1. Introduction
Bananas are the world's four widely grown fruits in southern China. According to the China Statistical Yearbook 2021, China produced 11.513 million tons of bananas in 2020 [
1], of which banana peels accounted for 30-40% [
2]. Banana peel and its residue contain nutrients and phytochemicals [
3,
4]. Banana peel is a potent source of dietary fiber [
5] and pectin [
6]. These banana by-products have been developed into animal feeds [
7]. The literature also reported the application of banana peel in ruminant feeds [
8].
Microbial fermentation of banana peel can improve its palatability and enhance nutritional value. Caicedo [
9] reported the addition of natural yogurt, whey, and molasses in banana silage fermentation. The physicochemical, biological, and sensory indexes of these fermented banana silages were determined, and the results showed that these silages were suitable for pig breeding. The experimental meal-fed (20% fermented banana peel) Siamese catfish had a similar feed consumption, specific growth rate, feed efficiency, fat retention, and energy retention compared to the control group feeding with the commercial diet [
10]. However, there are limited reports on the effects of adding fermented banana peel and pulp residue into poultry feeds, especially the research on feed quality and flavor components of poultry meat.
Limited studies on the application of fermented fruit peels to poultry feeds have been reported in the literature. No report on feeding poultries with fermented fruit peels for improving slaughter performance and the meat quality is available. No comparison has been made for the meat nutritional parameters between the fermented and unfermented fruit peel fed chicken. Therefore, the effects of adding fermented banana peels and pulp residues to the poultry feeds on slaughter performance, meat quality, and flavor components were evaluated. The data could provide a theoretical basis for utilizing banana by-products as a high-quality silage.
2. Materials and Methods
2.1. Fermented Banana Peel and Banana Residue
The banana variety used in this experiment was Guijiao 6 (Musa AAA). It was the main variety cultivated in the Guangxi region. The fruit was cultivated in a local plantation of Guangxi. The harvested unripe bananas were kept at room temperature in the laboratory before further processing. The ripen fruit peel was separated. They were then cut into smaller pieces and inoculated with a fermentation agent. After homogenization, the fruit peels were packed and sealed and then kept at a room temperature of 25 °C for 15 days. After partial fermentation, 0.8% pectinase and 0.5% cellulase were added onto it, and left to stand for 2 h. The enzymatically hydrolyzed peel samples were filtered, and the filtrate was collected. On the other hand, the pulp residues were inoculated with the fermentation agent, packed, and sealed before fermenting at room temperature for 15 days.
Ash content (%) of the fermented banana samples was determined by ashing the oven-dried banana samples at 550 °C for 3 h, whereas the dry matter (%) was determined by oven-dried at 105 °C until a constant weight obtained [
11]. The carbohydrate content was estimated using the anthrone-sulfuric acid method [
12], and the crude protein content was determined using the Kjeldahl method [
13]. The pH values of the sample homogenates (1:9, w/v, diluted with normal saline) were measured using a pH meter, and its titratable acidity was determined by titrating with titrate calibrated sodium hydroxide solution. The amounts of lactic acid bacteria (LAB) in the sample homogenates were determined according to the standard plate count method [
14]. The chemical constituents of these fermented samples are shown in
Table 1.
2.2. Poultry Feeding Experiment
The poultry experimentation had obtained ethical approval (GXAAS/AEEIF/005) from the Animal Care and Use Committee of Guangxi Academy of Agricultural Sciences, Nanning, China. Before the feeding experiment, 120 Guangxi Partridge chickens (Gallus gallus domesticus) were acclimatized at the Tiandong poultry farm in Guangxi. These chickens are 12 weeks old, with an average body weight of 1.25±0.02 g. They were randomly assigned to five experiment groups (n=24), including a control group (CK) and four supplementation groups.
All experimental chickens had free access to the experimental feeds and tap water ad libitum for 140 days. The CK group was fed a basal diet, while the other four supplementation groups were fed diets supplemented with 10% fermented banana peel (PE-10), 20% fermented banana peel (PE-20), 10% fermented banana pulp residue (PU-10), and 20% fermented banana pulp residue (PU-20). During the feeding period, all chickens had access to tap water ad-libitum. The poultry house was cleaned weekly and ventilated with ambient temperatures ranging from 27 °C to 33 °C.
Corn and soybean mixed meal was used as the standard daily feed. This feed consisted 57.5% corn, 25% soybean meal, 5% wheat bran, 2.5% soy oil, and 10% premix. It contained 17% crude protein. The two essential amino acids were lysine (0.8%) and methionine (0.4%). Its energy content was 2.75 MC/kg.
2.3. Preparation of Samples
At the end of the feeding experiment (32 weeks of age), all fasted chickens had their blood drawn before slaughtering. These poultries were fasted for 12 h before being sacrificed according to GB/T 19478-2018 procedure [
15]. After slaughtering via jugular vein exsanguination, the carcass was blanched in boiling water, and the feathers were removed by pulling off from the body. The weights of all organs were measured, and the pectoral and thigh muscles were collected for further analysis. The layers of abdominal fats around the muscular stomach and abdomen were also separated and weighed. The pectoral muscles along the sternum ridge were obtained with the chicken skin removed, whereas the thigh muscles were collected after removing the skin, subcutaneous fat, and bones. The slaughter performance of these chickens was presented as the carcass weights and percentages of pectoral and thigh muscles.
2.4. Nutritional Compositions and Color Values of Chicken Chest Meats
The pectoral muscle, commonly called chest meat, was determined for its moisture content and fatty acid (FA) composition. These parameters were analyzed according to the Chinese standard analytical methods. They were GB 5009.3—2016 [
16] and GB5009.168—2016 [
17], respectively. L*, a*, and b* values were analyzed in 45 min after slaughter. The values were determined using a chromometer (CR400/410, Minolta, Japan) in triplicate [
18].
2.5. Shear Force and Water Holding Capacity of Chicken Chest Meats
The shear force test and water loss analysis were performed for the chest meat samples within 24 h after slaughter. The shear force value of the meat samples was determined according to the method described in the literature [
19]. The meat samples were initially treated in a water bath at a constant temperature of 80 °C. The meat samples were cut into pieces of 2.5 cm in length, 1.0 cm in width, and 0.25 cm in height. The Meat Tenderness Tester RH-N50 (Runhu Instrument Co., Ltd., Guangzhou, China) was used to measure the shear force value at three different points to obtain an average value. The water-holding capacity of the meat samples was measured using an RH-1000 Water Holding Capacity Tester (Runhu Instrument Co., Ltd., Guangzhou, China). In brief, the meat sample was pressurized to 35 kg in weight, maintained for 5 min, and then weighed.
2.6. Determination of Flavor Compounds in Chicken Chest Meats
The analysis was performed by gas chromatography-ion migration spectrometry (GC-IMS). The GC analysis of flavor substances were determined using the method described by the literature with some modifications [
20]. In brief, 2.0 g chest meat samples were weighed and placed in a 20-mL headspace bottle, incubated at 90 °C for 15 min, and analyzed by a FlavourSpec® GC-IMS flavor analyzer (Dortmund, Germany). GC-IMS analytical conditions are as follows: FS-SE-54-CB-1 capillary column (15 m, ID: 0.53 mm) as stationary phase, column temperature of 60 °C, IMS temperature of 45 °C, injection volume of 500 μL, high-purity nitrogen as a carrier gas, and a total run time of 30 min. The gradient gas flow conditions were 0-2 min, 2 mL/min; 2-10 min, 2-10 mL/min; 10-20 min, 10-100 mL/min; 20-30 min, 100-150 mL/min.
2.7. Statistical Analysis
SPSS (version 20.0, SPSS, Inc., Chicago, USA) was used for the statistical analysis. The data were analyzed using one-way ANOVA, where P < 0.05 indicated a significant difference. The qualitative, quantitative, and fingerprints of the flavor compounds in the meat samples were determined using VOCal, Reporter, Gallery Plot, and Dynamic PCA plug-in. SIMCA was used to analyze the flavor compounds by applying the orthogonal partial least squares method and cluster analysis.
