Submitted:
21 September 2024
Posted:
24 September 2024
You are already at the latest version
Abstract
Keywords:
1. Background
2. Methodology
3. Results/Discussion
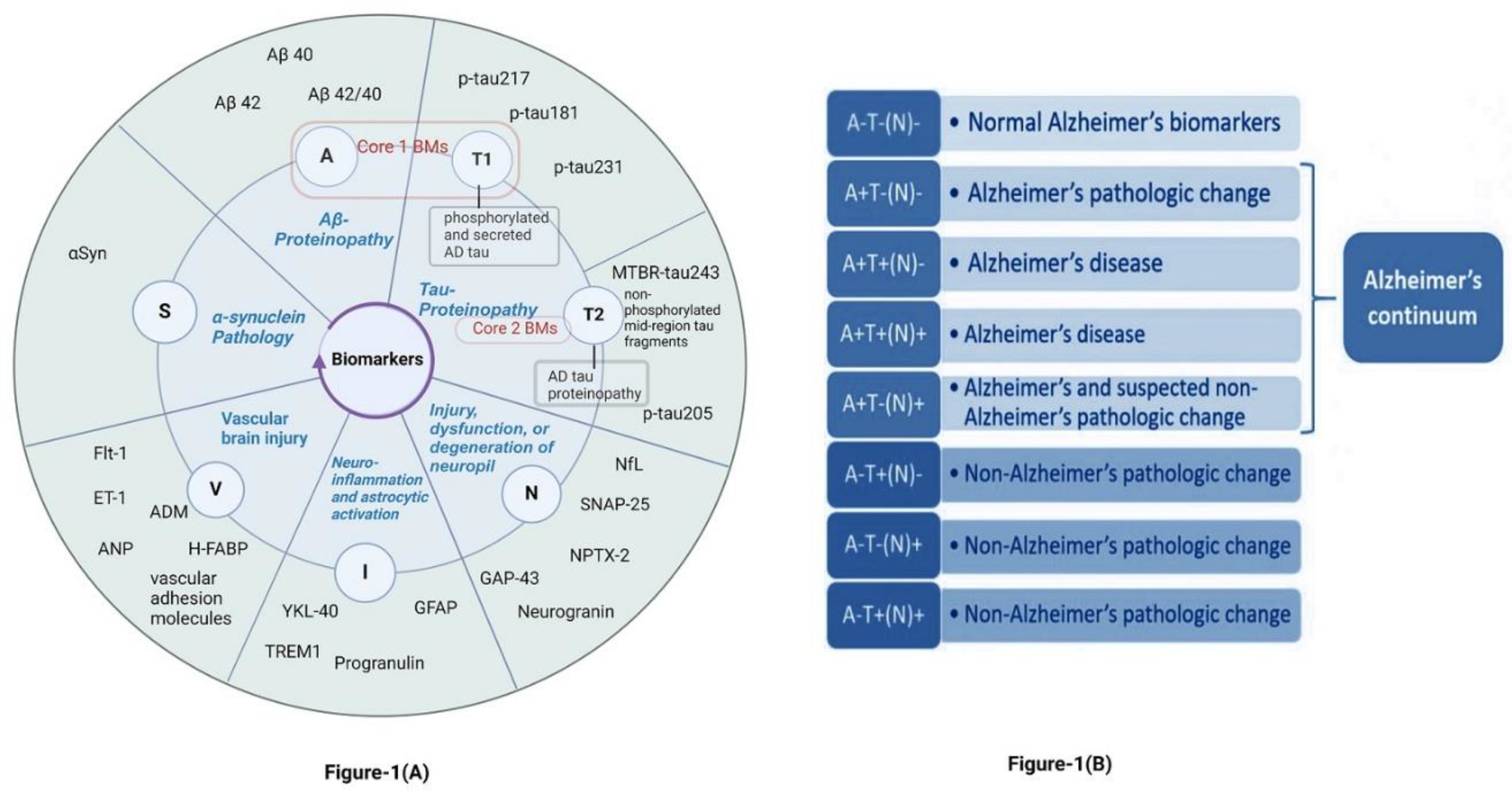
3.1. Current Insights into Different Biomarker Categorizations.
- Core biomarkers of AD neuropathological changes
- Non-specific biomarkers that are important in AD pathogenesis but are also involved in other brain diseases
