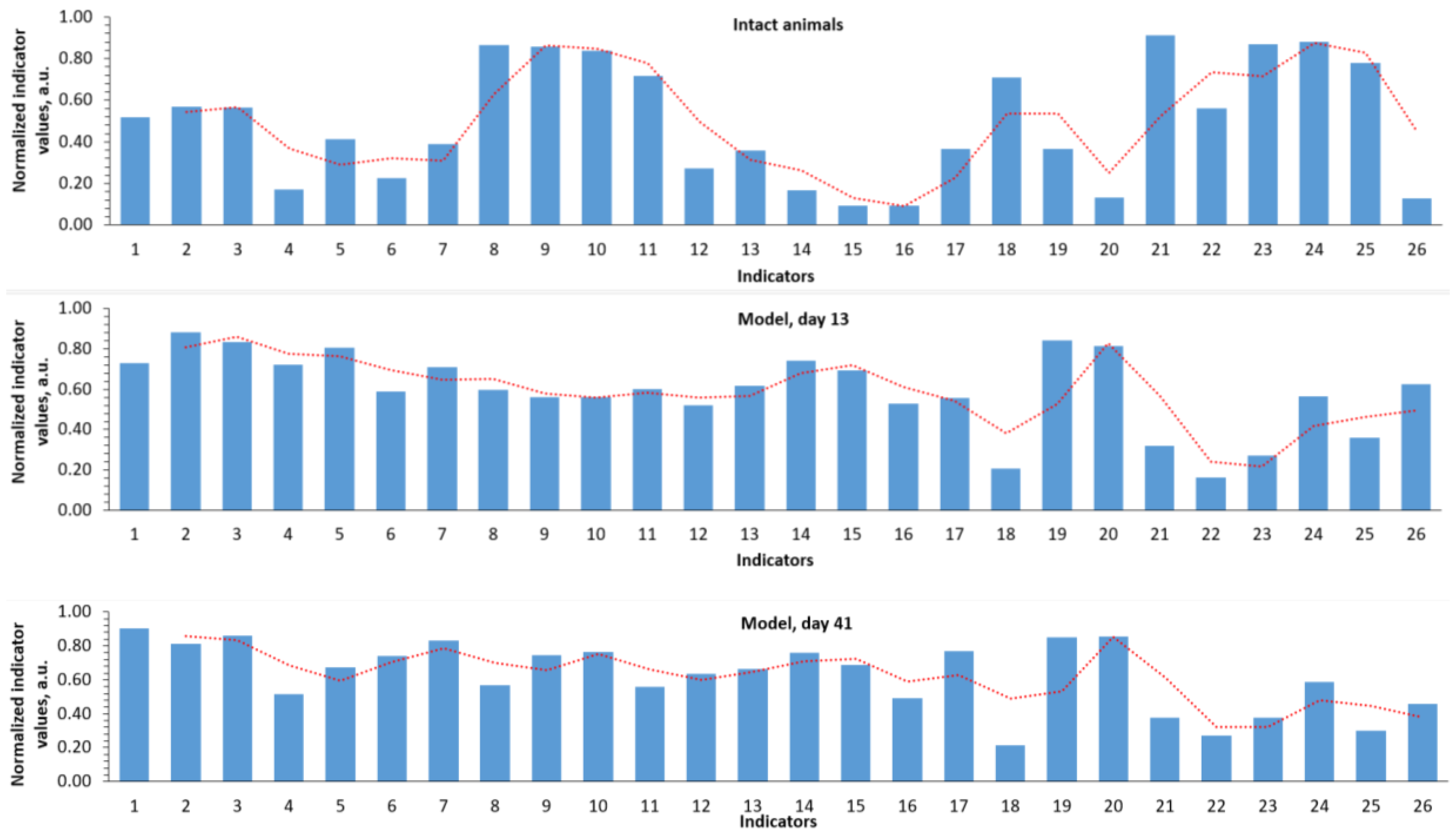
Figure 1.
Normalized profiles of biochemical indicator values during reproduction of the developed NAFLD model. The red dashed-dotted line describes the profile of changes in all indicators/parameters as a whole. 1 - Ferritin, μg/L; 2 - Transferrin saturation coefficient with iron, %; 3 - Serum iron, μmol/L; 4 - Reticulocytes, %; 5 - Reticulocytes, bln/L; 6 - Reticulocyte fraction, %; 7 - Leukocytes, bln/L; 8 - Erythrocytes, trln/L; 9 - Hemoglobin, g/L; 10 - Hematocrit, %; 11 - Mean corpuscular volume, fL; 12 - Mean hemoglobin content in erythrocytes, pg; 13 - Mean hemoglobin concentration in erythrocytes, g/L; 14 - Platelets, bln/l; 15 - Mentzer index; 16 - Sirdah index; 17 - folates, nmol/l; 18 - Total serum protein, g/l; 19 - AST, U/l; 20 - ALT, U/l; 21 - Serum creatinine, μmol/l; 22 – Glomerular filtration rate; 23 - Total bilirubin, μmol/l; 24 - Direct bilirubin, μmol/l; 25 - Vitamin B12, pg/ml; 26 - Kupffer cells, whose cytoplasm contains Prussian blue.
