Submitted:
20 September 2024
Posted:
23 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Program
2.1. Materials and Method
2.2. Test Methods
2.2.1. Setting Time
2.2.2. Mechanical Properties
2.2.3. Alkali-Silica Reaction
2.2.4. Autogenous Shrinkage
2.2.5. Particle Size Distribution
2.2.6. Scanning Electron Microscope
2.2.7. Thermogravimetric Analysis
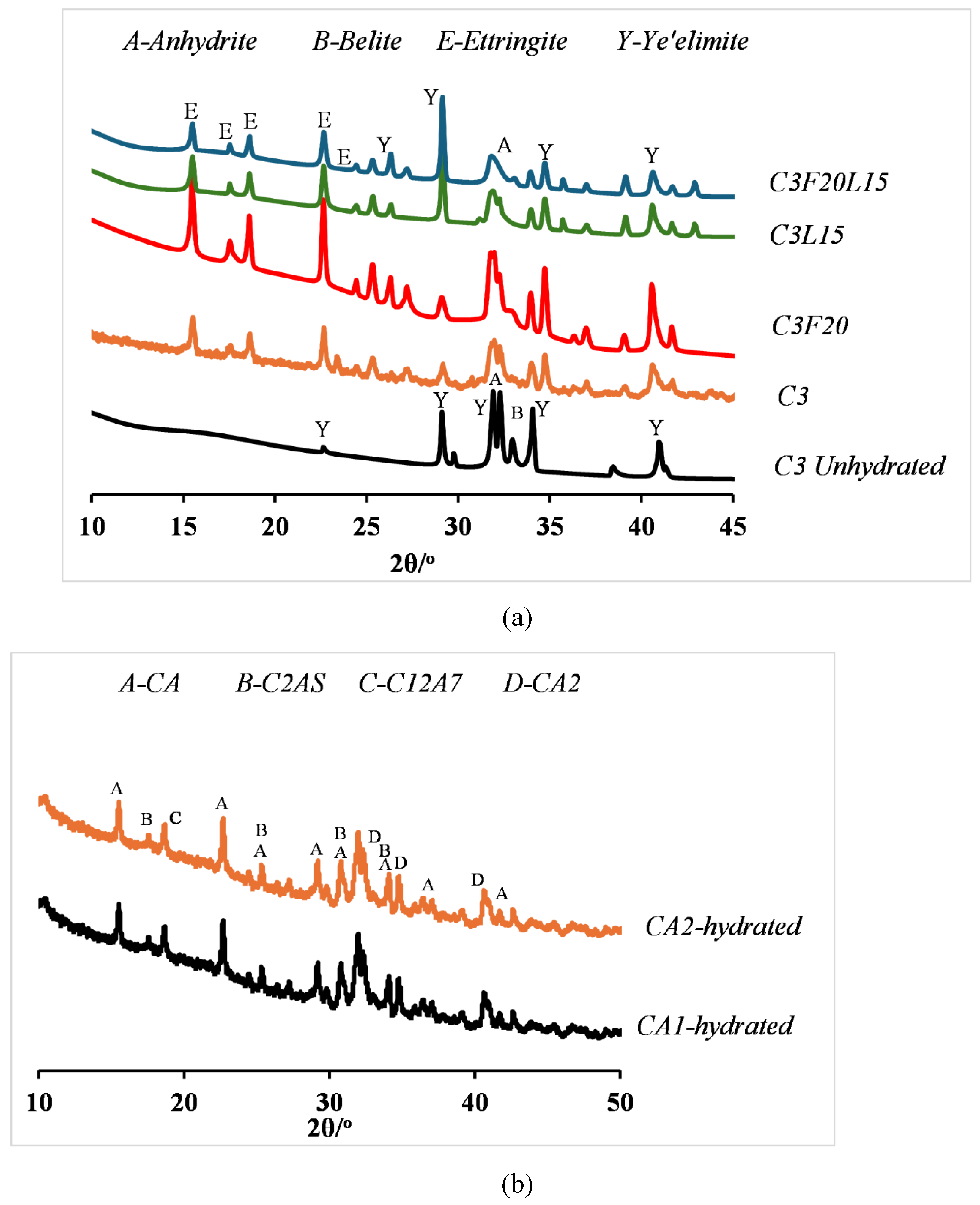
2.2.8. X-ray Diffraction
3. Results and Discussion
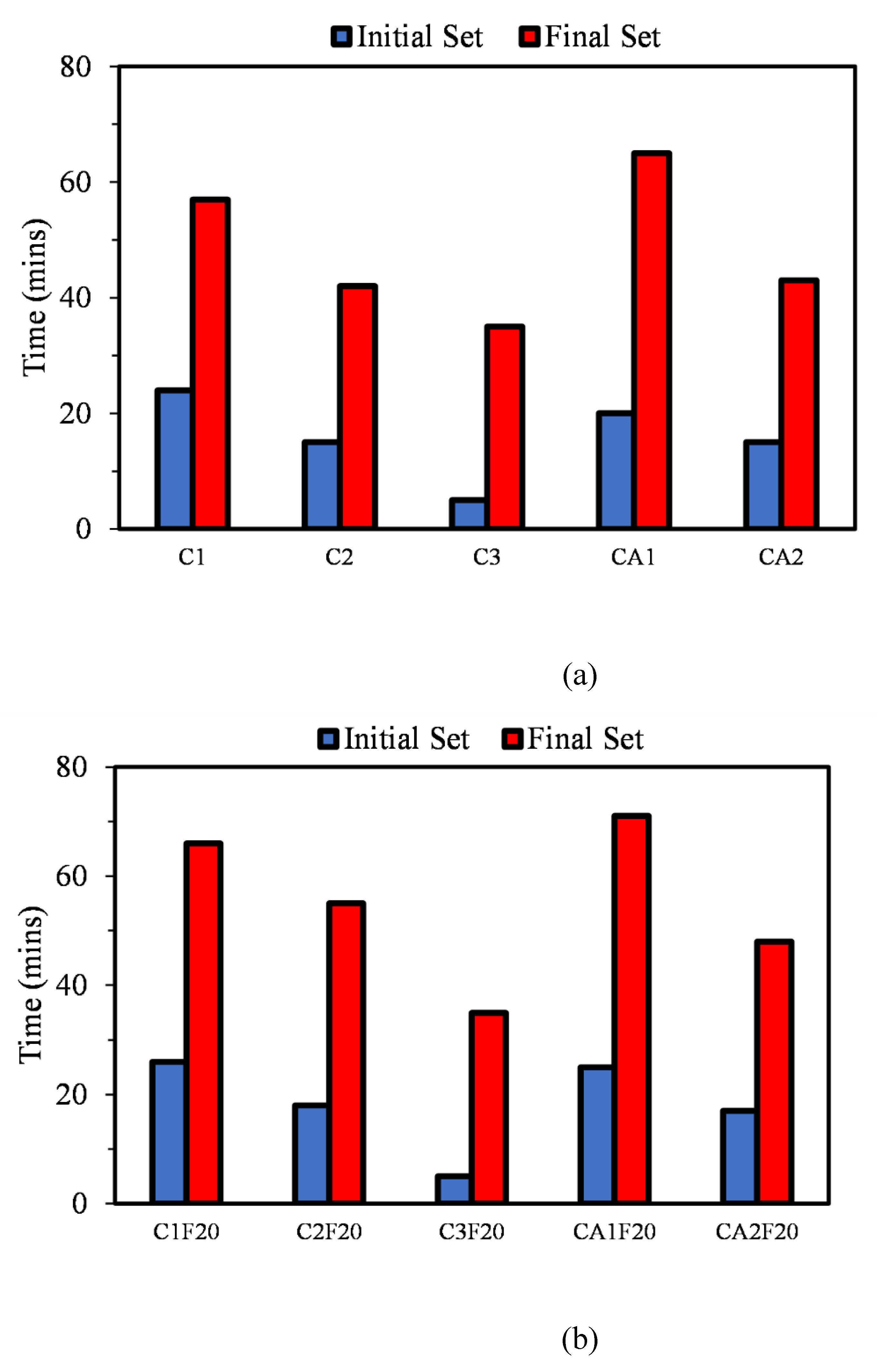
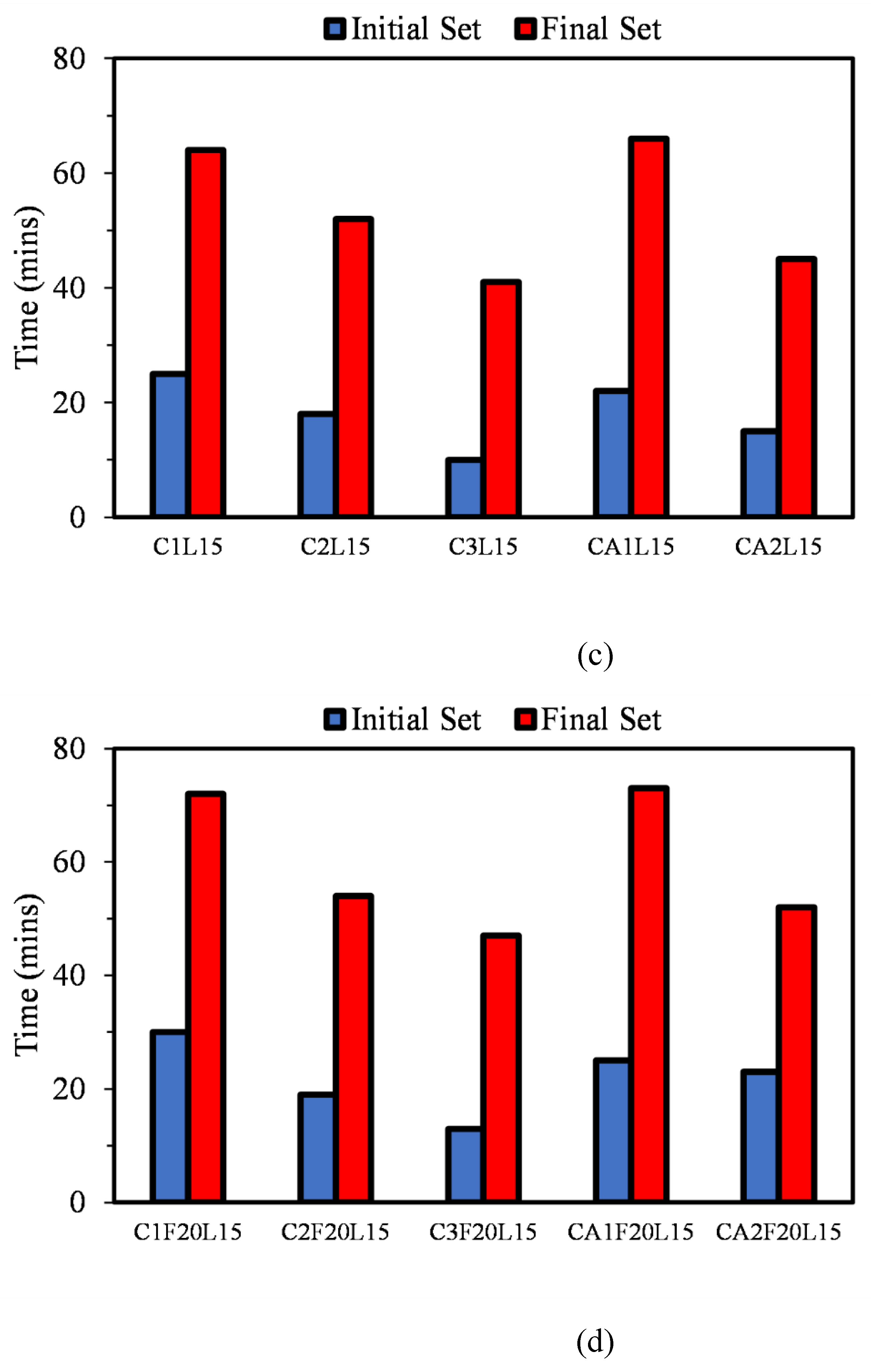
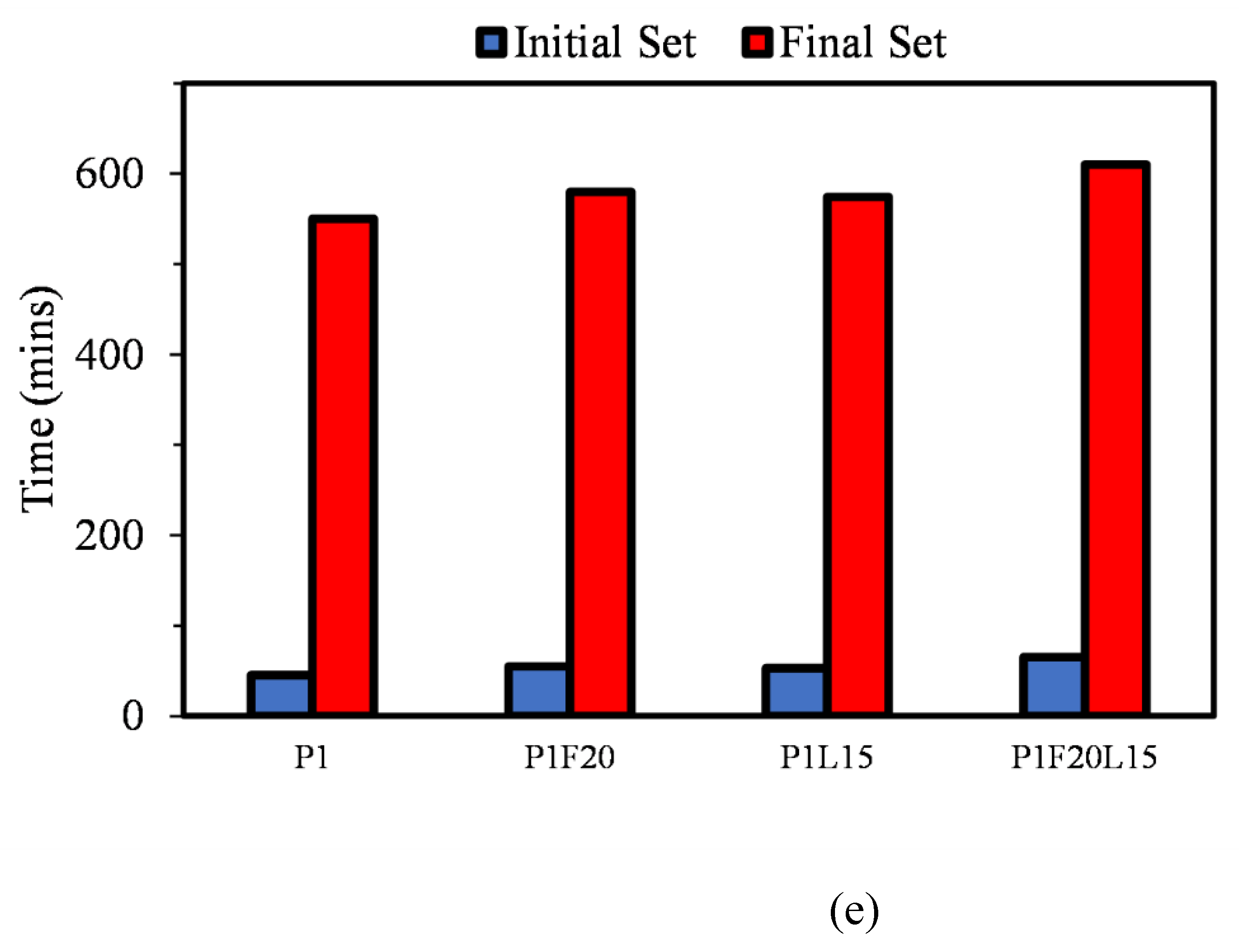
3.1. Initial and Final Setting Time
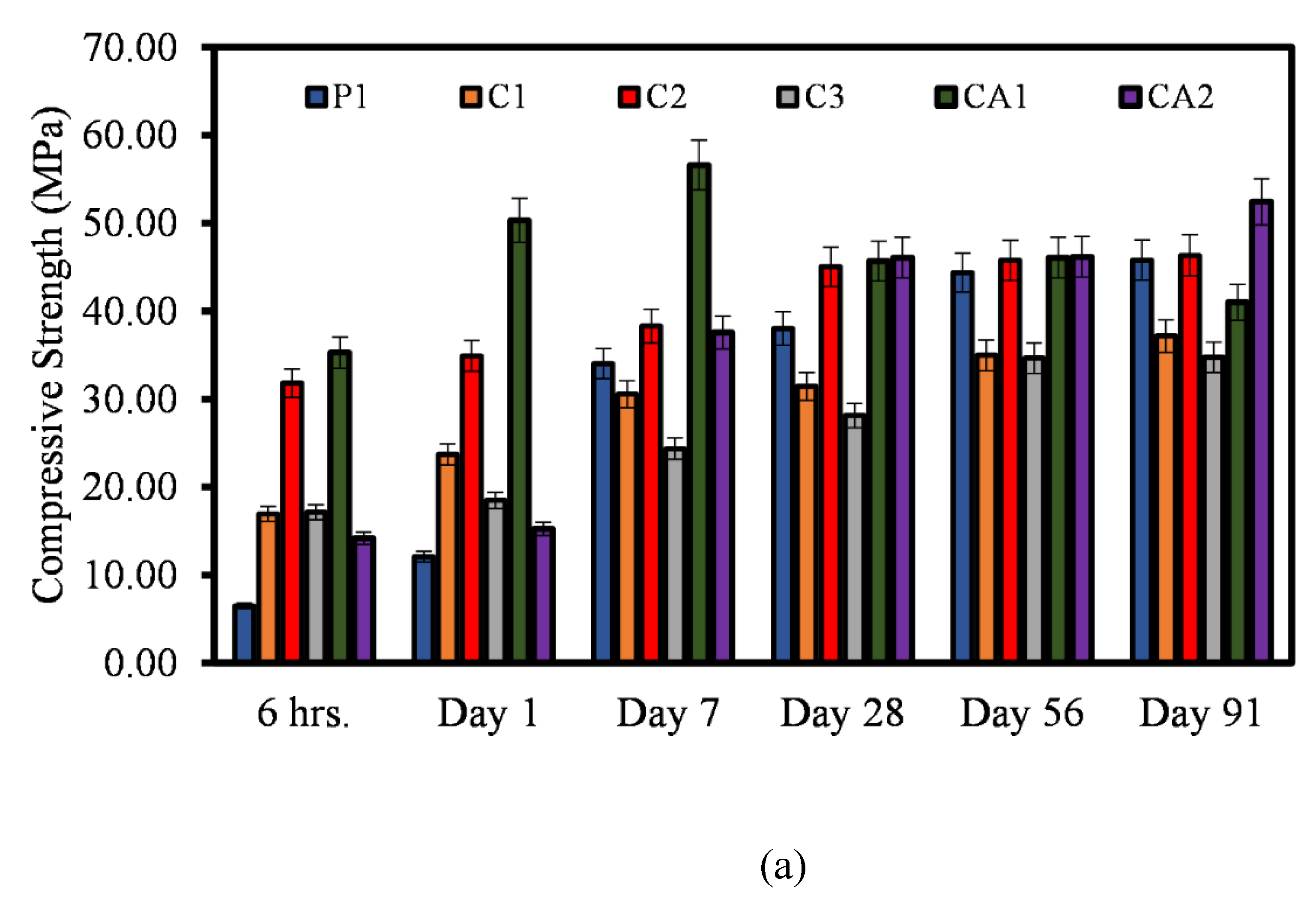
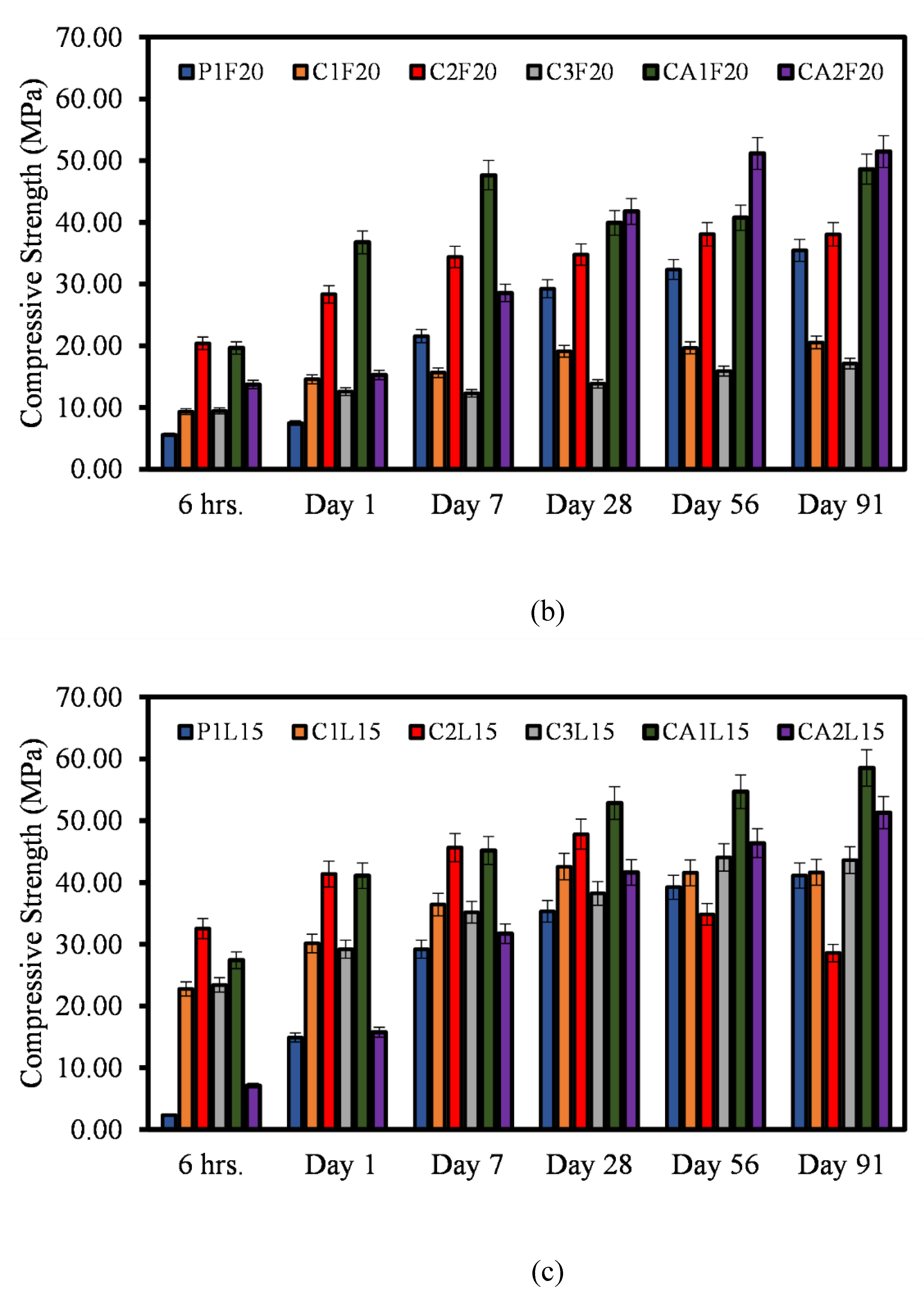
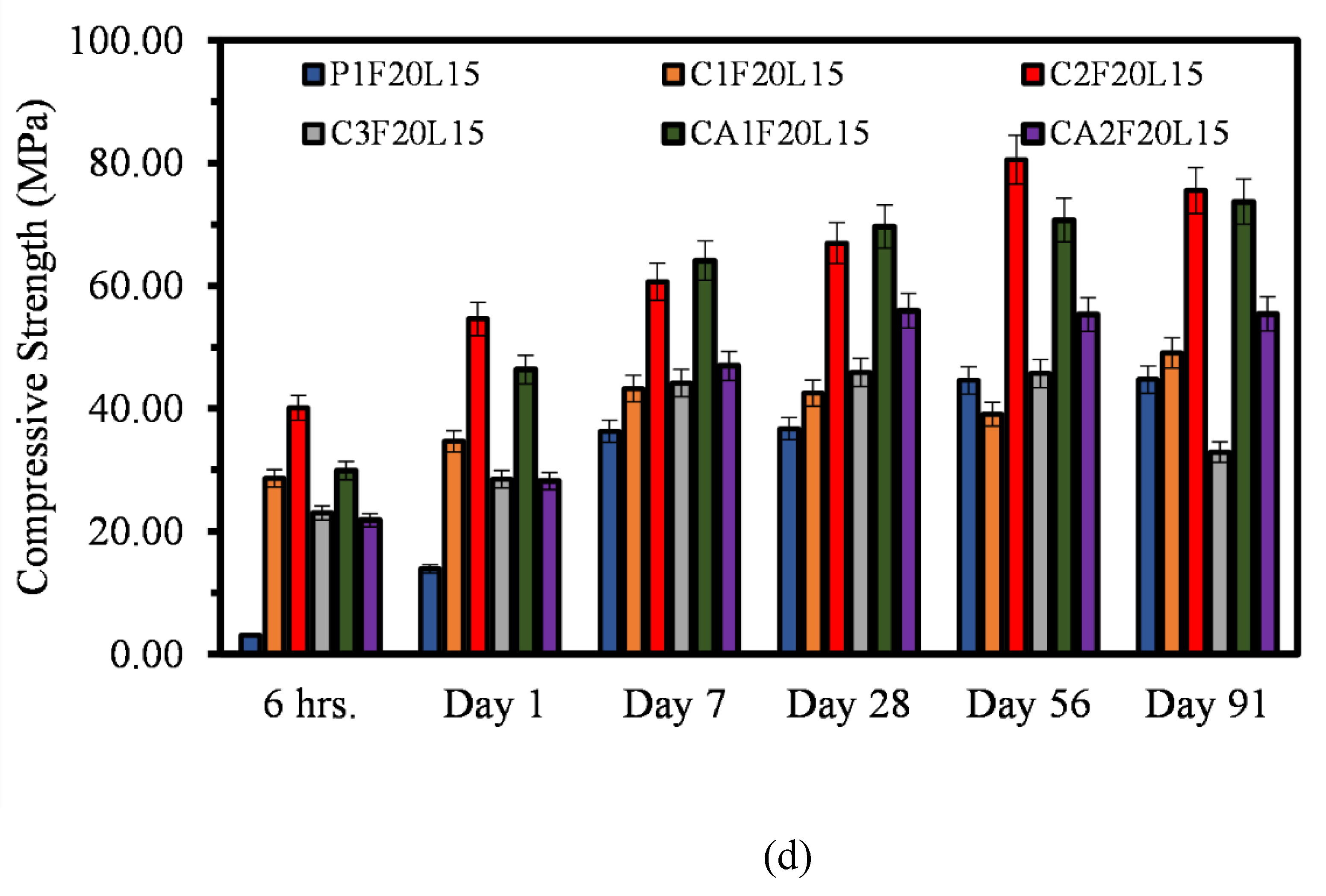
