1. Introduction
1.1. Henoch–Schönlein Purpura Incidence and Clinical Manifestations
Immunoglobulin A (IgA) vasculitis (IgAV), classically known as Henoch–Schönlein purpura (HSP), is a type of non-thrombocytopenic small-vessel vasculitis [
1]. HSP is the most frequent kind of systemic vasculitis in children, characterized by purpura, arthritis or arthralgia, gastrointestinal pain, and kidney dysfunction [
2]. Systemic IgAV can occur at any age; however, it most commonly affects children aged 3 to 15 years. In total, 90% of childhood-onset diseases develop before the age of ten years [
1,
3,
4]. The incidence of the pathology ranges from 3 to 27 cases per 100,000 children [
5,
6]. It is relatively uncommon among infants [
5].
IgAV typically causes palpable purpura on the skin, as well as in the joints, kidneys, and gastrointestinal tract. Incomplete or atypical symptoms, such as involvement of the central and peripheral nervous system or pulmonary complications, necessitate a differential diagnostic workup [
7,
8]. An atypical cutaneous manifestation can be mistaken for papular urticaria, systemic lupus erythematosus, meningococcemia, or dermatitis herpetiformis. It may also be mistaken for acute hemorrhagic edema of infancy, which many specialists regard as a form of HSP [
9]. This disorder is characterized by purpuric lesions on the face, ears, extremities, and scrotum in children under two years old who otherwise display normal serologic results [
10]. Its clinical symptoms are widely established, including non-thrombocytopenic palpable purpura, arthritis, and the involvement of internal organs such as the gastrointestinal tract and kidney [
11]. Although the precise pathogenesis of HSP is undetermined, pathological and laboratory findings, such as a vascular deposition of IgA-dominant immune complex, the infiltration of small blood vessels with polymorphonuclear leukocytes, and the presence of leukocyteoclasia, increased serum IgA, and proinflammatory cytokines, indicate that HSP is an immune-mediated disease [
11,
12].
1.2. Immunoglobulin A’s Role and Characterization in Pathogenesis
IgA, a member of the human immunoglobulin family, serves as an essential component of mucosal defense, primarily by neutralizing bacterial products, agglutinating microorganisms, and interfering with bacterial movement [
13,
14,
15]. IgA, a key immunoglobulin class found in serum and mucosal secretions, plays an important role in mucosal immunity [
16,
17]. IgA is produced in greater quantities than all other immunoglobulin classes combined due to its high mucosal production and short half-life of 5–6 days [
18,
19]. Humans present two subclasses of immunoglobulin A (IgA): IgA1 and IgA2. Each subclass consists of a fundamental molecular unit made up of two identical heavy chains (HCs) and two identical light chains (LCs) [
20]. Each chain starts with a variable region at the N-terminus, followed by a consistent region. The LCs are identical in both subclasses, but the HCs differ in their constant sections, which are encoded by different genes. In addition, two allotypic variations of human IgA2 have been identified: IgA2m(1) and IgA2m(2) [
21]. IgA1 is the first to be produced between the two IgA subclasses and varies from IgA2. IgA1 contains only 13–17 amino acid sequence in its hinge region [
22,
23]. Increased IgA synthesis could be linked to the antigen exposure processed by the mucosal immune system. Bacteria, viruses, or parasitic agents were hypothesized to initiate the HSP disease in genetically predisposed individuals, but causal agents and factors have yet to be found [
24,
25]. During the acute stage of HSP, a rise in IgA may indicate aberrant immunological responses; IgA1 accounts for over 90% of IgA in the blood, while IgA2 accounts for less than 10% [
13,
26,
27].
1.3. Potential Etiological Factors for Henoch–Schönlein Purpura
As mentioned before, the pathophysiology of HSP is poorly known; however, numerous medical professionals are aware that it is usually associated with a variety of viral disorders, as well as exposure to insect bites, immunizations, medicines, or food allergies [
25,
28,
29,
30]. Although the etiology of the disease remains unknown, it is obvious that the IgA system plays an important part in the pathogenesis [
31]. A variety of triggers have been proposed, including infections and medicines [
7,
32,
33]. Moreover, the hallmark pathogenic feature (the deposition of IgA-containing immune complexes within tiny artery walls and renal mesangium) suggests the involvement of mucosal infections [
16,
34,
35]. IgAV has a seasonal tendency, with fewer instances occurring during the summer months, confirming the hypothesis that viral precipitants cause the start of this disease [
36,
37].
Our research aimed to evaluate clinical data from pediatric patients diagnosed with HSP and investigate the association between infectious diseases and the described pathology. Additionally, our retrospective study examined demographic factors (including sex, area/environment, and age) and their impact on the pediatric HSP population. This study will emphasize the key clinical aspects of HSP in the studied population.
2. Materials and Methods
2.1. Study Design
The clinical research was conducted as a hospital-based retrospective study, including 144 children (1 to 18 years of age), all of whom were patients admitted to the “Grigore Alexandrescu” Emergency Clinical Hospital for Children in Bucharest. We searched the electronic medical database of the hospital for relevant records from 1 January 2017 to 31 December 2023. All children were previously referred for evaluation due to HSP. The children were diagnosed with HSP using the usual diagnostic criteria, EULAR/PRINTO/PRES for HSP [
5].
The samples for the quantitative measurement of the immunological panel (IgA, IgM, IgG, and IgE), C3, C4, C-reactive protein, fibrinogen, and VSH were obtained from venous blood. An anticoagulant-free vacutainer with or without separating gel served as the collecting container. Levels of immunological panel elements were measured with an enzyme-linked immunosorbent assay (ELISA).
The collected patient files were entered into a database. The hospital database was used to collect information such as age, gender, environment, immunological panel (IgA, IgM, IgG, and IgE), C3, C4, C-reactive protein, fibrinogen and VSH, clinical diagnosis, and other associated diagnosis. The data were statistically evaluated, and the statistical tests applied are detailed in the 'Descriptive Analysis of the Patients' Series' section.
The diagnosis of HSP and the assessment of disease severity (mild, moderate, or severe) were determined in accordance with the guidelines set forth by the European League Against Rheumatism (EULAR), the Pediatric Rheumatology International Trials Organization (PRINTO), and the Pediatric Rheumatology European Society (PRES). These organizations have delineated the current diagnostic criteria for systemic IgA vasculitis (IgAV) [
5,
33,
38]. In a pediatric patient presenting with purpura, characterized as round or oval and retiform, predominantly on the lower limbs, the diagnosis is confirmed if at least one of the following four criteria is met: (1) abdominal pain, (2) histologically confirmed IgA deposits, (3) arthritis or arthralgia, or (4) renal impairment [
5,
33,
38].
2.2. Descriptive Analysis of the Patients’ Series
The data were statistically analyzed using IBM’s Statistical Analysis Software Package (SPSS) version 29 (2022) and Microsoft Excel 2016 (Redmon, WA, USA). The investigation included descriptive statistics, tests to assess normal distribution (Kolmogorov–Smirnov and Shapiro–Wilks), tests to compare quantitative indicators in different groups (comparison of means), correlation analyses (Pearson correlation coefficient, PCC), ROC curves, positive predictive value (PPV), negative predictive value (NPV), and sensitivity and specificity. The chosen significance level was α = 0.05 and 0.01 for PCC. Thus, if the significance level is not reached for values of p < α, the null hypothesis is rejected.
2.2.1. Inclusion Criteria
The patients included in the study were of both sexes, with an age range between 0 and 18 years, admitted to the Pediatric Clinic of “Grigore Alexandrescu” Emergency Clinical Hospital for Children in Bucharest. The children were previously diagnosed with HSP and had their immunological panel (IgA, IgM, IgG, and IgE), C3, C4, C-reactive protein, fibrinogen, and VSH tested. Only patients who had no prior COVID-19 infection were accepted into the research study due to the complex treatment scheme for the disease.
