1. Introduction
Shingrix is a two-booster recombinant vaccine released by the FDA in 2017 to prevent shingles (herpes zoster) in patients aged 50 and older who are at risk. The vaccine combines the varicella zoster virus glycoprotein E antigen with the AS01B adjuvant system [
1]. Glycoprotein-E antigen is expressed during VSV infection and antibodies are readily detected in adults with prior varicella infection [
1]. To enhance cellular immune response, the adjuvant AS01B, which contains QS21, a plant derived saponin, and monophosphoryl lipid A (MPL), a Toll-like receptor 4 agonist, was added. These components stimulate antibody production and enhance the glycoprotein-E specific cell mediated immunity [
1]. The shingles vaccine is administered as a two-dose series. For most healthy patients the booster is administered 2 to 6 months after the first dose, while immunocompromised patients may receive the booster 1 to 2 months after the first dose [
1].
The estimated lifetime incidence of herpes zoster is around 10 to 20 percent [
2]. Mortality from herpes zoster is rare, with a reported incidence of 0 to 0.47 per 100,000 people per year [
2]. Although the mortality for herpes zoster is low, the virus has been attributed to causing a painful vesicular rash [
3]. More importantly, the virus has the potential to cause post-herpetic neuralgia, a complication of shingles in 10-18% of affected patients that causes severe neuralgia, which may or may not resolve over time. According to the Centers for Disease Control and Prevention (CDC), Shingrix is 97% effective in preventing shingles in adults 70 years and older [
4]. Among the side effects reported by the CDC, the most common include mild or moderate pain, redness and effusion at the injection site, fatigue, headache, myalgias, fever, abdominal pain, gastrointestinal symptoms, and nausea. In addition, severe symptoms that may develop include an allergic reaction – skin rash, itching, flushing, effusion, and anaphylaxis [
4]. Although rare, neuropathy has been recognized as an adverse event of the Shingrix vaccine [
5]. Neurological adverse events of the vaccine have been thought to be attributed to the QS-21 component in the AS01 adjuvant in the Shingrix vaccine [
