1. Introduction
Multiple Myeloma (MM) is a hematologic malignancy comprised of the explosive proliferation of clonal plasma cells in the bone marrow. It typically begins as a precancerous asymptomatic condition called monoclonal gammopathy of undetermined significance (MGUS) and is prevalent mainly in patients >65 [
1]. The diagnosis of MM requires the presence of at least one Myeloma defining event (e.g., CRAB criteria) in addition to the presence of ≥10% plasma cells on bone marrow exam or biopsy-proven plasmacytoma [
2]. Induction therapy on diagnosis consists of combination therapy with an injectable proteasome inhibitor bortezomib, an oral immunomodulatory agent lenalidomide, dexamethasone (VRd), and/or a monoclonal antibody (mAbs) daratumumab given for approximately 3-4 cycles followed by autologous stem cell transplantation (ASCT) in transplant eligible patients [
3,
4]. Transplant-ineligible patients are treated with VRd for approximately 8-12 cycles, followed by maintenance lenalidomide therapy or kept on daratumumab, lenalidomide, and dexamethasone (DRd) until progression [
3,
4].
Transplant eligibility depends on the patient’s age, performance status, disease stage, presence of renal insufficiency and comorbidity, and ability to tolerate pre-transplant chemotherapy [
5]. Geriatric assessment is used to determine eligibility in the elderly [
6,
7].
Despite advances in treatment, MM mortality remains high. With 5-year overall survival estimated at 61.1%, MM remains an incurable illness with poor patient prognosis [
8]. The introduction of mAbs, notably those targeting CD38 and SLAMF7, in the treatment of MM aimed to improve effectiveness and median survival in these patients [
9]. Daratumumab (DARA) is an immunoglobulin G1 kappa (IgG1k) human monoclonal antibody targeting the transmembrane surface glycoprotein CD38, which is highly expressed in myeloma cells [
10].
Our study aims to evaluate the effects of daratumumab combination therapy in transplant-eligible (TE) and ineligible (TIE) patient populations to determine if therapy confers any benefits regarding disease progression or survival.
2. Materials and Methods
Search Strategy
We performed a meta-analysis using the preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [
11]. A systematic search was done in PubMed, Cochrane Library, and ClinicalTrials.gov database for relevant clinical trials published from 2000 to 2024. Following MeSH terms were used to conduct a preliminary search: “daratumumab” or “Darzalex” and “multiple myeloma” and “randomized clinical trials.” After removing duplicate and irrelevant studies, two investigators examined each potential article independently (BF, AA) to determine if it met the inclusion criteria. Any disagreement was settled through discussion.
Study Selection
Studies that included a comparison of Daratumumab in newly detected multiple myeloma patients, irrespective of their transplant eligibility, were selected. Only randomized controlled trials (RCTs) using an adequate method of allocation concealment (e.g., sealed opaque envelopes) and studies that were double-blind, single-blind, or unblind were considered.
RCTs of Daratumumab comparing it with ongoing chemotherapy, namely Bortezomib, Dexamethasone, Caffezomib, Melphalan, prednisone, or Poloxamer in 18-year-olds or older with newly diagnosed multiple myeloma were taken into consideration. Daratumumab was given to the included patients in the dosage range of 16 mg/kg intravenous weekly for eight weeks, every two weeks for 16 weeks, and every four weeks after that. Studies with Daratumumab with no placebo arm, outcomes measured in the graphical presentation, unpublished research work or trials, observational studies, and preclinical studies were excluded from the analysis.
All the relevant data were extracted from studies that met the criteria. In some studies, the corresponding authors were contacted for more information if data could not be obtained. Data on the study design, the treatment comparator, the placebo used, the dosage, and the treatment response rate were collected.
Main Outcome Variables
Daratumumab versus the ongoing treatment in patients with newly detected multiple myeloma was considered. The primary endpoint was the events of the progression of disease or deaths. Secondary endpoints were hazard ratio for progression or death, overall response rate (ORR), complete response (CR), and rate of minimal residual disease (MRD)-negative.
Risk of Bias Assessment
Two independent reviewers (BF, AA) assessed the risk of bias for the included RCTs according to the Cochrane Handbook for Systematic Reviews of Interventions guidelines [
12]. Different parameters, such as selection bias, allocation bias, blinding, incomplete data reporting, and any other type of bias, were graded as low, unclear, or high risk.