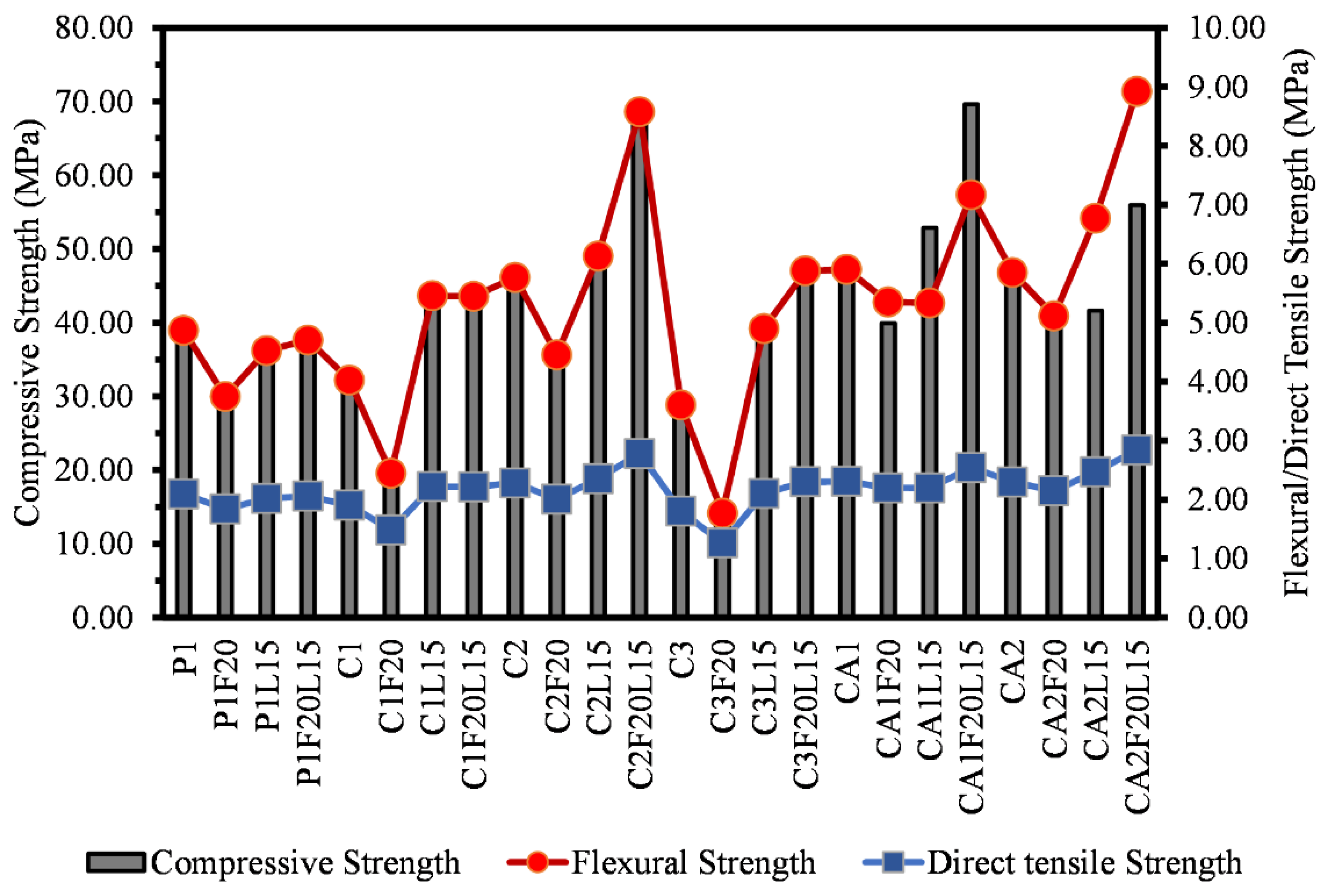
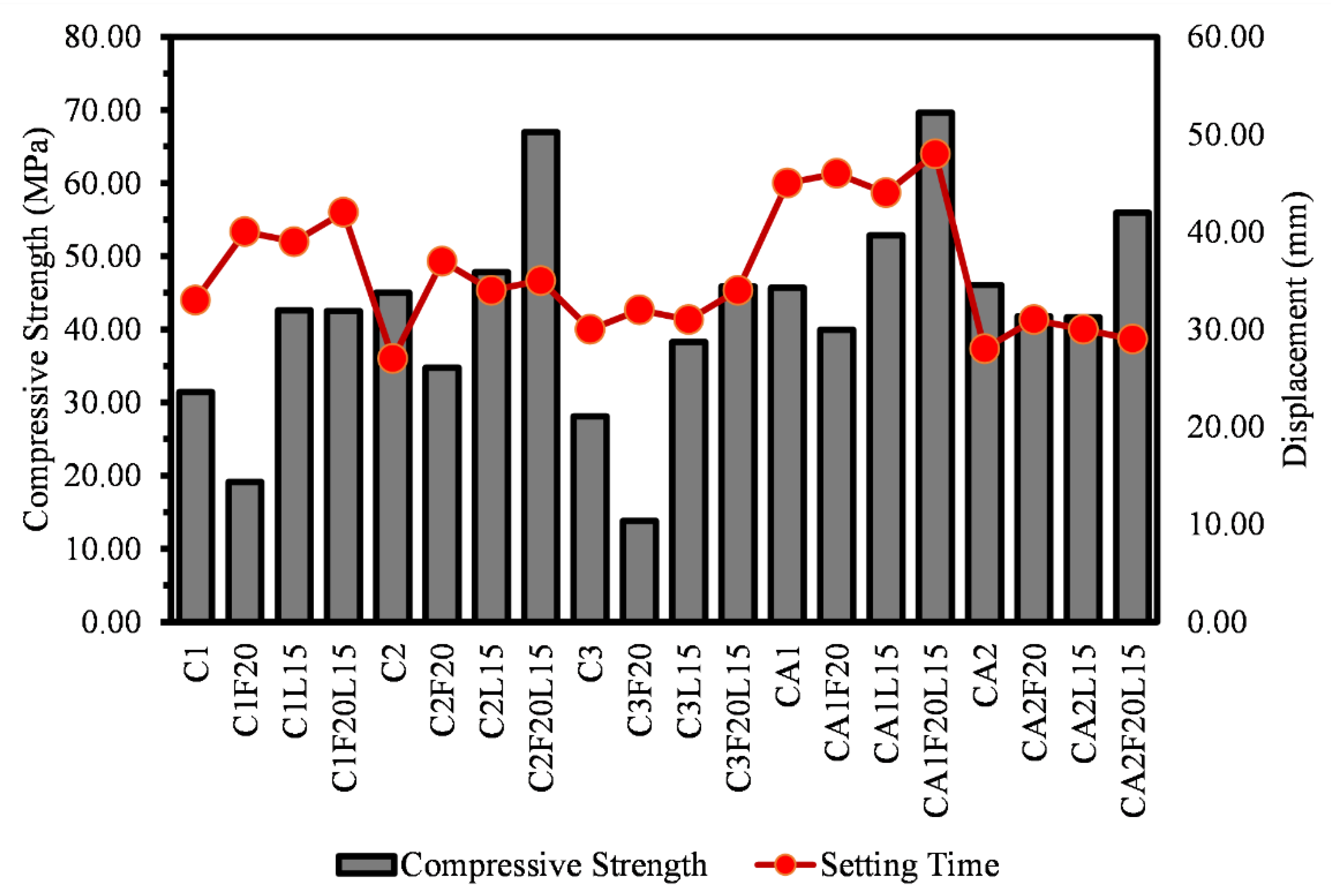
3.2. Compressive Strength
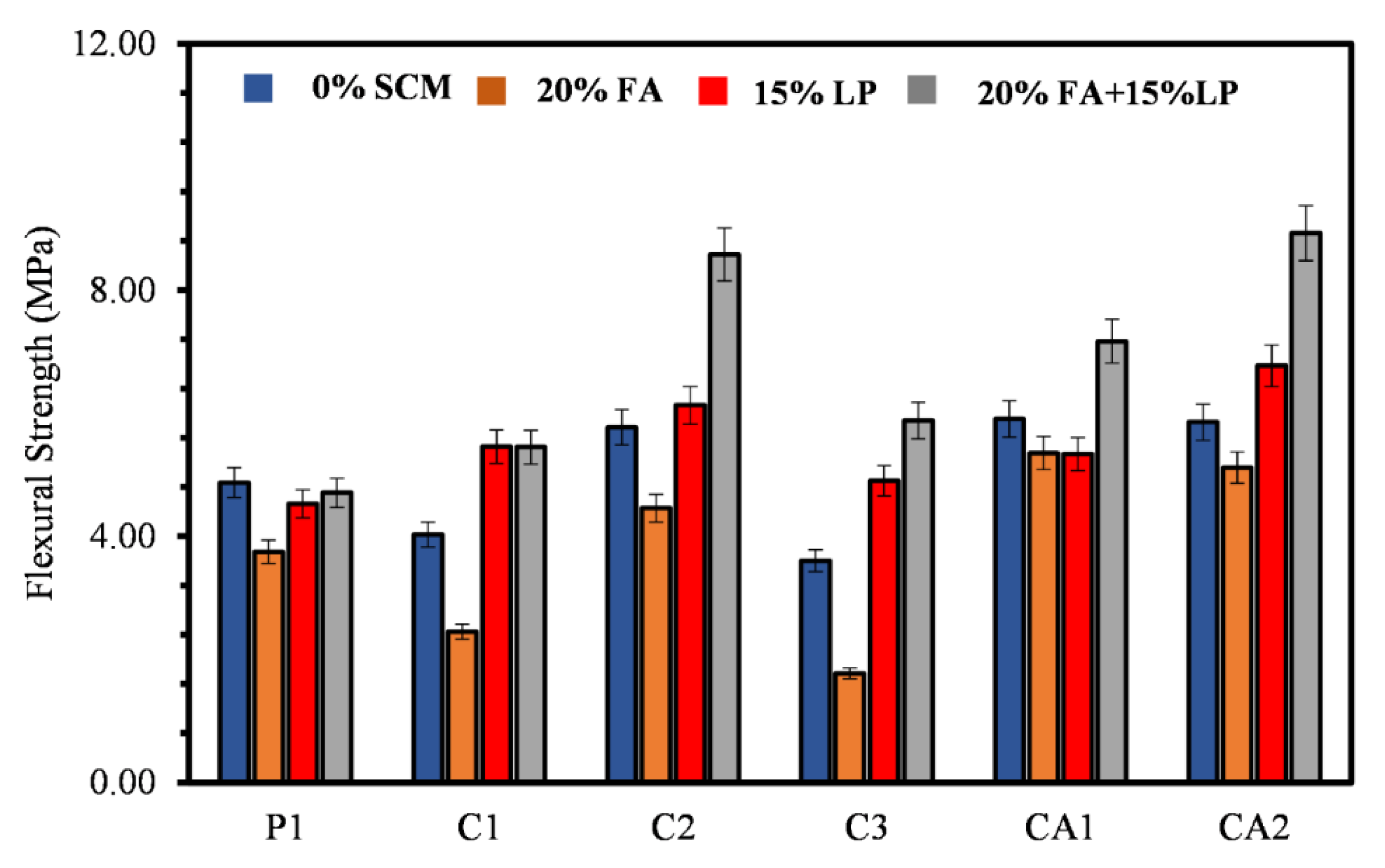
3.3. Flexural Strength
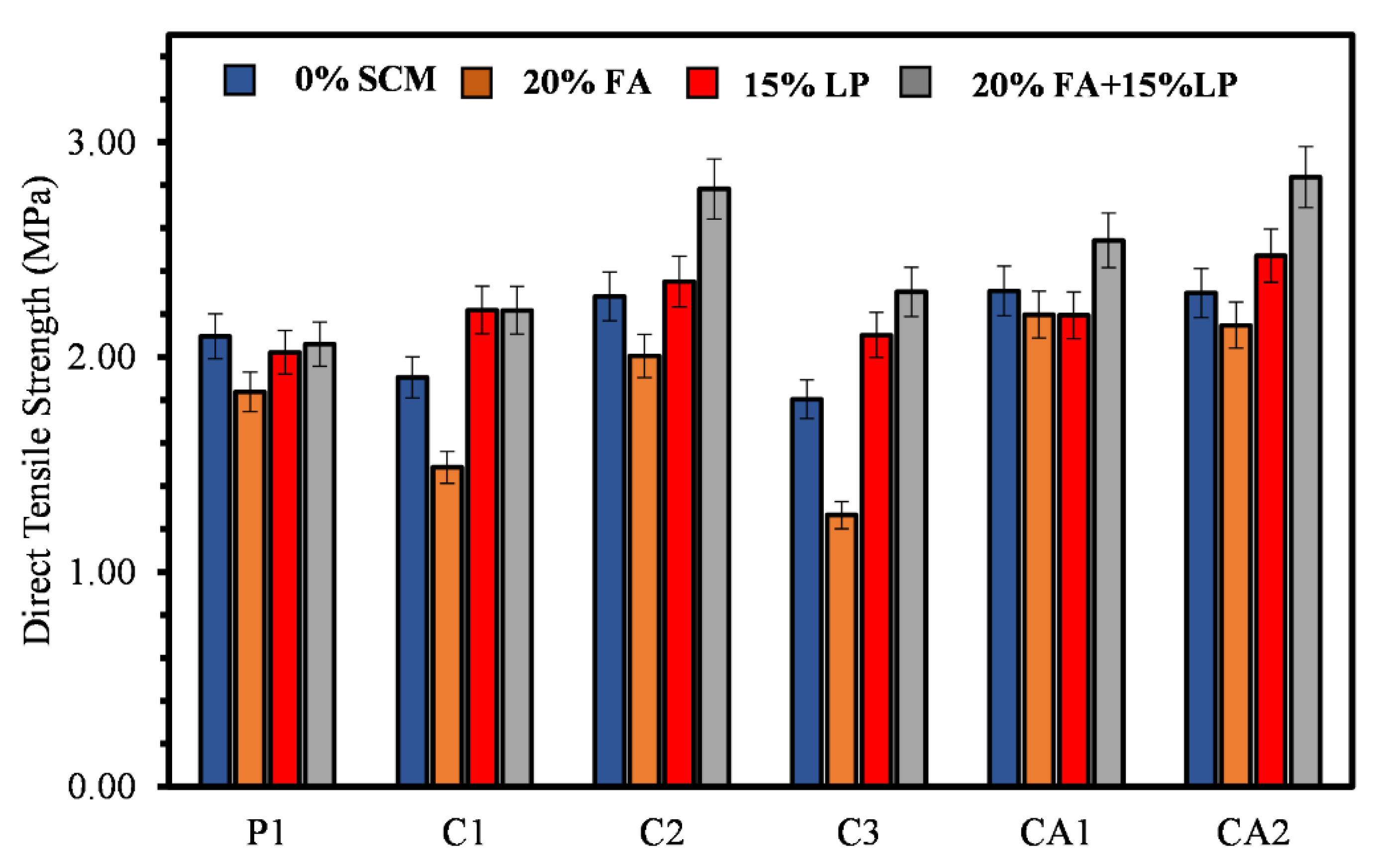
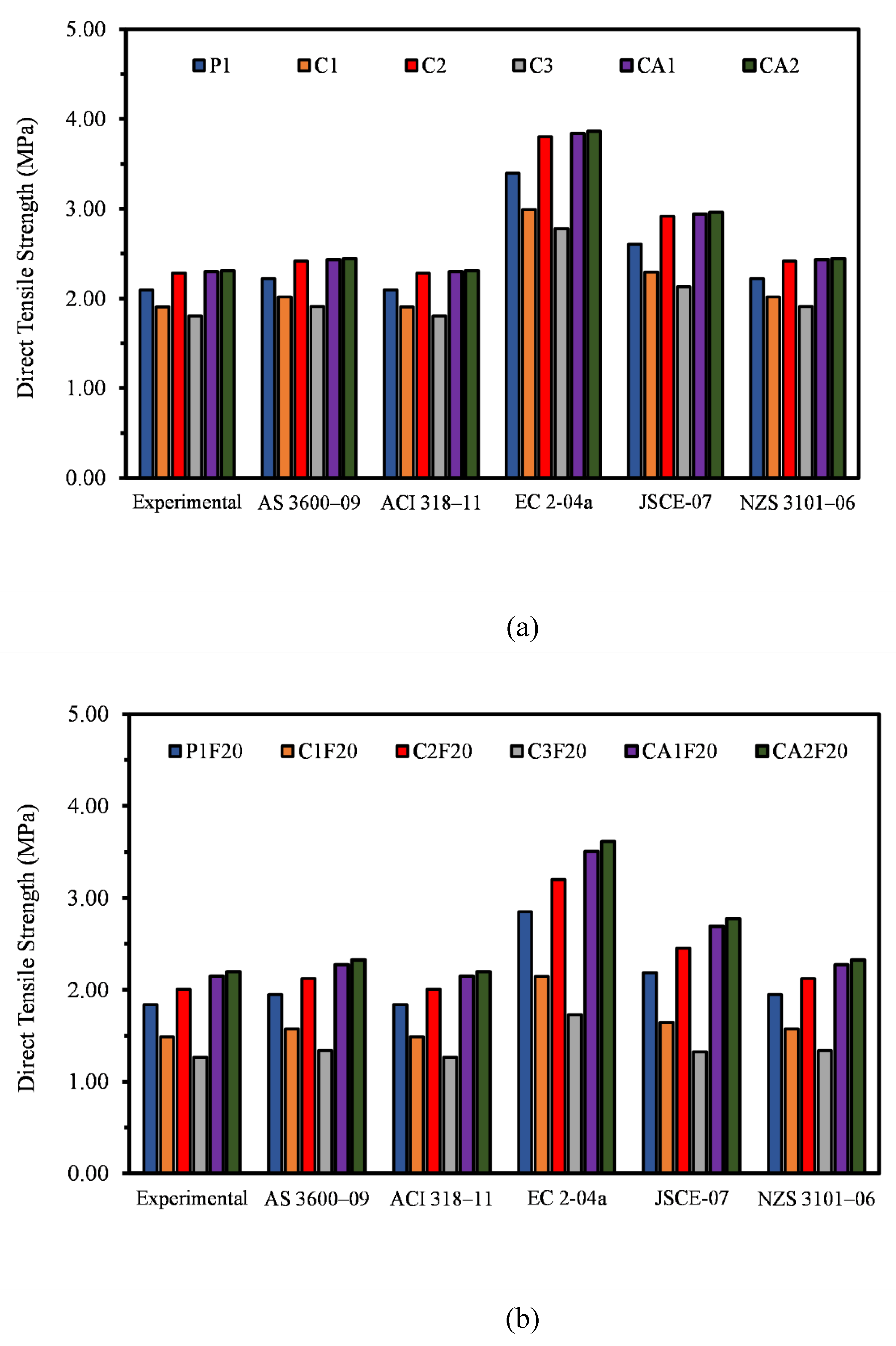
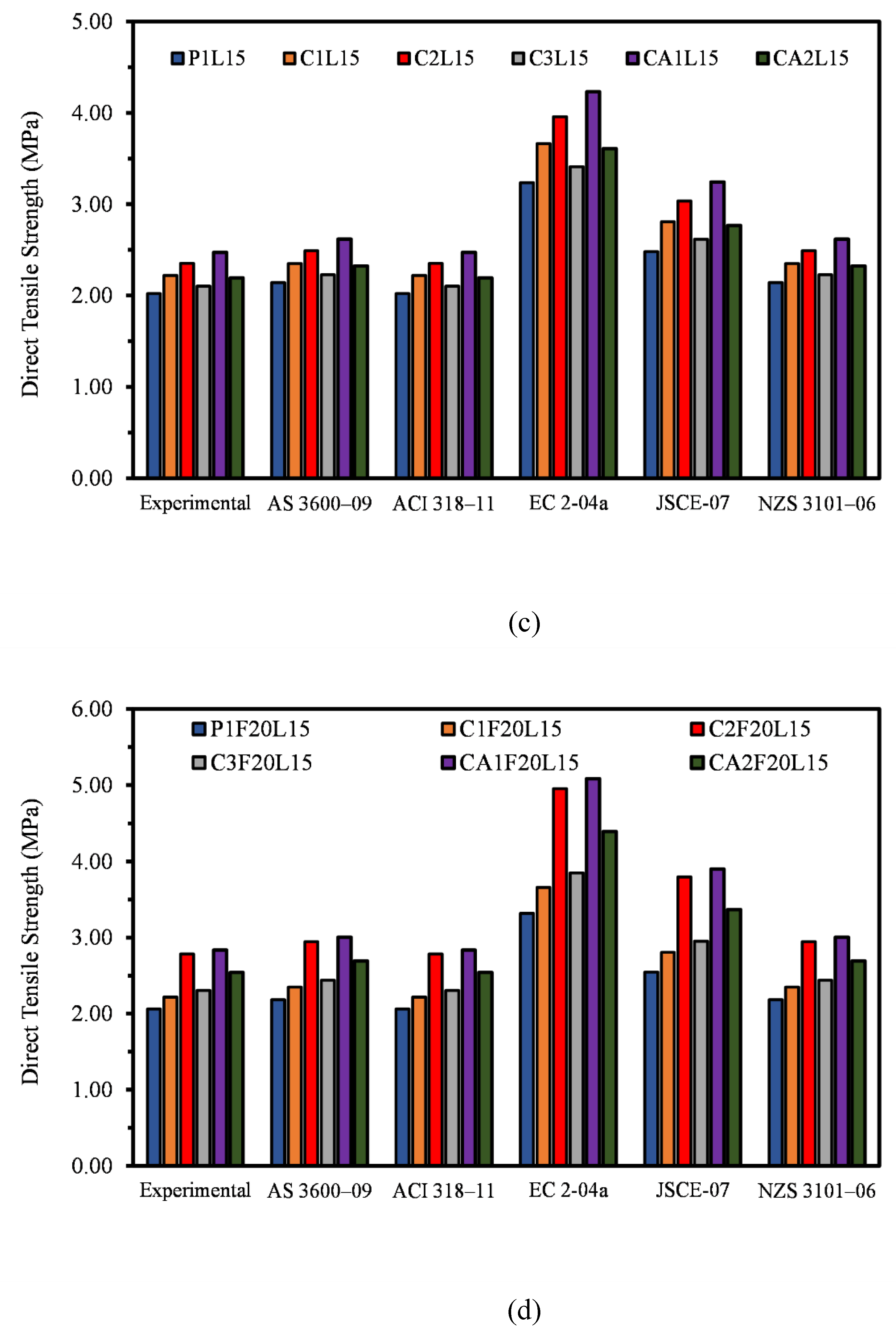
3.4. Direct Tensile Strength
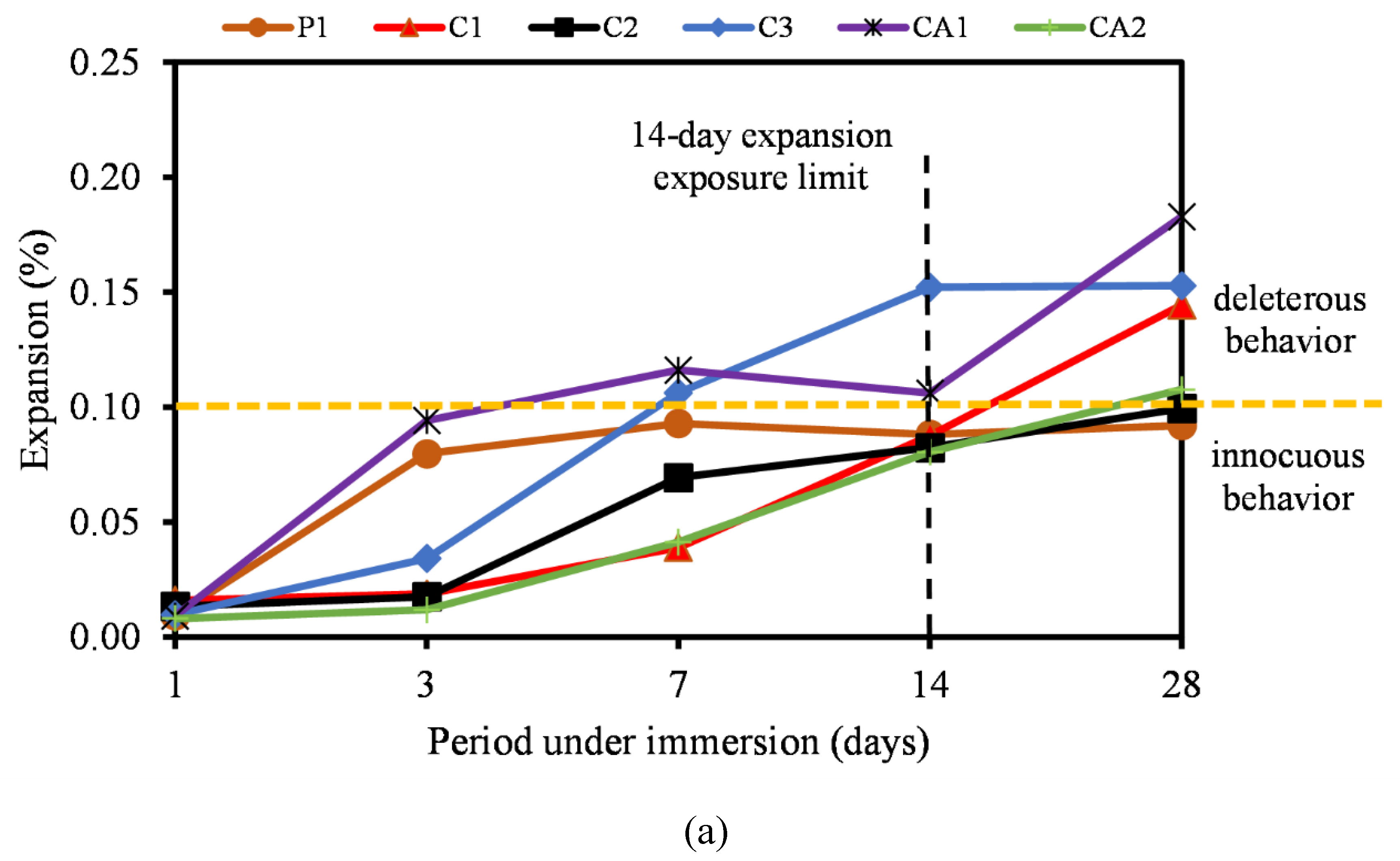
