1. Introduction
Mahoney Lake is located in South-Central British Columbia, Canada. Originally named as such in 1936 [
1], it was first examined for its properties in 1969. It has a surface area of 18 ha, depth of 18 m and is located 470 m above sea level [
2]. There is no water in or out flow with levels primarily regulated through precipitation and evaporation [
2]. The lake is meromictic, containing two water layers that do not mix: the bottom monimolimnion and the top mixolimnion. They are separated by a sharp chemical density gradient called the chemocline. This lake is commonly characterized as euxinic because it is both anoxic and sulfidic in the bottom layer [
1] and is concentrated in dissolved solids (up to 85 000 mg/L) [
2]. In contrast, the mixolimnion is oxygenated with a gradual decrease in levels until 8 m in depth at which oxygen is not present [
3] and contains significantly less dissolved solids (10 000 mg/L) [
2]. The saline waters of the lake are uniquely rich in MgSO
4 compared to marine environments that are primarily dominated by NaCl [
4]. Mahoney Lake formed a great interest to microbiologists with the discovery that purple sulfur bacteria constitute most of the primary productivity [
5], specifically,
Thiohalocapsa sp. ML1 [
6]. As such, studies on the microbial community continued to take place [
3,
7,
8,
9,
10,
11,
12,
13], which has resulted in the taxonomic characterization of new species and genera [
12,
13,
14,
15]. One bacterial group studied in the mixolimnion of the lake was aerobic anoxygenic phototrophs (AAP) [
3]. In this publication, 33 groups of AAP and purple non-sulfur bacteria were isolated and characterized [
3].
AAP are bacteria that require oxygen not only for respiration, but also to conduct anoxygenic photosynthesis, a system primarily used anaerobically by other anoxygenic phototrophs. In addition, they cannot produce RuBisCo to survive autotrophically by fixing CO
2 [
16]. The energy derived from harvesting light allows them to grow competitively alongside other heterotrophs and surviving low nutrient conditions. These core features differentiate the group, which primarily spans the α-, β- and γ-
Proteobacteria.
Salinarimonadaceae is a relatively new family in
Hyphomicrobiales [
17]. Currently, the family contains 4 species,
Salinarimonas rosea,
Salinarimonas ramus,
Salinarimonas soli and
Saliniramus fredricksonii [
18]. Most species have been isolated from saline environments including a salt mine [
19], soil [
20] and a lake [
17]. The exception is
Salinarimonas soli, which came from soil in the republic of South Korea and contrastingly has a very low salt tolerance, only growing in media containing up to 0.5 % (w/v) NaCl [
21]. The genus
Salinarimonas is a group of gram-negative, rod-shaped bacteria that are facultative anaerobic or obligate aerobic, motile, produce pink colonies, catalase positive and contain Q-10 as the primary ubiquinone. The major cellular polar lipids are diphosphatidylglycerol, phosphatidylglycerol, phosphatidylmethylethanolamine and phosphatidylcholine [
19]. Photosynthesis had been alluded to possibly be present in the genus with the discovery that they all contained genes for the photosynthetic reaction center (RC) [
20,
21]. However, only
Salinarimonas ramus had been physically tested, with pigment extracts lacking bacteriochlorophyll
a (Bchl
a), possibly due to the conditions not being suited for photoheterotrophic growth [
20]. Here, we describe a new AAP isolated from the mixolimnion of a meromictic lake, which produces Bchl
a, forms a RC and light-harvesting I (LHI) complex. Strain ML10
T, represents a new species of the genus
Salinarimonas for which the name
Salinarimonas chemoclinalis, is proposed.
2. Materials and Methods
2.1. Cultivation
ML10
T was isolated on medium N2 from a sample collected 3 m below the surface of Mahoney Lake (49° 17′ N, 119° 35′ W) [
3]. For subsequent characterization, it was grown for 7 days in the dark at 28 °C on a shaking incubator, unless otherwise indicated. For long term storage, cells were cryopreserved at -75 °C in modified N2 with 10 % (w/v) organics and 30 % (v/v) glycerol.
2.2. Pigment Analysis
Photosynthetic complexes from whole cells and extracted Bchl
a were detected using absorbance spectra in the 300 – 1100 nm region with the Hitachi U-2010 spectrophotometer [
16]. For whole cell reads, ML10
T from N2 plates was suspended in 0.3 ml 20 mM TRIS-HCl buffer (pH 7.8) and 0.7 ml glycerol to minimize light scattering [
12]. Pigments from cells grown under the same condition were extracted using acetone/methanol (7:2, v:v) [
16].
2.3. Morphology and Physiology Tests
Cell shape and size were determined after 7 days of growth and motility after 1 day via phase contrast microscopy (Zeiss Axioskop 2). Gram staining [
22] was done alongside a KOH hydrolysis [
23] for confirmation. Colony morphology was evaluated after 7 days of growth. Microscopy of negative-stained and TEM of ultra-thin section of cells as described [
3].
All liquid culture physiological tests were inoculated with a 5 % (v/v) of starter ML10
T cells. Temperature optimum and range was evaluated at the following (°C): 4, 8, 12, 16, 20, 24, 28, 32, 37 and 42. Growth at pH of 5.0 to 12.5 with 0.5 increments as well as NaCl and NaSO
4 % (w/v) range from 0 to 20 % at 1 % intervals were studied. Utilization of complex (bactopeptone, casamino acids and yeast extract) and single carbon sources such as organic Na salts (acetate, butyrate, citrate, formate, glutamate, malate, pyruvate, succinate), sugars (fructose, glucose, lacotse) and simple alcohols (ethanol and methanol) were determined at 0.5 % concentration in liquid N2 cultures modified to exclude any other organics. Oxidase, catalase, nitrate reduction and indole tests as well as hydrolysis of tween 20, 40, 60 and 80, starch, gelatin and agar, were assessed [
24]. Antibiotic susceptibility was evaluated using disk diffusion for the following (μg): ampicillin (10), chloramphenicol (30), erythromycin (15), imipenem (10), kanamycin (30), penicillin G (10 IU), polymyxin B (300 IU), nalidixic acid (30), streptomycin (10), and tetracycline (30). If no inhibition of growth was observed, ML10
T was considered resistant.
