Submitted:
23 September 2024
Posted:
24 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sampling Locations
2.2. Sampling Method
2.3. Incubation and Identification
2.4. Microscopic Examination
2.5. Molecular Analysis
2.6. Data Analysis
3. Results and Discussion
3.1. The Concentration and Diversity of Fungal Isolates from Indoor Air
3.2. Human Health Risks Associated with Indoor Fungi
3.3. Monitoring and Controlling Indoor Air Quality
3.4. Airborne Fungi Treatment
4. Conclusion
Acknowledgments
References
- Agarwal: R., Muthu, V., & Sehgal, I.S. (2024). Clinical Manifestation and Treatment of Allergic Bronchopulmonary Aspergillosis. Semin Respir Crit Care Med, 45(01), 114-127.
- Al Hallak, M., Verdier, T., Bertron, A., Roques, C., & Bailly, J.-D. (2023). Fungal Contamination of Building Materials and the Aerosolization of Particles and Toxins in Indoor Air and Their Associated Risks to Health: A Review. Toxins, 15(3), 175. [CrossRef]
- Alghamdi, R.G., Zabermawi, N.M., Altihani, F.A., Bokhari, F.M., Makki, R.M., Hassoubah, S.A., Sharawi, Z.W., & Najjar, A.A. (2023). Diversity and Density of Fungi Isolated from Dried Fruits. Journal of Biochemical Technology, 14(4), 45-55.
- Andersson, M.; Varga, A.; Mikkola, R.; Vornanen-Winqvist, C.; Salo, J.; Kredics, L.; Kocsubé, S.; Salonen, H. Aspergillus Was the Dominant Genus Found during Diversity Tracking of Potentially Pathogenic Indoor Fungal Isolates. 2022. Pathogens. 10(2), 22-34.
- American Air and Water (2020) Mold, mold spores and indoor air quality. American Air and Water. Available at https:// www. ameri canai randw ater. com/ mold Accessed on: 8 June 2020.
- Avilés-Robles, M., Gómez-Ponce, C., Reséndiz-Sánchez, J., Rodríguez-Tovar, A.V., Ceballos-Bocanegra, A., & Martínez-Rivera, Á. (2016). Disseminated penicilliosis due to Penicillium chrysogenum in a pediatric patient with Henoch–Schönlein syndrome. International Journal of Infectious Diseases, 51, 78-80.
- Belizario, J.A., Lopes, L.G., & Pires, R.H. (2021). Fungi in the indoor air of critical hospital areas: A review. Aerobiologia, 37, 379-394.
- Chawla, H., Anand, P., Garg, K., Bhagat, N., Varmani, S.G., Bansal, T., McBain, A.J., & Marwah, R.G. (2023). A comprehensive review of microbial contamination in the indoor environment: Sources, sampling, health risks, and mitigation strategies. Frontiers in Public Health, 11(2), 128-133.
- Chen, X., Wang, Y., Wang, Y., Zhang, Y., Shen, Y., He, X., & Xiao, C. (2023). A natural moisture gradient affects soil fungal communities on the south shore of Hulun Lake, Inner Mongolia, China. Journal of Fungi, 9(5), 549. [CrossRef]
- De Aguiar, C., R. (2020). Pocket Guide to Mycological Diagnosis (1st ed.). Taylor and Francis. CRC Press.
- Engel, A., Simler-Williamson, A., Ravenscraft, A., Bittleston, L., & de Graaf, M.-A. (2024). Interactive effects of fungal community structure and soil moisture on Wyoming big sagebrush performance. Plant and Soil. [CrossRef]
- Espinosa, K.C.S., Chávez, M.A., Duarte-Escalante, E., Flores, T.I.R., Frías-De-León, M.G., & Reyes-Montes, M.R. (2021). Phylogenetic Identification, Diversity, and Richness of Aspergillus from Homes in Havana, Cuba. Microorganisms, 9(1), 115. [CrossRef]
- Kumar, P., Kausar, M.A., Singh, A.B., & Singh, R. (2021). Biological contaminants in the indoor air environment and their impacts on human health. Air Quality, Atmosphere & Health, 14, 1723–1736.