3. Results and Discussion
3.1. Slaughter Performance
Slaughter performance is one of the quality indicators of poultry farming because feeding these poultries with different standard daily feed could promote optimum growth. In this study, the addition of the fermented banana peels and pulp residues promoted growth of the experimental chicken (
Table 2). The results showed that the pectoral muscle percentages of the chicken in supplementation groups were significantly higher than the CK group after adding different proportions of fermented banana peel and banana residue in their daily feed, except for the Pu-20 group (P < 0.05). The percentages of thigh muscle of chicken in the Pe groups were remarkably higher than in the control group. There were no significant differences in the other tested parameters of the chicken in the supplementation groups compared with the control group (P < 0.05), except for the carcass weight and abdominal fat content of the chicken in the Pe-10 group and Pu-10 group, respectively.
The literature reported that adding fermented plant products such as fermented leaves and fruit peels in poultry feeds could improve the slaughter performance of broiler chicken and their meat quality. Experimental broiler chickens fed with the fermented and unfermented grape skin had their slaughter performances better than the control group, but no significant differences were found between the fermented and unfermented sample groups [
21]. The broiler chicken fed fermented mulberry leaf powder had improved slaughter performance compared to those given the unfermented mulberry leaf powder [
22]. Moringa leaf extract also increased the growth performance of broilers [
23]. The previous studies supported our findings that, to some extent, poultry fed with fermented plant products had improved slaughter performance and meat quality.
3.2. Quality Parameters of Chicken Chest Meats
3.2.1. Meat brightness and colors
The main quality parameters of the chest meat samples of all chicken are presented in
Table 3. Meat color is one of the indicators of meat quality. It included brightness (L*), redness (a*), and yellowness (b*). The results showed that their L*, a*, and b* values were significantly different among these experiment groups. The meat samples of the chicken in the Pu-20 group had the remarkably highest L* value (P < 0.05), whereas the L* values of the other experimental chicken meats were not notably higher but also lower than in the CK group. The meat sample in the Pu-10 group was darker than the control group. The meat samples in the Pe-10 and Pu-10 groups also had the significantly highest a* and b* values, respectively. The meat samples in Pu-20 group had low a* and b* values, although they had the highest L* value. Moreover, the meat samples in the Pe-10 group had the least b* value with the highest a* value.
The literature reports that a higher a* value shows a higher red hue, whereas a higher b* value indicates a higher yellow hue [
24]. The chest meat samples of the chicken in the Pe-10 group had intense redness and lesser yellow hues with moderate brightness. It indicated that the meat sample in this group had a better meat quality than the other groups. The low meat redness of the chicken in the CK group shows that the supplementation of fermentation banana samples effectively improved the meat quality. The decrease in meat redness could be due to the oxidation of myoglobin [
25]. Other factors such as temperature, oxygen partial pressure, pH value, light, osmotic pressure, and surface microbial activity might alter the morphology of myoglobin, thus resulting in the color difference [
26].
The meat’s tenderness reflects its quality. It is conventionally expressed as a shear force value. A high shear force of a meat sample indicates that the meat sample has reduced fresh, thus reduced its chewiness. It is because fresh meat is always tender. As shown in
Table 3, the shear forces of the meat samples of the chicken in the supplementation groups were significantly lower than those in the CK group. The meat sample of the chicken in the Pe-10 group was the remarkably lowest (P < 0.05). This study showed that adding 10% fermented banana samples to the poultry feeds resulted in a better meat quality than the 20% fermented banana samples. The literature also reported that mixing fermented soybean meal to the poultry feeds improved the meat quality of the broiler chicken [
27].
3.2.2. Moisture content and water-holding capacity
The results showed that the moisture content of chest meat samples in these supplementation and control groups was higher than 70%. The moisture content of the meat samples in the Pe groups was similar to the CK group, whereas the meat samples in the Pu groups had moisture content significantly lower than in the CK group (P < 0.05). The moisture content of the meat sample in the Pu-20 group was the lowest. Besides its low moisture content, the meat sample in the Pu-20 group had a water-holding capacity lesser than the other supplementation groups. The water-holding capacity was the highest in the Pe-10 group.
Adding different amounts of fermented banana samples to the poultry feeds did not remarkably affect the meat quality, especially meat color, tenderness, and water-holding capacity. The water holding capacity of meat refers to the ability of postmortem animal muscles to retain their original moisture under the action of external forces, which affect the meat freshness and tenderness of the meat [
28]. The animal muscle with a high water-holding capacity was more tender and juicy. In this study, the meat samples with a high water holding capacity had a high moisture content. The literature also showed that the poultry fed with probiotics improved the water-holding capacity of its meat [
29].
3.3. Fatty Acid Compositions of Chicken Chest Meats
The fatty acid (FA) compositions of chest meat samples in these experimental groups are presented in
Table 4. The results showed that 12, 19, 22, 13, and 15 FAs were detected in the meat samples of CK, Pe-10, Pe-20, Pu-10, and Pu-20 groups, respectively. They were saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs). The meat samples in the Pe groups had the highest total fatty acids content, followed by Pu and CK groups. The meat sample in the CK group had the highest total SFA content, followed by Pe and Pu groups. The Pu groups had higher total PUFA content than in the Pe groups, but their total MUFA content was lower than the Pe groups. These FAs content was not significantly different between these experimental groups.
The SFAs mainly consist of palmitic acid (C16:0) and stearic acid (C18:0). The main MUFA in these meat samples was oleic acid (C18:1n9c), whereas the PUFA was linoleic acid (C18:2n6c). The palmitic, stearic, oleic, and linoleic acids content of the meat samples in Pe-10, Pe-20, Pe-20, and Pe-20 groups were the significantly highest (P < 0.05), respectively. The levels of these fatty acids in all supplementation groups were significantly higher than in the CK group, except for the Pu-10 group (P < 0.05). Also, the oleic acid content in the Pe groups was significantly higher than that in the Pu groups (P < 0.05). On the other hand, the trans-oleic acid (C18:1n9t, elaidic acid) was detected in all meat samples, while only the meat sample in the CK group had trans-linoleic acid (C18:2n6t, linolelaidic acid). The meat samples of the chickens fed with Pe samples had a significantly higher trans-oleic acid content than those chickens fed with Pu samples. The meat sample in Pu-10 had the lowest trans-oleic acid content.
Although the meat sample in Pe-20 had the highest total FAs content, pentadecanoic acid (C15:0), docosanoic acid (C22:0, behenic acid), and cis-15-tetracosenoic acid (C24:1, nervonic acid) were not detected in this meat sample. Pentadecanoic acid is a FA typically found in egg, milk, poultry, and ruminant meats. In this study, pentadecanoic acid was detected solely in the meat sample of the Pe-10 group, whereas nervonic acid was detected in Pe-10 and Pu-20. Undecanoic acid (C11:0), tridecanoic acid (C13:0), and icosanoic acid (C20:0) were only detected in the meat samples of Pe-20 group. Medium-chain fatty acids were also not determined in the chicken fed with the fermented banana pulp residue (Pu-10 and Pu-20 groups) and control groups. Among the long-chain FAs, C15:0 and C20:0 were not detected in Pu-10, Pu-20, and CK groups.
