1. Introduction
The solar energy sector has experienced significant growth in recent years. Solar PV technology is now widely considered as one of the most promising routes to decarbonizing the energy industry and combating climate change. As the implementation of photovoltaic technology expands, so does the consumption of silicon, silver and other resources required in the manufacture of the solar panels. The rapid increase in solar panel deployment has led to a significant accumulation of end-of-life photovoltaic waste, posing challenges in waste management and resource recovery due to the complexity and hazardous nature of the materials involved, e.g. lead [
1]. The current production capacities of the resources will not satisfy current market demand especially with regards to fulfilling the Paris agreement climate goals. Further, although solar panels have a user-life of 25-30 years, upgrades in technology have made way for a build-up in waste of discarded or damaged panels and this will only get worse in the foreseeable future [
2]. This situation requires efficient resource management [
3] and more importantly, cradle-to-cradle recycling methods that will see the bulk of the materials recycled back into the manufacturing of new panels. Currently, there is no recycling process that fulfills these cradle-to-cradle principles. The RETRIEVE project aims to solve this challenge by innovating the circularity in the PV industry via developing and improving state-of-the-art recycling processes in the upstream industry. RETRIEVE will develop cradle-to-cradle recycling processes for components of end-of-life (EoL) PV panels that will upcycle their quality to meet the current requirements of newly manufactured Made in Europe PV panels.
As part of this project, this study focuses on the recovery of silver (Ag) and aluminum (Al) from the cell fraction along with recommended methods for recovering valuable metals from EoL silicon. Thus far, several studies [
4,
5,
6,
7,
8,
9,
10,
11,
12] have been made on metal recovery from recycled PV panels at lab and at pilot scale. The focus was generally on recovering silicon, copper and aluminum as silver recovery needs intensive extractive metallurgical methods [
13]. Silver has the most economic value among the metals. However, the handling of other metals, particularly Pb is of great importance as it is toxic and hence an environmental threat. To establish an innovative, environmentally friendly, and high yield process of metals, the regeneration and re-use of process chemicals must also be considered along with the mitigation of the hazardous impurities. The study will evaluate the leaching and recovery of Ag and Al, combined with methods to remove Pb, resulting in a flowsheet that not only recovers the valuable metals and removes hazardous elements, but one that is innovative, environmentally friendly, and sustainable; incorporating process chemicals can be possibly regenerated for re-use or processes that produce useful by-products.
Nitric acid is widely used in the leaching of silver from various feed materials [
14,
15,
16,
17]. It is proposed that the Ag is leached in nitric acid, recovered as AgCl and reduced to metallic silver using hydrazine [
16,
17,
18]. The process occurs according to equations 1-3.
The above process is also expected to co-leach other elements, specifically toxic ones like Pb. This study incorporates a two-stage leaching system in which HCl will be used as a pre-leach step to extract Al prior to the leaching of Ag. Furthermore, the study also investigates the use of ion exchange (IX) resins in removing Pb from solution in favor of the conventional method PbSO
4 precipitation [
19,
20]. The IX resins do not add chemicals to the process, and removing the Pb from the resins with acid, regenerates them for continued use.
2. Materials and Methods
The sample used for test work was silicon solar cells supplied by the Soltek department at the Institute of Energy Technology (IFE, Norway). The solar cells were milled down to 400 µm for test work and analyses. The chemical composition of the solar cells was identified by as follows: A sample of the cells was completely digested in acids and afterwards diluted for analysis by high resolution ICP-MS (Element XR, Thermo Fisher Scientific). The same procedure was used to quantify the elements in leached solar cell samples.
Table 1 shows the main elements except for silicon and possibly other very minor elements. The elements of significance were Al, Ag and Pb.
