Submitted:
23 September 2024
Posted:
24 September 2024
You are already at the latest version
Abstract
Keywords:
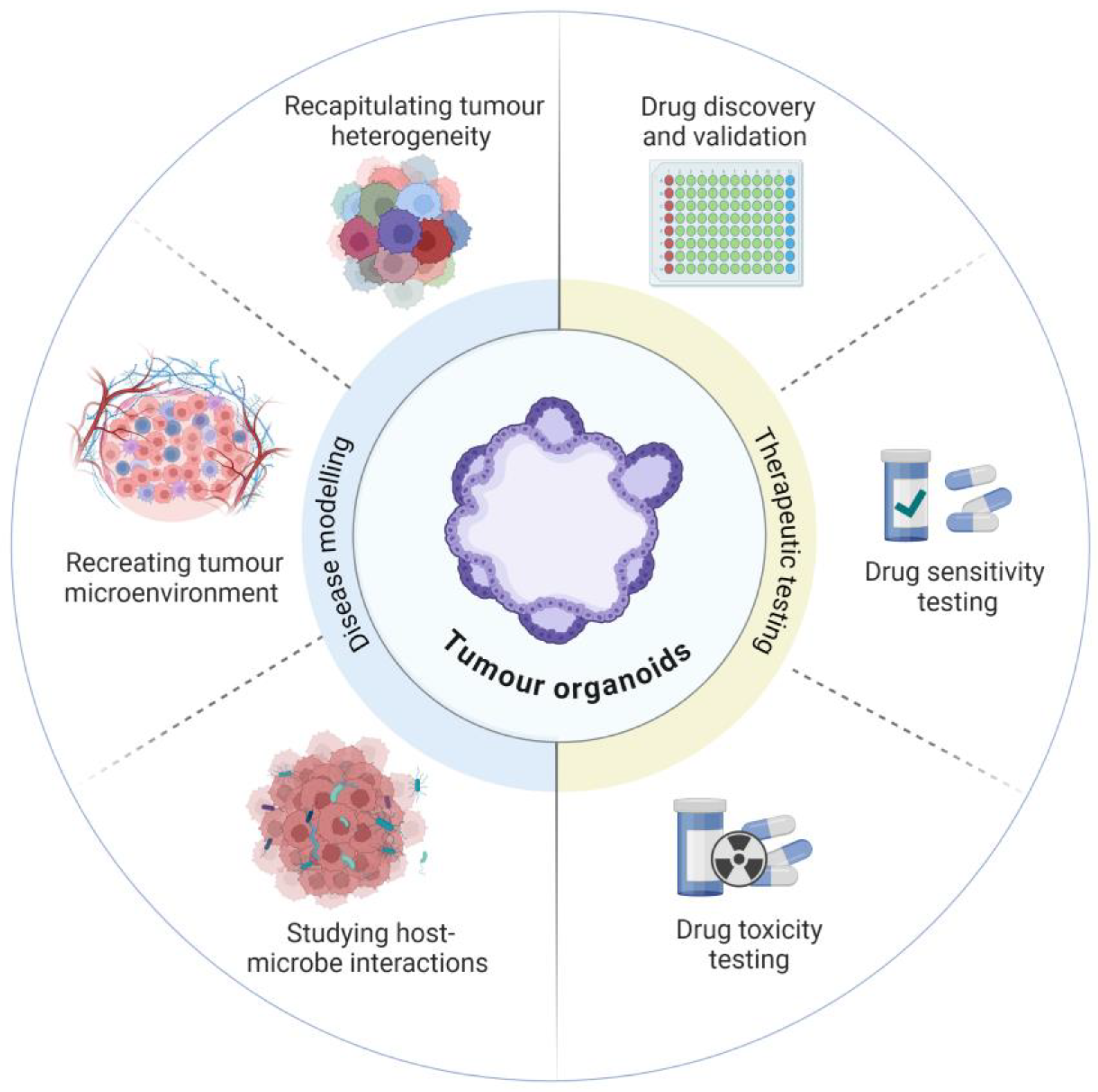
1. Introduction
2. Ex Vivo Epithelial Organoids
3. Developing Model Complexity
3.1. Replicating the Tumour Microenvironment
3.2. Host-Microbial Interactions
3.3. Intestine-on-a-Chip
4. Conclusions
5. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Morgan, E.; et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Blakely, T.; et al. Patterns of cancer care costs in a country with detailed individual data. Med Care 2015, 53, 302–9. [Google Scholar] [CrossRef]
- Laudicella, M.; et al. Cost of care for cancer patients in England: evidence from population-based patient-level data. Br J Cancer 2016, 114, 1286–92. [Google Scholar] [CrossRef]
- Mariotto, A.B.; et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst 2011, 103, 117–28. [Google Scholar] [CrossRef]
- Mishra, J.; et al. Prospective of colon cancer treatments and scope for combinatorial approach to enhanced cancer cell apoptosis. Crit Rev Oncol Hematol 2013, 86, 232–50. [Google Scholar] [CrossRef]
- Ciombor, K.K., C. Wu, and R.M. Goldberg, Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med 2015, 66, 83–95. [Google Scholar] [CrossRef]
- Xie, Y.-H., Y. -X. Chen, and J.-Y. Fang, Comprehensive review of targeted therapy for colorectal cancer. Signal Transduction and Targeted Therapy 2020, 5, 22. [Google Scholar] [CrossRef]
- Jonker, D.J.; et al. Cetuximab for the Treatment of Colorectal Cancer. New England Journal of Medicine 2007, 357, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E. , Integration of the anti-EGFR agent panitumumab into clinical practice in metastatic colorectal cancer. Clin Adv Hematol Oncol 2007, 5, 611–3. [Google Scholar]
- Chen, M.-H.; et al. How May Ramucirumab Help Improve Treatment Outcome for Patients with Gastrointestinal Cancers? Cancers 2021, 13, 3536. [Google Scholar] [CrossRef]
- Hwang, T.J.; et al. Failure of Investigational Drugs in Late-Stage Clinical Development and Publication of Trial Results. JAMA Intern Med 2016, 176, 1826–1833. [Google Scholar]
- Dowden, H. and J. Munro, Trends in clinical success rates and therapeutic focus. Nat. Rev. Drug Discov 2019, 18, 495–496. [Google Scholar] [CrossRef]
- Wong, C.H., K. W. Siah, and A.W. Lo, Estimation of clinical trial success rates and related parameters. Biostatistics 2019, 20, 273–286. [Google Scholar] [CrossRef]
- Sato, T.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–5. [Google Scholar] [CrossRef]
- Sato, T.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 2011, 141, 1762–72. [Google Scholar] [CrossRef]
- Co, J.Y.; et al. Toward Inclusivity in Preclinical Drug Development: A Proposition to Start with Intestinal Organoids. Advanced Biology 2023, 7, 2200333. [Google Scholar] [CrossRef]
- Du, Y.; et al. Development of a miniaturized 3D organoid culture platform for ultra-high-throughput screening. J Mol Cell Biol 2020, 12, 630–643. [Google Scholar] [CrossRef]
- Gilazieva, Z.; et al. Promising Applications of Tumor Spheroids and Organoids for Personalized Medicine. Cancers (Basel) 2020, 12. [Google Scholar] [CrossRef]
- Kaushik, G., M. P. Ponnusamy, and S.K. Batra, Concise Review: Current Status of Three-Dimensional Organoids as Preclinical Models. Stem Cells 2018, 36, 1329–1340. [Google Scholar] [CrossRef]