2.2.2. Exclusion Criteria
The following patients were excluded from the study: patients below 1 year of age, patients not previously diagnosed with HSP, and patients who had no IgA and IgE level tests performed. Children who had previously been diagnosed with COVID-19 or had at least one positive COVID-19 test during their stay in the hospital were also excluded from the study.
3. Results
3.1. Descriptive Statistics
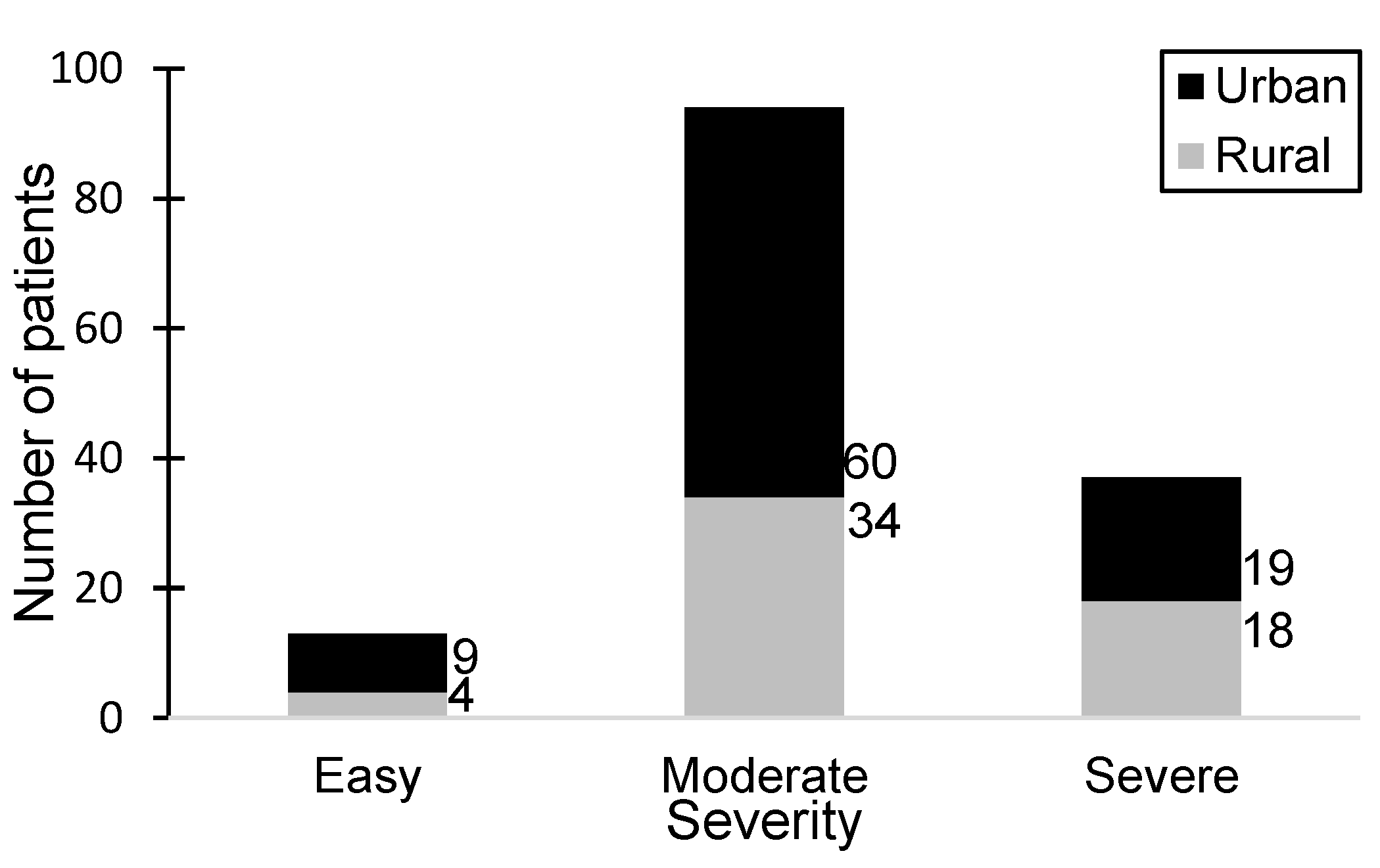
A retrospective hospital-based study was conducted over a five-year period, during which 144 patients were diagnosed with HSP. Of the 144 total children, 87 (60.4%) were boys and 57 (39.6%) were girls. The boys were slightly younger than the girls, with a mean age of 7.18 ± 3.907 years vs. 8.00 ± 4.044 years, respectively. According to the Kolmogorov–Smirnov and Shapiro–Wilk statistical tests, neither gender had a normal age distribution (p values were less than 0.01 for both sexes). Overall, the mean age of the group was 7.51 ± 3.96 years. There was a 1.57:1 ratio between the urban and rural patients (urban/rural, 88:56).
Among the 144 patients included in the study, a significant proportion (65.27%) exhibited a moderate disease status, particularly within the urban patient group, while the smallest group of patients (9.02%) exhibited the mildest symptoms. The urban-to-rural patient ratio stood at 2.25:1. Notably, severe manifestations were observed in 25.69% of patients, with a nearly equal distribution between urban and rural areas. Among patients diagnosed with severe HSP, 63.8% were male.
Figure 1 illustrates the distribution of patients with varying severity levels of HSP alongside the environmental distribution.
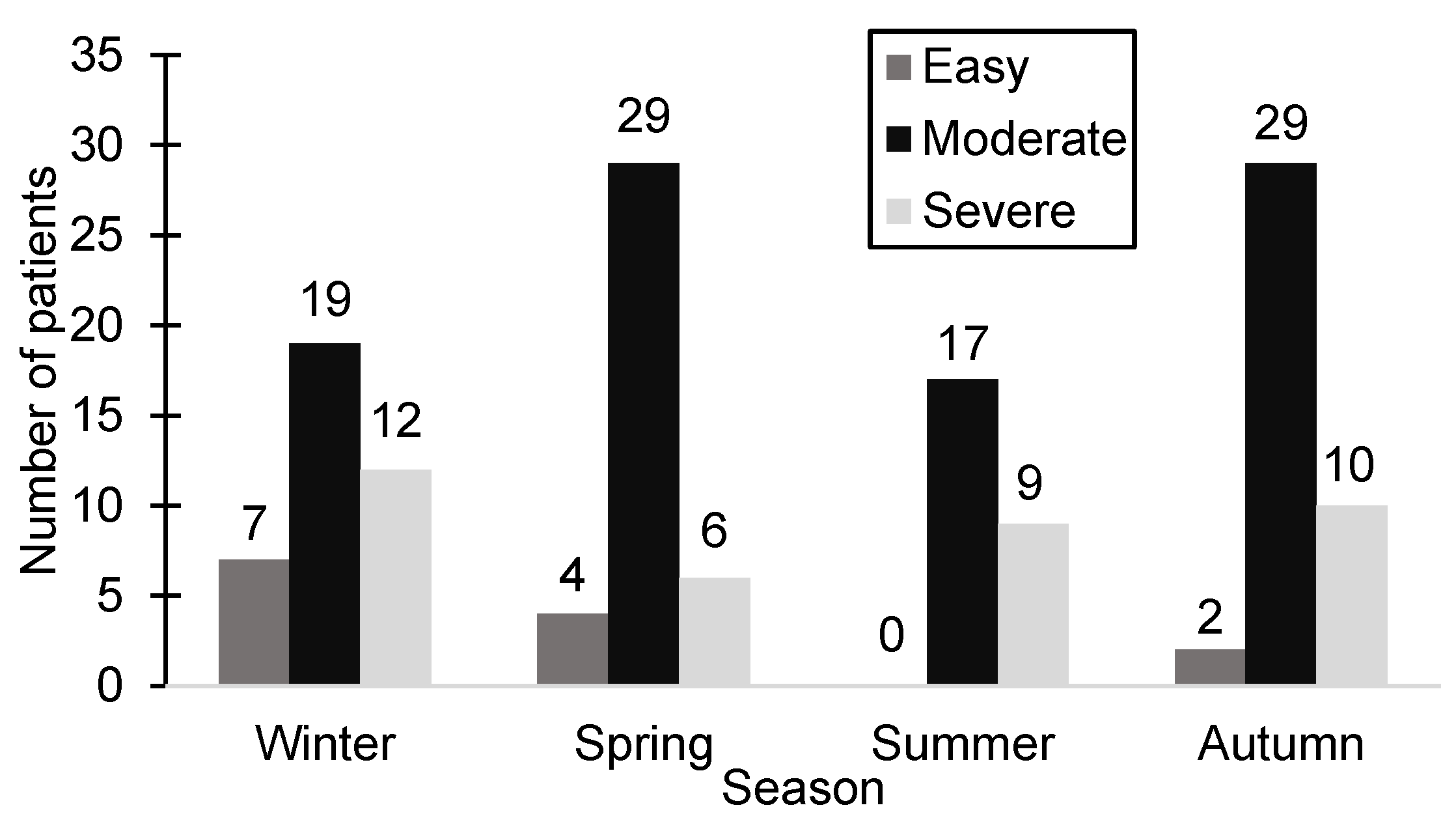
The clinical study documented the date of hospitalization, revealing that during autumn, the highest patient count across all seasons was recorded (41 patients, 28.47%). In contrast, the number of hospitalized patients during the winter and spring was nearly equal (38 patients during the winter and 39 patients during the spring). Interestingly, summer had the lowest hospitalization rate (26 patients, 18.05%), with no patients exhibiting mild-severity symptoms during that season.
