Submitted:
20 September 2024
Posted:
23 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
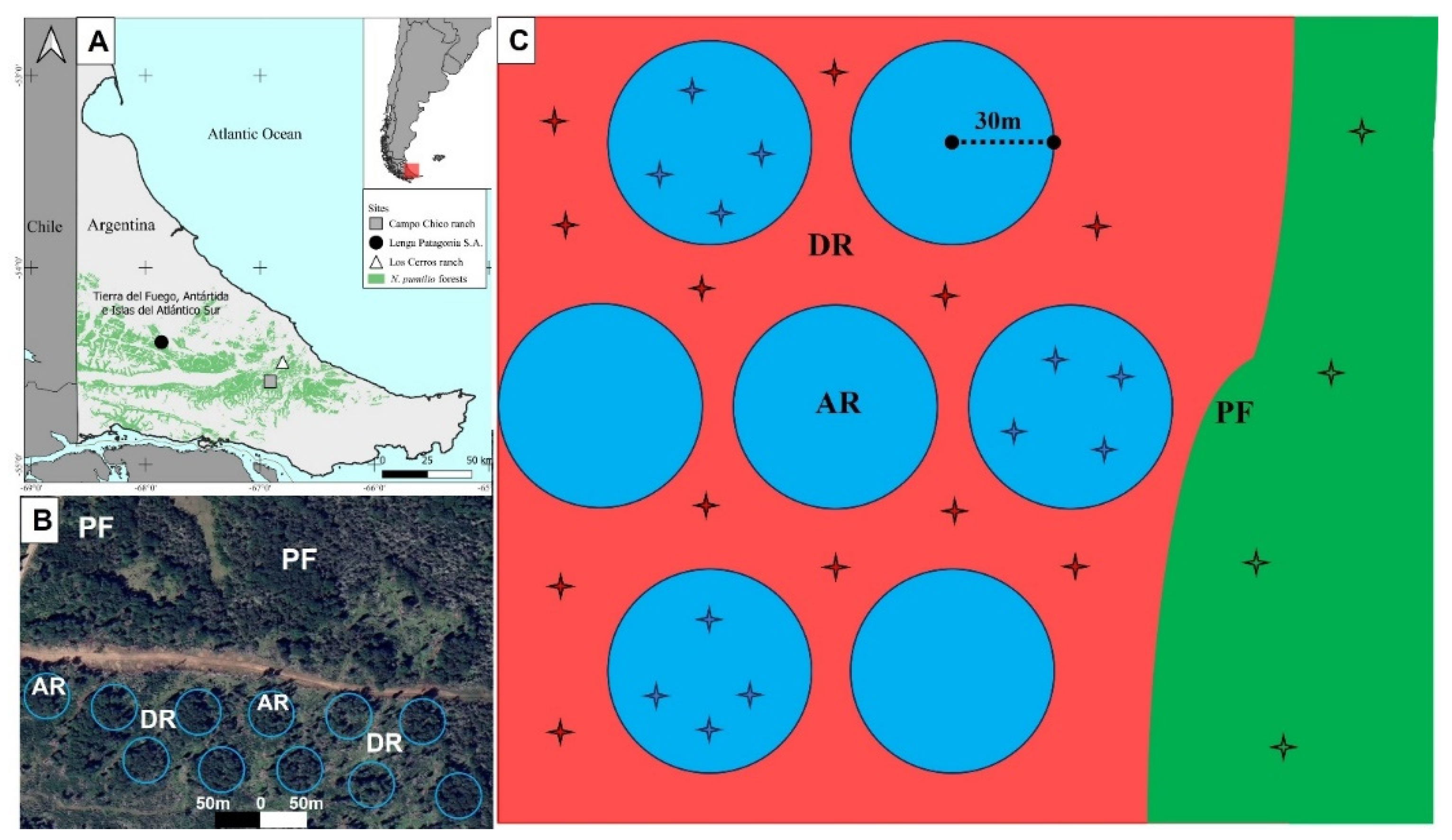
2.1. Study Area and Experimental Design
2.2. Characterization of Environmental and Vegetation Variables
2.3. Soil Microbial Biomass and Activity
2.4. Statistical Analyses
3. Results
3.1. Environmental and Vegetation Variables of the Studied Forest Areas
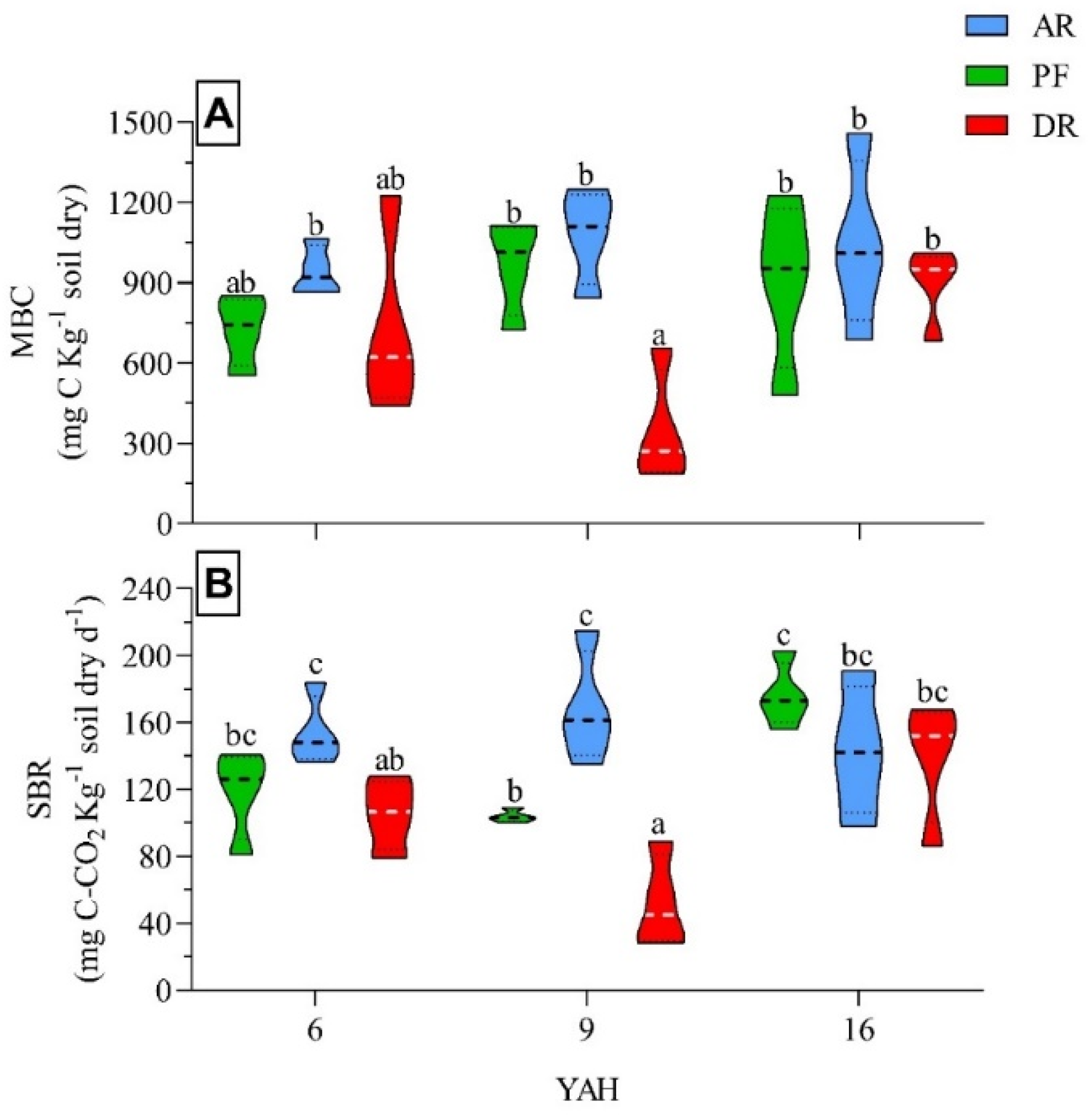
3.2. Response of Soil Microbial Variables to Retention Types and Years-after-Harvesting
