Submitted:
20 September 2024
Posted:
24 September 2024
You are already at the latest version
Abstract
Keywords:
Background
Case 1
Case 2
Discussion
References
- De Baetselier I, Smet H, Kehoe K, Loosen I, Reynders M, Mansoor I, et al. Estimation of antimicrobial resistance of Mycoplasma genitalium, Belgium, 2022. Eurosurveillance. 2024;29(7):2300318.
- Jensen J, Cusini M, Gomberg M, Moi H, Wilson J, Unemo M. 2021 European guideline on the management of Mycoplasma genitalium infections. Journal of the European Academy of Dermatology and Venereology. 2022;36(5):641-50.
- Li JM, Cosler LE, Harausz EP, Myers CE, Kufel WD. Methenamine for urinary tract infection prophylaxis: a systematic review. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2024;44(2):197-206.
- Petri, W. Sulfonamides, trimethoprim, sulfamethoxazole, quinolones, and agents for urinary tract infections. Goodman & Gilman’s the pharmacological basis of therapeutics New York (NY): McGraw Hill. 2006:1111-25.
- McGowin CL, Popov VL, Pyles RB. Intracellular Mycoplasma genitalium infection of human vaginal and cervical epithelial cells elicits distinct patterns of inflammatory cytokine secretion and provides a possible survival niche against macrophage-mediated killing. BMC microbiology. 2009;9:1-11.
- Pitt R, Boampong D, Day M, Jensen JS, Cole M. Challenges of in vitro propagation and antimicrobial susceptibility testing of Mycoplasma genitalium. Journal of Antimicrobial Chemotherapy. 2022;77(11):2901-7.
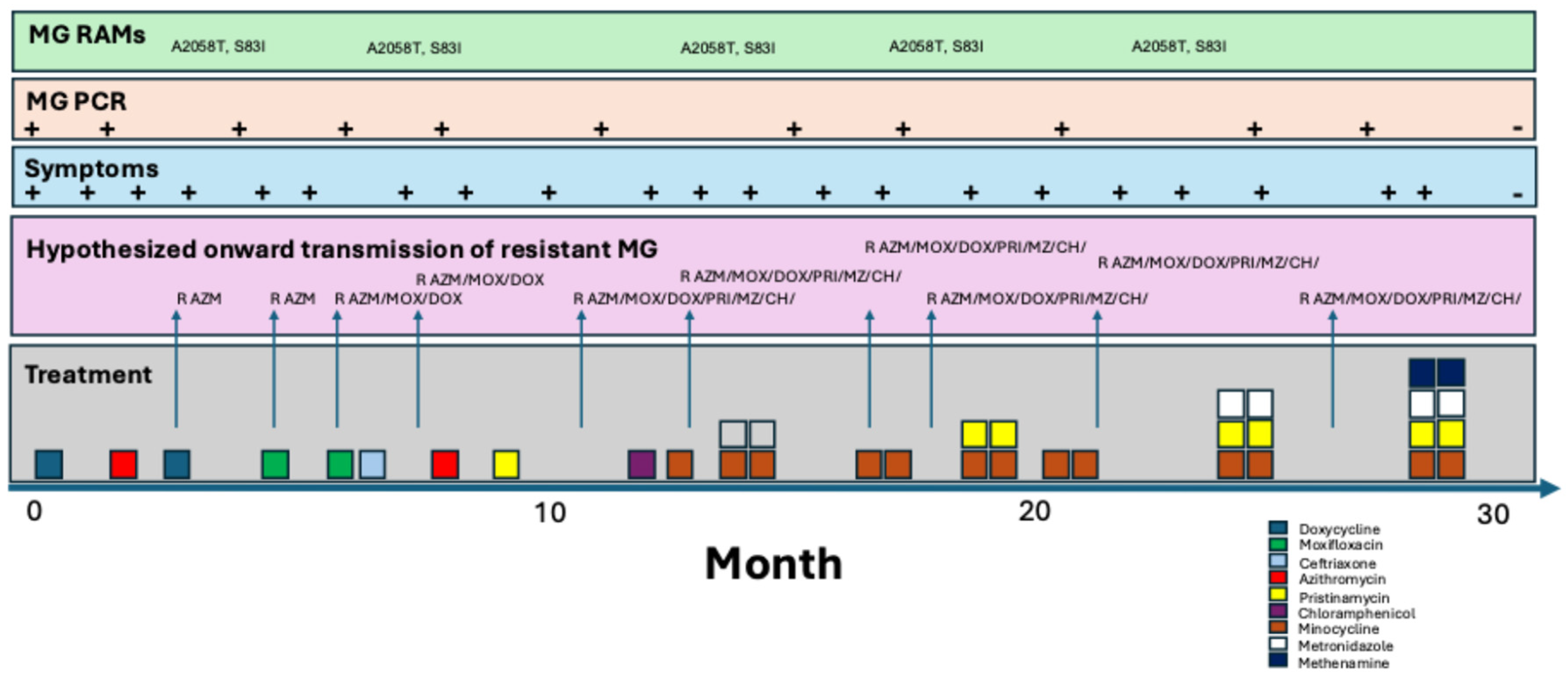
| Date | Symptoms | Micro. (PMN/HPF) | PCR MG | RAMs | Treatment | Outcome |
|---|---|---|---|---|---|---|
| 10/2021 | Subtle DC, dysuria | + | Doxycycline 100mg BID x 7d | Initial improvement but symptoms return 2 days post treatment | ||
| 02/2022 | Subtle DC, dysuria | 5-10 | + | Azithromycin 500mg d1 and 250mg d2-5 | No improvement | |
| 06/2022 | DC, dysuria | + | A2058T, S83I | Doxycycline 100mg BID x 21d | No improvement | |
| 07/2022 | DC, dysuria | + | A2058T, S83I | Moxifloxacin 500mg BID x 10d | Symptoms return 10days post treatment | |
| 08/2022 | DC, dysuria | + | A2058T, S83I | Moxifloxacin 500mg BID x 10d + ceftriaxone 1g IMI (partner had NG) | No improvement | |
| 09/2022 | DC, dysuria | + | Azithromycin 500mg d1 and 250mg d2-5 +BPG 2.4mu (partner had syphilis) | No improvement | ||