- Biomarkers of common non-AD pathologies
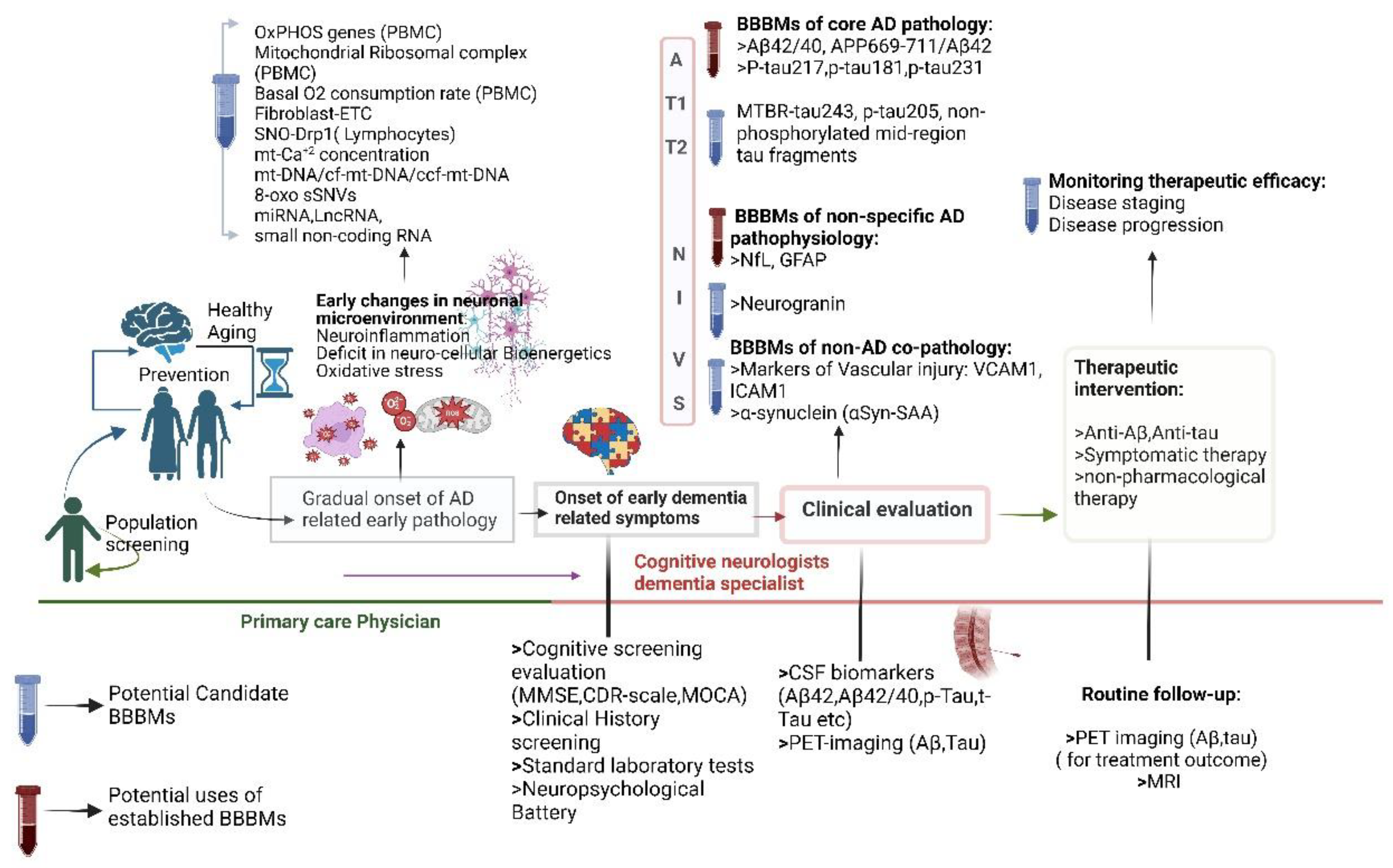
3.2. The In-Hitherto Context for Developing Blood-Based Biomarkers in AD
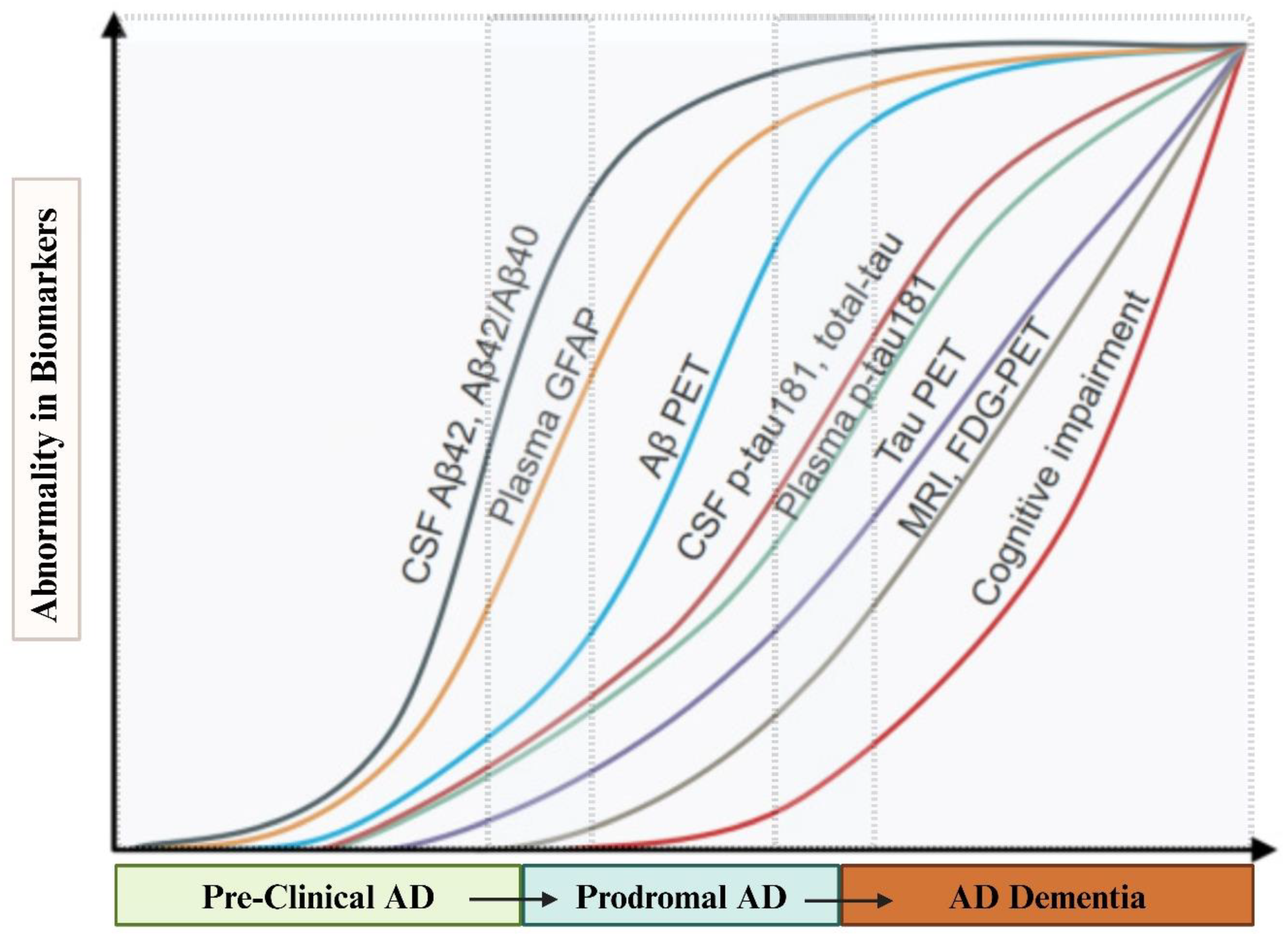
3.3. BBBMs Related to Proteinopathy in Early Detection of AD
3.3.1. Plasma Proteinopathy-Related Biomarkers of Core AD Pathology:
3.3.1.1. Aβ and Its Variations in Plasma:
3.3.1.2. Plasma p-Tau:
3.3.2. Proteinopathy-Related Biomarkers of Non-Core AD Pathology
3.3.2.1. Biomarker of TAR-DNA Binding Proteinopathy:
3.3.2.2. Blood-Based Biomarkers of Synuclein Pathology:
3.3.2.3. Serum Dickkopf-1(DKK1) as Candidate Blood-Based Biomarker in AD:
3.3.2.4. Plasma Visinin-Like Protein-1 (VILIP-1):
3.4. Blood-Based Biomarkers of Neuronal and Synaptic Injury
3.4.1. Plasma Neurofilaments (NfL) as AD Diagnostic and Disease Progression Biomarker:
3.4.2. BBBMs Related to Pre-Synaptic Dysfunction:
3.4.3. BBBMs Related to Post-Synaptic Protein Dysfunction:
3.5. Blood-Based AD-Related Biomarkers of Vascular Pathology.
3.5.1. Fms-Like Tyrosine Kinase-1 (Flt-1) in AD-Related Vascular Changes:
3.5.2. Role of Endothelin 1 (ET-1) in AD-Associated Vascular Pathology:
3.5.3. Alteration of Adrenomedullin (ADM) in AD
3.5.4. Role of Atrial Natriuretic Peptide (ANP) in AD-Related Vascular Alterations
3.5.5. Vascular Immune Interaction and Gamma Interferon/Chemokine (C-X-C) Ligand 9 (MIG/CXCL-9) in AD
3.5.6. Role of Heart-Type Fatty Acid Binding Protein (H-FABP) in AD-Related Vascular Pathology:
3.5.7. Alteration of Vascular Adhesion Molecule (AM) Expression and Endothelial Dysfunction in AD
3.6. Blood-Based Biomarkers of Oxidative Stress and Bioenergetics
3.6.1. BBBMs Related to Oxidative Stress:
3.6.2. Blood-Based Bioenergetic Profiling
3.7. Blood-Based Biomarkers of Neuroinflammation and Immune Dysregulation
3.8. Blood-Based Epigenetic Biomarkers Related to Early Detection and Prognosis of AD
3.8.1. DNA Methylation-Based Markers:
3.8.2. Potential Blood-Based microRNA Biomarkers for AD
3.8.3. Potential Blood-Based Long Non-Coding RNA (lncRNA) Biomarkers for AD
3.8.4. Markers Related to Histone Modification and DNA Alteration
3.8.5. Circular RNA (circRNA) Related Biomarkers
3.9. Plasma Exosome-Based AD-Related Biomarkers
4. Future Directions and Conclusion:
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Vanessa de Jesus, R.; Guimarães, F.M.; Diniz, B.S.; Forlenza, O.V. Neurobiological pathways to Alzheimer’s disease: Amyloid-beta, TAU protein or both? Dementia neuropsychologia 2009, 3, 188. [Google Scholar]
- Holtzman, D.M.; Morris, J.C.; Goate, A.M. Alzheimer’s disease: the challenge of the second century. Science translational medicine 2011, 3, sr1–sr77. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.F. Early-onset Alzheimer disease. Neurologic clinics 2017, 35, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Jack Jr, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimer's dementia 2018, 14, 535–562. [Google Scholar] [PubMed]
- Klyucherev, T.O.; Olszewski, P.; Shalimova, A.A.; Chubarev, V.N.; Tarasov, V.V.; Attwood, M.M.; Syvänen, S.; Schiöth, H.B. Advances in the development of new biomarkers for Alzheimer’s disease. Translational neurodegeneration 2022, 11, 25. [Google Scholar]
- Angioni, D.; Delrieu, J.; Hansson, O.; Fillit, H.; Aisen, P.; Cummings, J.; Sims, J.; Braunstein, J.; Sabbagh, M.; Bittner, T. Blood biomarkers from research use to clinical practice: What must be done? A report from the EU/US CTAD Task Force. The journal of prevention of Alzheimer's disease 2022, 9, 569–579. [Google Scholar] [CrossRef]
- Schreiner, T.G.; Croitoru, C.G.; Hodorog, D.N.; Cuciureanu, D.I. Passive Anti-Amyloid Beta Immunotherapies in Alzheimer’s Disease: From Mechanisms to Therapeutic Impact. Biomedicines 2024, 12, 1096. [Google Scholar] [CrossRef]
- Hyman, B.T.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Carrillo, M.C.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E. National Institute on Aging–Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimer's dementia 2012, 8, 1–13. [Google Scholar] [CrossRef]
- Jack Jr, C.R.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E. Revised criteria for diagnosis and staging of Alzheimer's disease: Alzheimer's Association Workgroup. Alzheimer's Dementia 2024. [Google Scholar] [CrossRef]
- Arslan, B.; Zetterberg, H.; Ashton, N.J. Blood-based biomarkers in Alzheimer’s disease–moving towards a new era of diagnostics. Clinical Chemistry Laboratory Medicine 2024, 62, 1063–1069. [Google Scholar] [CrossRef]
- Assfaw, A.D.; Schindler, S.E.; Morris, J.C. Advances in blood biomarkers for Alzheimer disease (AD): A review. The Kaohsiung Journal of Medical Sciences 2024, 40, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.C.; Kinney, J.W.; Ritter, A.; Andrews, R.D.; Toledano Strom, E.N.; Lukic, A.S.; Koenig, L.N.; Revta, C.; Fillit, H.M.; Zhong, K. Relationships between plasma biomarkers, tau PET, FDG PET, and volumetric MRI in mild to moderate Alzheimer's disease patients. Alzheimer's & Dementia: Translational Research & Clinical interventions 2024, 10, e12490. [Google Scholar]
- Brand, A.L.; Lawler, P.E.; Bollinger, J.G.; Li, Y.; Schindler, S.E.; Li, M.; Lopez, S.; Ovod, V.; Nakamura, A.; Shaw, L.M. The performance of plasma amyloid beta measurements in identifying amyloid plaques in Alzheimer’s disease: a literature review. Alzheimer's research therapy 2022, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Yi, S.; Han, J.-y.; Park, S.Y.; Jang, J.-W.; Chun, I.K.; Kim, S.E.; Lee, B.S.; Kim, G.J.; Yu, J.S.J.A. s. r. ; therapy, Oligomeric forms of amyloid-β protein in plasma as a potential blood-based biomarker for Alzheimer’s disease. Alzheimer's research therapy 2017, 9, 1–10. [Google Scholar] [CrossRef]
- Li, D.; Mielke, M.M. An update on blood-based markers of Alzheimer’s disease using the SiMoA platform. Neurology therapy 2019, 8 (Suppl 2), 73–82. [Google Scholar] [CrossRef]
- Wang, R.; Sweeney, D.; Gandy, S.E.; Sisodia, S.S. The Profile of Soluble Amyloid β Protein in Cultured Cell Media: detection and quantification of amyloid β protein and variants by immunoprecipitation-mass spectrometry. Journal of Biological Chemistry 1996, 271, 31894–31902. [Google Scholar] [CrossRef]
- Korecka, M.; Shaw, L.M. Mass spectrometry-based methods for robust measurement of Alzheimer's disease biomarkers in biological fluids. Journal of neurochemistry 2021, 159, 211–233. [Google Scholar] [CrossRef]
- Blennow, K.; Zetterberg, H. Cerebrospinal fluid biomarkers for Alzheimer's disease. Journal of Alzheimer's Disease 2009, 18, 413–417. [Google Scholar] [CrossRef]
- Guo, Y.; Shen, X.-N.; Wang, H.-F.; Chen, S.-D.; Zhang, Y.-R.; Chen, S.-F.; Cui, M.; Cheng, W.; Dong, Q.; Ma, T. The dynamics of plasma biomarkers across the Alzheimer’s continuum. Alzheimer's Research Therapy 2023, 15, 31. [Google Scholar] [CrossRef]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Doré, V.; Fowler, C.; Li, Q.-X.; Martins, R.; Rowe, C. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef]
- Schindler, S.E.; Bollinger, J.G.; Ovod, V.; Mawuenyega, K.G.; Li, Y.; Gordon, B.A.; Holtzman, D.M.; Morris, J.C.; Benzinger, T.L.; Xiong, C. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019, 93, e1647–e1659. [Google Scholar] [CrossRef] [PubMed]
- Ovod, V.; Ramsey, K.N.; Mawuenyega, K.G.; Bollinger, J.G.; Hicks, T.; Schneider, T.; Sullivan, M.; Paumier, K.; Holtzman, D.M.; Morris, J.C. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimer's Dementia 2017, 13, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, W.; Chen, Y.; Lin, Y.; Wang, B.; Guo, Q.; Miao, Y. Plasma Aβ as a biomarker for predicting Aβ-PET status in Alzheimer’s disease: A systematic review with meta-analysis. Journal of Neurology, Neurosurgery Psychiatry 2022, 93, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Kirmess, K.M.; Meyer, M.R.; Holubasch, M.S.; Knapik, S.S.; Hu, Y.; Jackson, E.N.; Harpstrite, S.E.; Verghese, P.B.; West, T.; Fogelman, I. The PrecivityAD™ test: Accurate and reliable LC-MS/MS assays for quantifying plasma amyloid beta 40 and 42 and apolipoprotein E proteotype for the assessment of brain amyloidosis. Clinica Chimica Acta 2021, 519, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Qiao, Y.; Choi, J.; Raman, R.; Ringman, J.M.; Shi, Y.; Initiative, A. s. D. N. Enhanced association of tau pathology and cognitive impairment in mild cognitive impairment subjects with behavior symptoms. Journal of Alzheimer's Disease 2022, 87, 557–568. [Google Scholar] [CrossRef]
- Kent, S.A.; Spires-Jones, T.L.; Durrant, C.S. The physiological roles of tau and Aβ: implications for Alzheimer’s disease pathology and therapeutics. Acta neuropathologica 2020, 140, 417–447. [Google Scholar] [CrossRef]
- Brickman, A.M.; Manly, J.J.; Honig, L.S.; Sanchez, D.; Reyes-Dumeyer, D.; Lantigua, R.A.; Lao, P.J.; Stern, Y.; Vonsattel, J.P.; Teich, A.F. Plasma p-tau181, p-tau217, and other blood-based Alzheimer's disease biomarkers in a multi-ethnic, community study. Alzheimer's Dementia 2021, 17, 1353–1364. [Google Scholar] [CrossRef]
- Palmqvist, S.; Janelidze, S.; Quiroz, Y.T.; Zetterberg, H.; Lopera, F.; Stomrud, E.; Su, Y.; Chen, Y.; Serrano, G.E.; Leuzy, A. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. Jama 2020, 324, 772–781. [Google Scholar] [CrossRef]