The trans-FAs in the chest meat samples could be due to the de-feathering process which involving the blanching in boiling water. Heating unsaturated FA-containing chicken muscles resulted in the oxidation of FAs [
30]. The high temperature could help to release these volatiles [
31]. Hence, FA oxidation occurred in the meat samples and increased the levels of flavor substances. This FA oxidation might alter the meat flavor [
32]. The higher levels of trans-fat determined in the meat samples of Pe groups could be due to banana peel having lower antioxidant content than banana pulp. The ingestion of antioxidant-rich feeds by the broiler chicken could also help to maintain oxidative stability in their muscles [
33]. Thus, reducing oxidation of FAs in the skeletal muscles. The results obtained in this study were supported by the finding reported in the literature that the experimental rabbit fed with antioxidant-rich extract had trans-FA levels lower than the control rabbits [
34].
3.4. Flavor Compounds of Chicken Chest Meats
Identification and analysis of flavor compounds in the chest meat samples were performed using HS-GC-IMS. The HS-GC-IMS analytical parameters are shown in
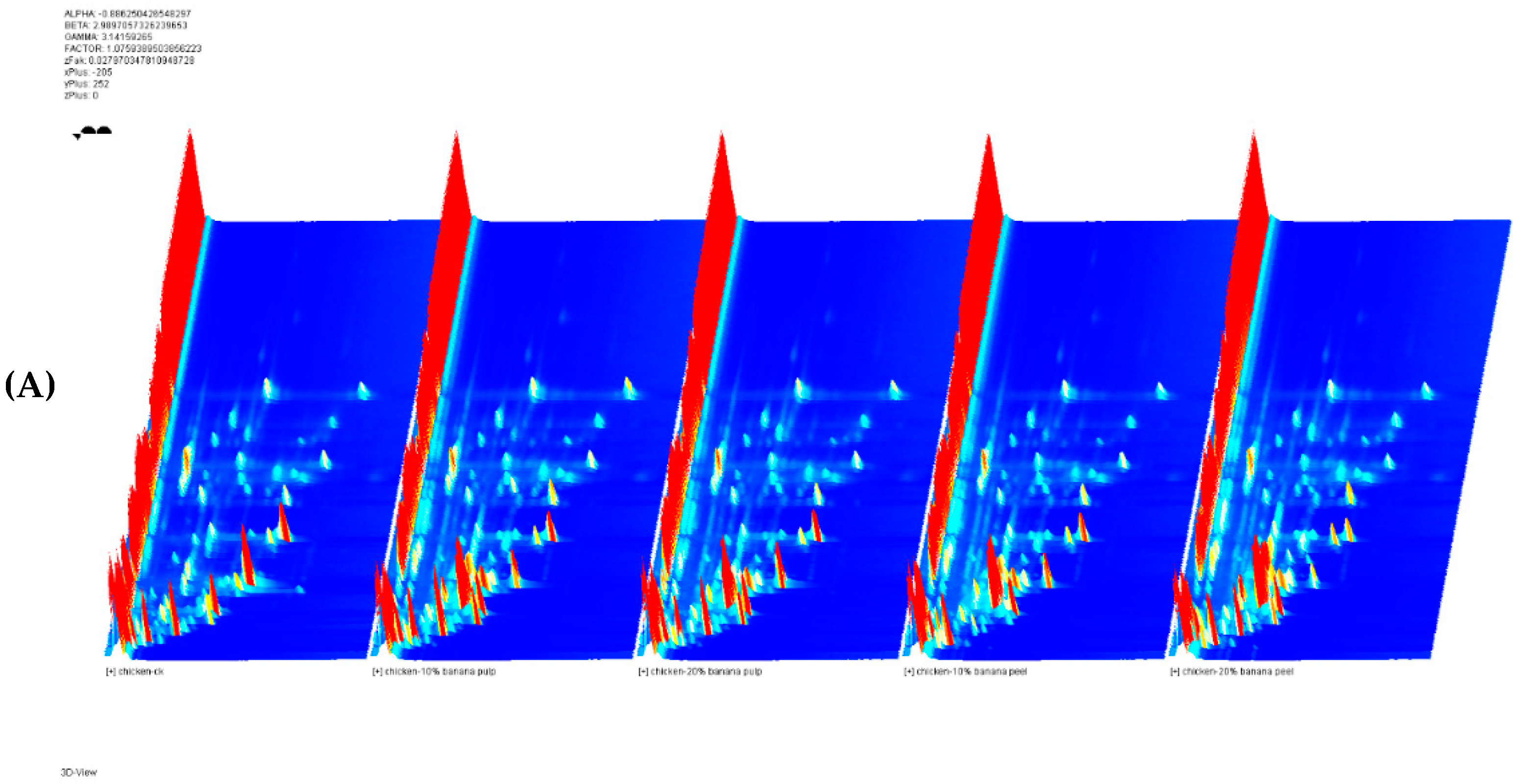
Table 5. A total of 60 flavor compounds were detected in the meat samples. They were identified using the GC-IMS library, including 22 aldehydes, 14 alcohols, six ketones, two acids, one furan, and 15 undefined compounds. They are depicted in
Figure 1 as the topographical plots. The X-axis represents the ion migration time, and Y-axis represents the GC retention time. The peak intensity on the Z-axis. The three-dimensional (3D) spectra of gas-ion migration showed the flavor composition of these meat samples (
Figure 1A). They had similar patterns for flavor compositions. The result showed that 3-hydroxy-2-butanone was only detected in the meat samples of these supplemented chicken but not for the meat samples in the CK group.
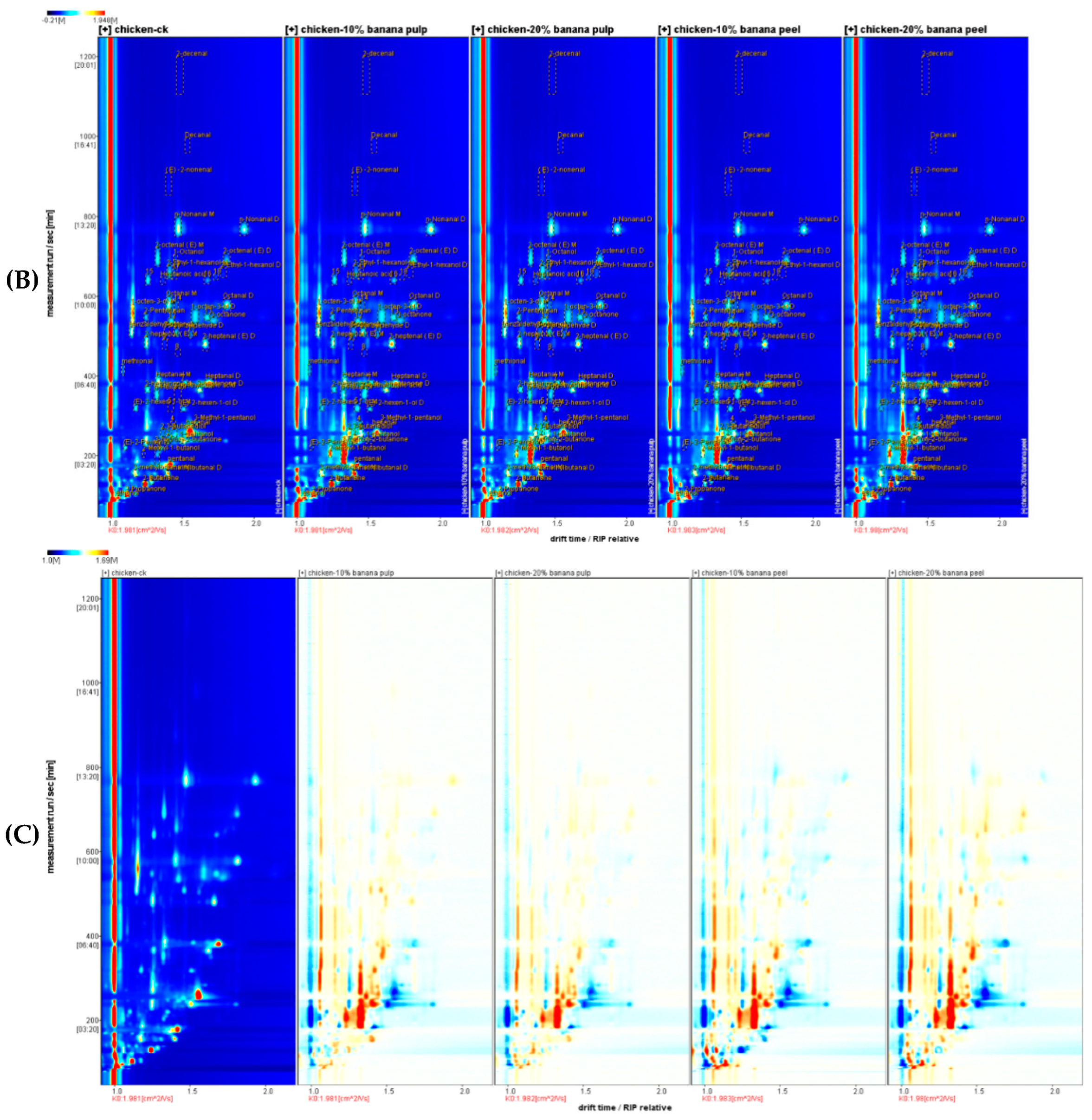
Figure 1B-C shows the two-densional (2D)-spectrum of gas-ion migration of the meat samples. These reactive ion peak in the plots were normalized. Each point on the right of the reactive ion peak represented a flavor compounds in the meat samples. The red points indicated a higher signal intensity, whereas the white spots had a low signal intensity. The signal intensities represented the concentrations of these flavor compounds. The meat sample in the CK group was applied for reference. The ratio of a flavor compound in the meat samples of the supplementary group was the same as the reference ratio, where it was shown as a white spot after the deduction. The red and blue points denoted the ratios higher than the reference value.