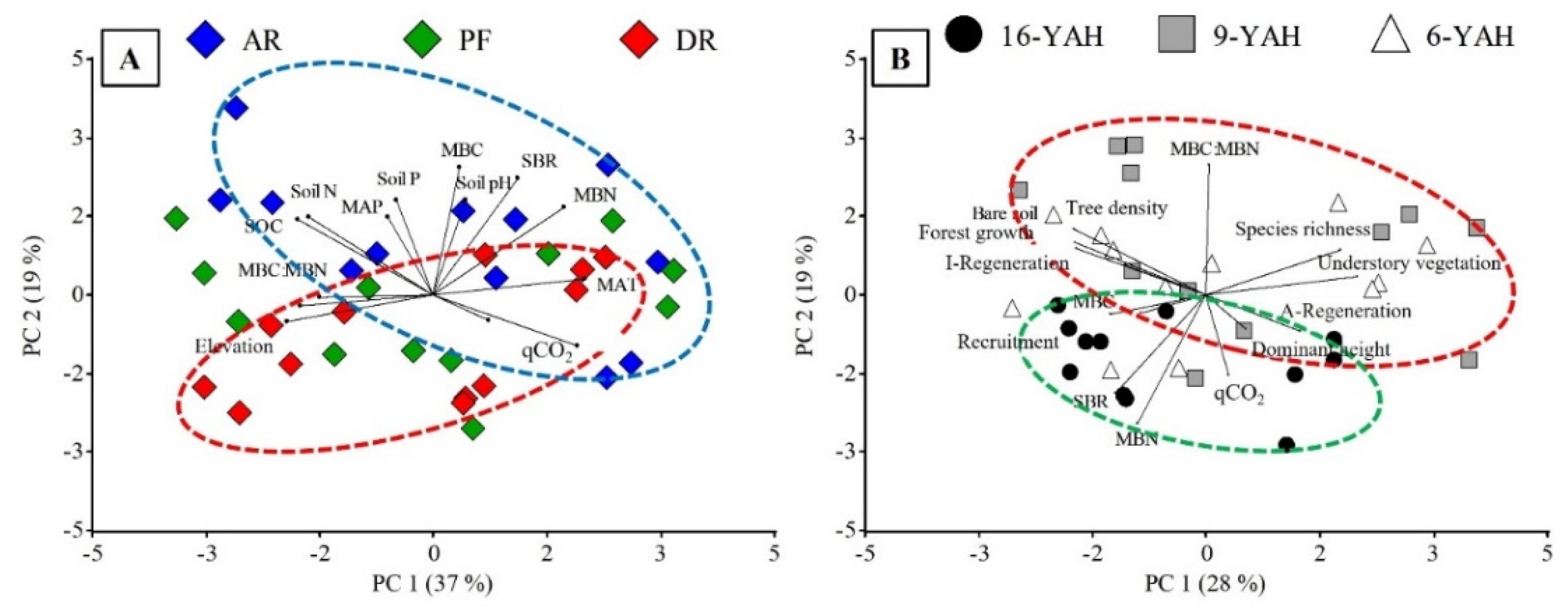
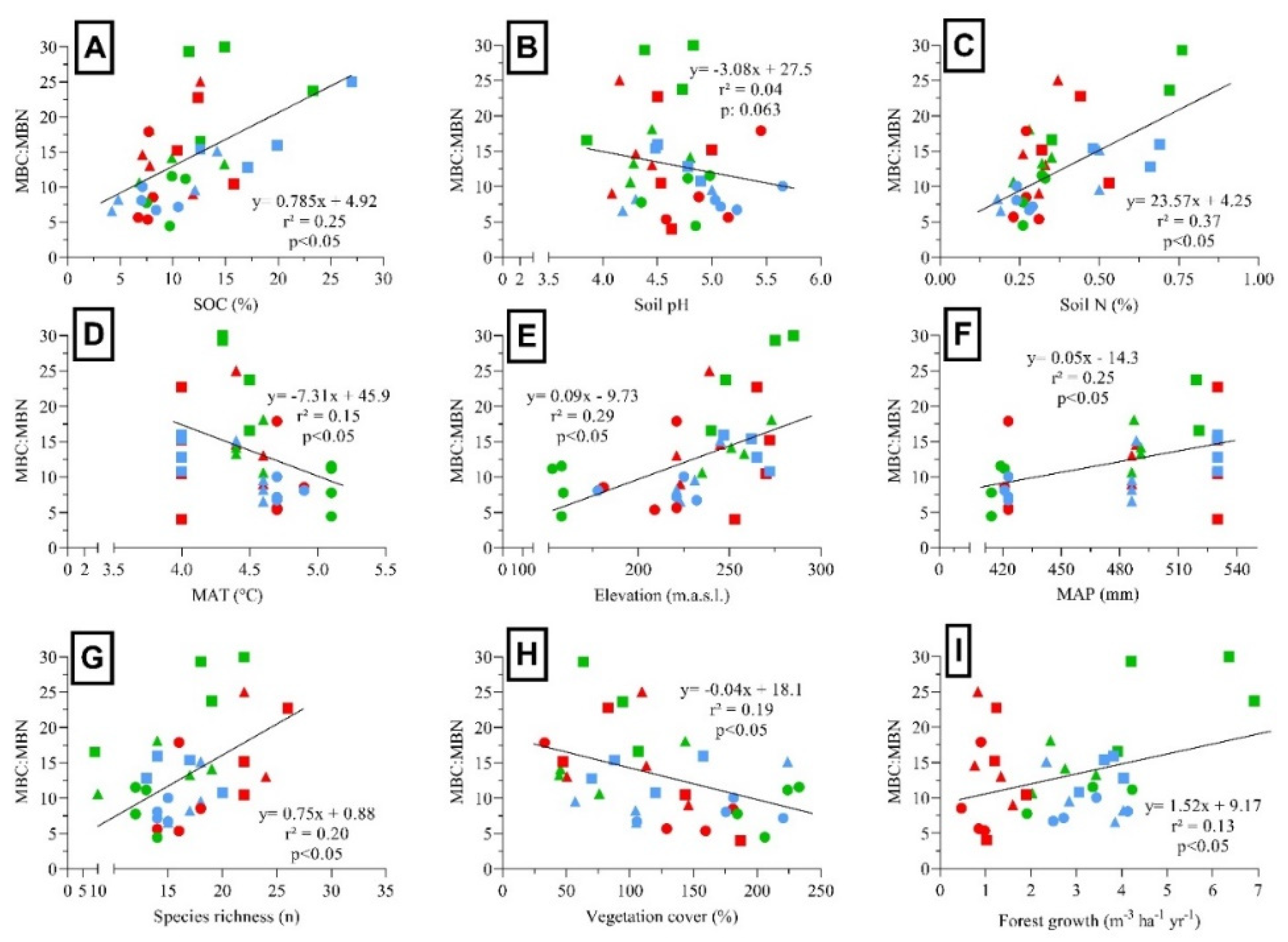
3.3. Relationships of Soil Microbial Variables with Environmental Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendixes
References
- Peri, P.L.; Martínez Pastur, G.; Schlichter, T. Uso sustentable del bosque: Aportes desde la silvicultura Argentina; Ministerio de Ambiente y Desarrollo Sostenible de la Nación Argentina: Buenos Aires, Argentina, 2021. http://hdl.handle.net/20.500.12123/10343.