Photo- and chemo- heterotrophic anaerobic growth was checked in screw-capped tubes incubated in light and dark with liquid N2 and purple non-sulfur bacteria media (PNSb) [
25]. The latter was supplemented with 0.3 g/L Na-Succinate [
26] as an additional organic electron donor and 1.5 % NaCl to match the salinity of N2. Further anaerobic growth testing was assessed with different inorganic electron donors by substituting the amino acids (cysteine and methionine) in PNSb with Na
2S (1 mM) or Na
2S
2O
3 (1 mM) [
14]. Anaerobic fermentation of individual carbon sources was done in the dark using screw-capped tubes filled with liquid minimal N2 and the following sugars (0.5 % w/v): glucose, fructose, lactose maltose and sucrose. Durham vials were added to detremine if CO
2 was produced. Aerobic photoautotrophy was evaluated in liquid, organic-free PNSb, supplemented with (g/L): 1.5 NaHCO
3 and 0.5 Na
2S
2O
3 as an inorganic carbon and sulfur source/electron donor, respectively [
27] and grown in the presence of an incandescent light bulb. Two subsequent cell transfers were conducted if growth occured to account for organics that remained in the initial inoculum.
2.4. Chemotaxonomy
ML10
T was grown on N2 plates for 3 days at 28°C in the dark. Cells were collected in triplicate, lipid extractions and purification were performed using Folch’s method [
28], then analyzed with gas chromatography to identify fatty acid composition [
29].
2.5. 16 S rRNA and Genomic Sequencing
DNA was extracted and the partial 16S rRNA gene was Sanger sequenced with universal primers 27F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 1492R (5’-GGTTACCTTGTTACGACTT-3’). Chromatograms were processed on DNA Baser Assembler v4.36.0 (Heracle BioSoft SRL, Romania). Most related type species for ML10
T based on this gene was identified through a standard nucleotide BLAST search [
30]. A 16S rRNA phylogenetic tree was constructed with MEGA X software [
31], using neighbour joining alignment and 1000 bootstrap replicates. The evolutionary history was inferred with the Maximum Likelihood method. The Tamura 3-parameter model [
32] selected for the tree was determined with the MEGA X ‘find best DNA model’ tool. To measure evolutionary rate differences among sites, a Gamma distribution (+G, parameter = 0.5022) with 5 rate categories was applied and it was assumed a certain fraction of sites are evolutionarily invariable ([+I], 35.61% sites). Final dataset had 36 nucleotide sequences and 1522 positions.
To obtain the whole genome sequence, extracted DNA was prepared using the Illumina DNA Library Prep Kit. From this, 500 µL of 1.8 pM was paired-end (2 x 150 bp) sequenced in the Illumina MiniSeq system. This generated 862.27 Mbp comprising 5,710,366 reads. The dataset was quality checked with FASTQC (version 1.0.0) using a k-mer size of 5 and contamination filtering for overrepresented sequences against the default contamination list. FASTQ Toolkit (version 2.2.6) was applied to clean the sequences. This included trimming of: N’s from 3’ end before identifying adapters; PCR common sequence; bases at 3’ and 5’ ends with a Q score < 30. The finalized reads were assembled de novo using unicycler (version 0.5.0) [
33] through the BV-BRC Comprehensive Genome Analysis tool [
34]. G + C content (mol %) was determined using the genome sequence. Annotation was completed with the Prokaryotic Genome Annotation Pipeline [
35]. A phylogenetic tree based on 467 single-copy PATRIC global protein families (PGFam) genes found in 24 genomes of ML10
T’s closely related species within Hyphomicrobiales was constructed using RAxML (version 8.2.11) within the BV-BRC Bacterial Genome Tree Tool [
34,
36]. The program’s Fast Bootstrapping were ran for branch support (100 rounds).
Digital DNA-DNA hybridization (dDDH) and confidence intervals were calculated with the Genome-to-Genome Distance Calculator (v 4.0) applying the recommended settings [
37,
38] on the Type (Strain) Genome Server (TYGS) [
37]. Average nucleotide identity (ANI) was assessed with JSpeciesWS [
39].
The 16S rRNA partial gene was deposited in GenBank under accession number PQ133587. This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession JBGMWH000000000 and is the version used in this work.