Figure 1.
Normalized profiles of biochemical indicator values during reproduction of the developed NAFLD model. The red dashed-dotted line describes the profile of changes in all indicators/parameters as a whole. 1 - Ferritin, μg/L; 2 - Transferrin saturation coefficient with iron, %; 3 - Serum iron, μmol/L; 4 - Reticulocytes, %; 5 - Reticulocytes, bln/L; 6 - Reticulocyte fraction, %; 7 - Leukocytes, bln/L; 8 - Erythrocytes, trln/L; 9 - Hemoglobin, g/L; 10 - Hematocrit, %; 11 - Mean corpuscular volume, fL; 12 - Mean hemoglobin content in erythrocytes, pg; 13 - Mean hemoglobin concentration in erythrocytes, g/L; 14 - Platelets, bln/l; 15 - Mentzer index; 16 - Sirdah index; 17 - folates, nmol/l; 18 - Total serum protein, g/l; 19 - AST, U/l; 20 - ALT, U/l; 21 - Serum creatinine, μmol/l; 22 – Glomerular filtration rate; 23 - Total bilirubin, μmol/l; 24 - Direct bilirubin, μmol/l; 25 - Vitamin B12, pg/ml; 26 - Kupffer cells, whose cytoplasm contains Prussian blue.
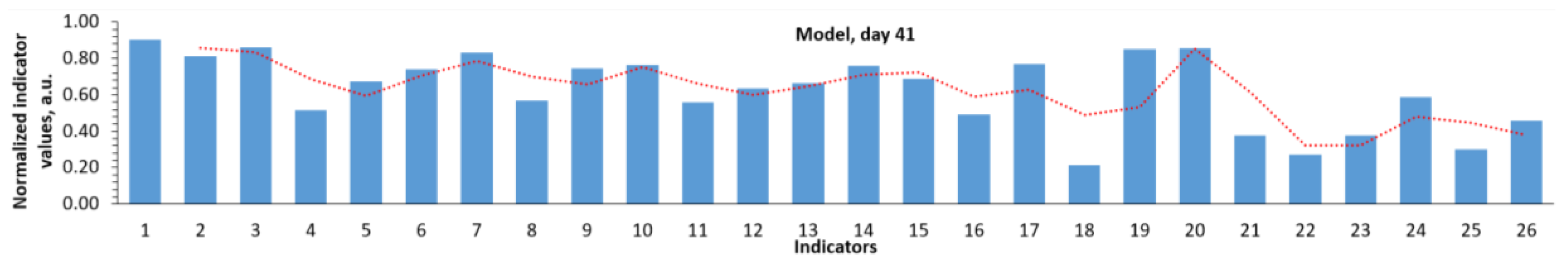
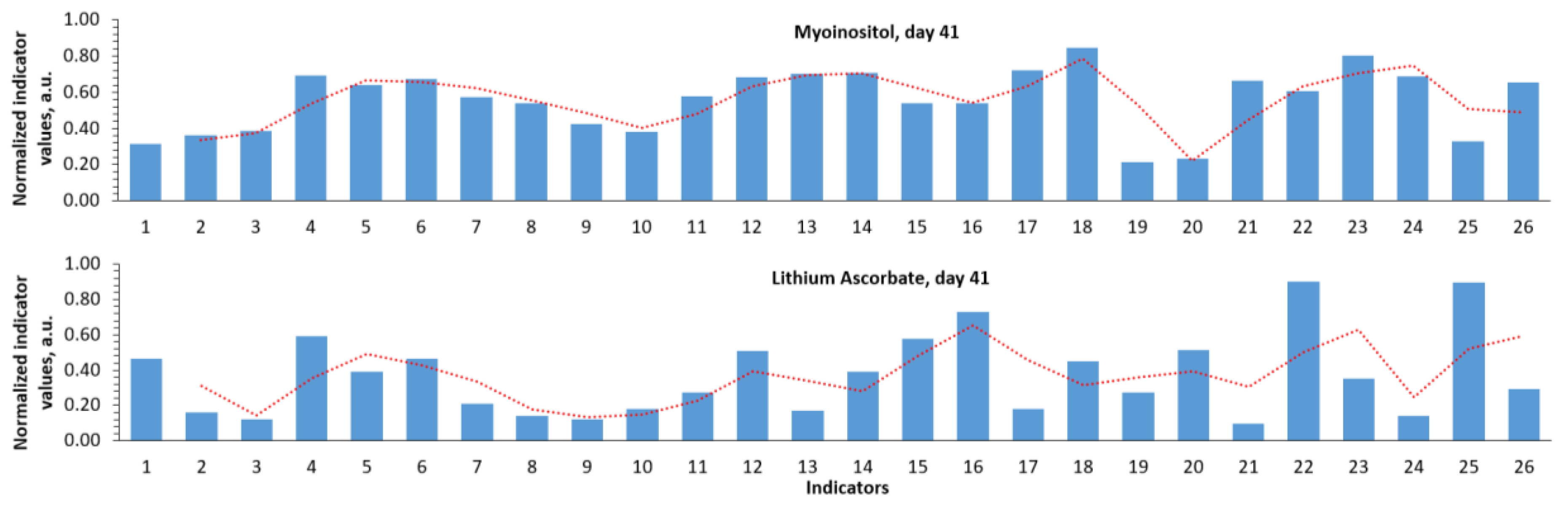
Figure 2.