- Li, X., A. Ootani, and C. Kuo, An Air-Liquid Interface Culture System for 3D Organoid Culture of Diverse Primary Gastrointestinal Tissues. Methods Mol Biol 2016, 1422, 33–40. [Google Scholar]
- Wang, X.; et al. Cloning and variation of ground state intestinal stem cells. Nature 2015, 522, 173–178. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, E.M.F.; et al. Modeling Colorectal Cancer Progression Through Orthotopic Implantation of Organoids. Methods Mol Biol 2020, 2171, 331–346. [Google Scholar]
- Co, J.Y.; et al. Controlling Epithelial Polarity: A Human Enteroid Model for Host-Pathogen Interactions. Cell Rep 2019, 26, 2509–2520. [Google Scholar] [CrossRef]
- Duleba, M.; et al. An Efficient Method for Cloning Gastrointestinal Stem Cells From Patients via Endoscopic Biopsies. Gastroenterology 2019, 156, 20–23. [Google Scholar] [CrossRef]
- van de Wetering, M.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–45. [Google Scholar] [CrossRef]
- Pauli, C.; et al. Personalized In Vitro and In Vivo Cancer Models to Guide Precision Medicine. Cancer Discov 2017, 7, 462–477. [Google Scholar] [CrossRef]
- Kondo, J.; et al. High-throughput screening in colorectal cancer tissue-originated spheroids. Cancer Sci 2019, 110, 345–355. [Google Scholar] [CrossRef]
- Yan, H.H.N.; et al. Organoid cultures of early-onset colorectal cancers reveal distinct and rare genetic profiles. Gut 2020, 69, 2165–2179. [Google Scholar] [CrossRef]
- Sargent, D.J.; et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010, 28, 3219–26. [Google Scholar] [CrossRef]
- Bolck, H.A.; et al. Cancer Sample Biobanking at the Next Level: Combining Tissue With Living Cell Repositories to Promote Precision Medicine. Front Cell Dev Biol 2019, 7, 246. [Google Scholar] [CrossRef]
- Luo, Z.; et al. Establishment of a large-scale patient-derived high-risk colorectal adenoma organoid biobank for high-throughput and high-content drug screening. BMC Medicine 2023, 21, 336. [Google Scholar] [CrossRef]
- Fujii, M.; et al. Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell 2018, 23, 787–793. [Google Scholar] [CrossRef]
- Fujii, M.; et al. A Colorectal Tumor Organoid Library Demonstrates Progressive Loss of Niche Factor Requirements during Tumorigenesis. Cell Stem Cell 2016, 18, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Roerink, S.F.; et al. Intra-tumour diversification in colorectal cancer at the single-cell level. Nature 2018, 556, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Farin, H.F.; et al. Colorectal Cancer Organoid-Stroma Biobank Allows Subtype-Specific Assessment of Individualized Therapy Responses. Cancer Discov 2023, 13, 2192–2211. [Google Scholar] [CrossRef]
- Engel, R.M.; et al. Modeling colorectal cancer: A bio-resource of 50 patient-derived organoid lines. Journal of Gastroenterology and Hepatology 2022, 37, 898–907. [Google Scholar] [CrossRef]
- He, X.; et al. Patient-derived organoids as a platform for drug screening in metastatic colorectal cancer. Frontiers in Bioengineering and Biotechnology 2023, 11. [Google Scholar] [CrossRef]
- Mo, S.; et al. Patient-Derived Organoids from Colorectal Cancer with Paired Liver Metastasis Reveal Tumor Heterogeneity and Predict Response to Chemotherapy. Advanced Science 2022, 9, 2204097. [Google Scholar] [CrossRef]
- Derouet, M.F.; et al. Towards personalized induction therapy for esophageal adenocarcinoma: organoids derived from endoscopic biopsy recapitulate the pre-treatment tumor. Sci Rep 2020, 10, 14514. [Google Scholar] [CrossRef]