As shown in
Figure 1C, most of the signals visualized in the range of retention times between 50 and 800 s and the drift times ranging from 1.0 to 1.8. Flavor compound such as 2-ethylhexanol in the meat samples were remarkably differrent between different groups.
Table 5 shows the qualitative analytical results of flavor compounds in chest muscle samples. Aldehydes were the most important flavor compounds in chicken meat because of their low flavor threshold and influence the overall meat flavor [
35]. Noleau and Toulemonde (1986) [
36] have shown that the removal of aldehydes made chicken meats lost its distinctive flavor, and its aroma became akin to beef. The aldehydes detected in the chest meat samples, such as hexanal, octanal, heptanal, nonanal, trans-2-pentenal, benzaldehyde, trans-2-nonenal, were also detected in chicken meat samples reported in the literature [
37,
38]. The literature also showed that most aldehydes, such as hexanal, octanal, octenal, nonanal, 2, 4-heptadienal, 2-heptenal, heptanal, are derived from lipid oxidation reactions [
39,
40]. Hexanal is the most abundant aldehyde compound in chicken. It is the primary product of linoleic acid oxidation reaction [
37]. It also has delicate smell of grass [
41].
Alcohols and ketones are the other products of lipid metabolism besides aldehydes. The flavor thresholds of alcohol are relatively high, and their contribution to the chicken meat flavor is relatively low. The flavor thresholds of ketones are lower than aldehydes, and their contribution to the meat flavor is lower than aldehydes. Among the alcohols detected in the chicken meat, 1-octene-3-ol was the flavor compound that affected the meat flavor [
38]. Relevant studies have shown that 1-octene-3-ol has a flavor similar to mushrooms [
42]. It is a delicious soup flavor. Ketones are also considered an important flavor of meat products [
43].
Several biochemical pathways are related to the formation of flavor substances, including the Maillard reaction, lipid oxidation, and thiamine degradations [
44]. Maillard reaction is a non-enzymatic browning process that produces pyridine, pyrrole, pyrazine, thiophene, thiazole, and furanone [
37]. Lipid degradation includes oxidation and hydrolysis of esters to form flavor compounds such as alcohols, aldehydes, ketones, esters, and furans [
38,
45]. These lipid oxidation products can also interact with Maillard reaction products to produce new flavor compounds. Thiamine degradation is the degradation of nitrogen-containing and sulfur-containing bicyclic compounds that generate a variety of nitrogen-containing, sulfur-containing, and heterocyclic compounds such as thiophenes, furans, and thiazoles.
3.5. Characteristic Fingerprint of Flavor Compounds in Chicken Chest Meats
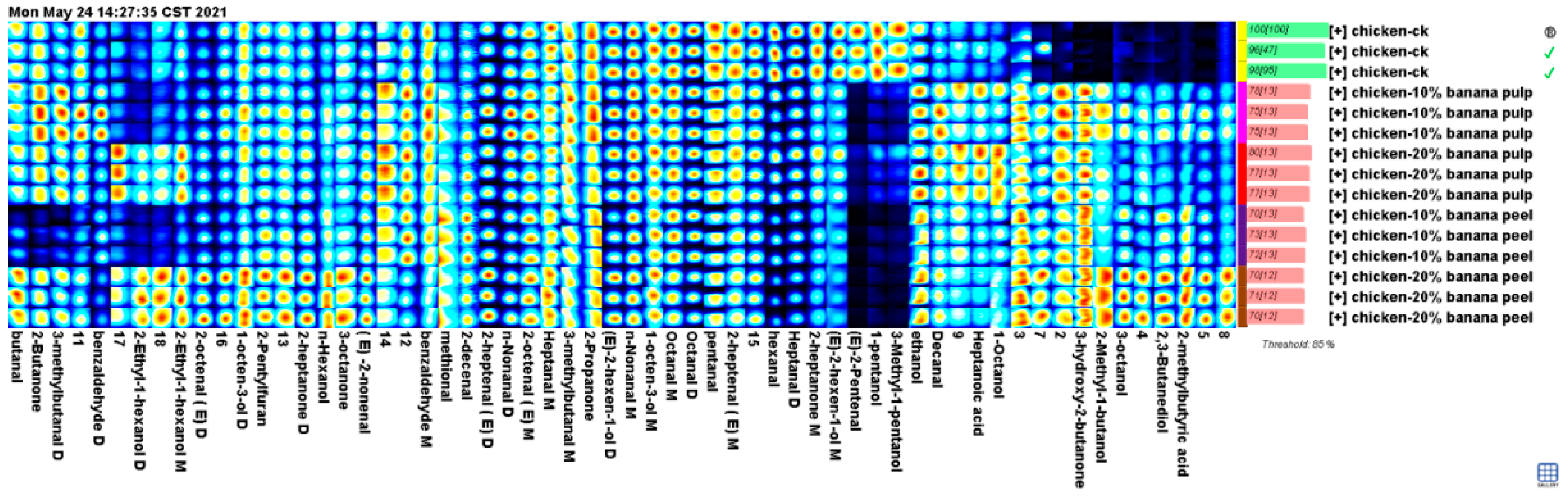
To comprehensively analyze the differences in flavor composition of the chest meat samples, all flavor compound peaks identified in the spectrograms of these samples were selected to form fingerprints (
Figure 2). The results showed that the meat samples in Pu-10, Pu-20, Pe-10, and Pe-20 groups contained some of the unique flavor compounds. They are 3-hydroxy-2-butanone, 2-methylbutyric acid, 3-octanol, 2,3-butanediol, and 2-methyl-1-butanol. The meat samples in the Pe-20 group had the highest levels of these flavor compounds. The ethanol, 1-octanol, decanal, and heptanoic acid levels in the meat samples of the Pu-10, Pu-20, Pe-10, and Pe-20 groups were higher than those in the control group. On the contrary, the levels of pentanal, (E)-2-pentenal, hexanal, heptanal, octanal, 1-pPentanol, 3-methyl-1-pentanol, and (E)-2-hexen-1-ol in the meat samples of the control group were higher than the supplementary groups. However, the levels of these flavor compounds in the meat samples of the supplementary groups varied.