All leaching tests were conducted in a three-neck 250 ml round bottomed flask using 200 ml of solution. Technical reagent grade 37% HCl and 65% HNO3 were used to make all the leaching solutions. 20 g samples of ball milled solar cells were leached with the desired solution, with the mixture being agitated using a magnetic stirrer and heated to the desired temperature using a heating mantle. For tests at temperatures higher than room temperature, the temperature was monitored using a thermometer while the slurry volume was maintained using a water-cooled condenser placed at the central neck of the flask. For the tests at elevated temperature, the time it took for the temperature to reach the desired point was included in the duration of the test. Samples were withdrawn, and syringe filtered with a 0.45 µm membrane, at various intervals of each test to be analyzed via XRF to determine the extent of metal extraction at that point. After each analysis, the sample was returned to the flask. On completion of the tests, the slurry was allowed to cool when necessary, and filtered using a porosity 4 funnel filter and then the filtrate was further filtered using 0.45 µm syringe filters. While still in the filter funnel, the solid residues were washed thoroughly by filtering through tap and then finally distilled water. The residues were either immediately used in the next stage leaching (from HCl leaching) or oven-dried at 65°C overnight at the end of the second stage leach for analysis by means of ICP-MS, XRF or XRD.
Metal extractions were calculated using the following two methods:
- 2
by analyzing the solid residues to determine the amounts left in the residue
For the Al recovery tests, the leachate from the HCl leach was placed in a beaker with a pH probe. Granular NaOH was added 2 g at a time while pH was monitored. Solution samples were analyzed via XRF at various intervals to determine when all the Al had been precipitated. When this was achieved, the slurry was filtered using a porosity 4 funnel filter. While in the filter, the Al precipitate was washed with water, collected in a beaker and oven dried at 65°C overnight for XRF and XRD analysis. The filtrate was further filtered using a 0.45 µm syringe filter for XRF analysis. This was to further confirm the Al had been completely precipitated.
The Ag recovery from the HNO3 leachate was conducted by adding 1 ml of 37% HCl at room temperature to the leachate in a 250 ml beaker. This created a milky solution which was allowed to settle for easy filtration for 4 hrs. A sample was taken from the supernatant solution, filtered using a 0.45 µm syringe filter and analyzed via XRF. The analysis confirmed that most of the Ag had precipitated. The supernatant was allowed to sit over night before being decanted to separate it from the AgCl precipitate, and further filtered with a 0.45 µm syringe filter. The precipitate was washed with water. A solution of 5 ml hydrazine and 2 g of NaOH dissolved in 50 ml of deionized water was prepared. The solution was added to the AgCl in a beaker and stirred for a few minutes. This was used to reduce the silver chloride to metallic Ag. The solution was decanted, and the solid residue washed thoroughly with distilled water. It was then oven dried over night at 65°C for analysis via XRF and XRD.
Pb removal from the decanted and filtered HNO3 solution above, after the Ag precipitation, was investigated using Puromet™MTS9300H resin, supplied by Purolite, an Ecolab Company (France). 50 ml of leachate was placed in an Erlenmeyer flask. 1 g of resin was added, and the flask was shaken at a rate of 600 rpm using a platform shaker for 1 hour. A sample was withdrawn and analyzed via XRF to determine the extent of Pb removal.
