1. Introduction
Hearing loss has been one of the major debilitating disorders leading to significant losses in the health and wealth of population globally. According to estimates from the World Health Organization (WHO), approximately 400 million people have disabling hearing loss (HL), mostly as a result of cochlear abnormalities that may be age-related, noise-induced, or hereditary in origin. Despite the prevalence of diverse factors that influence hearing loss including genetics, climate, occupation, and age, albeit there is a sever paucity in high quality reports in the region. This study aims to understand the frequency and rates of hearing loss in this region and the potential factors and reasons that cause the disorder.
Hearing loss is a common problem that impacts people of all ages. It’s defined as the inability to hear the sound within the normal hearing threshold 20 dB which can affect one ear or both ears [
1]. Three types of deafness are categorized as follows: mixed, sensorineural, and conductive. A malfunction in the ear's sound-conducting system results in the development of conductive deafness. The lesion could be located anywhere from the external auditory canal to the stapes footplate [
2] An impairment in the cochlea, auditory nerve, neural pathway, or their central connections to the auditory cortex results in sensorineural deafness [
3]. However, there are several factors that cause hearing loss. For instance, it has been found that the relationship between hearing loss and climate conditions can directly proportional and increase the progress of hearing loss gradually. Combined exposure to environmental conditions such as noise and heat may enhance the risk of hearing loss for employees in noisy settings by 1.39 times compared to those who only work in noisy environments. [
4] Wiebke Schmidt et al. suggest that Weather changes affect the severity of symptoms in Ménière's disease (MD). In addition, it has been suggested that lower atmospheric pressure was related to a higher risk of an attack and significantly increased levels of vertigo, tinnitus, and aural completion in people enduring MD. Furthermore, high humidity also additionally expanded the chances of experiencing an attack.[
4]
Global estimates in hearing losses vary widely depending on geographic locations and local genetic population structures and environmental factors. Worldwide estimates showed that 16% of cases involving disabling hearing loss in adults were attributed to occupational noise exposure [
5,
6,
7]. Occupational Noise-Induced Hearing Loss (ONIHL) is characterized by partial or complete hearing loss in one or both ears due to work-related factors. This typically develops gradually over several years [
8] depending on influencing factor(s). Research suggests that in the United States, individuals in the construction, manufacturing, mining, agriculture, utility, transportation, military, and music industries face the highest risk of ONIHL [
9,
10,
11]. In Saudi Arabia, the occurrence of hearing impairment among workers exposed to high noise levels varies between 15.8% and 57% [
12,
13,
14]. Notably, older individuals with mild hearing loss have twice the risk of developing dementia as a result. On the other hand, severe hearing loss increases that risk fivefold [
15] and develop into sequalae of other cognitive disfunctions. The ONIHL is a complex yet preventable condition, which underscores the importance of understanding its mechanisms and affected populations for the development of effective preventive measures.
Reduced oxygen (hypoxia) and atmospheric pressure (hypobaria) are the primary characteristics of high altitude (HA), which is defined as an altitude higher than 2500 m above sea level [
16,
17]. Changes in air pressure during flight can cause ear-drum pain, vertigo, hearing loss, and ear-drum perforation, among other consequences on the middle ear[
18]. The type of aircraft, altitude, and passenger characteristics all affect the frequency of symptoms. According to a point prevalence survey, 10% of adults and 22% of children exhibited otoscopic evidence of injury to the ear drum, and 20% of adult and 40% of child passengers experienced negative pressure in the middle ear following their flight [
19]. Data on the frequency of perforation, which appears to be incredibly uncommon in travelers on commercial aircraft, is limited. Furthermore, hearing loss has been reported as a result of ear infections. This includes the herpes virus family's beta subfamily, such as the cytomegalovirus (CMV)[
3]. Vertical transmission, sexual transmission, and contact transmission are all conceivable strategies of spreading CMV. Roughly 10-20% of children with hearing loss are influenced by inherent CMV (cCMV). An infection that causes non-genetic SNH (sensorineural hearing loss) and neurodevelopmental issues [
20]. An essential source of transmission of HSV-2 is sexual contact, or through contact with the mother's vaginal tract, whereas, a essential source of transmission of HSV-1 is hint contact. In children with chance variables, schedule hearing tests ought to be performed between the ages of 2 a long time and 30 months. A positive HSV culture postpartum disease and an in-utero disease stay chance variables for lasting intrinsic, postponed, or dynamic hearing loss in childhood. HSV contamination in newborn children can result in one-sided or two-sided hearing loss, mild to severe SNHL, and genuine neurological complications. The SARS-CoV-2 infection, of the Coronavirus family could, could lead to several malfunctioning in the upper respiratory tract. The affiliation between SARS-CoV-2 and sudden sensorineural HL (SSNHL) has been detailed in several case instances, numerous SARS-CoV-2 varieties can result in ontological symptoms [
21]. Unfortunately, high quality data on the prevalence of hearing loss before and after the pandemic is limited. Since the whole Ha’il population was vaccinated, the study may also account for the effect of vaccines.