Different amounts of the fermented banana samples added to the poultry feeds also affected the levels of flavor compounds in the meat samples. Hence, butanal, 3-methyl-butanal, (E)-2-heptenal, (E)-2-octenal, (E)-2-nonenal, N-nonanal, n-hexanol, 2-methyl-1-butanol, 2-ethyl-1-hexanol, 1-octen-3-ol, 3-octanol, 2,3-butanediol, 2-butanone, 2-heptanone, 3-octanone, 2-methylbutyric acid, and 2-pentylfuran increased with a higher proportion of fermented banana peel added to the poultry feeds. However, the levels of some other flavor compounds in the meat samples of the chicken fed with 20% fermented banana samples were lower than in the chicken given 10% fermented banana samples.
3.6. Multivariate Analysis of Flavor Compounds in Chicken Chest Meats
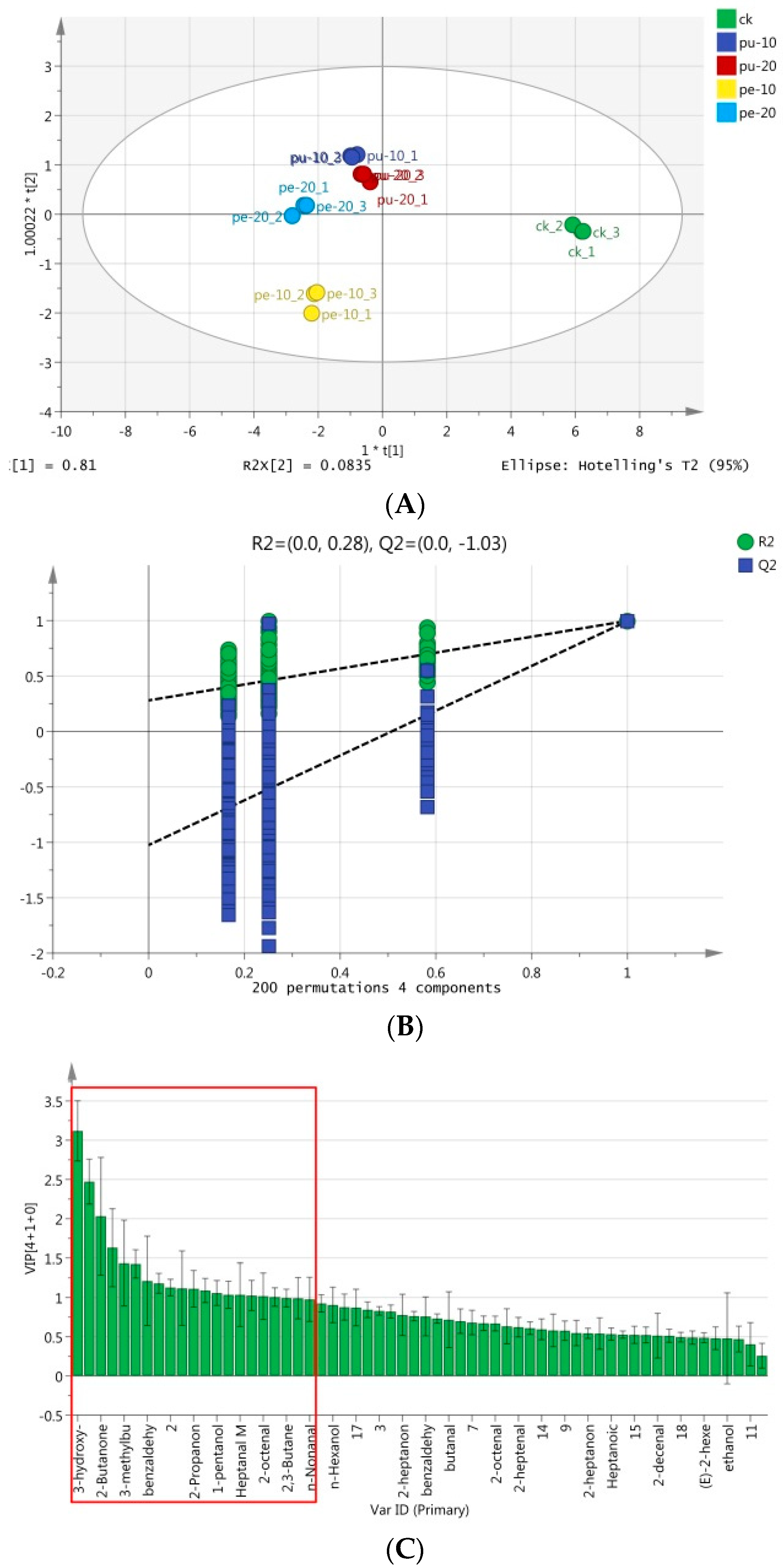
Principal component analysis (PCA) is one of the dimensionality-reduction methods. It was applied to analyze the main chemical components of the chest meat samples. PCA reduced multiple indicators into a smaller numbers of the multivariate data for the best comprehensive simplification of the multivariate statistical analysis [
46]. As shown in
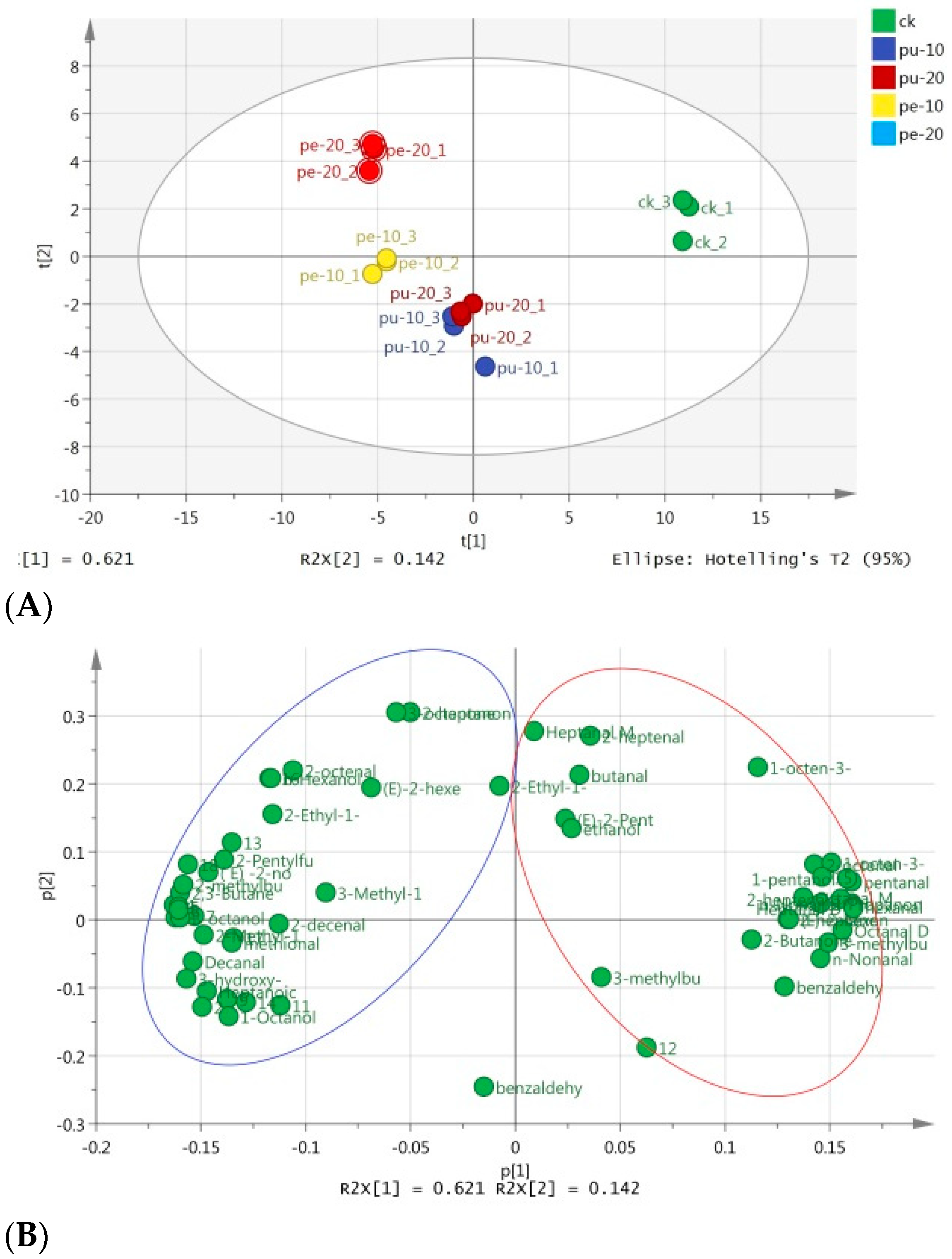
Figure 3A, PC1 and PC2 accounted for 62.1% and 14.2% of the total variances, respectively. The cumulative variance contribution rate of the two principal components is 75.3%. This result shows that the two principal components explained 75.3% of the principal components. It retained most of the original information of the two PCs. Also, the analytical effect of the main components was good.