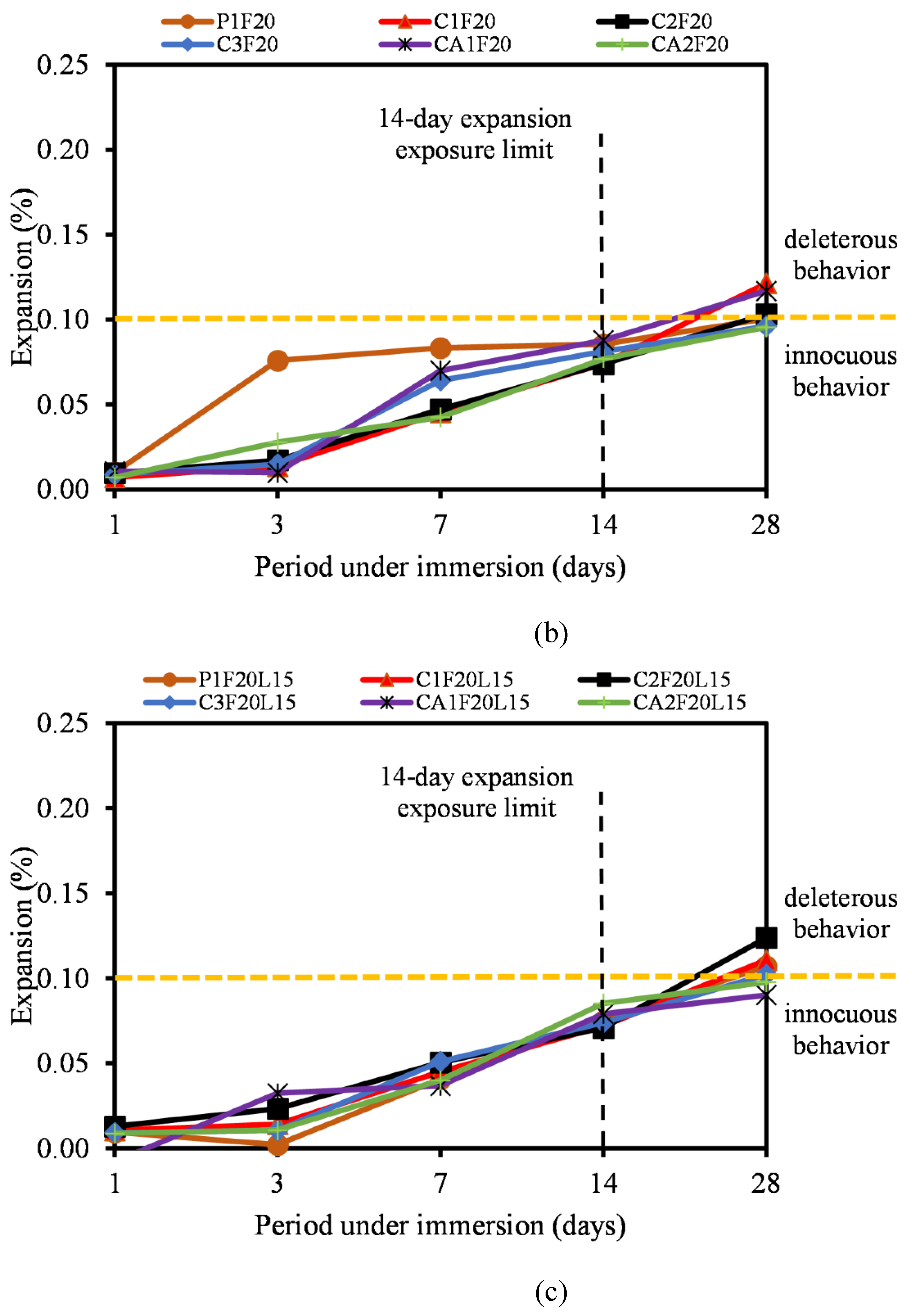
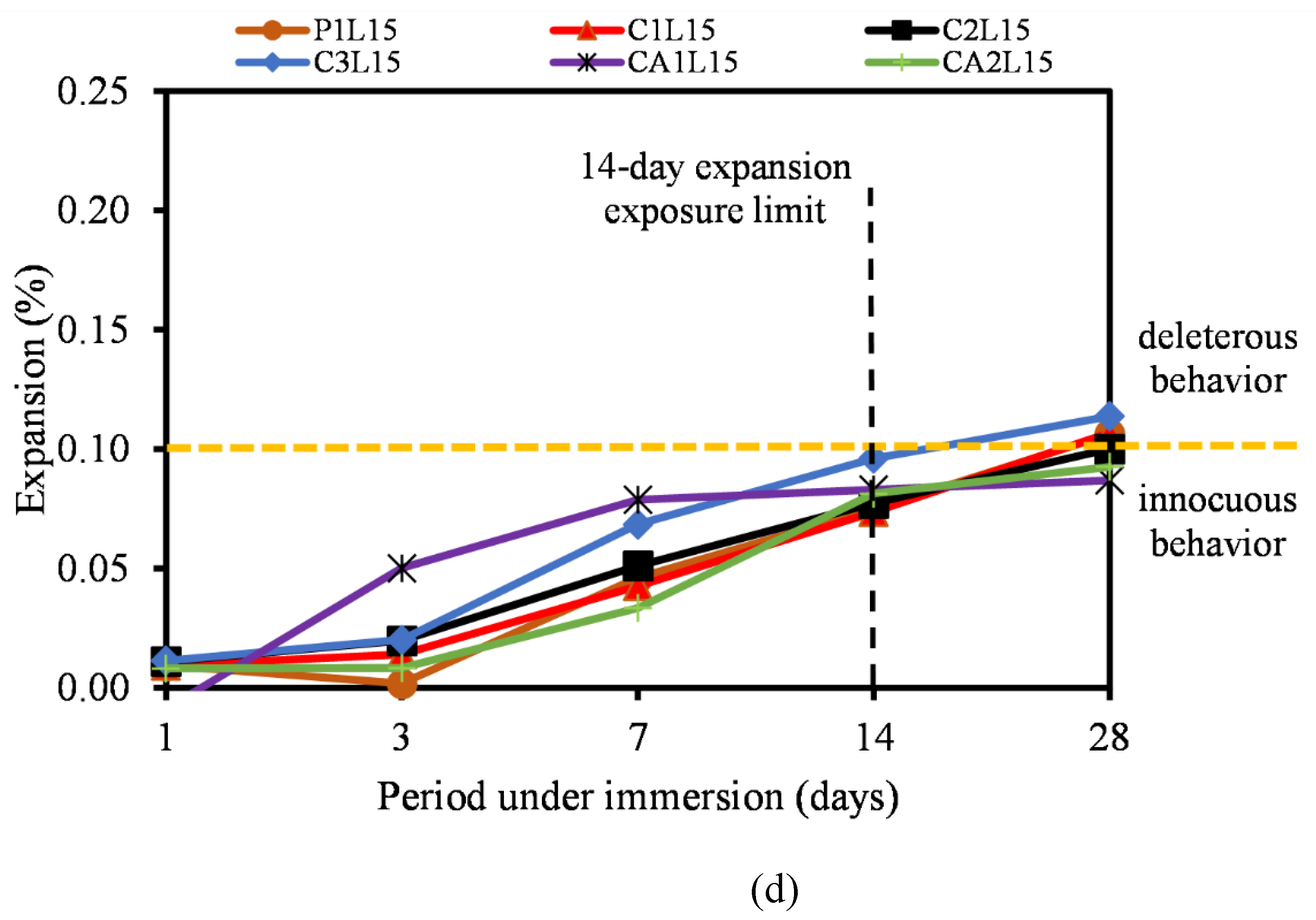
3.5. Alkali-Silica Reaction
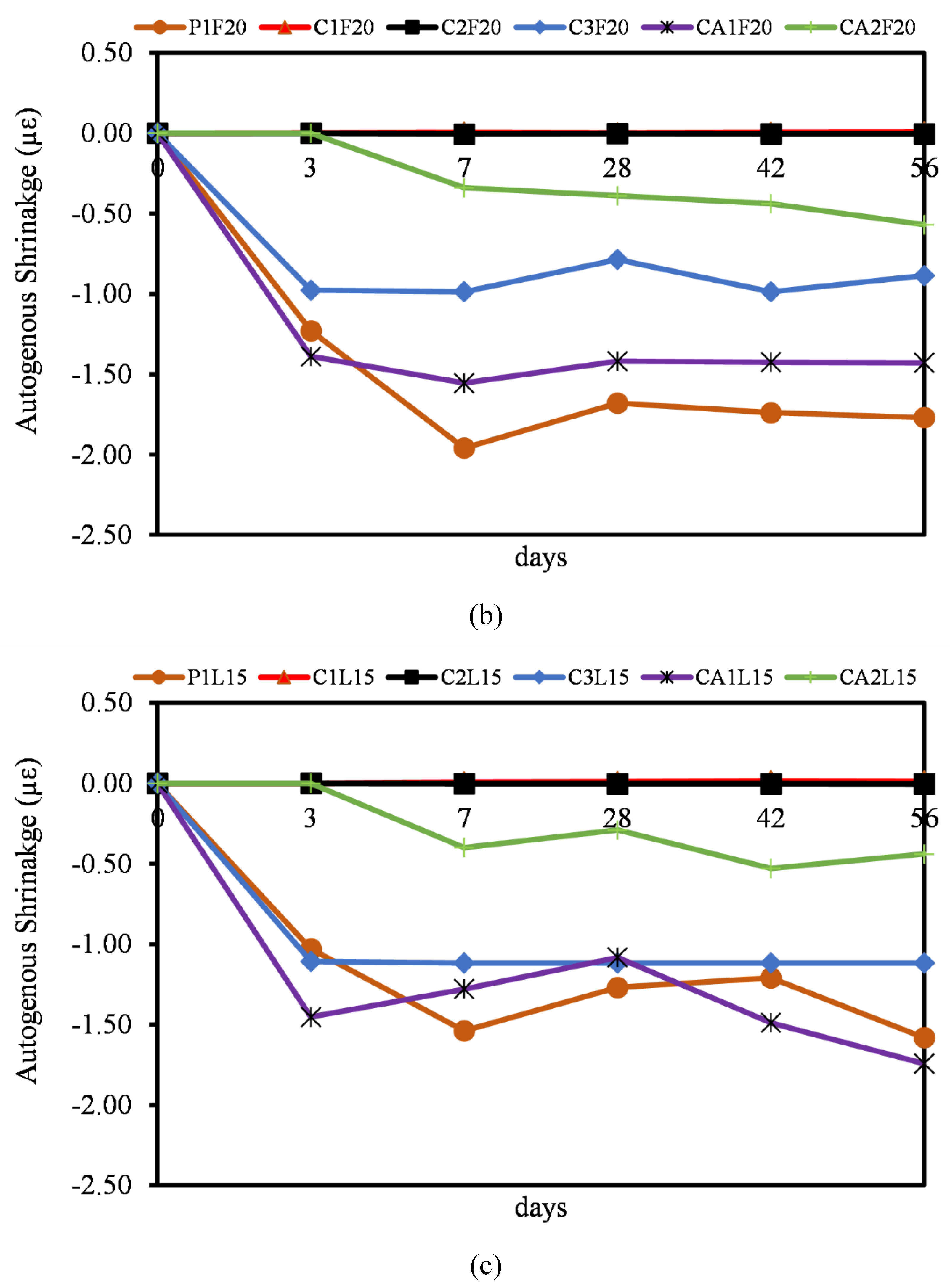
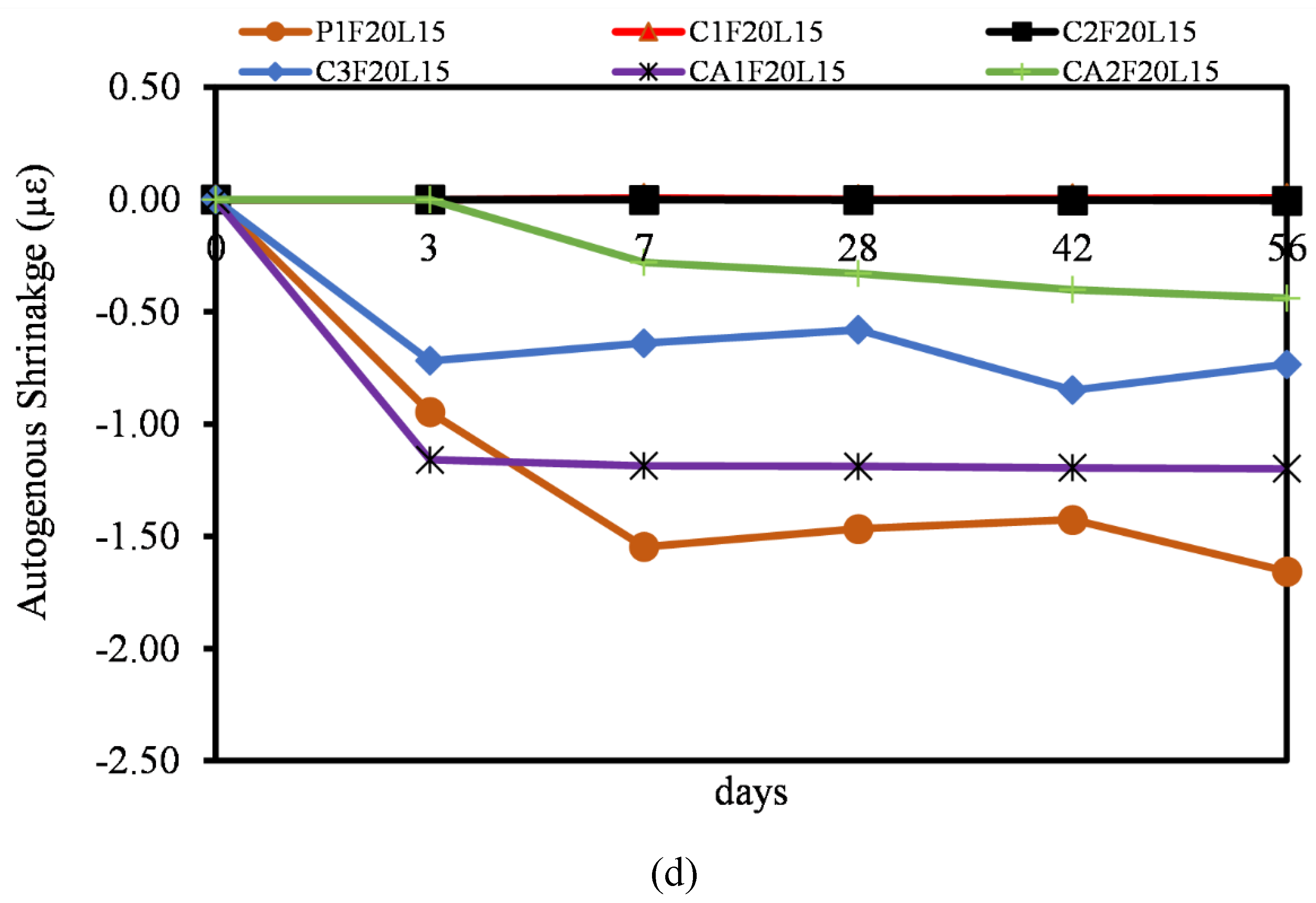
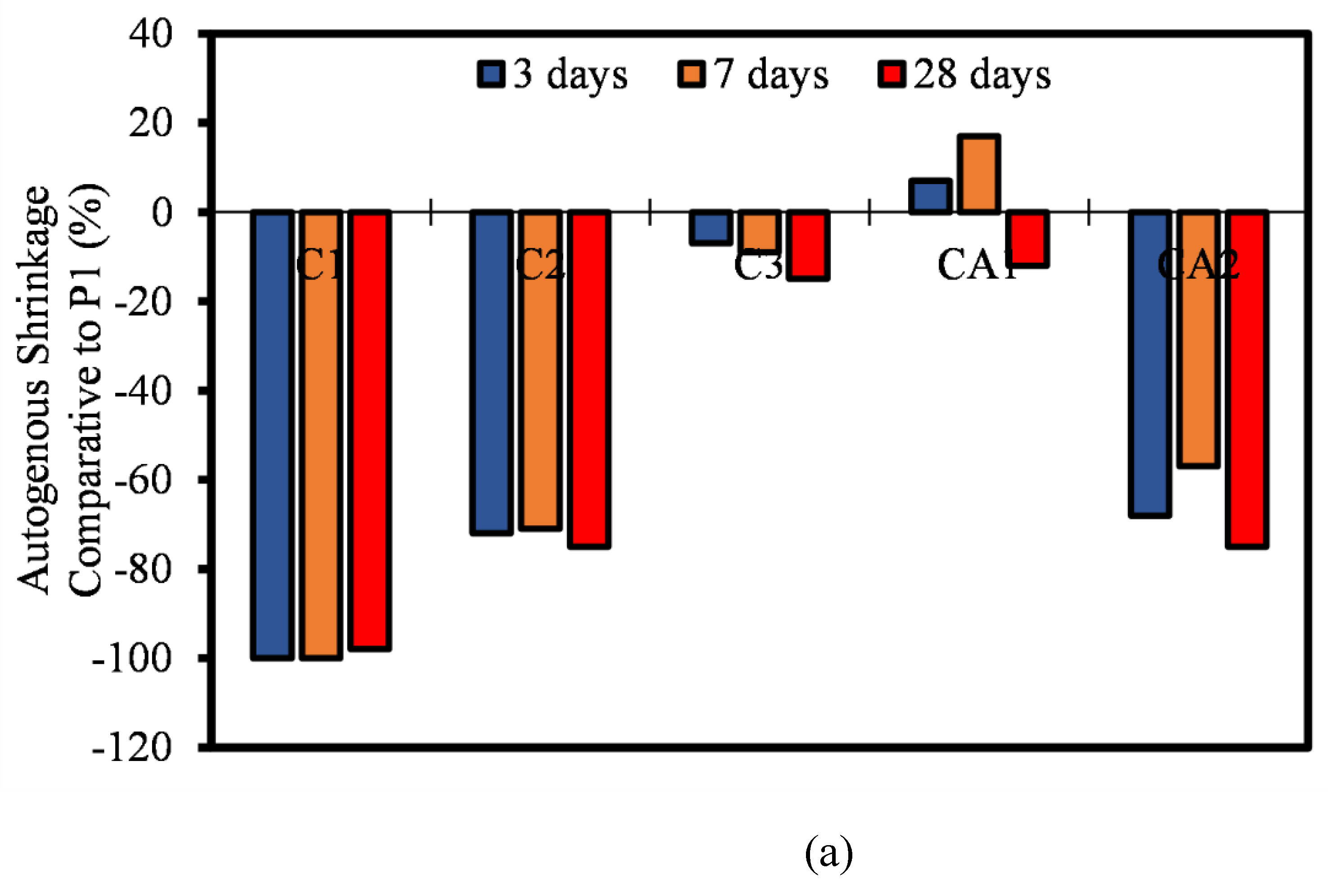
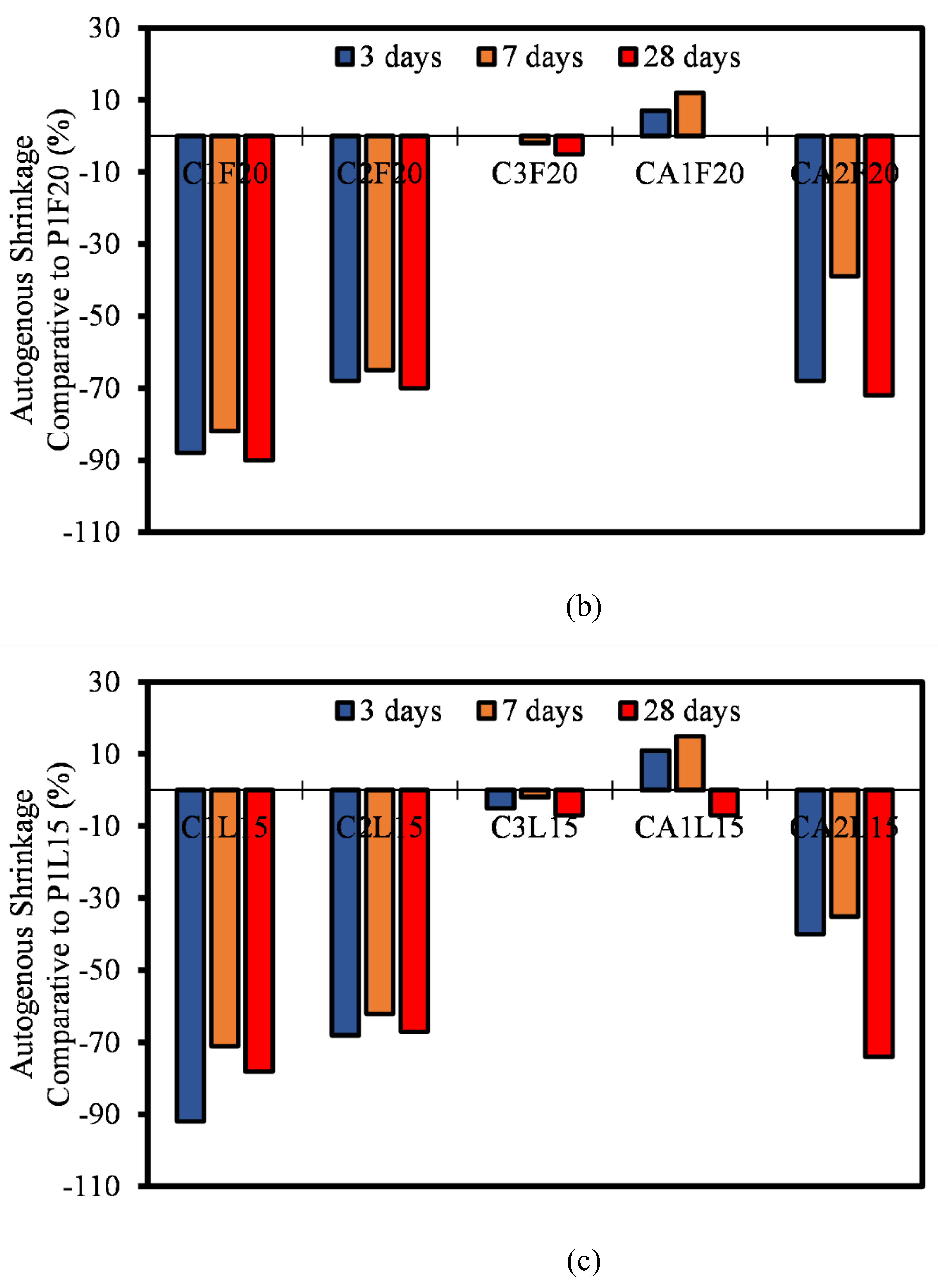
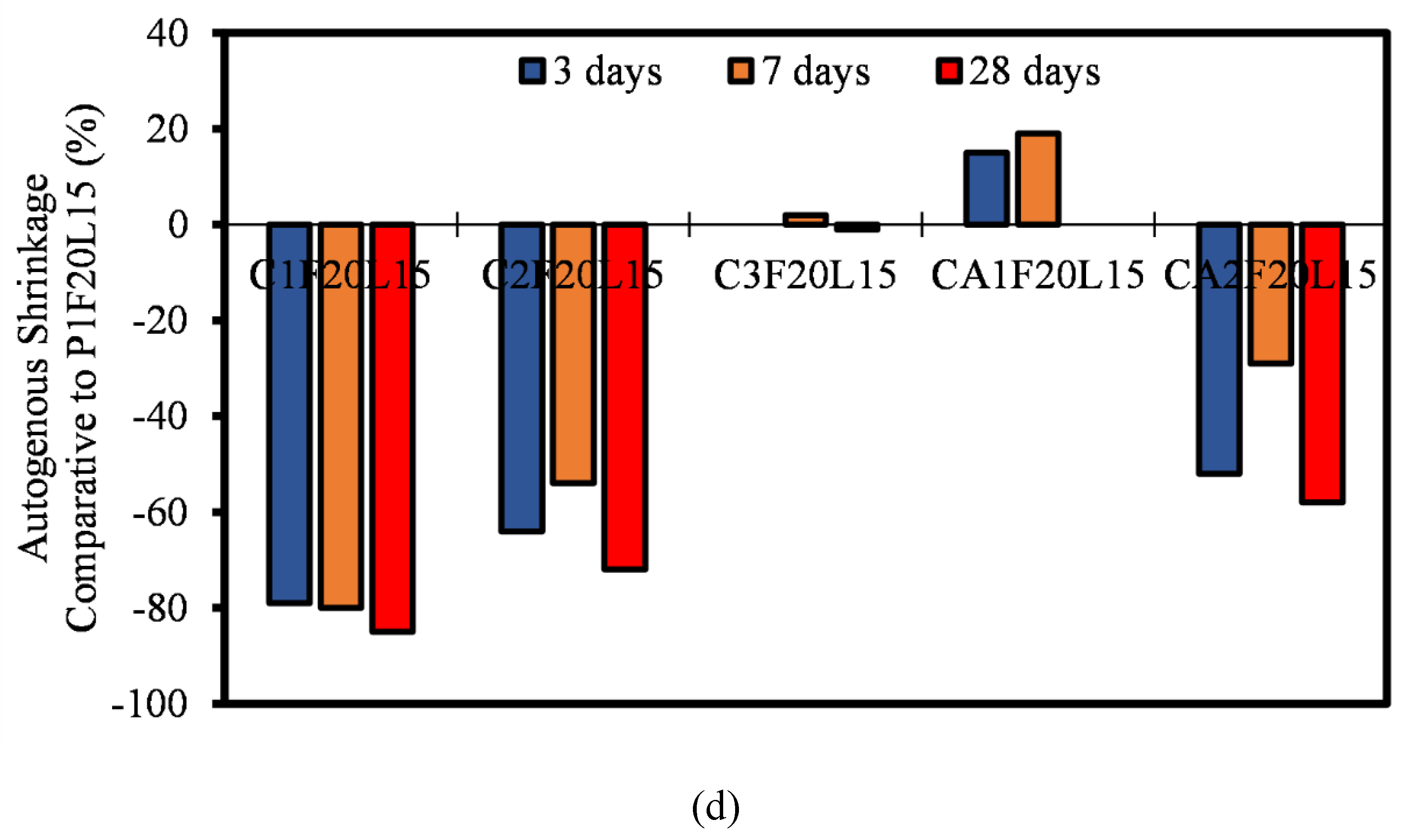
3.6. Autogenous Shrinkage
3.7. Microstructural Analysis
4. Conclusions
- The findings show that there is a correlation between the mechanical properties of both CSA and CAC cement mortars. There is a clear correlation between the compressive strength, flexural and the direct tensile strengths of the mortars. Similar relationship can be said for the compressive strength and the setting time of the mortar systems.
