1. Introduction
Crohn´s disease (CD) is a chronic, immune-mediated, inflammatory bowel disorder (IBD). The incidence is increasing globally with reports of 10.6-29.3 per 100000 people in the western world [
1] and 0.09-3.91 per 100000 people in low- and middle-income countries [
2]. Young adults are primarily affected, and the disease has a large impact on morbidity and quality of life [
1]. Its pathogenesis is complex and involves immune dysfunction, genes, the microbiome, and environmental factors [
3,
4,
5]. Current treatments for CD aim to inhibit or modulate the immune response in the gut using drugs that affect different targets in the immune system [
6].
In recent years, several biological drugs (tumor necrosis factor-a (TNF)-inhibitors, a4b7 integrin, Interleukin (IL) 12/23 and IL23 inhibitors) and drugs targeting small molecules (Janus kinase (JAK)-inhibitors, and sphingosine-1-phosphate (S1P) modulators) have been introduced in clinical practice, and an increasing number of patients with CD have achieved satisfactory disease control. However, many patients do not improve and/or tolerate available treatments. Therefore, there is a need for alternative therapeutic methods.
Extracorporeal photopheresis (ECP) with the photosensitizer 8-methoxypsoralen (8-MOP) is a well-known and well-tolerated treatment for several T-cell-mediated diseases, including chronic graft versus host disease (cGvHD), and cutaneous T-cell lymphoma (CTCL)[
7]. ECP combines the collection of leukocytes with the addition of 8-MOP as a photosensitizing agent before illumination with ultraviolet light A (UV-A). Since 8-MOP binds to the DNA of activated (diseased) and resting (normal) T cells, traditional ECP damages both these types of cells[
8]. As CD is mediated by T-cells [
9], the use of ECP for the treatment of CD has been explored in several small, uncontrolled studies with some promising results in steroid-dependent and medically refractory cases [
10,
11,
12].
The use of 5-Aminolevulinic acid (ALA), another photosensitizer precursor, may be an alternative to 8-MOP with ECP since it selectively targets cells, such as activated T-cells or cancer cells and may improve treatment effect [
13]. ALA leads to the accumulation of the photoactive porphyrin protoporphyrin-IX (PpIX) and this porphyrin accumulation occurs to a lesser extent in resting or normal cells, which are unharmed after light exposure[
14,
15,
16]. The absorption peak of PpIX is in the visible blue light range (400-420 nm)[
17], which is the rational for the use of blue light instead of UV-A light. Photodynamic diagnosis using ALA is a well-known option for the diagnosis of brain tumors and bladder cancer[
18,
19,
20,
21]. Further, photodynamic therapy with topical ALA application is a widely used topical treatment method for nonmelanoma skin cancer [
22,
23]. We have previously explored the use of ECP combined with ALA and UV-A light for patients with cGvHD or CTCL, and the results indicated that ALA-ECP treatment was safe [
24,
25].
Therefore, the present study investigated the safety and efficacy of ECP combined with ALA and blue light (405 nm) in patients with active CD who have failed or were intolerant to advanced therapy. In addition, we wanted to perform immunohistochemistry on biopsies from ileum and sick and presumed healthy colon in the patients before and after treatment.
2. Materials and Methods
2.1. Study Design and Approvals
This study is the first-in-human open-label phase I/II study to explore the safety and efficacy of ALA-ECP in patients with active CD. The study was approved by the ethics committee (REK – reference number 15685), the Norwegians Medicines Agency (NOMA – EudraCT 2018-002422-23, NOMA reference 18/10491), and the Data Protection Official (DPO, reference number 78_2019) at the Akershus University Hospital. It was registered on ClinicalTrials.gov (NCT04164849).
2.2. Patient Population
Patients with active CD and failure or intolerance to advanced therapy at Akershus University Hospital from November 2019 to March 2023 were considered for study inclusion. Active CD was defined as fecal calprotectin (FC) > 250 and/or serum C-reactive protein (CRP) > 5 in addition to Harvey Bradshaw Index[
26] (HBI) > 5 and endoscopy with simple endoscopic score for Crohn´s Disease [
27] (SES-CD) ≥ 6 or ≥ 4 if only isolated ileitis was present. Informed consent for study participation was obtained before inclusion. The patients were assessed using colonoscopy, the HBI, clinical workup, blood samples and the Inflammatory Bowel Disease Quality of Life Questionnaire (IBDQ) [
28,
29] at screening. The washout period for biological drugs was 2 months, and steroids were tapered for at least 2 weeks before entering the study. Women of childbearing potential (WOCBP) were obliged to use highly effective contraceptives throughout the study and perform a pregnancy test at screening and before all treatment visits.
The exclusion criteria for the study were as follows: patients with photosensitive comorbidities, porphyria, or known hypersensitivity to 5-aminolevulinic acid or porphyrins; individuals with aphakia; pregnant or breastfeeding women (a negative urine pregnancy test was required for women of child-bearing potential at the screening visit and before each treatment); patients with ongoing cardiac or pulmonary diseases, or abnormal ASAT, ALAT, bilirubin, or INR values (≥ 3x upper limit of normal), or clinically significant ECG findings; individuals with polyneuropathy; uncontrolled infection or fever; history of heparin-induced thrombocytopenia, absolute neutrophil count <1×109/L, or platelet count <20×109/L; body weight below 40 kg; subjects deemed unlikely to comply with study procedures by the investigator; and those with other gastrointestinal diseases that could potentially influence study outcomes.