The trends of flavor composition of the meat samples in the supplementary groups were differed from the control group. Also, the flavor composition and levels of the meat samples in the Pu groups were similar. As depicted in
Figure 3B, the aromatic substances shown on the right side of the PCA loading plot were positively correlated with PC1. A positive PC1 score was measured for these compounds in the CK group. On the contrary, the aromatic substances shown on the left side of the PCA loading plot were negatively correlated with PC1, where these flavor compounds in the Pe groups had negative PC1 scores.
OPLS-DA model was applied in the analysis to further understanding the distribution pattern of these flavor compounds in the meat samples of different experimental groups [
47]. As a supervised multivariate statistical analysis method, OPLS-DA was characterized by removing the data variation in the independent variable X, in which these data were unrelated to the categorical variable Y, and the information was also analyzed as one principal component. Therefore, the model becomes simple and easy to interpret. Its discriminant effect and the visualization effect of the principal component score plot are known. In this study, the OPLS-DA model was used to select the most statistically significant variables based on the data matrix regression modeling of the flavor compound peaks detected in the chest meat samples and then identify the specific marker compounds that caused the difference in flavor.
The peak information of flavor compounds in chest meat samples was initially standardized to remove the unit limitation of the data and then converted into a pure value without dimension. It could achieve the role of the lower peak index without weakening the flavor value and ensuring the result’s reliability. The results of the OPLS-DA analysis of these substances in the meat samples are shown in
Figure 4. The total variance was 89.35%, R2Y = 99.1%, and Q2 = 94.2%. The flavor compounds in meat samples were distributed in three of out the four quadrants, with Pu-10, Pu-20, and Pe-20 groups in the second quadrant (except Pe-20-2), Pe-10 group in the first quadrant, and CK group in the fourth quadrant (
Figure 4A). During the analysis, a permutation test was performed. The experiment data was then randomly rearranged by changing the sort order of the categorical variable (Y), and Q2Y was randomly assigned up to 200 times to validate the model.
Figure 4B shows the results of the permutation test result, and the regression line at the Q2 point intersects the vertical axis below zero, indicating that the discriminant model was not overfitting the data [
48]. Therefore, the initial model was superior to the random arrangement model. The 18 characteristic flavor compounds in different groups were also selected according to the variable projection importance index (VIP) that greater than 1 (
Figure 4C). These substances were 3-hydroxy-2-butanone, hexanal, 2-butanone, 2-ethyl-1-hexanol M, 3-methylbutanal D, heptanal D, benzaldehyde D, 2-methylbutyric acid, (E)-2,2-heptenal D, 2-propanone, pentanal, 1-pentanol, 1-octen-3-ol M, hepatanal M, 2-methyl-1-butanol, (E)-2-octenal D, 2,3-butanediol, and n-nonanal M.
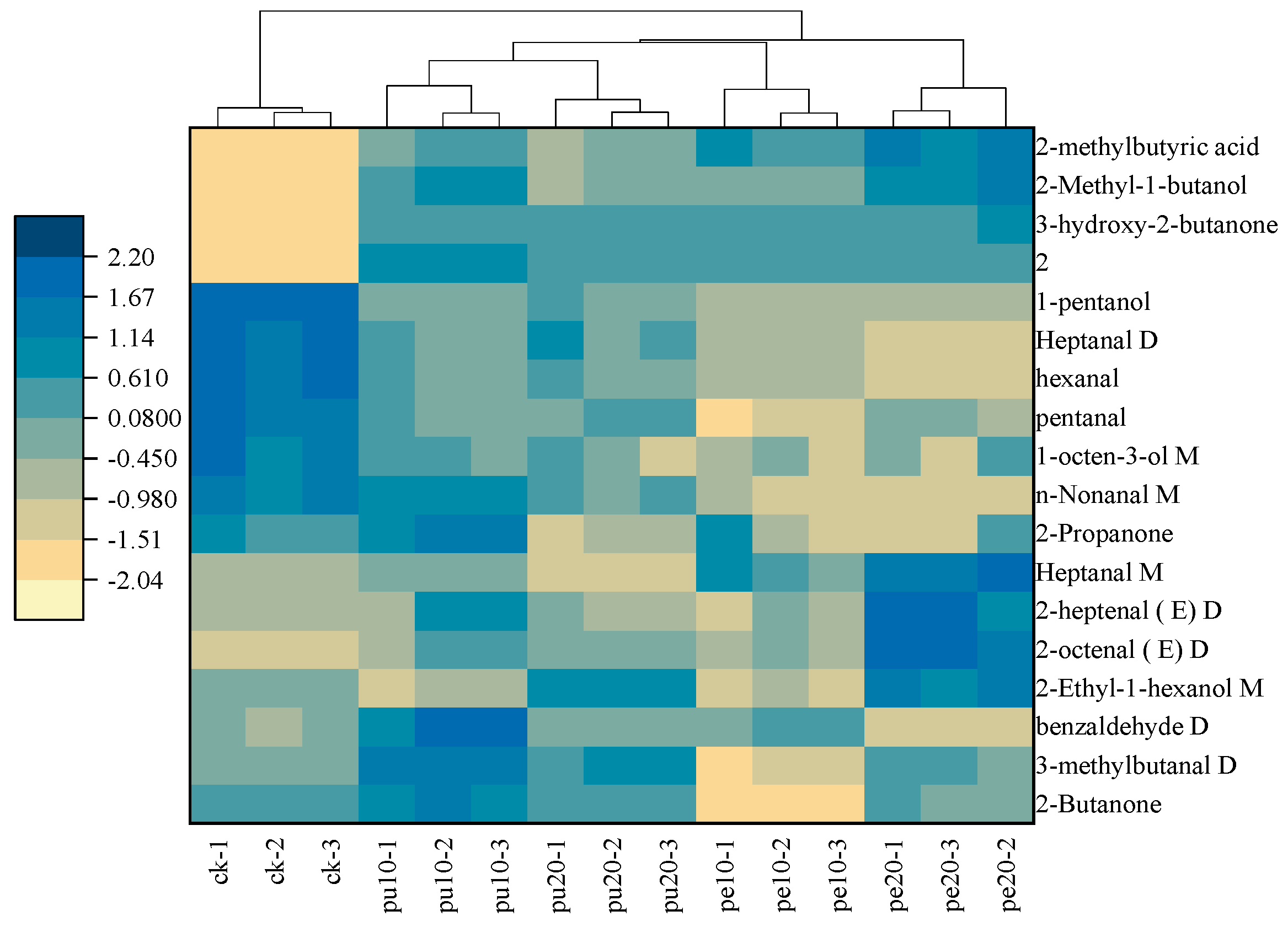
3.7. Cluster Heat Map of Flavor Compounds in Chicken Chest Meats
The OPLS-DA model was used to analyze the characteristic flavor compounds of the chest meat samples. The hierarchical cluster analysis was considered for the 18 characteristic flavor compounds (VIP score >1). The heat map of these flavor substances was presented to show the clustering of the flavor compounds (
Figure 5). The light to dark-colored squares of the heat map indicated the relative levels of characteristic flavor compounds from low to high. The hierarchical cluster analysis (HCA) divided the five sample groups into two categories, where the first category was the control group (CK), and the second category was the supplementary groups (Pu-10, Pu-20, Pe-10, and Pe-20).