Normalized profiles of biochemical parameter values using different approaches to therapy. Parameter designations are as in
Figure 1; the slide for day 41 of the model was repeated from Figure1 for the sake of convenience of visual comparisons.
Figure 2.
Normalized profiles of biochemical parameter values using different approaches to therapy. Parameter designations are as in
Figure 1; the slide for day 41 of the model was repeated from Figure1 for the sake of convenience of visual comparisons.
Figure 3.
Profiles of biochemical parameters of the studied groups. (a) Iron metabolism parameters, (b) general blood test parameters, (c) multiple organ dysfunction parameters.
Figure 3.
Profiles of biochemical parameters of the studied groups. (a) Iron metabolism parameters, (b) general blood test parameters, (c) multiple organ dysfunction parameters.
Figure 4.
Histological analysis of various organs of intact animals. All slides magnified x480. Stained with Perls reaction (A), hematoxylin and eosin (B-E). A) Structure of an unchanged liver lobule with trabecular arrangement of hepatocytes. B) Single lymphocytes in the stroma of the portal tract against the background of uniform perfusion of all parts of the liver lobule. C) The renal glomerulus has a lumen of the capsule and free mesangium, epithelial cells of the proximal and distal convoluted tubules with homogeneous staining of the cytoplasm. D) Pyramidal neurons of the usual configuration with uniform distribution of Nissl lumps in the cytoplasm. E) Contractile fibers of uniform staining and thickness, anisotropic disks are visible.
Figure 4.
Histological analysis of various organs of intact animals. All slides magnified x480. Stained with Perls reaction (A), hematoxylin and eosin (B-E). A) Structure of an unchanged liver lobule with trabecular arrangement of hepatocytes. B) Single lymphocytes in the stroma of the portal tract against the background of uniform perfusion of all parts of the liver lobule. C) The renal glomerulus has a lumen of the capsule and free mesangium, epithelial cells of the proximal and distal convoluted tubules with homogeneous staining of the cytoplasm. D) Pyramidal neurons of the usual configuration with uniform distribution of Nissl lumps in the cytoplasm. E) Contractile fibers of uniform staining and thickness, anisotropic disks are visible.
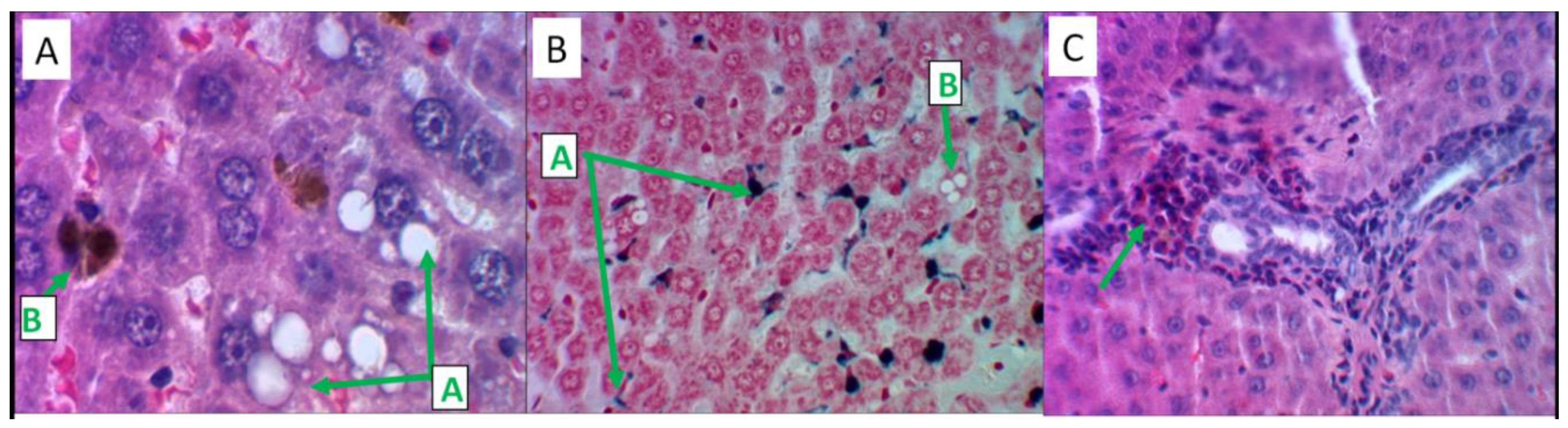
Figure 5.
