Submitted:
21 September 2024
Posted:
24 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Insect Rearing Procedures
2.2. Test site and weather conditions
2.3. Tested Host Fruit Species and Fruit Handling
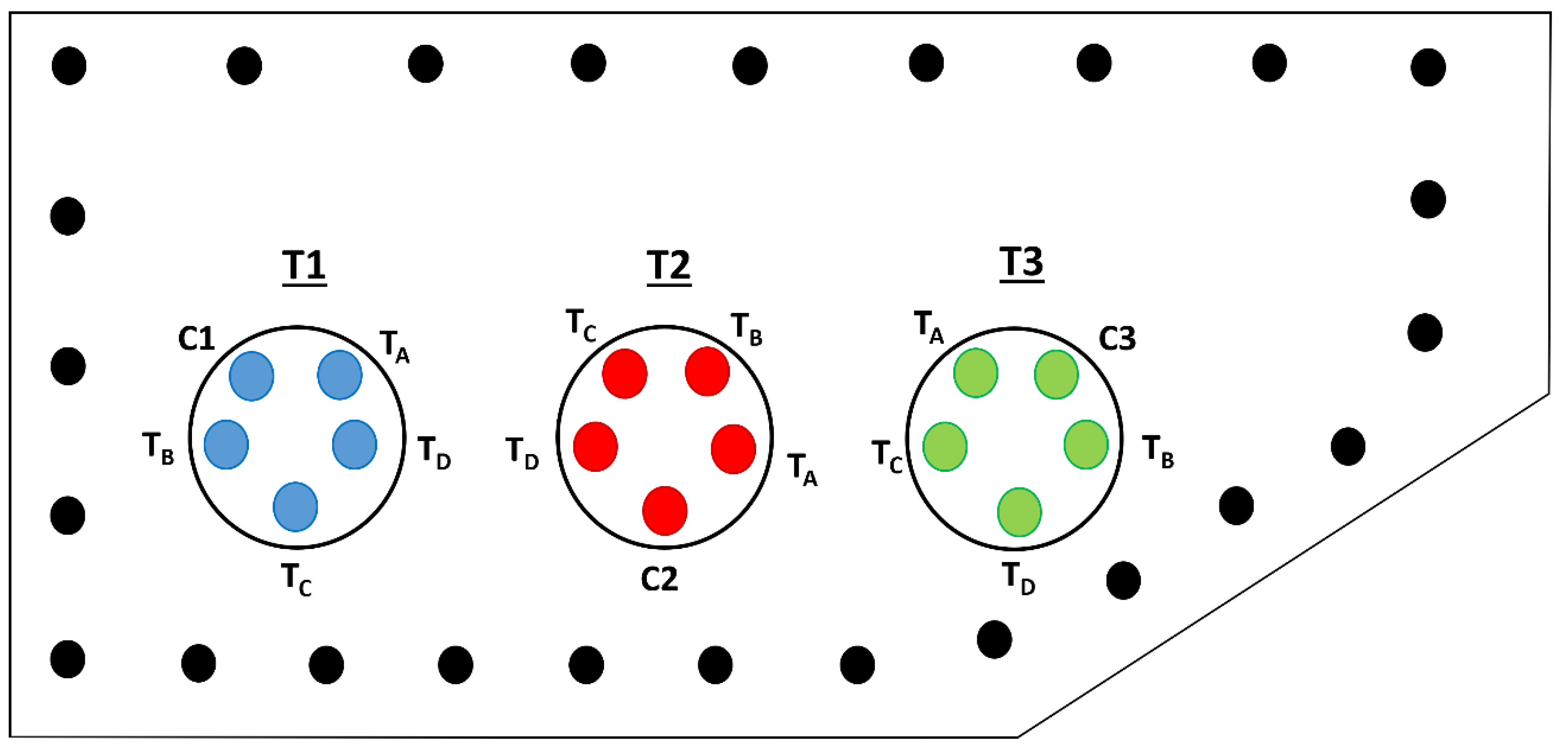
2.4. Experimental Setup and Procedure
2.5. Data Analysis
3. Results
3.1. Tested Host Fruit Species
