1. Introduction
Toxoplasma gondii is an obligate intracellular protozoan parasite that can infect humans and all other mammals [1, 2}. This parasite forms tissue cysts in various organs especially in the brain and establishes a long-lasting chronic infection in these hosts [
1,
2]. Since the tissue cysts can persist in immunocompetent hosts for long periods of time during the chronic stage of infection, it was considered that the immune system is unable to detect or attack the tissue cysts of this parasite. However, our recent studies uncovered that CD8
+ T cells have the capability to detect the host cells harboring
T.
gondii cysts and destroy them through their perforin-mediated effector activity [
3,
4]. Furthermore, we identified that CD8
+ T cells penetrate into the cysts using their perforin-mediated activity and induce morphological deterioration and destruction of the cysts, which is followed by an accumulation of large numbers of phagocytes [
4]. When mRNA levels for the immunity-related 734 molecules were compared in the brains of chronically infected SCID mice that had received CD8
+ T cells from infected wild-type (WT) and perforin-deficient (Prf1
-/-) mice, mRNA levels for only 6 molecules were identified to be significantly greater in the brains of the recipients of the WT CD8
+ T cells than those of Prf1
-/- CD8
+ T cells [
5]. These six molecules were two T cell costimulatory molecules (inducible costimulator [ICOS] and its ligand [ICOSL]); two chemokine receptors (C-X-C motif chemokine receptor 3 [CXCR3] and CXCR6); and two molecules related to an activation of microglia and macrophages (interleukin 18 receptor 1 [IL-18R1] and chitinase-like 3) [
5].
The ICOS is a prominent costimulatory molecule that belongs to CD28 receptor family for supporting the activities of both CD4
+ and CD8
+ T cells. However, whereas CD28 is expressed on most T cells including naïve T cells, ICOS expression on CD8+ T cells is induced only after their activation through their T cell receptor engagement with target antigens presented by the MHC class I molecules [
6,
7]. In several bacterial and viral infections, blocking or deficiency of ICOS resulted in reduced numbers of the pathogen-specific [
8,
9] or IFN-γ
+ [
10,
11] CD8
+ T cells. Reduced IFN-γ production and cytotoxic activity of CD8
+ T cells were also observed in ICOS
-/- mice infected with
Salmonella enterica serovar Typhimurium [
12]. In contrast, in persistent infection with
Plasmodium chabaude chabaude AS, an intracellular protozoan parasite that proliferates within red blood cells, ICOS
-/- mice displayed increased numbers of IFN-γ
+ CD8
+ T cells [
13]. Therefore, It is important to determine the roles of ICOS specifically on the cytotoxic effector activity of CD8
+ T cells against
T.
gondii cysts during chronic infection with this intracellular protozoan parasite.
In the present study, we examined the roles of ICOS on the effector activity of CD8+ T cells against T. gondii cysts by transferring CD8+ immune T cells from infected wild-type (WT) and ICOS-deficient (ICOS-/-) mice into infected SCID mice lacking T cells to determine the effects of ICOS deficiency on the effector function of those CD8+ T cells to remove the tissue cysts from the brains of the recipient SCID mice. Unexpectedly, we found that ICOS-/- CD8+ T cells eliminated T. gondii cysts from the brains of the recipients more efficiently than WT CD8+ T cells did, whereas fewer ICOS-/- T cells migrated into the brains of the recipients than the WT T cells. We identified that the ICOS-/- CD8+ T cells expressed greater levels of CD28 on their surface than WT CD8+ T cells did, and a blocking of CD28 signaling pathway with a combination of anti-CD80 and anti-CD86 antibodies abolished increased secretion of GzmB by ICOS-/- CD8+ T cells in response to T. gondii antigens in vitro, indicating that the upregulated expression of CD28 compensated the absence of ICOS and maintained the cytotoxic effector activity of CD8+ T cells against T. gondii during chronic infection.
4. Discussion
The present study with adoptive transfer of CD8
+ T cells from ICOS
-/- and WT mice chronically infected with
T.
gondii to infected SCID mice revealed that a deficiency of ICOS during chronic infection with this parasite results in increased efficiency of the CD8
+ T cells to eliminate tissue cysts of this parasite from the brains of the recipients. Our previous studies identified that anti-cyst activity of CD8
+ T cells is operated by their perforin-dependent cytotoxic activity [
3,
4]. Consistently, the present study also revealed that relative expression levels of perforin mRNA in ratios to CD8β mRNA in the brains of the recipients of ICOS
-/- CD8
+ T cells are significantly greater than those of the recipients of WT CD8
+ T cells, suggesting that ICOS
-/- CD8
+ T cells express greater levels of perforin mRNA in the brains of the recipient SCID mice than did WT CD8
+ T cells. Furthermore, the present study identified that CD8
+ T cells from infected ICOS
-/- mice secrete much greater amounts of Gzm B in response to
T.
gondii antigens
in vitro than did CD8
+ T cells from infected WT mice. GzmB is a key effector molecule, in addition to perforin, in the cytotoxic activity of CD8
+ T cells. In relation to our findings, a recent study with persistent infection with
Plasmodium chabaude chabaude AS, an intracellular protozoan parasite closely related to
T.
gondii, demonstrated that infected ICOS
-/- mice displayed increased numbers of IFN-γ
+ CD8
+ T cells when compared to infected WT mice [
13]. In contrast, previous studies using infections with viruses [
10,
28], bacteria [
8,
11,
12] showed that the absence of ICOS costimulatory signaling pathway, either by genetic deletion of ICOS or blocking of its functions by anti-ICOS mAb or ICOS-Ig (a fusion protein of ICOS and the Fc region of human IgG1), downregulates [
8,
10,
11,
12] or does not affect [
28] the cytotoxic activity and/or IFN-γ production of CD8
+ T cells during those microbial infections. The present study provides a new insight that ICOS deficiency induces an upregulation of the cytotoxic activity and the effector function of CD8
+ T cells against
T.
gondii during chronic infection with this protozoan parasite. Therefore, the effects of the absence of ICOS on the functions of CD8
+ T cells during microbial infections most likely differ depending of the types of pathogens.