- Soler, R.M.; Lorenzo, C.; González, J.; Carboni, L.; Delgado, J.; Díaz, M.; Huertas Herrera, A. The politics behind scientific knowledge: Sustainable forest management in Latin America. For. Pol. Econ. 2021, 131, e102543. [CrossRef]
- Muys, B. Forest ecosystem services. In Life on land: Encyclopedia of the UN Sustainable Development Goals; Leal Filho, W., Azul, A.M., Brandli, L., Lange Salvia, A., Wall, T., Eds.; Springer: Cham, Switzerland, 2021. [CrossRef]
- Peri, P.L.; Martínez Pastur, G.; Nahuelhual, L. Ecosystem services in Patagonia: A multi-criteria approach for an integrated assessment; Springer Nature: Charm, Switzerland, 2021. [CrossRef]
- Lencinas, M.V.; Martínez Pastur, G.; Gallo, E.; Cellini, J.M. Alternative silvicultural practices with variable retention to improve understory plant diversity conservation in southern Patagonian forests. For. Ecol. Manage. 2011, 262, 1236-1250. [CrossRef]
- Martínez Pastur, G.; Cellini, J.M.; Lencinas, M.V.; Barrera, M.; Peri, P.L. Environmental variables influencing regeneration of Nothofagus pumilio in a system with combined aggregated and dispersed retention. For. Ecol. Manage. 2011, 261, 178-186. [CrossRef]
- Gallo, E.A.; Lencinas, M.V.; Martínez Pastur, G. Site quality influences over understory plant diversity in old-growth and harvested Nothofagus pumilio forests. For. Syst. 2013, 22, 25-38. [CrossRef]
- Soler, R.M.; Schindler, S.; Lencinas, M.V.; Peri, P.L.; Martínez Pastur, G. Why biodiversity increases after variable retention harvesting: A meta-analysis for southern Patagonian forests. For. Ecol. Manage. 2016, 369, 161-169. [CrossRef]
- Lencinas, M.V.; Sola, F.J.; Martínez Pastur, G. Variable retention effects on vascular plants and beetles along a regional gradient in Nothofagus pumilio forests. For. Ecol. Manage. 2017, 406, 251-265. [CrossRef]
- Huertas Herrera, A.; Cellini, J.M.; Barrera, M.D.; Lencinas, M.V.; Martínez Pastur, G. Environment and anthropogenic impacts as main drivers of plant assemblages in forest mountain landscapes of Southern Patagonia. For. Ecol. Manage. 2018, 430, 380-393. [CrossRef]
- Toro Manríquez, M.D.; Cellini, J.M.; Lencinas, M.V.; Peri, P.L.; Peña Rojas, K.; Martínez Pastur, G. Suitable conditions for natural regeneration in variable retention harvesting of southern Patagonian Nothofagus pumilio forests. Ecol. Processes 2019, 8, 1-12. [CrossRef]
- Foote, J.A.; Boutton, T.W.; Scott, D.A. Soil C and N storage and microbial biomass in US southern pine forests: Influence of forest management. For. Ecol. Manage. 2015, 355, 48-57. [CrossRef]
- Lewandowski, T.E.; Forrester, J.A.; Mladenoff, D.J.; D’Amato, A.W.; Palik, B.J. Response of the soil microbial community and soil nutrient bioavailability to biomass harvesting and reserve tree retention in northern Minnesota aspen-dominated forests. Appl. Soil Ecol. 2016, 99, 110-117. [CrossRef]
- Lewandowski, T.E.; Forrester, J.A.; Mladenoff, D.J.; D’Amato, A.W.; Fassnacht, D.S.; Padley, E.; Martin, K.J. Do biological legacies moderate the effects of forest harvesting on soil microbial community composition and soil respiration. For. Ecol. Manage. 2019, 432, 298-308. [CrossRef]
- Singh, S.B.; Saha, S.; Dutta, S.K.; Singh, A.R.; Boopathi, T. Impact of secondary forest fallow period on soil microbial biomass carbon and enzyme activity dynamics under shifting cultivation in North Eastern Hill region, India. Catena 2017, 156, 10-17. [CrossRef]
- Churchland, C.; Bengtson, P.; Prescott, C.E.; Grayston, S.J. Dispersed variable-retention harvesting mitigates N losses on harvested sites in conjunction with changes in soil microbial community structure. Front. For. Glob. Change 2021, 3, e609216. [CrossRef]
- Zak, D.R.; Holmes, W.E.; White, D.C.; Peacock, A.D.; Tilman, D. Plant diversity, soil microbial communities, and ecosystem function: are there any links? Ecology 2003, 84(8), 2042-2050. [CrossRef]
- Li, F.; Zi, H.; Sonne, C.; Li, X. Microbiome sustains forest ecosystem functions across hierarchical scales. Eco-Environ. Health 2023, 2(1), 24-31. [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192-211. [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54(4), 655-670. [CrossRef]
- Bardgett, R.D.; Freeman, C.; Ostle, N.J. Microbial contributions to climate change through carbon cycle feedbacks. ISME J. 2008, 2(8), 805-814. [CrossRef]
- Manzoni, S.; Porporato, A. Soil carbon and nitrogen mineralization: Theory and models across scales. Soil Biol. Biochem. 2009, 41(7), 1355-1379. [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Comm. 2016, 7, e10541. [CrossRef]
- Gargaglione, V.; Gonzalez Polo, M.; Birgi, J.; Toledo, S.; Peri, P.L. Silvopastoral use of Nothofagus antarctica forests in Patagonia: Impact on soil microorganisms. Agrofor. Syst. 2022, 96(7), 957-968. [CrossRef]
- Marcos, M.S.; Olivera, N.L. Microbiological and biochemical indicators for assessing soil quality in drylands from Patagonia. In Biology and biotechnology of Patagonian microorganisms; Olivera, N., Libkind, D., Donati, E., Eds.; Springer: Cham, Switzerland, 2016. [CrossRef]
- Schloter, M.; Nannipieri, P.; Sørensen, S.J.; van Elsas, J.D. Microbial indicators for soil quality. Biol. Fert. Soils 2018, 54(1), 1-10. [CrossRef]
- Fierer, N.; Wood, S.A.; de Mesquita, C.P.B. How microbes can, and cannot, be used to assess soil health. Soil Biol. Biochem. 2021, 153, e108111. [CrossRef]
- Siira-Pietikäinen, A.; Pietikäinen, J.; Fritze, H.; Haimi, J. Short-term responses of soil decomposer communities to forest management: Clear felling versus alternative forest harvesting methods. Can. J. For. Res. 2001, 31(1), 88-99. [CrossRef]
- Hannam, K.D.; Quideau, S.A.; Kishchuk, B.E. Forest floor microbial communities in relation to stand composition and timber harvesting in northern Alberta. Soil Biol. Biochem. 2006, 38(9), 2565-2575. [CrossRef]
- Lindo, Z.; Visser, S. Microbial biomass, nitrogen and phosphorus mineralization, and mesofauna in boreal conifer and deciduous forest floors following partial and clear-cut harvesting. Can. J. For. Res. 2003, 33(9), 1610-1620. [CrossRef]
- Jones, M.D.; Twieg, B.D.; Durall, D.M.; Berch, S.M. Location relative to a retention patch affects the ECM fungal community more than patch size in the first season after timber harvesting on Vancouver Island, British Columbia. For. Ecol. Manage. 2008, 255(3-4), 1342-1352. [CrossRef]
- Hartmann, M.; Lee, S.; Hallam, S.J.; Mohn, W.W. Bacterial, archaeal and eukaryal community structures throughout soil horizons of harvested and naturally disturbed forest stands. Environ. Microbiol. 2009, 11(12), 3045-3062. [CrossRef]
- Kranabetter, J.M.; De Montigny, L.; Ross, G. Effectiveness of green-tree retention in the conservation of ectomycorrhizal fungi. Fungal Ecol. 2013, 6(5), 430-438. [CrossRef]
- Philpott, T.J.; Barker, J.S.; Prescott, C.E.; Grayston, S.J. Limited effects of variable-retention harvesting on fungal communities decomposing fine roots in coastal temperate rainforests. Appl. Environ. Microbiol. 2018, 84(3), e02061-17. [CrossRef]
- Sultaire, S.M.; Benucci, G.; Longley, R.; Kroll, A.; Verschuyl, J.; Bonito, G.; Roloff, G. Using high-throughput sequencing to investigate summer truffle consumption by chipmunks in relation to retention forestry. For. Ecol. Manage. 2023, 549, e121460. [CrossRef]
- Martínez Pastur, G.; Cellini, J.M.; Peri, P.L.; Vukasovic, R.; Fernández, M.C. Timber production of Nothofagus pumilio forests by a shelterwood system in Tierra del Fuego (Argentina). For. Ecol. Manage. 2000, 134(1-3), 153-162. [CrossRef]
- Martínez Pastur, G.; Lencinas, M.V.; Cellini, J.M.; Peri, P.L.; Soler, R. Timber management with variable retention in Nothofagus pumilio forests of Southern Patagonia. For. Ecol. Manage. 2009, 258, 436-443. [CrossRef]
- Gea Izquierdo, G.; Martínez Pastur, G.; Cellini, J.M.; Lencinas, M.V. Forty years of silvicultural management in southern Nothofagus pumilio (Poepp. et Endl.) Krasser primary forests. For. Ecol. Manage. 2004, 201(2-3), 335-347. [CrossRef]
- Martínez Pastur, G.; Peri, P.L.; Fernández, M.C.; Staffieri, G.; Rodríguez, D. Desarrollo de la regeneración a lo largo del ciclo del manejo forestal de un bosque de Nothofagus pumilio: Incidencia de la cobertura y el aprovechamiento o cosecha. Bosque 1999, 20(2), 39-46. [CrossRef]
- Rosenfeld, J.M.; Cerrillo, R.N.; Alvarez, J.G. Regeneration of Nothofagus pumilio (Poepp. et Endl.) Krasser forests after five years of seed tree cutting. J. Environ. Manage. 2006, 78(1), 44-51. [CrossRef]
- Franklin, J.F.; Berg, D.R.; Thornburgh, D.A.; Tappeiner, J.C. Alternative silvicultural approaches to timber harvesting: Variable retention systems. In Creating a forestry for the 21st century: The science of ecosystem management; Kohm, K.A., Franklin, J.F., Eds.; Island Press: Washington, USA, 1997; pp. 111-139.