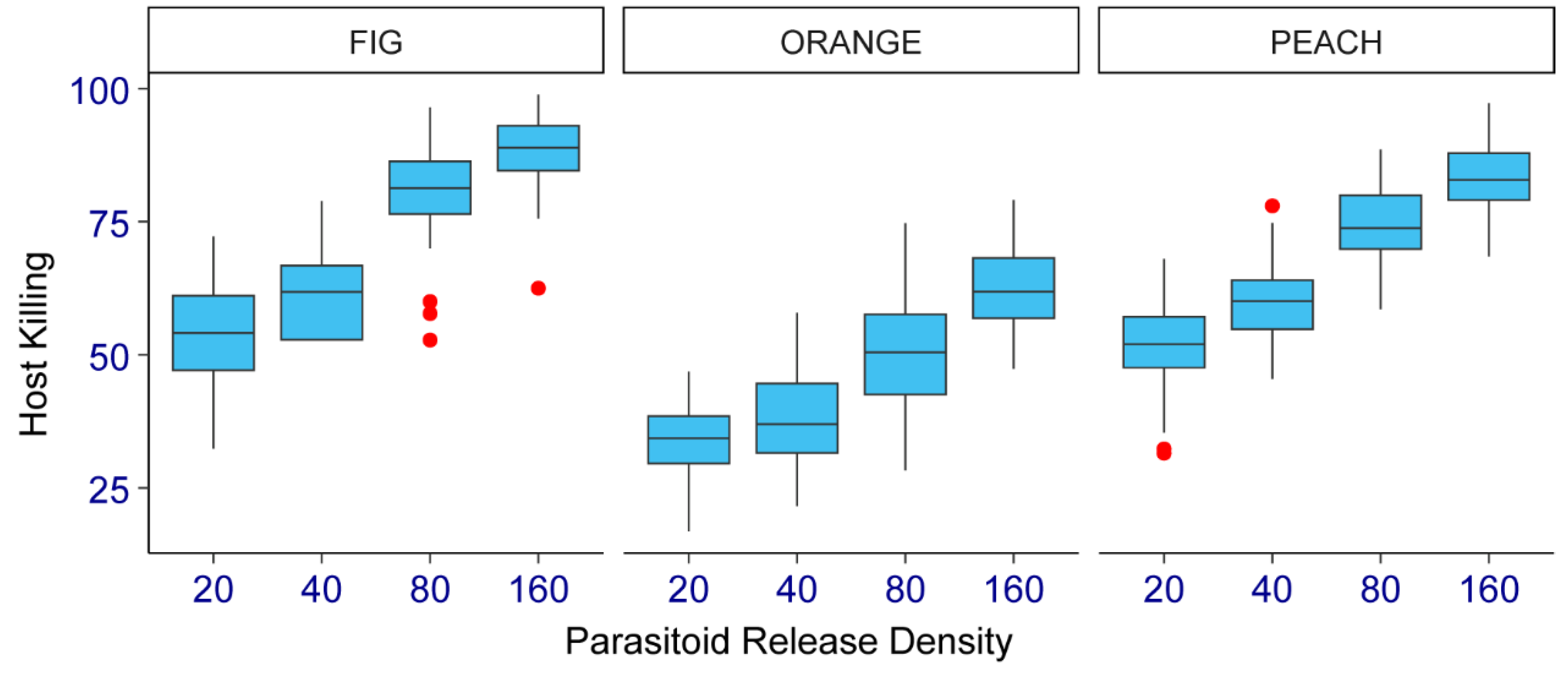
3.2. Host-Killing Parasitoid Capacity
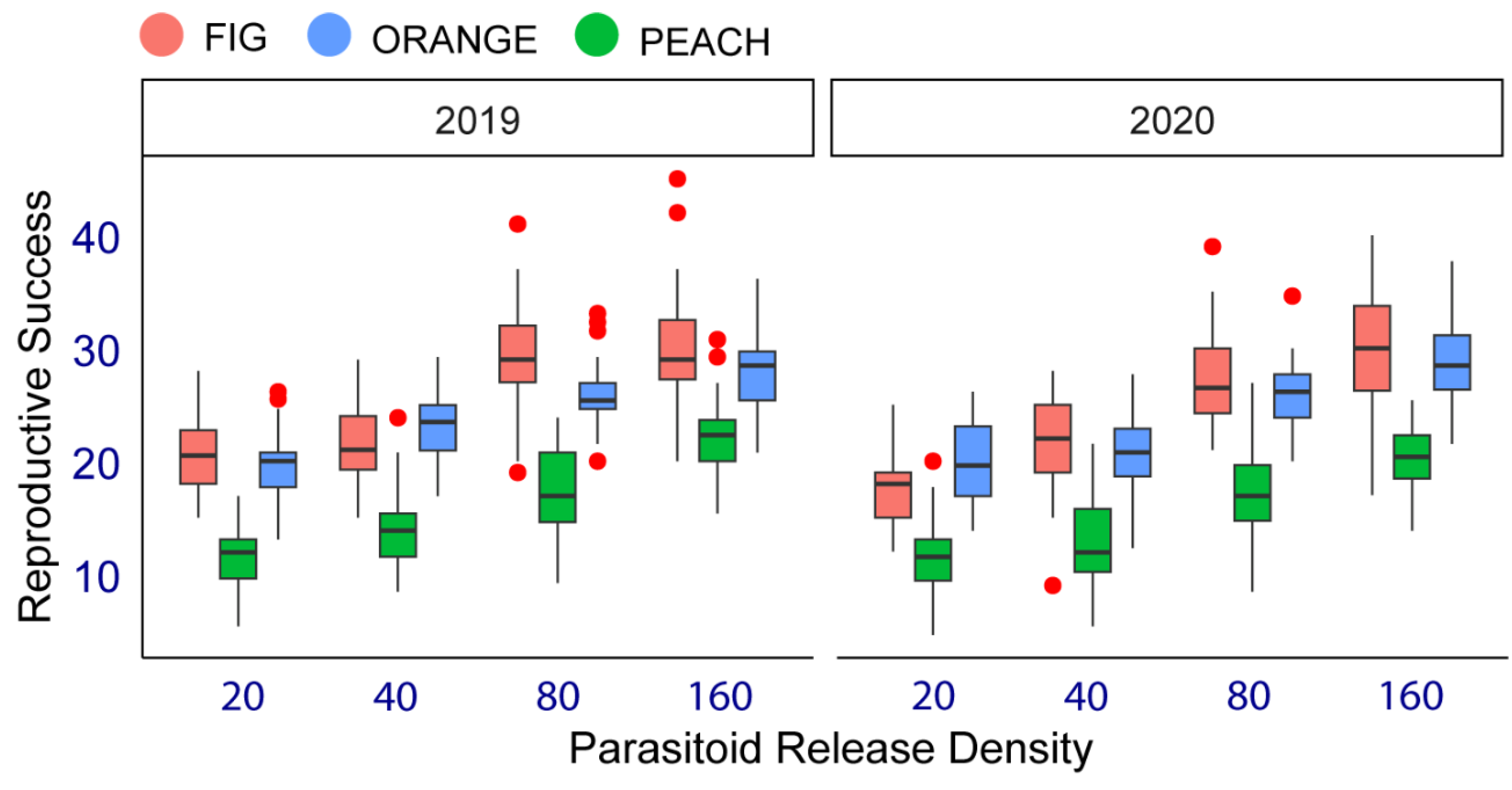
3.3. Parasitoid Reproductive Success
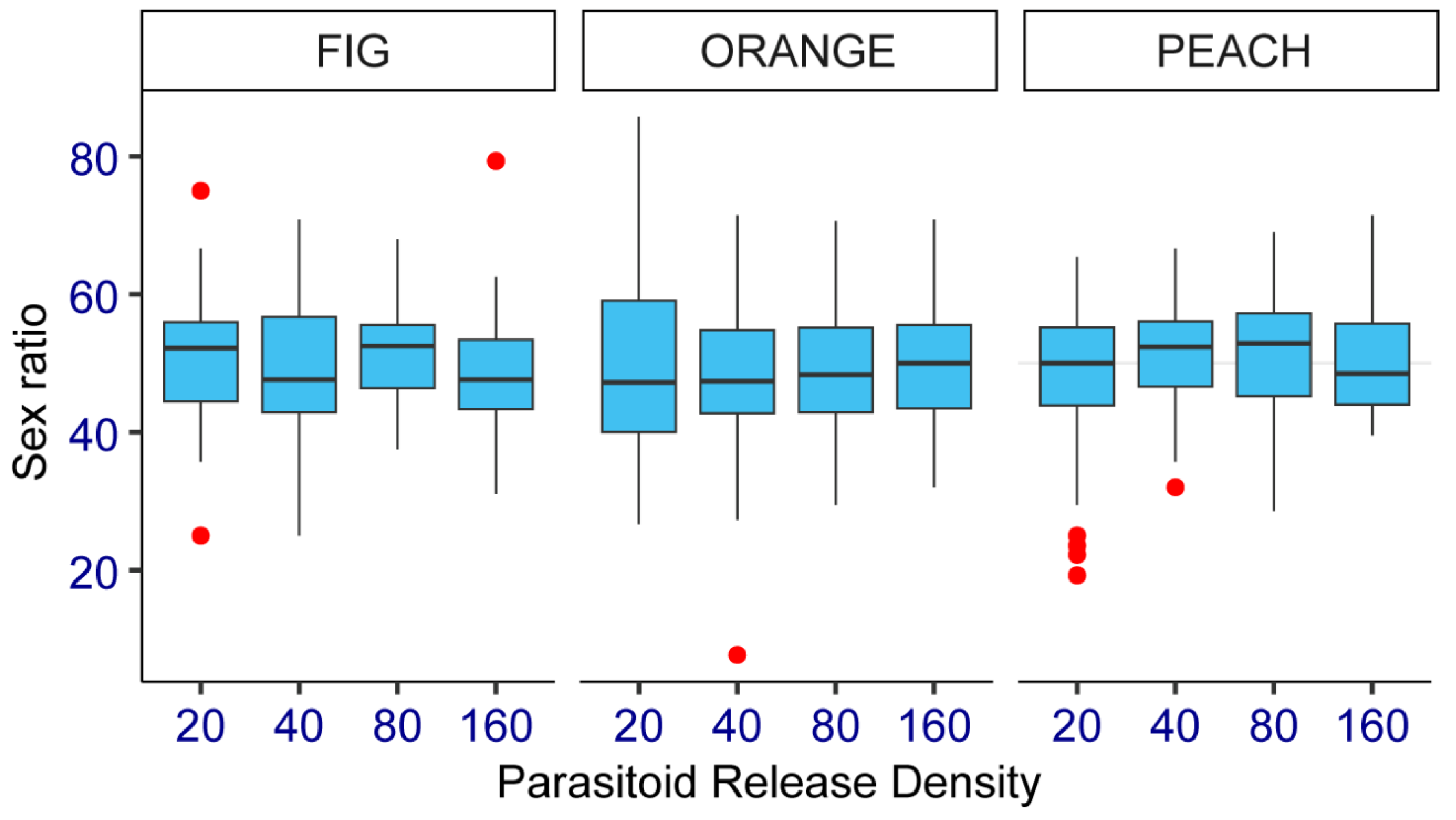
3.4. Parasitoid Offspring Sex Ratio
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Meyer, M.; Robertson, M.P.; Peterson, A.T.; Mansell, M.W. Ecological niches and potential geographical distributions of Mediterranean fruit fly (Ceratitis capitata) and Natal fruit fly (Ceratitis rosa). J. Biogeo. 2008, 35, 270–281. https://doi.org/ 10.1111/j.1365-2699.2007.01769.x. [CrossRef]
- Malacrida, A.R.; Gomulski, L.M.; Bonizzoni, M.; Bertin, S.; Gasperi, G.; Guglielmino, C.R. Globalization and fruit fly invasion and expansion: the medfly paradigm. Genetica 2007, 131, 1-9. https://doi.org/ 10.1007/s10709-006-9117-2. [CrossRef]
- Liquido, N.J.; Shinoda, L.A.; Cunningham, R.T. 1991. Host plants of the Mediterranean fruit fly (Diptera: Tephritidae): an annotated world review. Misc. Publ. Entomol. Soc. America 1991, 77, 1-52.