History of any clinically significant disease or disorder which in the opinion of the investigator, may either put the patient at risk because of participation in the study, or influence the result or the patient’s ability to participate in the study.
2.3. Treatment Drug
ALA (Gliolan®, Photonamic GmbH & Co, KG, Pinneberg, Germany) was obtained in accordance with Good Clinical Practice (GCP). ALA was mixed on a laminar flow bench by adding 50 mL of sterile 0.9% sodium chloride (Fresenius Kabi, Hamburg, Germany) to a vial followed by gentle shaking. After mixing, the vial was stored at 4 °C prior to use before 6 pm the next day.
2.4. Treatment Procedures
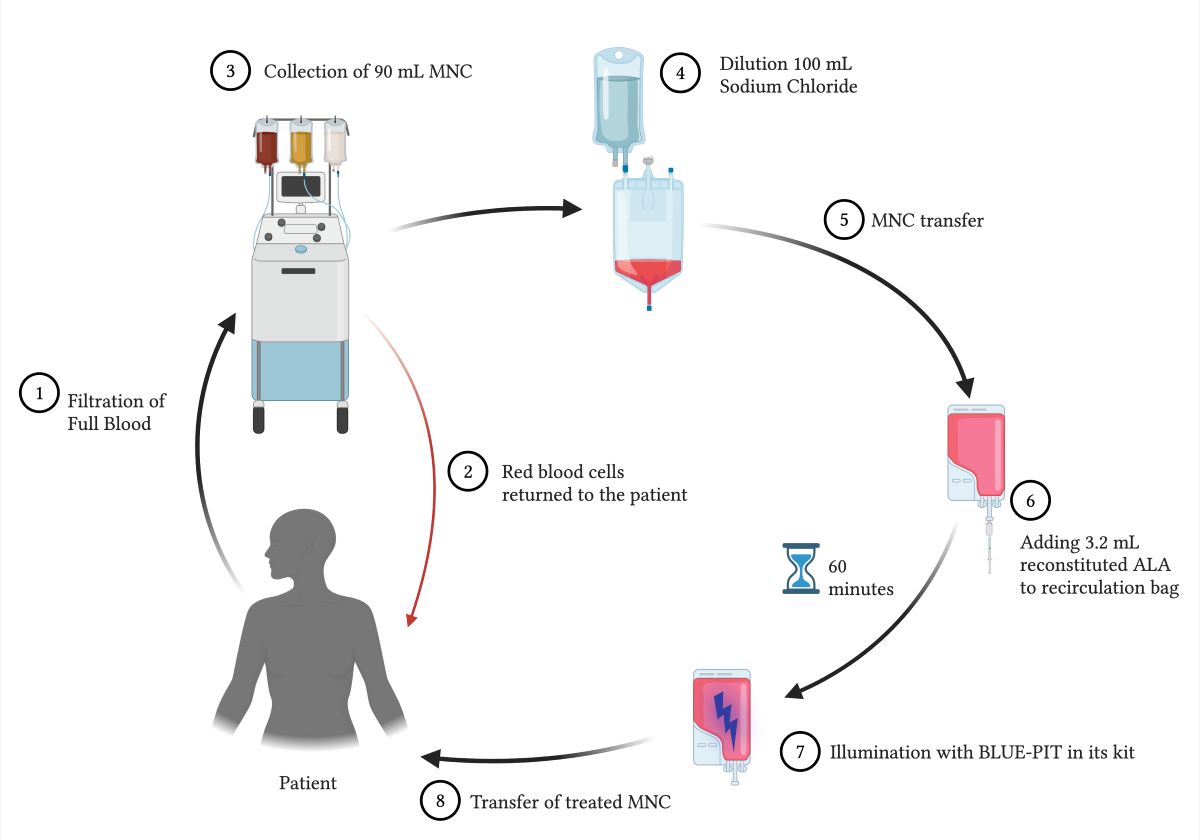
Treatment was performed using a Spectra Optia
® apheresis system (Terumo BCT, Tokyo, Japan) with a continuous mononuclear cell (CMNC) collection protocol. A volume of 90 mL of mononuclear cells (MNCs) was collected and diluted with 100 mL of 0.9% sodium chloride. A total of 190 mL of the diluted MNC solution was transferred to a recirculation bag connected to the BLUE-PIT light kit (PIT Medical Systems, Cadolzburg, Germany). The recirculation bag and lines were shaded from room light. Upon transfer and throughout the rest of the procedure, the patient remained connected to the Spectra Optia
®, and no lines were cut to maintain a closed-circuit system. A volume of 3.2 mL of the reconstituted ALA was added to the MNC solution in the recirculation bag through a sterile filter. Because the light dose in the BLUE-PIT light device is automatically determined by the hematocrit concentration in the MNC solution, a standardized high hematocrit of 1.5% was set in the device to ensure sufficient light exposure in all treatments. The BLUE-PIT device slowly circulated the MNC solution that was mixed with ALA for 1-hour to produce intracellular PpIX before the BLUE-PIT light device automatically illuminated the MNCs with blue light (405 nm). The ALA dose and exposure time were set according to our previous research [
13,
16]. After light exposure, the treated MNCs were transfused back to the patient through a standard transfusion filter (CODAN Medizinische Geräte GmbH & Co., Lensahn, Germany). The transfusion bag remained covered from room light until all MNCs were transfused back to the patient.
Figure 1 shows a schematic overview of the treatment procedure.