Histological analysis of liver tissues in the reproduction of the "iron sulfate + palm oil + fructose solution" model. Hematoxylin and eosin staining – slides B, E, F, G; Perls’ reaction staining – slides A, C, D. Slides B, C, F – magnification x480, slides A, D, E, G – magnification x1200. A) Intracellular and extracellular deposition of Prussian blue granules on the periphery of the liver lobule. B) Fine-droplet obesity of hepatocytes in the center of the liver lobule. C) The cytoplasm of stellate reticuloendotheliocytes (Kupffer cells) is saturated with Prussian blue. D) Focal accumulation of Prussian blue in the liver capsule. E) Large-droplet obesity of hepatocytes in the center of the liver lobule. F) Inflammatory cell infiltration of the stroma of the portal tract. G) The infiltrate contains T-lymphocytes (marked with letter “A”) and eosinophils (“B”).Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
Figure 5.
Histological analysis of liver tissues in the reproduction of the "iron sulfate + palm oil + fructose solution" model. Hematoxylin and eosin staining – slides B, E, F, G; Perls’ reaction staining – slides A, C, D. Slides B, C, F – magnification x480, slides A, D, E, G – magnification x1200. A) Intracellular and extracellular deposition of Prussian blue granules on the periphery of the liver lobule. B) Fine-droplet obesity of hepatocytes in the center of the liver lobule. C) The cytoplasm of stellate