3. Results and Discussion
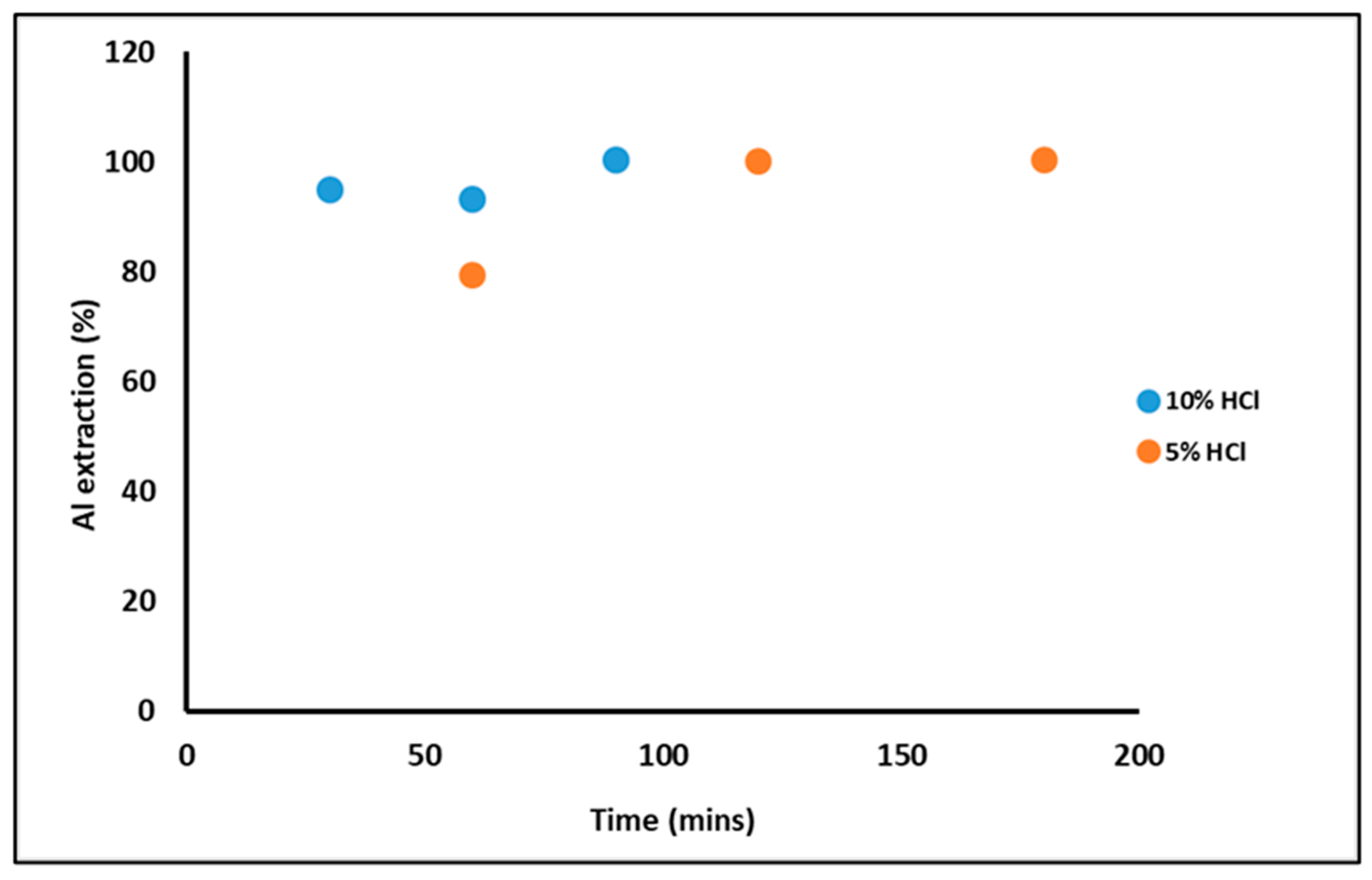
From several tests conducted, results presented and discussed will be mostly from two tests (Sample 1 and Sample 2). The HCl pre-leach achieved nearly 100% extraction of Al in tests, as confirmed by XRF analysis of the solution. Using a 5 % HCl (Sample 1) requires more time than a 10 % HCl (Sample 2) solution but shows the same extraction results of Al (
Figure 1).