Depending on the gender apportionment at birth, there's a better frequency of intrinsic HL in full-term newborn children (1.8 per 1000 live births) in males than in females (1.2 per 1000 live births) among newborn children with hearing disabilities [
22]. Male-dominated work environments, the nature of occupations [
23] and undesirable behaviors like smoking have been connected to this. It is widely known that smokers are more prone to hearing issues than non smoker usually when they smoke for extended periods [
24]. On the other hand, there is a common notion that women are frequently more noise-resistant than men [
25]. As such, there are striking gender incongruities in hearing patterns over the sound-related work, with premenopausal women regularly predominate compared to men of the same age. Besides, premenopausal women display inconstancy in their capacity to listen amid the menstrual cycle, with their capacity to listen is best at the top of estradiol or when the proportion of estradiol to progesterone falls. Women start to lose their capacity to listen around menopause; this loss is more discernible and happens more rapidly than in males of the same age [
26]. Men may be more subject to hearing loss, communicate better socially, and those who have wider social status are regularly more prone to hearing loss [
27]. This may clarify the tall rate of hearing loss in men.
The affiliation between advanced age and the type of hearing loss shows that patients within the more senior age groups were more likely to have sensorineural hearing loss, while conductive hearing loss was more among youth. The populace inhabiting a location for a long time was found more likely to have blended hearing loss [
28]. In any case, the most common cause of hearing loss among Saudi Arabia's youth populace is conductive hearing loss usually after ear diseases; [
29] whereas, sensorineural hearing loss is more prevalent within the more seasoned populace [
30] . Similarly, reports indicate that the conductive HL types are more common in youngsters [
31].
The worldwide predominance rates of hearing loss have been expanding relentlessly. A comprehensive consider on point-by-point data of hearing loss from the Worldwide Burden of Infection think about between 1990 and 2019 was detailed. Normal yearly rate changes (AAPCs) in hearing loss age-standardized predominance rate (ASPR), by sex, locale, and category, were calculated to measure the temporal predominance patterns. Within the previously mentioned ponder, around the world rates of hearing loss bounced from 7514.97×105 in 1990 to 14566.62×105 in 2019 whereas ASPR rose from 173.33×102 per 100,000 in 1990 to 177.56×102 per 100,000 in 2019. Time went through in hearing incapacity (a long time lived with incapacity YLD) increased from 220080.97×102 in 1990 to 402353.05×102 in 2019. The AAPC was 83.27 (95% CI 70.66, 95.88) ×10-3 in predominance and -72.87 (95% CI -92.18, -53.56) ×10-3 in YLD. Critical correlations of AAPCs with ASPR (r=-0.60, p<0.001), and age-standardized YLD (r=-0.43, p=0.0012 for YLD<455, r=0.32, p<0.001 for YLD≥455) were recognized. The YLDs of HL owing to word related noise (HLOON) expanded from 39334.39 (95% UI 26881.04, 55999.67) ×102 in 1990 to 70014.49 (98% UI 47605.62, 100593.43) ×102 in 2019, and the expanding AAPC was watched for females and matured between 15 and 49 a long time ancient in worldwide and most locales. The age impact was beneath zero in 7 age groups, the period effect of hearing loss prevalence was increasing and the birth cohort effect was decreasing with the time advance. The number of cases and ASPR of hearing loss within the is still growing. Efforts to control HL, especially HLOON, are imminent [
32]. Despite the steadily rising incidence rates within the regions including Saudi Arabia, there's severe lack of high-quality data regarding the causes and participating factors of hearing loss across all age groups; Thus, such research is therefore desperately needed.
2. Method and Material
2.1. Study Design
This study was a cross-sectional design using a web-based online public questionnaire sent out to different regions of Saudi Arabia; namely Eastern, Western, Northen, and Southern. All messages were sent simultaneously at the same time frame. A panel of reviewers ENT specialists from the King Khalid Hospital and University of Ha’il clinics reviewed the questionnaire and provided comments that were considered. Since over 97% of government utilities and services are now on digital electronic platforms and that most of Saudi population is at young age, the bias towards young and exclusion of less technologically inclined populations would not make significant bias. For this, we tested the questionnaire on a sample of different age groups and was user friendly and suitable for all.
2.2. Sample Size and Sampling Procedure
The sample was a self-administered non-probability sample of social media users in Saudi Arabia. The web-based data collection tool was designed using Google forms and was distributed via social media applications i.e., mainly WhatsApp, Twitter, and others. The invitation letter was sent via WhatsApp groups and was posted on some community groups on Twitter. The invitation letter explained the aim of the study and the approximate time required to complete the questionnaire. Participants were asked to distribute the survey further among their social networks. A total of 789 participants were screened from responses.