| 10/2022 | DC, dysuria | + | A2058T, S83I | Pristinamycin 1g QID x 10d | Symptoms return 21 days post treatment | |
| 12/2022 | DC, dysuria | + | A2058T, S83I | None | ||
| 02/2023 | DC, dysuria | + | Ibuprofen for pain | |||
| 03/2023 | DC, dysuria | 8 | + | Chloramphenicol 1g QID x 14d | ||
| 04/2023 | DC, dysuria | 4 | + | Minocycline 100mg BID x 14d | Symptoms return 20 days post treatment | |
| 05/2023 | DC, dysuria | 10 | + | A2058T, S83I | Minocycline 100mg BID x 14d then metronidazole 500mg TID x 14d | All symptoms resolve except light dysuria x 30 days |
| 08/2023 | DC, dysuria | + | Minocycline 100mg BID x 14d then Pristinamycin 1g QID x 10d | No response | ||
| 11/2023 | DC, dysuria | + | Minocycline 100mg BID x 14d | |||
| 01/2024 | Dysuria | + | Minocycline 100mg BID x 14d | |||
| 02/2024 | DC, dysuria | + | Minocycline 100mg BID x 14d + metronidazole 500mg TID x 14d + Pristinamycin 1g QID x 14d | Symptoms return 1 day post treatment | ||
| 03/2024 | DC, dysuria | + | Minocycline 100mg BID x 14d + metronidazole 500mg TID x 14d + Pristinamycin 1g QID x 14d + methenamine-amygdalate 1g QID x 28d | Symptoms resolve within one week | ||
| 07/2024 | None | 0 | Neg | No symptoms | ||
| 09/2024 | None | No symptoms |
| Date | Symptoms | Micro. (PMN/HPF) | PCR MG | RAMs | Treatment | Outcome |
|---|---|---|---|---|---|---|
| 01/2024 | DC, dysuria | 15+ | + | Doxycycline 100mg BID x 7d then azithromycin 500mg d1 and 250mg d2-5 | No improvement | |
| 02/2024 | DC, dysuria | 5-10 | + | Doxycycline 100mg BID x 21d, then then Moxifloxacin 500mg BID x 7d | Symptoms return 1 day post treatment | |
| 03/2024 | DC, dysuria | + | Azithromycin 2.5g over 4d then Moxifloxacin 500mg BID x 7d | No improvement | ||
| 04/2024 | DC, dysuria | Doxycycline 100mg BID x 7d then Moxifloxacin 500mg BID x 10d | Symptoms return 10 days post treatment | |||
| 05/2024 | DC, dysuria | + | Doxycycline 100mg BID x 14d then Azithromycin 500mg d1 and 250mg d2-5 then metronidazole 500mg TID x 7d | No improvement | ||
| 06/2024 | DC, dysuria | + | Doxycycline 100mg BID x 28d | No improvement | ||
| 07/2024 | DC, dysuria | + | Minocycline 100mg BID x 14d + metronidazole 500mg TID x 14d + Pristinamycin 1g QID x 14d + methenamine-amygdalate 1g QID x 28d | Symptoms resolve within 10days of starting treatment | ||
| 09/2024 | None | 0 | - | None | No symptoms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