The treatment was performed once every two weeks for 10 weeks in accordance with the guidelines for clinical studies on CD [
30]. Thirteen weeks after the first treatment, the patients were evaluated using the screening visit as a reference.
2.5. Safety Assessments
Safety was monitored using the frequency and severity of adverse events (AEs), vital signs (blood pressure, pulse, and body temperature before treatment, after apheresis but before transfusion and after transfusion), physical examination and hematological and clinical laboratory measurements. Urine was examined using a dipstick for erythrocytes, glucose, ketone, leukocytes, nitrite, pH, and protein. A 12-lead electrocardiogram (ECG) was recorded at screening and week 13.
Additionally, the patients were monitored with a 3-lead ECG during all treatments.
2.6. Treatment Assessments
Our primary endpoint was clinical response (HBI reduction > 3 points) at week 13.
Our secondary endpoints were clinical remission (HBI <5 points) and any reduction in FC, and CRP from baseline in addition to IBDQ at week 13. We also performed ileocolonoscopy with the SES-CD at screening and 13 weeks after treatment to assess the endoscopic response. Endoscopic effect was defined as ≥ 50 % reduction from baseline. Biopsies were also obtained: 4 from the ileum and 4 from endoscopically inflamed and endoscopically normal mucosa in the colon. The biopsies were immediately frozen and stored at -150°C for the immunohistochemistry analysis of CD4 for T helper cells, and FOXP3 for regulatory T cells (Tregs).
2.7. Immunohistochemistry
The frozen tissue biopsies were thawed then subjected to formalin fixation and paraffin embedding. Three-micron-thick paraffin-embedded tissue sections were cut and mounted on positively charged glass microscope slides. Deparaffinization was performed using PT-link and EnVisionTM FLEX target retrieval solution (High pH) (Dako, Glostrup, Denmark). To block endogenous peroxidase activity, the sections were treated for 5 min with EnVision Peroxidase-Blocking reagent (Dako) prior to a 30-min incubation with the mouse anti-CD4 or anti-FOXP3 primary antibody (Dako IR649 and Abcam ab20034). The sections were treated for 15 min with EnVision FLEX+ mouse (SM804) then 30 min with horseradish peroxidase (HRP)-labelled anti-mouse secondary antibody followed by 10 min with 3’3 diaminobenzidine tetrahydrochloride (DAB) before staining with Hagen’s hematoxylin. The tissue sections were also stained with routine hematoxylin and eosin (H&E). The sections were dehydrated and mounted in Pertex from Histolab.
2.8. Statistics
The data are presented as mean (minimum-maximum). Vital signs (heart rate, systolic and diastolic blood pressure, and temperature) are presented in violin plots before apheresis, after apheresis, and after transfusion of illuminated MNCs to the patient. The violin plots were created using Stata/SE 18.0 for Mac (StataCorp, Lakeway, USA).
3. Results
3.1. Patients and Treatment
Seven patients were included in the study, of which 4 completed the 10-week treatment.
Demographics, disease extent according to the Montreal Classification [
31] and information about biologics used for these 4 patients at screening are shown in
Table 1.
3.2. Treatment Data
The four patients received 6 treatments each, giving a total of 24 treatments. The mean (minimum-maximum) treatment time from the start of MNC collection to the return of the treated MNCs to the patient was 232.5 minutes (200-295). Ninety milliliters of MNCs were collected, and 100 mL of 0.9% sodium chloride was added to the MNC solution. The light dose was standardized to 13.7 Joules for 23 of the 24 treatments by setting of the same 1.5% hematocrit to the BLUE-PIT. In the first treatment of the first patient, the light dose was 6 Joules due to the setting of a measured hematocrit of only 0.5%.
The differential counts and hematocrit of the diluted MNCs were measured before the addition of ALA in all treatments and contained high levels of lymphocytes and monocytes and low levels of red blood cells (
Table 2).
Three patients did not complete all treatments due to technical difficulties, medical reasons or withdrawal of informed consent. The three received one, three or five treatments respectively.
3.3. Safety
Analysis of blood samples at all time points showed small variations within the reference values. There were no significant changes over time in the four patients. The values of liver-tests and creatinine are presented in
Table 3.
The results are presented with screening values compared to values before each treatment visit and assessment at week 13. The week 0-13 data are presented as mean (minimum-maximum). The bolded parameters (GT, creatinine) were slightly less (GT) and above (creatinine) the reference values and were assessed as not clinically significant. Sc.=Screening, ALT = alanine aminotransferase, AST = aspartate aminotransferase, ALP = alkaline phosphatase, GT = glutamyl transferase, INR = international normalized ratio.