2.3. Data Collection Tool
The questionnaire comprised of the following demographic characteristics including consent form, age, gender, health status and treatment history, region of residence, job type, if any, and other related factors prone to influence hearing loss. The questionnaire was prepared in the English language, then translated into the Arabic language. Language validity was undertaken by retranslating the Arabic version of the questionnaire into English to ensure that the original meaning of the questions was preserved (back translation) that is done by the authors, who are influential native speakers in both English and Arabic languages.
2.4. Ethics Approval
The Ethics Approval was obtained from the Research Ethics Standing Committee, University of Hail #UOH 2021-631, dated 4/3/2024, number H-2024-067. All participants were asked for their consent before participation in this study.
2.5. Statistical Analysis
Updated Statistical Package for Social Sciences software (IBM SPSS; Version 24 SPSS version 23.0 for Windows (SPSS, Inc., Chicago, IL, USA) was used for the analysis of data collected. Descriptive and stratified analyses were conducted; we present absolute numbers, proportions, and graphical distributions. We conducted exact statistical tests for proportions and showed p-values where appropriate (a p-value <0.05 was considered statistically significant).
3. Results
In this study we provided the frequency of hearing loss among different patients demographics across different regions in Saudi Arabia. In addition, to patients’ demographics, family history, and genetics, climate, urbanization, and industrialization are the major differences between difference provinces that are prone to influence hearing loss. The study surveyed 789 sample revealed that a small proportion (6.6%) reported being diagnosed with hearing loss. Additionally, the sociodemographic analysis showed a diverse age distribution, with the majority falling between 11 to 50 years old, and a slight female predominance (59.1%). The largest proportion of participants resided in the Eastern province (62.4%) and were either employed (41.2%) or students (31.7%) [
Table 1].
Iindividuals aged over 50 years had a higher prevalence of hearing loss compared to younger age groups (p<0.001). Specifically, among individuals aged 51-60 years, the prevalence of hearing loss was 17.2%, which is notably higher than in younger age categories. Moreover, the prevalence appears to increase with advancing age, with individuals aged 61-70 years showing a prevalence of 0.0% and those aged 71-80 years showing a prevalence of 25.0%. Among females, 30 out of 466 individuals were diagnosed with hearing loss, accounting for 6.4% of the total female population. Similarly, among males, 22 out of 323 individuals were diagnosed with hearing loss, accounting for 6.8% of the total male population. However there was no significant difference in prevalence of hearing loss observed (p=0.838) [
Table 2].
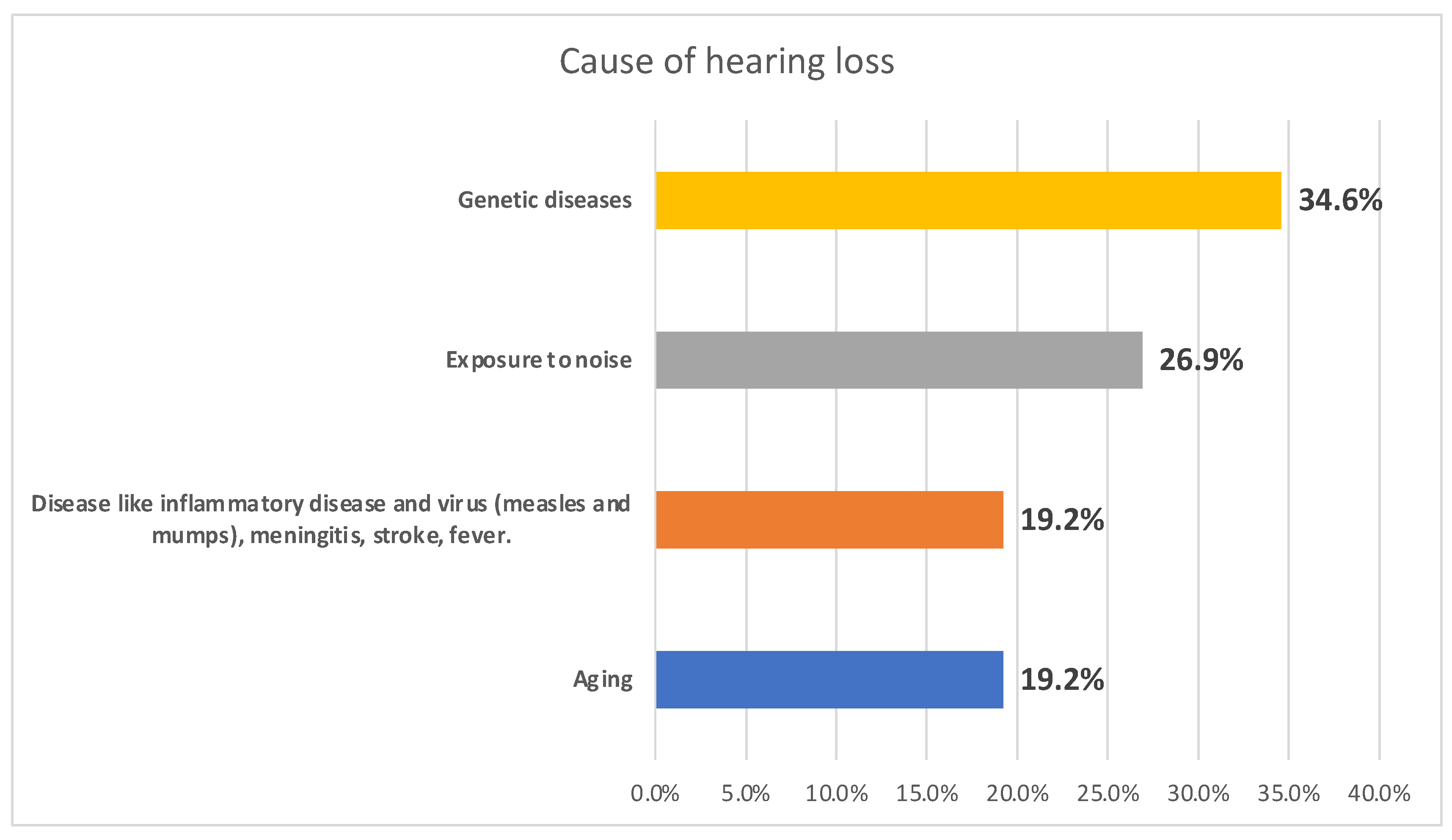
Figure 1 illustrates the causes of hearing loss among the surveyed individuals. Genetic diseases emerged as a potential cause, accounting for 34.6% of cases, followed closely by exposure to noise at 26.9%. Disease-related factors, including inflammatory conditions, viral infections such as measles and mumps, meningitis, stroke, and fever, as well as aging, each contributed to 19.2% of cases.