The present study was performed during the chronic stage of
T.
gondii infection, in which WT and ICOS
-/- mice were infected for at least 2 months.
T. gondii resides and proliferate within the parasitophorous vacuole (PV) in infected host cells. The PV prevents its fusion with lysosomes and protects the parasite from their elimination [
29,
30]. Similarly,
Mycobacterium tuberculosis, an intracellular bacterium, also resides within phagosomes in infected cells, and prevents their fusion with lysosomes [
31,
32,
33]. In infection with
Mycobacterium tuberculosis, the bacterial loads in the spleen did not differ between ICOS
-/- and WT mice during the first 40 days of the infection, but the pathogen loads become significantly less in the former than the latter at 60 and 120 days after infection (11). Of interest, the significantly reduced bacterial loads in the ICOS
-/- mice during the later time points of the infection are associated with increased numbers of IFN-γ
+ CD4
+ T cells in the spleens of these mice [
11]. Therefore, the effects of ICOS deficiency on CD4
+ and CD8
+ T cells could differ depending on the time periods that the hosts have been infected with certain pathogens.
There are notable differences in resistance and susceptibility to chronic infection with
T.
gondii among inbred strains of mice [
34,
35,
36]. Mice with the H-2
b (e.g. C57BL/6) and H-2
k haplotypes (e.g. C3H/He) are susceptible and develop progressive and ultimately fatal toxoplasmic encephalitis during the later stage of infection, whereas mice with the H-2
d haplotype (e.g. BALB/c) are resistant and maintain a latency of the chronic infection in their brains [
34,
35,
36]. The present study was performed in the genetically resistant BALB/c-background mice. A previous study by others [
37] using the BALB/c-background ICOS
-/- mice showed that whereas percentages of IFN-γ
+ cells in CD4
+ T cells in the spleens were reduced in ICOS
-/- mice when compared to WT mice during the acute stage (day 7) of infection, percentages of IFN-γ
+ cells in CD8
+ T cells did not differ between ICOS
-/- and WT mice in their spleens during the early stage of infection and in their brains during a later stage (weeks 4-6) of infection. In contrast, the present study revealed increased cytotoxic effector activity of CD8
+ T cells against
T.
gondii cysts in BALB/c-background ICOS
-/- than WT mice during the chronic stage of infection. Therefore, it may be possible that IFN-γ production and cytotoxic activity of CD8
+ T cells are controlled in a different manner through ICOS-mediated pathways.
In contrast to the genetically resistant BALB/c mice, mice in genetically susceptible C57BL/6-background displayed that blocking of ICOS signaling by anti-ICOSL mAb or a genetic deletion of ICOS increases numbers of CD4
+ and CD8
+ T cells and IFN-γ
+ CD8
+ T cells in the spleens and brains during 5-6 weeks after infection, but significantly greater numbers of
T.
gondii cysts were detected in the brains of the infected ICOS
-/- than WT mice [
38,
39]. In the present study with genetically resistant BALB/c-background mice, we identified that numbers of ICOS
-/- CD8
+ T cells in the brains of the SCID mice that had received those T cells were fewer than those in the brains of the recipients of WT CD8
+ T cells, but the former eliminated
T.
gondii cysts from the brains of the recipients more efficiently than the latter did. It is most likely that the roles of ICOS on the protective activities of CD8
+ T cells against
T.
gondii differ depending of the genetic resistance/susceptibility of the hosts to the infection.
Previous studies using infections with vaccinia virus [
40], influenza virus [
9], and
Listeria monocytogenes [
41] demonstrated a requirement of CD28 for optimal recall responses of CD8
+ T cells. Notably, the present study using flow cytomotry identified that the absence of ICOS becomes compensated by the upregulation of CD28 expression levels on splenic CD8
+ T cells during the chronic stage of
T.
gondii infection. Consistently, the present study also identified that the ratios of CD28 mRNA levels to CD8β mRNA levels in the brains of infected SCID mice that had received ICOS
-/- splenic CD8
+ T cells were significantly greater than those in those of the recipients of WT splenic CD8
+ T cells. Furthermore,
in vitro stimulation of CD8
+ T cells purified from the spleens of infected ICOS
-/- and WT mice with
T.
gondii antigens revealed that upregulated CD28 expression mediates the increased cytotoxic effector activity of the ICOS
-/- CD8
+ T cells in their recall responses to the pathogen.