3. Results and Discussion
3.1. Spectral Analysis
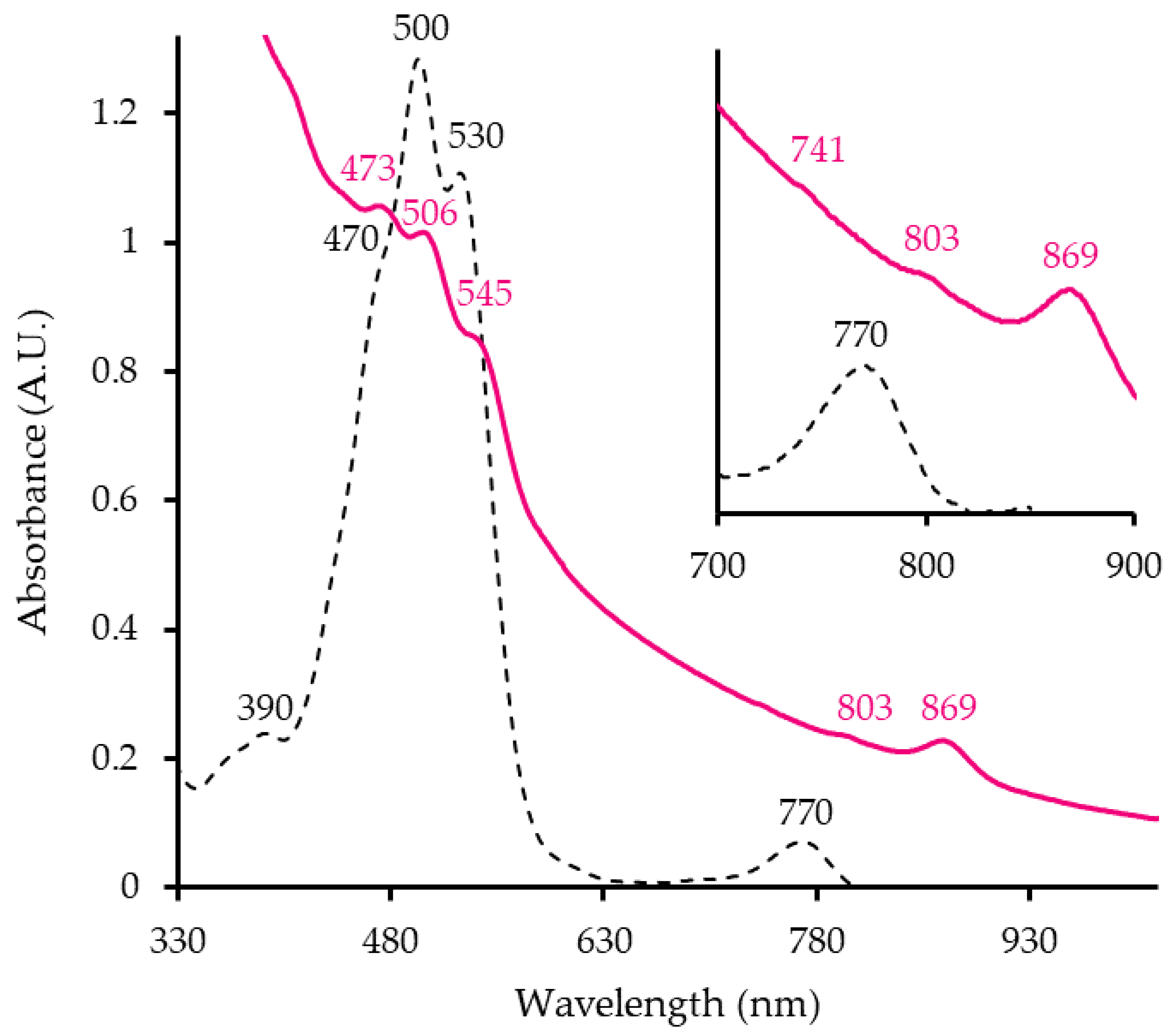
The absorbance spectrum of ML10
T whole cells shows typical peaks of an anoxygenic photosynthetic apparatus (
Figure 1). The strain has an LHI complex, indicated at the 869 nm peak (
Figure 1, pink line) as well as RC (803 nm, 741 nm). Hence ML10
T has membrane-bound photosynthetic-pigment complex. Peaks in the 400-550 nm range are responsible for cells’ pink hue. The pigment extract absorption spectrum (
Figure 1 black, dashed line) shows that it is synthesizing Bchl
a (770 nm), although at a much lower proportion relative to the carotenoids (470 nm, 500 nm, 530 nm). This ratio is reflective of AAP since only a small portion of secondary pigments is usually associated with LH complexes and the majority is found across the membrane, presumably helping the cell deal with oxidative stress [
16]. These findings are valuable as it is the first evidence of photosynthesis taking place in
Salinarimonadaceae. Previously, no Bchl
a was detected in the analysis of
Salinarimonas ramus [
20]. However, in past studies, genes for the RC proteins were identified in the genomes of all
Salinarimonas spp., [
20,
21]. Therefore, it is possible that
Salinarimonas ramus and other members can use light to produce chemical energy (ATP) for cell activities.
3.2. Culture and Cell Features
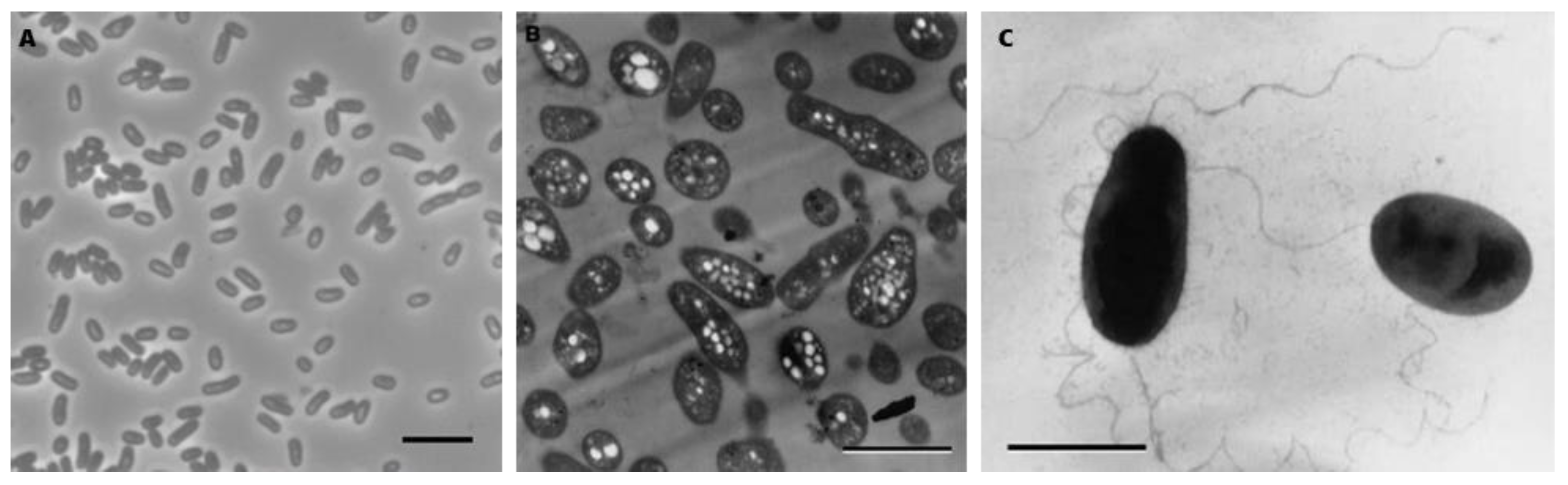
When grown aerobically on N2 for 7 days, ML10
T forms raised, convex, circular, glossy punctiform colonies with a soft texture. Like most members of
Salinarimonadaceae, they have a pink apperance. It is gram-negative, confirmed with staining, KOH test and electron microscopy [
3]. Cells are ovoid rods (
Figure 2A) and after 7 days of growth are 2.1±0.4 µm in length and 1.0±0.1 µm in width. In electron micrographs, it is shown that they accumulate clear intracellular granules, potentially containing polyhydroxyalkanotae(s) (PHA) (
Figure 2B) [
3]. Similar compounds have also been detected in
Salinarimonas rosea and
Salinarimonas ramus [
20]. Furthermore, PHA accumulation is commonly found in AAP, with production depending on light availability and carbon source [
40]. They are motile, powered by single or possibly multiple polar wavy flagella (
Figure 2C).