- McGrath, E.R.; Beiser, A.S.; O’Donnell, A.; Yang, Q.; Ghosh, S.; Gonzales, M.M.; Himali, J.J.; Satizabal, C.L.; Johnson, K.A.; Tracy, R.P. Blood phosphorylated tau 181 as a biomarker for amyloid burden on brain PET in cognitively healthy adults. Journal of Alzheimer's Disease 2022, 87, 1517–1526. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, F.; Ferreira, P.C.; González-Escalante, A.; Montoliu-Gaya, L.; Ortiz-Romero, P.; Kac, P.R.; Turton, M.; Kvartsberg, H.; Ashton, N.J.; Zetterberg, H. A novel ultrasensitive assay for plasma p-tau217: performance in individuals with subjective cognitive decline and early Alzheimer's disease. Alzheimer's Dementia 2024, 20, 1239–1249. [Google Scholar] [CrossRef]
- Ashton, N.J.; Brum, W.S.; Di Molfetta, G.; Benedet, A.L.; Arslan, B.; Jonatis, E.; Langhough, R.E.; Cody, K.; Wilson, R.; Carlsson, C.M. Diagnostic accuracy of the plasma ALZpath pTau217 immunoassay to identify Alzheimer’s disease pathology. medRxiv 2023. [Google Scholar]
- Palmqvist, S.; Tideman, P.; Cullen, N.; Zetterberg, H.; Blennow, K.; Initiative, A. s. D. N.; Dage, J.L.; Stomrud, E.; Janelidze, S.; Mattsson-Carlgren, N. Prediction of future Alzheimer’s disease dementia using plasma phospho-tau combined with other accessible measures. Nature medicine 2021, 27, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Berron, D.; Smith, R.; Strandberg, O.; Proctor, N.K.; Dage, J.L.; Stomrud, E.; Palmqvist, S.; Mattsson-Carlgren, N.; Hansson, O. Associations of plasma phospho-tau217 levels with tau positron emission tomography in early Alzheimer disease. JAMA neurology 2021, 78, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ortiz, F.; Turton, M.; Kac, P.R.; Smirnov, D.; Premi, E.; Ghidoni, R.; Benussi, L.; Cantoni, V.; Saraceno, C.; Rivolta, J. Brain-derived tau: a novel blood-based biomarker for Alzheimer’s disease-type neurodegeneration. Brain 2023, 146, 1152–1165. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; van der Kant, R.; Hansson, O. Tau biomarkers in Alzheimer's disease: towards implementation in clinical practice and trials. The Lancet Neurology 2022, 21, 726–734. [Google Scholar] [CrossRef]
- Cabrera-Rodríguez, R.; Pérez-Yanes, S.; Montelongo, R.; Lorenzo-Salazar, J.M.; Estévez-Herrera, J.; García-Luis, J.; Íñigo-Campos, A.; Rubio-Rodríguez, L.A.; Muñoz-Barrera, A.; Trujillo-González, R. Transactive response DNA-binding protein (TARDBP/TDP-43) regulates cell permissivity to HIV-1 infection by acting on HDAC6. International journal of molecular sciences 2022, 23, 6180. [Google Scholar] [CrossRef]
- Gatignol, A.; Duarte, M.; Daviet, L.; Chang, Y.-N.; Jeang, K.-T. Sequential steps in Tat trans-activation of HIV-1 mediated through cellular DNA, RNA, and protein binding factors. Gene expression 1996, 5, 217. [Google Scholar]
- Meneses, A.; Koga, S.; O’Leary, J.; Dickson, D.W.; Bu, G.; Zhao, N. TDP-43 pathology in Alzheimer’s disease. Molecular neurodegeneration 2021, 16, 1–15. [Google Scholar] [CrossRef]
- Lopez, O.L.; Kofler, J.; Chang, Y.; Berman, S.B.; Becker, J.T.; Sweet, R.A.; Nadkarni, N.; Patira, R.; Kamboh, M.I.; Cohen, A.D. Hippocampal sclerosis, TDP-43, and the duration of the symptoms of dementia of AD patients. Annals of clinical translational neurology 2020, 7, 1546–1556. [Google Scholar] [CrossRef]
- Katisko, K.; Huber, N.; Kokkola, T.; Hartikainen, P.; Krüger, J.; Heikkinen, A.-L.; Paananen, V.; Leinonen, V.; Korhonen, V.E.; Helisalmi, S. Serum total TDP-43 levels are decreased in frontotemporal dementia patients with C9orf72 repeat expansion or concomitant motoneuron disease phenotype. Alzheimer's research therapy 2022, 14, 151. [Google Scholar] [CrossRef]
- Cordts, I.; Wachinger, A.; Scialo, C.; Lingor, P.; Polymenidou, M.; Buratti, E.; Feneberg, E. TDP-43 proteinopathy specific biomarker development. Cells 2023, 12, 597. [Google Scholar] [CrossRef] [PubMed]
- Sephton, C.F.; Cenik, B.; Cenik, B.K.; Herz, J.; Yu, G. TDP-43 in central nervous system development and function: clues to TDP-43-associated neurodegeneration. Biological chemistry 2012, 393, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Wilhite, R.; Sage, J.M.; Bouzid, A.; Primavera, T.; Agbas, A. Platelet phosphorylated TDP-43: an exploratory study for a peripheral surrogate biomarker development for Alzheimer's disease. Future Science OA 2017, 3, FSO238. [Google Scholar] [CrossRef] [PubMed]
- Twohig, D.; Nielsen, H.M. α-synuclein in the pathophysiology of Alzheimer’s disease. Molecular neurodegeneration 2019, 14, 23. [Google Scholar] [PubMed]
- Stefanis, L. α-Synuclein in Parkinson's disease. Cold Spring Harbor perspectives in medicine 2012, 2, a009399. [Google Scholar] [CrossRef]
- Barbour, R.; Kling, K.; Anderson, J.P.; Banducci, K.; Cole, T.; Diep, L.; Fox, M.; Goldstein, J.M.; Soriano, F.; Seubert, P. Red blood cells are the major source of alpha-synuclein in blood. Neurodegenerative diseases 2008, 5, 55–59. [Google Scholar] [CrossRef]
- Kasuga, K.; Tokutake, T.; Ishikawa, A.; Uchiyama, T.; Tokuda, T.; Onodera, O.; Nishizawa, M.; Ikeuchi, T. Differential levels of α-synuclein, β-amyloid42 and tau in CSF between patients with dementia with Lewy bodies and Alzheimer's disease. Journal of Neurology, Neurosurgery Psychiatry 2010, 81, 608–610. [Google Scholar] [CrossRef]
- Kasuga, K.; Nishizawa, M.; Ikeuchi, T. α-Synuclein as CSF and Blood Biomarker of Dementia with Lewy Bodies. International Journal of Alzheimer’s Disease 2012, 2012, 437025. [Google Scholar] [CrossRef]
- Daniele, S.; Baldacci, F.; Piccarducci, R.; Palermo, G.; Giampietri, L.; Manca, M.L.; Pietrobono, D.; Frosini, D.; Nicoletti, V.; Tognoni, G. α-Synuclein heteromers in red blood cells of Alzheimer’s disease and Lewy body dementia patients. Journal of Alzheimer's Disease 2021, 80, 885–893. [Google Scholar] [CrossRef]
- Laske, C.; Fallgatter, A.J.; Stransky, E.; Hagen, K.; Berg, D.; Maetzler, W. Decreased α-synuclein serum levels in patients with Lewy body dementia compared to Alzheimer’s disease patients and control subjects. Dementia geriatric cognitive disorders 2011, 31, 413–416. [Google Scholar] [CrossRef]
- Clinton, L.K.; Blurton-Jones, M.; Myczek, K.; Trojanowski, J.Q.; LaFerla, F.M. Synergistic interactions between Aβ, tau, and α-synuclein: acceleration of neuropathology and cognitive decline. Journal of Neuroscience 2010, 30, 7281–7289. [Google Scholar] [CrossRef] [PubMed]
- Baldacci, F.; Daniele, S.; Piccarducci, R.; Giampietri, L.; Pietrobono, D.; Giorgi, F.S.; Nicoletti, V.; Frosini, D.; Libertini, P.; Lo Gerfo, A. Potential diagnostic value of red blood cells α-synuclein heteroaggregates in Alzheimer’s disease. Molecular Neurobiology 2019, 56, 6451–6459. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Gu, X.; Li, H.; Lei, S.; Wang, Z.; Wang, J.; Yin, P.; Zhang, C.; Wang, F.; Liu, C. The role of DKK1 in Alzheimer’s disease: a potential intervention point of brain damage prevention? Pharmacological Research 2019, 144, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Caricasole, A.; Copani, A.; Caraci, F.; Aronica, E.; Rozemuller, A.J.; Caruso, A.; Storto, M.; Gaviraghi, G.; Terstappen, G.C.; Nicoletti, F. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer's brain. Journal of Neuroscience 2004, 24, 6021–6027. [Google Scholar] [CrossRef] [PubMed]
- Purro, S.A.; Galli, S.; Salinas, P.C. Dysfunction of Wnt signaling and synaptic disassembly in neurodegenerative diseases. Journal of molecular cell biology 2014, 6, 75–80. [Google Scholar] [CrossRef]
- Seib, D.R.; Corsini, N.S.; Ellwanger, K.; Plaas, C.; Mateos, A.; Pitzer, C.; Niehrs, C.; Celikel, T.; Martin-Villalba, A. Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell stem cell 2013, 12, 204–214. [Google Scholar] [CrossRef]
- Marzo, A.; Galli, S.; Lopes, D.; McLeod, F.; Podpolny, M.; Segovia-Roldan, M.; Ciani, L.; Purro, S.; Cacucci, F.; Gibb, A. Reversal of synapse degeneration by restoring Wnt signaling in the adult hippocampus. Current Biology 2016, 26, 2551–2561. [Google Scholar] [CrossRef]
- Tay, L.; Leung, B.; Yeo, A.; Chan, M.; Lim, W.S. Elevations in Serum Dickkopf-1 and disease progression in community-dwelling older adults with mild cognitive impairment and mild-to-moderate Alzheimer’s disease. Frontiers in Aging Neuroscience 2019, 11, 278. [Google Scholar] [CrossRef]
- Tarawneh, R.; D'Angelo, G.; Macy, E.; Xiong, C.; Carter, D.; Cairns, N.J.; Fagan, A.M.; Head, D.; Mintun, M.A.; Ladenson, J.H. Visinin-like protein-1: diagnostic and prognostic biomarker in Alzheimer disease. Annals of neurology 2011, 70, 274–285. [Google Scholar] [CrossRef]
- Halbgebauer, S.; Steinacker, P.; Riedel, D.; Oeckl, P.; Anderl-Straub, S.; Lombardi, J.; von Arnim, C.A.; Nagl, M.; Giese, A.; Ludolph, A.C. Visinin-like protein 1 levels in blood and CSF as emerging markers for Alzheimer’s and other neurodegenerative diseases. Alzheimer's research therapy 2022, 14, 175. [Google Scholar] [CrossRef]
- Mavroudis, I.A.; Petridis, F.; Chatzikonstantinou, S.; Karantali, E.; Kazis, D. A meta-analysis on the levels of VILIP-1 in the CSF of Alzheimer’s disease compared to normal controls and other neurodegenerative conditions. Aging Clinical Experimental Research 2021, 33, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Rao, M.V.; Nixon, R.A. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harbor perspectives in biology 2017, 9, a018309. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Shi, M.; Li, Y.; Zhao, X. Elevated plasma neurofilament light was associated with multi-modal neuroimaging features in Alzheimer’s Disease signature regions and predicted future tau deposition. Research Square 2024. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, N.; Andreasson, U.; Zetterberg, H.; Blennow, K.; neurology, A.s.D.N.I.J.J. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. 2017, 74, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Kuhle, J.; Barro, C.; Andreasson, U.; Derfuss, T.; Lindberg, R.; Sandelius, Å.; Liman, V.; Norgren, N.; Blennow, K.; Zetterberg, H. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clinical Chemistry Laboratory Medicine 2016, 54, 1655–1661. [Google Scholar] [CrossRef]