Analysis of the leached residues after the HNO
3 leach showed that up to 99% of the Al had been leached in both cases. It should be noted that it was not possible to analyze the residue after the HCl leach as at least half the sample was required for the analysis. Further, the material could not be returned for the second stage leach test. Negligible quantities of Ag, in the range of 20 ppm, were leached along with some Pb in the range of 70 ppm. It was noted that at higher temperatures (50°C) more Pb was leached, however in the follow up HNO
3 acid leach, the Ag extraction was considerably poor. It is hypothesized that at high temperature, a small amount of the Ag is leached and immediately precipitates as insoluble AgCl coating the rest of the Ag. This passivates the Ag and prevents it from leaching in the HNO
3 acid. It was thus decided that the optimal leaching temperature for the first stage was room temperature. For the Al precipitation using NaOH, it was found that all the Al precipitated at near neutral pH of 6.54. For the nearly 200 ml of HCl leachate solution, 12 g of NaOH was required to achieve this pH and precipitate all the Al. The process was suspected to occur as follows:
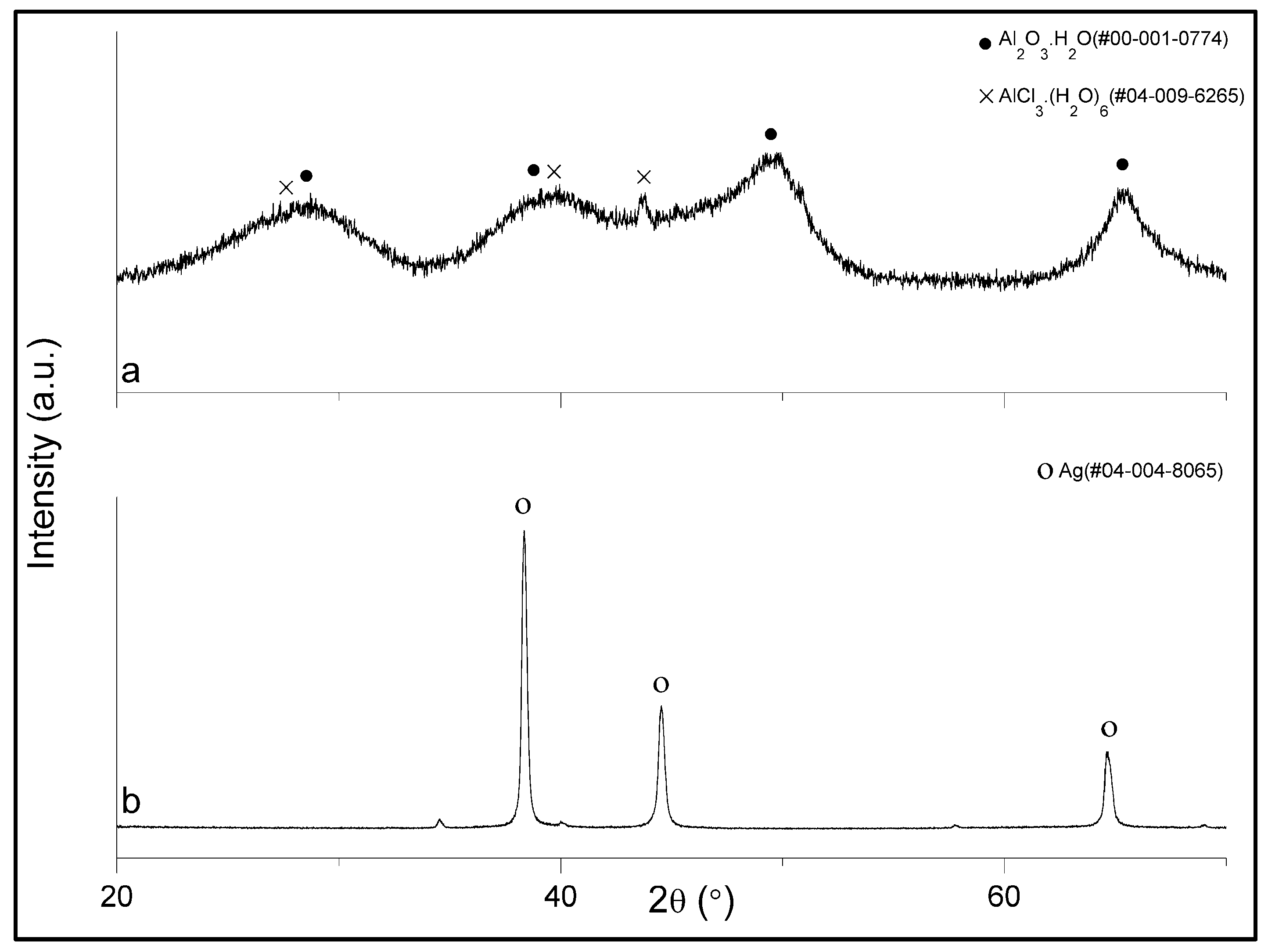
XRF analysis confirmed the presence of Al with some Cl and possible Fe, Cu, Zn and Pb impurities which could not be quantified. From this it was suspected that the precipitate was AlCl
3 with some impurities. However, XRD analysis on this amorphous precipitate showed that the precipitate was composed of mostly Al-oxides, with possibly various contamination of Cl, Fe, Cu, Zn and Pb (
Figure 2). It should be noted that except for Pb, the other elements were present in the HCl leachate solution in quantities below detection limit, according to the XRF analysis. Further, XRF analysis of the solution after Al-precipitation did not detect any heavy elements. It is thus concluded that the solution is mostly a brine. It might be possible to consider that reverse osmosis (RO) be explored to recover the water and produce NaCl as a saleable product. This process can be referred to as desalination and has long been established at industrial level [
21,