Analysis
The Mantel-Haenszel formula with a random effect model was used to directly compare daratumumab with the ongoing chemotherapy to calculate the pooled risk ratio (RR) at a 95% confidence interval (CI) for dichotomous data, and a forest plot was created. An RR of one means that the daratumumab and the ongoing treatment group have equivalent effects. If improvement is associated with higher scores on the outcome measure, an RR of greater than one indicates the degree to which treatment is more efficacious than an ongoing treatment, and an RR of less than one indicates the degree to which treatment is less productive than an ongoing treatment group. Meta-analysis was performed using RevMan version 5.4. Statistical heterogeneity across studies was reported using I2 statistics. A P-value of <0.05 was considered significant.
3. Results
A total of 11 studies were identified through an electronic or manual search and selected for a full-text review based on the title and abstract details. However, four studies were eventually excluded because they did not have a placebo arm, and one was excluded because it was a conference paper. Ultimately, six RCTs involving 3664 patients met the inclusion criteria [
13,
14,
15,
16,
17,
18]. All the RCTs provided data related to the primary and secondary endpoint events.
Table 1 provides the relevant features of the RCTs included in the meta-analysis.
3.1. Primary endpoint
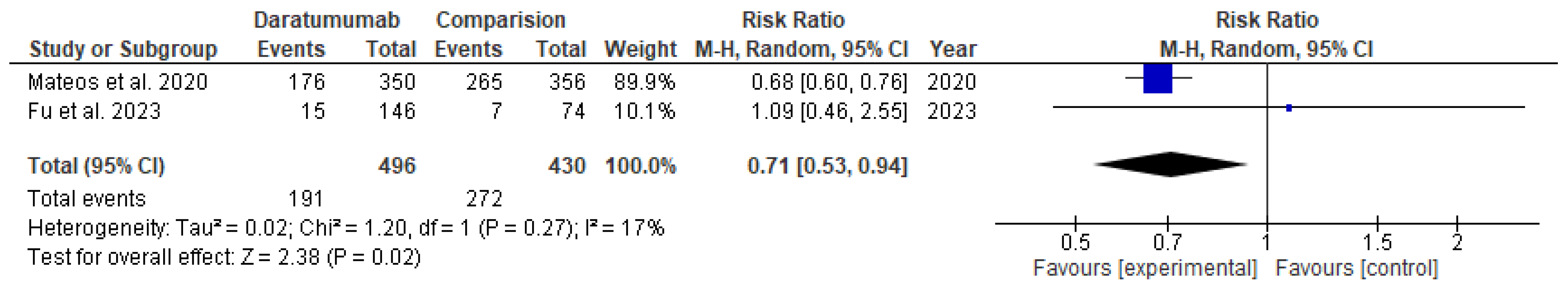
The use of daratumumab was associated with 39% fewer events of progression of disease or deaths in the transplant-eligible group (RR= 0.61; 95% CI= 0.39 to 0.93; p=0.02) and 29% lower in the transplant-ineligible group (RR= 0.71; 95% CI= 0.53 to 0.94; p=0.02), as shown in
Figure 1 and
Figure 2, respectively.