- Mitchell, S.J.; Beese, W.J. The retention system: Reconciling variable retention with the principles of silvicultural systems. For. Chron. 2002, 78(3), 397-403. [CrossRef]
- Elíades, L.A.; Cabello, M.N.; Pancotto, V.; Moretto, A.; Ferreri, N.A.; Saparrat, M.C.; Barrera, M.D. Soil mycobiota under managed and unmanaged forests of Nothofagus pumilio in Tierra del Fuego, Argentina. NZ J. For. Sci. 2019, 49, e7. [CrossRef]
- Martínez Pastur, G.; Vanha-Majamaa, I.; Franklin, J.F. Ecological perspectives on variable retention forestry. Ecol. Processes 2020, 9, 1-6. [CrossRef]
- Chaves, J.E.; Aravena Acuña, M.C.; Rodríguez-Souilla, J.; Cellini, J.M.; Rappa, N.J.; Lencinas, M.V.; Martínez Pastur, G. Carbon pool dynamics after variable retention harvesting in Nothofagus pumilio forests of Tierra del Fuego. Ecol. Processes 2023, 12(1), e5. [CrossRef]
- Jerabkova, L.; Prescott, C.E.; Titus, B.D.; Hope, G.D.; Walters, M.B. A meta-analysis of the effects of clearcut and variable-retention harvesting on soil nitrogen fluxes in boreal and temperate forests. Can. J. For. Res. 2011, 41(9), 1852-1870. [CrossRef]
- Gustafsson, L.; Baker, S.C.; Bauhus, J.; Beese, W.J.; Brodie, A.; Kouki, J.; Lindenmayer, D.B.; Lõhmus, A.; Martínez Pastur, G.; Messier, C.; Neyland, M.; Palik, B.; Sverdrup-Thygeson, A.; Volney, A.; Wayne, A.; Franklin, J.F. Retention forestry to maintain multifunctional forests: A world perspective. BioScience 2012, 62(7), 633-645. [CrossRef]
- Martínez Pastur, G.; Aravena Acuña, M.C.; Chaves, J.E.; Cellini, J.M.; Silveira, E.M.; Rodríguez-Souilla, J.; Peri, P.L. Nitrogenous and phosphorus soil contents in Tierra del Fuego forests: Relationships with soil organic carbon, climate, vegetation and landscape metrics. Land 2023, 12(5), e983. [CrossRef]
- Paterson, E. Importance of rhizodeposition in the coupling of plant and microbial productivity. Eur. J. Soil Sci. 2003, 54(4), 741-750. [CrossRef]
- Luoma, D.L.; Stockdale, C.A.; Molina, R.; Eberhart, J.L. The spatial influence of Pseudotsuga menziesii retention trees on ectomycorrhiza diversity. Can. J. For. Res. 2006, 36(10), 2561-2573. [CrossRef]
- Bach, L.H.; Grytnes, J.A.; Halvorsen, R.; Ohlson, M. Tree influence on soil microbial community structure. Soil Biol. Biochem. 2010, 42(11), 1934-1943. [CrossRef]
- Mitchell, R.J.; Campbell, C.D.; Chapman, S.J.; Cameron, C.M. The ecological engineering impact of a single tree species on the soil microbial community. J. Ecol. 2010, 98(1), 50-61. [CrossRef]
- Hartmann, M.; Howes, C.G.; Van Insberghe, D.; Yu, H.; Bachar, D.; Christen, R.; Mohn, W.W. Significant and persistent impact of timber harvesting on soil microbial communities in Northern coniferous forests. ISME J. 2012, 6(12), 2199-2218. [CrossRef]
- Teste, F.P.; Lieffers, V.J.; Strelkov, S.E. Ectomycorrhizal community responses to intensive forest management: Thinning alters impacts of fertilization. Plant Soil 2012, 360, 333-347. [CrossRef]
- Churchland, C.; Grayston, S.J.; Bengtson, P. Spatial variability of soil fungal and bacterial abundance: Consequences for carbon turnover along a transition from a forested to clear-cut site. Soil Biol. Biochem. 2013, 63, 5-13. [CrossRef]
- Bardgett, R.D.; Bowman, W.D.; Kaufmann, R.; Schmidt, S.K. A temporal approach to linking aboveground and belowground ecology. Trends Ecol. Evol. 2005, 20(11), 634-641. [CrossRef]
- Fierer, N.; Strickland, M.S.; Liptzin, D.; Bradford, M.A.; Cleveland, C.C. Global patterns in belowground communities. Ecol. Let. 2009, 12(11), 1238-1249. [CrossRef]
- Bartelt-Ryser, J.; Joshi, J.; Schmid, B.; Brandl, H.; Balser, T. Soil feedbacks of plant diversity on soil microbial communities and subsequent plant growth. Perspec. Plant Ecol. Evol. Syst. 2005, 7(1), 27-49. [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Clim. 2017, 37(12), 4302-4315. [CrossRef]
- Farr, T.G.; Rosen, P.A.; Caro, E.; Crippen, R.; Duren, R.; Hensley, S.; Kobrick, M.; Paller, M.; Rodríguez, E.; Roth, L.; Seal, D.; Shaffer, S.; Shimada, J.; Umland, J.; Werner, M.; Oskin, M.; Burbank, D.; Alsdorf, D. The shuttle radar topography mission. Rev. Geophys. 2007, 45, RG2004. [CrossRef]
- Bitterlich, W. The Relascope Idea: Relative Measurements in Forestry; CAB: London, England, 1984.
- Martínez Pastur, G.; Rosas, Y.M.; Toro Manríquez, M.; Huertas Herrera, A.; Miller, J.A.; Cellini, J.M.; Lencinas, M.V. Knowledge arising from long-term research of variable retention harvesting in Tierra del Fuego: Where do we go from here? Ecol. Processes 2019, 8, 1-16. [CrossRef]
- Levy, E.G.; Madden, E.A. The point method of pasture analyses. NZ J. Agric. 1933, 46, 267-379.
- Moore, D. Flora of Tierra del Fuego; Anthony Nelson: London, England, 1983.
- Correa, M.N. Flora Patagónica; INTA, Buenos Aires, Argentina, 1969-1998.
- IUSS Working Group WRB. World reference base for soil resources 2014, update 2015: International soil classification system for naming soils and creating legends for soil maps; World Soil Resources Reports, FAO, Rome, Italy, 2015; n° 106.
- Peña-Rodríguez, S.; Moretto, A.S.; Pontevedra-Pombal, X.; Oro, N.; García Rodeja Gayoso, E.; Rodríguez-Salgado, I.; Rodríguez-Racedo, J.; Escobar, J.; Nóvoa-Muñoz, J.C. Trends in nutrient reservoirs stored in uppermost soil horizons of subantarctic forests differing in their structure. Agrofor. Syst. 2013, 87, 1273-1128. [CrossRef]
- Peri, P.L.; Rosas, Y.M.; Ladd, B.; Toledo, S.; Lasagno, R.G.; Martínez Pastur, G. Modelling soil carbon content in South Patagonia and evaluating changes according to climate, vegetation, desertification and grazing. Sustainability 2018, 10(2), e438. [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of soil analysis: Part 2, chemical and microbiological properties; Page, A.L., Ed.; Agronomy Monographs, American Society of Agronomy: NY, USA, 1983. [CrossRef]
- Olsen, S.R. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US Department of Agriculture: NY, USA, 1954; n° 939.