3.3. Physiology and Biochemistry
ML10
T was isolated from a saline environment like
Salinarimonas rosea and
Salinarimonas ramus. As such, the NaCl range and optimum of the strain is similar to those members (
Table 1) and significantly different from
Salinarimonas soli. Mahoney Lake is sulfate-rich, therefore the Na
2SO
4 tolerance was also determined. The strain could grow in 0 to 9 %, with optimum being at 8 %. The ideal temperature for mesophilic ML10
T is the highest among the genus at 37 °C, with the others preferring around 28-30 °C (
Table 1). It also has a greater range of alkali tolerance and lower optimal pH. ML10
T could neither survive in any of the anoxic conditions tested, including fermentation of sugars, nor could it grow photoautotrophically. For this reason, it is classified as an AAP, since it demonstrates the core features: Bchl
a production, oxygen requirement and photoheterotrophy.
Salinarimonas soli is also an obligate aerobe however, most related
Salinarimonas rosea and
Salinarimonas ramus, are facultative anaerobes (
Table 1).
ML10T was positive for oxidase, catalase and gelatinase, the latter of which has not been observed in Salinarimonas. It could not hydrolyze tweens, was negative for indole, amylase and nitrate reductase. As most AAP, the strain can use a variety of carbon sources including acetate, pyruvate, glutamate, malate, succinate, lactate, glucose and yeast extract. It is susceptible to all antibiotics tested except nalidixic acid and polymyxin B.
Fatty acid analysis highlighted differences in the lipid composition of ML10
T and other
Salinarimonadaceae (
Table 2). It shared its most prevalent fatty acid, C
18:1 ω7c with all
Salinarimonas spp. as well as C
16:0 and C
18:0. Alternatively, it did not have C
14:0 3-OH/iso-C
16:1, C
16:1 ω7c/C
16:1 ω6c, C
19:0 cyclo ω8c or C
20:1 ω7c as the other members of the genus did. The strain had C
18:1, C
16:1 in common with only
Saliniramus fredricksonii, although in different proportions. C
17:0, C
20:4, C
20:5 are exclusive to ML10
T.
3.4. Genome Characteristics and Phylogeny
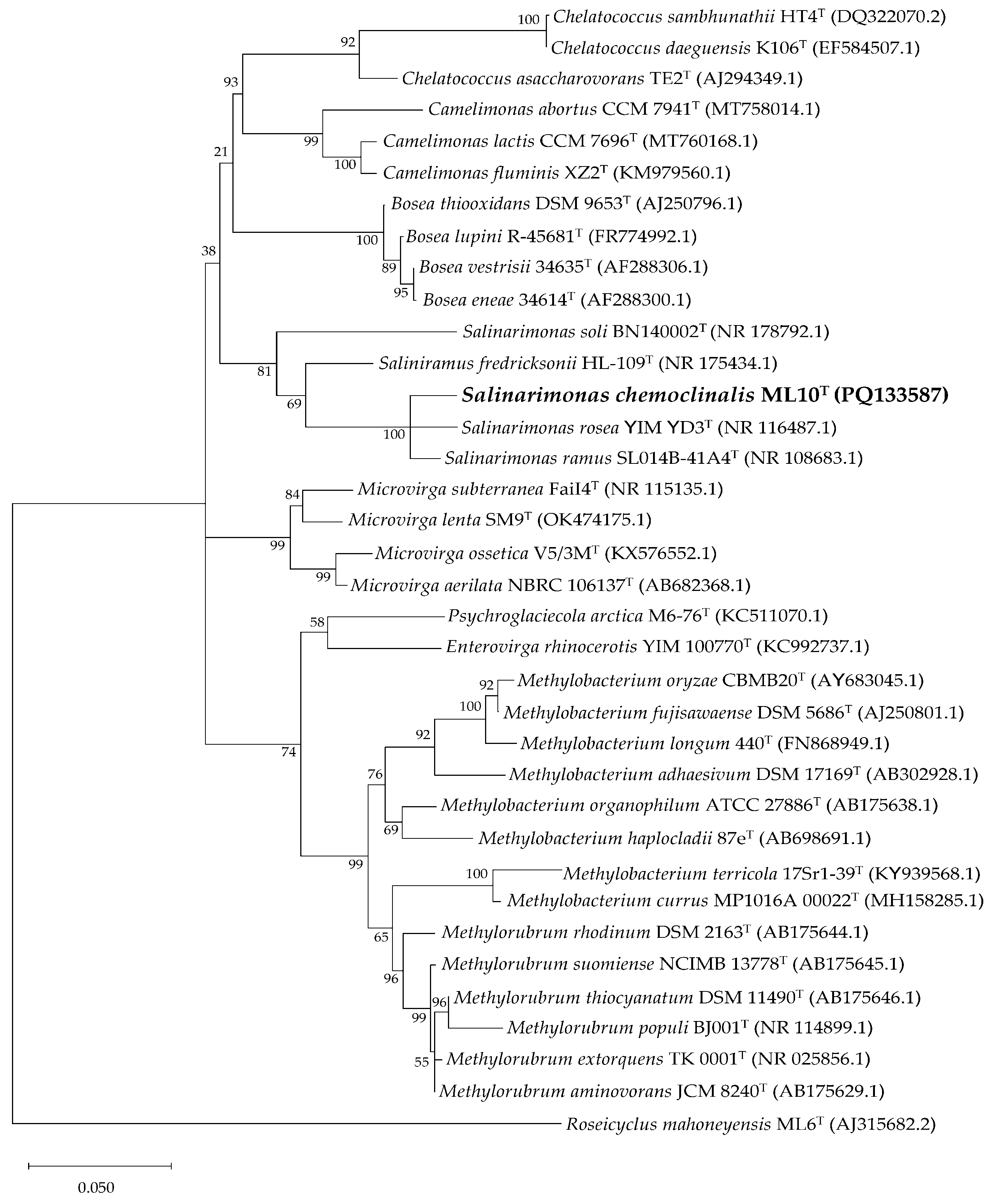
According to standard nucleotide BLAST results of the partial 16S rRNA gene sequence of ML10
T (1,398 bp), the most related species were
Salinarimonas rosea (97.92 %),
Salinarimonas ramus (97.85 %)
Saliniramus fredricksonii (94.61 %). This aligned with the observed similarities in their physiology. Including these and other 16S rRNA fragments from closely related genera, a maximum-likelihood tree was constructed (
Figure 3). Interestingly,
Saliniramus fredricksonii more closely related to ML10
T, than the other member of the genus,
Salinarimonas soli.
ML10
T’s genome is 4,753,738 bp making it slightly smaller than the other
Salinarimonas (