- Furthermore, the ASR of most CSA and CAC was lower than the 0.10% ASR recommendation. Most of the mixtures remained under innocuous conditions at 14 days of testing and only C1F20 and CA1F20 (FA incorporated samples) showed a slight expansion increase at 28 days of measurement.
- The incorporation of FA into CSA cement mortar serves a multifaceted role in mitigating ASR, a destructive chemical process leading to the expansion and cracking of the mortar. LP also reduced the risk of ASR by helping to mitigate length expansion and deleterious behavior of the mixtures as reported. Similarly, the reactivity of the fine aggregate used was regarded as low, hence could be attributed to the low reactivity of the cementitious binders.
- Most of the autogenous shrinkage for both CAC and CSA mortar mixtures had a lower autogenous shrinkage relative to OPC. The influence of the SCMs (FA and LP) was evident, even though there wasn’t significant reduction in the rate of autogenous shrinkage. It can be concluded that, at early ages, the reaction in autogenous shrinkage is usually only dependent on the hydration and mechanism of only the pure binders. The effect of FA and LP is believed to come into play after 28 days of age.
- Finally, these findings are significant for the cement and concrete industries, highlighting the potential of CAC and CSA mortars incorporating FA and LP in creating more sustainable, durable, and resilient building materials. This study not only provides valuable data on the mechanical and durability properties of these different mortar systems but also emphasizes areas for future research, especially in understanding the long-term effects of SCMs on CSA and CAC mortar performance. Such knowledge advances contribute to current literature and the capacity to construct sustainably and effectively, addressing global environmental issues while ensuring structural integrity.
References
- P. Hawkins, P. Tennis, R. Detwiler, The Use of Limestone in Portland Cement: A State-of-the-Art Review, 2003.
- J.B.S. Eikeland Per Ove, Corporate Responses to EU Emissions Trading: Resistance, Innovation or Responsibility?, Routledge, London, 2016. [CrossRef]
- Oliveira, I.; Ortega, F.; Pandolfelli, V. Hydration of CAC cement in a castable refractory matrix containing processing additives. Ceram. Int. 2009, 35, 1545–1552. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Pace, M.; Tomasulo, M.; Valenti, G.; Monteiro, P. A hydration study of various calcium sulfoaluminate cements. Cem. Concr. Compos. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- Gartner, E. Industrially interesting approaches to ‘low-CO2’ cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Sharp, J.H.; Lawrence, C.D.; Yang, R. Calcium sulfoaluminate cements—low-energy cements, special cements or what? Adv. Cem. Res. 1999, 11, 3–13. [Google Scholar] [CrossRef]
- Glasser, F.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate–belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Zhang, L.; Glasser, F.P. Investigation of the microstructure and carbonation of CS¯A-based concretes removed from service. Cem. Concr. Res. 2005, 35, 2252–2260. [Google Scholar] [CrossRef]
- Gastaldi, D.; Paul, G.; Marchese, L.; Irico, S.; Boccaleri, E.; Mutke, S.; Buzzi, L.; Canonico, F. Hydration products in sulfoaluminate cements: Evaluation of amorphous phases by XRD/solid-state NMR. Cem. Concr. Res. 2016, 90, 162–173. [Google Scholar] [CrossRef]
- García-Maté, M.; De la Torre, A.; León-Reina, L.; Aranda, M.; Santacruz, I. Hydration studies of calcium sulfoaluminate cements blended with fly ash. Cem. Concr. Res. 2013, 54, 12–20. [Google Scholar] [CrossRef]
- Fan, W.; Zhuge, Y.; Ma, X.; Chow, C.W.; Gorjian, N. Strain hardening behaviour of PE fibre reinforced calcium aluminate cement (CAC) – Ground granulated blast furnace (GGBFS) blended mortar. Constr. Build. Mater. 2020, 241, 118100. [Google Scholar] [CrossRef]
- Voegel, C.; Giroudon, M.; Bertron, A.; Patapy, C.; Matthieu, P.L.; Verdier, T.; Erable, B. Cementitious materials in biogas systems: Biodeterioration mechanisms and kinetics in CEM I and CAC based materials. Cem. Concr. Res. 2019, 124. [Google Scholar] [CrossRef]
- J. Bizzozero, Hydration and dimensional stability of calcium aluminate cement based systems, EPFL, 2014. [CrossRef]
- Calcium aluminate, expansive and other cements, Default Book Series (n.d.). [CrossRef]
- Midgley, H.G.; Midgley, A. The conversion of high alumina cement. Mag. Concr. Res. 1975, 27, 59–77. [Google Scholar] [CrossRef]
- Shirani, S.; Cuesta, A.; De la Torre, A.; Diaz, A.; Trtik, P.; Holler, M.; Aranda, M. Calcium aluminate cement conversion analysed by ptychographic nanotomography. Cem. Concr. Res. 2020, 137, 106201. [Google Scholar] [CrossRef]
- Pelletier-Chaignat, L.; Winnefeld, F.; Lothenbach, B.; Müller, C.J. Beneficial use of limestone filler with calcium sulphoaluminate cement. Constr. Build. Mater. 2012, 26, 619–627. [Google Scholar] [CrossRef]
- Martin, L.H.; Winnefeld, F.; Müller, C.J.; Lothenbach, B. Contribution of limestone to the hydration of calcium sulfoaluminate cement. Cem. Concr. Compos. 2015, 62, 204–211. [Google Scholar] [CrossRef]
- Hargis, C.W.; Telesca, A.; Monteiro, P.J. Calcium sulfoaluminate (Ye'elimite) hydration in the presence of gypsum, calcite, and vaterite. Cem. Concr. Res. 2014, 65, 15–20. [Google Scholar] [CrossRef]
- Živica, V. Properties of blended sulfoaluminate belite cement. Constr. Build. Mater. 2000, 14, 433–437. [Google Scholar] [CrossRef]
- De Weerdt, K.; Ben Haha, M.; Le Saout, G.; Kjellsen, K.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Baert, G.; Hoste, S.; De Schutter, G.; De Belie, N. Reactivity of fly ash in cement paste studied by means of thermogravimetry and isothermal calorimetry. J. Therm. Anal. Calorim. 2008, 94, 485–492. [Google Scholar] [CrossRef]
- B. Lothenbach, K. Scrivener, R. Hooton, Supplementary cementitious materials, Cement and Concrete Research 41 (2011) 1244–1256.
- Rahhal, V.; Talero, R. Calorimetry of Portland cement with metakaolins, quartz and gypsum additions. J. Therm. Anal. Calorim. 2008, 91, 825–834. [Google Scholar] [CrossRef]
- Hwang, C.-L.; Shen, D.-H. The effects of blast-furnace slag and fly ash on the hydration of portland cement. Cem. Concr. Res. 1991, 21, 410–425. [Google Scholar] [CrossRef]
- Ioannou, S.; Reig, L.; Paine, K.; Quillin, K. Properties of a ternary calcium sulfoaluminate–calcium sulfate–fly ash cement. Cem. Concr. Res. 2014, 56, 75–83. [Google Scholar] [CrossRef]
- Ma, B.; Li, X.; Shen, X.; Mao, Y.; Huang, H. Enhancing the addition of fly ash from thermal power plants in activated high belite sulfoaluminate cement. Constr. Build. Mater. 2014, 52, 261–266. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements — Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2009, 40, 1239–1247. [Google Scholar] [CrossRef]
- Andac, M.; Glasser, F.P. Pore solution composition of calcium sulfoaluminate cement. Adv. Cem. Res. 1999, 11, 23–26. [Google Scholar] [CrossRef]
- Martin, L.H.; Winnefeld, F.; Tschopp, E.; Müller, C.J.; Lothenbach, B. Influence of fly ash on the hydration of calcium sulfoaluminate cement. Cem. Concr. Res. 2017, 95, 152–163. [Google Scholar] [CrossRef]
- Collepardi, M.; Monosi, S.; Piccioli, P. The influence of pozzolanic materials on the mechanical stability of aluminous cement. Cem. Concr. Res. 1995, 25, 961–968. [Google Scholar] [CrossRef]
- López-Zaldívar, O.; Lozano-Díez, R.; Verdú-Vázquez, A.; Llauradó-Pérez, N. Effects of the addition of inertized MSW fly ash on calcium aluminate cement mortars. Constr. Build. Mater. 2017, 157, 1106–1116. [Google Scholar] [CrossRef]
- Pyatina, T.; Sugama, T. Acid resistance of calcium aluminate cement–fly ash F blends. Adv. Cem. Res. 2016, 28, 433–457. [Google Scholar] [CrossRef]
- Standard Guide for Reducing the Risk of Deleterious Alkali-Aggregate Reaction in Concrete, (n.d.). https://www.astm.org/c1778-23.html (accessed February 21, 2024).
- Ma, B.; Ma, M.; Shen, X.; Li, X.; Wu, X. Compatibility between a polycarboxylate superplasticizer and the belite-rich sulfoaluminate cement: Setting time and the hydration properties. Constr. Build. Mater. 2014, 51, 47–54. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Bullerjahn, F.; Ben Haha, M. Effect of retarders on the early hydration of calcium-sulpho-aluminate (CSA) type cements. Cem. Concr. Res. 2016, 84, 62–75. [Google Scholar] [CrossRef]
- Brien, J.V.; Henke, K.R.; Mahboub, K.C. OBSERVATIONS OF PEAK STRENGTH BEHAVIOR IN CSA CEMENT MORTARS. J. Green Build. 2013, 8, 97–115. [Google Scholar] [CrossRef]
- Zhang, L.; Glasser, F.P. Hydration of calcium sulfoaluminate cement at less than 24 h. Adv. Cem. Res. 2002, 14, 141–155. [Google Scholar] [CrossRef]
- Sahu, S.; Havlica, J.; Tomková, V.; Majling, J. Hydration behaviour of sulphoaluminate belite cement in the presence op various calcium sulphates. Thermochim. Acta 1991, 175, 45–52. [Google Scholar] [CrossRef]
- Cheung, J.; Jeknavorian, A.; Roberts, L.; Silva, D. Impact of admixtures on the hydration kinetics of Portland cement. Cem. Concr. Res. 2011, 41, 1289–1309. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Zajac, M.; Skocek, J.; Ben Haha, M. The role of boron during the early hydration of belite ye’elimite ferrite cements. Constr. Build. Mater. 2019, 215, 252–263. [Google Scholar] [CrossRef]
- Zou, F.; Tan, H.; Guo, Y.; Ma, B.; He, X.; Zhou, Y. Effect of sodium gluconate on dispersion of polycarboxylate superplasticizer with different grafting density in side chain. J. Ind. Eng. Chem. 2017, 55, 91–100. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, J.; Qi, Q.; Li, H.; Zhang, N.; Mu, R. Influence of Fly Ash on Mechanical Properties and Hydration of Calcium Sulfoaluminate-Activated Supersulfated Cement. Materials 2020, 13, 2514. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Hu, W.; Wu, K. Durability and microstructure of CSA cement-based materials from MSWI fly ash. Cem. Concr. Compos. 2013, 46, 26–31. [Google Scholar] [CrossRef]
- Markosian, N.; Tawadrous, R.; Mastali, M.; Thomas, R.J.; Maguire, M. Performance Evaluation of a Prestressed Belitic Calcium Sulfoaluminate Cement (BCSA) Concrete Bridge Girder. Sustainability 2021, 13, 7875. [Google Scholar] [CrossRef]
- Dunster, I. Holton, A laboratory study of the resistance of CAC concretes to chemical attack by sulphate and alkali carbonate solutions, in: 2001: pp. 333–348.