6]. Through molecular mimicry from vaccine administration, it is hypothesized that patients experiencing adverse reactions may express immune cross-reactivity, leading to autoimmune-like symptoms [
7]. Although instances of temporary neuropathy have been reported, this vaccine's chronic neurological side effects have not been well documented in the VAERS database or listed by the CDC.
While Shingrix has demonstrated efficacy against herpes zoster, documenting and thoroughly investigating any possible long-term adverse symptoms remains critical for ongoing pharmacovigilance and improving patient safety profiles, especially among vulnerable groups. Although research has been carried out on the long-term effects, further studies are essential to enhance patient prognosis, focusing on preventive measures and therapeutic approaches [
8]. This case is one of multiple in a series in which a patient developed long-lasting side effects (over six months) after receiving the Shingrix vaccine.
2. Case Presentation
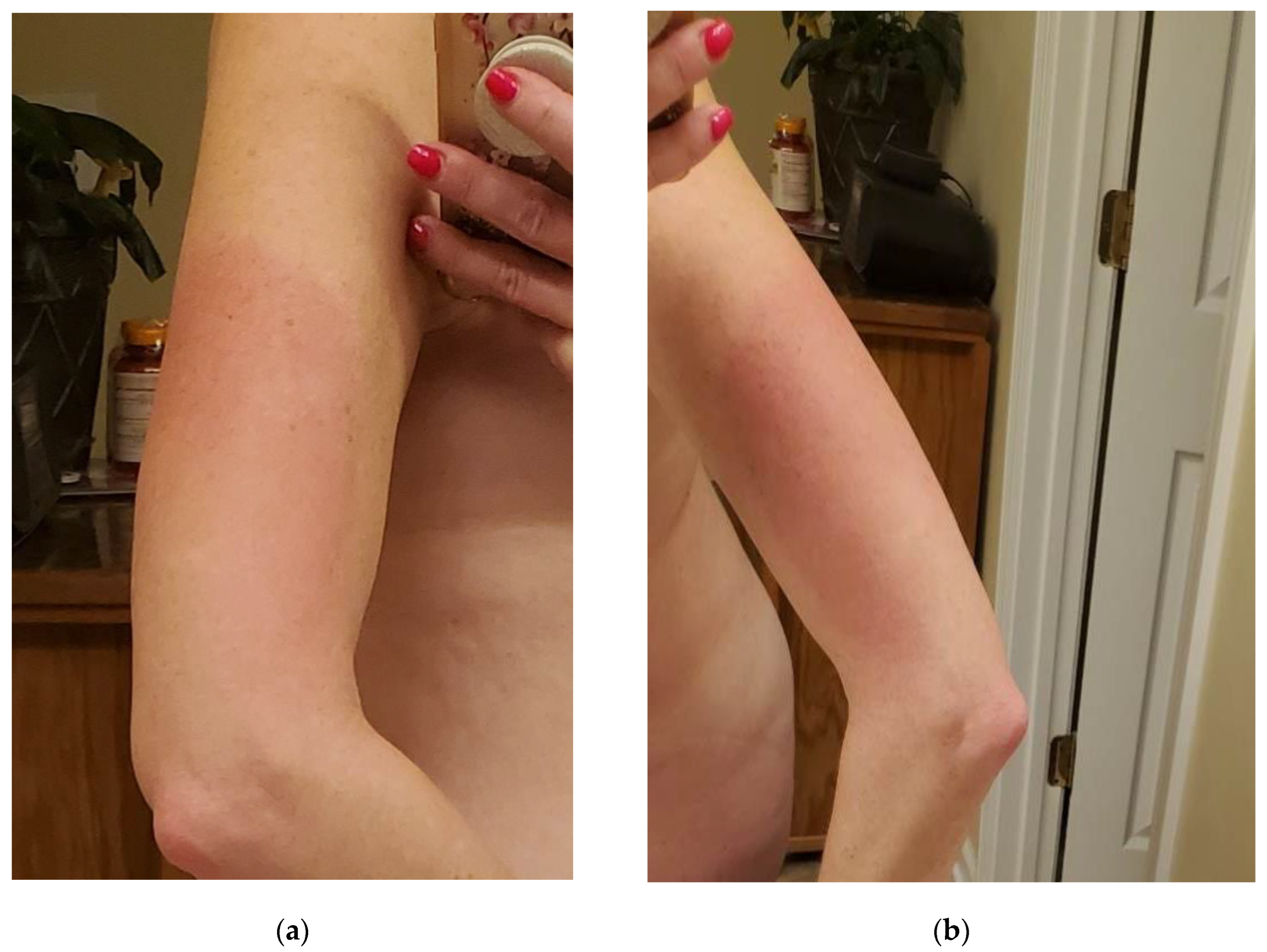
The patient in this case study is a 50-year-old female with a history of Crohn's disease and right knee pain secondary to sports injury who received the Shingrix vaccine at her primary care office in October 2022. Several hours after receiving the Shingrix vaccine in her left deltoid, the patient developed generalized flu-like symptoms, including fever, general myalgias and arthralgias, and fatigue described as "worse pain than intense exercising." The day after her injection (day 2), the patient developed nausea and decreased appetite, as well as her first incident of a tingling sensation accompanied by a burning sensation in her arms bilaterally (L>R) along with her left anterior thigh. The injection site on the left deltoid was significantly enlarged, warm, and erythematous (see
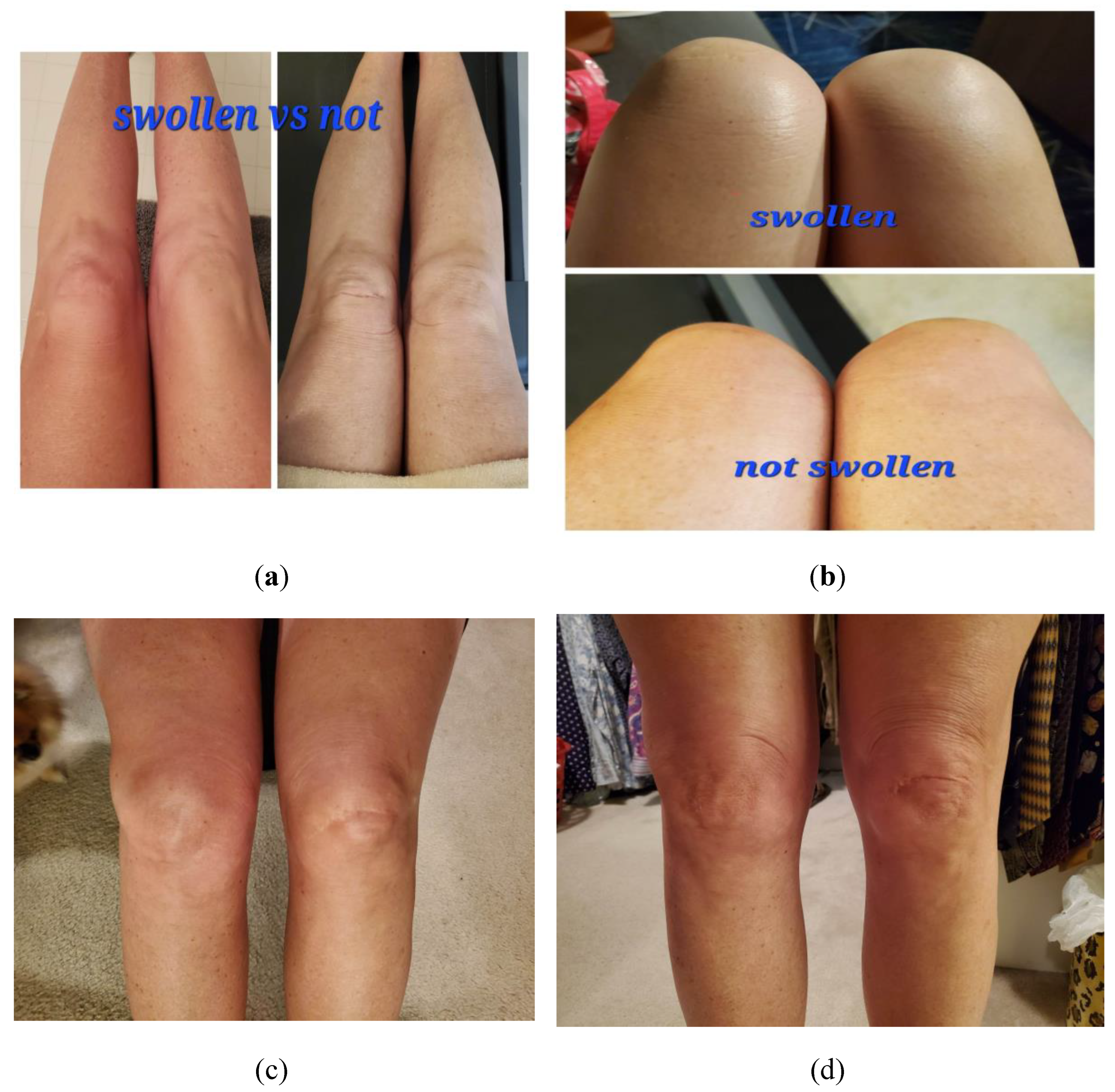
Figure 1 below) and lasted approximately two weeks before complete resolution. On day three her fever, nausea/decreased appetite, myalgias, and fatigue resolved; however, she had lingering pain, described as an intense "ache” in her joints, including her elbows, ankles, and knees bilaterally, along with effusion in these affected joints (see