reticuloendotheliocytes (Kupffer cells) is saturated with Prussian blue. D) Focal accumulation of Prussian blue in the liver capsule. E) Large-droplet obesity of hepatocytes in the center of the liver lobule. F) Inflammatory cell infiltration of the stroma of the portal tract. G) The infiltrate contains T-lymphocytes (marked with letter “A”) and eosinophils (“B”).Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
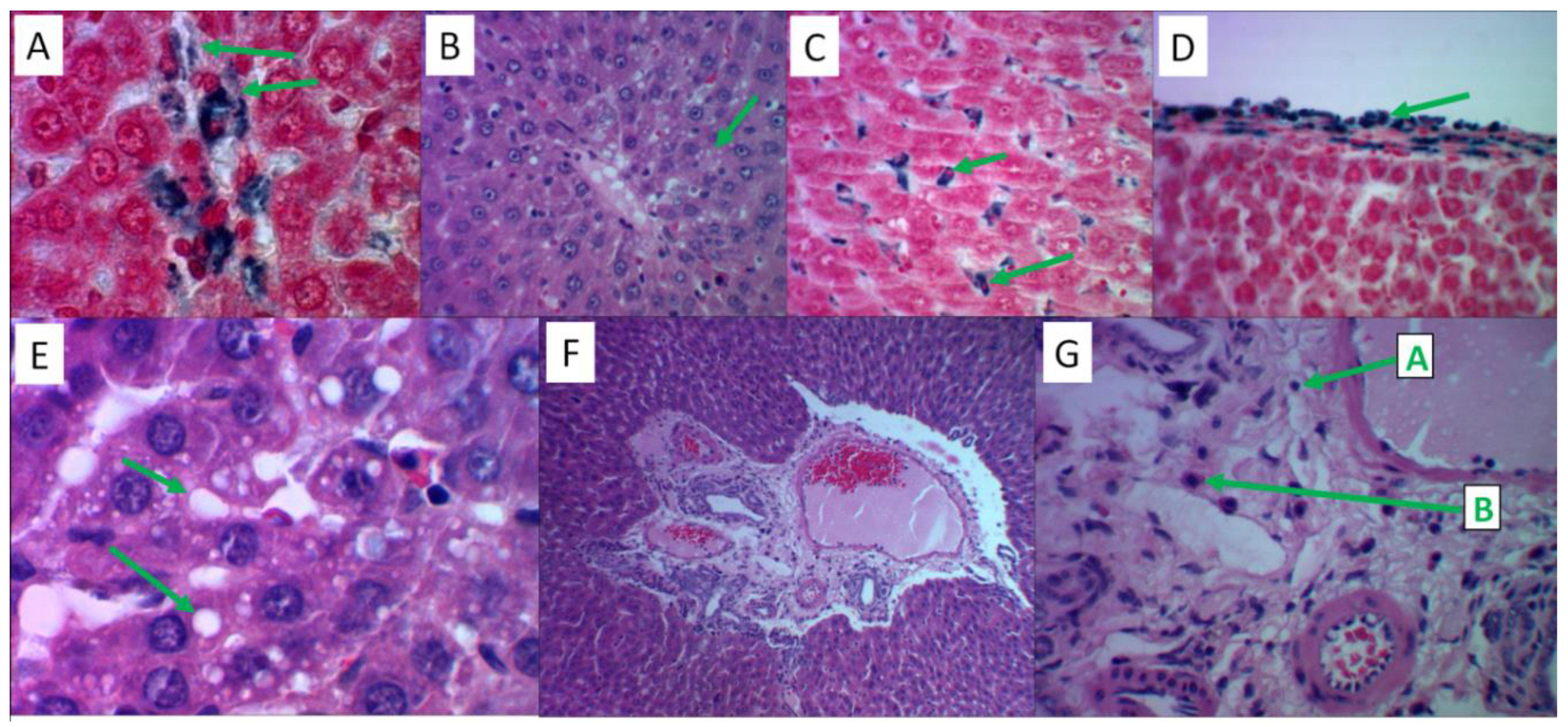
Figure 6.
Histological analysis of MI effects on liver tissue. Hematoxylin and eosin staining – slides A, B; Perls reaction staining – slide B. Slides B, C – magnification x480, slide A – magnification x1200. A) Large-droplet obesity of hepatocytes in the centers of lobules (label "A"), iron-containing substrate in the cytoplasm of Kupffer cells (label "B"). B) Prussian blue in Kupffer cells, Disse space (label "A"), fatty degeneration of hepatocytes (label "B"). C) Predominance of eosinophilic leukocytes in the inflammatory infiltrate of the stroma of the portal tract.
Figure 6.
Histological analysis of MI effects on liver tissue. Hematoxylin and eosin staining – slides A, B; Perls reaction staining – slide B. Slides B, C – magnification x480, slide A – magnification x1200. A) Large-droplet obesity of hepatocytes in the centers of lobules (label "A"), iron-containing substrate in the cytoplasm of Kupffer cells (label "B"). B) Prussian blue in Kupffer cells, Disse space (label "A"), fatty degeneration of hepatocytes (label "B"). C) Predominance of eosinophilic leukocytes in the inflammatory infiltrate of the stroma of the portal tract.