- Bäckström, D.; Linder, J.; Jakobson Mo, S.; Riklund, K.; Zetterberg, H.; Blennow, K.; Forsgren, L.; Lenfeldt, N. NfL as a biomarker for neurodegeneration and survival in Parkinson disease. Neurology 2020, 95, e827–e838. [Google Scholar] [CrossRef]
- Kivisäkk, P.; Carlyle, B.C.; Sweeney, T.; Quinn, J.P.; Ramirez, C.E.; Trombetta, B.A.; Mendes, M.; Brock, M.; Rubel, C.; Czerkowicz, J. Increased levels of the synaptic proteins PSD-95, SNAP-25, and neurogranin in the cerebrospinal fluid of patients with Alzheimer’s disease. Alzheimer's research therapy 2022, 14, 58. [Google Scholar] [CrossRef]
- Halbgebauer, S.; Steinacker, P.; Hengge, S.; Oeckl, P.; Rumeileh, S.A.; Anderl-Straub, S.; Lombardi, J.; Von Arnim, C.A.; Giese, A.; Ludolph, A.C. CSF levels of SNAP-25 are increased early in Creutzfeldt-Jakob and Alzheimer’s disease. Journal of Neurology, Neurosurgery Psychiatry 2022, 93, 1059–1065. [Google Scholar] [CrossRef]
- Agliardi, C.; Guerini, F.R.; Zanzottera, M.; Bianchi, A.; Nemni, R.; Clerici, M. SNAP-25 in serum is carried by exosomes of neuronal origin and is a potential biomarker of Alzheimer’s disease. Molecular neurobiology 2019, 56, 5792–5798. [Google Scholar] [CrossRef]
- Libiger, O.; Shaw, L.M.; Watson, M.H.; Nairn, A.C.; Umaña, K.L.; Biarnes, M.C.; Canet-Avilés, R.M.; Jack Jr, C.R.; Breton, Y.A.; Cortes, L. Longitudinal CSF proteomics identifies NPTX2 as a prognostic biomarker of Alzheimer's disease. Alzheimer's Dementia 2021, 17, 1976–1987. [Google Scholar] [CrossRef]
- Saunders, T.S.; Gadd, D.A.; Spires-Jones, T.L.; King, D.; Ritchie, C.; Muniz-Terrera, G. Associations between cerebrospinal fluid markers and cognition in ageing and dementia: A systematic review. European Journal of Neuroscience 2022, 56, 5650–5713. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Q.; Skudder-Hill, L.; Toyota, T.; Wei, W.; Adachi, H. CSF GAP-43 as a biomarker of synaptic dysfunction is associated with tau pathology in Alzheimer’s disease. Scientific Reports 2022, 12, 17392. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhu, M.; Kong, C.; Pang, Y.; Zhang, H.; Qiu, Q.; Wei, C.; Tang, Y.; Wang, Q.; Li, Y. Blood neuro-exosomal synaptic proteins predict Alzheimer's disease at the asymptomatic stage. Alzheimer's Dementia 2021, 17, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, T.; Blandino, V.; Maniscalco, L.; Matranga, D.; Graziano, F.; Guajana, F.; Agnello, L.; Lo Sasso, B.; Gambino, C.M.; Giglio, R.V. Biomarkers related to synaptic dysfunction to discriminate alzheimer’s disease from other neurological disorders. International Journal of Molecular Sciences 2022, 23, 10831. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Gerges, N.Z. Neurogranin and synaptic plasticity balance. Communicative integrative biology 2010, 3, 340–342. [Google Scholar] [CrossRef]
- Kvartsberg, H.; Portelius, E.; Andreasson, U.; Brinkmalm, G.; Hellwig, K.; Lelental, N.; Kornhuber, J.; Hansson, O.; Minthon, L.; Spitzer, P. Characterization of the postsynaptic protein neurogranin in paired cerebrospinal fluid and plasma samples from Alzheimer’s disease patients and healthy controls. Alzheimer's research therapy 2015, 7, 1–9. [Google Scholar] [CrossRef]
- Wellington, H.; Paterson, R.W.; Portelius, E.; Törnqvist, U.; Magdalinou, N.; Fox, N.C.; Blennow, K.; Schott, J.M.; Zetterberg, H. Increased CSF neurogranin concentration is specific to Alzheimer disease. Neurology 2016, 86, 829–835. [Google Scholar] [CrossRef]
- He, M.; Sun, L.; Cao, W.; Yin, C.; Sun, W.; Liu, P.; Tan, L.; Xu, Z.; Zhao, W. Association between plasma exosome neurogranin and brain structure in patients with Alzheimer’s disease: A protocol study. BMJ Open 2020, 10, e036990. [Google Scholar] [CrossRef]
- Shibuya, M.J.G. cancer, Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti-and pro-angiogenic therapies. 2011, 2, 1097–1105. [Google Scholar]
- Ceci, C.; Lacal, P.M.; Barbaccia, M.L.; Mercuri, N.B.; Graziani, G.; Ledonne, A. The VEGFs/VEGFRs system in Alzheimer’s and Parkinson’s diseases: Pathophysiological roles and therapeutic implications. J Pharmacological Research 2024, 107101. [Google Scholar]
- Lau, S.-F.; Cao, H.; Fu, A.K.; Ip, N.Y. Single-nucleus transcriptome analysis reveals dysregulation of angiogenic endothelial cells and neuroprotective glia in Alzheimer’s disease. Proceedings of the National Academy of Sciences 2020, 117, 25800–25809. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.; Ashby, E.L.; Wellington, D.; Barrow, V.M.; Palmer, J.C.; Kehoe, P.G.; Esiri, M.M.; Love, S. Pathophysiology of white matter perfusion in Alzheimer’s disease and vascular dementia. Brain 2014, 137, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.C.; Tayler, H.M.; Love, S. Endothelin-converting enzyme-1 activity, endothelin-1 production, and free radical-dependent vasoconstriction in Alzheimer's disease. Journal of Alzheimer's Disease 2013, 36, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Geven, C.; Kox, M.; Pickkers, P. Adrenomedullin and adrenomedullin-targeted therapy as treatment strategies relevant for sepsis. Frontiers in Immunology 2018, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A. Inflammation and white matter damage in vascular cognitive impairment. Stroke 2009, 40 (3_suppl_1), S20–S23. [Google Scholar] [CrossRef]
- Ferrero, H.; Larrayoz, I.M.; Martisova, E.; Solas, M.; Howlett, D.R.; Francis, P.T.; Gil-Bea, F.J.; Martínez, A.; Ramírez, M. Increased levels of brain adrenomedullin in the neuropathology of Alzheimer’s disease. J Molecular Neurobiology 2018, 55, 5177–5183. [Google Scholar] [CrossRef]
- Noda, M.; Matsuda, T. Central regulation of body fluid homeostasis. Proceedings of the Japan Academy, Series B 2022, 98, 283–324. [Google Scholar] [CrossRef]
- Mahinrad, S.; Sabayan, B.; Garner, C.R.; Lloyd-Jones, D.M.; Sorond, F.A. N-terminal pro brain, N-terminal pro atrial natriuretic peptides, and dynamic cerebral autoregulation. Journal of the American Heart Association 2020, 9, e018203. [Google Scholar] [CrossRef]
- Qi, X.-m.; Ma, J.-f. The role of amyloid beta clearance in cerebral amyloid angiopathy: more potential therapeutic targets. Translational neurodegeneration 2017, 6, 1–12. [Google Scholar] [CrossRef]
- Mahinrad, S.; Bulk, M.; Van Der Velpen, I.; Mahfouz, A.; van Roon-Mom, W.; Fedarko, N.; Yasar, S.; Sabayan, B.; Van Heemst, D.; Van Der Weerd, L. Natriuretic peptides in post-mortem brain tissue and cerebrospinal fluid of non-demented humans and Alzheimer’s disease patients. Frontiers in Neuroscience 2018, 12, 864. [Google Scholar] [CrossRef]
- Hong, J.; Cheng, H.; Wang, P.; Wu, Y.; Lu, S.; Zhou, Y.; Wang, X.B.; Zhu, X. CXCL9 may serve as a potential biomarker for primary Sjögren’s syndrome with extra-glandular manifestations. Arthritis Research Therapy 2024, 26, 26. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, T.K.; Dunachie, S.J.; Todryk, S.; Hill, A.V.; Fletcher, H.A. MIG (CXCL9) is a more sensitive measure than IFN-γ of vaccine induced T-cell responses in volunteers receiving investigated malaria vaccines. Journal of immunological methods 2009, 340, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Sun, Y.; Xie, X.; Zhao, Y. Blood and CSF chemokines in Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis. Alzheimer's research therapy 2023, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Olsson, B.; Hertze, J.; Ohlsson, M.; Nägga, K.; Höglund, K.; Basun, H.; Annas, P.; Lannfelt, L.; Andreasen, N.; Minthon, L. Cerebrospinal fluid levels of heart fatty acid binding protein are elevated prodromally in Alzheimer's disease and vascular dementia. Journal of Alzheimer's Disease 2013, 34, 673–679. [Google Scholar] [CrossRef]
- Desikan, R.S.; Thompson, W.K.; Holland, D.; Hess, C.P.; Brewer, J.B.; Zetterberg, H.; Blennow, K.; Andreassen, O.A.; McEvoy, L.K.; Hyman, B.T. Heart fatty acid binding protein and Aβ-associated Alzheimer’s neurodegeneration. Molecular neurodegeneration 2013, 8, 1–9. [Google Scholar] [CrossRef]
- Sashindranath, M.; Nandurkar, H.H. Endothelial dysfunction in the brain: setting the stage for stroke and other cerebrovascular complications of COVID-19. Stroke 2021, 52, 1895–1904. [Google Scholar] [CrossRef]
- Jickling, G.C.; Ander, B.P.; Zhan, X.; Stamova, B.; Hull, H.; DeCarli, C.; Sharp, F.R. Progression of cerebral white matter hyperintensities is related to leucocyte gene expression. Brain 2022, 145, 3179–3186. [Google Scholar] [CrossRef]
- Chen, J.; Dai, A.-X.; Tang, H.-L.; Lu, C.-H.; Liu, H.-X.; Hou, T.; Lu, Z.-J.; Kong, N.; Peng, X.-Y.; Lin, K.-X. Increase of ALCAM and VCAM-1 in the Plasma Predicts the Alzheimer’s Disease. Frontiers in Immunology 2023, 13, 1097409. [Google Scholar] [CrossRef]
- Papasavvas, E.; Azzoni, L.; Pistilli, M.; Hancock, A.; Reynolds, G.; Gallo, C.; Ondercin, J.; Kostman, J.R.; Mounzer, K.; Shull, J. Increased soluble vascular cell adhesion molecule-1 plasma levels and soluble intercellular adhesion molecule-1 during antiretroviral therapy interruption and retention of elevated soluble vascular cellular adhesion molecule-1 levels following resumption of antiretroviral therapy. Aids 2008, 22, 1153–1161. [Google Scholar]
- Austin, S.A.; Katusic, Z.S. Partial loss of endothelial nitric oxide leads to increased cerebrovascular beta amyloid. Journal of Cerebral Blood Flow Metabolism 2020, 40, 392–403. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattsson, N.; Stomrud, E.; Lindberg, O.; Palmqvist, S.; Zetterberg, H.; Blennow, K.; Hansson, O. CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology 2018, 91, e867–e877. [Google Scholar] [CrossRef]