Figure 2 below).
For the month of November 2022, the pain and effusion persisted. Symptoms were reported to be worse in the mornings, with her pain rated to be an 8/10, slightly improving as the day progressed to a 6/10. The patient felt that the pain significantly impacted her gait and mobility, limiting her range of motion with daily activities. At this point, the patient contacted her primary care physician, who recommended the use of over-the-counter NSAIDs. Despite daily use as directed, no improvements were noted. Additionally, the patient continued to experience intermittent numbness and tingling, as described in the locations above.
Between November 2022 and January 2023, the patient continued to experience daily pain and effusion in joints without any improvements despite daily OTC ibuprofen. The patient also reported intermittent tingling in her arms bilaterally (L>R) and left lower extremity. The patient spoke with her primary care physician during that time and was referred for a nerve conduction study, which showed the presence of carpal tunnel, otherwise, no significant findings were reported. In January 2023, the patient discontinued daily ibuprofen use and began the application of topical herbal substances – helichrysum and frakenson's oil- along with using wraps and kinetic tape. The patient reported mild improvements in her discomfort and effusion with daily application. Following the results, her PCP suggested that she see orthopedics for further evaluation. The patient underwent x-ray imaging in February 2023, which revealed no abnormal findings. The patient's PCP also ordered labs around that time, which were negative for any abnormalities in her inflammatory markers (see
Table 1 below). Repeat labs completed in April 2023 showed no abnormalities in her inflammatory markers.
By May 2023, her joint pain and effusion had gradually improved with the nightly use of topical oils. By now, the patient no longer needed to wrap her joints daily - only as needed. The patient reported recurrent and increasing episodes of pain and effusion in her joints if treatments were missed. The frequency of episodes had significantly improved to once a month compared to daily several months prior.