The transcription factor T-bet plays critical roles for the cytotoxic activities of CD8
+ T cells [
24,
25,
26]. The present study identified that the degrees of increases in relative expression levels of mRNA for CD28 in ratios to CD8β mRNA levels strongly correlate with the degrees of increases in ratios of T-bet mRNA levels to CD8β mRNA levels in the brains of the recipients of ICOS
-/- and WT CD8
+ T cells. In addition, the degrees of increases in the ratios of T-bet mRNA levels/CD8β mRNA levels in the brains of the recipients of those CD8
+ T cells strongly correlated with the degrees of increases in the ratios of perforin mRNA levels/ CD8β mRNA levels in those mice. Therefore, enhanced costimulatory signal through the increased expression of CD28 in ICOS
-/- CD8
+ T cells most likely induced upregulation of perforin mRNA levels through their increased expression of T-bet transcription factor and enhanced the efficiency of elimination of
T.
gondii cysts through their cytotoxic activity. To our knowledge, an upregulation of CD28 expression on CD8
+ T cells in compensation of a deficiency of ICOS and an enhancement of their cytotoxic effector activity through the upregulated CD28 expression have not been reported before.
In relation to our finding on the compensation of the absence of ICOS by an upregulation of CD28 in the recall responses of the cytotoxic activity of CD8
+ T cells against
T.
gondii, a previous study with infections with Lymphocytic choriomeningitis virus and vesicular stomatitis virus demonstrated that blocking of ICOS signaling by ICOS-Ig markedly impaired IFN-γ production of CD4
+ T cells against the viruses in CD28
-/- mice, whereas ICOS-Ig treatment in WT mice had only a limited downregulatory effect on their IFN-γ production [
28]. Thus, it would be possible that not only a compensation of the absence of ICOS signaling by upregulation of CD28 expression but also a compensation of the absence of CD28 by upregulation of ICOS could be operated in maintaining the effector functions of not only CD8
+ T cells but also in CD4
+ T cells during microbial infections, although the interactions between ICOS and CD28 on regulating the functions of these T cell populations could differ depending on the pathogens as discussed earlier.
The present study provided a novel insight on a notable capability of the immune system to secure the protective activities of CD8
+ T cells by utilizing compensatory interactions between two important costimulatory molecules, ICOS and CD28, for host resistance during chronic infection with
T.
gondii. The results of the present study may also suggest that under the presence of CD28 expression, increased expression of ICOS in WT CD8
+ T cells as detected in our recent study [
5] could enhances their anti-cyst effector activity to eliminate
T.
gondii cysts.
Figure 1.
CD8+ immune T cells from ICOS-/- mice chronically infected with T. gondii possess an increased capability to eliminate T. gondii cysts. CD8+ T cells purified from the spleens from chronically infected WT and ICOS-/- mice were injected (2 x 106 cells/mouse) intravenously into chronically infected (infected and treated with sulfadiazine) SCID mice. As a control, two groups of the SCID mice did not receive any T cells. Seven days later (Day 7), the brains of the T cell recipients and one group of the control mice with no T cell transfer were obtained for measuring mRNA levels for (A) bradyzoite (cyst)-specific BAG1 by RT-PCR. Brain samples from another group of the control mice with no the T cell transfer were obtained on the day of the T cell transfer (Day 0) for the RT-PCR. (B) Numbers of T. gondii cysts per a sagittal section of the brain on Day 7 after their immunohistological staining. Three sections with 16 or 20 μm distance were counted for each mouse, and the mean value from the counts from the three sections was used for each mouse. (C) CD8+ T cells detected in a total of 10 randomly selected fields at X200 magnification of a sagittal section the brain of each of the recipients of CD8+ T cells from ICOS-/- or WT mice were counted microscopically after their immunohistological staining. A representative image (X200 magnification) of CD8+ T cells (stained in brown, some are arrowed) detected in a sagittal section of the brains of (D) WT CD8+ T cell recipients and (E) ICOS-/- CD8+ T cell recipients. (F) A representative image (X200 magnification) of a T. gondii cyst (stained in red, arrowed) attacked by WT CD8+ T cells (stained in brown). (G) CD8β mRNA levels in the brains of the recipients of WT and ICOS-/- CD8+ T cells, (H) the efficiency of cyst removal by CD8+ T cells that migrated into the brains of the recipients (the ratios of BAG1 mRNA level reduction by the CD8+ T cell transfer [differences between the mean value of BAG1 mRNA levels in the control mice with no T cell transfer and BAG1 mRNA levels in each of the recipients of WT or ICOS-/- CD8+ T cells] to amounts of CD8β mRNA in the brain of each recipient mouse). There were four SCID mice in each of the groups that received WT or ICOS-/- CD8+ T cells. (I) Frequencies of CD44highCD62Llow effector memory population in the splenic CD8+ T cells of chronically infected ICOS-/- and WT mice. There were three or four SCID mice in the control group without any T cell transfer at each of Day 0 and Day 7. In regard to the donors of the CD8+ T cells, there were three or four mice in each of infected WT and ICOS-/- mice, and their spleen cells were pooled within the same experimental group for purifying CD8+ T cells. Two independent experiments were performed. Panels A, G, and H show the RT-PCR results combined from the two independent experiments, which provided 7-8 mice in each experimental group. *P<0.05, **P<0.01, ***P<0.001, N.S., Not significant.
Figure 1.