- Zuliani, G.; Cavalieri, M.; Galvani, M.; Passaro, A.; Munari, M.; Bosi, C.; Zurlo, A.; Fellin, R. Markers of endothelial dysfunction in older subjects with late onset Alzheimer's disease or vascular dementia. Journal of the neurological sciences 2008, 272, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.D.; Chambers, A.B.; Ott, B.R.; Daiello, L.A.; Initiative, A. s. D. N. Peripheral markers of vascular endothelial dysfunction show independent but additive relationships with brain-based biomarkers in association with functional impairment in Alzheimer’s disease. Journal of Alzheimer's Disease 2021, 80, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
- Farrall, A.J.; Wardlaw, J.M. Blood–brain barrier: ageing and microvascular disease–systematic review and meta-analysis. Neurobiology of aging 2009, 30, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Popescu, B.O.; Toescu, E.C.; Popescu, L.M.; Bajenaru, O.; Muresanu, D.F.; Schultzberg, M.; Bogdanovic, N. Blood-brain barrier alterations in ageing and dementia. Journal of the neurological sciences 2009, 283, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Fan, L.M.; Liu, F.; Smith, C.; Li, J.-M. Nox2 dependent redox-regulation of microglial response to amyloid-β stimulation and microgliosis in aging. Scientific reports 2020, 10, 1582. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Kanski, J. Methionine residue 35 is critical for the oxidative stress and neurotoxic properties of Alzheimer’s amyloid β-peptide 1–42. Peptides 2002, 23, 1299–1309. [Google Scholar] [CrossRef]
- Lovell, M.; Robertson, J.; Teesdale, W.; Campbell, J.; Markesbery, W. Copper, iron and zinc in Alzheimer's disease senile plaques. Journal of the neurological sciences 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Bartzokis, G.; Sultzer, D.; Cummings, J.; Holt, L.E.; Hance, D.B.; Henderson, V.W.; Mintz, J. In vivo evaluation of brain iron in Alzheimer disease using magnetic resonance imaging. Archives of general psychiatry 2000, 57, 47–53. [Google Scholar] [CrossRef]
- Söderberg, M.; Edlund, C.; Kristensson, K.; Dallner, G. Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids 1991, 26, 421–425. [Google Scholar] [CrossRef]
- Markesbery, W.; Lovell, M. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiology of aging 1998, 19, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Selley, M.; Close, D.; Stern, S. The effect of increased concentrations of homocysteine on the concentration of (E)-4-hydroxy-2-nonenal in the plasma and cerebrospinal fluid of patients with Alzheimer’s disease. Neurobiology of aging 2002, 23, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Greilberger, J.; Koidl, C.; Greilberger, M.; Lamprecht, M.; Schroecksnadel, K.; Leblhuber, F.; Fuchs, D.; Oettl, K. Malondialdehyde, carbonyl proteins and albumin-disulphide as useful oxidative markers in mild cognitive impairment and Alzheimer's disease. Free radical research 2008, 42, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D.; Clark, C.M.; Lee, V.M.Y.; Trojanowski, J.Q.; Rokach, J.; FitzGerald, G.A. Increased 8, 12-iso-iPF2α-VI in Alzheimer's disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Annals of neurology 2000, 48, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, M.; Yao, Y.; Hyman, B.; Growdon, J.; Pratico, D. Plasma F2A isoprostane levels in Alzheimer’s and Parkinson’s disease. Neurodegenerative Diseases 2007, 4, 403–405. [Google Scholar] [CrossRef]
- Cecchi, C.; Fiorillo, C.; Sorbi, S.; Latorraca, S.; Nacmias, B.; Bagnoli, S.; Nassi, P.; Liguri, G. Oxidative stress and reduced antioxidant defenses in peripheral cells from familial Alzheimer’s patients. Free Radical Biology Medicine 2002, 33, 1372–1379. [Google Scholar] [CrossRef]
- Rao, A.; Bharani, M.; Pallavi, V. Role of antioxidants and free radicals in health and disease. Adv Pharmacol Toxicol 2006, 7, 29–38. [Google Scholar]
- Butterfield, D.A.; Reed, T.T.; Perluigi, M.; De Marco, C.; Coccia, R.; Keller, J.N.; Markesbery, W.R.; Sultana, R. Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: implications for the role of nitration in the progression of Alzheimer's disease. Brain research 2007, 1148, 243–248. [Google Scholar] [CrossRef]
- Ahmed, N.; Ahmed, U.; Thornalley, P.J.; Hager, K.; Fleischer, G.; Münch, G. Protein glycation, oxidation and nitration adduct residues and free adducts of cerebrospinal fluid in Alzheimer's disease and link to cognitive impairment. Journal of neurochemistry 2005, 92, 255–263. [Google Scholar] [CrossRef]
- Yu, H.L.; Chertkow, H.M.; Bergman, H.; Schipper, H.M. Aberrant profiles of native and oxidized glycoproteins in Alzheimer plasma. Proteomics 2003, 3, 2240–2248. [Google Scholar] [CrossRef]
- Polidori, M.C.; Mattioli, P.; Aldred, S.; Cecchetti, R.; Stahl, W.; Griffiths, H.; Senin, U.; Sies, H.; Mecocci, P. Plasma antioxidant status, immunoglobulin g oxidation and lipid peroxidation in demented patients: relevance to Alzheimer disease and vascular dementia. Dementia geriatric cognitive disorders 2004, 18, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; Polidori, M.C.; Cherubini, A.; Ingegni, T.; Mattioli, P.; Catani, M.; Rinaldi, P.; Cecchetti, R.; Stahl, W.; Senin, U. Lymphocyte oxidative DNA damage and plasma antioxidants in Alzheimer disease. Archives of neurology 2002, 59, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, E.; Sardas, S.; Aslan, S.; Isik, E.; Karakaya, A.E. Detection of oxidative DNA damage in lymphocytes of patients with Alzheimer's disease. Biomarkers 2004, 9, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Rivière, S.; Birlouez-Aragon, I.; Nourhashémi, F.; Vellas, B. Low plasma vitamin C in Alzheimer patients despite an adequate diet. International journal of geriatric psychiatry 1998, 13, 749–754. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Bayer, A.J.; Johnston, J.; Warner, C.; Maxwell, S.R. Altered plasma antioxidant status in subjects with Alzheimer's disease and vascular dementia. International journal of geriatric psychiatry 1998, 13, 840–845. [Google Scholar] [CrossRef]
- Perez Ortiz, J.M.; Swerdlow, R.H. Mitochondrial dysfunction in Alzheimer's disease: Role in pathogenesis and novel therapeutic opportunities. British journal of pharmacology 2019, 176, 3489–3507. [Google Scholar] [CrossRef]
- Gao, R.; Ma, S.L. Is mitochondria DNA variation a biomarker for AD? Genes 2022, 13, 1789. [Google Scholar] [CrossRef]
- Mahapatra, G.; Gao, Z.; Bateman III, J.R.; Lockhart, S.N.; Bergstrom, J.; DeWitt, A.R.; Piloso, J.E.; Kramer, P.A.; Gonzalez-Armenta, J.L.; Amick, K.A. Blood-based bioenergetic profiling reveals differences in mitochondrial function associated with cognitive performance and Alzheimer's disease. Alzheimer's Dementia 2023, 19, 1466–1478. [Google Scholar] [CrossRef]
- Bhatia, S.; Rawal, R.; Sharma, P.; Singh, T.; Singh, M.; Singh, V. Mitochondrial dysfunction in Alzheimer’s disease: opportunities for drug development. Current Neuropharmacology 2022, 20, 675. [Google Scholar] [CrossRef]
- Maynard, S.; Hejl, A.-M.; Dinh, T.-S. T.; Keijzers, G.; Hansen, Å.M.; Desler, C.; Moreno-Villanueva, M.; Bürkle, A.; Rasmussen, L.J.; Waldemar, G. Defective mitochondrial respiration, altered dNTP pools and reduced AP endonuclease 1 activity in peripheral blood mononuclear cells of Alzheimer's disease patients. Aging 2015, 7, 793. [Google Scholar] [CrossRef]
- Coskun, P.; Helguera, P.; Nemati, Z.; Bohannan, R.C.; Thomas, J.; Samuel, S.E.; Argueta, J.; Doran, E.; Wallace, D.C.; Lott, I.T. Metabolic and growth rate alterations in lymphoblastic cell lines discriminate between Down syndrome and Alzheimer’s disease. Journal of Alzheimer's Disease 2017, 55, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Veitinger, M.; Varga, B.; Guterres, S.B.; Zellner, M. Platelets, a reliable source for peripheral Alzheimer’s disease biomarkers? Acta neuropathologica communications 2014, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Toledo, G.; Silva-Lucero, M.-d.-C.; Herrera-Díaz, J.; García, D.-E.; Arias-Montaño, J.-A.; Cardenas-Aguayo, M.-d.-C. Patient-derived fibroblasts with presenilin-1 mutations, that model aspects of Alzheimer’s disease pathology, constitute a potential object for early diagnosis. Frontiers in Aging Neuroscience 2022, 14, 921573. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.M.; Burgess, T.; Lee, J.; Blackburn, D.J.; Allen, S.P.; Mortiboys, H. Peripheral glycolysis in neurodegenerative diseases. International journal of molecular sciences 2020, 21, 8924. [Google Scholar] [CrossRef]
- Bossy, B.; Petrilli, A.; Klinglmayr, E.; Chen, J.; Lütz-Meindl, U.; Knott, A.B.; Masliah, E.; Schwarzenbacher, R.; Bossy-Wetzel, E. S-Nitrosylation of DRP1 does not affect enzymatic activity and is not specific to Alzheimer's disease. Journal of Alzheimer's disease 2010, 20 (s2), S513–S526. [Google Scholar] [CrossRef]
- Huang, D.-X.; Yu, X.; Yu, W.-J.; Zhang, X.-M.; Liu, C.; Liu, H.-P.; Sun, Y.; Jiang, Z.-P. Calcium signaling regulated by cellular membrane systems and calcium homeostasis perturbed in Alzheimer’s disease. Frontiers in Cell Developmental Biology 2022, 10, 834962. [Google Scholar] [CrossRef]
- Trumpff, C.; Michelson, J.; Lagranha, C.J.; Taleon, V.; Karan, K.R.; Sturm, G.; Lindqvist, D.; Fernström, J.; Moser, D.; Kaufman, B.A. Stress and circulating cell-free mitochondrial DNA: A systematic review of human studies, physiological considerations, and technical recommendations. Mitochondrion 2021, 59, 225–245. [Google Scholar] [CrossRef]