As of August 2023, the patient continued to experience intermittent tingling in the arms and lateral aspect of the left lower extremity and intermittent joint pain and effusion despite daily herbal oil application. The patient reported seeing multiple primary care providers who could not explain the cause and recurrence of episodes of pain, effusion, and tingling in the areas as described in detail above. After experiencing these symptoms, the patient declined the administration of the second booster as recommended by her current primary care provider with concerns that this may do further harm than good.
3. Discussion
Reviewing this complex case highlights several opportunities to optimize care for patients with prolonged, difficult-to-diagnose symptoms following vaccination. For example, while repeated lab work and imaging were appropriately negative in this case, referral to specialists like rheumatology or neurology could have provided additional expert assessment of her joint and neurological symptoms. Enhanced continuity of care with her primary provider may have led to an earlier multidisciplinary approach. The patient reported seeing multiple providers over a year with little continuity of care. The patient also admitted the reluctance of these physicians to accept whether the cause of her symptoms was indeed the result of the vaccine.
This case is unique because the patient's symptoms, as described above, have not been reported among the listed side effects of the Shingrix vaccine. The most common adverse events reported in clinical trials were pain at the injection site (78.7%), myalgias (45.4%), fatigue (45%), headache (38.1%), shivering (31.9%), fever (30.5%), and gastrointestinal symptoms (26.8%) [
8]. More importantly, no long-term side effects have been reported in experienced trials, as evidenced by the continuation of this patient's symptoms almost one year after her vaccination. While initial safety data on vaccines is under extensive review and obtained via pre-licensure clinical trials and short-term marketing surveillance, ongoing monitoring for adverse events over longer periods of time are crucial to determine their benefits and risks in the patient population. Rare risks of vaccines may only surface after millions of doses have been administered, and further adverse events may present themselves months or even years after vaccination. Therefore, it is essential to have strong systems in place for continuous safety monitoring, including the Vaccine Adverse Event Reporting System (VAERS). Healthcare providers must monitor patients’ health post-vaccination and report any potentially concerning events. This requires openness to the possibility of rare vaccine-associated effects, even without established causality. Patient concerns and experiences warrant careful documentation regardless of presumed etiology. Collecting long term data for vaccines such as Shingrix may provide a further understanding of their safety profile. Increased reporting of adverse events can also strengthen post-marketing surveillance to refine usage guidelines and identify high-risk populations that may benefit from more tailored vaccine recommendations. More studies are also needed to determine the causal relationship between which patient populations are susceptible to developing long-term adverse side effects after receiving the Shingrix vaccine. Going forward, this case reinforces the need for further long-term pharmacovigilance and studies to better characterize the incidence of long-term side effects after Shingrix, especially in vulnerable subgroups.
Although we have discussed the possibility of adverse events, including neuropathy associated with the Shingrix vaccine, the morbidity and mortality of shingles in unvaccinated individuals remain significantly more substantial. Each year, approximately 1 million Americans are affected by shingles. Complications, including post-herpetic neuralgia, occur in roughly 13-40% of shingles cases. Shingles can cause extreme acute pain, resulting in significant negative impacts to quality of life. In some instances post-herpetic pain is a lifelong complication requiring the necessity of pain medication. Despite concerns regarding post-vaccination the incidence of vaccine side effects is generally low. Shingrix reduces shingles risk by 97% and lessen complications to individuals who do develop shingles post-vaccination [
1]. As a result, we can conclude that benefits far outweigh the risks. Close monitoring for adverse events and comparisons to shingles morbidity and mortality are crucial, but currently the benefits of vaccination outweigh the potential harms.
It is crucial to emphasize that this case report, which highlights a concerning adverse event following Shingrix vaccination, should not be interpreted as anti-vaccine literature. Documenting and comprehending safety signals, even those that are rare, enables proper education and well-informed assessment of benefits and risks. Extensive data overwhelmingly support the significant public health advantages of the Shingrix vaccine for appropriate populations [
10]. Nevertheless, maintaining transparency regarding complete safety profiles is equally essential. We caution against drawing broad conclusions or exaggerating implications based on limited case reports. Healthcare providers should continue to recommend this vaccine in accordance with guidelines. Monitoring for potential risks does not diminish vaccine confidence or effectiveness.