22]. The XRF analysis also showed no heavy metals in the solution. This means that the water produced from the proposed RO process can be recycled back into the main process, specifically in the HCl leaching. At a larger operating scale, if heavy metals are present and they accumulate to high levels or it is decided the water needs to be discharged, methods such as precipitation, ion exchange resins, solvent extraction, or adsorption [
23] can be explored to remove them.
In the HNO
3 leach, XRF analysis of the solution samples confirmed that 0.5 M HNO
3 at 80°C for 2 hrs was sufficient to extract 99% of the Ag. This was further confirmed by analysis of the solid residue samples (
Table 3).
The leaching patterns were identical for both Samples 1 and 2 as constructed from XRF analysis of the solution samples.
Figure 3 shows the leaching pattern for Sample 2 where it is seen that a dramatic increase occurs in Ag extraction from 55 to 72°C. Some co-leaching of Pb in the concentration of 160 ppm was also experienced. However, Ag was the element leached in the most abundance in the range of 1300 ppm. Furthermore, there were around 280 ppm Al in the leachate solution. This varied with different tests, as some did not have any. This small amount of Al compared to the HCl leachate which had 14
,000 ppm confirms the bulk of the Al is leached in the HCl step even though the residue could not be analyzed after that leaching step as a large portion of the residue is required for the analysis. The follow-up precipitation process recovered 99% of the Ag in solution based on XRF analysis of the solution before and after precipitation. Overall, this represents a 98% recovery of Ag from silicon. The reduction process produced a product that according to XRD analysis, was metallic and mostly silver (
Figure 3). XRF analysis showed the Ag was of >99.9% purity with some possible contamination of Pb and Fe. Around 160 ppm Pb precipitated, based on XRF analysis of the solution after precipitation. The resulting effluent is thus mostly regenerated and unreacted HNO
3, as per equation 1, and 160 ppm of Pb. It is worth noting that the solution can be re-used in the process. When the Pb accumulates to unacceptable levels, or it is decided to discharge the solution, IX resin system can be used to remove the Pb. This study achieved successful Pb removal using the Puromet™MTS9300H resin. XRF analysis of the solution showed the concentration dropped from 160 ppm to 7 ppm after an hour of treatment with the resin.