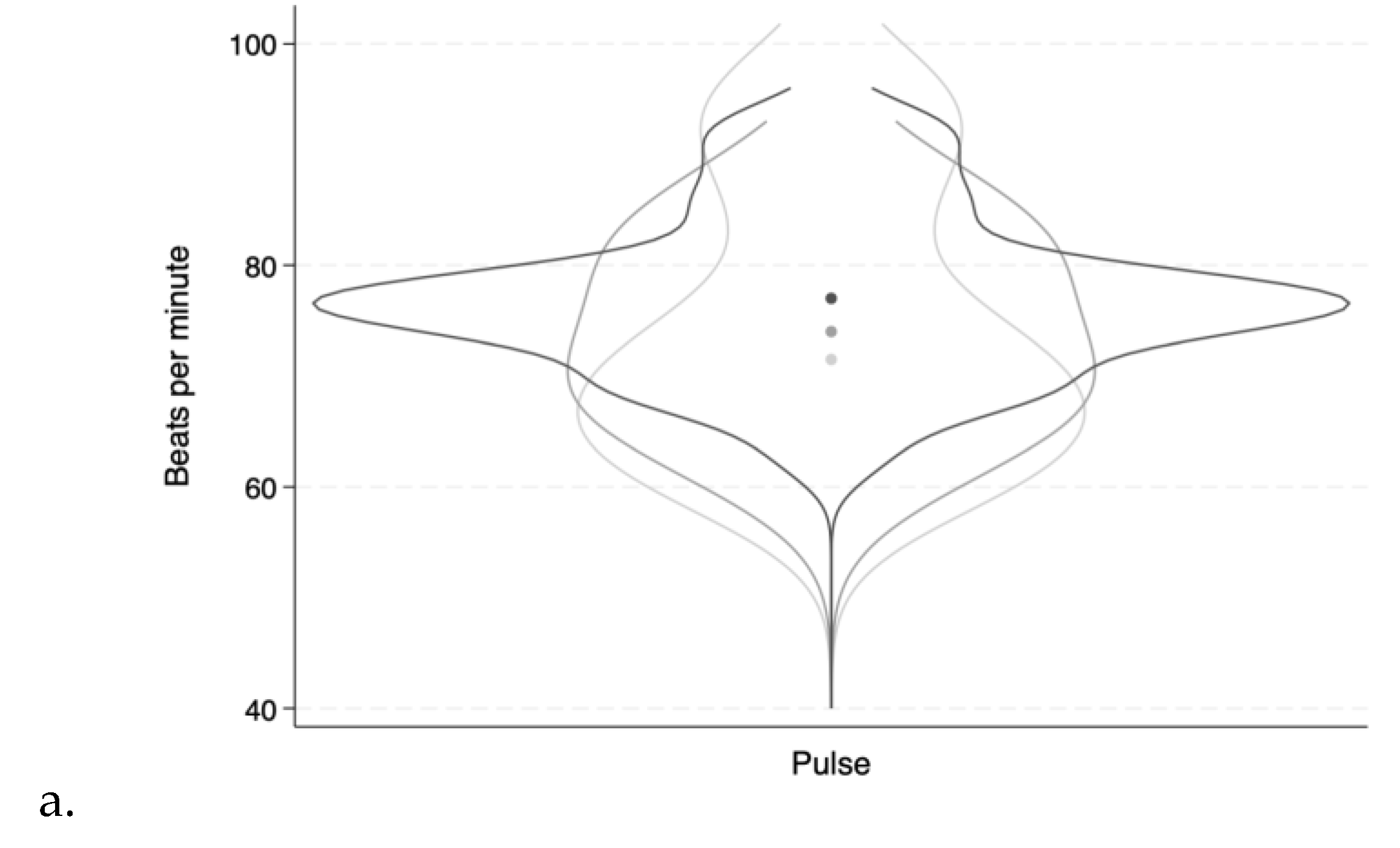
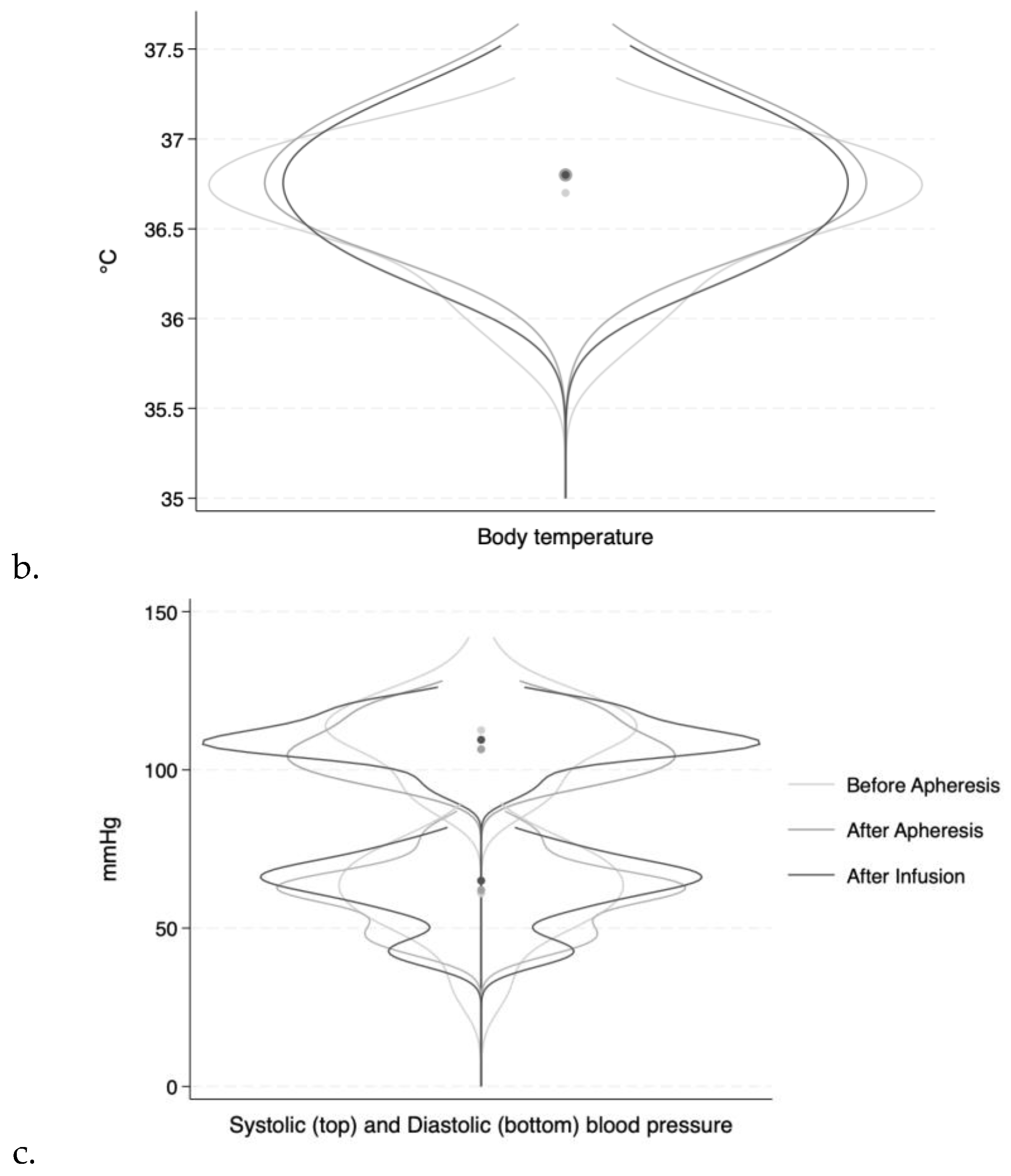
Heart rate, blood pressure, and body temperature varied slightly within each patient during treatment.