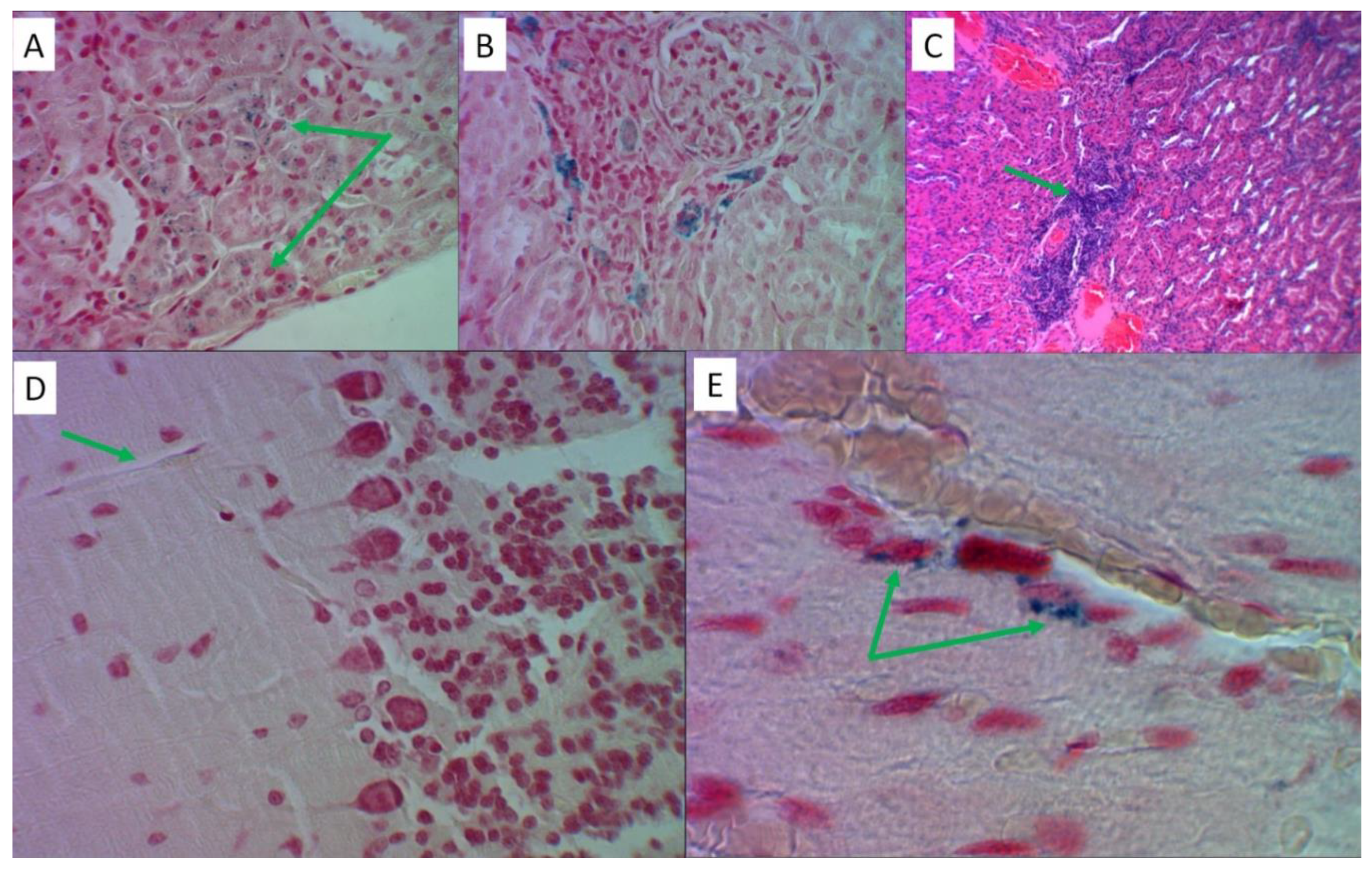
Figure 7.
Histological analysis of MI effects on kidney, brain and myocardial tissues. Hematoxylin and eosin staining – slide C; Perls reaction staining – slides A, B, D, E. Slides A, B, D – magnification x480, slides C, E – magnification x1200. A) Prussian blue in proximal tubular nephrocytes. B) Iron-containing products in nephrocytes and in the lumens of distal tubules. C) Focal lymphocytic infiltration of the renal stroma. D) Moderate perivascular edema of the cerebellar nervous tissue in the absence of iron-containing products. E) Perivascular zone cardiomyocytes contain Prussian blue granules.
Figure 7.
Histological analysis of MI effects on kidney, brain and myocardial tissues. Hematoxylin and eosin staining – slide C; Perls reaction staining – slides A, B, D, E. Slides A, B, D – magnification x480, slides C, E – magnification x1200. A) Prussian blue in proximal tubular nephrocytes. B) Iron-containing products in nephrocytes and in the lumens of distal tubules. C) Focal lymphocytic infiltration of the renal stroma. D) Moderate perivascular edema of the cerebellar nervous tissue in the absence of iron-containing products. E) Perivascular zone cardiomyocytes contain Prussian blue granules.