- Nims, R.W. and P. J. Price, Best practices for detecting and mitigating the risk of cell culture contaminants. In Vitro Cell Dev Biol Anim 2017, 53, 872–879. [Google Scholar]
- Aref, A.R.; et al. 3D microfluidic ex vivo culture of organotypic tumor spheroids to model immune checkpoint blockade. Lab Chip 2018, 18, 3129–3143. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, K.K.; et al. Challenges in Establishing Pure Lung Cancer Organoids Limit Their Utility for Personalized Medicine. Cell Rep 2020, 31, 107588. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014, 159, 176–187. [Google Scholar] [CrossRef]
- Karthaus, W.R.; et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 2014, 159, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Wallaschek, N.; et al. Establishing Pure Cancer Organoid Cultures: Identification, Selection and Verification of Cancer Phenotypes and Genotypes. J Mol Biol 2019, 431, 2884–2893. [Google Scholar] [CrossRef]
- Lannagan, T.R.M.; et al. Genetic editing of colonic organoids provides a molecularly distinct and orthotopic preclinical model of serrated carcinogenesis. Gut 2019, 68, 684–692. [Google Scholar] [CrossRef]
- Wilding, J.L. and W.F. Bodmer, Cancer cell lines for drug discovery and development. Cancer Res 2014, 74, 2377–84. [Google Scholar] [CrossRef]
- Berzins, S.P.; et al. A Role for MAIT Cells in Colorectal Cancer. Front Immunol 2020, 11, 949. [Google Scholar] [CrossRef]
- Dijkstra, K.K.; et al. Generation of Tumor-Reactive T Cells by Co-culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell 2018, 174, 1586–1598. [Google Scholar] [CrossRef]
- Harter, M.F.; et al. Analysis of off-tumour toxicities of T-cell-engaging bispecific antibodies via donor-matched intestinal organoids and tumouroids. Nature Biomedical Engineering 2024, 8, 345–360. [Google Scholar] [CrossRef]
- Schnalzger, T.E.; et al. 3D model for CAR‐mediated cytotoxicity using patient‐derived colorectal cancer organoids. The EMBO Journal 2019, 38, e100928. [Google Scholar]
- Neal, J.T.; et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972–1988. [Google Scholar] [CrossRef]
- Grebenyuk, S. and A. Ranga, Engineering Organoid Vascularization. Front Bioeng Biotechnol 2019, 7, 39. [Google Scholar] [CrossRef]
- Rajasekar, S.; et al. IFlowPlate-A Customized 384-Well Plate for the Culture of Perfusable Vascularized Colon Organoids. Adv Mater 2020, 32, e2002974. [Google Scholar] [CrossRef]
- de Vos, W.M.; et al. Gut microbiome and health: mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- O'Hara, A.M. and F. Shanahan, The gut flora as a forgotten organ. EMBO Rep 2006, 7, 688–93. [Google Scholar] [CrossRef]
- Sogari, A.; et al. Tolerance to colibactin correlates with homologous recombination proficiency and resistance to irinotecan in colorectal cancer cells. Cell Rep Med 2024, 5, 101376. [Google Scholar] [CrossRef]
- Iftekhar, A.; et al. Genomic aberrations after short-term exposure to colibactin-producing E. coli transform primary colon epithelial cells. Nat Commun 2021, 12, 1003. [Google Scholar] [CrossRef]
- Pleguezuelos-Manzano, C.; et al. Mutational signature in colorectal cancer caused by genotoxic pks(+) E. coli. Nature 2020, 580, 269–273. [Google Scholar] [CrossRef]
- Allen, J.; et al. Colon Tumors in Enterotoxigenic Bacteroides fragilis (ETBF)-Colonized Mice Do Not Display a Unique Mutational Signature but Instead Possess Host-Dependent Alterations in the APC Gene. Microbiol Spectr 2022, 10, e0105522. [Google Scholar] [CrossRef]