- Wernicke, M.; Egartner, A.; Blümel, S.; Moraiti, C. A.; Papadopoulos, N. T. Overwintering potential of the Mediterranean fruit fly (Diptera: Tephritidae) in Austria. J. Econ. Entomol. 2024, toae180, https://doi.org/10.1093/jee/toae180. [CrossRef]
- He, Y.; Xu, Y.; Chen, X. Biology, ecology and management of tephritid fruit flies in China: a review. Insects 2023, 14, 196. ; https://doi.org/10.3390/insects14020196. [CrossRef]
- Vergani, A. R. La mosca del mediterráneo. Publ. Inst. Sanidad Vegetal de Argentina Serie B, 1952, 22, 1-12.
- Villarreal, P.; Mongabure, A.; Borges, C. A.; Gómez-Segade, C. Evaluación del impacto económico del Programa Nacional de Control y Erradicación de Mosca de los Frutos, PROCEM Patagonia. FunBaPa Ediciones: Viedma, Río Negro, Argentina, 2023, p. 88.
- Guillén, D.; Sánchez, R. Expansion of the National Fruit Fly Control Programme in Argentina. In Area-Wide Control of Insects Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 653–660.
- Bouvet, J.P.; Segade, G. Arándanos y Cítricos: Anastrepha fraterculus (Wiedemann) y Ceratitis capitata (Wiedemann). In Plagas Cuarentenarias de frutales de la República Argentina: Avances en los Resultados; Rossini, M., Agostini, J.P., Dummel, D.M., Eds.; Ediciones del Instituto Nacional de Tecnología Agropecuaria, Centro Regional Patagonia Norte, Estación Experimental Agropecuaria Alto Valle: Río Negro, Argentina, 2015; pp. 31–43.
- Ernst, B. Fruticultura Argentina: características e importancia. 2020. Available online: https://www.revistainternos.com.ar/v2/wpcontent/uploads/2021/04/FRUTICULTURA-ARGENTINA-Estudio-Comparativo-2009vs.2019-2-COMPLETO-FDA.pdf (accessed on 2 September 2024).
- SENASA; Servicio Nacional de Sanidad y Calidad Agroalimentaria. Moscas de los Frutos. 2017. Available online: http://www.senasa.gob.ar/cadena-vegetal/frutales/produccion-primaria/programas-fitosanitarios/mosca-de-los-frutos-0 (accessed on 2 September 2024).
- Quiroga, D.; Ramirez, W.; Ruiz, C. National Fruit Fly Control and Eradication Program (PROCEM) Argentina. In Proceedings of the 8th International Symposium on Fruit Flies of Economic Importance, Valencia, Spain, 26 September–1 October 2010; Sabater-Muñóz, B., Navarro-Llopis, V., Urbaneja, A., Eds.; Editorial Universitat Politécnica de Valéncia: Valencia, Spain, 2010; p. 100. Available online: http://hdl.handle.net/10251/11200 (accessed on 2 September 2024).
- SENASA; Servicio Nacional de Sanidad y Calidad Agroalimentaria. Control de 1 Millón de Hectáreas para Prevenir la Mosca de los Frutos. 2018. Available online: http://www.senasa.gob.ar/senasa-comunica/infografias/control-de-1-millon-de-hectareaspara-prevenir-la-mosca-de-los-frutos (accessed on 2 September 2024).
