Submitted:
20 September 2024
Posted:
23 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Preoperative Assessment
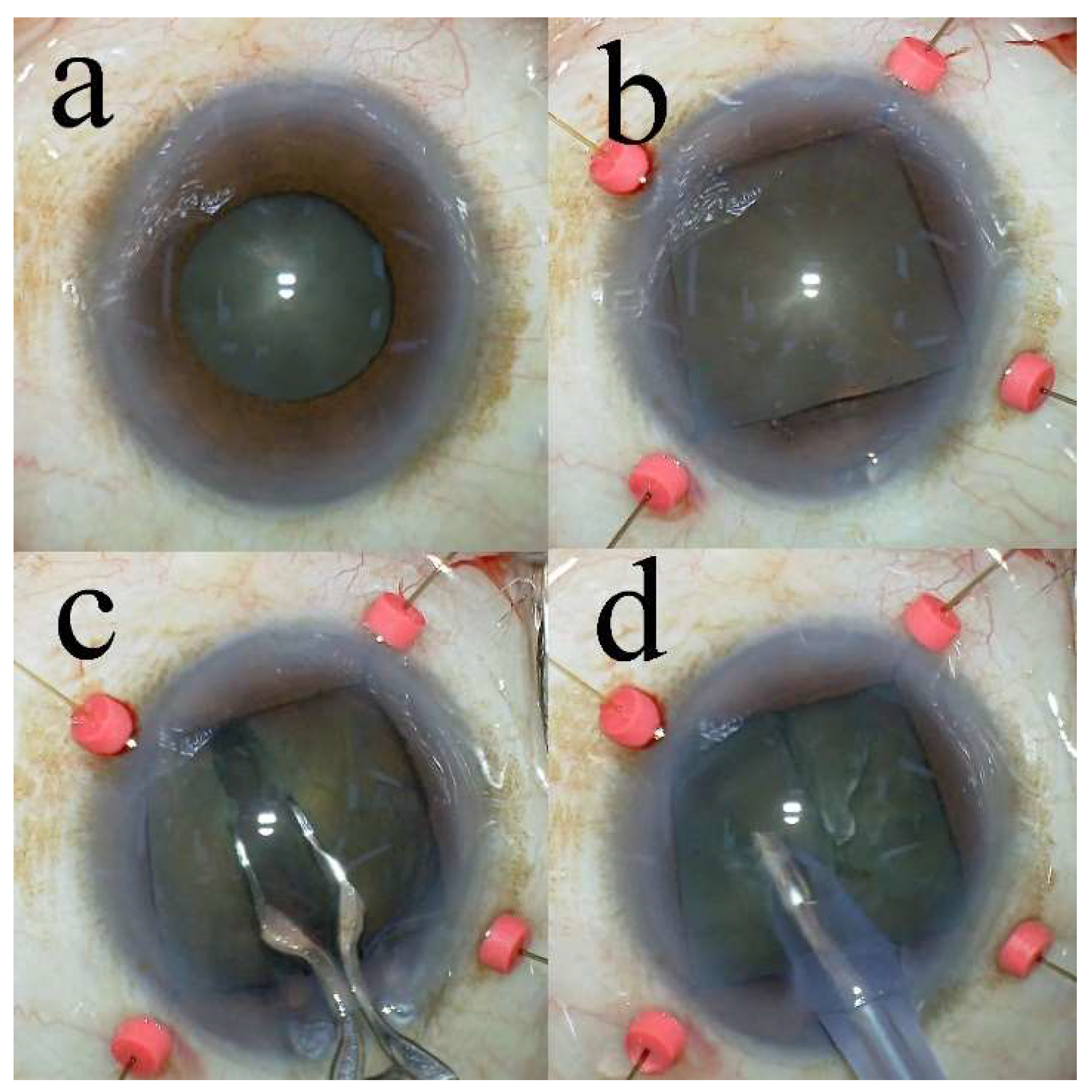
2.3. New Surgical Instruments
2.4. Surgical Technique
2.5. Outcome Measures and Data Collection
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forooghian, F.; Agrón, E.; Clemons, T.E.; Ferris, F.L., 3rd; Chew, E.Y.; Age-Related Eye Disease Study Research Group. Visual acuity outcomes after cataract surgery in patients with age-related macular degeneration: age-related eye disease study report no. 27. Ophthalmology 2009, 116, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Nderitu, P.; Ursell, P. Iris hooks versus a pupil expansion ring: operating times, complications, and visual acuity outcomes in small pupil cases. J Cataract Refract Surg 2019, 45, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Vasavada, A.; Singh, R. Phacoemulsification in eyes with a small pupil. J Cataract Refract Surg 2000, 26, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Seyedian, M.A.; Mohammadpour, M. Small pupil and cataract surgery. Curr Opin Ophthalmol 2015, 26, 3–9. [Google Scholar] [CrossRef]
- Balal, S.; Jbari, A.S.; Nitiahpapand, R.; Cook, E.; Akhtar, W.; Din, N.; Sharma, A. Management and outcomes of the small pupil in cataract surgery: iris hooks, Malyugin ring or phenylephrine? Eye (Lond) 2021, 35, 2714–2718. [Google Scholar] [CrossRef]
- Halkiadakis, I.; Chatziralli, I.; Drakos, E.; Katzakis, M.; Skouriotis, S.; Patsea, E.; Mitropoulos, P.; Kandarakis, A. Causes and management of small pupil in patients with cataract. Oman J Ophthalmol 2017, 10, 220–224. [Google Scholar]
- Akman, A.; Yilmaz, G.; Oto, S.; Akova, Y.A. Comparison of various pupil dilatation methods for phacoemulsification in eyes with a small pupil secondary to pseudoexfoliation. Ophthalmology 2004, 111, 1693–1698. [Google Scholar] [CrossRef]
- Sato, T. Efficacy and safety of the eight-chop technique in phacoemulsification for patients with cataract. J Cataract Refract Surg 2023, 49, 479–484. [Google Scholar] [CrossRef]
- Emery, J.M. Kelman phacoemulsification; patient selection. In Extracapsular cataract surgery; Emery, J.M., Mclyntyre, D.J., Eds.; CV Mosby: St Louis, USA, 1983; pp. 95–100. [Google Scholar]
- Miyata, K.; Nagamoto, T.; Maruoka, S.; Tanabe, T.; Nakahara, M.; Amano, S. Efficacy and safety of the soft-shell technique in cases with a hard lens nucleus. J Cataract Refract Surg 2002, 28, 1546–1550. [Google Scholar] [CrossRef]
- Vasavada, A.R.; Desai, J.P.; Singh, R. Measuring structures within the eye. J Cataract Refract Surg 1996, 22, 5–6. [Google Scholar] [CrossRef]
- Poley, B.J.; Lindstrom, R.L.; Samuelson, T.W. Long-term effects of phacoemulsification with intraocular lens implantation in normotensive and ocular hypertensive eyes. J Cataract Refract Surg 2008, 34, 735–742. [Google Scholar] [CrossRef]