Table 3). It contained 42 contigs and a 177X average fold coverage. No plasmids were found. The G+C content at 72.06 mol % is higher than the others, but still within reasonable range for the genus. The full 16S rRNA sequence was identified in the genome (1,491 bp) A total of 4,629 coding sequences were identified, matching expected outputs for this genome size. ML10
T had 89 annotated protein sequences, which are absent in the other
Salinarimonadaceae genomes. Majority were hypothetical, however the CRISPR-associated Cas9 and Cas2 as well as phage-related genes were present. This suggest ML10
T may have the capability to combat bacteriophage infections with the CRISPR system. Like other AAP and
Salinarimonas members, it did not encode RuBisCo [
41]. As observed in the absorbance spectra, (
Figure 1), it also contained all the genes required for anoxygenic photosynthesis. Analysis of the other species in the genus, showed they also have these genes, increasing the likelihood that they can also harness light energy for ATP production. Definitive proof is still needed, especially as
Salinarimonas ramus and
Salinarimonas rosea are facultative anaerobes and their oxygen requirement for the expression of anoxygenic photosynthesis would affect whether they are characterized as a new type of AAP or as a purple non-sulfur bacteria. Although
Saliniramus fredricksonii was reported to have no photosynthetic genes [
17], it encodes a xanthorhodopsin and therefore potentially utilizes light energy. PHA synthase and Polyhydroxybutyrate (PHB) depolymerase sequences in ML10
T’s genome supports the conclusion that the intracellular granules accumulated in cells are a PHA.
The ANI for ML10
T alongside all species in the family is lower than 95 % [
42] and dDDH is below the 70 % cut-off for species designation (
Table 4) [
38,43].
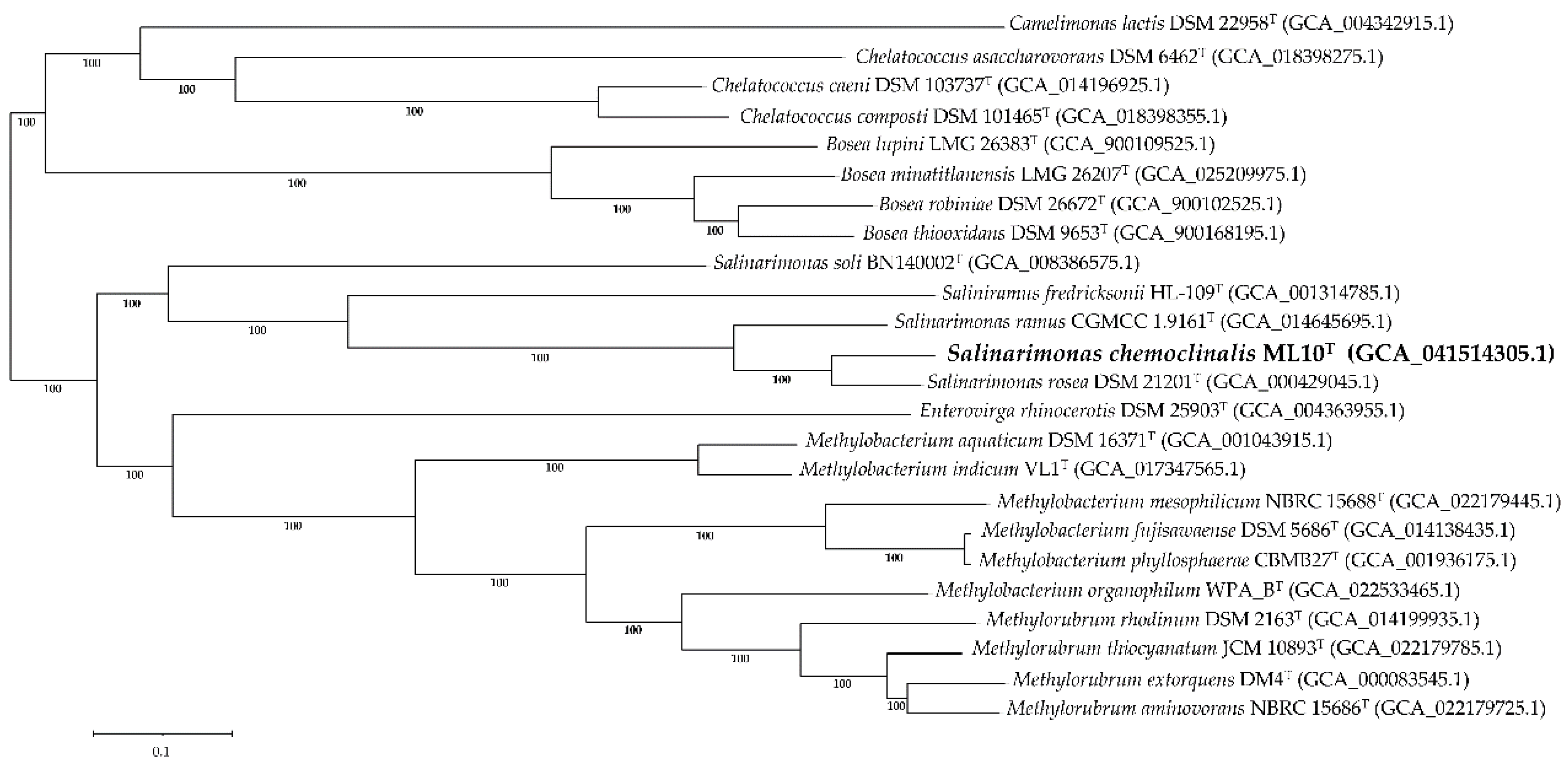
Similar to the 16S rRNA tree, a genome-based phylogenetic tree found that ML10
T formed a distinct lineage among the
Salinarimonadaceae (
Figure 4), supporting classification of the strain as a new species.
Salinarimonas soli was also the most divergent from the other family members despite
Saliniramus fredricksonii residing in a different genus. To resolve this contradiction, a separate study with further analysis of the relationship between the species in
Salinarimonadaceae must be conducted.
4. Conclusion
Polyphasic analysis of ML10T supports its classification as a new species within Salinarimonas. As such, we propose the new name Salinarimonas chemoclinalis, with ML10T as the type strain.
4.1. Emended description of Salinarimonas genus (Liu et al. 2010) [19]
It is as published by Liu et al. [
19] with the following amendments. Genus includes facultative anaerobes as well as obligate aerobes. All members contain genes for bacteriochlorophyll
a, carotenoid and light-harvesting complex synthesis required for anoxygenic photosynthesis as well as for polyhydroxyalkanoate intracellular accumulation.