- Suárez, L.; Biancheri, M.J.B.; Murúa, F.; Ordano, M.; Wang, X.; Cancino, J.; Garcia, F.R.M.; Sánchez, G.; Beltrachini, S.; Kulichevsky, L.E.; Ovruski, S.M. Medfly population suppression through augmentative release of an introduced parasitoid in an irrigated multi-fruit orchard of central–western Argentina. Insects 2023a, 14, 387. https://doi.org/10.3390/insects14040387. [CrossRef]
- Sánchez, G.; Murúa, F.; Suárez, L.; Van Nieuwenhove, G.; Taret, G.; Pantano, V.; Ovruski, S.M. Augmentative releases of Diachasmimorpha longicaudata (Hymenoptera: Braconidae) for Ceratitis capitata (Diptera: Tephritidae) control in a fruit-growing region of Argentina. Biol. Control 2016, 103, 101–107. https://doi.org/10.1016/j.biocontrol.2016.08.002. [CrossRef]
- Suárez, L.; Buonocore Biancheri, M.J.; Murúa, F.; Bilbao, M.; García, M.; Cancino, J.; Martín, O.; Molina, D.; Laría, O.; Ovruski, S.M. Effects of host age and radiation dose in Diachasmimorpha longicaudata (Hymenoptera: Braconidae) mass reared on Medfly larvae of the tsl Vienna 8 genetic sexing strain. Biol. Control 2019, 130, 51–59. https://doi.org/10.1016/j.biocontrol.2018.12.013. [CrossRef]
- Saabna, N.; Keasar, T. Parasitoids for biological control in dryland agroecosystems. Curr. Opin. Insect Sci., 2024, 64, 101226. https://doi.org/10.1016/j.cois.2024.101226. [CrossRef]
- Suárez, L.; Murúa, F.; Lara, N.; Escobar, J.; Taret, G.; Rubio, J.L.; Van Nieuwenhove, G.; Bezdjian, L.; Schliserman, P.; Ovruski, S.M. Biological control of Ceratitis capitata (Diptera: Tephritidae) in Argentina: releases of Diachasmimorpha longicaudata (Hymenoptera: Braconidae) in fruit-producing semi-arid areas of San Juan. Nat. Sci. 2014, 6, 664–675. https://doi.org/10.4236/ns.2014.69066. [CrossRef]
- Cancino, J.; Ruiz, L.; López E.; Aguilar, E.; Galvez, C.; Montoya, P.; Liedo, P. Suppression of Ceratitis capitata (Wied.) (Diptera: Tephritidae) populations in coffee in the Mexico-Guatemala border region through the augmentative releases of Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biocontrol Sci. Tech. 2019, 29, 822–826. https://doi.org/10.1080/09583157.2019.1608507. [CrossRef]
- Hoffmeister, T. Factors determining the structure and diversity of parasitoid complexes in tephritids fruit flies. Oecologia 1992, 89, 288-297. https://doi.org/10.1007/BF00317230. [CrossRef]
- López, M.; Aluja, M.; Sivinski, J. Hymenopterous larval-pupal and pupal parasitoids of Anastrepha flies (Diptera: Tephritidae) in Mexico. Biol. Control 1999, 15, 119–129. https://doi.org/10.1006/BCON.1999.0711. [CrossRef]
- Wang, X-G.; Johnson, M.W.; Daane, K.M.; Yokoyama, V.Y. Larger Olive Fruit Size Reduces the Efficiency of Psyttalia concolor as a parasitoid of the Olive fruit fly. Biol. Control 2009, 49, 45-51. https://doi.org/10.1016/j.biocontrol.2009.01.004. [CrossRef]
- Purcell, M.F. Contribution of biological control to integrated pest management of tephritid fruit flies in the tropics and subtropics. Integ. Pest. Manag. Rev. 1998, 3, 63–83. https://doi.org/10.102 3/a:1009647429498. [CrossRef]
- Messing, R.H.; Jang, E.B. (1992) Response of the fruit fly parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) to host-fruit stimuli. Environ. Entomol. 1992, 21, 1189-1195. https://doi. org/10.1603/0046-225X-34.3.576.