Figure 2 illustrates the distribution of patients according to season and the severity of HSP.
3.2. Immunologic and Paraclinical Results
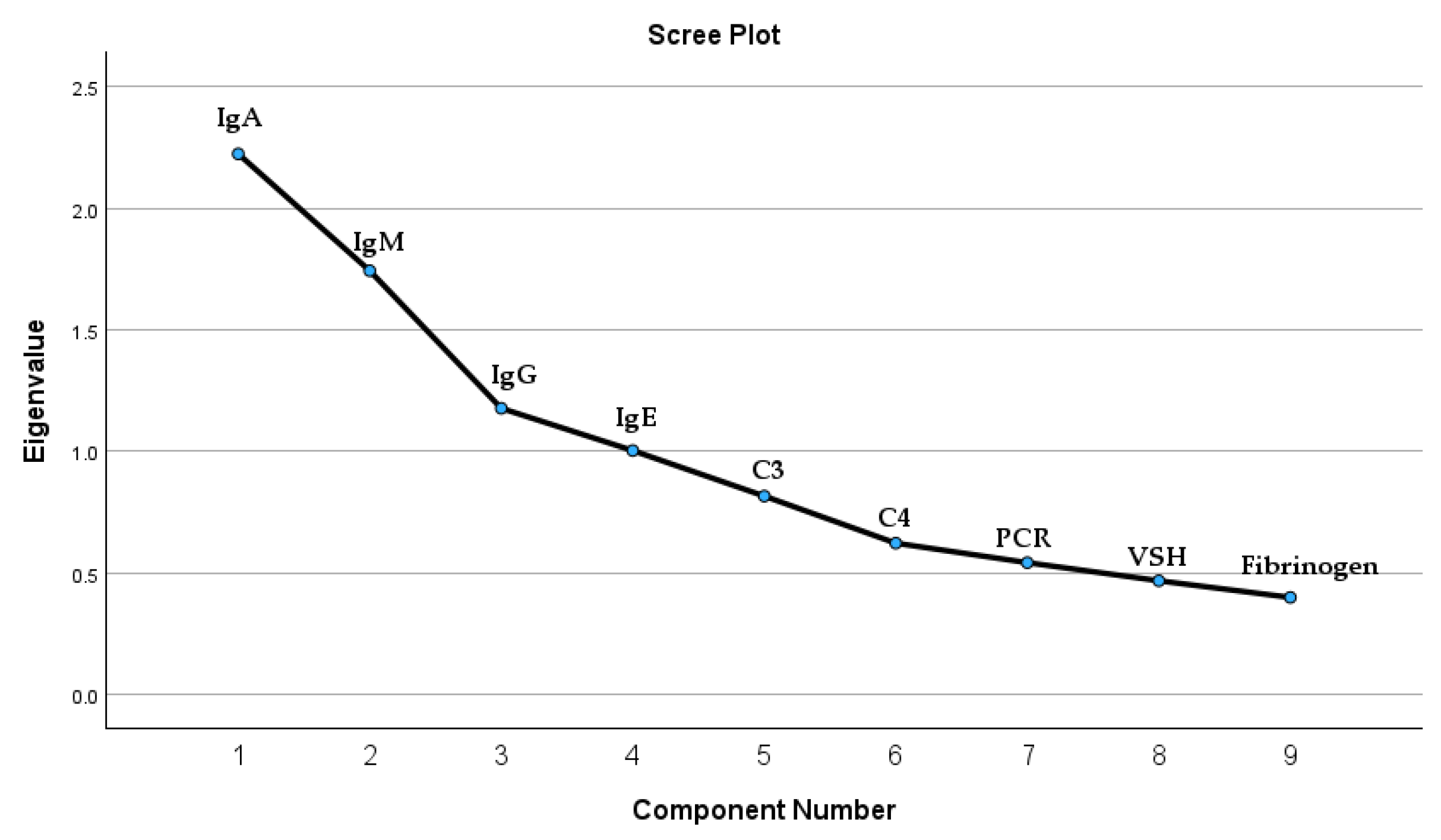
We investigated potential correlations between IgA serum levels and immunological panel elements within a patient cohort. The immunological panel analysis included, besides IgA, immunoglobulin G (IgG), immunoglobulin M (IgM), and immunoglobulin E (IgE). Moreover, we included in the Principal Component Analysis (PCA) the inflammation markers such as complement 3 (C3), complement 4 (C4), fibrinogen, sedimentation velocity of hematite (VSH), and protein C reactive (PCR). The test results indicated underlying correlations that exist in the set of variables; the Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy was 0.636, and Bartlett’s test of sphericity yielded a chi-square value of 188.711 with 36 degrees of freedom (p < 0.001). According to the test results, there are four extracted components that satisfy the condition of a correlation coefficient greater than or equal to 1. The four components extracted are all the immunological panel elements. These results are detailed in
Figure 3 and
Table A1.
The Pearson correlation coefficient (PCC) quantifies the linear association between two data sets. In our study, we applied the PCC to explore the potential correlations among elements in the immunological panel. Our findings indicate moderate positive correlations between IgA and IgG (PCC = 0.238, p-value = 0.01), as well as between IgA and IgM (PCC = 0.443, p-value = 0.01). Additionally, a weaker positive correlation exists between IgG and IgM (PCC = 0.222, p-value = 0.01).
Table A2 covers all correlations. It also includes relationships that, while not statistically significant (p-values greater than 0.01), are provided for transparency.
According to the immunological panel, 33.33% of patients exhibit high IgA levels, 34.02% exhibit high IgM levels, only 14.58% exhibit high IgG levels, and 45.13% present high IgE levels. Aside from purpura, the most common clinical symptoms were joint involvement (76%), followed by digestive issues (48%). Renal impairment occurred in 13.9% of patients, followed by testicular involvement with acute orchepididymitis in 8.5% of cases. Severe forms of the disease more frequently presented thrombocytosis (30.55% of all patients and 48.80% of severe cases) and hypoalbuminemia (11.11% of all patients and 17.02% of severe cases) at admission, while leukocytosis (47.22% of all patients) at admission was associated with renal impairment. Meanwhile, those who had elevated IgA levels exhibited edema and renal impairment.