Other clinical characteristics related to hearing loss are given in
Table 3. Results indicate varied durations since diagnosis, with the largest proportion experiencing hearing loss for more than 5 years (36.5%). A minority were programmed and fitted with hearing aids or cochlear implants (21.2%). Additionally, 19.2% of participants reported having diabetes mellitus, while 51.9% had a family history of hearing loss and/or hearing problems. Few individuals reported work involving travel by air or sea (7.7%), a history of head injury or trauma (11.5%), or relocation between low and high-altitude areas (21.2%). Approximately a quarter (25.0%) were currently taking medications, with aspirin being the most common (9.6%).
Table 4 shows the relationship between travel history and hearing loss within the surveyed population. The data show no significant association between frequent travel by plane (more than 3 times per year) and the diagnosis of hearing loss (p = 0.628). Among those who did not frequently travel by plane, 93.2% had no hearing loss, compared to 6.8% who did. Similarly, among frequent travelers, 94.3% had no hearing loss, while 5.7% did.
A logistic regression model was done to predict the risk of potential factor for hearing loss among the participants [
Table 5]. Individuals aged over 50 years have a significantly elevated risk (OR = 1.43, 95% CI: 0.78-2.81, p = 0.032), while those engaged in certain occupations seem to have a reduced risk (OR = 0.32, 95% CI: 0.02-0.73, p = 0.862). Frequent exposure to noisy environments (OR = 1.54, 95% CI: 0.83-3.01, p = 0.048) and regular use of medications like Aspirin or NSAIDs (OR = 2.15, 95% CI: 1.03-3.32, p = 0.017) are also associated with higher odds of hearing loss. Additionally, individuals responded with a history of genetic disorder (
P = 0.0432), although these would need experimental testing to confirm, and those with a history of inflammatory conditions (OR = 0.72, 95% CI: 0.32-1.87, p = 0.229) show notable associations, though the latter is not statistically significant. Other factors, such as gender, travel frequency by plane, diabetes, family history of hearing loss, occupation involving navigation across the sky or sea, and a history of head injury, do not show statistically significant associations with hearing loss in this study.
Discussion
In this study we provided the rates of hearing loss among different patient’s demographics across different regions in Saudi Arabia. In addition, to patients’ demographics, family history, and genetics, climate, urbanization, and industrialization are the major differences between difference provinces that are prone to influence hearing loss. Hearing loss induce many negative effects in populations including mental health and cognitive function were based on epidemiologic evidence their association exists between hearing loss and increased risk of isolation, depression, increased risk of dementia, poorer balance, falls, hospitalizations, fatigue, and early mortality[
33]. Saudi Arabia is one of the most young populations globally; however, estimates of hearing loss still remains significant.