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703-707. [CrossRef]
- Joergensen, R.G.; Mueller, T. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEN value. Soil Biol. Biochem. 1996, 28(1), 33-37. [CrossRef]
- Robertson, G.P.; Wedin, D.; Groffman, P.M.; Blair, J.M.; Holland, E.A.; Nadelhoffer, K.J.; Harris, D. Soil Carbon and nitrogen availability nitrogen mineralization, nitrification and soil respiration potentials. In Standard soil methods for long term ecological research; Robertson, G.P., Coleman, D.C. Bledsoes, C.S., Sollins, P., Eds.; Oxford University Press: NY, USA, 1999.
- Anderson, T.H.; Domsch, K.H. Application of eco-physiological quotients (qCO2 and qMC) on microbial biomasses from soils of different cropping histories. Soil Biol. Biochem. 1990, 22(2), 251-255. [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochem. 2007, 85, 235-252. [CrossRef]
- Sun, Y.; Liao, J.; Zou, X.; Xu, X.; Yang, J.; Chen, H.Y.; Ruan, H. Coherent responses of terrestrial C:N stoichiometry to drought across plants, soil, and microorganisms in forests and grasslands. Agric. For. Meteorol. 2020, 292, e108104. [CrossRef]
- Wan, P.; He, R.; Wang, P.; Cao, A. Implementation of different forest management methods in a natural forest: Changes in soil microbial biomass and enzyme activities. For. Ecol. Manage. 2022, 520, e120409. [CrossRef]
- Wang, M.; Xu, Z.; Huang, Z. Soil carbon accrual under harvest residue retention modulated by the copiotroph-oligotroph spectrum in bacterial community. J. Soils Sed. 2022, 22, 2459-2474. [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; González, L.; Tablada, M.; Robledo, C.W. Infostat; Grupo InfoStat, Universidad Nacional de Córdoba, Córdoba, Argentina, 2013.
- Chatterjee, A.; Vance, G.F.; Pendall, E.; Stahl, P.D. Timber harvesting alters soil carbon mineralization and microbial community structure in coniferous forests. Soil Biol. Biochem. 2008, 40(7), 1901-1907. [CrossRef]
- Das, S.; Deb, S.; Sahoo, S.S.; Sahoo, U.K. Soil microbial biomass carbon stock and its relation with climatic and other environmental factors in forest ecosystems: A review. Acta Ecol. Sinica 2023, 43(6), 933-945. [CrossRef]
- Toledo, S.; Bondaruk, V.F.; Yahdjian, L.; Oñatibia, G.R.; Loydi, A.; Alberti, J.; Peri, P.L. Environmental factors regulate soil microbial attributes and their response to drought in rangeland ecosystems. Sci. Tot. Environ. 2023, 892, e164406. [CrossRef]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytologist 2013, 198(3), 656-669. [CrossRef]
- Jevon, F.V.; Polussa, A.; Lang, A.K.; William Munger, J.; Wood, S.A.; Wieder, W.R.; Bradford, M.A. Patterns and controls of aboveground litter inputs to temperate forests. Biogeochem. 2022, 161(3), 335-352. [CrossRef]
- Morikawa, Y.; Hayashi, S.; Negishi, Y.; Masuda, C.; Watanabe, M.; Watanabe, K.; Seiwa, K. Relationship between the vertical distribution of fine roots and residual soil nitrogen along a gradient of hardwood mixture in a conifer plantation. New Phytol. 2022, 235(3), 993-1004. [CrossRef]
- Zhang, S.; Yang, X.; Li, D.; Li, S.; Chen, Z.; Wu, J. A meta-analysis of understory plant removal impacts on soil properties in forest ecosystems. Geoderma 2022, 426, e116116. [CrossRef]
- Xiao, R.; Man, X.; Duan, B.; Cai, T.; Ge, Z.; Li, X.; Vesala, T. Changes in soil bacterial communities and nitrogen mineralization with understory vegetation in boreal larch forests. Soil Biol. Biochem. 2022, 166, e108572. [CrossRef]
- Fernández, M.; Vincent, G.; Dorr, E.; Bakker, S.; Lerch, T.Z.; Leloup, J.; Bazot, S. Does forest stand density affect soil microbial communities? Appl. Soil Ecol. 2024, 195, e105244. [CrossRef]
- Finér, L.; Ohashi, M.; Noguchi, K.; Hirano, Y. Fine root production and turnover in forest ecosystems in relation to stand and environmental characteristics. For. Ecol. Manage. 2011, 262(11), 2008-2023. [CrossRef]
- Freschet, G.T.; Cornwell, W.K.; Wardle, D.A.; Elumeeva, T.G.; Liu, W.; Jackson, B.G.; Cornelissen, J.H. Linking litter decomposition of above- and below-ground organs to plant–soil feedbacks worldwide. J. Ecol. 2013, 101(4), 943-952. [CrossRef]
- Prescott, C.E.; Grayston, S.J. TAMM review: Continuous root forestry, living roots sustain the belowground ecosystem and soil carbon in managed forests. For. Ecol. Manage. 2023, 532, e120848. [CrossRef]
- Högberg, M.N.; Briones, M.J.; Keel, S.G.; Metcalfe, D.B.; Campbell, C.; Midwood, A.J.; Högberg, P. Quantification of effects of season and nitrogen supply on tree below-ground carbon transfer to ectomycorrhizal fungi and other soil organisms in a boreal pine forest. New Phytol. 2010, 187(2), 485-493. [CrossRef]
- Simard, S.W.; Roach, W.J.; Defrenne, C.E.; Pickles, B.J.; Snyder, E.N.; Robinson, A.; Lavkulich, L.M. Harvest intensity effects on carbon stocks and biodiversity are dependent on regional climate in Douglas-fir forests of British Columbia. Front. For. Glob. Change 2020, 3, e88. [CrossRef]
- Liu, J.; Wang, Q.; Ku, Y.; Zhang, W.; Zhu, H.; Zhao, Z. Precipitation and soil pH drive the soil microbial spatial patterns in the Robinia pseudoacacia forests at the regional scale. Catena 2022, 212, e106120. [CrossRef]
- Chen, Y.L.; Ding, J.Z.; Peng, Y.F.; Li, F.; Yang, G.B.; Liu, L.; Yang, Y.H. Patterns and drivers of soil microbial communities in Tibetan alpine and global terrestrial ecosystems. J. Biogeogr. 2016, 43(10), 2027-2039. [CrossRef]
- Busse, M.D.; Beattie, S.E.; Powers, R.F.; Sanchez, F.G.; Tiarks, A.E. Microbial community responses in forest mineral soil to compaction, organic matter removal, and vegetation control. Can. J. For. Res. 2006, 36(3), 577-588. [CrossRef]
- Mummey, D.L.; Clarke, J.T.; Cole, C.A.; O’Connor, B.G.; Gannon, J.E.; Ramsey, P.W. Spatial analysis reveals differences in soil microbial community interactions between adjacent coniferous forest and clearcut ecosystems. Soil Biol. Biochem. 2010, 42(7), 1138-1147. [CrossRef]
- Rodríguez-Souilla, J.; Cellini, J.M.; Lencinas, M.V.; Roig, F.A.; Chaves, J.E.; Aravena Acuña, M.C.; Martínez Pastur, G. Variable retention harvesting and climate variations influence over natural regeneration dynamics in Nothofagus pumilio forests of Southern Patagonia. For. Ecol. Manage. 2023, 544, e121221. [CrossRef]
- Webster, K.L.; Wilson, S.A.; Hazlett, P.W.; Fleming, R.L.; Morris, D.M. Soil CO2 efflux and net ecosystem exchange following biomass harvesting: Impacts of harvest intensity, residue retention and vegetation control. For. Ecol. Manage. 2016, 360, 181-194. [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137(3-4), 253-268. [CrossRef]
- Bingham, A.H.; Cotrufo, M.F. Organic nitrogen storage in mineral soil: implications for policy and management. Sci. Total Environ. 2016, 551, 116-126. [CrossRef]
- Soares, M.; Rousk, J. Microbial growth and carbon use efficiency in soil: links to fungal-bacterial dominance, SOC-quality and stoichiometry. Soil Biol. Biochem. 2019, 131, 195-205. [CrossRef]
- Ponder, F.; Tadros, M. Phospholipid fatty acids in forest soil four years after organic matter removal and soil compaction. Appl. Soil Ecol. 2002, 19(2), 173-182. [CrossRef]
- Moore-Kucera, J.; Dick, R.P. PLFA profiling of microbial community structure and seasonal shifts in soils of a Douglas-fir chronosequence. Microbial Ecol. 2008, 55, 500-511. [CrossRef]
- Kramer, C.; Gleixner, G. Soil organic matter in soil depth profiles: Distinct carbon preferences of microbial groups during carbon transformation. Soil Biol. Biochem. 2008, 40(2), 425-433. [CrossRef]
- Burton, J.; Chen, C.; Xu, Z.; Ghadiri, H. Soil microbial biomass, activity and community composition in adjacent native and plantation forests of subtropical Australia. J. Soils Sed. 2010, 10, 1267-1277. [CrossRef]
- Busse, M.D.; Sanchez, F.G.; Ratcliff, A.W.; Butnor, J.R.; Carter, E.A.; Powers, R.F. Soil carbon sequestration and changes in fungal and bacterial biomass following incorporation of forest residues. Soil Biol. Biochem. 2009, 41(2), 220-227. [CrossRef]
- Varenius, K.; Lindahl, B.D.; Dahlberg, A. Retention of seed trees fails to lifeboat ectomycorrhizal fungal diversity in harvested Scots pine forests. FEMS Microbiol. Ecol. 2017, 93(9), e105. [CrossRef]
- Moretto, A.S.; Martínez Pastur, G. Litterfall and leaf decomposition in Nothofagus pumilio forests along an altitudinal gradient in Tierra del Fuego, Argentina. J. For. Sci. 2014, 60(12), 500-510. https://10.17221/74/2014-JFS.