Regarding the initial rash localization, the lower limb was the most common site of purpuric eruption (84.61%). The cutaneous signs were most typically found on the calves (84.81%), thighs (65.03%), ankles (67.13%), and foot (67.13%). Notably, only 60.13% of patients (86 people) had cutaneous eruptions over their entire lower limb. The buttocks (24.47%) and upper limb (15.37%) were the next most affected locations for purpura. There were 14 patients (9.79%) who presented with an unusually confined purpuric eruption onset. These individuals developed IgAV in the left ear (two patients), thorax (one patient), right wrist joint and left arm (two patients), and upper abdomen (two patients). Two individuals had generalized HSP localization, whereas five others had the eruption on their scalp, submentum, or face. The most frequent combination of IgAV manifestations was observed on the lower and upper limbs (15 patients, 10.48%), and on the lower limbs and buttocks (24 patients, 16.78%). Approximately one-third of patients (46 patients) had relapses, with 26.6% experiencing moderate symptoms and 58.3% suffering from severe symptoms. The average number of relapses was 1.5 episodes (range: 1 to 5), requiring hospitalization.
Corticosteroid therapy was used to relieve symptoms in 113 patients (79.02%), antihistamine therapy was used in 142 patients (99.30%), nonsteroidal anti-inflammatory medications (NSAIDs) were used in 49 patients (34.26%), and immunological therapy was necessary in just 3 patients. In addition, 78 patients (54.54%) were treated with antibiotics after being identified with bacterial infections.
3.3. Patients with Infectious Diseases as Secondary Diagnoses
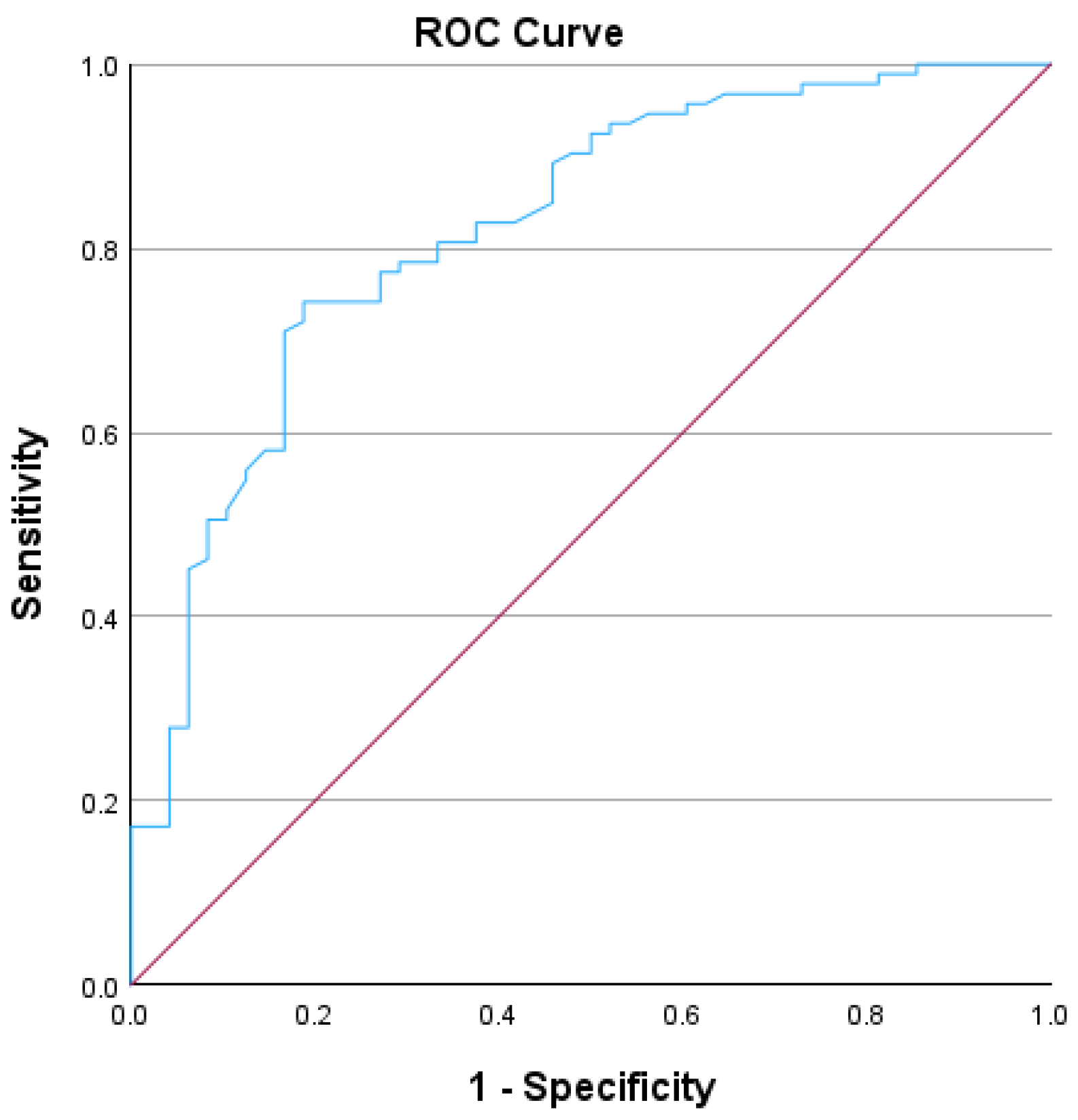
In this study, we used the Receiver Operating Characteristic (ROC) curve to assess the diagnostic performance of serum IgA levels in distinguishing between patients who had experienced infectious diseases as a secondary diagnosis during admission and those who had not. The ROC curve results indicated that IgA levels achieved statistical significance, with an AUC of 0.821 and a standard error of 0.037, demonstrating a strong discriminative capacity for diagnosing normal IgA levels in patients with infections, as detailed in
Table 1 and in the graphic representation presented in
Figure 4. The ROC curve analysis was conducted on a cohort of 141 patients included in the study; the remaining patients did not undergo testing for their total IgA serum levels. The patients who did not undergo testing for total IgA serum levels presented very mild symptoms; thus, the hospital protocol did not include the IgA serum levels. Patients with normal IgA levels and infection as secondary clinical diagnoses were classified as true positives (43 patients), whereas those with high IgA levels but no clinical infectious disease diagnoses were classified as false negatives (31 patients). True negatives included patients with normal IgA levels and no infection as secondary clinical diagnoses (50 patients), and false positives comprised patients with high IgA levels and infection as secondary diagnoses (17 patients). The sensitivity was calculated to be 58.10%, and the specificity was 74.62%. These findings indicate that the model is modest as a screening tool (the model correctly detects approximately 58.10% of positive cases) and reliable as a confirmatory test (the model accurately identifies approximately 74.62% of true negative cases). The positive predictive value (PPV) was 71.66%, suggesting that more than two-thirds of the patients with a positive screening test had normal IgA levels with an infectious disease. The negative predictive value (NPV) was 61.72%, indicating that nearly two-thirds of patients with a negative screening test (high IgA levels) were free of infectious diseases.
Among all the patients enrolled in the study, 26% (37 patients) had a recent history of, or an infection associated with, Group A beta-hemolytic streptococcus. Additionally, apart from infections as secondary diagnoses, patients also exhibited persistent nephropathy (6.3%) and inflammatory bowel disease (4.9%).