- Reid, D.M.; Barber, R.C.; Jones, H.P.; Thorpe Jr, R.J.; Sun, J.; Zhou, Z.; Phillips, N.R. Integrative blood-based characterization of oxidative mitochondrial DNA damage variants implicates Mexican American’s metabolic risk for developing Alzheimer’s disease. Scientific reports 2023, 13, 14765. [Google Scholar] [CrossRef]
- Moya, G.E.; Rivera, P.D.; Dittenhafer-Reed, K.E. Evidence for the role of mitochondrial DNA release in the inflammatory response in neurological disorders. International journal of molecular sciences 2021, 22, 7030. [Google Scholar]
- Miao, J.; Ma, H.; Yang, Y.; Liao, Y.; Lin, C.; Zheng, J.; Yu, M.; Lan, J. Microglia in Alzheimer’s disease: Pathogenesis, mechanisms, and therapeutic potentials. Frontiers in aging neuroscience 2023, 15, 1201982. [Google Scholar]
- Frost, G.R.; Li, Y.-M. The role of astrocytes in amyloid production and Alzheimer's disease. Open biology 2017, 7, 170228. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, G.; Burgaletto, C.; Bellanca, C.M.; Munafò, A.; Bernardini, R.; Cantarella, G. Role of microglia and astrocytes in Alzheimer’s disease: from neuroinflammation to Ca2+ homeostasis dysregulation. Cells 2022, 11, 2728. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Shin, K.Y.; Chang, K.-A. GFAP as a potential biomarker for Alzheimer’s disease: a systematic review and meta-analysis. Cells 2023, 12, 1309. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, T.; König, T.; Wurm, R.; Silvaieh, S.; Altmann, P.; Klotz, S.; Rommer, P.S.; Furtner, J.; Regelsberger, G.; Lehrner, J. Real-world applicability of glial fibrillary acidic protein and neurofilament light chain in Alzheimer’s disease. Frontiers in Aging Neuroscience 2022, 14, 887498. [Google Scholar] [CrossRef] [PubMed]
- Benedet, A.L.; Milà-Alomà, M.; Vrillon, A.; Ashton, N.J.; Pascoal, T.A.; Lussier, F.; Karikari, T.K.; Hourregue, C.; Cognat, E.; Dumurgier, J. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer disease continuum. JAMA neurology 2021, 78, 1471–1483. [Google Scholar] [CrossRef]
- Domingues, C.; AB da Cruz e Silva, O.; Henriques, A. Impact of cytokines and chemokines on Alzheimer's disease neuropathological hallmarks. Current Alzheimer Research 2017, 14, 870–882. [Google Scholar] [CrossRef]
- Perea, J.R.; Lleó, A.; Alcolea, D.; Fortea, J.; Ávila, J.; Bolós, M. Decreased CX3CL1 levels in the cerebrospinal fluid of patients with Alzheimer’s disease. Frontiers in Neuroscience 2018, 12, 609. [Google Scholar] [CrossRef]
- Vacínová, G.; Vejražkova, D.; Rusina, R.; Holmerová, I.; Vaňková, H.; Jarolímová, E.; Včelák, J.; Bendlová, B.; Vaňková, M. Regulated upon activation, normal T cell expressed and secreted (RANTES) levels in the peripheral blood of patients with Alzheimer’s disease. Neural Regeneration Research 2021, 16, 796–800. [Google Scholar] [CrossRef]
- Martens, L.H.; Zhang, J.; Barmada, S.J.; Zhou, P.; Kamiya, S.; Sun, B.; Min, S.-W.; Gan, L.; Finkbeiner, S.; Huang, E.J. Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury. The Journal of clinical investigation 2012, 122, 3955–3959. [Google Scholar] [CrossRef]
- Mendsaikhan, A.; Tooyama, I.; Walker, D.G. Microglial progranulin: involvement in Alzheimer’s disease and neurodegenerative diseases. Cells 2019, 8, 230. [Google Scholar] [CrossRef]
- Vergallo, A.; Lista, S.; Lemercier, P.; Chiesa, P.A.; Zetterberg, H.; Blennow, K.; Potier, M.-C.; Habert, M.-O.; Baldacci, F.; Cavedo, E. Association of plasma YKL-40 with brain amyloid-β levels, memory performance, and sex in subjective memory complainers. Neurobiology of aging 2020, 96, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Hua, F.; Zhang, L.; Lin, Y.; Fang, P.; Chen, S.; Ying, J.; Wang, X. Dual roles of interleukin-33 in cognitive function by regulating central nervous system inflammation. Journal of Translational Medicine 2022, 20, 369. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; Marventano, I.; Piancone, F.; La Rosa, F.; Galimberti, D.; Fenoglio, C.; Scarpini, E.; Clerici, M. IL-33 and its decoy sST2 in patients with Alzheimer’s disease and mild cognitive impairment. Journal of neuroinflammation 2020, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Casati, M.; Ferri, E.; Gussago, C.; Mazzola, P.; Abbate, C.; Bellelli, G.; Mari, D.; Cesari, M.; Arosio, B. Increased expression of TREM 2 in peripheral cells from mild cognitive impairment patients who progress into Alzheimer's disease. European journal of neurolog 2018, 25, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Tan, M.-S.; Yu, J.-T.; Sun, L.; Tan, L.; Wang, Y.-L.; Jiang, T.; Tan, L. Increased expression of TREM2 in peripheral blood of Alzheimer's disease patients. Journal of Alzheimer's Disease 2014, 38, 497–501. [Google Scholar] [CrossRef]
- Lu, Q.; Xie, Y.; Qi, X.; Yang, S. TREM1 as a novel prognostic biomarker and tumor immune microenvironment evaluator in glioma. Medicine (Baltimore) 2023, 102, e36410. [Google Scholar] [CrossRef]
- Španić Popovački, E.; Babić Leko, M.; Langer Horvat, L.; Brgić, K.; Vogrinc, Ž.; Boban, M.; Klepac, N.; Borovečki, F.; Šimić, G. Soluble TREM2 concentrations in the cerebrospinal fluid correlate with the severity of neurofibrillary degeneration, cognitive impairment, and inflammasome activation in Alzheimer’s disease. Neurology international 2023, 15, 842–856. [Google Scholar] [CrossRef]
- AlMansoori, M.E.; Jemimah, S.; Abuhantash, F.; AlShehhi, A. Predicting early Alzheimer’s with blood biomarkers and clinical features. Scientific Reports 2024, 14, 6039. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, J.; Yang, Y.; Zhang, Z.; Zhong, H.; Zeng, G.; Zhou, D.; Nowakowski, R.S.; Long, J.; Wu, C. Identification of candidate DNA methylation biomarkers related to Alzheimer’s disease risk by integrating genome and blood methylome data. Translational psychiatry 2023, 13, 387. [Google Scholar] [CrossRef]
- Nagaraj, S.; Zoltowska, K.M.; Laskowska-Kaszub, K.; Wojda, U. microRNA diagnostic panel for Alzheimer’s disease and epigenetic trade-off between neurodegeneration and cancer. Ageing research reviews 2019, 49, 125–143. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Tarazona-Sánchez, A.; Braza-Boils, A.; Balaguer, A.; Ferré-González, L.; Cañada-Martínez, A.J.; Baquero, M.; Cháfer-Pericás, C. Plasma microRNAs as potential biomarkers in early Alzheimer disease expression. Scientific Reports 2022, 12, 15589. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Tordera, L.; Papandreou, C.; Novau-Ferré, N.; García-González, P.; Rojas, M.; Marquié, M.; Chapado, L.A.; Papagiannopoulos, C.; Fernàndez-Castillo, N.; Valero, S. Exploring small non-coding RNAs as blood-based biomarkers to predict Alzheimer’s disease. Cell Bioscience 2024, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Sadlon, A.; Takousis, P.; Evangelou, E.; Prokopenko, I.; Alexopoulos, P.; Udeh-Momoh, C.-M.; Price, G.; Middleton, L.; Perneczky, R.; Initiative, A. s. D. N. Association of Blood MicroRNA Expression and Polymorphisms with Cognitive and Biomarker Changes in Older Adults. The Journal of Prevention of Alzheimer's Disease 2024, 11, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Yuen, S.C.; Liang, X.; Zhu, H.; Jia, Y.; Leung, S.-w. Prediction of differentially expressed microRNAs in blood as potential biomarkers for Alzheimer’s disease by meta-analysis and adaptive boosting ensemble learning. Alzheimer's research therapy 2021, 13, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Khodayi, M.; Khalaj-Kondori, M.; Feizi, M.A.H.; Bonyadi, M.J.; Talebi, M. Plasma lncRNA profiling identified BC200 and NEAT1 lncRNAs as potential blood-based biomarkers for late-onset Alzheimer’s disease. EXCLI journal 2022, 21, 772. [Google Scholar]
- Feng, L.; Liao, Y.-T.; He, J.-C.; Xie, C.-L.; Chen, S.-Y.; Fan, H.-H.; Su, Z.-P.; Wang, Z. Plasma long non-coding RNA BACE1 as a novel biomarker for diagnosis of Alzheimer disease. BMC neurology 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Santana, D.A.; Smith, M. d. A. C.; Chen, E.S. Histone modifications in Alzheimer’s disease. Genes 2023, 14, 347. [Google Scholar] [CrossRef]
- De Plano, L.M.; Saitta, A.; Oddo, S.; Caccamo, A. Epigenetic Changes in Alzheimer’s Disease: DNA Methylation and Histone Modification. Cells 2024, 13, 719. [Google Scholar] [CrossRef]
- Salameh, Y.; Bejaoui, Y.; El Hajj, N. DNA methylation biomarkers in aging and age-related diseases. Frontiers in Genetics 2020, 11, 480672. [Google Scholar] [CrossRef]
- Thrush, K.L.; Bennett, D.A.; Gaiteri, C.; Horvath, S.; van Dyck, C.H.; Higgins-Chen, A.T.; Levine, M.E. Aging the brain: multi-region methylation principal component based clock in the context of Alzheimer’s disease. Aging 2022, 14, 5641. [Google Scholar] [CrossRef]
- Ren, Z.; Chu, C.; Pang, Y.; Cai, H.; Jia, L. A circular RNA blood panel that differentiates Alzheimer’s disease from other dementia types. Biomarker Research 2022, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, X.; Chen, Y.-H.; Zhang, K. Identification of circular RNA hsa_Circ_0003391 in peripheral blood is potentially associated with Alzheimer's disease. Frontiers in Aging Neuroscience 2020, 12, 601965. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Morikawa, S.; Nakashima, M.; Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Suga, N.; Tsuji, A.; Matsuda, S. CircRNAs and RNA-binding proteins involved in the pathogenesis of cancers or central nervous system disorders. Non-coding RNA 2023, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, F.; He, A.T.; Yang, B.B. Circular RNAs: expression, localization, and therapeutic potentials. Molecular therapy 2021, 29, 1683–1702. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, S.; Jedrychowski, M.P.; Tatebe, H.; DeLeo, A.M.; Ikezu, S.; Tokuda, T.; Gygi, S.P.; Stern, R.A.; Ikezu, T. Proteomic profiling of extracellular vesicles isolated from cerebrospinal fluid of former national football league players at risk for chronic traumatic encephalopathy. Frontiers in neuroscience 2019, 13, 1059. [Google Scholar] [CrossRef]