- Spake, R.; Ezard, T.H.; Martin, P.A.; Newton, A.C.; Doncaster, C.P. A meta-analysis of functional group responses to forest recovery outside of the tropics. Conserv. Biol. 2015, 29(6), 1695-1703. [CrossRef]
- Holden, S.R.; Treseder, K.K. A meta-analysis of soil microbial biomass responses to forest disturbances. Front. Microbiol. 2013, 4, e52720. [CrossRef]
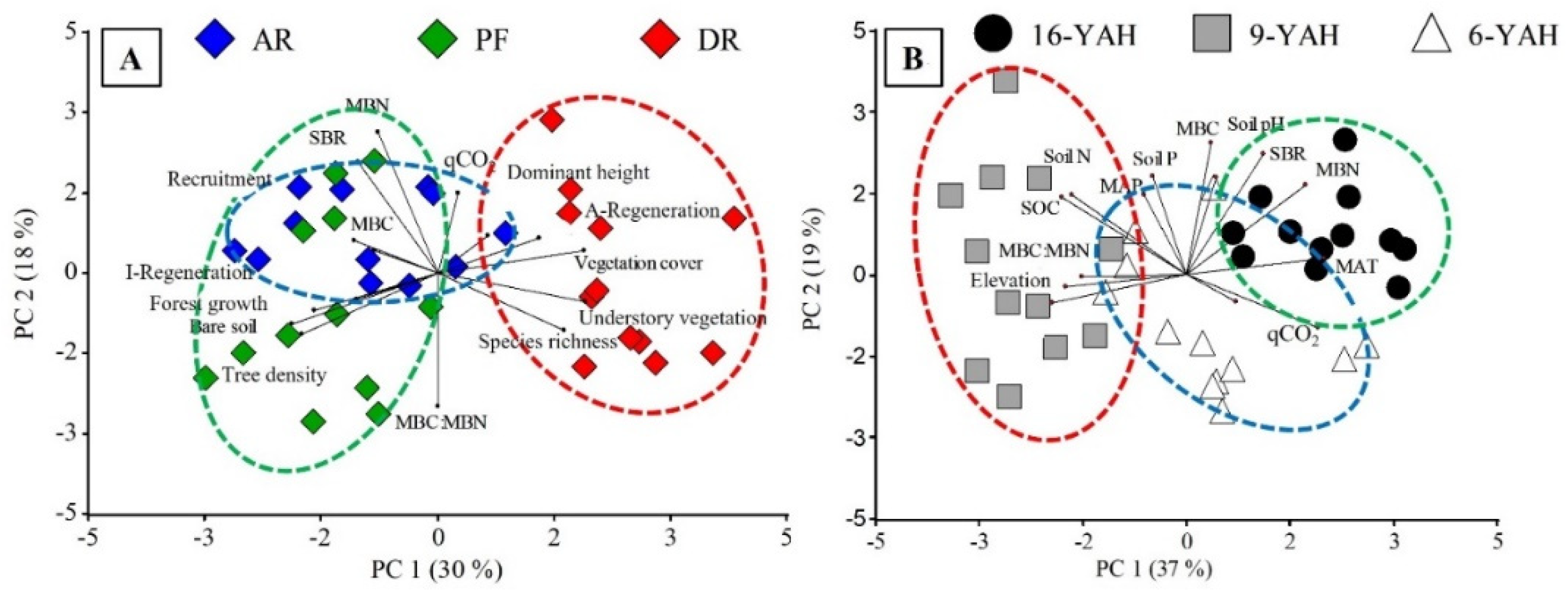
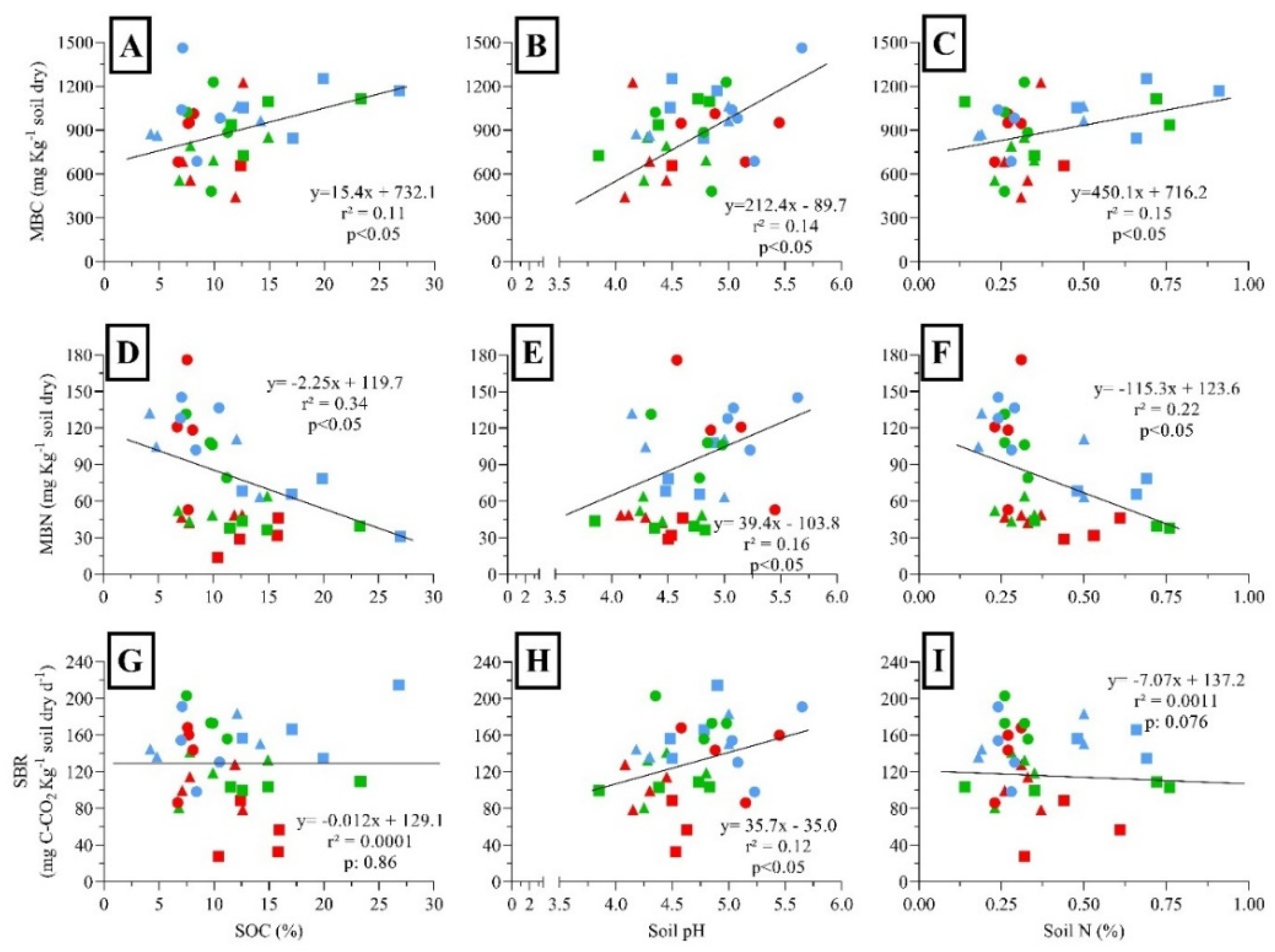
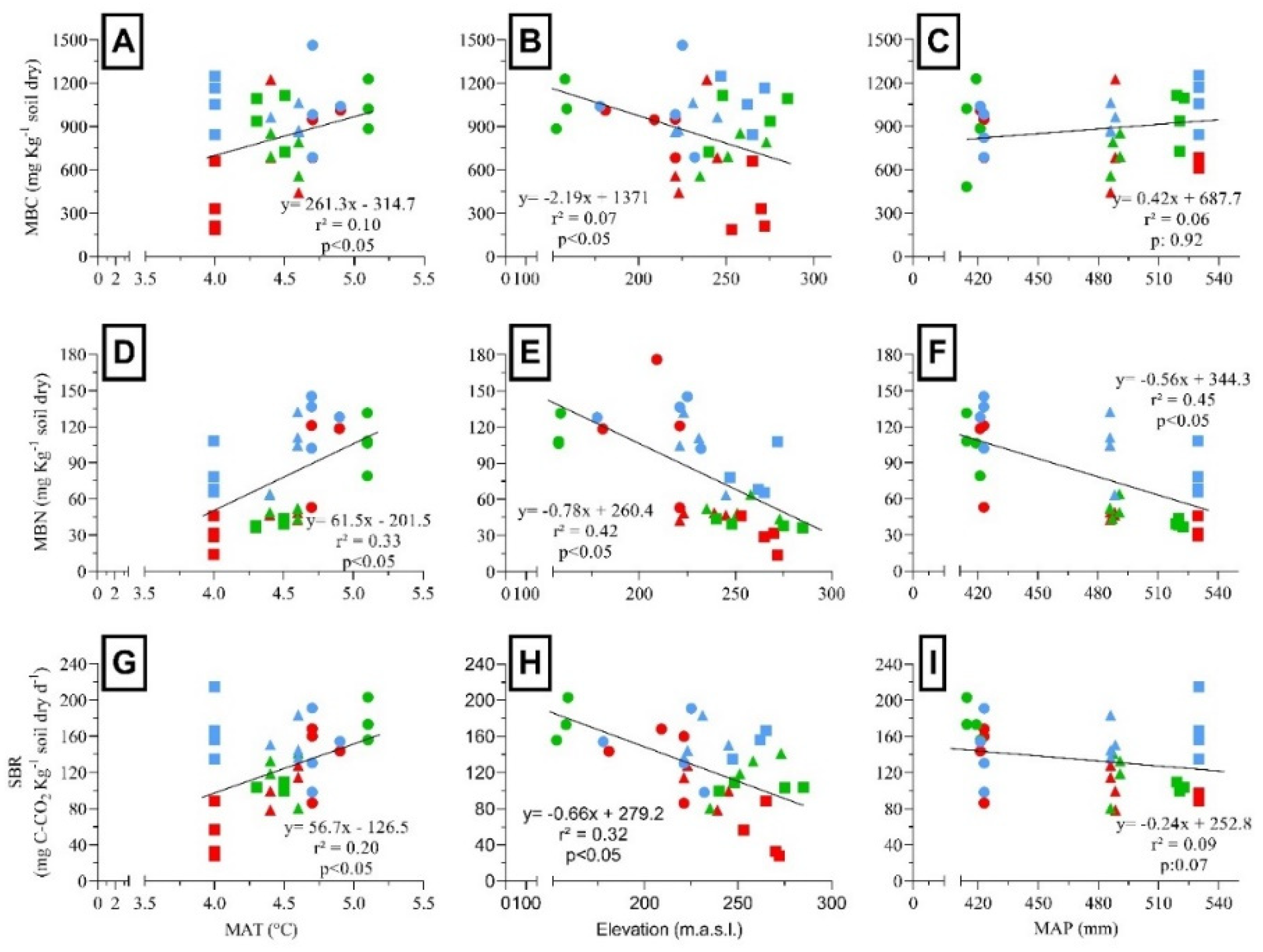
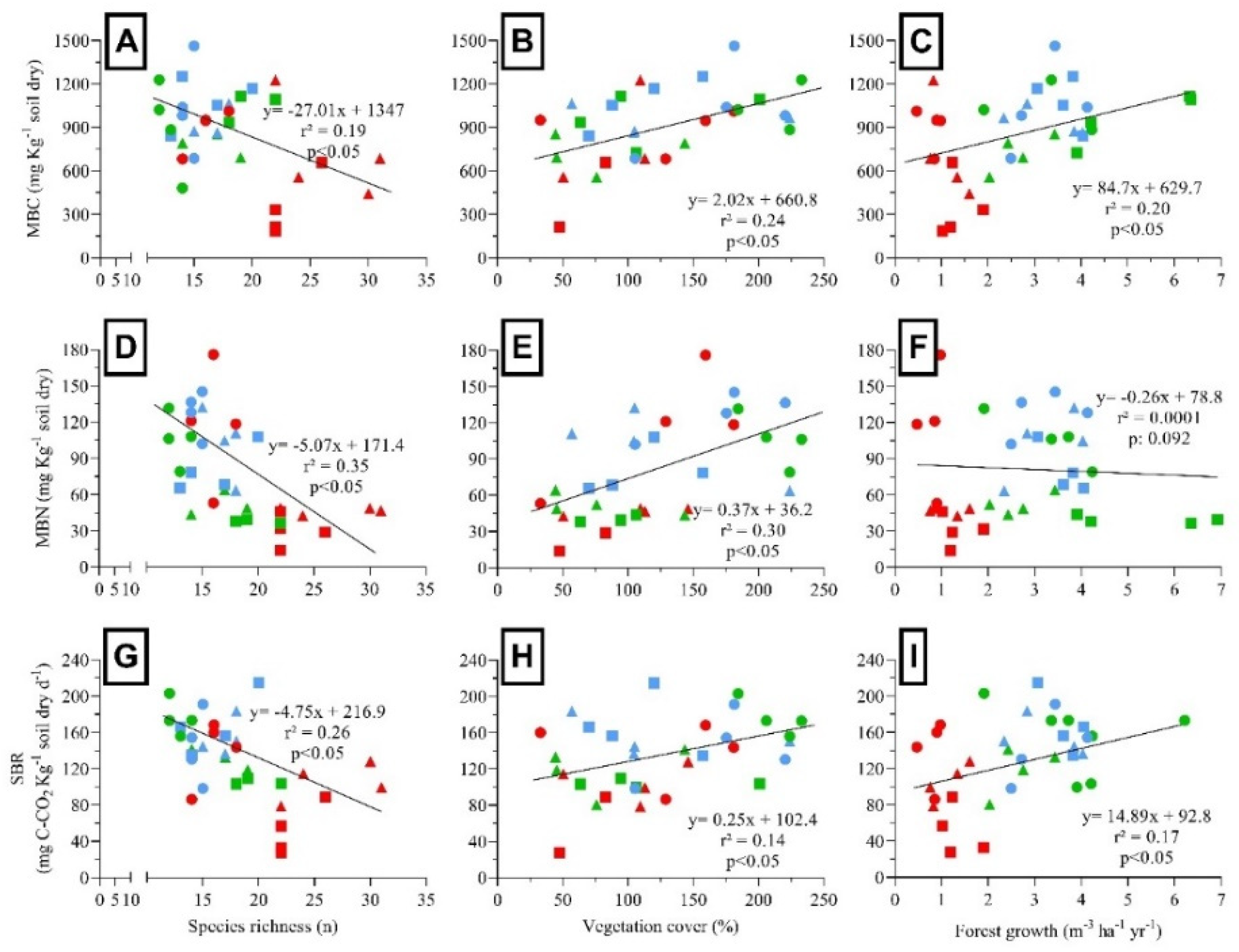
| Factor | 6-YAH | 9-YAH | 16-YAH | |||||||
| AR | DR | PF | AR | DR | PF | AR | DR | PF | ||
| Latitude | -54.483 | -54.480 | -54.482 | -54.580 | -54.581 | -54.569 | -54.382 | -54.381 | -54.365 | |
| Longitude | -66.828 | -66.823 | -66.802 | -66.907 | -66.907 | -66.927 | -67.864 | -67.862 | -67.869 | |
| Climate | MAP | 486.6 ± 1.7 | 487.2 ± 1.2 | 488.7 ± 2.1 | 530.0 ± 1.1 | 530.0 ± 1.1 | 520.6 ± 1.3 | 422.4 ± 1.0 | 425.4 ± 1.0 | 427.1 ± 3.6 |
| MAT | 4.7 ± 0.1 | 4.6 ± 0.1 | 4.6 ± 0.1 | 4.0 ± 0.1 | 4.0 ± 0.1 | 4.4 ± 0.1 | 4.7 ± 0.1 | 4.7 ± 0.1 | 5.0 ± 0.1 | |
| ELE | 230 ± 11 | 232 ± 12 | 254 ± 16 | 261 ± 10 | 265 ± 8 | 262 ± 21 | 214 ± 24 | 208 ± 19 | 157 ± 3 | |
| Forest Structure | DH | 23.6 ± 3.9 | 21.9 ± 1.9 | 19.1 ± 1.6 | 23.3 ± 2.2 | 24.4 ± 2.1 | 22.4 ± 2.9 | 21.7 ± 2.0 | 21.7 ± 1.3 | 22.7 ± 2.7 |