4. Discussion
4.1. Demographic Characteristics Correlated with HSP Manifestations
HSP is the most frequent kind of connective tissue disease, and its cause is unknown. HSP is a leukocytoclastic vasculitis that mostly affects the skin, gastrointestinal tract, joints, and kidneys [
39]. The current etiopathogenic paradigm for IgAV involves an aberrant immune response produced by a variety of antigenic stimuli in genetically vulnerable individuals [
6,
40]. Given the average age of our study group, we may conclude that IgAV primarily affects children under the age of ten in over 70% of cases, with the peak incidence occurring between the ages of four and seven [
41]. This relates to the time when children start school and are exposed to communicable diseases [
41]. In this research and prior publications, symptoms of infection, primarily in the upper respiratory tract, were observed in the majority of cases within days after the onset of IgAV symptoms [
42,
43]. When comparing genders, males seem to be more susceptible than females [
35].
According to our statistics, HSP onset occurred throughout the year, with autumn having the highest prevalence. Furthermore, consistent with previous research, we discovered a lower incidence of IgAV throughout the summer months, which corresponds to a lower incidence of respiratory viral infections and less bacterial infection transmission during school closures [
43,
44,
45]. The interaction of environmental factors (urban and rural areas) and many genes has been considered the most important inner workings of HSP formation, reflecting epidemiologic disparities among ethnicities, cases of family aggregation, and interindividual variance in disease prognosis [
46,
47]. According to the study results, the majority of patients who developed HSP are from urban areas, where the density of population is highly elevated compared to rural areas, thus having a higher risk of contracting an infectious disease [
48].
Gastrointestinal signs, which occur several days or even a week before cutaneous symptoms, might cause clinical perplexity until the rash appears; in severe cases, it requires immediate immunosuppressive treatment [
49]. Approximately two-thirds of patients have gastrointestinal system involvement, which usually manifests as colicky abdominal pain due to bowel angina [
50]. Renal involvement is frequently asymptomatic, necessitating active screening. It is seen in less than 30% of patients, with the majority having a moderate renal course that resolves on its own [
49,
51]. Microscopic hematuria is the most prevalent finding on urinalysis, followed by proteinuria with no edema [
51]. The rash appears as a symmetrical erythematous petechial or purpuric eruption that primarily affects the lower limbs and buttocks [
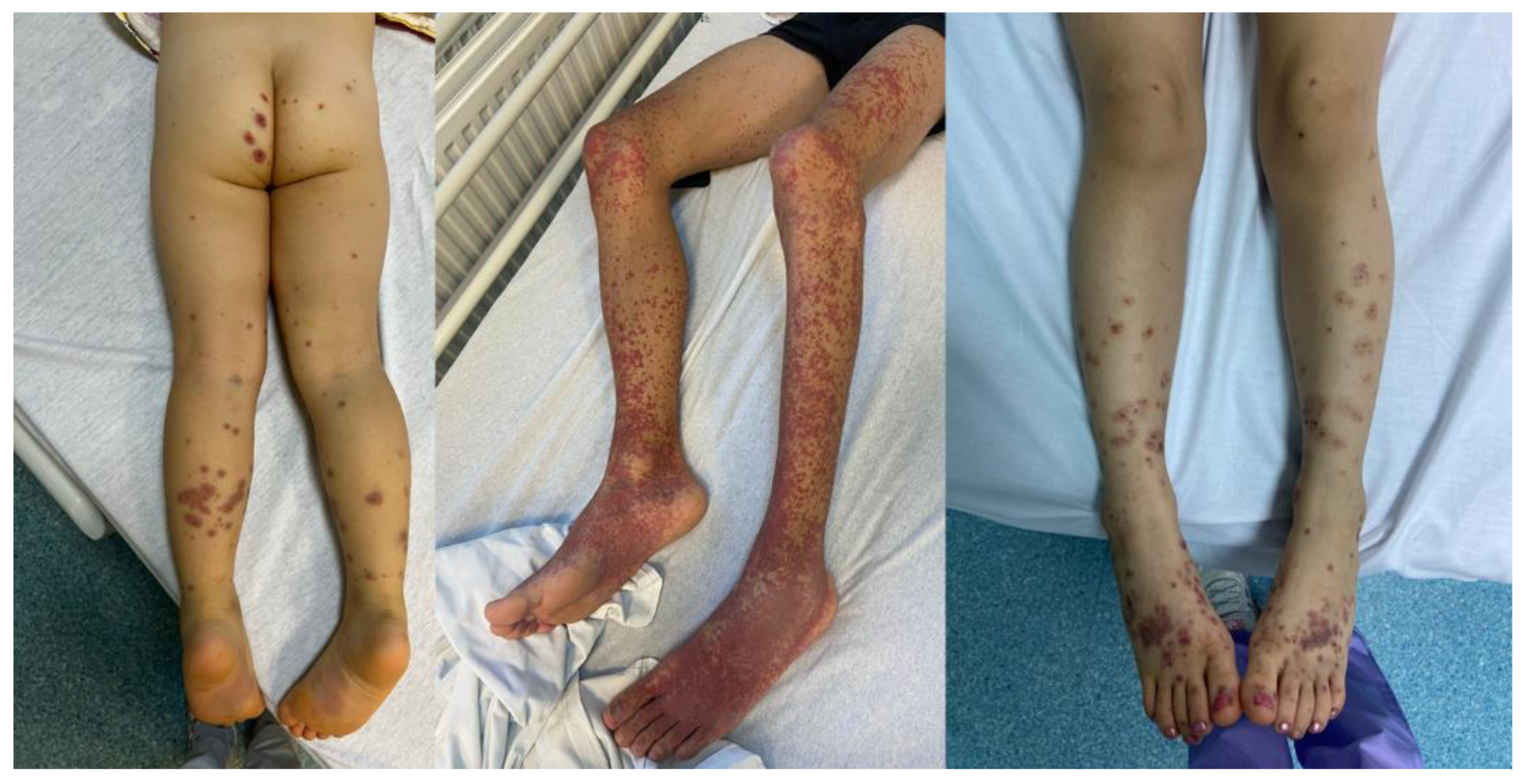
36]. Patients may have interwoven bruises along with the purpura and, in rare cases, necrotic lesions or bullae as presented in
Figure A1 [
33,
52]. Palpable purpura is a typical feature, and the rash can spread to the arms but less frequently to the torso. Skin edema can surround purpuric lesions, and face involvement is extremely rare but possible in severe cases [
33]. During the acute manifestation of HSP, more than 70% of patients present musculoskeletal involvement manifesting as either arthralgia or arthritis [
51]. The frequency of arthritis is lower, at around 60% [
47]. Arthritis tends to have an oligo-articular pattern (four or fewer joints) with a predilection to joints of the lower limb. The joints of the feet and ankles are most commonly involved, followed by the knees, wrists, elbows, and hands [
36,
37,
40,
52]. Furthermore, younger patients, under 7 years old, have more musculoskeletal and stomach complaints, whereas older patients, above 7 years old have more renal involvement. Although the exact mechanism is unknown for now, aging is thought to be a risk factor for renal involvement. In conclusion, HSP in older patients has a higher chance of progression to renal involvement; hence, more aggressive therapy and extended follow-up with repeated urinalysis are generally required in older HSP patients, despite initially benign renal results [
53]. The majority of IgAV cases in children resolve on their own and do not require any specific therapy other than supportive care. In more severe cases, therapy options are partly determined by the type and severity of organ involvement [
36].