3.2. Secondary endpoints
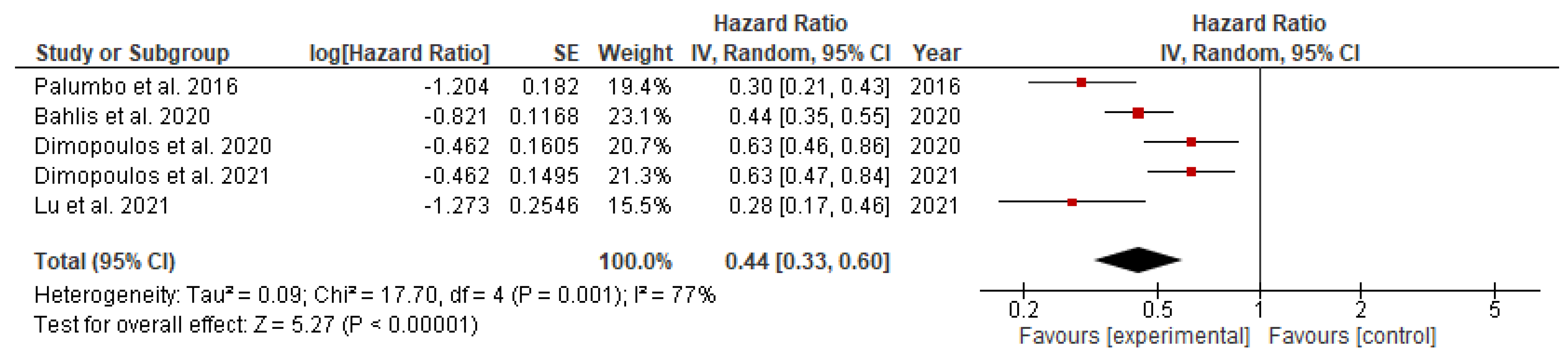
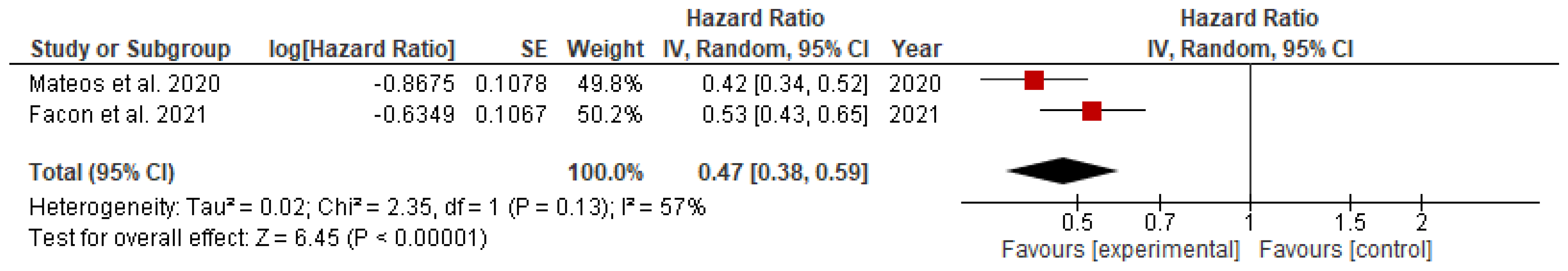
The hazard ratio for the progression of disease or death among patients with newly detected multiple myeloma on daratumumab versus the ongoing chemotherapy was significantly lower in both transplant eligible (HR= 0.44; 95% CI= 0.35 to 0.57; p<0.00001) and transplant-ineligible (HR= 0.47; 95% CI= 0.38 to 0.59; p<0.00001) groups, as shown in
Figure 3 and
Figure 4.
Daratumumab significantly increased overall response rate (ORR) (RR= 1.20; 95% CI= 1.11 to 1.30 ; p<0.00001) and complete response (CR) (RR= 1.34; 95% CI= 1.02 to 1.76; p=0.04) in the TIE group only. Interestingly, the rate of minimal residual disease (MRD)-negative was increased only in the TIE group (RR= 4.09; 95% CI= 2.81 to 5.94; p<0.00001) with the use of daratumumab compared to the TE group (RR= 1.07; 95% CI= 0.68 to 1.67; p=0.78).
4. Discussion
This study provides an updated and thorough evaluation of the predictive benefits of adding Daratumumab to existing combination therapy compared to combination therapy alone in both TE and TIE patients with newly diagnosed MM patients. The following conclusions were drawn based on data from six phase III randomized controlled trials (RCTs) that met our inclusion criteria. The use of daratumumab was associated with significant reduction, measured as relative risk, in disease progression or death by 39% in the TE group (RR=0.61) and 29% in the TIE group (RR=0.53). The hazard ratio for these incidents was also significantly lower in the TE (HR=0.44) and TIE (HR=0.47) groups. Additionally, daratumumab significantly increased the overall response rate (RR=1.20) and complete response rate (RR=1.34), along with the rate of minimal residual disease (RR=4.09) in the TIE group.
Other studies investigating the potential of daratumumab therapy also show the benefits of combination therapy. Firstly, preclinical trials studying the effects of daratumumab demonstrate synergistic activity with Lenalidomide, given that lenalidomide increases NK cell activity and daratumumab utilizes NK cells for antibody mediated cytotoxicity [
19]. A phase 1b trial studying the effects of daratumumab alongside other MM regimens showed promising results in newly diagnosed TIE patients treated with bortezomib-prednisone-melphalan and newly diagnosed patients, irrespective of transplant eligibility treated with bortezomib-dexamethasone or bortezomib- thalidomide-dexamethasone [
20]. Another research studying the effect of adding daratumumab to antineoplastic therapy for TIE patients reveals benefits in overall survival, progression-free survival, and quality of life [
21]. Furthermore, coupling subcutaneous daratumumab with bortezomib-lenalidomide-dexamethasone combination therapy for induction and consolidation and with lenalidomide for maintenance therapy in TE patients significantly improved progression-free survival [
15].