In this study we found 7% as the overall hearing loss among the examined population in this study. Although this is relatively small, it is an improvement from 2003 survey (18.6%) in ages 10 to 20 [
34]; however, this rate is still significant when blotted against the whole population and the impact this will have on lifestyle. Nevertheless, the ratio of slight increase in hearing loss among gender differences in this study (female 59%, male 41%) remained same for decades since 2003 [
34] and it is in fact related to the fact that female respondents were higher with that rate. This is further confirmed by the fact that the gender difference in the test diagnosed ration between females (6.4%) and male (6.8%) was not statistically significant (p=0.838). This is consistent with earlier findings that there was no statistically significant association between gender and different attributes of hearing loss among the Saudi population [
35]. The Eastern provinces merit with industrialized lifestyle in addition to significant climate change towards more humid weather year-round; this justifies the increase in hearing loss in this region (62.4%) in agreement with earlier studies[
36]. Despite the significance, only 21% sought diagnosis and hearing aid or cochlear implants and quarter (25.0%) were currently taking medications, with aspirin being the most common (9.6%). This implied negligence and lack of awareness.
Consistent with widely published data, hearing impairment appears to increase incrementally in those over 50 years old and higher compared to younger age groups with significant value (p<0.001)[
37]. However, genetic disorders (34.6%) and family history (52%) were revealed as causes; however, these findings would need to be substantiated and confirmed by genetic testing. These were followed closely by exposure to noise at 26.9% while other factors such as inflammatory conditions, viral infections such as measles and mumps, meningitis, stroke, and fever, as well as aging, each contributed to 19.2% of cases. Genetic and congenital influence is commonly associated to developed countries; particularly, with uniform population genetic structure such as Japan[
38,
39] however, while Saudi Arabia is fascinatingly homogeneous, genetic studies is limited that reveal experimentally confirmed data. Infection and inflammation are common causes of hearing loss worldwide; an example is Malawi where HIV clinical studies are intensively conducted where 24% (n 380) had hearing loss due to AIDS[
40]. It is widely known that diabetes plays significant role in hearing loss [
41]and Saudi Arabia has a high rate of the disease [
42]where 19.2% of participants in this study reported having diabetes mellitus.
Interestingly, among the 52 patients with hearing loss, 10 (19.2%) were diabetic. Subjects with type 2 diabetes show a higher rate of altered evoked otoacoustic emissions (e-OAEs) compared to healthy individuals. E-OAE dysfunction is associated neither with a lesion of the auditory nerve pathway nor with diabetic microvasculopathy[
43]
. Therefore, diabetes per se, regardless of microvascular and neuropathic complications, is related to potential hearing loss. While travelling, injuries and trauma, and other factors were lower, and may not have high impact, 21.2% of respondents reported change in altitude from low to high or vise versa.
To predict hearing loss among respondents, a logistic regression analysis was conducted. This confirmed that age groups over 50 years showed a significantly higher risk (OR = 1.43, 95% CI: 0.78-2.81, p = 0.032). In addition, this test identified that exposure to noise (OR = 1.54, 95% CI: 0.83-3.01, p = 0.048) and medications like Aspirin or NSAIDs (OR = 2.15, 95% CI: 1.03-3.32, p = 0.017) were also associated with elevated odds of hearing loss. Furthermore, potential genetic disorder(P = 0.432), and family history of inflammation and infections (P = 0.229) showed slight associations. However, an important finding was that other factors such as gender, travel frequency by plane, diabetes, family history of hearing loss, occupation involving navigation across the sky or sea, and a history of head injury, did not strongly indicate statistically significant associations with hearing loss in this study.
Conclusion
We have examined hearing profiles and patterns of 789 responses from across four different provinces for hearing loss where 6.6 % had the impairment. The diverse sociodemographic surveillance included wide age distribution with the majority falling between 11 to 50 years old with a slight female predominance (59.1%). The association with advances in age irrespective of gender differences as well as the wide time lag since diagnosis have significant clinical and public health implications in geriatric healthcare and management. Furthermore, the high impact of chronic diseases such as diabetes and use of medications implied significance of early screening and specific diagnosis and treatment strategies. Importantly, family history of infections, genetic disorder, noisy environment, although require further confirmation, emerged as potential causes of hearing loss. Thus, while other occupational health parameters can also affect, this study reveals frequences of hearing loss and the potential predisposing factors as age-related, chronic diseases, noise-induced, or hereditary in origin. These findings have significant clinical implications in geriatric management strategies and population genetic structure, family, and public health issues. Future large cohort multicenter study would gain more insights; particularly, in the humid and industrialized Eastern regions that showed increased incidences in this study. The study was limited by the surveillance questionnaire used for the lack of objective clinical data that address the factual factors and precise diagnosis of the degree and type of the HL in the country. However, as a baseline study, important trends in public opinion were revealed that need to be substantiated by experiments and objective data. In addition, since this was a short-term study, the sample size may not represent the breadth of the regional and national trends of the national population in the HL.