- Sayed, I.M.; et al. The DNA Glycosylase NEIL2 Suppresses Fusobacterium-Infection-Induced Inflammation and DNA Damage in Colonic Epithelial Cells. Cells 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; et al. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut 2019, 68, 289–300. [Google Scholar] [CrossRef]
- Engevik, M.A.; et al. Fusobacterium nucleatum Secretes Outer Membrane Vesicles and Promotes Intestinal Inflammation. mBio 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, W.; et al. Cytolethal Distending Toxin Promotes Replicative Stress Leading to Genetic Instability Transmitted to Daughter Cells. Front Cell Dev Biol 2021, 9, 656795. [Google Scholar] [CrossRef]
- Miyakawa, Y.; et al. Gut Bacteria-derived Membrane Vesicles Induce Colonic Dysplasia by Inducing DNA Damage in Colon Epithelial Cells. Cell Mol Gastroenterol Hepatol 2024, 17, 745–767. [Google Scholar] [CrossRef]
- Zhang, L.; et al. Enterococcus faecalis promotes the progression of colorectal cancer via its metabolite: biliverdin. J Transl Med 2023, 21, 72. [Google Scholar] [CrossRef]
- Holst, L.M.; et al. Fecal Luminal Factors from Patients with Gastrointestinal Diseases Alter Gene Expression Profiles in Caco-2 Cells and Colonoids. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Zhang, Y.G.; et al. Vitamin D Receptor Protects Against Dysbiosis and Tumorigenesis via the JAK/STAT Pathway in Intestine. Cell Mol Gastroenterol Hepatol 2020, 10, 729–746. [Google Scholar] [CrossRef]
- Tang, Q.; et al. Endogenous Coriobacteriaceae enriched by a high-fat diet promotes colorectal tumorigenesis through the CPT1A-ERK axis. NPJ Biofilms Microbiomes 2024, 10, 5. [Google Scholar] [CrossRef]
- Mowat, C.; et al. Short chain fatty acids prime colorectal cancer cells to activate antitumor immunity. Front Immunol 2023, 14, 1190810. [Google Scholar] [CrossRef]
- Sugimura, N.; et al. Lactobacillus gallinarum modulates the gut microbiota and produces anti-cancer metabolites to protect against colorectal tumourigenesis. Gut 2021, 71, 2011–21. [Google Scholar] [CrossRef]
- Iwama, T.; et al. Bacteria-derived ferrichrome inhibits tumor progression in sporadic colorectal neoplasms and colitis-associated cancer. Cancer Cell Int 2021, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Mackie, G.M.; et al. Bacterial cancer therapy in autochthonous colorectal cancer affects tumor growth and metabolic landscape. JCI Insight 2021, 6. [Google Scholar] [CrossRef]
- Gao, Y.; et al. Fusobacterium nucleatum enhances the efficacy of PD-L1 blockade in colorectal cancer. Signal Transduct Target Ther 2021, 6, 398. [Google Scholar] [CrossRef]
- Williamson, I.A.; et al. A High-Throughput Organoid Microinjection Platform to Study Gastrointestinal Microbiota and Luminal Physiology. Cell Mol Gastroenterol Hepatol 2018, 6, 301–319. [Google Scholar] [CrossRef]
- Sasaki, N.; et al. Development of a Scalable Coculture System for Gut Anaerobes and Human Colon Epithelium. Gastroenterology 2020, 159, 388–390. [Google Scholar] [CrossRef]
- Sunuwar, L.; et al. Mechanical Stimuli Affect Escherichia coli Heat-Stable Enterotoxin-Cyclic GMP Signaling in a Human Enteroid Intestine-Chip Model. Infect Immun 2020, 88. [Google Scholar] [CrossRef]
- Mitrofanova, O.; et al. Bioengineered human colon organoids with in vivo-like cellular complexity and function. Cell Stem Cell 2024. [Google Scholar] [CrossRef]