Figure 2 shows the variations presented as overlaying violin plots.
No serious adverse events were recorded for the patients during the study period. The total number of AEs was 23, all considered low grade according to the Common Terminology Criteria for AEs (CTCAE version 4.03).
Adverse events occurring in the different patients (number 1-4) throughout the study and whether deemed related to ALA. Grade according to CTCAE (1-5).
There were no serious adverse events recorded in the patients not completing treatment.
3.4. Efficacy
Three of the four patients who completed treatment experienced some clinical improvement, but clinical remission was not achieved. A reduction in SES-CD ≥ 50% and lower FC from baseline was demonstrated in two of these three patients. In Patient 1, no clinical or endoscopic changes were observed after 6 treatments. The changes in the IBDQ score were small and not significant but correlated with the treatment effects (
Table 5).
3.5. Immunohistochemistry
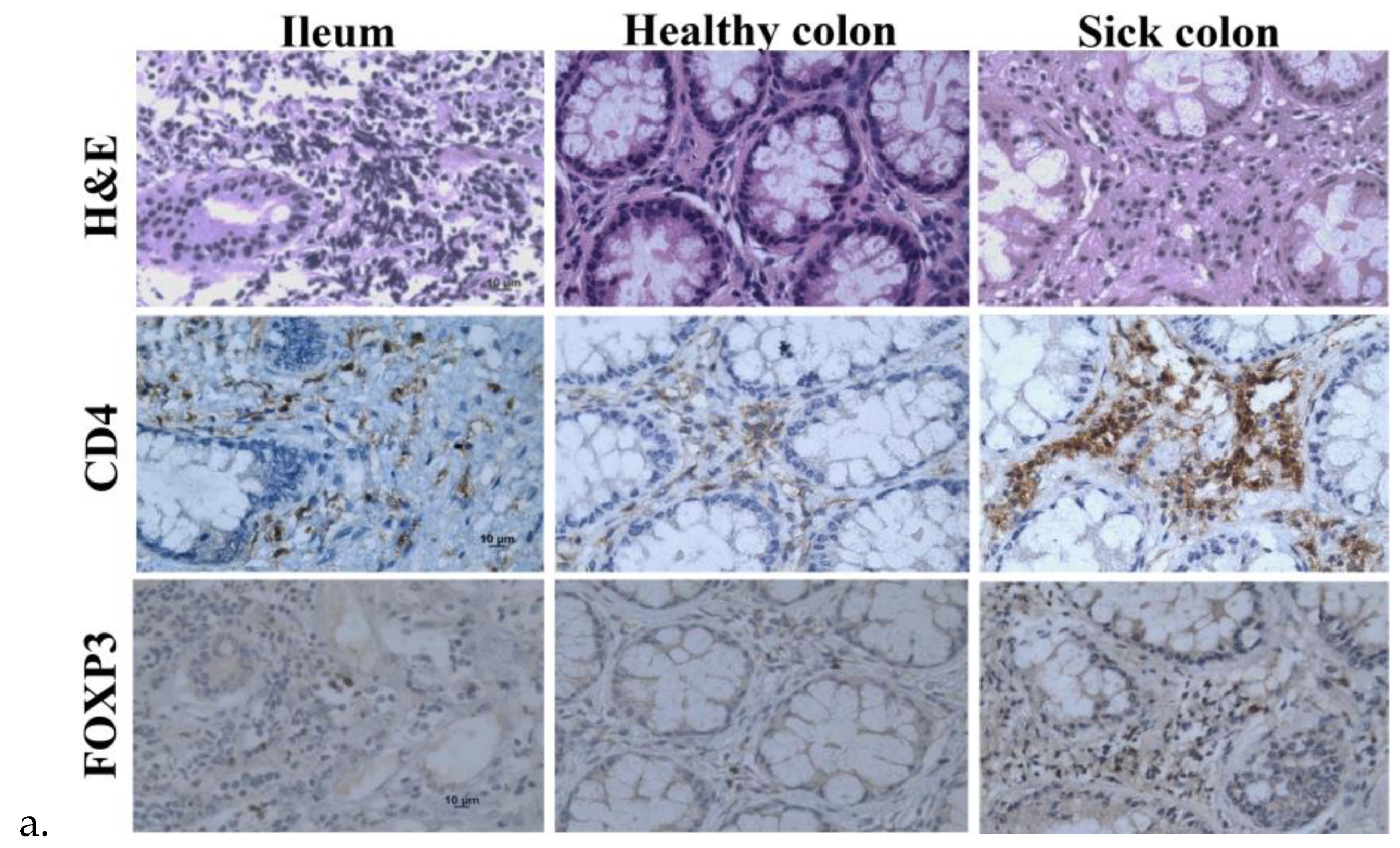
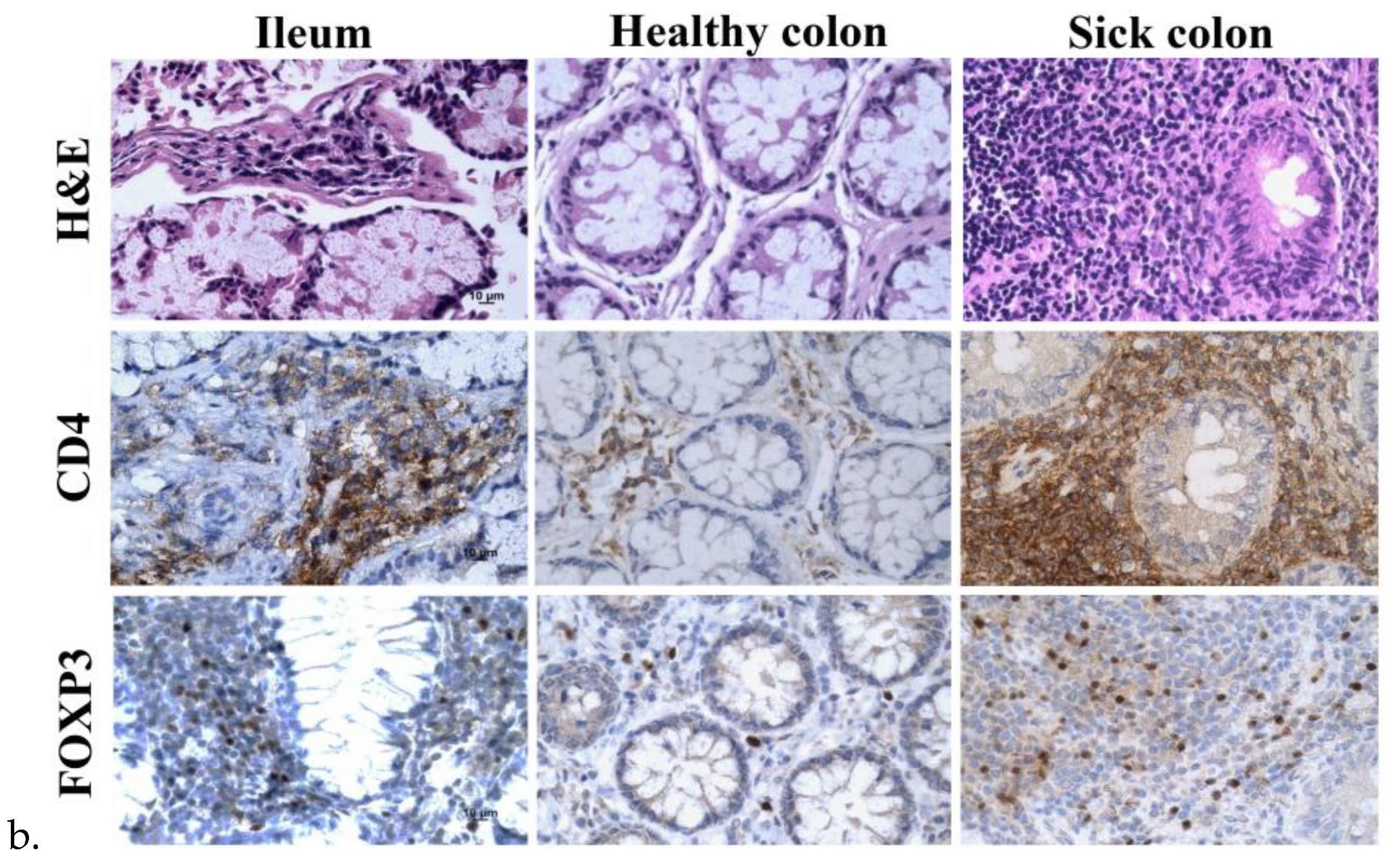
The tissue sections of the ileum and healthy and sick colon exhibited lymphoid infiltration with lymph follicles in the lamina propria that likely extended into the submucosa through the incomplete muscularis, but there was less lymphocyte infiltration in the healthy colon than the ileum and sick colon (
Figure 3a). Immunohistochemistry of the surface marker CD4 for helper T cells and the transcription factor forkhead box protein 3 (FOXP3) for regulatory T cells (Tregs) confirmed the infiltration of CD4
+ T cells and Tregs largely in the lymph follicles. Because there was more lymphoid infiltration in the ileum and sick colon, there were more CD4
+ cells and Tregs in these samples. The samples from the ileum and sick colon taken 13 weeks after treatment appeared to have more CD4
+ T cells and Tregs than the samples taken before treatment (
Figure 3b). However, there was large variation in the amounts and distribution patterns of these cells in different biopsies of the same area and various areas of the same and different patients.