4.2. Description of Salinarimonas chemoclinalis sp. nov.
Salinarimonas chemoclinalis (che.mo.cli.na’lis. N.L. fem. adj. chemoclinalis referring to chemocline zone in meromictic Mahoney Lake)
Cells are ovoid, motile with a single or possibly multiple polar flagella and are approximately 2.1±0.4 µm in length and 1.0±0.1 µm in width after 7 days of growth. Gram-negative, oxidase –positive, catalase-negative. Grows in the following conditions (optimum): temperature range of 12 to 41 °C (37 °C); pH of 6.0 to 10.0 (6.5); NaCl (% w/v) at 0 to 10 % (6 %); and NaSO4 (% w/v) at 0 to 9 % (8 %). After growing for 7 days on N2 medium, colonies are pink, raised, convex, circular, glossy and punctiform with a soft texture. Bacteriochlorophyll a, carotenoids, photosynthetic reaction center and light-harvesting I complex are produced, indicative of aerobic anoxygenic photosynthesis. Does not have nitrate reductase, amylase or tryptophanase. Can hydrolyze gelatin, but not tween 20, 40, 60 or 80. Is able to use acetate, pyruvate, glutamate, malate, succinate, lactate, glucose and yeast extract, but not butyrate, citrate, formate, fructose, lactose, methanol, ethanol, bactopeptone or casamino acids as carbon sources. Cells are sensitive to (μg): ampicillin (10), chloramphenicol (30), erythromycin (15), imipenem (10), kanamycin (30), penicillin G (10 IU), streptomycin (10), and tetracycline (30) but resistant to polymyxin B (300 IU) and nalidixic acid (30). Major fatty acids (> 4 %) are C18:1 ω7c, C16:1, C16:0 and minor fatty acids are C14:0, C17:0, C18:0, C18:1, C20:4 and C20:5. The genome is 4.75 Mbp and has a G+C content of 72.06 mol %.
The type strain is ML10T (= NCIMB 15586T = DSM 118510T). It was isolated from Mahoney lake, a saline- and sulfate-rich meromictic lake in British Columbia, Canada. The GenBank accession numbers for the 16S rRNA gene sequence and genome assembly of ML10T is PQ133587 and GCA_041514305.1, respectively.
Author Contributions
Conceptualization, Katia Messner and Vladimir Yurkov; Data curation, Katia Messner, John Kyndt and Vladimir Yurkov; Formal analysis, Katia Messner, John Kyndt and Vladimir Yurkov; Funding acquisition, John Kyndt and Vladimir Yurkov; Investigation, Katia Messner, John Kyndt and Vladimir Yurkov; Methodology, Katia Messner, John Kyndt and Vladimir Yurkov; Project administration, Vladimir Yurkov; Resources, John Kyndt and Vladimir Yurkov; Supervision, John Kyndt and Vladimir Yurkov; Validation, Katia Messner, John Kyndt and Vladimir Yurkov; Visualization, Katia Messner and Vladimir Yurkov; Writing – original draft, Katia Messner; Writing – review & editing, Katia Messner, John Kyndt and Vladimir Yurkov. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant 1501 and a University of Manitoba GETS grant, both held by V. Yurkov; and the Wilson Enhancement Fund for Applied Research in Science at Bellevue University, given to J. A. Kyndt.