- Wang, J.D.; Zhang, J.S.; Li, M.; Mao, Y.Y.; Mayinuer, Y.; Wan, X.H. Comparison of different pupil dilatation methods for phacoemulsification in eyes with a small pupil. BMC Ophthalmol 2022, 22, 173. [Google Scholar] [CrossRef]
- Helvacioglu, F.; Yeter, C.; Tunc, Z.; Sencan, S. Outcomes of torsional microcoaxial phacoemulsification performed by 12-degree and 22-degree bent tips. J Cataract Refract Surg 2013, 39, 1219–1225. [Google Scholar] [CrossRef]
- Igarashi, T.; Ohsawa, I.; Kobayashi, M.; Umemoto, Y.; Arima, T.; Suzuki, H.; Igarashi, T.; Otsuka, T.; Takahashi, H. Effects of hydrogen in prevention of corneal endothelial damage during phacoemulsification: a prospective randomized clinical trial. Am J Ophthalmol 2019, 207, 10–17. [Google Scholar] [CrossRef]
- Upadhyay, S.; Sharma, P.; Chouhan, J.K.; Goyal, R. Comparative evaluation of modified crater (endonucleation) chop and conventional crater chop techniques during phacoemulsification of hard nuclear cataracts: a randomized study. Indian J Ophthalmol 2022, 70, 794–798. [Google Scholar] [CrossRef]
- Sato, M.; Sakata, C.; Yabe, M.; Oshika, T. Soft-shell technique using Viscoat and Healon 5: a prospective, randomized comparison between a dispersive-viscoadaptive and a dispersive-cohesive soft-shell technique. Acta Ophthalmol 2008, 86, 65–70. [Google Scholar] [CrossRef]
- Park, J.; Yum, H.R.; Kim, M.S.; Harrison, A.R.; Kim, E.C. Comparison of phaco-chop, divide-and-conquer, and stop-and-chop phaco techniques in microincision coaxial cataract surgery. J Cataract Refract Surg 2013, 39, 1463–1469. [Google Scholar] [CrossRef]
- Wirbelauer, C.; Anders, N.; Pham, D.T.; Wollensak, J.; Laqua, H. Intraocular pressure in nonglaucomatous eyes with pseudoexfoliation syndrome after cataract surgery. Ophthalmic Surg Lasers 1998, 29, 466–471. [Google Scholar] [CrossRef]
- Shingleton, B.J.; Gamell, L.S.; O’Donoghue, M.W.; Baylus, S.L.; King, R. Long-term changes in intraocular pressure after clear corneal phacoemulsification: normal patients versus glaucoma suspect and glaucoma patients. J Cataract Refract Surg 1999, 25, 885–890. [Google Scholar] [CrossRef]
- Irak-Dersu, I.; Nilson, C.; Zabriskie, N.; Durcan, J.; Spencer, H.J.; Crandall, A. Intraocular pressure change after temporal clear corneal phacoemulsification in normal eyes. Acta Ophthalmol 2010, 88, 131–134. [Google Scholar] [CrossRef]
- Shingleton, B.J.; Pasternack, J.J.; Hung, J.W.; O’Donoghue, M.W. Three and five year changes in intraocular pressures after clear corneal phacoemulsification in open angle glaucoma patients, glaucoma suspects, and normal patients. J Glaucoma 2006, 15, 494–498. [Google Scholar] [CrossRef]