Pertinent side-effects associated with daratumumab therapy are infusion reactions, neutropenia causing increased susceptibility to infections, and thrombocytopenia with subcutaneous administration shower fewer infusion reaction rates than intravenous formulations [
22,
23]. However, the most important side-effect of long-term daratumumab therapy remains the development of drug resistance due to the gradual loss of CD38 antigen expression [
24]. As premature treatment discontinuation due to adverse effects can negatively impact a patient’s quality of life and prognostic outcome, it is necessary to tailor treatment combinations and dosages according to individual needs, focusing on frailty and comorbidity.
Since daratumumab binds to CD38 on erythrocytes and may interfere with blood compatibility testing, patients must be screened for alloantigens before transfusions [
25]. All patients on DARA should be regularly assessed for risk of infection, mainly upper respiratory symptoms and prevention, and vaccination coverage should be offered to avoid infections [
26]. Steroids, antipyretics, and antihistamines can be provided to prevent infusion reactions [
27]. Pretreatment with All-trans-retinoic acid has shown enhanced DARA cytotoxicity through increased CD38 expression and signifies the importance of discovering combinations that counter drug resistance [
28].
As with any meta-analysis, there comes an inherent heterogeneity bias, as in our study. Additionally, we only focused on the efficacy of daratumumab without considering its adverse effects, which could have affected its clinical use. Nevertheless, this meta-analysis had the strength of 3,664 patients, which could provide more accurate data than individual clinical trials by potentially increasing the statistical power and independent analysis of the efficacy of daratumumab in both transplant-eligible and transplant-ineligible patients with newly detected multiple myeloma. Efforts should be made to explore new drug combinations with daratumumab, gain insight into resistance mechanisms and adverse effects, create strategies to counter them, and identify patient profiles most suited to benefit from these treatments.
5. Conclusions
Daratumumab showed benefits in both TE and TIE patients with newly diagnosed multiple myeloma (NDMM). It significantly reduced the risk of disease progression or death and improved clinical outcomes regarding ORR and CR. Interestingly, the rate of minimal residual disease (MRD)-negative was increased only in the TIE group with the use of daratumumab compared to the TE group. Whereas, the most notable improvements were observed in the TIE-NDMM group, suggesting an effective treatment option in these patient groups.
Author Contributions
Conceptualization, B.F., V.S., M.A., R.M., P.M, and V.N.; methodology, V.S., V.N., A.K., S.G., M.G., & S.P.; software, V.S., V.N.; formal analysis, V.S., V.N., A.K., S.G., A.A., S.P., & M.G.; data curation, J.S., S.G., M.M., M.G., S.P., A.A., J.S., A.K., S.G., V.P., & V.N.; writing—original draft preparation, B.F., V.S., M.A., R.M., P.M., J.S., & S.G; writing—review and editing, M.M., M.G., S.P., A.A., J.S., A.K., S.G., V.P., & V.N.; supervision, V.N. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Silberstein J, Tuchman S, Grant SJ: What is multiple myeloma? JAMA. 2022, 327, 497. [CrossRef] [PubMed]
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. : International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–48. [Google Scholar] [CrossRef] [PubMed]
- Cowan AJ, Green DJ, Kwok M, et al. : Diagnosis and management of multiple myeloma: A review: A review. JAMA. 2022, 327, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar SV: Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022, 97, 1086–1107. [CrossRef]
- Devarakonda S, Efebera Y, Sharma N: Role of stem cell transplantation in multiple myeloma. Cancers 2021, 13, 863. [CrossRef]
- Jayani R, Rosko A, Olin R, Artz A: Use of geriatric assessment in hematopoietic cell transplant. J Geriatr Oncol. 2020, 11, 225–236. [CrossRef]
- Rosko AE, Huang Y, Benson DM, et al. : Use of a comprehensive frailty assessment to predict morbidity in patients with multiple myeloma undergoing transplant. J Geriatr Oncol. 2019, 10, 479–485. [Google Scholar] [CrossRef]