4. Discussion
In this first-in-human study, clinical and endoscopic efficacy was demonstrated in patients with active CD treated with ALA/blue light-based ECP. All participants had previously failed advanced therapies due to intolerance or perceived contraindication. No safety or tolerability concerns were observed.
Overall, the patients tolerated the treatment very well. However, two episodes of nausea, which is a common side-effect of ALA, were recorded, and there were also a few episodes of headache. All the reported side-effects were self-limiting without medical intervention.
Although conventional 8-MOP/UV-A-based ECP has been investigated in some small studies for the treatment of CD with effects for steroid-refractory cases and patients intolerant or unresponsive to other options [
10,
11,
12,
32], a disadvantage of DNA binding of 8-MOP is the ability to kill both activated (diseased) and resting (normal) cells with no selectivity. Since our previous research has revealed greater selective killing effects on T cells after treatment with ALA/blue light than after treatment with 8-MOP/UV-A [
13,
16], a two-step approach of ECP was used in this study with the possibility of using Spectra-Optia
® for MNC collection followed by illumination with a separate 405-nm blue light source suitable for the absorption peak of PpIX.
Disadvantages of using ALA/blue light was the treatment time of approximately 4 hours in addition to patient logistics, including transportation and gaining of intravenous access. Regular ECP using 8-MOP with UV-A is faster [
33,
34], partially due to the ALA incubation. However, the ALA/blue light ECP method may in the future be improved by simplifying the procedure by e.g. dividing the treatment into three parts: collection, incubation, and transfusion. After collection, the patient is free during incubation period, which could also be longer. This longer incubation would accumulate more PpIX [
16], which suggests a better photodynamic effect.
Overall, autoimmune diseases, including CD and graft versus host disease (GvHD), represent common immune-mediated diseases with diverse clinical presentations. These diseases share common underlying pathogenetic features but present their own clinical immune phenotypes. Tregs produce suppressive messengers, such as TGF-beta and IL-10, to inhibit the activation, proliferation, and cytokine production of other cells in the immune system. They regulate immune responses to self-antigens and foreign antigens and help prevent immune-mediated diseases. Therefore, Tregs are indispensable for the establishment and maintenance of immunological self-tolerance and homeostasis. FOXP3 is an essential molecular marker in the development and function of Tregs [
35]. Because the reduced number and functional abnormalities of tolerogenic Tregs may cause immune-mediated diseases [
36], it is tempting to investigate whether Tregs are involved in the treatment of CD with ALA-ECP. A decrease in Tregs may be associated with active IBD [
37]. There were more CD4
+ T cells and Tregs in the ileum and sick colon at week 13 after ALA-ECP than before treatment, which suggests that ALA-ECP enhances the infiltration of Tregs into diseased tissues and improves Treg infiltration. However, there is large variation in the amounts and distribution patterns of Tregs in different biopsies of the same area of the same patient and between different patients. Further studies with more CD patients are needed to confirm these findings.
The main limitations in our study includes the small samples size which limits the conclusions that can be drawn from this study. Further limitations are the lack of a control group and the lack of a blinded follow-up. . In this study, we experienced a long inclusion period. This was due to the COVID pandemic, which led to the postponement of the inclusion of patients and to the change in work tasks for the study team. Previously, no randomized clinical study on ECP in patient with CD has been performed, although ECP guidelines list it as an available option [
7,
38]. On the other hand, the European Crohn’s and Colitis Organization (ECCO) guidelines do not mention this treatment method as an option[
6]. Even so, we argue that ECP could have a place in CD treatment. With the increasing prevalence of CD, more patients will fail or be intolerant to conventional and/or advanced therapy. A particularly important group of patients are the patients with a history of malignancies for whom treatment with immunosuppressive drugs is often perceived as contraindicated. Additionally, ECP has few side effects, including lower risk of infection, which is a common adverse effect associated with biologics.
Furthermore, combination therapy for CD is a topic of significant international interest [
1,
39]. ALA/blue light ECP may be an option in induction therapy for hospitalized critically ill patients to help reboot the immune system, but this possible treatment option must be explored in clinical studies.
In conclusion, the results of this study suggest that ALA/blue light-based ECP is both safe and well-tolerated, with potential to provide clinical efficacy in patients with CD. These findings provide a basis for further investigation into the use of ALA-ECP in the treatment of CD and other T-cell-mediated disorders.
Author Contributions
K.E., E.C., A.A., A.U., T.W., A.K., S.D., P.J., V.V., Q.P., and J.J.: methodology. K.E., E.C., T.W., Q.P., and J.J.; conceptualization. Q.P., and J.J.: acquired funding. K.E., E.C., Q.P., and J.J.: writing the original draft. K.E., and Q.P.: formal analysis and interpretation of the data. K.E., A.A., and A.U.: patient treatment and assessment. E.C., Q.P., and J.J.: project administration and supervision of K.E. All authors have approved the final draft for submission.
Funding
The South-Eastern Norway Regional Health Authority (Project numbers: 2016092, 2017058, 2020069), the Norwegian Cancer Society (Project number: 190397), the Norwegian Radium Hospital Research Foundation (Project number: SE1701), and Internal Funds of Akershus University Hospital 2019 (project number 080).