- Loukou, E., Jensen, N.F., Rohde, L., & Andersen, B. (2024). Damp buildings: Associated fungi and how to find them. Journal of Fungi, 10(2), 108. [CrossRef]
- Lu, R.; Pørneki, A.D.; Lindgreen, J.N.; Li, Y.; Madsen, A.M. Species of Fungi and Pollen in the PM1 and the Inhalable Fraction of Indoor Air in Homes. Atmosphere 2021, 12, 404. [CrossRef]
- Nafis, M.M.H., Quach, Z.M., Al-Shaarani, A.A.Q.A., Muafa, M.H.M., & Pecoraro, L. (2023). Pathogenicity of Aspergillus Airborne Fungal Species Collected from Indoor and Outdoor Public Areas in Tianjin, China. Pathogens, 12(9), 1154. [CrossRef]
- Navale, V., Vamkudoth, K.R., Ajmera, S. and Dhuria, V. (2021). Navale, V., Vamkudoth, K.R., Ajmera, S. and Dhuria, V. (2021). Aspergillus derived mycotoxins in food and the environment: Prevalence, detection, and toxicity. Toxicology Reports, 8, 1008-1030. [CrossRef]
- Polizzi, V., Adams, A., Picco, A.M., Adriaens, E., Lenoir, J., Van Peteghem, C., De Saeger, S., & De Kimpe, N. (2011). Influence of environmental conditions on production of volatiles by Trichoderma atroviride in relation with the sick building syndrome. Building and Environment, 46(4), 945-954.
- Sánchez Espinosa, K.C., Rodríguez Davydenko, S., Rojas Flores, T.I., Venero Fernández, S.J., & Almaguer, M. (2022). Indoor air quality and diversity of fungi inside and outside residences of children with a history of allergy in Cuba. Grana, 61(4), 284-295.
- Sánchez Espinosa, K.C., Rodríguez Davydenko, S., Rojas Flores, T.I., Fernández-González, M., & Almaguer, M. (2024). Xerophilic and cellulolytic fungi in the indoor air of houses in Havana. International Biodeterioration & Biodegradation, 188, 105730.
- Sham, N.M., Ahmad, N.I., Pahrol, M.A., & Leong, Y.-H. (2021). Fungus and mycotoxins studies in hospital environment: A scoping review. Building and Environment, 193, 107626.
- Tabatabaei, Z., Rafiee, A., Abbasi, A., Mehdizadeh, A., Morovati, R., & Hoseini, M. (2020). Investigation of fungal contamination in indoor air and on surfaces of traditional public baths in a historical city. Journal of Environmental Health Science and Engineering, 18(2), 925–932. [CrossRef]
- Tang, J.W. (2015). Investigating the airborne transmission pathway – different approaches with the same objectives. Indoor Air, 25(2), 119–124. [CrossRef]
- Tian, X.G., Bao, D.F., Karunarathna, S.C., Jayawardena, R.S., Hyde, K.D., Bhat, D.J., Luo, Z.L., Elgorban, A.M., Hongsanan, S., Rajeshkumar, K.C., Maharachchikumbura, S.S.N., Suwannarach, N., Dawoud, T.M., Lu, Y.Z., Han, J.J., Xiao, Y.P., Du, T.Y., Lu, L., Xu, R.F., Dai, D.Q., Liu, X.F., Liu, C., & Tibpromma, S. (2024). Taxonomy and phylogeny of ascomycetes associated with selected economically important monocotyledons in China and Thailand. Mycosphere, 15(1), 1–274.
- Xing, H., Wang, J., Sun, Y., & Wang, H. (2022). Recent Advances in the Allergic Cross-Reactivity between Fungi and Foods. Journal of Immunology Research, 2022, Article ID 7583400. [CrossRef]
- WHO (2020) Coronavirus disease (COVID-19) World Health Organization situation reports 142. Available on: https:// www. who. int/docs/ defau lt- source/ coron aviru se/ situa tion- repor ts Accessed on-10 June 2020.
- Zhou, H., Xia, X., Wang, Y., Ma, Y., Zhao, Y., Wang, P., Tang, C., & Wang, P. (2023). A rare case of refractory facial phaeohyphomycosis caused by Cladosporium halotolerans successfully treated with aminolevulinic acid photodynamic therapy. Photodiagnosis and Photodynamic Therapy, 42, 103347.