- Bein, A.; et al. Microfluidic Organ-on-a-Chip Models of Human Intestine. Cellular and Molecular Gastroenterology and Hepatology 2018, 5, 659–668. [Google Scholar] [CrossRef]
- Gonçalves, I.M.; et al. Organ-on-a-Chip Platforms for Drug Screening and Delivery in Tumor Cells: A Systematic Review. Cancers 2022, 14, 935. [Google Scholar] [CrossRef]
- Workman, M.J.; et al. Enhanced Utilization of Induced Pluripotent Stem Cell–Derived Human Intestinal Organoids Using Microengineered Chips. Cellular and Molecular Gastroenterology and Hepatology 2018, 5, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Probst, C., S. Schneider, and P. Loskill, High-throughput organ-on-a-chip systems: Current status and remaining challenges. Current Opinion in Biomedical Engineering 2018, 6, 33–41. [Google Scholar] [CrossRef]
- Sánchez-Salazar, M.G.; et al. 3D-Printed Tumor-on-Chip for the Culture of Colorectal Cancer Microspheres: Mass Transport Characterization and Anti-Cancer Drug Assays. Bioengineering 2023, 10, 554. [Google Scholar] [CrossRef] [PubMed]
| Primary intestinal cells (Transwell support) | Patient-derived organoids | Organ-on-a-Chip | Patient derived xenografts | Patient derived explants | |
|---|---|---|---|---|---|
| Represents in vivo system — Native organ structures? | Crypt-like formation in collagen-based gels with ALI [20,21] | “Spherical“ (3D), with crypt-like formation, differentiated cell types present [15] | Organoid, Tubular (3D) & planar (2D) | “Spherical” (3D) [22] | High fidelity, overall architecture retained, mixed mucosal cell types |
| Preservation of Intra-tumoural heterogeneity | Low | Medium | Variable | Medium | High |
| Clonogenicity | High, >70% in stem cell culture, low in ALI [21] | Low, possibly <2% under differentiating conditions [21] | NA | NA | Low, differentiation & maturation close to normal |
| Accessible lumen | No | Yes, [23] | Organoids: no Planar/tubular cultures: yes | No | Yes |
| Long-term culture | Stem cells repeat passages | Yes | Possible, likely depends on ECM stability | Viability diminished after 3–5 passages | Static culture: Viability declines after 7 days medium perfusion: Bioreactor: 30 days |
| Throughput | Low-throughput format | Scalable, grown in multiwell format, up to 1536-well plates | Generally low to medium | Difficult to achieve — Labour and costs prohibitive even for organoid grafts | Non-scalable — limited by size of starting material |
| Biobanking | Stem cell banking [21,24] | High success rate, existing CRC organoid banks [25] | NA | Tumours can be banked | Can be cryopreserved but not expandable |
| Genetic manipulation | Yes | Yes | Yes | Direct from tumour: No Organoid grafts: Yes | No |
| Trial number | Study Title | Study status | Conditions | Interventions | Country |
|---|---|---|---|---|---|
| NCT05669586 | Organoids Predict Therapeutic Response in Patients With Multi-line Drug-resistant Lung Cancer | Recruiting | Lung Cancer|Organoid | phase 2 | China |
| NCT04768270 | The Culture of Ovarian Cancer Organoids and Drug Screening | Recruiting | Ovarian Cancer | observational, patient registry | China |
| NCT05092009 | Lung Cancer Organoids and Patient Derived Tumor Xenografts | Recruiting | Lung Cancer | observational | Netherlands |
| NCT05290961 | The Culture of Advanced or Recurrent Ovarian Cancer Organoids and Drug Screening | Recruiting | Ovarian Neoplasms | observational, patient registry | China |
| NCT06064682 | An Organoid-based Functional Precision Medicine Trial in Osteosarcoma | Recruiting | Osteosarcoma | observational, standard of care biopsy | USA |