- Poley, B.J.; Lindstrom, R.L.; Samuelson, T.W.; Schulze, R., Jr. Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and nonglaucomatous eyes: evaluation of a causal relationship between the natural lens and open-angle glaucoma. J Cataract Refract Surg 2009, 35, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Storr-Paulsen, A.; Jørgensen, J.S.; Norregaard, J.C.; Thulesen, J. Corneal endothelial cell changes after cataract surgery in patients on systemic sympathetic α-1a antagonist medication (tamsulosin). Acta Ophthalmol 2014, 92, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Hargitai, J.; Vezendi, L.; Vigstrup, J.; Eisgart, F.; Lundbye-Christensen, S.; Hargitai, B.; Vorum, H. Comparing the efficacy of mydriatic cocktail-soaked sponge and conventional pupil dilation in patients using tamsulosin - a randomized controlled trial. BMC Ophthalmol 2013, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.F. Use of Malyugin pupil expansion device for intraoperative floppy-iris syndrome: results in 30 consecutive cases. J Cataract Refract Surg 2008, 34, 835–841. [Google Scholar] [CrossRef]
- Papaconstantinou, D.; Kalantzis, G.; Brouzas, D.; Kontaxakis, A.; Koutsandrea, C.; Diagourtas, A.; Georgalas, I. Safety and efficacy of phacoemulsification and intraocular lens implantation through a small pupil using minimal iris manipulation. Clin Interv Aging 2016, 11, 651–657. [Google Scholar]
- Kopsachilis, N.; Carifi, G. Phacoemulsification using 8 flexible iris hooks in a patient with a short eye, small pupil, and phacodonesis. J Cataract Refract Surg 2014, 40, 1408–1411. [Google Scholar] [CrossRef]
- Salimi, A.; Abu-Nada, M.; Harasymowycz, P. Matched cohort study of cataract surgery with and without trabecular microbypass stent implantation in primary angle-closure glaucoma. Am J Ophthalmol 2021, 224, 310–320. [Google Scholar] [CrossRef]
- Hayashi, K.; Hayashi, H.; Nakao, F.; Hayashi, F. Effect of cataract surgery on intraocular pressure control in glaucoma patients. J Cataract Refract Surg 2001, 27, 1779–1786. [Google Scholar] [CrossRef]
- Iancu, R.; Corbu, C. Intraocular pressure after phacoemulsification in patients with uncontrolled primary open angle glaucoma. J Med Life 2014, 7, 11–16. [Google Scholar]
- Falck, A.; Hautala, N.; Turunen, N.; Airaksinen, P.J. A four-year prospective study on intraocular pressure in relation to phacoemulsification cataract surgery. Acta Ophthalmol 2011, 89, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.M.; Taylor, H.; Qureshi, K.; Smith, R.; Birnie, K.; Johnston, R.L. The cataract national dataset electronic multi-centre audit of 55,567 operations: risk indicators for monocular visual acuity outcomes. Eye (Lond) 2012, 26, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Narendran, N.; Jaycock, P.; Johnston, R.L.; Taylor, H.; Adams, M.; Tole, D.M.; Asaria, R.H.; Galloway, P.; Sparrow, J.M. The cataract national dataset electronic multicentre audit of 55,567 operations: risk stratification for posterior capsule rupture and vitreous loss. Eye (Lond) 2009, 23, 31–37. [Google Scholar] [CrossRef] [PubMed]