- Soroka, I.; Stern, N. Calcareous fillers and the compressive strength of portland cement. Cem. Concr. Res. 1976, 6, 367–376. [Google Scholar] [CrossRef]
- L. Amathieu, T. Bier, K. Scrivener, Mechanisms of set acceleration of Portland cement through CAC addition, International Conference on Calcium Aluminate Cements (2001) 303–317.
- Ali, M.; Gopal, S.; Handoo, S. Studies on the formation kinetics of calcium sulphoaluminate. Cem. Concr. Res. 1994, 24, 715–720. [Google Scholar] [CrossRef]
- Rodrıguez, R.; Gutierrez, I. Correlation between nanoindentation and tensile propertiesInfluence of the indentation size effect. Mater. Sci. Eng. A 2003, 361, 377–384. [Google Scholar] [CrossRef]
- Brien, J.V.; Henke, K.R.; Mahboub, K.C. INFLUENCE OF LATEX POLYMER ADDITION ON THE BEHAVIOR OF MATERIALS CONTAINING CSA CEMENT CURED AT LOW HUMIDITY. J. Green Build. 2013, 8, 94–109. [Google Scholar] [CrossRef]
- Dashti, J.; Nematzadeh, M. Compressive and direct tensile behavior of concrete containing Forta-Ferro fiber and calcium aluminate cement subjected to sulfuric acid attack with optimized design. Constr. Build. Mater. 2020, 253, 118999. [Google Scholar] [CrossRef]
- AS 3600:2018 | Concrete Structures, Steel & Tendons | SAI Global, (n.d.). https://infostore.saiglobal.com/en-us/Standards/AS-3600-2018-98877_SAIG_AS_AS_207930/ (accessed January 25, 2024).
- ACI: Building code requirements for structural concrete... - Google Scholar, (n.d.). https://scholar.google.com/scholar_lookup?title=%E2%80%9C318-11%3A+Building+Code+Requirements+for+Structural+Concrete+and+Commentary+%28318%E2%80%9311%29%2C%E2%80%9D+ed%3A+American+Concrete+Institute&author=A.+Aci&publication_year=2011&inst=6114818187226770759 (accessed January 25, 2024).
- Européen: Eurocode 2: Design of concrete structures—pa... - Google Scholar, (n.d.). https://scholar.google.com/scholar_lookup?title=Eurocode+2%3A+Design+of+concrete+structures%3A+Part+1%E2%80%931%3A+General+rules+and+rules+for+buildings&author=B.S.+Institution&publication_year=2004&inst=6114818187226770759 (accessed January 25, 2024).
- Uomoto, T.; Ishibashi, T.; Nobuta, Y.; Satoh, T.; Kawano, H.; Takewaka, K.; Uji, K. Standard Specifications for Concrete Structures-2007 by Japan Society of Civil Engineers. Concr. J. 2008, 46, 3–14. [Google Scholar] [CrossRef]
- NZS 3101.1:2006 (inc A1, A2, A3) Concrete structures standard. - Part 1: The design of concrete structures. - SPONSORED. | Building CodeHub, (n.d.). https://codehub.building.govt.nz/resources/nzs-3101-12006-inc-a1-a2-a3/ (accessed January 25, 2024).
- Cofired biomass fly ashes in mortar: Reduction of Alkali Silica Reaction (ASR) expansion, pore solution chemistry and the effects on compressive strength | Semantic Scholar, (n.d.). https://www.semanticscholar.org/paper/Cofired-biomass-fly-ashes-in-mortar%3A-Reduction-of-Wang/374c86198c39c5004c259671e812ec2ff2ea5807 (accessed March 19, 2024).
- H. Tariq, R. Azam, A. Ahmed, W. Abbass, S. Abbas, THE EFFECT OF ALKALI CONTENT OF CEMENT ON ASR EXPANSION: EVALUATION AND ITS MITIGATION USING FLY ASH, (n.d.).
- Chopperla, K.S.T.; Smith, J.A.; Ideker, J.H. The efficacy of portland-limestone cements with supplementary cementitious materials to prevent alkali-silica reaction. Cement 2022, 8, 100031. [Google Scholar] [CrossRef]
- Lindgård, J.; Andiç-Çakır, Ö.; Fernandes, I.; Rønning, T.F.; Thomas, M.D.A. Alkali–silica reactions (ASR): Literature review on parameters influencing laboratory performance testing. Cem. Concr. Res. 2012, 42, 223–243. [Google Scholar] [CrossRef]
- R. Detwiler, The Role of Fly Ash Composition in Reducing Alkali-Silica Reaction, (n.d.).
- Shehata, M.H.; Thomas, M.D.A. The effect of fly ash composition on the expansion of concrete due to alkali–silica reaction. Cem. Concr. Res. 2000, 30, 1063–1072. [Google Scholar] [CrossRef]
- He, P.; Zhang, B.; Lu, J.-X.; Poon, C.S. A ternary optimization of alkali-activated cement mortars incorporating glass powder, slag and calcium aluminate cement. Constr. Build. Mater. 2020, 240, 117983. [Google Scholar] [CrossRef]
- Gołaszewski, J.; Gołaszewska, M. Properties of mortars with Calcium Sulfoaluminate cements with the addition of Portland cement and limestone. Arch. Civ. Eng. 2021, 67, 425–435. [Google Scholar] [CrossRef]
- V.K. Harish, P.R. Rangaraju, INVESTIGATIONS INTO ALKALI-SILICA REACTION IN CALCIUM SULFO- ALUMINATE CEMENT MORTARS AND ITS BLENDS WITH POZZOLANS, (n.d.).
- Kleib, J.; Aouad, G.; Louis, G.; Zakhour, M.; Boulos, M.; Rousselet, A.; Bulteel, D. The use of calcium sulfoaluminate cement to mitigate the alkali silica reaction in mortars. Constr. Build. Mater. 2018, 184, 295–303. [Google Scholar] [CrossRef]
- Bizzozero, J.; Scrivener, K.L. Limestone reaction in calcium aluminate cement–calcium sulfate systems. Cem. Concr. Res. 2015, 76, 159–169. [Google Scholar] [CrossRef]
- Termkhajornkit, P.; Nawa, T.; Nakai, M.; Saito, T. Effect of fly ash on autogenous shrinkage. Cem. Concr. Res. 2005, 35, 473–482. [Google Scholar] [CrossRef]
- Burris, L.E.; Kurtis, K.E. Influence of set retarding admixtures on calcium sulfoaluminate cement hydration and property development. Cem. Concr. Res. 2018, 104, 105–113. [Google Scholar] [CrossRef]
- Sirtoli, D.; Wyrzykowski, M.; Riva, P.; Lura, P. Autogenous and drying shrinkage of mortars based on Portland and calcium sulfoaluminate cements. Mater. Struct. 2020, 53, 1–14. [Google Scholar] [CrossRef]
- Kumarappa, D.B.; Peethamparan, S.; Ngami, M. Autogenous shrinkage of alkali activated slag mortars: Basic mechanisms and mitigation methods. Cem. Concr. Res. 2018, 109, 1–9. [Google Scholar] [CrossRef]
- Wu, L.; Farzadnia, N.; Shi, C.; Zhang, Z.; Wang, H. Autogenous shrinkage of high performance concrete: A review. Constr. Build. Mater. 2017, 149, 62–75. [Google Scholar] [CrossRef]