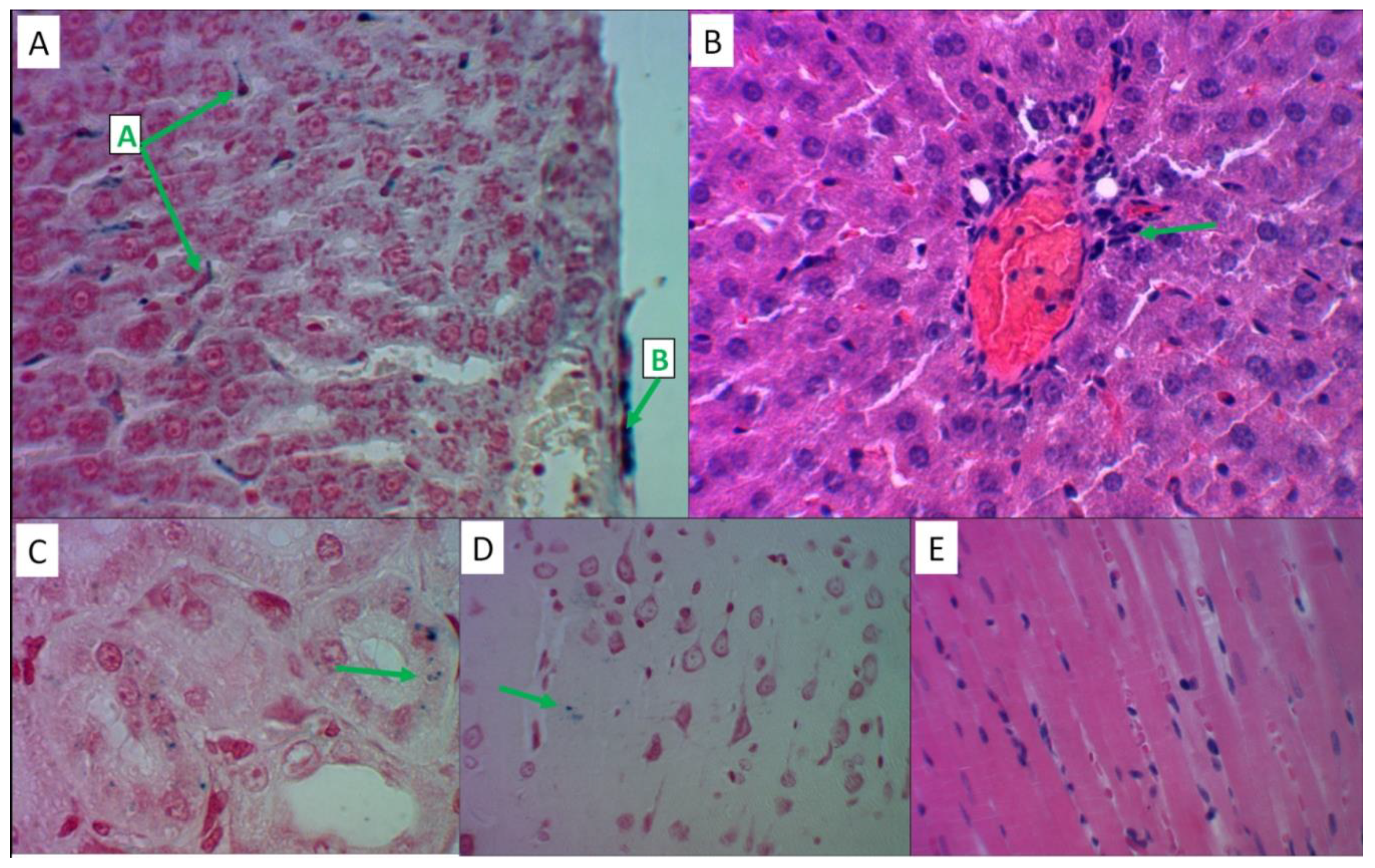
Figure 8.
Histological analysis of the effects of lithium ascorbate. Hematoxylin and eosin staining – slides B, E; Perls reaction staining – slides A, C, D. Slides A, B, D - magnification x480, slides C, E - magnification x1200. A) An insignificant amount of iron-containing products in the cytoplasm of Kupffer cells (label "A"), liver capsule (label "B"). B) Single lymphocytes in the stroma of the portal tract. C) Grains of Prussian blue in nephrocytes of the distal convoluted tubules. D) Small lumps of Prussian blue in the perivascular space of the nervous tissue. E) Cardiomyocytes are uniformly stained, contractile fibers with transverse striation, capillary perfusion is preserved.
Figure 8.
Histological analysis of the effects of lithium ascorbate. Hematoxylin and eosin staining – slides B, E; Perls reaction staining – slides A, C, D. Slides A, B, D - magnification x480, slides C, E - magnification x1200. A) An insignificant amount of iron-containing products in the cytoplasm of Kupffer cells (label "A"), liver capsule (label "B"). B) Single lymphocytes in the stroma of the portal tract. C) Grains of Prussian blue in nephrocytes of the distal convoluted tubules. D) Small lumps of Prussian blue in the perivascular space of the nervous tissue. E) Cardiomyocytes are uniformly stained, contractile fibers with transverse striation, capillary perfusion is preserved.
Table 1.
Results of chemoreactomic analysis of the lithium salts and myoinositol in rats. "Const.", The common name for the type of investigational pharmacological constant ("IC50"); "Error" - the error of the estimated biological constant; "Unit", units of measurement.
Table 1.