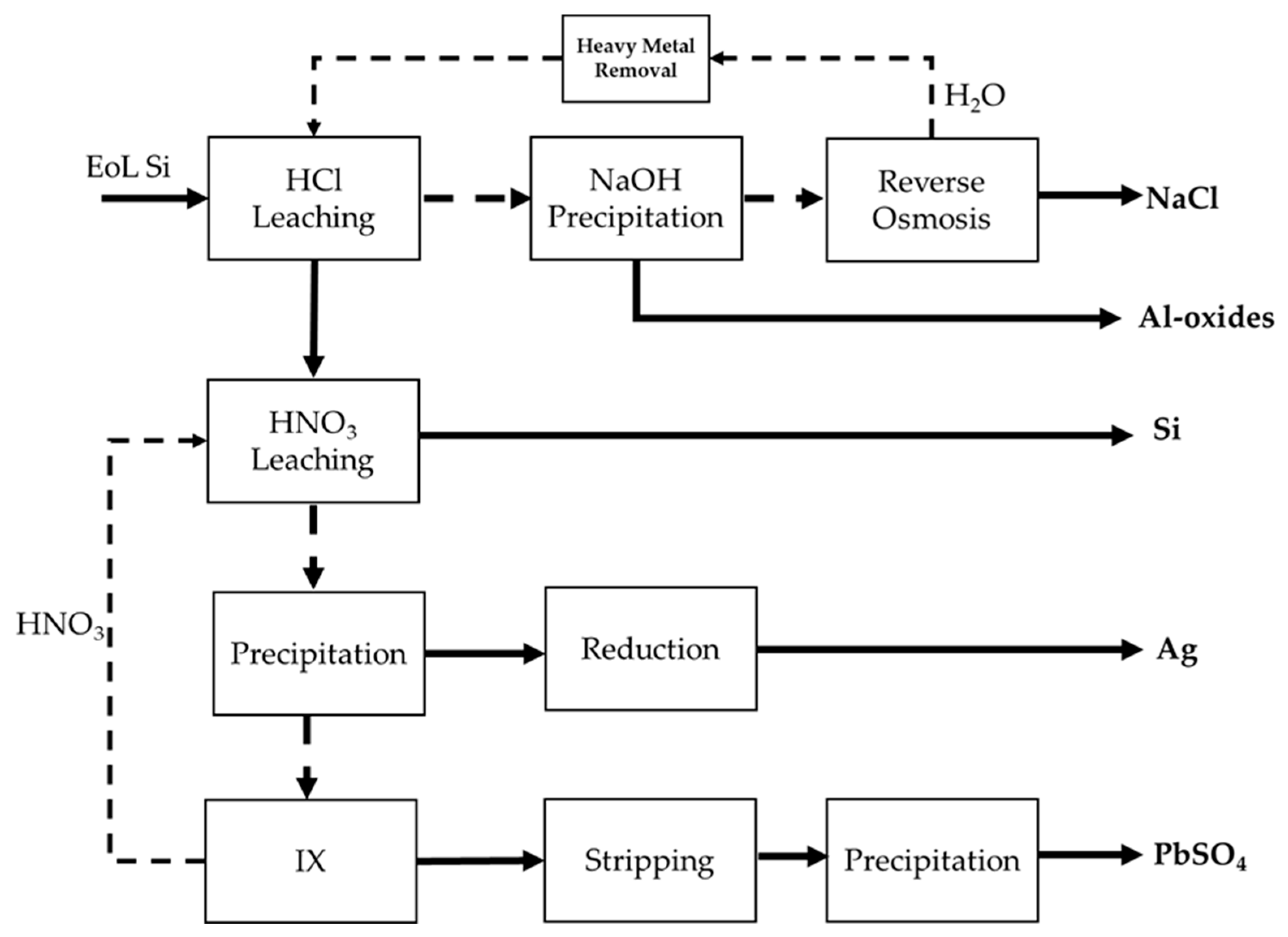
The above experimental results are presented and summarized in a flowsheet,
Figure 4 and
Table 3. The flowsheet incorporates elements tested and proven in the laboratory, such as Ag and Al extraction and recovery and Pb removal. Other elements of the flowsheet are conceptual and are yet to be tested such as the RO stage, heavy metal removal and Pb stripping and resin regeneration.