| TD | 229 ± 111 | 120 ± 87 | 376 ± 89 | 234 ± 172 | 61 ± 37 | 671 ± 172 | 354 ± 170 | 45 ± 20 | 350 ± 114 | |
| BA | 51.0 ± 10.4 | 18.3 ± 6.8 | 55.5 ± 5.2 | 49.5 ± 7.5 | 14.9 ± 3.0 | 63.8 ± 11.1 | 51.0 ± 11.5 | 12.8 ± 3.2 | 50.3 ± 9.0 | |
| FG | 3.3 ± 0.8 | 1.1 ± 0.4 | 2.7 ± 0.6 | 3.6 ± 0.4 | 1.3 ± 0.4 | 5.3 ± 1.5 | 3.2 ± 0.7 | 0.8 ± 0.2 | 3.3 ± 1.0 | |
| I-R | 603 ± 220 | 101 ± 71 | 1078 ± 357 | 446 ± 95 | 94 ± 40 | 195 ± 31 | 181 ± 61 | 31 ± 21 | 170 ± 39 | |
| A-R | 0 ± 0 | 0 ± 0 | 0 ± 0 | 2 ± 1 | 3.3 ± 2.9 | 0 ± 0 | 0 ± 0 | 4.0 ± 2.7 | 0 ± 0 | |
| REC | 198 ± 27 | 21 ± 13 | 56 ± 30 | 0 ± 0 | 3 ± 1 | 0 ± 0 | 31 ±18 | 0 ± 0 | 138 ± 25 | |
| Understory vegetation | SR | 17 ± 1 | 27 ± 4 | 15 ± 4 | 16 ±3 | 23 ± 2 | 17 ± 6 | 15 ± 1 | 16 ± 2 | 13 ± 1 |
| BIO | 406 ± 131 | 3613 ± 178 | 779 ± 236 | 1771 ± 487 | 2493 ± 1216 | 859 ± 184 | 611 ± 267 | 1968 ± 727 | 524 ± 136 | |
| DS | OSDE, VIMA, POPR | GAAP, OSDE, TAOF | OSDE, VIMA, TAOF | OSDE, SCRA, PHAL | OSDE, SCRA, VIMA | MAGR, SCRA, VIMA | GAAP, OSDE, TAOF | OSDE SCRA, HIRE | GAAP, OSDE, FEMA | |
| COV | 103.2 ± 39.2 | 141.5 ± 34.0 | 78.5 ± 38.1 | 174.2 ± 49.3 | 209.0 ± 24.6 | 89.7 ± 56.0 | 63.0 ± 14.8 | 189.0 ± 33.6 | 107.5 ± 27.9 | |
| BS | 25.5 ± 13.7 | 16.0 ± 7.3 | 37.5 ± 17.9 | 7.5 ± 2.4 | 5.5 ± 2.5 | 33.0 ± 22.1 | 35.5 ± 6.2 | 2.5 ± 1.6 | 21.5 ± 10.6 | |