- Muraoka, S.; Jedrychowski, M.P.; Yanamandra, K.; Ikezu, S.; Gygi, S.P.; Ikezu, T. Proteomic profiling of extracellular vesicles derived from cerebrospinal fluid of Alzheimer’s disease patients: a pilot study. Cells 2020, 9, 1959. [Google Scholar] [CrossRef]
- Guo, M.; Yin, Z.; Chen, F.; Lei, P. Mesenchymal stem cell-derived exosome: a promising alternative in the therapy of Alzheimer’s disease. Alzheimer's Research Therapy 2020, 12, 1–14. [Google Scholar]
- Colombo, E.; Borgiani, B.; Verderio, C.; Furlan, R. Microvesicles: novel biomarkers for neurological disorders. Frontiers in physiology 2012, 3, 63. [Google Scholar] [CrossRef]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimer's Dementia 2015, 11, 600–607. e1. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Nogueras-Ortiz, C.; Mustapic, M.; Mullins, R.J.; Abner, E.L.; Schwartz, J.B.; Kapogiannis, D. Deficient neurotrophic factors of CSPG4-type neural cell exosomes in Alzheimer disease. The FASEB Journal 2019, 33, 231. [Google Scholar] [CrossRef]
- Kapogiannis, D.; Boxer, A.; Schwartz, J.B.; Abner, E.L.; Biragyn, A.; Masharani, U.; Frassetto, L.; Petersen, R.C.; Miller, B.L.; Goetzl, E.J. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. The FASEB Journal 2015, 29, 589. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J. Advancing medicine for Alzheimer’s disease: A plasma neural exosome platform. The FASEB Journal 2020, 34, 13079–13084. [Google Scholar] [CrossRef] [PubMed]
- Frühbeis, C.; Fröhlich, D.; Kuo, W.P.; Amphornrat, J.; Thilemann, S.; Saab, A.S.; Kirchhoff, F.; Möbius, W.; Goebbels, S.; Nave, K.-A. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte–neuron communication. PLoS biology 2013, 11, e1001604. [Google Scholar] [CrossRef] [PubMed]
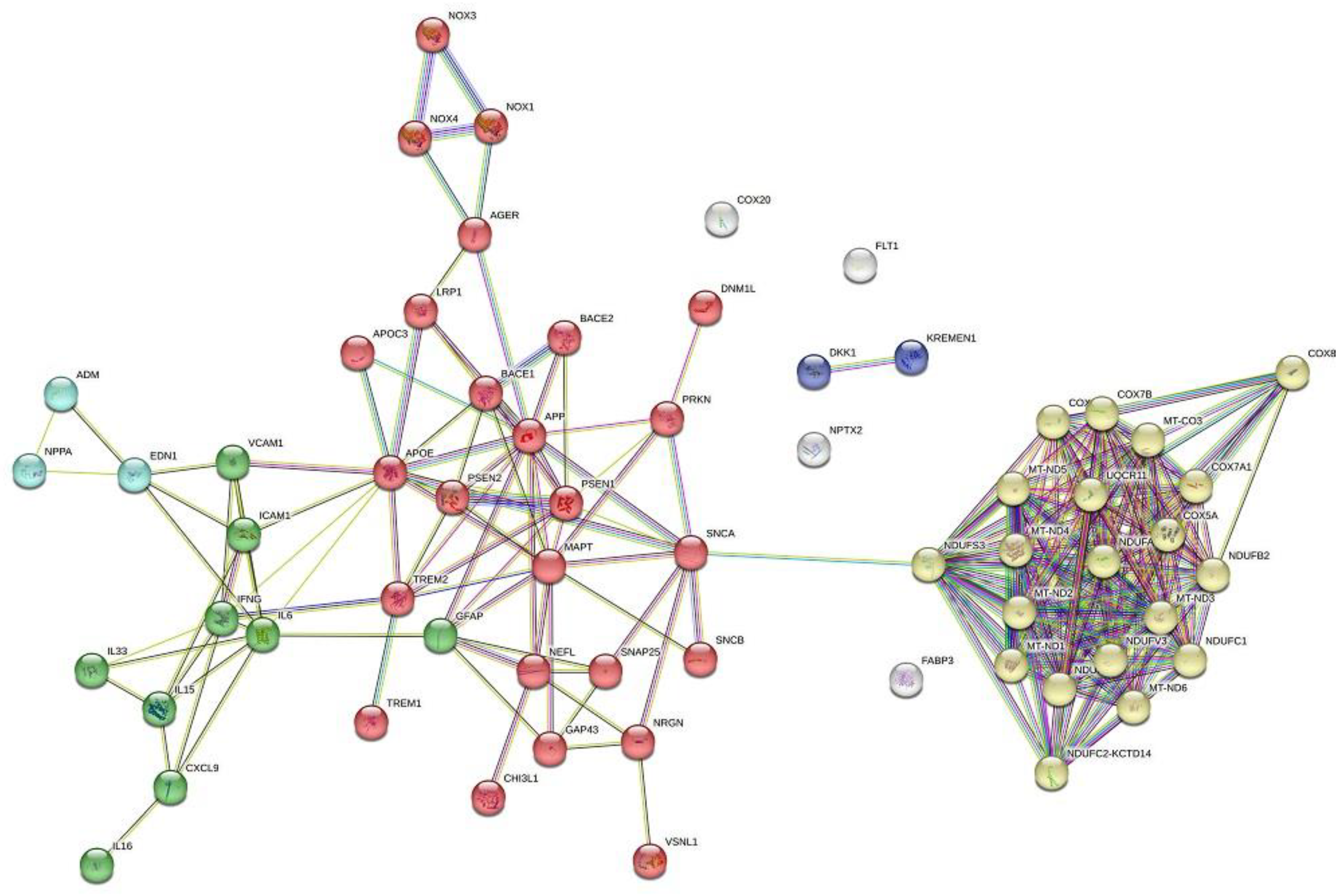
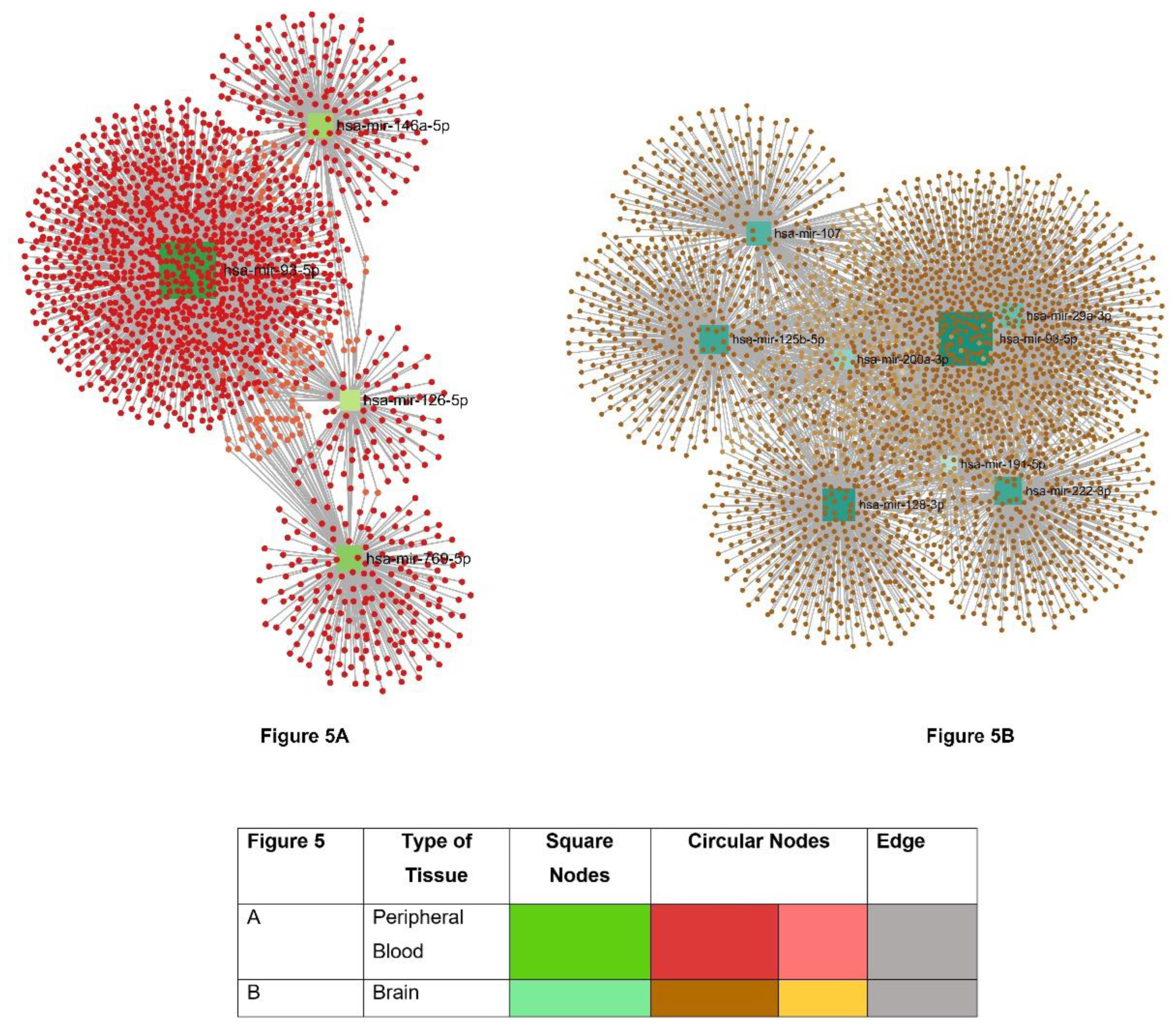
| Name of the BBMs | Underlying Pathophysiology |
Current Categorization of BBBMs (NIA-AA'2024) | Relevance in AD | Trend of the biomarker in Plasma | Assessment techniques | |
|---|---|---|---|---|---|---|
| Amyloid β | Aβ42 | Plasma proteinopathy- Amyloidogenic | Core 1 biomarker (A) |
Early detection of AD in asymptomatic individuals, with progression from normal cognition to MCI or AD | Decreased in AD and MCI in comparison to controls | 1: ELISA 2: Luminex xMAP Technology 3: SIMOA 4: LCMS 5: Immunoprecipitation Mass Spectrometry |
| Aβ40 | Plasma proteinopathy- Amyloidogenic | Core 1 biomarker (A) |
Early detection of AD in asymptomatic individuals, with progression from normal conition to MCI or AD | Decreased in AD and MCI in comparison to controls | ||
| Aβ42/40 | Plasma proteinopathy- Amyloidogenic | Core 1 biomarker (A) |
Identify early stages of AD and predict cognitive decline in concordance with CSF and neuroimaging biomarkers. | Decreased Aβ42/Aβ40 Ratio in AD and MCI compared to control | ||
| Tau | p-tau217 | Plasma proteinopathy-Tauopathy phosphorylated and secreted AD tau | Core 1 biomarker (T1) |
Early detection of AD in people without symptoms -Accurately predict the progression of individuals from subjective cognitive decline (SCD) and MCI to dementia when combined with other risk factors. |
Increased in AD and MCI in compared to controls | 1: ELISA 2: Luminex xMAP Technology 3: SIMOA 4: LCMS 4: Immunoprecipitation Mass Spectrometry |
| p-tau181 | Plasma proteinopathy-Tauopathy phosphorylated and secreted AD tau | Core 1 biomarker (T1) |
Early detection of AD in people without symptoms -Distinguishes between Aβ-PET (+) and Aβ-PET (-) individuals, along with the disease progression to dementia and tau-burdened brain areas with AD-related atrophic changes. |
Increased in AD and MCI compared to controls | ||
| p-tau 231 | Plasma proteinopathy-Tauopathy phosphorylated and secreted AD tau | Core 1 biomarker (T1) |
-Early detection of AD in people without symptom -Discriminates patients with and without AD pathology during post-mortem assessment. |
Increased in AD and MCI compared to controls | ||
| MTBR-tau243 | Plasma proteinopathy-Tauopathy AD tau proteinopathy |
Core 2 biomarker (T2) |
Elevated in later stages of AD Staging of biological disease severity along with Core 1 biomarker -strongly associated with tau-PET and disease progression. |
Increased in AD and MCI compared to controls | ||
| Non-phosphorylated mid-region tau fragments |
Plasma proteinopathy-Tauopathy AD tau proteinopathy |
Core 2 biomarker (T2) |
Elevated in later stages of AD -staging of biological disease severity along with Core 1 biomarker. |
Increased in AD and MCI compared to controls | ||
| α-synuclein (αSyn) | αSyn/tau | Proteinopathy-related biomarkers of non-core AD pathology Synuclein pathology | Biomarkers of non-AD co-pathology (S) |
Total α-synuclein levels in the blood may not differ significantly between patients with neurodegenerative diseases. The oligomeric or phosphorylated form of α-synuclein accelerates cognitive dysfunction. |
Decreased in AD and MCI compared to controls | 1: Seed Amplification Assays: -Protein Misfolding Cyclic Amplification (PMCA) -Real-Time Quaking-Induced Conversion (RT-QuIC) 2: ELISA and Western blotting 3: Quantitative Mass Spectrometry 4: Luminex xMAP Technology 5: SPR- DLS 6: Immuno-PCR |
| αSyn /Aβ 42 | Biomarkers of non-AD co-pathology | Increased in AD and MCI compared to controls | ||||
| DKK-1 or Dickkopf-1 | Proteinopathy-related biomarkers of non-core AD pathology. | Research Biomarker | Elevated levels correlate with disease severity, particularly cognitive decline and synaptic loss and help differentiate AD from other neurodegenerative conditions |
Increased in AD | 1: ELISA and Western blotting 2: Luminex xMAP Technology 3: Immuno-PCR 4: Mass Spectrometry |
|
| VILIP-1 | Proteinopathy-related biomarkers of non-core AD pathology. | Research Biomarker | Increased levels are seen in AD, but there is no significant difference in concentrations with AD-MCI patients and other neurodegenerative groups. |
Increased in AD | 1. ELISA and Western blotting 2. Luminex xMAP Technology 3. Immuno-PCR 4. Mass Spectrometry |
|
| Plasma Neurofilaments (NfL) | Injury, dysfunction, or degeneration of neuropil | Biomarkers of non-specific processes involved in AD pathophysiology (N) |
Increased levels in Aβ-positive patients with AD and MCI are associated with the degree of cognitive impairment as well as used as monitoring biomarkers to indicate the severity of neurodegeneration. | Increased in AD and MCI vs controls | 1. ELISA (Enzyme-Linked Immunosorbent Assay) 2. Luminex xMAP Technology 3. ECLIA 4. Mass Spectrometry 5. SIMOA |
|
| SNAP-25 | Neuronal and synaptic injury-pre synaptic dysfunction | Biomarkers of non-specific processes involved in AD pathophysiology (N) |
CSF concentrations can distinguish between various neurodegenerative diseases like AD, Parkinson’s dieases, and ALS. | Decreased in AD compared to controls | 1. ELISA and Western blotting 2. Luminex xMAP Technology 3.Immuno-PCR 4. Mass Spectrometry |
|
| Neuronal pentraxin 2 (NPTX-2) | Neuronal and synaptic injury-pre synaptic dysfunction | Biomarkers of non-specific processes involved in AD pathophysiology (N) |
Potential as a probable biomarker for early detection of AD. | Decreased in AD compared to control | 1. ELISA and Western blotting 2. Luminex xMAP Technology 3.Immuno-PCR 4. Mass Spectrometry |
|
| Growth-associated protein (GAP-43) | Neuronal and synaptic injury-pre synaptic dysfunction | Biomarkers of non-specific processes involved in AD pathophysiology (N) |
Potential as a probable biomarker for early detection of AD. | Increased in AD compared to control | 1. ELISA and Western blotting 2. Luminex xMAP Technology 3.Immuno-PCR 4. Mass Spectrometry |
|
| Neurogranin (NG) | Neuronal and synaptic injury- post-synaptic protein dysfunction | Biomarkers of non-specific processes involved in AD pathophysiology (N) |
Potential as a probable biomarker for early detection of AD. | Decreased in AD compared to control | 1. ELISA 2. Luminex xMAP Technology 3. ECLIA 4. Mass Spectrometry 5. SIMOA |
|
| Fms-like tyrosine kinase-1 (Flt-1) | Vascular Damages related to AD | Research Biomarker (V) |
Assess total vascular involvement and early detection of vascular changes associated with AD. |
Increased in AD compared to control | 1. ELISA and Western blotting 2. Luminex xMAP Technology 3.Immuno-PCR 4. Mass Spectrometry |
|
| Endothelin 1 (ET-1) | Vascular Damages related to AD | Research Biomarker (V) |
Indicates vascular impairment in AD. | Increased in AD compared to AD | 1. ELISA 2. Luminex xMAP Technology 3. Immuno-PCR 4. Mass Spectrometry |
|
| Atrial natriuretic peptide (ANP) | Vascular Damage related to AD | Research Biomarker (V) |
Causes reduced cerebral blood flow and impairment of neurovascular health. . |
Increased in AD compared to control | 1. ELISA 2. Luminex xMAP Technology 3.Immuno-PCR 4. Mass Spectrometry |
|
| MIG/CXCL-9 | Vascular Damage related to AD | Research Biomarker (V) |
Indicate ongoing chronic neuroinflammatory processes. | Increased in AD compared to control | 1. ELISA 2. Luminex xMAP Technology 3.Immuno-PCR 4. Mass Spectrometry |
|
| heart-type fatty acid binding protein (H-FABP) | Vascular Damage related to AD | Research Biomarker (V) |
Potential as a probable biomarker for early detection of AD as it was found to be elevated in the pre-clinical phase of AD dementia. | Increased in AD compared to control | 1. ELISA and Western blotting 2. Luminex xMAP Technology 3.Immuno-PCR 4. Mass Spectrometry |