| NCT05577689 | Novel Therapy Target in Metastatic Prostate Cancer | Not yet recruiting | Prostate Neoplasms | observational | China |
| NCT05832398 | Precision Chemotherapy Based on Organoid Drug Sensitivity for Colorectal Cancer | Recruiting | Colorectal Cancer | interventional | China |
| NCT04931394 | Organoid-Guided Adjuvant Chemotherapy for Pancreatic Cancer | Recruiting | Pancreatic Cancer | interventional, phase 3 | China |
| NCT04931381 | Organoid-Guided Chemotherapy for Advanced Pancreatic Cancer | Recruiting | Advanced Pancreatic Cancer | interventional, phase 3 | China |
| NCT06268652 | Patient Derived Organoid-guided Personalized Treatment Versus Treatment of Physician's Choice in Breast Cancer | Recruiting | Breast Cancer|Refractory Breast Carcinoma | interventional, phase 3 | China |
| NCT05024734 | Guiding Instillation in Non Muscle-invasive Bladder Cancer Based on Drug Screens in Patient Derived Organoids | Recruiting | Bladder Cancer|Non-muscle Invasive | interventional, phase 2 | Switzerland |
| NCT05725200 | Study to Investigate Outcome of Individualized Treatment in Patients With Metastatic Colorectal Cancer | Recruiting | Metastatic Colorectal Cancer | interventional, phase 2 | Norway |
| NCT06468527 | Clinical Trial to Evaluate the Efficacy and Safety of Dirocaftor/Posenacaftor/Nesolicaftor in Adults With CF | Recruiting | Cystic Fibrosis | interventional, phase 2 | Netherlands |
| NCT06102824 | Organoid-based Functional Precision Therapy for Advanced Breast Cancer | Recruiting | HER2-negative Breast Cancer|Advanced Breast Cancer | interventional, phase 2 | China |
| NCT05352165 | The Clinical Efficacy of Drug Sensitive Neoadjuvant Chemotherapy Based on Organoid Versus Traditional Neoadjuvant Chemotherapy in Advanced Rectal Cancer | Not yet recruiting | Neoadjuvant Therapy | interventional | China |
| NCT06227065 | Precise Neoadjuvant Chemoresection of Low Grade NMIBC | Not yet recruiting | Bladder Cancer, Non-muscle Invasive Bladder Cancer | interventional, phase 2 | Switzerland |
| NCT03979170 | Patient-derived Organoids of Lung Cancer to Test Drug Response | Recruiting | Lung cancer | observational, patient registry | Switzerland |
| NCT03283527 | Chemoradioresistance in Prospectively Isolated Cancer Stem Cells in Esophageal Cancer-Organoid: RARE STEM-Organoid | Recruiting | Esophageal cancer | observational | Netherlands |
| 387579 (ACTRN12624000684527p) | FORECAST-II Feasibility of using Organoid Response to inform treatments for patients with Colorectal cancer staring first-line therapy | Not yet recruiting | Colorectal Cancer | Diagnosis / Prognosis |
Australia |
| 386544 (ACTRN12623001136695) | ORganoId GuIded N-of-1 (ORIGIN-1) Trial: A phase 4 study to investigate whether people with cystic fibrosis (CF) with rare cystic fibrosis transmembrane regulator (CFTR) mutations who have an in vitro response to Trikafta will also have a clinically meaningful response to Trikafta versus placebo | Not yet recruiting | Cystic Fibrosis | interventional, phase 4 | Australia |
| 380279 (ACTRN12620001353987) | FORECAST 1. Feasibility of using Organoid Response to find Effective Treatments for patients with Colorectal cancer After failure of Standard Therapy | Recruitment closed | Metastatic Colorectal Cancer | interventional | Australia |
| NCT03544255 | Drug Screening of Pancreatic Cancer Organoids Developed From EUS-FNA Guided Biopsy Tissues | Unknown status | Pancreatic Cancer | observational | China |