CD8+ immune T cells from ICOS-/- mice chronically infected with T. gondii possess an increased capability to eliminate T. gondii cysts. CD8+ T cells purified from the spleens from chronically infected WT and ICOS-/- mice were injected (2 x 106 cells/mouse) intravenously into chronically infected (infected and treated with sulfadiazine) SCID mice. As a control, two groups of the SCID mice did not receive any T cells. Seven days later (Day 7), the brains of the T cell recipients and one group of the control mice with no T cell transfer were obtained for measuring mRNA levels for (A) bradyzoite (cyst)-specific BAG1 by RT-PCR. Brain samples from another group of the control mice with no the T cell transfer were obtained on the day of the T cell transfer (Day 0) for the RT-PCR. (B) Numbers of T. gondii cysts per a sagittal section of the brain on Day 7 after their immunohistological staining. Three sections with 16 or 20 μm distance were counted for each mouse, and the mean value from the counts from the three sections was used for each mouse. (C) CD8+ T cells detected in a total of 10 randomly selected fields at X200 magnification of a sagittal section the brain of each of the recipients of CD8+ T cells from ICOS-/- or WT mice were counted microscopically after their immunohistological staining. A representative image (X200 magnification) of CD8+ T cells (stained in brown, some are arrowed) detected in a sagittal section of the brains of (D) WT CD8+ T cell recipients and (E) ICOS-/- CD8+ T cell recipients. (F) A representative image (X200 magnification) of a T. gondii cyst (stained in red, arrowed) attacked by WT CD8+ T cells (stained in brown). (G) CD8β mRNA levels in the brains of the recipients of WT and ICOS-/- CD8+ T cells, (H) the efficiency of cyst removal by CD8+ T cells that migrated into the brains of the recipients (the ratios of BAG1 mRNA level reduction by the CD8+ T cell transfer [differences between the mean value of BAG1 mRNA levels in the control mice with no T cell transfer and BAG1 mRNA levels in each of the recipients of WT or ICOS-/- CD8+ T cells] to amounts of CD8β mRNA in the brain of each recipient mouse). There were four SCID mice in each of the groups that received WT or ICOS-/- CD8+ T cells. (I) Frequencies of CD44highCD62Llow effector memory population in the splenic CD8+ T cells of chronically infected