4. Conclusions
This case serves as a reminder of the imperative for continued monitoring and research into longitudinal vaccine outcomes. The persistence of somatic and neurological symptoms in this patient, despite standard interventions and negative diagnostic findings, highlights the need for heightened awareness among healthcare providers regarding the possibility of vaccine-related complications. Documentation and reporting of individual experiences, as exemplified in this case, can facilitate the early detection of rare adverse events and contribute to refining safety screening protocols to safeguard vulnerable patient populations. Enhanced reporting mechanisms and interdisciplinary collaboration can further strengthen post-marketing surveillance, ultimately optimizing patient safety and informing tailored vaccine recommendations. We advocate for increased awareness and vigilance around rare, but serious adverse events associated with vaccines. We promote the use of VAERS as well as patient-provider communication and education to improve outcomes and time to diagnosis.
Author Contributions
Conceptualization, B.D.B.; methodology, S.H.; validation, S.H. and M.W.; formal analysis, S.H. M.W., and A.K.; investigation, S.H.; resources, M.W. and A.V; data curation, S.H.; writing—original draft preparation, S.H., M.W., and A.K.; writing—review and editing, S.H. M.W., and A.K.; visualization, S.H..; supervision, B.D.B.; project administration, B.D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of ROCKY VISTA UNIVERSITY (protocol code 2024-154 and approval date of 15 August 2024).
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy concerns.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Levin, M.J. , Weinberg A. Adjuvanted Recombinant Glycoprotein E Herpes Zoster Vaccine. Clinical Infectious Diseases. 2019. [CrossRef]
- JohnJohnson, R.W.; Alvarez-Pasquin, M.J.; Bijl, M.; Franco, E.; Gaillat, J.; Clara, J.G.; Labetoulle, M.; Michel, J.-P.; Naldi, L.; Sanmarti, L.S.; et al. Herpes Zoster Epidemiology, Management, and Disease and Economic Burden in Europe: A Multidiscipli-nary Perspective. Ther. Adv. Vaccines 2015, 3, 109–120. [Google Scholar] [CrossRef] [PubMed]
- James, S.F.; Chahine, E.B.; Sucher, A.J.; Hanna, C. Shingrix: The New Adjuvanted Recombinant Herpes Zoster Vaccine. Ann. Pharmacother. 2018, 52, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Dooling, K. , Guo, A., Patel, M., Lee, G., Moore, K., Belongia, E., … & Harpaz, R. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morbidity and Mortality Weekly Report. 2018, 67(3), 103–108. [Google Scholar] [CrossRef] [PubMed]
- Goud, R.; Lufkin, B.; Duffy, J.; Whitaker, B.; Wong, H.L.; Liao, J.; Lo, A.-C.; Parulekar, S.; Agger, P.; Anderson, S.; et al. Risk of Guillain-Barré Syndrome Following Recombinant Zoster Vaccine in Medicare Beneficiaries. JAMA Intern. Med. 2021, 181, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Hesse, E.M.; Shimabukuro, T.T.; Su, J.R.; Hibbs, B.F.; Dooling, K.L.; Goud, R.; Lewis, P.; Ng, C.S.; Cano, M.V. Postlicensure Safety Surveillance of Recombinant Zoster Vaccine (Shingrix)—United States, October 2017–June 2018. Morb. Mortal. Wkly. Rep. 2019, 68, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Segal, Y.; Shoenfeld, Y. Vaccine-Induced Autoimmunity: The Role of Molecular Mimicry and Immune Crossreaction. Cell. Mol. Immunol. 2018, 15, 586–594. [Google Scholar] [CrossRef] [PubMed]
- JohnJohnson, R.W.; Alvarez-Pasquin, M.J.; Bijl, M.; Franco, E.; Gaillat, J.; Clara, J.G.; Labetoulle, M.; Michel, J.-P.; Naldi, L.; Sanmarti, L.S.; et al. Herpes Zoster Epidemiology, Management, and Disease and Economic Burden in Europe: A Multidiscipli-nary Perspective. Ther. Adv. Vaccines 2015, 3, 109–120. [Google Scholar] [CrossRef] [PubMed]
- James, Stephanie & Chahine, Elias & Sucher, Allana & Hanna, Cassandra. (2018). Shingrix: The New Adjuvanted Recombinant Herpes Zoster Vaccine. Annals of Pharmacotherapy. 52. 106002801875843. [CrossRef]
- Famuyiro, T. , Smith, S.T., & Raji, M. (2018). Making the case for universal herpes zoster vaccination in older adults. Annals of Long-term Care, 26(2), 27-31.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).