- Montoya, P.; Liedo, P.; Benrey, B.; Barrera, J.F.; Cancino, J.; Sivinski, J.; Aluja, M. Biological control of Anastrepha spp. (Diptera: Tephritidae) in mango orchards through augmentative releases of Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biol. Control 2000, 18, 212–224. https://doi.org/10.1006/bcon.2000.0819. [CrossRef]
- Montoya, P.; López, P.; Cruz Bustos, J. Effect of Diachasmimorpha longicaudata releases on the native parasitoid guild attacking Anastrepha spp. larvae in disturbed zones of Chiapas, Mexico. BioControl 2017, 62, 581-593. https://doi.org/10.1007/s10526-017-9826-8. [CrossRef]
- Garcia, F.R.M.; Ovruski, S.M.; Suárez, L.; Cancino, J.; Liburd, O.E. Biological control of tephritid fruit flies in the Americas and Hawaii: A review of the use of parasitoids and predators. Insects 2020, 11, 662. https://doi.org/10.3390/insects11100662. [CrossRef]
- Montoya, P.; Cancino, J.; Zenil, M.; Santiago, G.; Gutierrez, J.M. The augmentative biological control component in the Mexican national campaign against Anastrepha spp. fruit flies. In Area-Wide Control of Insect Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 661–670.
- de Pedro, L.; Tormos, J.; Harbi, A.; Ferrara, F.; Sabater-Muñoz, B.; Asís, J.D.; Beitia, F. Combined use of the larvo-pupal parasitoids Diachasmimorpha longicaudata and Aganaspis daci for biological control of the medfly. Ann. Appl. Biol. 2019, 174, 40–50. https://doi.org/10.1111/aab.12468. [CrossRef]
- Sivinski, J. Augmentative biological control: research and methods to help make it work. CAB Rev. 2013, 8, 1–11. https://doi.org/10.1079/PAVSNNR20138026. [CrossRef]
- Dias, N.P.; Montoya, P.; Nava, D. E. A 30-year systematic review reveals success in tephritid fruit fly biological control research. Entomol Exp Appl. 2022, 170, 370–384. https://doi.org/10.1111/eea.13157. [CrossRef]
- Cáceres, C. Mass rearing of temperate sensitive genetic sexing strains in the Mediterranean fruit fly (Ceratitis capitata). Genetica 2002, 116, 107–116. https://doi.org/10.1023/A:1020967810703. [CrossRef]
- Campos, V. E.; Gatica, G.; Andino, N.; Fernández Maldonado, V. N.; Cardús, A. Land surface temperature in an arid city: assessing spatio-temporal changes. 2023. Available online: https://ri.conicet.gov.ar/bitstream/handle/11336/232349/CONICET_Digital_Nro.a5dc7937-042d-43de-9076-72ffe5c2679b_B.pdf?seq (accessed on 2 September 2024).
- García-Medel, D.; Sivinski, J.; Dıáz-Fleischer, F.; Ramirez-Romero, R.; Aluja, M. Foraging behavior by six fruit fly parasitoids (Hymenoptera: Braconidae) released as single- or multiple-species cohorts in field cages: influence of fruit location and host density. Biol. Control 2007, 43, 12–22. https://doi.org/10.1016/j.biocontrol.2007.06.008. [CrossRef]
- Harbi, A.; Beitia, F.; Ferrara, F.; Chermiti, B.; Sabater-Muñoz, B. Functional response of Diachasmimorpha longicaudata (Ashmead) over Ceratitis capitata (Wiedemann): influence of temperature, fruit location and host density. Crop. Prot. 2018, 109, 115–122. https://doi.org/10.1016/j.cropro.2018.03.010. [CrossRef]
- Rosenheim, J.A.; Hoy, M. A. Confidence intervals for the Abbott’s formula correction of bioassay data for control response. J. Econ. Entomol. 1989, 82, 331-335. https://doi.org/10.1093/jee/82.2.331. [CrossRef]
- Pinheiro, J.C.; Bates, D. M. nlme: Linear and Nonlinear Mixed Effects Models; R package version 3.1-166, 2024. Available online: https://CRAN.R-project.org/package=nlme (accessed on 10 September 2024).
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer, New York, USA, 2000. https://doi.org/10.1007/b98882. [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing, Vienna, Austria, 2024. Available online: https://www.R-project.org/ (accessed on 10 September 2024).