- Polat, R.; Demirboğa, R.; Khushefati, W.H. Effects of nano and micro size of CaO and MgO, nano-clay and expanded perlite aggregate on the autogenous shrinkage of mortar. Constr. Build. Mater. 2015, 81, 268–275. [Google Scholar] [CrossRef]
- Tao, Y.; Rahul, A.; Mohan, M.K.; De Schutter, G.; Van Tittelboom, K. Recent progress and technical challenges in using calcium sulfoaluminate (CSA) cement. Cem. Concr. Compos. 2023, 137. [Google Scholar] [CrossRef]
- D. Sirtoli, Shrinkage and creep of high-performance concrete based on calcium sulfoaluminate cement - ScienceDirect, (2019). https://www.sciencedirect.com/science/article/pii/S095894651831014X (accessed July 26, 2022).
- Khatib, J.M.; Ramadan, R.; Ghanem, H.; Elkordi, A.; Baalbaki, O.; Kırgız, M. Chemical shrinkage of paste and mortar containing limestone fines. Mater. Today: Proc. 2022, 61, 530–536. [Google Scholar] [CrossRef]
- Afroz, S.; Zhang, Y.; Nguyen, Q.D.; Kim, T.; Castel, A. Shrinkage of blended cement concrete with fly ash or limestone calcined clay. Mater. Struct. 2023, 56, 1–20. [Google Scholar] [CrossRef]
- Liu, J.; An, R.; Jiang, Z.; Jin, H.; Zhu, J.; Liu, W.; Huang, Z.; Xing, F.; Liu, J.; Fan, X.; et al. Effects of w/b ratio, fly ash, limestone calcined clay, seawater and sea-sand on workability, mechanical properties, drying shrinkage behavior and micro-structural characteristics of concrete. Constr. Build. Mater. 2022, 321, 126333. [Google Scholar] [CrossRef]
- Mohan, M.K.; Rahul, A.; De Schutter, G.; Van Tittelboom, K. Early age hydration, rheology and pumping characteristics of CSA cement-based 3D printable concrete. Constr. Build. Mater. 2021, 275, 122136. [Google Scholar] [CrossRef]
- Mohammed, T.; Torres, A.; Aguayo, F.; Okechi, I.K. Evaluating carbonation resistance and microstructural behaviors of calcium sulfoaluminate cement concrete incorporating fly ash and limestone powder. Constr. Build. Mater. 2024, 442. [Google Scholar] [CrossRef]
- Brien, J.V.; Henke, K.R.; Mahboub, K.C. OBSERVATIONS OF PEAK STRENGTH BEHAVIOR IN CSA CEMENT MORTARS. J. Green Build. 2013, 8, 97–115. [Google Scholar] [CrossRef]
- Jansen, D.; Goetz-Neunhoeffer, F.; Lothenbach, B.; Neubauer, J. The early hydration of Ordinary Portland Cement (OPC): An approach comparing measured heat flow with calculated heat flow from QXRD. Cem. Concr. Res. 2011, 42, 134–138. [Google Scholar] [CrossRef]
- F. Winnefeld, Interaction of superplasticizers with calcium sulfoaluminate cements, 2012.
- Winnefeld, F.; Martin, L.H.; Müller, C.J.; Lothenbach, B. Using gypsum to control hydration kinetics of CSA cements. Constr. Build. Mater. 2017, 155, 154–163. [Google Scholar] [CrossRef]
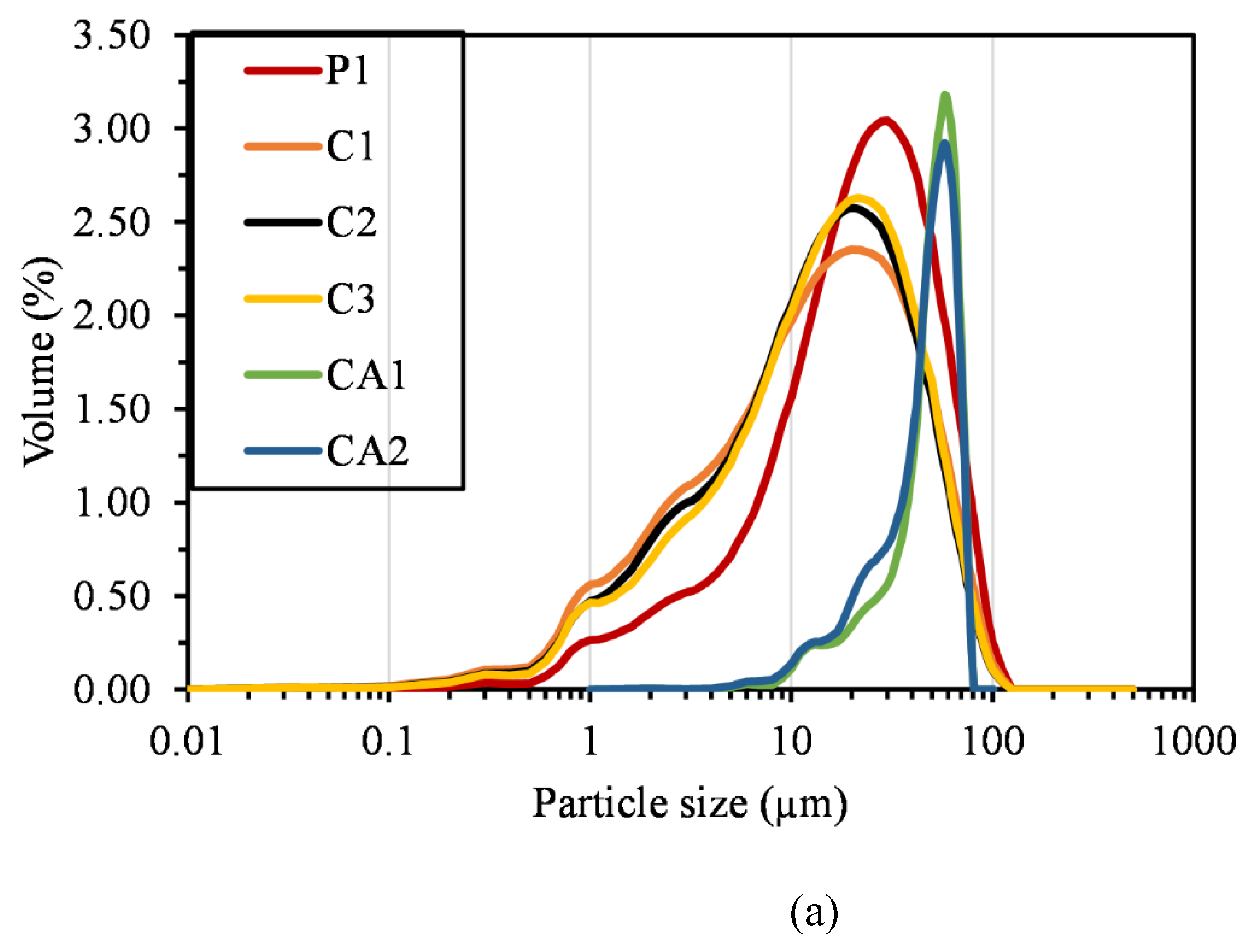
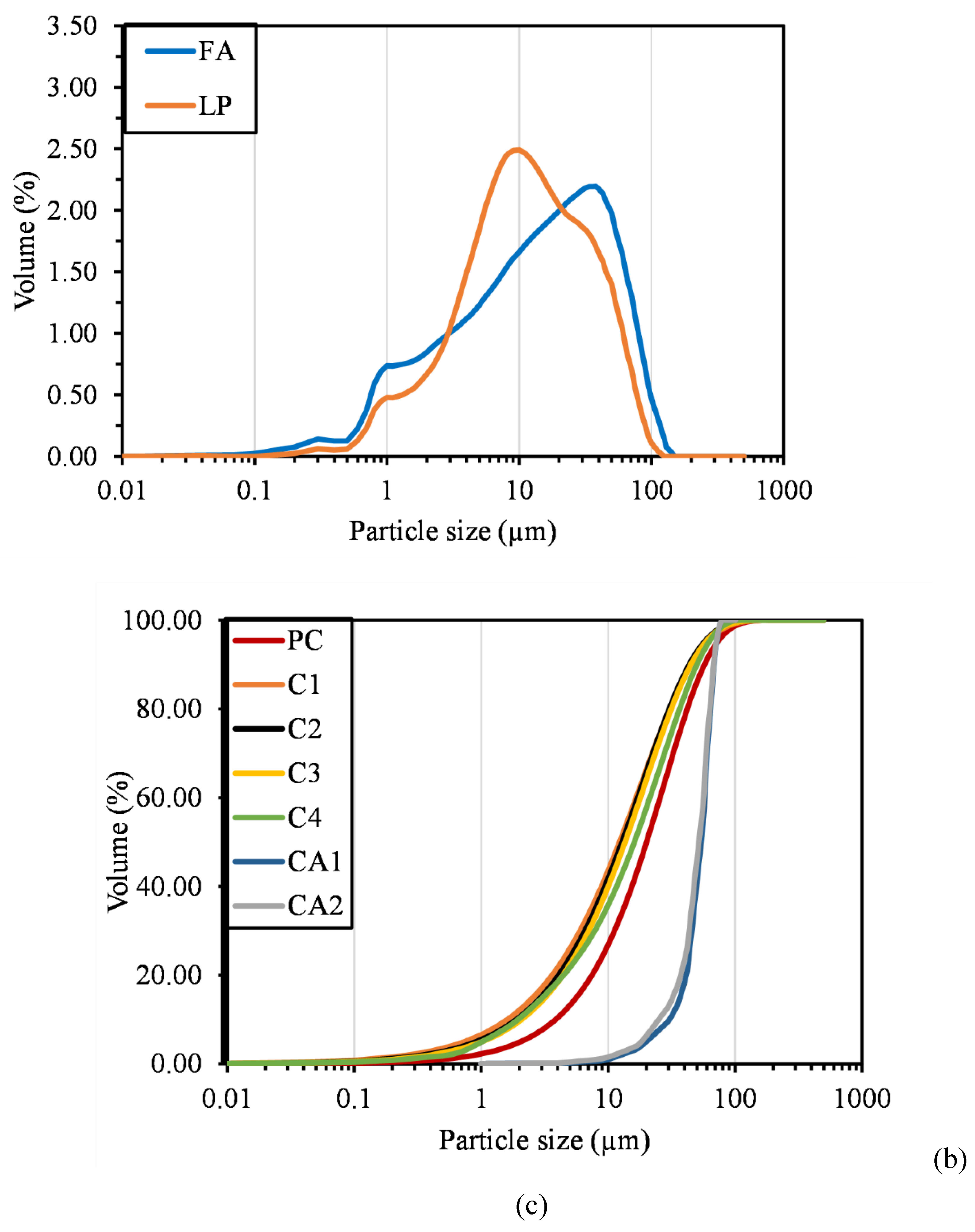
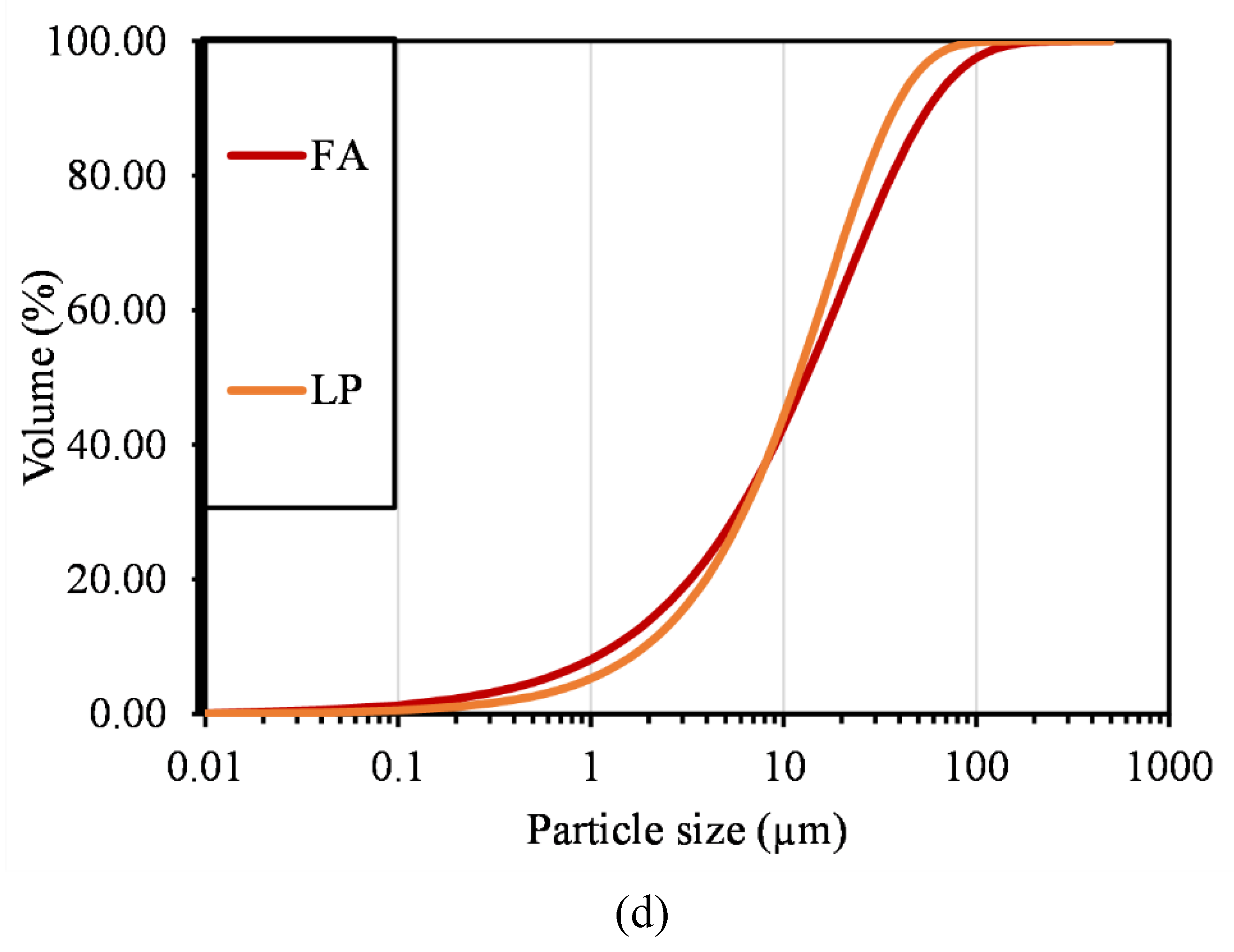
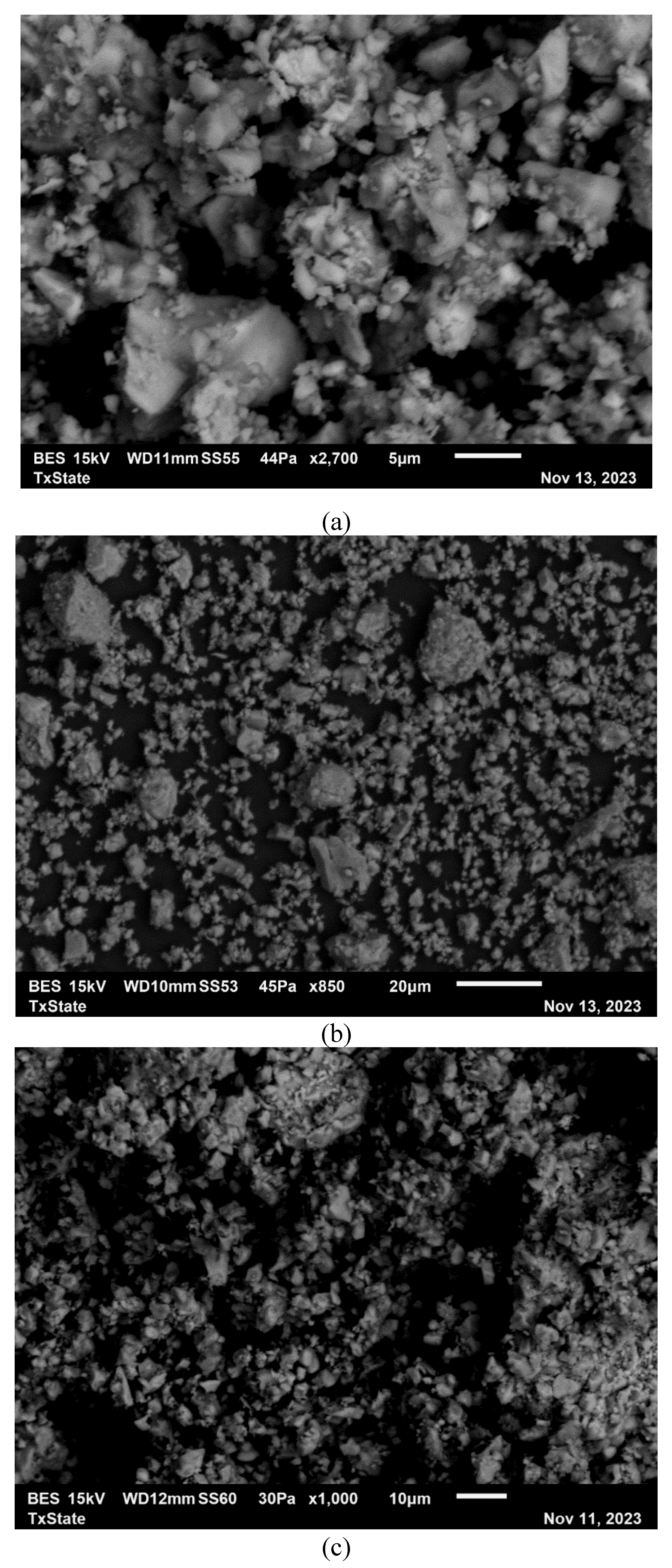


| Compound | P1 | C1 | C2 | C3 | CA1 | CA2 | FA | LP |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 19.60 | 20.56 | 13.63 | 14.72 | 4.34 | 13.46 | 34.87 | 4.50 |
| Al2O3 | 5.19 | 16.14 | 15.82 | 14.37 | 38.65 | 12.23 | 17.43 | - |
| Fe2O3 | 2.06 | 1.35 | 0.75 | 1.22 | 15.09 | 2.67 | 5.67 | - |
| CaO | 64.01 | 45.31 | 51.28 | 53.85 | 38.37 | 56.65 | 27.60 | - |
| MgO | 1.12 | 1.23 | 1.14 | 1.23 | 0.39 | 2.86 | 5.50 | - |
| SO3 | 3.86 | 14.73 | 16.62 | 14.40 | 0.16 | 9.90 | 2.27 | - |
| Na2O | 0.12 | 0.77 | 0.29 | 0.10 | 0.05 | 0.20 | 1.69 | - |
| K2O | 0.91 | 0.72 | 0.62 | 0.59 | 0.14 | 0.79 | 0.46 | - |
| Na2Oe | 0.72 | 1.24 | 0.69 | 0.49 | 0.14 | 0.72 | - | - |
| P2O5 | 0.13 | 0.16 | 0.15 | 0.15 | 0.12 | 0.11 | - | - |
| Cl | 0.01 | 0.02 | 0.02 | 0.02 | 0.00 | 0.01 | - | - |
| TiO2 | 0.24 | 0.76 | 0.72 | 0.65 | 1.82 | 0.60 | - | - |
| MnO | 0.03 | 0.01 | 0.01 | 0.04 | 0.11 | 0.14 | - | - |
| ZnO | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.07 | - | - |
| Cr2O3 | 0.01 | 0.02 | 0.02 | 0.02 | 0.11 | 0.04 | - | - |
| CaCO3 | - | - | - | - | - | - | - | 92.00 |
| MgCO3 | - | - | - | - | - | - | - | 2.50 |
| LOI | 3.80 | 4.74 | 3.06 | 3.39 | 1.55 | 1.21 | 0.42 | |
| CO2 | 2.49 | 1.81 | 1.28 | 1.76 | 0.64 | 0.54 | - | - |
| Systems | Mixture | Mix ID | PureBinder | FA | LP | Water/ Binder |
Sand | Flow (mm) |
|---|---|---|---|---|---|---|---|---|
| Pure | OPC | P1 | 1.00 | 0 | 0 | 0.40 | 2.75 | 25.75 |
| CSA1 | C1 | 1.00 | 0 | 0 | 0.40 | 2.75 | 33.50 | |
| CSA2 | C2 | 1.00 | 0 | 0 | 0.40 | 2.75 | 30.50 | |
| CSA3 | C3 | 1.00 | 0 | 0 | 0.40 | 2.75 | 27.25 | |
| CAC1 | CA1 | 1.00 | 0 | 0 | 0.40 | 2.75 | 30.25 | |
| CAC2 | CA2 | 1.00 | 0 | 0 | 0.40 | 2.75 | 27.75 | |
| Binary | OPC+20%FA | P1F20 | 0.80 | 0.20 | 0 | 0.40 | 2.75 | 30.00 |
| CSA1+20%FA | C1F20 | 0.80 | 0.20 | 0 | 0.40 | 2.75 | 29.00 | |
| CSA2+20%FA | C2F20 | 0.80 | 0.20 | 0 | 0.40 | 2.75 | 29.00 | |
| CSA3+20%FA | C3F20 | 0.80 | 0.20 | 0 | 0.40 | 2.75 | 28.50 | |
| CAC1+20%FA | CA1F20 | 0.80 | 0.20 | 0 | 0.40 | 2.75 | 31.25 | |
| CAC2+20%FA | CA2F20 | 0.80 | 0.20 | 0 | 0.40 | 2.75 | 29.50 | |
| OPC+15%LP | P1L15 | 0.85 | 0 | 0.15 | 0.40 | 2.75 | 27.25 | |
| CSA1+15%LP | C1L15 | 0.85 | 0 | 0.15 | 0.40 | 2.75 | 28.50 | |
| CSA2+15%LP | C2L15 | 0.85 | 0 | 0.15 | 0.40 | 2.75 | 28.25 | |
| CSA3+15%LP | C3L15 | 0.85 | 0 | 0.15 | 0.40 | 2.75 | 27.50 | |
| CAC1+15%LP | CA1L15 | 0.85 | 0 | 0.15 | 0.40 | 2.75 | 32.00 | |
| CAC2+15%LP | CA2L15 | 0.85 | 0 | 0.15 | 0.40 | 2.75 | 29.25 | |
| Ternary | OPC+20%FA+15%LP | P1F20L15 | 0.65 | 0.20 | 0.15 | 0.40 | 2.75 | 25.75 |
| CSA1+20%FA+15%LP | C1F20L15 | 0.65 | 0.20 | 0.15 | 0.40 | 2.75 | 28.75 | |
| CSA2+20%FA+15%LP | C2F20L15 | 0.65 | 0.20 | 0.15 | 0.40 | 2.75 | 30.50 | |
| CSA3+20%FA+15%LP | C3F20L15 | 0.65 | 0.20 | 0.15 | 0.40 | 2.75 | 29.25 | |
| CAC1+20%FA+15%LP | CA1F20L15 | 0.65 | 0.20 | 0.15 | 0.40 | 2.75 | 31.00 | |
| CAC2+20%FA+15%LP | CA2F20L15 | 0.65 | 0.20 | 0.15 | 0.40 | 2.75 | 29.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