Data Availability Statement
The ML10T 16S rRNA sequence has been deposited in GenBank with the accession number PQ133587. This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession JBGMWH000000000, which is used in this work.
Acknowledgments
We thank Dr. Aharon Oren for his assistance with checking Latin grammar and spelling of the new name as well as Dr. Steven B. Kuzyk for helping with techniques and procedures.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Heinrichs, M.L.; Walker, I.R.; Hall, K.J.; Overmann, J.; O’Beirne, M.D. Fifty Years of Limnology (1969-2019) at Mahoney Lake, British Columbia, Canada. Limnol. Rev. 2020, 20, 219–227. [Google Scholar] [CrossRef]
- BC Parks Mahoney Lake Ecological Reserve. Available online: https://bcparks.ca/mahoney-lake-ecological-reserve/ (accessed on 1 August 2024).
- Yurkova, N.; Rathgeber, C.; Swiderski, J.; Stackebrandt, E.; Beatty, J.T.; Hall, K.J.; Yurkov, V. Diversity, Distribution and Physiology of the Aerobic Phototrophic Bacteria in the Mixolimnion of a Meromictic Lake. FEMS Microbiol. Ecol. 2002, 40, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Northcote, T.G.; Hall, K.J. Limnological Contrasts and Anomalies in Two Adjacent Saline Lakes. Hydrobiologia 1983, 105, 179–194. [Google Scholar] [CrossRef]
- Northcote, T.G.; Hall, K.J. Vernal Microstratification Patterns in a Meromictic Saline Lake: Their Causes and Biological Significance. Hydrobiologia 1990, 197, 105–114. [Google Scholar] [CrossRef]
- Hamilton, T.L.; Bovee, R.J.; Thiel, V.; Sattin, S.R.; Mohr, W.; Schaperdoth, I.; Vogl, K.; Gilhooly, W.P.; Lyons, T.W.; Tomsho, L.P.; et al. Coupled Reductive and Oxidative Sulfur Cycling in the Phototrophic Plate of a Meromictic Lake. Geobiology 2014, 12, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Klepac-Ceraj, V.; Hayes, C.A.; Gilhooly, W.P.; Lyons, T.W.; Kolter, R.; Pearson, A. Microbial Diversity under Extreme Euxinia: Mahoney Lake, Canada. Geobiology 2012, 10, 223–235. [Google Scholar] [CrossRef]
- Coolen, M.J.L.; Overmann, J. Analysis of Subfossil Molecular Remains of Purple Sulfur Bacteria in a Lake Sediment. Appl. Environ. Microbiol. 1998, 64, 4513–4521. [Google Scholar] [CrossRef]
- Overmann, J.; Sandmann, G.; Hall, K.J.; Northcote, T.G. Fossil Carotenoids and Paleolimnology of Meromictic Mahoney Lake, British Columbia, Canada. Aquat. Sci. 1993, 55, 31–39. [Google Scholar] [CrossRef]
- Overmann, J.; Beatty, J.T.; Hall, K.J.; Pfennig, N.; Northcote, T.G. Characterization of a Dense, Purple Sulfur Bacterial Layer in a Meromictic Salt Lake. Limnol. Oceanogr. 1991, 36, 829–1070. [Google Scholar] [CrossRef]
- Overmann, J.; Thomas Beatty, J.; Hall, K.J. Photosynthetic Activity and Population Dynamics of Amoebobacter purpureus in a Meromictic Saline Lake. FEMS Microbiol. Ecol. 1994, 15, 309–319. [Google Scholar] [CrossRef]
- Rathgeber, C.; Yurkova, N.; Stackebrandt, E.; Schumann, P.; Beatty, J.T.; Yurkov, V. Roseicyclus mahoneyensis gen. nov., sp. nov., an Aerobic Phototrophic Bacterium Isolated from a Meromictic Lake. Int. J. Syst. Evol. Microbiol. 2005, 55, 1597–1603. [Google Scholar] [CrossRef]
- Rathgeber, C.; Yurkova, N.; Stackebrandt, E.; Schumann, P.; Humphrey, E.; Beatty, J.T.; Yurkov, V. Porphyrobacter meromictius sp. nov., an Appendaged Bacterium, That Produces Bacteriochlorophyll a. Curr. Microbiol. 2007, 55, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, S.B.; Jafri, M.; Humphrey, E.; Maltman, C.; Kyndt, J.A.; Yurkov, V. Prosthecate Aerobic Anoxygenic Phototrophs Photocaulis sulfatitolerans gen. nov. sp. nov. and Photocaulis rubescens sp. nov. Isolated from Alpine Meromictic Lakes in British Columbia, Canada. Arch. Microbiol. 2022, 204, 444. [Google Scholar]
- Overmann, J.; Fischer, U.; Pfennig, N. A New Purple Sulfur Bacterium from Saline Littoral Sediments, Thiorhodovibrio winogradskyi gen. nov. and sp. nov. Arch. Microbiol. 1992, 157, 329–335. [Google Scholar] [CrossRef]
- Yurkov, V.; Beatty, J.T. Aerobic Anoxygenic Phototrophic Bacteria. Microbiol. Mol. Biol. Rev. 1998, 62, 695–724. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.K.; Morton, B.R.; Cardamone, H.C.; Lake, H.R.R.; Dohnalkova, A.C.; Kim, Y.-M.; Kyle, J.E.; Maezato, Y.; Dana, K.L.; Metz, T.O.; et al. Corrigendum: Saliniramus fredricksonii gen. nov., sp. nov., a Heterotrophic Halophile Isolated from Hot Lake, Washington, a Member of a Novel Lineage (Salinarimonadaceae fam. nov.) within the Order Rhizobiales, and Reclassification of the Genus Salinarimonas Liu et al. 2010 into Salinarimonadaceae. Int. J. Syst. Evol. Microbiol. 2018, 68, 2116–2123. [Google Scholar]