4. Conclusions
This study demonstrates a two-step leaching process for efficiently recovering silver (Ag) and aluminum (Al) from the silicon (Si) of end-of-life (EoL) photovoltaic (PV) panels, resulting in the development of a conceptual flowsheet. In the first step, Al is leached using 5% HCl at low temperature for 3 hours, achieving a 99% recovery rate. The Al is precipitated as Al-oxides using NaOH, generating a brine effluent solution that could potentially be treated with reverse osmosis to produce NaCl and water for recycling within the process. The remaining Si residue is then leached with 0.5 M HNO₃ at 80°C for 2 hours to extract 99% Ag, which is subsequently recovered by precipitation with HCl and reduced to metallic Ag with a purity of >99.9% using hydrazine, achieving an overall Ag recovery of 98%. Additionally, lead (Pb) can be effectively removed from the HNO₃ solution after Ag precipitation using Puromet™ MTS9300H resins.
The integration of sequential leaching with ion exchange resins for Pb removal provides a novel approach that enhances metal recovery while minimizing environmental impact, distinguishing this method from existing recycling techniques. Although chemical regeneration is proposed for future research to further reduce waste and operational costs, the current study lays a strong foundation for sustainable, large-scale PV recycling. The promising results highlight the need for further evaluation and optimization through additional laboratory work to confirm scalability and economic feasibility, aligning with circular economy principles and sustainable resource management.
Author Contributions
“Conceptualization, J.M.M. and D.Y.; methodology, J.M.M.; software, J.M.M., S.M. and S.W.; validation, J.M.M., S.M. and S.W.; formal analysis, J.M.M., S.M. and S.W.; investigation, J.M.M.; resources, J.M.M., S.M., S.W. and D.Y.; data curation, J.M.M., S.M. and S.W.; writing—original draft preparation, J.M.M and D.Y.; writing—review and editing, J.M.M., S.M., S.W. and D.Y.; visualization, J.M.M., S.M., S.W. and D.Y.; supervision, D.Y.; project administration, J.M.M and D.Y.; funding acquisition, J.M.M and D.Y. All authors have read and agreed to the published version of the manuscript.”
Funding
This project has received funding from the European Union’s Horizon Europe research and innovation program under grant agreement No 101122332.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not Applicable.
Acknowledgments
We acknowledge the support of our project partners in the RETRIEVE project and colleagues in our respective organizations.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shrestha, N.; Zaman, A. 2024. Decommissioning and Recycling of End-of-Life Photovoltaic Solar Panels in Western Australia. Sustainability, 16, 526. [CrossRef]
- Ardente, F., Latunussa, C.E.L., Blengini, G.A. 2019. Resource efficient recovery of critical and precious metals from waste silicon PV panel recycling. Waste Management, Vol. 91, 15 May, 156-167. [CrossRef]
- Fiandra, V., Sannino, L., Andreozzi, C., Graditi, G. 2019. End-of-life of silicon PV panels: A sustainable materials recovery process. Waste Management, Vol. 84, 1 February 2019, Pages 91-101. [CrossRef]
- Dias, P., Javimczik, S., Benevit, M., Veit, H., Bernardes, A. M. 2016. Recycling WEEE: Extraction and concentration of silver from waste crystalline silicon photovoltaic modules. Waste Management, Vol. 57, 220-225. [CrossRef]
- Oliveira, L.S.S., Lima, M.T.W.D.C., Yamane, L.H., Siman, R.R. 2020. Silver recovery from end-of-life photovoltaic panels. Multidisciplinary J. of Waste Resources & Residues, Vol. 10, 62-74. 6.