Results of chemoreactomic analysis of the lithium salts and myoinositol in rats. "Const.", The common name for the type of investigational pharmacological constant ("IC50"); "Error" - the error of the estimated biological constant; "Unit", units of measurement.
| Activity |
Const |
Unit |
Error |
LiAsc |
MI |
Li2CO3
|
| Hepatoprotective activity against D-GalN-induced cytotoxicity in primary cultured rat hepatocytes at 30 uM dose |
- |
% |
8.2 |
20.8 |
25.5 |
0 |
| Hepatoprotective activity assessed as inhibition of D-galactosamine-induced cytotoxicity (100 uM of substance, MTT assay, % relative to untreated control) |
- |
% |
5.3 |
17.2 |
28.2 |
0 |
|
In vitro inhibition of Acyl coenzyme A:cholesterol acyltransferase in rat hepatic microsomes (AIV assay) |
IC50 |
nM |
50.7 |
225.4 |
41.8 |
>10000 |
|
In vitro inhibition of rat liver HMG-CoA reductase |
IC50 |
nM |
19.4 |
122.0 |
97.0 |
>10000 |
|
In vivo lowering of blood glucose levels in Zucker diabetic rats (postprandial glucose, day 3, after 300 uM/kg per os) |
- |
% |
7.2 |
46.2 |
46.0 |
0 |
Table 2.
Biochemical parameters (indicators) measured in this work
Table 2.
Biochemical parameters (indicators) measured in this work
| Abbreviation |
Biochemical indicator |
| Ferritin |
Ferritin, mcg/l |
| Sat.transferrin |
Transferrin iron saturation coefficient, % |
| Serum Iron |
Serum iron, µmol/l |
| Reticulocytes, % |
Reticulocytes, % |
| Reticulocytes, abs. |
Reticulocytes, bln/l |
| Frac. reticulocytes,% |
Reticulocyte fraction, % |
| Leukocytes, abs. |
Leukocytes, bln/l |
| Erythrocytes, abs. |
Erythrocytes, trln/l |
| Hemoglobin |
Hemoglobin, g/l |
| Hematocrit, % |
Hematocrit, % |
| V-erythrocyte |
Mean corpuscular volume, fL |
| Hemoglobin-erythrocyte |
Average hemoglobin content in erythrocytes, pg |
| Conc.hemoglobin erythrocytes |
Average hemoglobin concentration in red blood cells, g/l |
| Platelets, abs. |
Platelets, bln/l |
| Ind. Mentzer |
Mentzer Index |
| Ind. Sirdah |
Sirdah Index |
| Vitamin B9 |
Vitamin B9 (folic acid), nmol/l |
| Tot.protein |
Total protein in serum, g/l |
| AST |
Aspartate aminotransferase, U/L |
| ALT |
Alanine aminotransferase, U/L |
| Creatinine |
Serum creatinine, µmol/l |
| GFR |
Glomerular filtration rate, ml/min/1.73m^2 |
| Tot.bilirubin |
Total bilirubin, µmol/l |
| Dir.bilirubin |
Direct bilirubin, µmol/l |
| Vitamin B12 |
Vitamin B12 (cyanocobalamin), pg/ml |
| Kupffer cells |
Kupffer cells, which contain Prussian blue in their cytoplasm (after Perls reaction) |
Table 3.
Methods and equipment used to measure biochemical indicators.
Table 3.
Methods and equipment used to measure biochemical indicators.
| Indicator |
Method |
Equipment |
| Ferritin |
Immunoturbidimetry
|
Cobas 6000, Roche Diagnostics, Switzerland |
| Transferrin |
Immunoturbidimetry
|
Cobas 6000, Roche Diagnostics, Switzerland |
| Serum iron |
Colorimetric photometric method |
Cobas 6000, Roche Diagnostics, Switzerland |
| Reticulocytes |
Flow cytometry |
BC-6200, Mindray, China |
Leukocytes
Erythrocytes
Hemoglobin
Hematocrit
Mean corpuscular volume
Average hemoglobin content in red blood cells
Average hemoglobin concentration in red blood cells
Platelets |
Conductometric method based on SLS (sodium lauryl sulfate), flow cytometry
|
BC-6200, Mindray, China
|
Total protein
Total bilirubin
Direct bilirubin |
Colorimetric photometric method |
Cobas 6000, Roche Diagnostics, Switzerland |
Aspartate aminotransferase (AST)
Alanine aminotransferase (ALT) |
UV kinetic test |
Cobas 6000, Roche Diagnostics, Switzerland |
| Serum creatinine (with GFR determination) |
Jaffe's "kinetic" method
|
Cobas 6000, Roche Diagnostics, Switzerland |
| Vitamin B12
|
Immunochemiluminescent assay |
Cobas 6000, Roche Diagnostics, Switzerland |
| Vitamin B9
|
Competitive solid-phase chemiluminescent enzyme immunoassay |
Cobas 6000, Roche Diagnostics, Switzerland |