The results showed that the relative content of 2-methylbutyric acid, 2-methyl-1-butanol, 3-hydroxy-2-butanone, and compound 2 in the first category was lower than in the second category. However, the relative content of 1-pentanol, heptanal D, hexanal, pentanal, 1-octen-3-ol M, and N-nonanal M in the first category was higher than in the second category. Among them, hexanal and 1-octen-3-ol were the key flavor compounds in the chest meat samples [
38]. The aromas of these meat samples were attributed to the differences in flavor content.
4. Conclusions
The results of this study showed that the addition of different proportions of fermented banana peel and pulp residue in the daily feed increased the carcass weights and the percentages of chicken pectoral and thigh muscles, as well as improved moisture content, FAs, and flavor components of the chest meat samples. In addition, adding different proportions of fermented banana peel and banana residue in daily feed affected the type and content of flavor substances in chicken samples. In conclusion, adding different proportions of fermented banana peel and banana residue in the daily feed could improve the quality of chicken and affect its flavors.
Author Contributions
Zhichun Li: Investigation, Data curation, Writing – original draft, Funding acquisition. Xuemei He: Methodology, Resources, Project administration. Yayuan Tang: Methodology, Investigation. Ping Yi: Methodology, Investigation. Ying Yang: Formal analysis, Investigation. Jiemin Li: Methodology, Investigation. Dongning Ling: Methodology, Investigation. Bo Jie Chen: Software, Resources. Hock Eng Khoo: Methodology, Writing – review & editing. Jian Sun: Conceptualization, Supervision, Funding acquisition.
Funding
This study received financial support from the National Modern Agricultural Technology System Construction Special Fund of China (CARS-31).
Institutional Review Board Statement
In accordance with the ethical standards set forth by the relevant institutional review board, the necessity for ethical review and approval was waived for this study. This decision was made due to the fact that the animal subjects involved in the study were domesticated broiler chicken that were subsequently subjected to the commercial slaughtering procedure.
Data Availability Statement
All data are presented in this manuscript.
Acknowledgments
The authors would like to acknowledge the contributions of the research assistants and technical staffs at the laboratories of the Guangxi Academy of Agricultural Sciences and Guangxi State Farm Jinguang Dairy Co., Ltd., Nanning, China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- National Bureau of Statistics. China Statistical Yearbook. Peking: China Statistics Press. 2021.
- Oberoi, H.S.; Sandhu, S.K.; Vadlani, P.V. Statistical optimization of hydrolysis process for banana peels using cellulolytic and pectinolytic enzymes. Food Bioproc Tech, 2012, 90, 257–265. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Irfan, S.; Nadeem, M.; Mahmood, S. A comprehensive review on nutritional value, medicinal uses, and processing of banana. Food Rev. Int. 2022, 38, 199–225. [Google Scholar] [CrossRef]
- Shankar, G.; Jeevitha, P.; Shadeesh, L. Nutritional analysis of Musa acuminata. Res Rev J Food Dairy Technol, 2017, 5, 27–29. [Google Scholar]
- Wachirasiri, P.; Julakarangka, S.; Wanlapa, S. The effects of banana peel preparations on the properties of banana peel dietary fibre concentrate. Warasan Songkhla Nakharin 2009, 31, 605–611. [Google Scholar]
- Khamsucharit, P.; Laohaphatanalert, K.; Gavinlertvatana, P.; Sriroth, K.; Sangseethong, K. Characterization of pectin extracted from banana peels of different varieties. Food Sci. Biotechnol. 2018, 27, 623–629. [Google Scholar] [CrossRef]
- Álvarez, S.; Méndez, P.; Martínez-Fernández, A. Fermentative and nutritive quality of banana by-product silage for goats. J. Appl. Anim. Res. 2015, 43, 396–401. [Google Scholar] [CrossRef]
- Rigueira, J.P. S.; de Jesus, N.G.; Júnior, V.R. R.; Monção, F.P.; Costa, N.M.; David, G.S. S.; e Silva, F.V.; da Cunha Siqueira Carvalho, C. Effects of different banana crop wastes on nutrient intake and digestibility, microbial protein synthesis, feeding behavior, and animal performance of ¾ Holstein× Zebu heifers in a semiarid rangeland. Trop Anim Health Prod, 2021, 53, 1–13. [Google Scholar] [CrossRef]
- Caicedo, W.; Vargas, J.C.; Uvidia, H.; Samaniego, E.; Valle, S.; Flores, L.; Moyano, J.; Aguiar, S. Physicochemical, biological and organoleptic indicators in banana silage (Musa sapientum) for pig feeding. Cuba. J. Agric. Sci. 2017, 51. [Google Scholar]
- Aisyah, A.; Gustiningrum, A.S.; Al-Arif, M.A. Substitution of commercial feed with fermented banana peel flour (Musaceaea sp.) and fish meal to feed consumption level, specific growth rate, feed efficiency, fat retention, and energy retention in siam catfish (Pangasius hypophthalmus). IOP Conf. Ser. Earth Environ. Sci. 2021; 679. [Google Scholar] [CrossRef]
- Zhang, Y.; Nie, L.; Sun, J.; Hong, Y.; Yan, H.; Li, M.; You, X.; Zhu, L.; Fang, F. Impacts of environmental factors on pasting properties of cassava flour mediated by its macronutrients. Front. Nutr. 2020, 7, 598960. [Google Scholar] [CrossRef]
- Tang, Y.; Sheng, J.; He, X.; Sun, J.; Wei, Z.; Liu, G.; Li, C.; Lin, B.; Li, L. Novel antioxidant and hypoglycemic water-soluble polysaccharides from jasmine tea. Foods 2021, 10, 2375. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Y.; Wei, Z.; Deng, Z.; Li, Z.; Li, L.; He, X.; Sun, J. Study on quality change and processing suitability evaluation of the low-temperature vacuum frying of bananas. Foods 2023, 12, 1822. [Google Scholar] [CrossRef]
- Wen, L.; Lin, S.; Zhu, Q.; Wu, D.; Jiang, Y.; Zhao, M.; Sun, J.; Luo, D.; Zeng, S.; Yang, B. Analysis of Chinese olive cultivars difference by the structural characteristics of oligosaccharides. Food Anal. Methods 2013, 6, 1529–1536. [Google Scholar] [CrossRef]
- Deng, S.; Liu, R.; Li, C.; Xu, X.; Zhou, G. Meat quality and flavor compounds of soft-boiled chickens: effect of Chinese yellow-feathered chicken breed and slaughter age. Poult. Sci. 2022, 101, 102168. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, S.; Xiao, J.; Hu, Q.; Zhao, L. A Rapid and multi-element method for the determination of As, Cd, Ni, Pb, Sn, and Zn in scallops using high definition X-ray fluorescence (HDXRF) spectrometry. Food Anal. Methods 2022, 15, 2712–2724. [Google Scholar] [CrossRef]
- Li, Z.; Sun, J.; Wei, J.; Khoo, H.E.; Wei, Z.; Mo, R. Growth performance and beef quality of Xinjiang brown cattle fed with different dosages of selenized yeast. Sci. Asia 2021, 47. [Google Scholar] [CrossRef]