4.2. Relationship of Immune Parameters in Children with Henoch–Schönlein Purpura
During the acute stage of IgAV, complement components such as complement factor C3 and C4 complexes are frequently seen in skin and kidney biopsy specimens from patients, although serum complement levels are low [
31]. The IgA/C3 ratio is significantly higher in patients with progressive IgA nephropathy compared to those without nephropathy symptoms. As a result, we can conclude that the IgA/C3 serum level ratio may be a useful predictor of disease activity in HSP patients [
31]. The elevated IgA levels correlated with the high levels of IgM in the blood samples of the pediatric population with HSP, and are an indication of renal involvement. Serum IgM levels may be connected with pathogenic variables that contribute to renal involvement in HSP patients. While HSP nephritis is more common in adults and is associated with poorer results, pediatric patients recover more quickly [
54]. It is generally accepted that IgA vasculitis implies the involvement of the skin and kidneys. The current study found a significant correlation between elevated IgM serum levels and C3 deposition in the small-vessel vasculitis of patients with HSP [
55]. Based on our findings, serum IgA levels appear to be raised in fewer than 40% of HSP patients, particularly those with moderate or severe manifestations. Notably, the degree of serum IgG alterations in our pediatric patients was significantly influenced by infectious illnesses, which were a subsequent diagnosis. Previous investigations on serum IgG and IgE levels in HSP patients have produced mixed results, with some indicating declines, increases, or no significant changes.
4.3. The Correlation between Infectious Diseases and HSP Manifestations
Plasma cells in mucosa-associated lymphoid tissue (MALT) produce IgA, which is found in the nasopharynx, tonsils, and gastrointestinal mucosa. IgA–antibody immune complexes are formed in response to antigenic exposure from an infection or medication [
56]. They are then deposited in the small vessels (usually capillaries) of the skin, joints, kidneys, and gastrointestinal tract. This results in an influx of inflammatory mediators, such as prostaglandins [
57]. When the body is activated by pathogens, B cells develop into mature plasma cells, causing the secretion of more IgA polymers [
54,
58]. HSP is associated with a variety of childhood infections and genetic variables. Many clinicians agree that HSP may be followed by a series of viral infections or exposure to certain medicines, bug bites, immunizations, food allergies, and so on [
50]. According to the study results, IgA serum levels tend to be in the normal range when the patient is hospitalized with an infectious disease as a secondary diagnosis. Moreover, fulminant infectious disease manifestations tend to be the main factor in normalizing IgA serum levels. Elevated IgG and IgM levels in pediatric HSP patients are strongly linked to infectious diseases. Notably, patients who tested positive for pathogens in their IgG- or IgM-specific blood tests also had high IgG or IgM serum levels [
31,
59,
60].
The study group in the present study had a high prevalence of respiratory infectious illnesses, with β-hemolytic streptococcus being the primary pathogen. Group A β-hemolytic streptococcus is the most investigated pathogen associated with HSP, as it can be found in up to 50% of persons with acute HSP, either by serological testing or bacterial cultures [
61,
62]. However, several bacteria and viruses have been linked to the development of HSP.
5. Conclusions
The statistical results showed that there is a correlation between the frequency of infectious diseases and normal IgA levels in patients diagnosed with HSP. Furthermore, the environment plays an important role in the sensitization of the immune system. Thus, it is crucial to monitor the immunoglobulins involved in the immune response to infectious microorganisms, as well as their association with IgA levels.
As a key limitation, because this is a retrospective study, IgA levels were evaluated only once after clinical signs occurred. Thus, increased or normal IgA levels in patients presenting various types of HSP manifestations may have been impacted by recurring infections. To determine this connection, IgA levels could be evaluated prior to, during, and following infections. Moreover, this study was conducted in a pediatric clinic and consisted of an extensive evaluation of clinical observation sheets. The study did not involve an examination of IgA subcategories or socio-economic status due to the fact that the information was not previously recorded.
Author Contributions
Conceptualization, S.O.; methodology, V.G.N.; software, S.O.; validation, V.G.N., D.B.; investigation, E.I.I., D.A.U., C.L., A.N.; resources, M.G.; data curation, E.M.; writing—original draft preparation, S.O.; writing—review and editing, V.G.N.; supervision, E.M.;. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of ‘Grigore Alexandrescu’ Pediatric Emergency Hospital (the approval code is 19 from the 28 May 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study according to the ‘Grigore Alexandrescu’ Pediatric Emergency Hospital protocol.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to restrictions such as privacy and ethics.
Acknowledgments
Publication of this paper was supported by the University of Medicine and Pharmacy Carol Davila, through the institutional program Publish not Perish.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Table A1.
KMO and Bartlett's test results.
Table A1.
KMO and Bartlett's test results.
| Kaiser–Meyer–Olkin Measure of Sampling Adequacy |
.636 |
| Bartlett's Test of Sphericity |
Approx. Chi-Square |
188.711 |
| df |
36 |
| Sig. |
<.001 |
Table A2.
Correlation results according to PCC.
Table A2.
Correlation results according to PCC.
| |
IgA |
IgG |
IgM |
IgE |
| IgA |
Pearson Correlation |
1 |
.238**
|
.443**
|
-.016 |
| Sig. (two-tailed) |
|
.005 |
<.001 |
.857 |
| N |
137 |
137 |
137 |
137 |
| Bootstrapc
|
Bias |
0 |
.011 |
-.085 |
.000 |
| Std. Error |
0 |
.111 |
.261 |
.060 |
| 95% Confidence Interval |
Lower |
1 |
.052 |
-.087 |
-.132 |
| Upper |
1 |
.465 |
.746 |
.111 |
| IgG |
Pearson Correlation |
.238**
|
1 |
.222**
|
.015 |
| Sig. (two-tailed) |
.005 |
|
.009 |
.859 |
| N |
137 |
137 |
137 |
137 |
| Bootstrapc
|
Bias |
.011 |
0 |
.020 |
.001 |
| Std. Error |
.111 |
0 |
.116 |
.047 |
| 95% Confidence Interval |
Lower |
.052 |
1 |
.046 |
-.071 |
| Upper |
.465 |
1 |
.469 |
.110 |
| IgM |
Pearson Correlation |
.443**
|
.222**
|
1 |
-.008 |
| Sig. (two-tailed) |
<.001 |
.009 |
|
.925 |
| N |
137 |
137 |
137 |
137 |
| Bootstrapc
|
Bias |
-.085 |
.020 |
0 |
.001 |
| Std. Error |
.261 |
.116 |
0 |
.057 |
| 95% Confidence Interval |
Lower |
-.087 |
.046 |
1 |
-.108 |
| Upper |
.746 |
.469 |
1 |
.116 |
| IgE |
Pearson Correlation |
-.016 |
.015 |
-.008 |
1 |
| Sig. (two-tailed) |
.857 |
.859 |
.925 |
|
| N |
137 |
137 |
137 |
137 |
| Bootstrapc
|
Bias |
.000 |
.001 |
.001 |
0 |
| Std. Error |
.060 |
.047 |
.057 |
0 |
| 95% Confidence Interval |
Lower |
-.132 |
-.071 |
-.108 |
1 |
| Upper |
.111 |
.110 |
.116 |
1 |
| **. Correlation is significant at the 0.01 level (two-tailed). |
| c. Unless otherwise noted, bootstrap results are based on 1000 bootstrap samples. |
Figure A1.
Characteristic purpuric lesions (photographs taken from the archives of the Toxicology – Intensive Care Unit of ‘Grigore Alexandrescu’ Pediatric Emergency Hospital)
Figure A1.