ICOS-/- and WT mice. There were three or four SCID mice in the control group without any T cell transfer at each of Day 0 and Day 7. In regard to the donors of the CD8+ T cells, there were three or four mice in each of infected WT and ICOS-/- mice, and their spleen cells were pooled within the same experimental group for purifying CD8+ T cells. Two independent experiments were performed. Panels A, G, and H show the RT-PCR results combined from the two independent experiments, which provided 7-8 mice in each experimental group. *P<0.05, **P<0.01, ***P<0.001, N.S., Not significant.
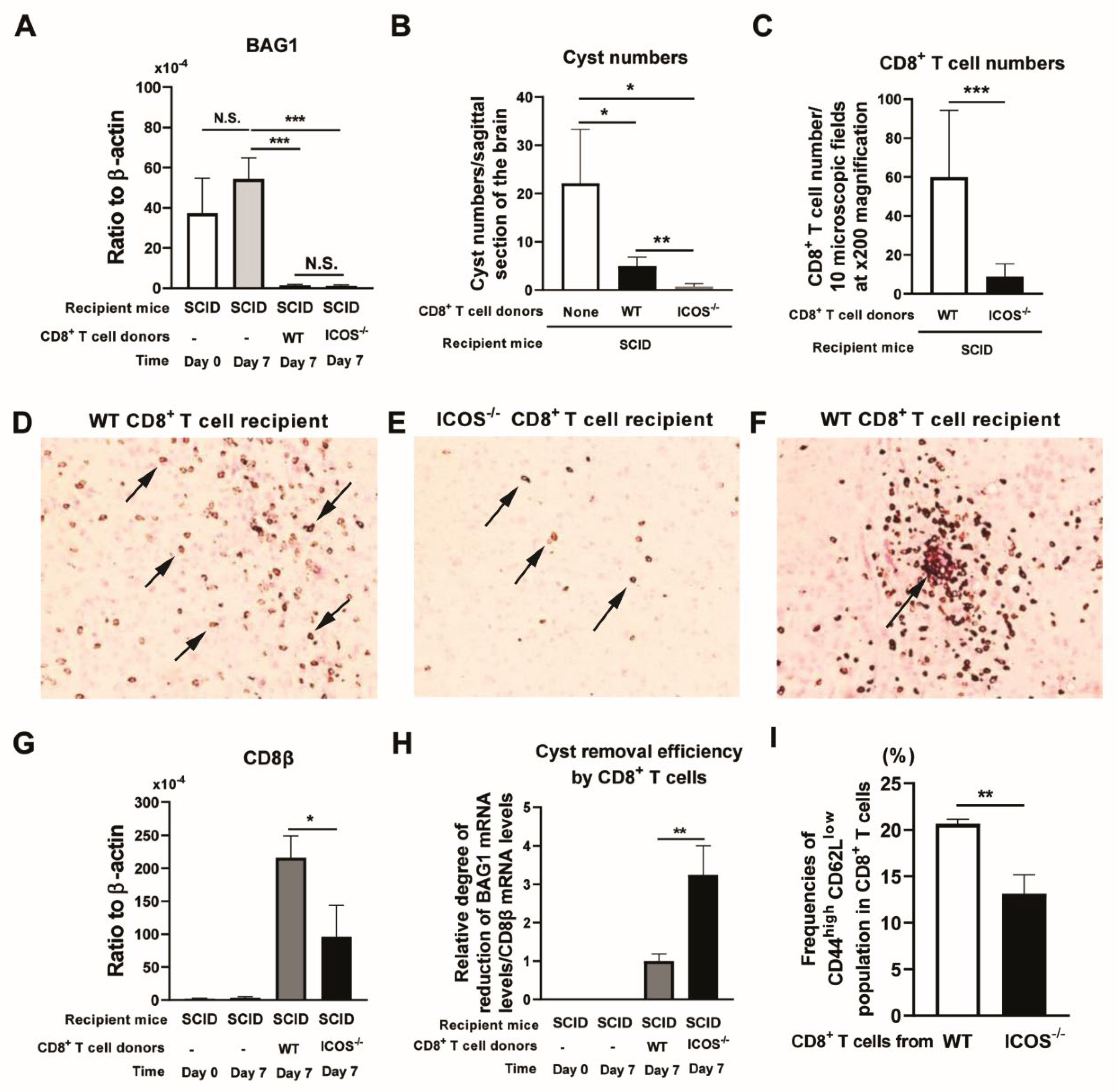
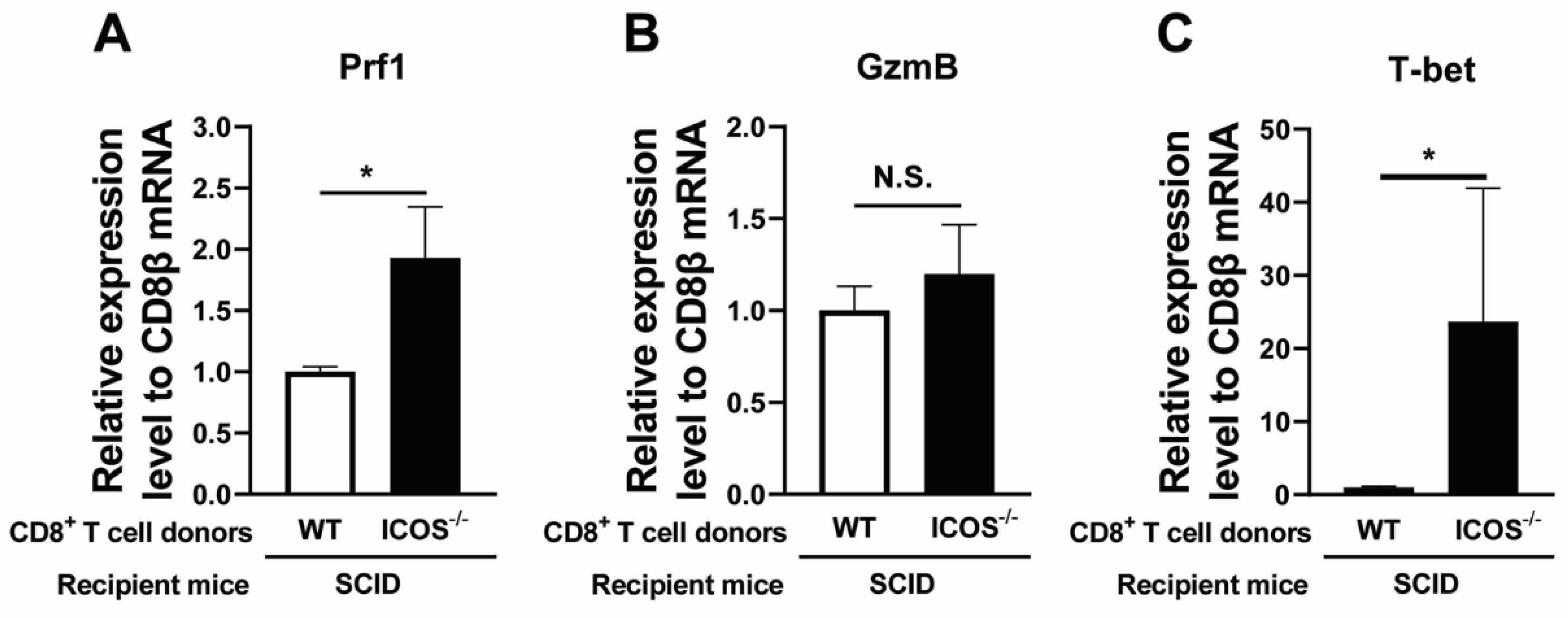
Figure 2.
Relative mRNA expression levels for perforin and T-bet in ratios to CD8β mRNA levels are greater in the brains of SCID mice that received ICOS-/- CD8+ T cells than those that received WT CD8+ T cells. CD8+ T cells purified from the spleens from chronically infected WT and ICOS-/- mice were injected (2 x 106 cells/mouse) intravenously into chronically infected (infected and treated with sulfadiazine) SCID mice. Seven days later (Day 7), the ratios of mRNA levels for (A) perforin, (B) GzmB, and (C) T-bet to mRNA levels to mRNA levels for CD8β were measured in the brains of those SCID mice by RT-PCR. There were four SCID mice in each of the groups. Two independent experiments were performed, and results combined from the two independent experiments (a total of 7-8 mice in each experimental group). *P<0.05, N.S., Not significant.
Figure 2.
Relative mRNA expression levels for perforin and T-bet in ratios to CD8β mRNA levels are greater in the brains of SCID mice that received ICOS-/- CD8+ T cells than those that received WT CD8+ T cells. CD8+ T cells purified from the spleens from chronically infected WT and ICOS-/- mice were injected (2 x 106 cells/mouse) intravenously into chronically infected (infected and treated with sulfadiazine) SCID mice. Seven days later (Day 7), the ratios of mRNA levels for (A) perforin, (B) GzmB, and (C) T-bet to mRNA levels to mRNA levels for CD8β were measured in the brains of those SCID mice by RT-PCR. There were four SCID mice in each of the groups. Two independent experiments were performed, and results combined from the two independent experiments (a total of 7-8 mice in each experimental group). *P<0.05, N.S., Not significant.