Author Contributions
All authors contributed equally in all parts of acquisition of data, analysis and interpretation as explained below: Conceptualization and design, Kamaleldin B Said; Abdullah D.Alotaibi; Data acquisition, curation, and formal analysis Abdullah D.Alotaibi, Kamaleldin B Said, Abdelhafiz I. Bashir3 Ruba Ahmed, Mona Alonazi, Bassam Alshammari, Lama B. Abdulkarim, Amal A. Al-Otaibi, Bashayr Alsuwayt, Wafi A. Alrashidi, Razan S Alsubaie, Manar A ALGhaslan, Arwa A Alotaibi2, Mohammad S. Alzughaibi2, Fahad M.Alshammary2; Salem A. Almijrad; Anwar E. Almallahi; Funding acquisition, Abdullah D.Alotaibi and Kamaleldin B Said; Investigation, Kamaleldin B Said; Abdullah D.Alotaibi; Methodology, Abdullah D.Alotaibi, Kamaleldin B Said, Abdelhafiz I. Bashir3 Ruba Ahmed, Mona Alonazi, Bassam Alshammari, Lama B. Abdulkarim, Amal A. Al-Otaibi, Bashayr Alsuwayt, Wafi A. Alrashidi, Razan S Alsubaie, Manar A ALGhaslan, Arwa A Alotaibi2, Mohammad S. Alzughaibi2, Fahad M.Alshammary2; Salem A. Almijrad; Anwar E. Almallahi; Project administration, Abdullah D.Alotaibi and Kamaleldin B Said; Resources, Abdullah D.Alotaibi, Kamaleldin B Said, Abdelhafiz I. Bashir3 Ruba Ahmed, Mona Alonazi, Bassam Alshammari, Lama B. Abdulkarim, Amal A. Al-Otaibi, Bashayr Alsuwayt, Wafi A. Alrashidi, Razan S Alsubaie, Manar A ALGhaslan, Arwa A Alotaibi2, Mohammad S. Alzughaibi2, Fahad M.Alshammary2; Salem A. Almijrad; Anwar E. Almallahi; Supervision, Kamaleldin B Said and Mona . Alonazi; Data normalization, visualization and Validation, Abdullah D.Alotaibi, Kamaleldin B Said, Abdelhafiz I. Bashir3 Ruba Ahmed, Mona Alonazi, Bassam Alshammari, Lama B. Abdulkarim, Amal A. Al-Otaibi, Bashayr Alsuwayt, Wafi A. Alrashidi, Razan S Alsubaie, Manar A ALGhaslan, Arwa A Alotaibi2, Mohammad S. Alzughaibi2, Fahad M.Alshammary2; Salem A. Almijrad; Anwar E. Almallahi; Writing – original draft, Kamaleldin B Saidi; Abdullah D.Alotaibi; Writing – substantially revised or critically reviewed the article: Abdullah D.Alotaibi, Kamaleldin B Said, Abdelhafiz I. Bashir3 Ruba Ahmed, Mona Alonazi, Bassam Alshammari, Lama B. Abdulkarim, Amal A. Al-Otaibi, Bashayr Alsuwayt, Wafi A. Alrashidi, Razan S Alsubaie, Manar A ALGhaslan, Arwa A Alotaibi2, Mohammad S. Alzughaibi2, Fahad M.Alshammary2; Salem A. Almijrad; Anwar E. Almallahi.
Funding
This research received no external funding.
Institutional Review Board Statement
The standard guidelines were followed during this research according to the IRB protocols. The ethical application for this study was reviewed and (Approved) by the Research Ethics Committee (REC) of the University of Ha’il (KSA)), dated 4/3/2024, REC# H-2024-067.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
no additional data is available elsewhere other than this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Deafness and hearing loss. https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss (accessed 2024-03-01).
- Sooriyamoorthy, T.; Jesus, O. De. Conductive Hearing Loss. Medicina 2023, 38, 245–252. [Google Scholar] [CrossRef]
- Tanna, R. J.; Lin, J. W.; De, O.; Affiliations, J. Sensorineural Hearing Loss. 2023.