Characteristic purpuric lesions (photographs taken from the archives of the Toxicology – Intensive Care Unit of ‘Grigore Alexandrescu’ Pediatric Emergency Hospital)
References
- Penido, M.; Palma, L.M.P. IgA vasculitis in children. J Bras Nefrol 2022, 44, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Barankin, B.; Leong, K.F. Henoch-Schönlein Purpura in Children: An Updated Review. Curr Pediatr Rev 2020, 16, 265–276. [Google Scholar] [CrossRef]
- Pillebout, E.; Sunderkötter, C. IgA vasculitis. Seminars in Immunopathology 2021, 43, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.R.; White, R.H.; Akuse, R.; Chantler, C. Long-term follow-up of childhood Henoch-Schönlein nephritis. Lancet 1992, 339, 280–282. [Google Scholar] [CrossRef]
- Ruperto, N.; Ozen, S.; Pistorio, A.; Dolezalova, P.; Brogan, P.; Cabral, D.A.; Cuttica, R.; Khubchandani, R.; Lovell, D.J.; O'Neil, K.M.; et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part I: Overall methodology and clinical characterisation. Ann Rheum Dis 2010, 69, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Piram, M.; Maldini, C.; Biscardi, S.; De Suremain, N.; Orzechowski, C.; Georget, E.; Regnard, D.; Koné-Paut, I.; Mahr, A. Incidence of IgA vasculitis in children estimated by four-source capture–recapture analysis: a population-based study. Rheumatology 2017, 56, 1358–1366. [Google Scholar] [CrossRef]
- Rose, K.; Turner, J.E.; Iking-Konert, C. [Immunoglobulin A vasculitis (IgAV)]. Z Rheumatol 2023, 82, 587–598. [Google Scholar] [CrossRef]
- Horgos, M.S.; Pop, O.L.; Sandor, M.; Borza, I.L.; Negrean, R.A.; Cote, A.; Neamtu, A.-A.; Grierosu, C.; Sachelarie, L.; Huniadi, A. Platelets Rich Plasma (PRP) Procedure in the Healing of Atonic Wounds. Journal of Clinical Medicine 2023, 12, 3890. [Google Scholar] [CrossRef]
- Crowe, M.A.; Jonas, P. Acute hemorrhagic edema of infancy. Cutis 1998, 62, 65–66. [Google Scholar]
- Lawee, D. Atypical clinical course of Henoch-Schonlein purpura. Can Fam Physician 2008, 54, 1117–1120. [Google Scholar]
- He, X.; Yu, C.; Zhao, P.; Ding, Y.; Liang, X.; Zhao, Y.; Yue, X.; Wu, Y.; Yin, W. The genetics of Henoch-Schönlein purpura: a systematic review and meta-analysis. Rheumatol Int 2013, 33, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Cîrnaţu, D.; Szentesi, S.G.; Cuc, L.D.; Ciurariu, E.; Bran, L.R.; Bâtcă-Dumitru, G.-C.; Joldes, C.S.R.; Pantea, M.F.; Pârvu, S. Investigation and Modeling of the Variables of the Decision to Vaccinate as the Foundation of an Algorithm for Reducing Vaccination Reluctance. Systems 2023, 11, 220. [Google Scholar] [CrossRef]
- Ruan, J.W.; Fan, G.Z.; Niu, M.M.; Jiang, Q.; Li, R.X.; Qiu, Z.; Hu, P. Serum immunoglobulin profiles in Chinese children with Henoch-Schönlein purpura. Scand J Immunol 2022, 96, e13191. [Google Scholar] [CrossRef] [PubMed]
- Aleyd, E.; Heineke, M.H.; van Egmond, M. The era of the immunoglobulin A Fc receptor FcαRI; its function and potential as target in disease. Immunol Rev 2015, 268, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.-M.; Margan, M.-M.; Anghel, M.; Mocanu, A.; Laitin, S.M.D.; Margan, R.; Capraru, I.D.; Tene, A.-A.; Gal-Nadasan, E.-G.; Cirnatu, D.; et al. Developing Prediction Models for COVID-19 Outcomes: A Valuable Tool for Resource-Limited Hospitals. International Journal of General Medicine 2023, Volume 16, 3053–3065. [Google Scholar] [CrossRef]
- Oruc, Z.; Oblet, C.; Boumediene, A.; Druilhe, A.; Pascal, V.; Le Rumeur, E.; Cuvillier, A.; El Hamel, C.; Lecardeur, S.; Leanderson, T.; et al. IgA Structure Variations Associate with Immune Stimulations and IgA Mesangial Deposition. J Am Soc Nephrol 2016, 27, 2748–2761. [Google Scholar] [CrossRef]
- Donadio, J.V.; Grande, J.P. IgA nephropathy. N Engl J Med 2002, 347, 738–748. [Google Scholar] [CrossRef]
- Kerr, M.A. The structure and function of human IgA. Biochem J 1990, 271, 285–296. [Google Scholar] [CrossRef]
- Trnka, P. <scp>H</scp>enoch–<scp>S</scp>chönlein purpura in children. Journal of Paediatrics and Child Health 2013, 49, 995–1003. [Google Scholar] [CrossRef]
- Chintalacharuvu, K.R.; Raines, M.; Morrison, S.L. Divergence of human alpha-chain constant region gene sequences. A novel recombinant alpha 2 gene. J Immunol 1994, 152, 5299–5304. [Google Scholar] [CrossRef]
- de Sousa-Pereira, P.; Woof, J.M. IgA: Structure, Function, and Developability. Antibodies (Basel) 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Berthoux, F.; Suzuki, H.; Thibaudin, L.; Yanagawa, H.; Maillard, N.; Mariat, C.; Tomino, Y.; Julian, B.A.; Novak, J. Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol 2012, 23, 1579–1587. [Google Scholar] [CrossRef]
- Rajasekaran, A.; Julian, B.A.; Rizk, D.V. IgA Nephropathy: An Interesting Autoimmune Kidney Disease. Am J Med Sci 2021, 361, 176–194. [Google Scholar] [CrossRef]
- Pillebout, E. Purpura rhumatoïde de l’adulte. La Presse Médicale 2008, 37, 1773–1778. [Google Scholar] [CrossRef]
- Rigante, D.; Castellazzi, L.; Bosco, A.; Esposito, S. Is there a crossroad between infections, genetics, and Henoch–Schönlein purpura? Autoimmunity Reviews 2013, 12, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Crago, S.S.; Kutteh, W.H.; Moro, I.; Allansmith, M.R.; Radl, J.; Haaijman, J.J.; Mestecky, J. Distribution of IgA1-, IgA2-, and J chain-containing cells in human tissues. J Immunol 1984, 132, 16–18. [Google Scholar] [CrossRef]
- Davin, J.C.; Ten Berge, I.J.; Weening, J.J. What is the difference between IgA nephropathy and Henoch-Schönlein purpura nephritis? Kidney Int 2001, 59, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Nakanishi, K.; Yoshizawa, N.; Iijima, K.; Yoshikawa, N. Group A streptococcal antigen in the glomeruli of children with henoch-schönlein nephritis. American Journal of Kidney Diseases 2003, 41, 366–370. [Google Scholar] [CrossRef]
- Farooq, H.; Aemaz Ur Rehman, M.; Asmar, A.; Asif, S.; Mushtaq, A.; Qureshi, M.A. The pathogenesis of COVID-19-induced IgA nephropathy and IgA vasculitis: A systematic review. Journal of Taibah University Medical Sciences 2022, 17, 1–13. [Google Scholar] [CrossRef]
- Jassim Aziz R., M.D., Moroşan (Dogaru) E. “Study Regarding The Influence Of Vitis ViniferaFruit (Muscat Of Hamburg Species) On Some Biochemical Parameters”. Farmacia 2010, pp. 332-340,.