| Characteristic/Parameter | Iris hooks group | Control group | p-Value |
|---|---|---|---|
| Number of eyes | 65 | 65 | |
| Age (y) | 76.8 ± 7.8 | 75.0 ± 6.2 | .16a |
| Gender: Men | 42 (65%) | 16 (25%) | <.01b |
| Women | 23 (35%) | 49 (75%) | |
| Diabetes mellitus | 15 | 10 | .27c |
| IFIS | 10 | 0 | <.01b |
| Preoperative pupil size (mm) | 5.34 ± 0.53 | 7.62 ± 0.55 | <.01d |
| Postoperative pupil size (mm) | 6.37 ± 0.68 | 7.33 ± 0.65 | <.01d |
| Iris hooking time (min) | 3.3 ± 1.37 | - | |
| Operative time (min) | 10.6 ± 2.32 | 4.6 ± 1.20 | <.01d |
| Phaco time (s) | 20.7 ± 6.4 | 16.2 ± 7.6 | <.01d |
| Aspiration time (s) | 101.1 ± 52.2 | 72.1 ± 18.6 | <.01d |
| CDE | 7.8 ± 2.44 | 7.0 ± 3.36 | .16a |
| Volume of fluid used (mL) | 38.0 ± 8.8 | 28.9 ± 9.2 | <.01d |
| Group/Parameter | Preoperatively | 7 weeks postoperatively |
19 weeks postoperatively |
p-Value |
|---|---|---|---|---|
| Iris hooks | ||||
| BCVA (logMAR) (n = 65) | 0.21 ± 0.33 | −0.011 ± 0.083 | −0.026 ± 0.078 | <.01a, <.01a, <.01a |
| CECD (cells/mm2) (n = 37) | 2503.6 ± 213.0 | 2437.4 ± 215.6 | 2448.8 ± 215.7 | <.01a, .51b, <.01a |
| CECD loss (%) | - | 2.6 ± 4.3 | 2.1 ± 4.4 | - |
| Control | ||||
| BCVA (logMAR) (n = 65) | 0.17 ± 0.29 | −0.042 ± 0.074 | −0.039 ± 0.075 | <.01a, .42b, <.01a |
| CECD (cells/mm2) (n = 65) | 2585.1 ± 236.2 | 2553.2 ± 254.6 | 2553.2 ± 246.0 | <.01a, .99b, <.01a |
| CECD loss (%) | - | 1.3 ± 3.2 | 1.2 ± 3.2 | - |
| p-Value | .47c, .09c | <.05d, <.05d | .38c, <.05d | |
| Mean IOP (mmHg) ± SD (% decrease) | |||||||
|---|---|---|---|---|---|---|---|
| Examination | Iris hooks group (n = 51) | Control group (n = 65) | p-Value | ||||
| Preoperatively | 14.5 ± 2.5 | - | 14.0 ± 2.2 | - | .33a | ||
| 7 weeks postoperatively |
13.0 ± 2.7 | (10.1 ± 15.4) | 11.8 ± 2.3 | (15.6 ± 12.3) | <.01b | <.01c | <.01d |
| 19 weeks postoperatively |
12.7 ± 2.6 | (12.0 ± 11.8) | 11.5 ± 2.4 | (18.1 ± 9.7) | <.01b | <.01 c | <.01d |
| Iris hooks group | ||||||
| Examination | IOP above 15 mmHg group (n = 26) | p-Value | IOP below 15 mmHg group (n = 25) | p-Value | ||
| Preoperatively | 16.5 ± 1.4 | - | 12.5 ± 1.6 | - | ||
| 7 weeks postoperatively | 14.6 ± 2.0 | (11.3 ± 10.8) | <.01a | 11.3 ± 2.4 | (8.9 ± 19.1) | <.01a |
| 19 weeks postoperatively | 14.4 ± 1.9 | (12.3 ± 11.2) | <.01a | 11.0 ± 2.0 | (11.6 ± 12.7) | <.01a |
| Control group | ||||||
| Examination | IOP above 15 mmHg group (n = 27) | p-Value | IOP below 15 mmHg group (n = 38) | p-Value | ||
| Preoperatively | 16.0 ± 0.9 | 12.7 ± 1.3 | ||||
| 7 weeks postoperatively | 12.8 ± 2.0 | (20.4 ± 12.1) | <.01a | 10.8 ± 1.9 | (14.9 ± 13.0) | <.01a |
| 19 weeks postoperatively | 12.8 ± 1.8 | (20.2 ± 10.7) | <.01a | 10.6 ± 1.8 | (16.7 ± 9.6) | <.01a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