- Parte, A.C.; Carbasse, J.S.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic Names with Standing in Nomenclature (LPSN) Moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, Y.X.; Zhang, X.X.; Wang, Z.G.; Chen, Y.G.; Wen, M.L.; Xu, L.H.; Peng, Q.; Cui, X.L. Salinarimonas rosea gen. nov., sp. nov., a New Member of the α-2 Subgroup of the Proteobacteria. Int. J. Syst. Evol. Microbiol. 2010, 60, 55–60. [Google Scholar] [CrossRef]
- Cai, M.; Wang, L.; Cai, H.; Li, Y.; Wang, Y.N.; Tang, Y.Q.; Wu, X.L. Salinarimonas ramus sp. nov. and Tessaracoccus oleiagri sp. nov., Isolated from a Crude Oil-Contaminated Saline Soil. Int. J.Syst. Evol. Microbiol. 2011, 61, 1767–1775. [Google Scholar] [CrossRef]
- Jin, C.Z.; Jin, L.; Liu, M.J.; Kang, M.K.; Park, S.H.; Park, D.J.; Kim, C.J. Salinarimonas soli sp. nov., Isolated from Soil. Int. J. Syst. Evol. Microbiol. 2022, 72, 005095. [Google Scholar] [CrossRef]
- Beveridge, T.J. Use of the Gram Stain in Microbiology. Biotech. Histochem. 2001, 76, 111–118. [Google Scholar] [CrossRef]
- Gregersen, T. Rapid Method for Distinction of Gram-Negative from Gram-Positive Bacteria. Eur. J. Appl. Microbiol. Biotech. 1978, 5, 123–127. [Google Scholar] [CrossRef]
- Yurkov, V. V.; Krieger, S.; Stackebrandt, E.; Beatty, J.T. Citromicrobium bathyomarinum, a Novel Aerobic Bacterium Isolated from Deep-Sea Hydrothermal Vent Plume Waters That Contains Photosynthetic Pigment- Protein Complexes. J. Bacteriol. 1999, 181, 4517–4525. [Google Scholar] [CrossRef] [PubMed]
- Bilyj, M.; Lepitzki, D.; Hughes, E.; Swiderski, J.; Stackebrandt, E.; Pacas, C.; Yurkov, V. V. Abundance and Diversity of the Phototrophic Microbial Mat Communities of Sulphur Mountain Banff Springs and Their Significance to the Endangered Snail, Physella Johnsoni. Open J. Ecol. 2014, 4, 488–516. [Google Scholar] [CrossRef]
- Csotonyi, J.T.; Swiderski, J.; Stackebrandt, E.; Yurkov, V. V. Novel Halophilic Aerobic Anoxygenic Phototrophs from a Canadian Hypersaline Spring System. Extremophiles 2008, 12, 529–539. [Google Scholar] [CrossRef]
- Csotonyi, J.T.; Stackebrandt, E.; Swiderski, J.; Schumann, P.; Yurkov, V. An Alphaproteobacterium Capable of Both Aerobic and Anaerobic Anoxygenic Photosynthesis but Incapable of Photoautotrophy: Charonomicrobium ambiphototrophicum, gen. nov., sp. nov. Photosynth. Res. 2011, 107, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Politz, M.; Lennen, R.; Pfleger, B. Quantification of Bacterial Fatty Acids by Extraction and Methylation. Bio. Protoc. 2013, 3, 950. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Tamura, K. Estimation of the Number of Nucleotide Substitutions When There Are Strong Transition-Transversion and G+C-Content Biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [PubMed]
- Wick, R.R; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed]
- Wattam, A.R.; Davis, J.J.; Assaf, R.; Boisvert, S.; Brettin, T.; Bun, C.; Conrad, N.; Dietrich, E.M.; Disz, T.; Gabbard, J.L.; et al. Improvements to PATRIC, the All-Bacterial Bioinformatics Database and Analysis Resource Center. Nucleic Acids Res. 2017, 45, D535–D542. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI Prokaryotic Genome Annotation Pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS Is an Automated High-Throughput Platform for State-of-the-Art Genome-Based Taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Carbasse, J.S.; Peinado-Olarte, R.L.; Göker, M. TYGS and LPSN: A Database Tandem for Fast and Reliable Genome-Based Classification and Nomenclature of Prokaryotes. Nucleic Acids Res. 2022, 50, D801–D807. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R.; Oliver Glöckner, F.; Peplies, J. JSpeciesWS: A Web Server for Prokaryotic Species Circumscription Based on Pairwise Genome Comparison. Bioinformatics 2016, 32, 929–931. [Google Scholar] [CrossRef]
- Xiao, N.; Jiao, N. Formation of Polyhydroxyalkanoate in Aerobic Anoxygenic Phototrophic Bacteria and Its Relationship to Carbon Source and Light Availability. Appl. Environ. Microbiol. 2011, 77, 7445–7450. [Google Scholar] [CrossRef] [PubMed]
- Yurkov, V.; Hughes, E. Aerobic Anoxygenic Phototrophs: Four Decades of Mystery. In Modern Topics in the Phototrophic Prokaryotes: Environmental and Applied Aspects; Springer International Publishing: Cham, Switzerland, 2017; pp. 193–214. [Google Scholar]
- Kim, M.; Oh, H.S.; Park, S.C.; Chun, J. Towards a Taxonomic Coherence between Average Nucleotide Identity and 16S RRNA Gene Sequence Similarity for Species Demarcation of Prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef]
- Goris, J.; Konstantinidis, K.T.; Klappenbach, J.A.; Coenye, T.; Vandamme, P.; Tiedje, J.M. DNA-DNA Hybridization Values and Their Relationship to Whole-Genome Sequence Similarities. Int. J. Syst. Evol. Microbiol. 2007, 57, 81–91. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).