- Audemard-Verger, A.; Pillebout, E.; Guillevin, L.; Thervet, E.; Terrier, B. IgA vasculitis (Henoch-Shönlein purpura) in adults: Diagnostic and therapeutic aspects. Autoimmun Rev 2015, 14, 579–585. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Ozen, S.; Ruperto, N.; Dillon, M.J.; Bagga, A.; Barron, K.; Davin, J.C.; Kawasaki, T.; Lindsley, C.; Petty, R.E.; Prieur, A.M.; et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis 2006, 65, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.K.; Barratt, J. Inherited IgA glycosylation pattern in IgA nephropathy and HSP nephritis: where do we go next? Kidney Int 2011, 80, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Gardner-Medwin, J.M.; Dolezalova, P.; Cummins, C.; Southwood, T.R. Incidence of Henoch-Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet 2002, 360, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Oni, L.; Sampath, S. Childhood IgA Vasculitis (Henoch Schonlein Purpura)-Advances and Knowledge Gaps. Front Pediatr 2019, 7, 257. [Google Scholar] [CrossRef]
- Garzoni, L.; Vanoni, F.; Rizzi, M.; Simonetti, G.D.; Goeggel Simonetti, B.; Ramelli, G.P.; Bianchetti, M.G. Nervous system dysfunction in Henoch-Schonlein syndrome: systematic review of the literature. Rheumatology (Oxford) 2009, 48, 1524–1529. [Google Scholar] [CrossRef]
- Ozen, S.; Pistorio, A.; Iusan, S.M.; Bakkaloglu, A.; Herlin, T.; Brik, R.; Buoncompagni, A.; Lazar, C.; Bilge, I.; Uziel, Y.; et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis 2010, 69, 798–806. [Google Scholar] [CrossRef]
- Salas-Cuestas, F.; Bautista-Molano, W.; Bello-Gualtero, J.M.; Arias, I.; Castillo, D.M.; Chila-Moreno, L.; Valle-Oñate, R.; Herrera, D.; Romero-Sánchez, C. Higher Levels of Secretory IgA Are Associated with Low Disease Activity Index in Patients with Reactive Arthritis and Undifferentiated Spondyloarthritis. Front Immunol 2017, 8, 476. [Google Scholar] [CrossRef]
- Saulsbury, F.T. Henoch-Schönlein purpura in children. Report of 100 patients and review of the literature. Medicine (Baltimore) 1999, 78, 395–409. [Google Scholar] [CrossRef]
- Hamdan, J.M.; Barqawi, M.A. Henoch-Schonlein purpura in children. Influence of age on the incidence of nephritis and arthritis. Saudi Med J 2008, 29, 549–552. [Google Scholar]
- Rigante, D.; Castellazzi, L.; Bosco, A.; Esposito, S. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev 2013, 12, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Calviño, M.C.; Llorca, J.; García-Porrúa, C.; Fernández-Iglesias, J.L.; Rodriguez-Ledo, P.; González-Gay, M.A. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore) 2001, 80, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Piram, M.; Mahr, A. Epidemiology of immunoglobulin A vasculitis (Henoch-Schönlein): current state of knowledge. Curr Opin Rheumatol 2013, 25, 171–178. [Google Scholar] [CrossRef]
- Gay, C.; Lavocat, M.P.; Blanc, J.P. [Incidence of rheumatoid purpura in children and frequency of associated nephropathy in the Loire region]. Arch Pediatr 1997, 4, 486–488. [Google Scholar] [CrossRef]
- Peru, H.; Soylemezoglu, O.; Bakkaloglu, S.A.; Elmas, S.; Bozkaya, D.; Elmaci, A.M.; Kara, F.; Buyan, N.; Hasanoglu, E. Henoch Schonlein purpura in childhood: clinical analysis of 254 cases over a 3-year period. Clin Rheumatol 2008, 27, 1087–1092. [Google Scholar] [CrossRef]
- Trapani, S.; Micheli, A.; Grisolia, F.; Resti, M.; Chiappini, E.; Falcini, F.; De Martino, M. Henoch Schonlein purpura in childhood: epidemiological and clinical analysis of 150 cases over a 5-year period and review of literature. Semin Arthritis Rheum 2005, 35, 143–153. [Google Scholar] [CrossRef]
- Maldini, C.; Seror, R.; Fain, O.; Dhote, R.; Amoura, Z.; De Bandt, M.; Delassus, J.L.; Falgarone, G.; Guillevin, L.; Le Guern, V. Epidemiology of primary Sjögren's syndrome in a French multiracial/multiethnic area. Arthritis care & research 2014, 66, 454–463. [Google Scholar]
- Nong, B.R.; Huang, Y.F.; Chuang, C.M.; Liu, C.C.; Hsieh, K.S. Fifteen-year experience of children with Henoch-Schönlein purpura in southern Taiwan, 1991-2005. J Microbiol Immunol Infect 2007, 40, 371–376. [Google Scholar]
- Liu, C.; Luo, L.; Fu, M.; Li, Z.; Liu, J. Analysis of children with Henoch–Schonlein purpura secondary to infection. Clinical Rheumatology 2022, 41, 803–810. [Google Scholar] [CrossRef]
- Jauhola, O.; Ronkainen, J.; Koskimies, O.; Ala-Houhala, M.; Arikoski, P.; Hölttä, T.; Jahnukainen, T.; Rajantie, J.; Ormälä, T.; Nuutinen, M. Clinical course of extrarenal symptoms in Henoch-Schonlein purpura: a 6-month prospective study. Arch Dis Child 2010, 95, 871–876. [Google Scholar] [CrossRef]
- Dolezalová, P.; Telekesová, P.; Nemcová, D.; Hoza, J. Incidence of vasculitis in children in the Czech Republic: 2-year prospective epidemiology survey. J Rheumatol 2004, 31, 2295–2299. [Google Scholar] [PubMed]
- Mao, Y.; Yin, L.; Huang, H.; Zhou, Z.; Chen, T.; Zhou, W. Henoch-Schönlein purpura in 535 Chinese children: clinical features and risk factors for renal involvement. J Int Med Res 2014, 42, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.; Harper, S.; Feehally, J. Origin and structure of pathogenic IgA in IgA nephropathy. Biochem Soc Trans 1997, 25, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Soma, Y.; Kawakami, T. IgM in lesional skin of adults with Henoch-Schönlein purpura is an indication of renal involvement. J Am Acad Dermatol 2010, 63, 1026–1029. [Google Scholar] [CrossRef] [PubMed]
- Negreş S., C. C., Moroşan E., Arsene A.L. „Experimental Pharmacological Model Of Diabetes Induction With Aloxan In Rat”. Farmacia 2013, 6, 10. [Google Scholar]
- Nikolaishvili, M.; Pazhava, A.; Di Lernia, V. Viral Infections May Be Associated with Henoch-Schönlein Purpura. J Clin Med 2023, 12. [Google Scholar] [CrossRef]
- Novak, J.; Moldoveanu, Z.; Julian, B.A.; Raska, M.; Wyatt, R.J.; Suzuki, Y.; Tomino, Y.; Gharavi, A.G.; Mestecky, J.; Suzuki, H. Aberrant glycosylation of IgA1 and anti-glycan antibodies in IgA nephropathy: role of mucosal immune system. Adv Otorhinolaryngol 2011, 72, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Bardana, E.J., Jr. Immunoglobulin E- (IgE) and non-IgE-mediated reactions in the pathogenesis of atopic eczema/dermatitis syndrome (AEDS). Allergy 2004, 59 Suppl 78, 25–29. [Google Scholar] [CrossRef]
- Oprițescu, S.; Nițescu, G.V.; Cîrnațu, D.; Trifunschi, S.; Munteanu, M.; Golumbeanu, M.; Boghițoiu, D.; Dărăban, A.M.; Ilie, E.I.; Moroșan, E. Elevated Immunoglobulin E Serum Levels: Possible Underlying Factors That Can Cause an Inborn Error of Immunity in the Pediatric Population with Recurrent Infections. Antibodies 2024, 13, 47. [Google Scholar] [CrossRef]
- Saulsbury, F.T. Clinical update: Henoch-Schönlein purpura. Lancet 2007, 369, 976–978. [Google Scholar] [CrossRef]
- Hovanet M.V., A. R., Dinu M., Oprea E., Budura E.A., Negreṣ S., Velescu B., Duṭu L., Anghel I., Ancu I, Moroṣan E., Seremet O. „Toxicity and anti-inflammatory activity of Ziziphus jujuba Mill. Leaves”. Farmacia 2016, 64, 7. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).