| No. | Fungal isolates | Classrooms | Staff offices | Microbiology laboratories | Toilets | Total(CFU) |
|---|---|---|---|---|---|---|
| 1 | Alternaria sp. | 24 | 0 | 12 | 0 | 36 |
| 2 | Aspergillus flavus | 28 | 10 | 22 | 36 | 96 |
| 3 | A. niger | 80 | 40 | 38 | 25 | 183 |
| 4 | A. ustus | 19 | 0 | 0 | 0 | 19 |
| 5 | Cladosporium sp. | 64 | 29 | 20 | 22 | 135 |
| 6 | Curvularia sp. | 10 | 0 | 0 | 0 | 10 |
| 7 | Fusarium sp. | 0 | 22 | 0 | 3 | 25 |
| 8 | Paecilomyces variotii | 50 | 41 | 18 | 11 | 120 |
| 9 | Penicillium sp.1 | 44 | 50 | 17 | 28 | 139 |
| 10 | Penicillium sp.2 | 31 | 11 | 22 | 19 | 83 |
| 11 | Trichoderma sp. | 50 | 36 | 12 | 27 | 125 |
| Total | 400* | 239* | 161* | 171* | 971 | |
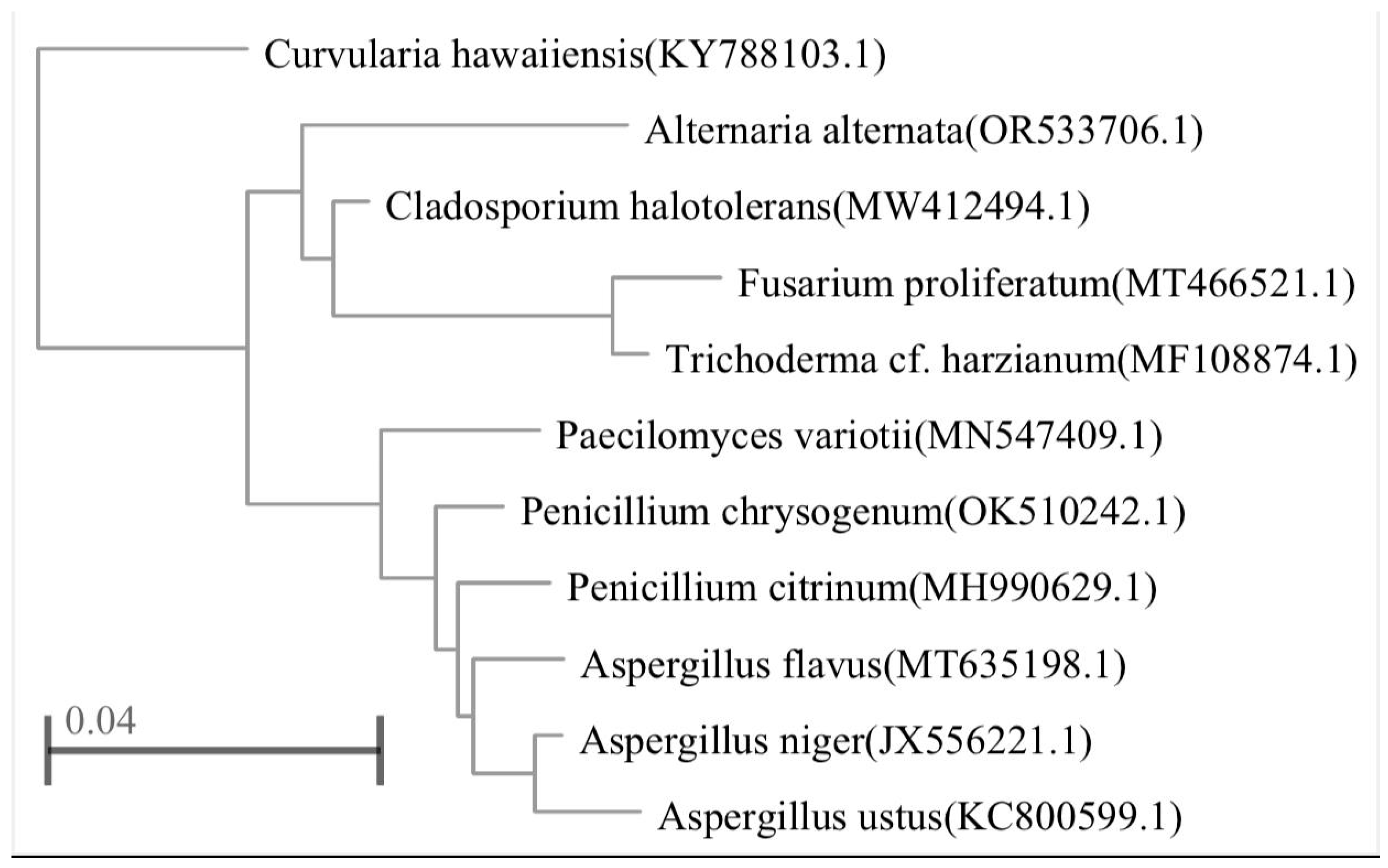
| Fungal accession numbers | Closest related species | Similarity (%) |
|---|---|---|
| Alternaria alternate | OR533706.1 | 100 |
| Aspergillus flavus | MT635198.1 | 89.29 |
| A. niger | JX556221.1 | 90 |
| A. ustus | KC800599.1 | 88.83 |
| Curvularia hawaiiensis | KY788103.1 | 87.32 |
| Fusarium proliferatum | MT466521.1 | 91.21 |
| Paecilomyces variotii | MN547409.1 | 88.57 |
| Penicillium chrysogenum | OK510242.1 | 89.69 |
| P. citrinum | MH990629.1 | 91.24 |
| Cladosporium halotolerans | MW412494.1 | 93.07 |
| Trichoderma harzianum | MF108874.1 | 91.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