- Jin, S.; Pang, Q.; Yang, H.; Diao, X.; Shan, A.; Feng, X. Effects of dietary resveratrol supplementation on the chemical composition, oxidative stability and meat quality of ducks (Anas platyrhynchos). Food Chem. 2021, 363, 130263. [Google Scholar] [CrossRef]
- Wang, T.; Li, J.; Shao, Y.; Yao, W.; Xia, J.; He, Q.; Huang, F. The effect of dietary garcinol supplementation on oxidative stability, muscle postmortem glycolysis and meat quality in pigs. Meat Sci. 2020, 161, 107998. [Google Scholar] [CrossRef]
- Fan, X.; Jiao, X.; Liu, J.; Jia, M.; Blanchard, C.; Zhou, Z. Characterizing the volatile compounds of different sorghum cultivars by both GC-MS and HS-GC-IMS. Food Res. Int. 2021, 140, 109975. [Google Scholar] [CrossRef]
- Nardoia, M.; Romero, C.; Brenes, A.; Arija, I.; Viveros, A.; Ruiz-Capillas, C.; Chamorro, S. Addition of fermented and unfermented grape skin in broilers’ diets: Effect on digestion, growth performance, intestinal microbiota and oxidative stability of meat. Animal 2020, 14, 1371–1381. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, X.; Yao, X.; Zhang, H.; Song, Z.; He, X.; Cao, R. Effects of feeding fermented mulberry leaf powder on growth performance, slaughter performance, and meat quality in chicken broilers. Animals 2021, 11, 3294. [Google Scholar] [CrossRef]
- Teteh, A.; Lawson, E.; Tona, K.; Decuypere, E.; Gbeassor, M. Moringa oleifera leave: hydro-alcoholic extract and effects on growth performance of broilers. Int. J. Poult. Sci. 2013, 12, 401–405. [Google Scholar] [CrossRef]
- Xu, H.; Qiao, P.; Pan, J.; Qin, Z.; Li, X.; Khoo, H.E.; Dong, X. CaCl2 treatment effectively delays postharvest senescence of passion fruit. Food Chem. 2023, 417, 135786. [Google Scholar] [CrossRef] [PubMed]
- Chatkitanan, T.; Harnkarnsujarit, N. Development of nitrite compounded starch-based films to improve color and quality of vacuum-packaged pork. Food Packag. Shelf Life 2020, 25, 100521. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, Y.; Cheng, Q.; Xv, J.; Hou, Y.; Wu, X.; Du, E.; Ding, B. Partial substitution of fermented soybean meal for soybean meal influences the carcass traits and meat quality of broiler chickens. Animals 2020, 10, 225. [Google Scholar] [CrossRef]
- Hughes, J.M.; Oiseth, S.K.; Purslow, P.P.; Warner, R.D. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef]
- Popova, T. Effect of probiotics in poultry for improving meat quality. Curr. Opin. Food Sci. 2017, 14, 72–77. [Google Scholar] [CrossRef]
- Azimah, R.; Azrina, A.; Khoo, H.E. Stability of blended palm oils during potato frying. Int Food Res, J. 2017, 24. [Google Scholar]
- Schindler, S.; Krings, U.; Berger, R.G.; Orlien, V. Aroma development in high pressure treated beef and chicken meat compared to raw and heat treated. Meat Sci. 2010, 86, 317–323. [Google Scholar] [CrossRef]
- Mancinelli, A.C.; Silletti, E.; Mattioli, S.; Dal Bosco, A.; Sebastiani, B.; Menchetti, L.; Koot, A.; Ruth, S.V.; Castellini, C. Fatty acid profile, oxidative status, and content of volatile organic compounds in raw and cooked meat of different chicken strains. Poult. Sci. 2021, 100, 1273–1282. [Google Scholar] [CrossRef]
- Aziza, A.E.; Quezada, N.; Cherian, G. Antioxidative effect of dietary Camelina meal in fresh, stored, or cooked broiler chicken meat. Poult. Sci. 2010, 89, 2711–2718. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.R.; Gokulakrishnan, P.; Giriprasad, R.; Yatoo, M.A. Fruit-based natural antioxidants in meat and meat products: A review. Crit Rev Food Sci Nutr. 2015, 55, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, H.; Tang, J.; Huang, M.; Zhao, J.; Zhang, J. Influence of partial replacement of NaCl with KCl on formation of volatile compounds in Jinhua ham during processing. Food Sci. Biotechnol. 2016, 25, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Noleau, I.; Toulemonde, B. Quantitative study of roasted chicken flavour. LWT 1986, 19, 122–125. [Google Scholar]
- Shahidi, F.; Hossain, A. Role of lipids in food flavor generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- Zhao, W.H.; Wang, G.Y.; Xun, W.; Yu, Y.R.; Ge, C.R.; Liao, G.Z. Characterisation of volatile flavour compounds in Chinese Chahua chicken meat using a spectroscopy-based non-targeted metabolomics approach. Int Food Res, J. 2021, 28, 763–779. [Google Scholar] [CrossRef]
- Ansorena, D.; Gimeno, O.; Astiasaran, I.; Bello, J. Analysis of volatile compounds by GC–MS of a dry fermented sausage: Chorizo de Pamplona. Food Res. Int. 2001, 34, 67–75. [Google Scholar] [CrossRef]
- Marco, A.; Navarro, J.L.; Flores, M. The influence of nitrite and nitrate on microbial, chemical and sensory parameters of slow dry fermented sausage. Meat Sci. 2006, 73, 660–673. [Google Scholar] [CrossRef]
- Zhang, C.; Ao, Z.; Chui, W.; Shen, C.; Tao, W.; Zhang, S. Characterization of the aroma-active compounds in Daqu: a tradition Chinese liquor starter. Eur. Food Res. Technol. 2012, 234, 69–76. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, H.; Wu, Q.; Hao, L.; Pan, D.; Dang, Y. The flavor quality of dried Lentinus edodes with different species and drying methods (charcoal roasting and naturally drying). J. Food Meas. Charact. 2020, 14, 613–622. [Google Scholar] [CrossRef]
- Purrinos, L.; Bermúdez, R.; Franco, D.; Carballo, J.; Lorenzo, J.M. Development of volatile compounds during the manufacture of dry-cured “Lacón,” a Spanish traditional meat product. J. Food Sci. 2011, 76, C89–C97. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; You, L.; Ji, C.; Zhang, T.; Wang, Y.; Geng, D.; Gao, S.; Bi, Y.; Luo, R. Formation of volatile flavor compounds, maillard reaction products and potentially hazard substance in China stir-frying beef sao zi. Food Res. Int. 2022, 159, 111545. [Google Scholar] [CrossRef]
- Sun, L.; Chen, J.; Li, M.; Liu, Y.; Zhao, G. Effect of star anise (Illicium verum) on the volatile compounds of Stewed chicken. J. Food Process Eng. 2014, 37, 131–145. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.L.; Mitchell, J.P. A comparison of soil quality indexing methods for vegetable production systems in Northern California. Agric Ecosyst Environ 2002, 90, 25–45. [Google Scholar] [CrossRef]
- Wang, X.; Fang, C.; He, J.; Dai, Q.; Fang, R. Comparison of the meat metabolite composition of Linwu and Pekin ducks using 600 MHz 1H nuclear magnetic resonance spectroscopy. Poult. Sci. 2017, 96, 192–199. [Google Scholar] [CrossRef]
- He, F.; Duan, J.; Zhao, J.; Li, H.; Sun, J.; Huang, M.; Sun, B. Different distillation stages Baijiu classification by temperature-programmed headspace-gas chromatography-ion mobility spectrometry and gas chromatography-olfactometry-mass spectrometry combined with chemometric strategies. Food Chem. 2021, 365, 130430. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).