| NCT03544047 | Clinical Study on Drug Sensitivity Verification or Prediction of Therapy for Breast Cancer by Patient-Derived Organoid Model | Unknown status | Breast cancer | interventional | China |
| Organoid co-culture models: pro-tumorigenesis mechanisms | |||
|---|---|---|---|
| Organoid type and species | Bacterial species | Effect shown | Reference |
| Human CRC organoids | Colibactin-producing E. coli DH10B | DNA damage (double-strand break (DSB)) | [57] |
| Murine colon organoids | pks+ E. coli | DSB, genomic instability, chromosomal aberrations andgenetic mutations | [58] |
| Human intestinal organoids | pks+ E. coli | DNA damage and oncogenic mutational signatures | [59] |
| Human intestinal organoids | Enterotoxigenic B. fragilis | Did not induce a unique mutational pattern | [60] |
| Mouse and human colon organoids | F. nucleatum, E. coli K12 strain DH10B, E. coli strain LF82 and Helicobacter pylori | F. nucleatum downregulated expression of DNA repair protein (NEIL2), increased the accumulation of DNA damage and production of the IL-8 | [61] |
| Murine intestinal organoids | Bacterial lysates of wild-type C. jejuni (WT) or C. jejuni mutcdtB | DNA damage | [62] |
| Human colon organoids | F. nucleatum conditioned media | Increased inflammatory responses characterised by increased secretion of TNF and activation of NF-κB, p-ERK, p-CREB signalling pathways | [63] |
| Human intestinal organoids | E. coli-derived cytolethal distending toxin | DNA damage | [64] |
| Human intestinal organoids | Actinomyces odontolyticus -derived lipoteichoic acid-rich membrane vesicles | DSB | [65] |
| Human CRC organoids | Biliverdin, a key metabolite produced by CRC-associated E. faecalis | Increased the expression of cell proliferation marker Ki67 | [66] |
| Human colon organoids | Faecal supernatant from colon cancer patients | Alterations in gene expression | [67] |
| Human and murine colon organoids | Faecal supernatant from a cancer mouse model lacking intestinal vitamin D receptor | Activation of JAK/STAT3 signalling and increase in PCNA and β-catenin expression | [68] |
| Organoid co-culture models: protective mechanisms | |||
| Murine colon organoids | Coriobacteriaceae (Cori.ST1911) and Lactobacillus murinus (La.mu730) | Upregulated expression of carnitine palmitoyltransferase 1A (CPT1A), and downregulated MUC2 protein. Lactobacillus murinus (La.mu730) reversed negative effect of Cori.ST1911 |
[69] |
| Human and murine CRC organoids | Short chain fatty acids | Upregulated expression of Type I IFN Stimulated Genes (CXCL10 and ISG15) which are important for anti-tumour immune response | [70] |
| Human CRC organoids | Lactobacillus gallinarum supernatant | Induction of apoptosis | [71] |
| Human adenoma and CRC organoids | Lactobacillus casei- derived ferrichrome | Tumour suppression response by upregulating the expression of DNA damage-inducible transcript 3 | [72] |
| Organoid co-culture models: mechanisms related to treatment response | |||
| Murine tumour organoids | Salmonella enterica serovar Typhimurium (aromatase A–deficient Salmonella Typhimurium (STmΔaroA) | Altered gene expression analysis including reduced expression of stem cell and EMT markers, increased expression of innate immunity proteins | [73] |
| Human CRC organoid | F. nucleatum | Enhanced efficacy of anti-PD-L1 immunotherapy | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