- Nieto MJ, Hedjar A, Locke M, Caro J, Saif MW: Analysis of updates in multiple myeloma treatment and management. J Clin Haematol. 2023, 4, 35–42. [CrossRef]
- Jelinek T, Hajek R: Monoclonal antibodies - A new era in the treatment of multiple myeloma. Blood Rev. 2016, 30, 101–110. [CrossRef]
- Sanchez L, Wang Y, Siegel DS, Wang ML: Daratumumab: a first-in-class CD38 monoclonal antibody for the treatment of multiple myeloma. J Hematol Oncol. 2016, 9, 51. [CrossRef]
- Page MJ, McKenzie JE, Bossuyt PM, et al. : Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Higgins JPT, Altman DG, Gøtzsche PC, et al. : The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Voorhees PM, Rodriguez C, Reeves B, et al. : Daratumumab plus RVd for newly diagnosed multiple myeloma: final analysis of the safety run-in cohort of GRIFFIN. Blood Adv. 2021, 5, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Moreau P, Attal M, Hulin C, et al. : Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019, 394, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld P, Dimopoulos MA, Boccadoro M, et al. : Daratumumab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2024, 390, 301–313. [Google Scholar] [CrossRef]
- Mateos M-V, Cavo M, Blade J, et al. : Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. Lancet. 2020, 395, 132–141. [Google Scholar] [CrossRef]
- Facon T, Kumar SK, Plesner T, et al. : Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 1582–1596. [Google Scholar] [CrossRef]
- Fu W, Bang S-M, Huang H, et al. : Bortezomib, melphalan, and prednisone with or without daratumumab in transplant-ineligible Asian patients with newly diagnosed multiple myeloma: The phase 3 OCTANS study. Clin Lymphoma Myeloma Leuk. 2023, 23, 446–455. [Google Scholar] [CrossRef]
- van der Veer MS, de Weers M, van Kessel B, et al. : Towards effective immunotherapy of myeloma: enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab. Haematologica. 2011, 96, 284–290. [Google Scholar] [CrossRef]
- Paper: Open-Label, Multicenter, Phase 1b Study of Daratumumab in Combination with Pomalidomide and Dexamethasone in Patients with at Least 2 Lines of Prior Therapy and Relapsed or Relapsed and Refractory Multiple Myeloma. Available online: https://ash.confex.com/ash/2015/webprogramscheduler/Paper79027.html (accessed on 23 September 2024).
- Langer P, John L, Monsef I, Scheid C, Piechotta V, Skoetz N: Daratumumab and antineoplastic therapy versus antineoplastic therapy only for adults with newly diagnosed multiple myeloma ineligible for transplant. Cochrane Database Syst Rev. 2024, 5, CD013595.
- Phipps C, Chen Y, Gopalakrishnan S, Tan D: Daratumumab and its potential in the treatment of multiple myeloma: overview of the preclinical and clinical development. Ther Adv Hematol. 2015, 6, 120–127. [CrossRef] [PubMed]
- San-Miguel J, Usmani SZ, Mateos M-V, et al. : Subcutaneous daratumumab in patients with relapsed or refractory multiple myeloma: Part 2 of the open-label, multicenter, dose-escalation phase 1b study (PAVO). Haematologica. 2021, 106, 1725–1732. [Google Scholar]
- Krejcik J, Frerichs KA, Nijhof IS, et al. : Monocytes and granulocytes reduce CD38 expression levels on myeloma cells in patients treated with daratumumab. Clin Cancer Res. 2017, 23, 7498–7511. [Google Scholar] [CrossRef] [PubMed]
- Chapuy CI, Nicholson RT, Aguad MD, et al. : Resolving the daratumumab interference with blood compatibility testing: DARATUMUMAB BLOOD BANK INTERFERENCE. Transfusion 2015, 55, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Geraldes C, Neves M, Chacim S, da Costa FL: Practical considerations for the daratumumab management in Portuguese routine clinical practice: Recommendations from an expert panel of hematologists. Front Oncol. 2021, 11, 817762.
- Barroso A, Estevinho F, Hespanhol V, Teixeira E, Ramalho-Carvalho J, Araújo A: Management of infusion-related reactions in cancer therapy: strategies and challenges. ESMO Open. 2024, 9, 102922.
- Nijhof IS, Casneuf T, van Velzen J, et al. : CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016, 128, 959–970. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).