- Tao et al. 2020. Major challenges and opportunities in silicon solar module recycling. Prog Photovolt, Res Appl., 1-12. [CrossRef]
- Kuczyńska-Łażewska, A., Klugmann-Radziemska, E., Sobcza, Z., Klimczuk, T. 2018. Recovery of silver metallization from damaged silicon cells. Solar Energy Materials and Solar Cells. Vol. 176, 190-195. [CrossRef]
- Nieland, S., Neuhaus, U., Pfaff, T., Rädlein, E. 2012. New approaches for component recycling of crystalline solar modules. 27th European Photovoltaic Solar Energy Conference and Exhibition, Frankfurt, January 2012.
- Palitzsch, W., Loser, U. 2012. Economic PV waste recycling solutions-Results from R&D and practice. 38th IEEE Photovoltaic Specialists Conference, Austin, TX, USA, 3-8 June 2012.
- Klugman-Radziemska, E., Ostrowski, P. 2010. Chemical treatment of crystalline silicon solar cells as a method of recovering pure silicon from photovoltaic modules. Renewable Energy, Vol. 35, Issue 8, 1751-1759. [CrossRef]
- Müller, A., Wambach, K., Alsema, E. 2005. Life cycle analysis of a solar module recycling process. 20th European Photovoltaic Solar Energy Conference, Barcelona, Spain 6-10 June 2005.
- Frission et al. Recent improvements in industrial PV module recycling. 2000. 16th European Photovoltaic Solar Energy Conference, Glasgow, UK, 1-5 May 2000.
- Azeumo, M. F., Germana, C., Ippolito, N. M., Franco, M., Luigi, P., Settimio, S. Photovoltaic module recycling, a physical and a chemical recovery process. Solar Energy Materials and Solar Cells, Vol. 193, 2019, 314-319. [CrossRef]
- Teknetzi, I., Holgersson, S., Ebin, B. 2023. Valuable metal recycling from thin film CIGS solar cells by leaching under mild conditions. Solar Energy Materials & Solar Cells 252 (2023) 112178. [CrossRef]
- Chen et al. 2020. Recovery of valuable metal from Photovoltaic solar cells through extraction. IOP Conf. Series: Materials Science and Engineering 720 (2020) 012007. [CrossRef]
- Tao, J., Yu, S. 2015. Review on feasible recycling pathways and technologies of solar photovoltaic modules, Solar Energy Mater. Solar Cells 141 Pages 108–124. [CrossRef]
- Potgieter, J.H., Potgieter, S.S., Mbaya, R.K.K., Teodorovic, A. 2004. Small-scale recovery of noble metals from jewellery wastes. The Journal of The South African Institute of Mining and Metallurgy. November 2004 104(10) 563-571.
- Hassan, S.M., Hussein, J.M.A., Ouda, S.A. 2016. The improved procedure of hydrazine dihydrochloride method to prepare Pt(II) chloride. Journal of Babylon University, Pure and Applied Sciences, No. 9, Vol. 24, 2554-2563.
- Luo et al. 2021. A comprehensive hydrometallurgical recycling approach for the environmental impact mitigation of EoL solar cells. Journal of Environmental Chemical Engineering, Vol. 9, Issue 6, 106830. [CrossRef]
- Neto, I.F.F., Soares, H.M.V.M. 2018. Sequential separation of Ag, Al, Cu and Pb from a multi-metal leached solution using a zero waste technology. Separation Science and Technology, Vol. 53, No. 18, 2961-2970. [CrossRef]
- Alkaisia, A., Mossad, R., Sharifian-Barforousha, A. 2017. A review of the water desalination systems integrated with renewable energy, Energy Procedia 110 (2017) 268 – 274. [CrossRef]
- Garud R. M., Kore S. V., Kore V. S., Kulkarni G. S. 2014. A Short Review on Process and Applications of Reverse Osmosis. Universal Journal of Environmental Research and Technology, Volume 1, Issue 3: 233-238.
- Rao K.S., Mohapatra M., Anand S., Venkateswarlu P. 2010. Review on cadmium removal from aqueous solutions. Int. J. of Eng., Sci. and Tech. Vol. 2, No. 7, 2010, 81-103. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).