Figure 3.
Relative mRNA levels for CD28 in ICOS-/- CD8+ T cells that migrated into the brains of the recipient SCID mice are greater than those of the WT CD8+ T cells that migrated into the brains of recipient SCID mice (A and B), and strong correlations are present between the increased relative mRNA expression levels for CD28 and those for T-bet (C) and between relative mRNA expression levels for T-bet and those for perforin (D) in the CD8+ T cells that migrated into the brains of the recipients during their elimination of T. gondii cysts. CD8+ T cells purified from the spleens from chronically infected WT and ICOS-/- mice were injected (2 x 106 cells/mouse) intravenously into chronically infected (infected and treated with sulfadiazine) SCID mice. Seven days later, their brains were obtained for measuring (A) the ratios of 4-1BB (TNFRSF9) mRNA levels to CD8β mRNA levels, and (B) the ratios of CD28 mRNA levels to CD8β mRNA by RT-PCR. Correlations of (C) the ratios of CD28 mRNA/ CD8β mRNA levels with T-bet mRNA/CD8β mRNA levels and (D) the ratios of T-bet mRNA/ CD8β mRNA levels with perforin mRNA/CD8β mRNA levels with were examined in the brains of the recipients of the ICOS-/- and WT CD8+ T cells. In these correlation analyses, the data from both the recipients of ICOS-/- CD8+ T cells and those of WT CD8+ T cells were included. Two independent experiments were performed, and the results from these two experiments were combined (a total of 7-8 mice in each experimental group). *P<0.05. .
Figure 3.
Relative mRNA levels for CD28 in ICOS-/- CD8+ T cells that migrated into the brains of the recipient SCID mice are greater than those of the WT CD8+ T cells that migrated into the brains of recipient SCID mice (A and B), and strong correlations are present between the increased relative mRNA expression levels for CD28 and those for T-bet (C) and between relative mRNA expression levels for T-bet and those for perforin (D) in the CD8+ T cells that migrated into the brains of the recipients during their elimination of T. gondii cysts. CD8+ T cells purified from the spleens from chronically infected WT and ICOS-/- mice were injected (2 x 106 cells/mouse) intravenously into chronically infected (infected and treated with sulfadiazine) SCID mice. Seven days later, their brains were obtained for measuring (A) the ratios of 4-1BB (TNFRSF9) mRNA levels to CD8β mRNA levels, and (B) the ratios of CD28 mRNA levels to CD8β mRNA by RT-PCR. Correlations of (C) the ratios of CD28 mRNA/ CD8β mRNA levels with T-bet mRNA/CD8β mRNA levels and (D) the ratios of T-bet mRNA/ CD8β mRNA levels with perforin mRNA/CD8β mRNA levels with were examined in the brains of the recipients of the ICOS-/- and WT CD8+ T cells. In these correlation analyses, the data from both the recipients of ICOS-/- CD8+ T cells and those of WT CD8+ T cells were included. Two independent experiments were performed, and the results from these two experiments were combined (a total of 7-8 mice in each experimental group). *P<0.05. .

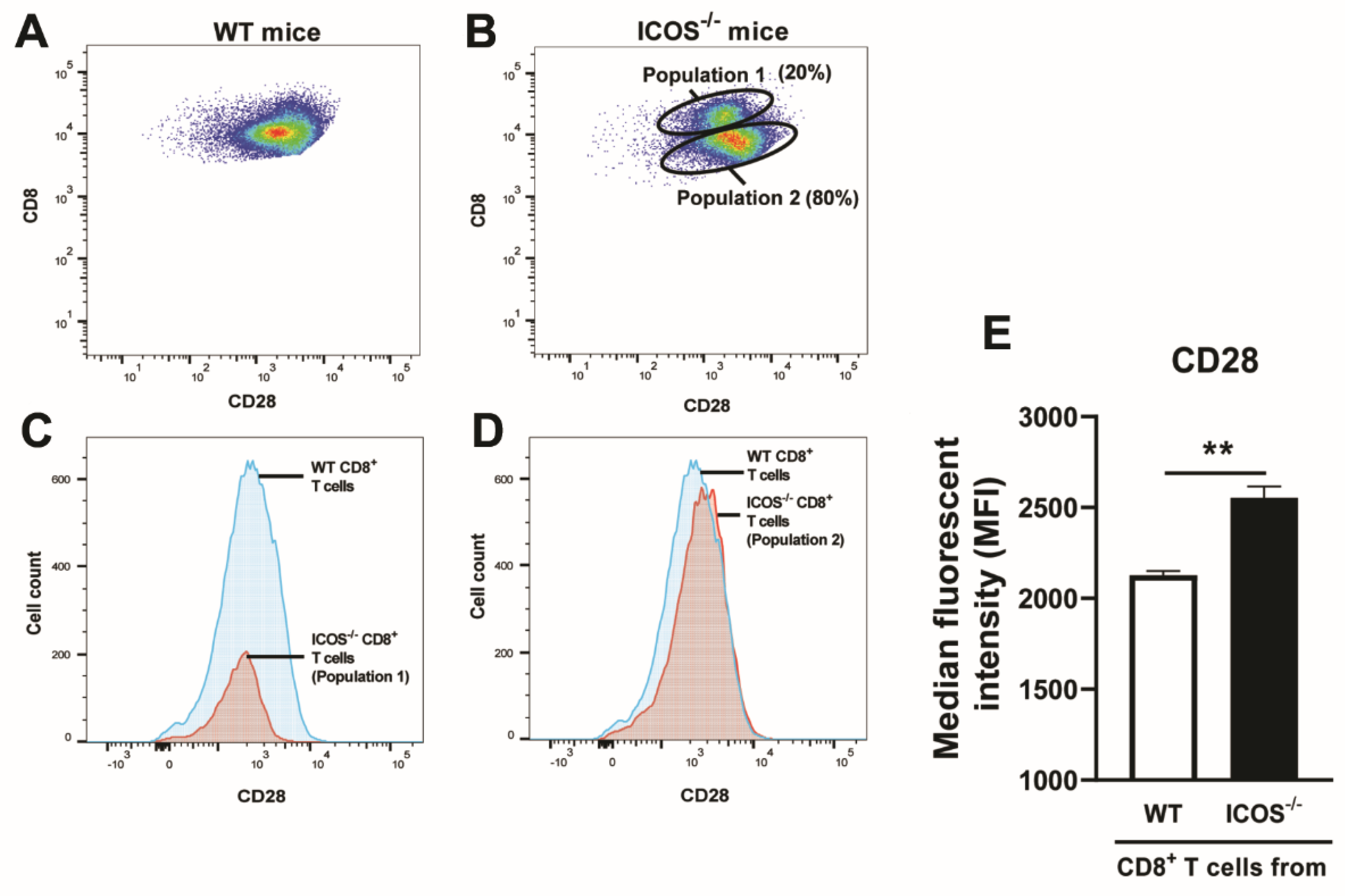
Figure 4.
Splenic CD8+ T cells of ICOS-/- mice chronically infected with T. gondii express greater levels of CD28 on their surfaces than CD8+ T cells of the infected WT mice. CD8+ T cells purified from the spleens of chronically infected WT and ICOS-/- mice were stained with FITC-labeled anti-mouse CD8α and PE-labeled anti-mouse CD28 mAbs and applied for flow cytometric analysis. For control, the cells were stained with FITC- and PE-labeled isotype control mAbs. A representative image of the FACS plots for expressions of CD8 and CD28 on CD8+ T cells from chronically infected (A) WT and (B) ICOS-/- mice. Comparisons of CD28 expression levels between (C) the population 1 of ICOS-/- CD8+ T cells and WT CD8+ T cells, and (D) between the population 2 of ICOS-/- CD8+ T cells and WT CD8+ T cells. (E) The median fluorescence intensity (MFI) of CD28 expressions on the population 2 of ICOS-/- CD8+ T cells and WT CD8+ T cells. *P<0.05, **P<0.01. N.S., Not significant.
Figure 4.
Splenic CD8+ T cells of ICOS-/- mice chronically infected with T. gondii express greater levels of CD28 on their surfaces than CD8+ T cells of the infected WT mice. CD8+ T cells purified from the spleens of chronically infected WT and ICOS-/- mice were stained with FITC-labeled anti-mouse CD8α and PE-labeled anti-mouse CD28 mAbs and applied for flow cytometric analysis. For control, the cells were stained with FITC- and PE-labeled isotype control mAbs. A representative image of the FACS plots for expressions of CD8 and CD28 on CD8+ T cells from chronically infected (A) WT and (B) ICOS-/- mice. Comparisons of CD28 expression levels between (C) the population 1 of ICOS-/- CD8+ T cells and WT CD8+ T cells, and (D) between the population 2 of ICOS-/- CD8+ T cells and WT CD8+ T cells. (E) The median fluorescence intensity (MFI) of CD28 expressions on the population 2 of ICOS-/- CD8+ T cells and WT CD8+ T cells. *P<0.05, **P<0.01. N.S., Not significant.