- Schmidt, W.; Sarran, C.; Ronan, N.; Barrett, G.; Whinney, D.J.; Fleming, L.E.; Osborne, N.J.; Tyrrell, J. The Weather and Ménière's Disease: A Longitudinal Analysis in the UK. Otol. Neurotol. 2017, 38, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.I.; Nelson, R.Y.; Concha-Barrientos, M.; Fingerhut, M. The global burden of occupational noise-induced hearing loss. Am. J. Ind. Med. 2005, 48, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Kerr, M.J.; Neitzel, R.L.; Hong, O.; Sataloff, R.T. Historical review of efforts to reduce noise-induced hearing loss in the United States. Am. J. Ind. Med. 2017, 60, 569–577. [Google Scholar] [CrossRef]
- Mirza, R.; Kirchner, D.B.; Dobie, R.A.; Crawford, J. Occupational Noise-Induced Hearing Loss. J. Occup. Environ. Med. 2018, 60, e498–e501. [Google Scholar] [CrossRef]
- Masterson, E.A.; Tak, S.; Themann, C.L.; Wall, D.K.; Groenewold, M.R.; Deddens, J.A.; Calvert, G.M. Prevalence of hearing loss in the United States by industry. Am. J. Ind. Med. 2013, 56, 670–681. [Google Scholar] [CrossRef]
- Tak, S.; Davis, R.R.; Calvert, G.M. Exposure to hazardous workplace noise and use of hearing protection devices among US workers—NHANES, 1999–2004. Am. J. Ind. Med. 2009, 52, 358–371. [Google Scholar] [CrossRef]
- Masterson, E.A.; Bushnell, P.T.; Themann, C.L.; Morata, T.C. Hearing Impairment Among Noise-Exposed Workers — United States, 2003–2012. Mmwr. Morb. Mortal. Wkly. Rep. 2019, 65, 389–394. [Google Scholar] [CrossRef]
- Ahmed, H.O.; Dennis, J.H.; Ballal, S.G. The accuracy of self-reported high noise exposure level and hearing loss in a working population in Eastern Saudi Arabia. Int. J. Hyg. Environ. Health 2004, 207, 227–234. [Google Scholar] [CrossRef]
- Alabdulwahhab, B.M.; I Alduraiby, R.; A Ahmed, M.; I Albatli, L.; Alhumain, M.S.; A Softah, N.; Saleh, S. Hearing loss and its association with occupational noise exposure among Saudi dentists: a cross-sectional study. BDJ Open 2016, 2, 16006. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A. H.; Alkharboush, G.; Almazyed, O.; Alhilal, S.; Alnajashi, I.; Al-Andejani, T.; Momani, M. Al; Hagr, A. Prevalence of Hearing Loss and Tinnitus with Correlation to the Usage of Protective Hearing Equipment among Airport Workers. Journal of Nature and Science of Medicine 2018, 1, 31–35. [Google Scholar] [CrossRef]
- Walling, A. D.; Dickson, G.M. Hearing Loss in Older Adults – Who’s Listening? JAMA : the journal of the American Medical Association 2012, 307, 1147. [Google Scholar] [CrossRef]
- Masè, M.; Viziano, A.; Strapazzon, G.; Alessandrini, M.; Micarelli, A. Auditory function in humans at high altitude. A scoping review. PLOS ONE 2023, 18, e0291919. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.H.; Newman, S.; Imray, C.H. The cerebral effects of ascent to high altitudes. Lancet Neurol. 2009, 8, 175–191. [Google Scholar] [CrossRef]
- Basu, A. Middle-Ear Pain and Trauma during Air Travel. BMJ Clin Evid 2007, 2007.
- Stangerup, S.-E.; Tjernström, .; Harcourt, J.; Klokker, M.; Stokholm, J. Barotitis in children after aviation; prevalence and treatment with Otovent®. J. Laryngol. Otol. 1996, 110, 625–628. [CrossRef]
- Ramzan, K.; Al-Owain, M.; Al-Numair, N.S.; Afzal, S.; Al-Ageel, S.; Al-Amer, S.; Al-Baik, L.; Al-Otaibi, G.F.; Hashem, A.; Al-Mashharawi, E.; et al. Identification of TMC1 as a relatively common cause for nonsyndromic hearing loss in the Saudi population. Am. J. Med Genet. Part B: Neuropsychiatr. Genet. 2020, 183, 172–180. [Google Scholar] [CrossRef]
- Shi, X.; Liu, X.; Sun, Y. The Pathogenesis of Cytomegalovirus and Other Viruses Associated with Hearing Loss: Recent Updates. Viruses 2023, 15, 1385. [Google Scholar] [CrossRef]
- Van Kerschaver, E.; Boudewyns, A.N.; Declau, F.; Van de Heyning, P.H.; Wuyts, F.L. Socio-demographic determinants of hearing impairment studied in 103 835 term babies. Eur. J. Public Health 2012, 23, 55–60. [Google Scholar] [CrossRef]
- Almaayeh, M.; Al-Musa, A.; Khader, Y.S. Prevalence of noise induced hearing loss among Jordanian industrial workers and its associated factors. Work 2018, 61, 267–271. [Google Scholar] [CrossRef]
- Alateeq, M.; Alnizari, O.; A Hafiz, T. Measuring the Effect of Smoking on Hearing and Tinnitus Among the Adult Population in the Kingdom of Saudi Arabia. Cureus 2023, 15, e39689. [Google Scholar] [CrossRef]