|
| Vascular adhesion molecular | sVCAM-1 | Vascular Damage related to AD | Research Biomarker (V) |
Indicate the burden of atherosclerosis in AD with elevated sVCAM, indicating a significant correlation between age and the severity of cognitive decline. |
Increased in AD compared to control | 1. ELISA and Western blotting 2. Luminex xMAP Technology 3.Immuno-PCR 4. Mass Spectrometry |
| Soluble intercellular adhesion molecule-1 | Vascular Damage related to AD | Research Biomarker (V) |
Indicate the burden of atherosclerosis in AD with elevated Soluble intercellu-lar adhesion molecule-1. | Increased in AD compared to control | 1. ELISA and Western blotting 2. Luminex xMAP Technology 3.Immuno-PCR 4. Mass Spectrometry |
|
| Metabolic products secondary to lipid peroxidation (LPO) | malondialdehyde (MDA) | Oxidative Stress | Research Biomarker | Increased levels in familial AD that carry APP and presenilin-1 gene mutations. | Increased in AD compared to control | 1. HPLC 2. LC-MS 3. ELISA 4. GC-MS |
| 4-hydroxynonenal (4-HNE) | Oxidative Stress | Research Biomarker | Increased levels in familial AD that carry APP and presenilin-1 gene mutations. | Increased in AD compared to control | ||
| increased F2-isoprostanes (F2-IsoPs) | Oxidative Stress | Research Biomarker | potential marker of oxidative stress during the MCI phase of AD, and its quantity correlates with the disease continuum from SCD to MCI to AD. | Increased in AD compared to control | ||
| Free radicals | ROS | Oxidative Damage | Research Biomarker | ROS modifies neuronal macromolecules and induces τ protein hyperphosphorylation during prodromal AD phases. | Increased in AD | 1. DCFDA 2. Electron Spin Resonance (ESR) Spectroscopy 3.Nitroblue Tetrazolium (NBT) Assay 4. Flow Cytometry with ROS-sensitive dyes |
| RNS | Oxidative damage | Research Biomarker | Nitrosylation of critical proteins in neurons impairs their function, promoting neurodegenerative processes. | Increased in AD | 1. Nitrotyrosine ELISA Electron Spin Resonance (ESR) Spectroscopy Western Blot for 3-Nitrotyrosine-modified Proteins |
|
| Nucleoside 8-hydroxyguanosine (8-OHG) | Oxidative Damage | Research Biomarker | It is notable for determining the gradient of DNA oxidative damage in AD patients. It allows us to determine oxidative damage to plasma DNA early in AD. | Increased in lymphocytes of AD patients compared to control | 1. ELISA 2. HPLC and Electrochemical detection 3. LC-MS 4. Western Blot using specific anti-5.8-OHG antibodies 6. Immunoprecipitation 7. GC-MS |
|
| Mitochondrial respiratory complex I-V genes (OxPHOS genes) | Bioenergetic abnormality | Research Biomarker | An imbalance in nuclear and mitochondrial genome-encoded OXPHOS transcripts may cause a negative feedback loop that lowers mitochondrial translation and compromises OXPHOS efficiency. This would likely result in the generation of harmful reactive oxygen species. |
Reduced expression in early AD patients | 1. qPCR Western Blot IHC Blue Native Gel Electrophoresis |
|
| SNO-Drp1 | Bioenergetic abnormality | Research Biomarker | SNO-Drp1 can result in increased mitochondrial fission, loss of synapses, and neuronal damage in mice models and primary neuronal culture, as well as in post-mortem tissue. | Increased in peripheral blood lymphocytes in AD patients. There are also contradictory findings that SNO-Drp1 does not differ significantly in AD compared to controls. | 1. Biotin Switch Assay Mass Spectrometry Nitroso-Proteome Profiling Immunoprecipitation and Western blot |
|
| mitochondrial DNA (mt-DNA) | Bioenergetic abnormality | Research Biomarker | mtDNA copy number acts as an indirect indicator for several functioning mitochondria and thus provides info regarding bioenergetics as a factor for AD progression. | Decreased in patients with AD | 1. qPCR Digital droplet PCR Southern Blotting |
|
| 8oxoG sSNVs | Bioenergetic abnormality | Research Biomarker | due to their inflammatory endophenotype, the circulating cf-mtDNA (ccf-mtDNA) 8oxoG variant can be used as an improved biomarker. | Increased in AD patients | 1. 8-oxoG DNA Glycosylase (OGG1) Assay Comet Assay with Fpg (Formamidopyrimidine-DNA Glycosylase) ELISA HPLC with electrochemical detection |
|
| circulating cf-mtDNA (ccf-mtDNA) | Bioenergetic abnormality | Research Biomarker | cellular mt-DNA copy number can be used as a potential biomarker of mitochondrial biogenesis and cellular energetics to reflect upon mitochondrial health in AD. | Increased in AD patients | qPCR Digital droplet PCR Southern Blotting |
|
| The intermediate filament glial fibrillary acidic protein (GFAP) | Neuroinflammation and Immune Dysregulation | Research Biomarker (I) |
The marker of astrogliosis can be seen in chronic inflammatory processes like progressing AD. | Increases in AD patients | 1. ELISA ECLIA mesoscale discovery immunoassay V-PLEX |
|
| CX3CL1 (Fractalkine) | Neuroinflammation and Immune Dysregulation | Research Biomarker (I) |
significantly elevated in the plasma of MCI and AD compared to other neuroinflammatory disease processes. | Increases in AD and MCI | 1. ELISA Western blot IHC Flow Cytometry Luminex |
|
| CCL23 | Neuroinflammation and Immune Dysregulation | Research Biomarker (I) |
Their plasma concentration has also been found to have a predictive value toward MCI-to-AD progression. | Increases in AD | 1. ELISA Western blot IHC Flow Cytometry Luminex |
|
| C-C chemokine ligands or RANTES | Neuroinflammation and Immune Dysregulation | Research Biomarker (I) |
Elevated in AD and correlate with the neuroinflammatory burden. | Increases in AD | ELISA Western blot IHC Flow Cytometry 5. Luminex |
|
| YKL-40 | Neuroinflammation and Immune Dysregulation | Research Biomarker (I) |
increasingly expressed in astrocytes during neuroinflammatory changes. Plasma YKL-40 level shown to have a positive correlation with the result of sensitive free and cued selective reminding test | Increases in AD | 1. ELISA 2. Western blot 3. IHC 4. Flow Cytometry 5. Luminex |
|
| Progranulin | Neuroinflammation and Immune Dysregulation | Research Biomarker (I) |
Studies have revealed that the increased progranulin-expressing gene GRN is in the blood of MCI and AD patients. | Increases in AD | 1. ELISA 2. Western blot 3. IHC 4. Flow Cytometry 5. Luminex |
|
| Triggering receptor expressed on myeloid cells 2 (TREM2) | Neuroinflammation and Immune Dysregulation | Research Biomarker (I) |
mRNA levels in peripheral mononuclear cells have been found to have the distinguishing ability between aMCI, AD, and healthy control individuals and to be dependent on the Apolipoprotein E genotype. | Increases in AD | 1. ELISA 2. Western blot 3. IHC 4. Flow Cytometry 5. Luminex |
|
| NDE | P-S396-tau | Tauopathy | Research Biomarker | Can predict the development of AD up to 10 years before the clinical onset of sporadic AD. | Increased in AD | Proteomic Analysis of the EV’s like ELISA |
| p-tau 181 | Tauopathy | Research Biomarker | Can predict the development of AD up to 10 years before the clinical onset of sporadic AD | Increased in AD and MCI vs healthy controls | 1 ELISA 2. Ultra-sensitive inhouse SIMOA |
|
| Synaptotagmin | Synaptopathy | Research Biomarker | Its impairment leads to decreased neurotransmission, neuroplasticity, and long-term potentiation, thus hampering memory formation. | Reduced in AD | 1. ELISA LC-MS SIMOA |
|
| synaptophysin | Synaptic loss and dysfunction | Research Biomarker | Loss of proper functioning synapse leads to impaired signal transmission and, thus, cognitive impairment. | Reduced in AD | 1. ELISA LC-MS SIMOA |
|
| P-S312-IRS-1 | Neuroinflammation and Insulin Resistance | Research Biomarker | Its increment promotes insulin resistance, leading to progressive neurodegeneration. | Increased in AD vs. controls | 1. ELISA LC-MS SIMOA |
|
| P-panY-IRS-1 | Insulin resistance and Synaptic dysfunction | Research Biomarker | Its reduction promotes insulin resistance, leading to progressive neurodegeneration. | Downregulated in AD | 1. ELISA LC-MS SIMOA |
|
| N-(1-carboxymethyl)-L-lysine | ROS mediated damage | Research Biomarker | can differentiate early to moderate AD. | Downregulated in AD | 1. ELISA LC-MS SIMOA |
|
| MDE | Tauopathy | Research Biomarker | When neurons absorb microglia-derived exosomes containing tau, it triggers further abnormal tau aggregation | Increases in AD | ELISA LC-MS |
|
| ADE | Neuroinflammation | Research Biomarker | Plasma levels of various complement components, such as C1q, C3b, and factor D, could serve as predictive biomarkers for the progression of MCI to AD | Increases in AD | 1. ELISA LC-MS |
|
| Source of the miRNA | Names of miRNA | Reference |
|---|---|---|
| Whole Blood | hsa-miR-107 | [160,161,162,164] |
| Plasma | hsa-miR-92a-3p, hsa-miR-486-5p, hsa-miR-29a-3p, hsa-miR-107, hsa-miR-128-3p, hsa-miR-132-3p, hsa-miR-34c-5p, hsa-let-7d-5p, hsa-miR-191-5p, hsa-miR-200a-3p, hsa-miR-483-5p, hsa-miR-486-5p, hsa-miR-502-3p, hsa-miR-548k, hsa-miR-339-5p, hsa-miR-221-5p, hsa-miR-144-5p, hsa-miR-382-5p, hsa-miR-146b-5p, hsa-miR-224-5p, hsa-miR-625-5p, hsa-miR-769-5p, hsa-miR-454-5p, hsa-miR-548d-5p, hsa-miR-877-5p, hsa-miR-146a, hsa-miR-125b, | |
| Serum | hsa-miR-106-b-3p, hsa-miR-22-3p, hsa-miR-126-5p, hsa-miR148b-5p, hsa-miR-181c-3p, hsa-miR-93-5p, hsa-miR-29c-3p, hsa-miR-132-3p, hsa-miR-222-3p, hsa-let-7d-5p, hsa-miR-191-5p, hsa-miR-146a, hsa-miR-125b, hsa-miR-135a | |
| PBMC | hsa-miR-128-3p, hsa-miR-34c-5p, |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