- Suárez, L.; Biancheri, M.J.B.; Díaz-Nieto, L.M.; Schliserman, P.; Murúa, F.; Rull, J.; Lasa, R.; Pantano, V.; Ovruski, S.M. Dynamic seasonal response of Ceratitis capitata (Diptera: Tephritidae) to fruit juice-based lures in fig orchards. Int. J. Pest Manag. 2023b, 64, 346-358. https://doi.org/10.1080/09670874.2021.1918360. [CrossRef]
- Sivinski, J.; Aluja, M. The evolution of ovipositor length in the Parasitic Hymenoptera and the search for predictability in biological control. Fla. Entomol. 2003, 86, 143–150. https://doi.org/10.1653/0015-4040(2003)086[0143:TEOOLI]2.0.CO;2. [CrossRef]
- Sivinski, J.; Aluja, M. The roles of parasitoid foraging for hosts, food and mates in the augmentative control of Tephritidae. Insects 2012, 3, 668–691. https://doi.org/10.3390/insects3030668. [CrossRef]
- Buonocore-Biancheri, M.J.; Wang, X.; Núñez-Campero, S.R.; Suárez, L.; Schliserman, P.; Ponssa, M.D.; Kirschbaum, D.S.; Garcia, F.R.M.; Ovruski, S.M. The population dynamics and parasitism rates of Ceratitis capitata, Anastrepha fraterculus, and Drosophila suzukii in non-crop hosts: Implications for the management of pest Fruit flies. Insects 2024, 15, 61. https://doi.org/10.3390/ insects15010061. [CrossRef]
- Segura, D.F.; Nussenbaum, A.L.; Viscarret, M.M.; Devescovi, F.; Bachmann, G.E.; Corley, J.C.; Ovruski, S.M.; Cladera, J.L. Innate host habitat preference in the parasitoid Diachasmimorpha longicaudata: functional significance and modifications through learning. PLoS One 2016, 11, e0152222. https://doi.org/10.1371/journal. pone.0152222. [CrossRef]
- Eben, A.; Benrey, B.; Sivinski, J.; Aluja, M. Host species and host plant effects on preference and performance of Diachasmimorpha longicaudata (Hymenoptera: Braconidae). Environ. Entomol. 2000, 29, 87–94. https://doi.org/10.1603/0046-225X-29.1.87. [CrossRef]
- Stuhl, C.; Sivinski, J.; Teal, P.; Aluja, M. Responses of multiple species of tephritid (Diptera) fruit fly parasitoids (Hymenoptera: Braconidae: Opiinae) to sympatric and exotic fruit volatiles. Fla. Entomol. 2012, 95, 1031–1039. https://doi.org/10.1653/024.095.0432. [CrossRef]
| Study year | Temperature (°C) |
Relative Humidity (%) |
Precipitation (mm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Average monthly | Min. | Max. | Average monthly | Accumulated rainfall | |||
| 2019 | 9.9 | 36.3 | 21.1 | 10 | 86 | 56.2 | 0.2 | ||
| 2020 | 10.9 | 36.5 | 22.8 | 23 | 89 | 57.1 | 0.3 | ||
| Host fruits | Fruit physical features (n = 30) (mean ± SE) |
Medfly larvae | ||||||
|---|---|---|---|---|---|---|---|---|
| Weight (g) |
Diameter (cm) |
Rind thickness (cm) | Pulp depth (cm) | Surface area (cm2) |
Density * (cm2) (mean ± SE) |
Larvae per fruit | ||
| Sweet orange | ||||||||
| 200.2 ± 4.8a | 7.1 ± 0.2a | 4.6 ± 0.2a | 3.1 ± 0.1a | 44.0 ± 8.0a | 3.08 ± 0.06a | 130 | ||
| Peach | 156.9 ± 1.5b | 6.6 ± 0.1b | 0.3 ± 0.1b | 2.5 ± 0.1b | 41.9 ± 0.4b | 3.10 ± 0.03a | 130 | |
| Fig | 57.2 ± 0.8c | 5.1 ± 0.2c | 0.1 ± 0.1c | 2.0 ± 0.1c | 32.3 ± 1.1c | 3.11 ± 0.01a | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