- Muñiz, J.F.; Ventura, A.M.; Algarra, J.M. Estudio de la correlación existente entre el efecto supresor contralateral y la fatiga auditiva mediante otoemisiones acústicas transitorias. Acta Otorrinolaringol. espanola 2006, 57, 199–203. [Google Scholar] [CrossRef]
- Aloufi, N.; Heinrich, A.; Marshall, K.; Kluk, K. Sex differences and the effect of female sex hormones on auditory function: a systematic review. Front. Hum. Neurosci. 2023, 17, 1077409. [Google Scholar] [CrossRef] [PubMed]
- Heine, C.; Browning, C.; Cowlishaw, S.; Kendig, H. Trajectories of older adults' hearing difficulties: Examining the influence of health behaviors and social activity over 10 years. Geriatr. Gerontol. Int. 2013, 13, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Lien, K.-H.; Yang, C.-H. Sex Differences in the Triad of Acquired Sensorineural Hearing Loss. Int. J. Mol. Sci. 2021, 22, 8111. [Google Scholar] [CrossRef]
- Khanal, P.; Acharya, S.; Lageju, N. Pattern of Hearing Loss Among Patients Attending ENT Department of a Tertiary Hospital in Nepal: A Retrospective Study. Indian J. Otolaryngol. Head Neck Surg. 2021, 74, 1–4. [Google Scholar] [CrossRef]
- Alqarny, M.; Assiri, A.M.; Alshehri, A.; Alharbi, S.M.; Alshahrani, E.H.; Alessa, H.; A Alghubishi, S. Patterns and Correlations of Hearing Loss Among Adolescents, Adults, and Elderly in Saudi Arabia: A Retrospective Study. Cureus 2021, 13. [CrossRef]
- Feder, K.P.; Michaud, D.; McNamee, J.; Fitzpatrick, E.; Ramage-Morin, P.; Beauregard, Y. Prevalence of Hearing Loss Among a Representative Sample of Canadian Children and Adolescents, 3 to 19 Years of Age. Ear Hear. 2017, 38, 7–20. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Z.; Lu, Z.; Ruan, W.; Yang, M.; Wang, D. The prevalence and global burden of hearing loss in 204 countries and territories, 1990–2019. Environ. Sci. Pollut. Res. 2022, 29, 12009–12016. [Google Scholar] [CrossRef]
- Davis, A.; McMahon, C.M.; Pichora-Fuller, K.M.; Russ, S.; Lin, F.; Olusanya, B.O.; Chadha, S.; Tremblay, K.L. Aging and Hearing Health: The Life-course Approach. Gerontol. 2016, 56, S256–S267. [Google Scholar] [CrossRef]
- A Al-Abduljawad, K.; Zakzouk, S.M. The prevalence of sensorineural hearing loss among Saudi children. Int. Congr. Ser. 2003, 1240, 199–204. [Google Scholar] [CrossRef]
- Alqarny, M.; Assiri, A.M.; Alshehri, A.; Alharbi, S.M.; Alshahrani, E.H.; Alessa, H.; A Alghubishi, S. Patterns and Correlations of Hearing Loss Among Adolescents, Adults, and Elderly in Saudi Arabia: A Retrospective Study. Cureus 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, J.M.; Burch, K.A.; Hanrahan, J.; Garza, J.L.; Dugan, A.G. Safety climate, hearing climate and hearing protection device use among transportation road maintainers: Hearing Climate and HPD Use. Am. J. Ind. Med. 2019, 62, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, B.; McMahon, C.; Tang, D.; Burlutsky, G.; Mitchell, P. Workplace noise exposure and the prevalence and 10-year incidence of age-related hearing loss. PLOS ONE 2021, 16, e0255356. [Google Scholar] [CrossRef] [PubMed]
- Usami, S.-I.; Nishio, S.-Y. The genetic etiology of hearing loss in Japan revealed by the social health insurance-based genetic testing of 10K patients. Hum. Genet. 2021, 141, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Korver, A.M.H.; Smith, R.J.H.; Van Camp, G.; Schleiss, M.R.; Bitner-Glindzicz, M.A.K.; Lustig, L.R.; Usami, S.-I.; Boudewyns, A.N. Congenital hearing loss. Nat. Rev. Dis. Prim. 2017, 3, 1–17. [Google Scholar] [CrossRef]
- Hrapcak, S.; Kuper, H.; Bartlett, P.; Devendra, A.; Makawa, A.; Kim, M.; Kazembe, P.; Ahmed, S. Hearing Loss in HIV-Infected Children in Lilongwe, Malawi. PLOS ONE 2016, 11, e0161421. [Google Scholar] [CrossRef]
- Maia, C.A.S.; de Campos, C.A.H. Diabetes mellitus as etiological factor of hearing loss. Braz. J. Otorhinolaryngol. 2015, 71, 208–214. [Google Scholar] [CrossRef]
- AlHarbi, M.; Othman, A.; Nahari, A.A.; Al-Jedai, A.H.; Cuadras, D.; Almalky, F.; AlAzmi, F.; Almudaiheem, H.Y.; AlShubrumi, H.; AlSwat, H.; et al. Burden of Illness of Type 2 Diabetes Mellitus in the Kingdom of Saudi Arabia: A Five-Year Longitudinal Study. Adv. Ther. 2024, 41, 1120–1150. [Google Scholar] [CrossRef]
- Sasso, F.C.; Salvatore, T.; Tranchino, G.; Cozzolino, D.; Caruso, A.A.; Persico, M.; Gentile, S.; Torella, D.; Torella, R. Cochlear dysfunction in type 2 diabetes: A complication independent of neuropathy and acute hyperglycemia. Metabolism 1999, 48, 1346–1350. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).