| Soil | pH | 4.62 ± 0.44 | 4.24 ± 0.17 | 4.44 ± 0.25 | 4.66 ± 0.21 | 4.66 ± 0.23 | 4.44 ± 0.44 | 5.24 ± 0.28 | 5.01 ± 0.37 | 4.74 ± 0.27 |
| SOC | 8.81 ± 5.10 | 9.85 ± 2.79 | 9.85 ± 3.62 | 19.11 ± 5.94 | 13.63 ± 2.69 | 15.59 ± 5.37 | 8.24 ± 1.62 | 7.52 ± 0.57 | 9.56 ± 1.55 | |
| SN | 0.34 ± 0.18 | 0.32 ± 0.04 | 0.29 ± 0.06 | 0.69 ± 0.17 | 0.48 ± 0.13 | 0.49 ± 0.30 | 0.26 ± 0.03 | 0.27 ± 0.03 | 0.29 ± 0.04 | |
| SP | 55.9 ± 36.7 | 52.1 ± 17.2 | 48.3 ± 34.5 | 66.7 ± 34.9 | 39.0 ± 21.8 | 62.3 ± 28.2 | 62.9 ± 17.9 | 58.4 ± 27.0 | 47.5 ± 9.6 | |
| Treatments | Levels | MBC | MBN | SBR | MBC:MBN | qCO2 | qMC |
| FT | PF | 865±228 ab | 66±33 a | 133±37 b | 13±9 b | 0.17±0.07 a | 0.81±0.29 a |
| AR | 1021±206 b | 104±42 b | 155±31 b | 10±4 a | 0.15±0.02 a | 1.09±0.60 a | |
| DR | 658±331 a | 65±50 a | 99±46 a | 10±8 a | 0.17±0.07 a | 0.75±0.44 a | |
| F(p) | 7.50(<0.05) | 8.62(<0.05) | 12.82(<0.05) | 5.58(<0.05) | 0.15(0.86) | 3.00(0.06) | |
| YAH | 6 | 835±226 a | 75±47 a | 129±30 ab | 11±7 ab | 0.17±0.05 a | 1.08±0.50 b |
| 9 | 712±378 a | 55±32 a | 109±55 a | 13±9 b | 0.16±0.06 a | 0.43±0.26 a | |
| 16 | 970±256 a | 122±39 b | 141±34 b | 8±4 a | 0.15±0.06 a | 1.26±0.40 b | |
| F(p) | 1.70(0.20) | 11.20(<0.05) | 8.28(<0.05) | 6.24(<0.05) | 0.67(0.52) | 9.38(<0.05) | |
| FT x YAH | 2.93(<0.05) | 0.47(0.75) | 5.36(<0.05) | 1.79(0.15) | 1.62(0.19) | 1.25(0.31) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