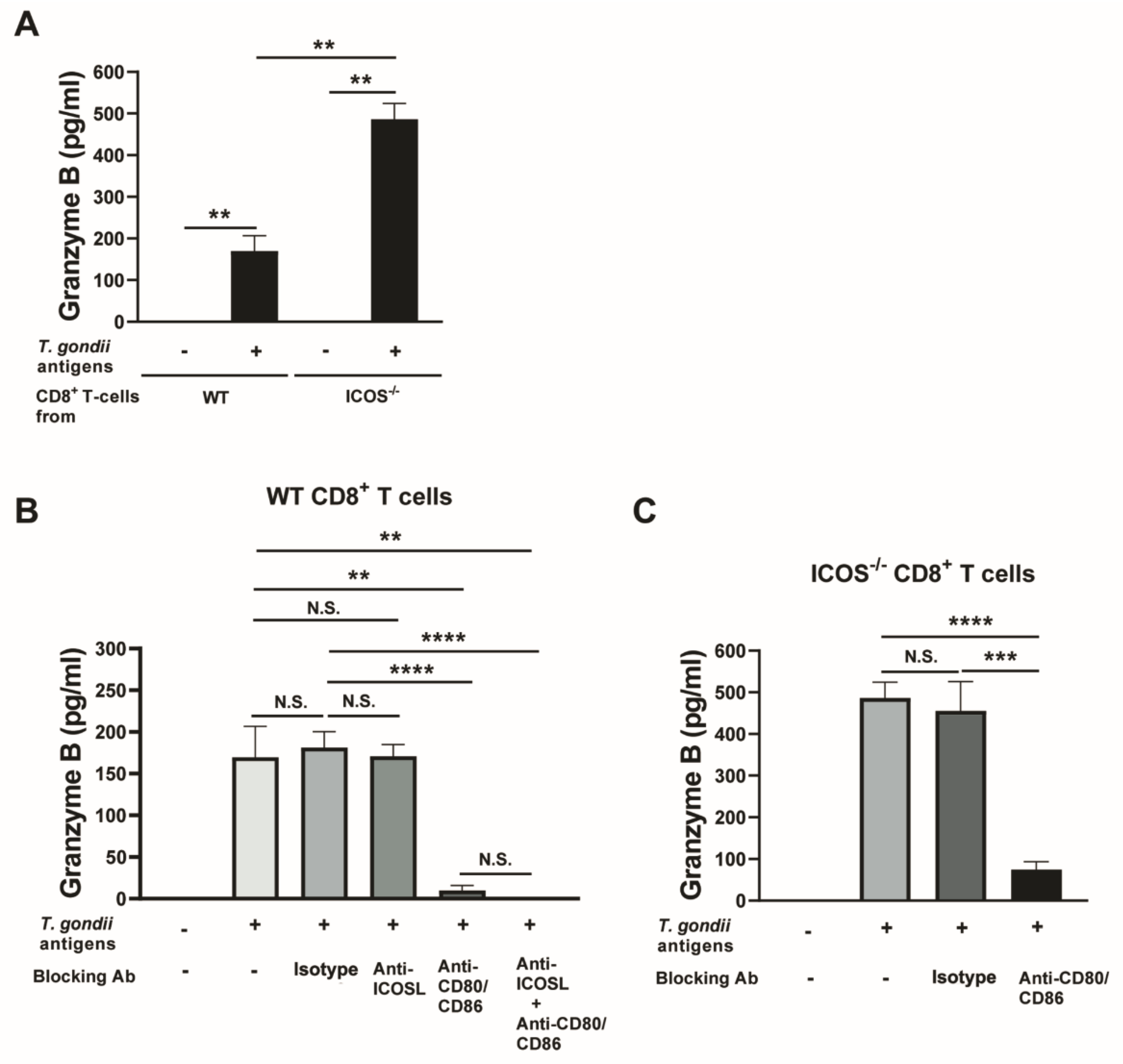
Figure 5.
Blockage of CD28−CD80/CD86 costimulatory pathway abolishes the cytotoxic functions of CD8+ T cells of ICOS-/- mice chronically infected with T. gondii. CD8+ T cells were purified from the spleens of chronically WT and ICOS-/- mice and cultured (3 x 105 cells/well) in 96 well culture plates with antigen-presenting cells (plastic-adherent cells) from the corresponding strain of mice in the presence or absence of T. gondii antigens (10 μg/ml) for 72 hrs. Blocking mAbs against ICOSL, both CD80 and CD86, or a combination of ICOSL, CD80, and CD86 were added at 10 μg/ml to a part of these cultures to block the ICOS-ICOSL, CD28-CD80/CD86, or both of these costimulatory pathways. As a control, isotype control mAbs were added in the same manner. Concentrations of GzmB in the culture supernatants in the cultures were measured by ELISA. (A) A comparison of GzmB levels in the culture supernatants of WT and ICOS-/- CD8+ T cells in the presence or absence of T. gondii antigens without any blocking mAbs. Comparisons of GzmB levels in the culture supernatants of (B) WT CD8+ T cells and (C) ICOS-/- CD8+ T cells in the presence and absence of the blocking mAbs against the ICOS-ICOSL or CD28-CD80/CD86 costimulatory pathways. There were 2 mice in each of infected WT and ICOS-/- mice, and their spleen cells were pooled within the same experimental group for purifying CD8+ T cells. There were 5 wells in each experimental group. **P<0.01, ***P<0.001, ****P<0.0001, N.S., Not significant.
Figure 5.
Blockage of CD28−CD80/CD86 costimulatory pathway abolishes the cytotoxic functions of CD8+ T cells of ICOS-/- mice chronically infected with T. gondii. CD8+ T cells were purified from the spleens of chronically WT and ICOS-/- mice and cultured (3 x 105 cells/well) in 96 well culture plates with antigen-presenting cells (plastic-adherent cells) from the corresponding strain of mice in the presence or absence of T. gondii antigens (10 μg/ml) for 72 hrs. Blocking mAbs against ICOSL, both CD80 and CD86, or a combination of ICOSL, CD80, and CD86 were added at 10 μg/ml to a part of these cultures to block the ICOS-ICOSL, CD28-CD80/CD86, or both of these costimulatory pathways. As a control, isotype control mAbs were added in the same manner. Concentrations of GzmB in the culture supernatants in the cultures were measured by ELISA. (A) A comparison of GzmB levels in the culture supernatants of WT and ICOS-/- CD8+ T cells in the presence or absence of T. gondii antigens without any blocking mAbs. Comparisons of GzmB levels in the culture supernatants of (B) WT CD8+ T cells and (C) ICOS-/- CD8+ T cells in the presence and absence of the blocking mAbs against the ICOS-ICOSL or CD28-CD80/CD86 costimulatory pathways. There were 2 mice in each of infected WT and ICOS-/- mice, and their spleen cells were pooled within the same experimental group for purifying CD8+ T cells. There were 5 wells in each experimental group. **P<0.01, ***P<0.001, ****P<0.0001, N.S., Not significant.