Institutional Review Board Statement
The study was approved by the ethics committee (REK – reference number 15685), the Norwegians Medicines Agency (NOMA – EudraCT 2018-002422-23, NOMA reference 18/10491), and the Data Protection Official (DPO, reference number 78_2019) at Akershus University Hospital. It was registered on ClinicalTrials.gov (
https://clinicaltrials.gov/study/NCT04164849).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We thank Koshala Kantharuban and Shazia Sahab from the Department of Immunology and Transfusion Medicine, Akershus University Hospital, Lorenskog, Norway; Synnøve Louise Aure from the Department of Gastroenterology, Akershus University Hospital, Lorenskog, Norway; and Phuong Mai Thi Nguyen from the Laboratory for Research Support, Department of Pathology, Oslo University Hospital. We also thank Photonamic GmbH & Co. (KG, Pinneberg, Germany) for providing Gliolan® (ALA) and PIT Medical Systems (Cadolzburg, Germany) for providing the BLUE-PIT and kits.
Conflicts of Interest
none to report.
References
- Torres, J., S. Mehandru, J.F. Colombel, and L. Peyrin-Biroulet, Crohn's disease. Lancet, 2017. 389(10080): p. 1741-1755. [CrossRef]
- Rajbhandari, R., S. Blakemore, N. Gupta, A.J. Adler, C.A. Noble, S. Mannan, K. Nikolli, A. Yih, S. Joshi, and G. Bukhman, Crohn's disease in low and lower-middle income countries: A scoping review. World Journal of Gastroenterology, 2020. 26(43): p. 6891-6908. [CrossRef]
- Baumgart, D.C. and W.J. Sandborn, Crohn's disease. Lancet, 2012. 380(9853): p. 1590-605. [CrossRef]
- Baumgart, D.C. and S.R. Carding, Inflammatory bowel disease: cause and immunobiology. Lancet, 2007. 369(9573): p. 1627-40. [CrossRef]
- Ocansey, D.K.W., L. Wang, J. Wang, Y. Yan, H. Qian, X. Zhang, W. Xu, and F. Mao, Mesenchymal stem cell-gut microbiota interaction in the repair of inflammatory bowel disease: an enhanced therapeutic effect. Clin Transl Med, 2019. 8(1): p. 31. [CrossRef]
- Torres, J., S. Bonovas, G. Doherty, T. Kucharzik, J.P. Gisbert, T. Raine, M. Adamina, A. Armuzzi, O. Bachmann, P. Bager, L. Biancone, B. Bokemeyer, P. Bossuyt, J. Burisch, P. Collins, A. El-Hussuna, P. Ellul, C. Frei-Lanter, F. Furfaro, C. Gingert, P. Gionchetti, F. Gomollon, M. González-Lorenzo, H. Gordon, T. Hlavaty, P. Juillerat, K. Katsanos, U. Kopylov, E. Krustins, T. Lytras, C. Maaser, F. Magro, J. Kenneth Marshall, P. Myrelid, G. Pellino, I. Rosa, J. Sabino, E. Savarino, A. Spinelli, L. Stassen, M. Uzzan, S. Vavricka, B. Verstockt, J. Warusavitarne, O. Zmora, G. Fiorino, o.b.o.t.E. Crohn’s, and C. Organisation, ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. Journal of Crohn's and Colitis, 2019. 14(1): p. 4-22. [CrossRef]
- Connelly-Smith, L., C.R. Alquist, N.A. Aqui, J.C. Hofmann, R. Klingel, O.A. Onwuemene, C.J. Patriquin, H.P. Pham, A.P. Sanchez, J. Schneiderman, V. Witt, N.D. Zantek, and N.M. Dunbar, Guidelines on the Use of Therapeutic Apheresis in Clinical Practice - Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Ninth Special Issue. Journal of Clinical Apheresis, 2023. 38(2): p. 77-278. [CrossRef]
- Goussetis, E., I. Varela, and P. Tsirigotis, Update on the mechanism of action and on clinical efficacy of extracorporeal photopheresis in the treatment of acute and chronic graft versus host disease in children. Transfusion and Apheresis Science, 2012. 46(2): p. 203-9. [CrossRef]
- Gulwani-Akolkar, B., P.N. Akolkar, A. Minassian, R. Pergolizzi, M. McKinley, G. Mullin, S. Fisher, and J. Silver, Selective expansion of specific T cell receptors in the inflamed colon of Crohn's disease. Journal of Clinical Investigation, 1996. 98(6): p. 1344-54. [CrossRef]
- Reinisch, W., R. Knobler, P.J. Rutgeerts, T. Ochsenkuhn, F. Anderson, C. von Tirpitz, M. Kaatz, C. Janneke van der Woude, D. Parenti, and P.J. Mannon, Extracorporeal photopheresis (ECP) in patients with steroid-dependent Crohn's disease: an open-label, multicenter, prospective trial. Inflammatory Bowel Diseases, 2013. 19(2): p. 293-300. [CrossRef]
- Abreu, M.T., C. von Tirpitz, R. Hardi, M. Kaatz, G. Van Assche, P. Rutgeerts, E. Bisaccia, S. Goerdt, S. Hanauer, R. Knobler, P. Mannon, L. Mayer, T. Ochsenkuhn, W.J. Sandborn, D. Parenti, K. Lee, and W. Reinisch, Extracorporeal photopheresis for the treatment of refractory Crohn's disease: results of an open-label pilot study. Inflammatory Bowel Diseases, 2009. 15(6): p. 829-36. [CrossRef]
- Reinisch, W., H. Nahavandi, R. Santella, Y. Zhang, C. Gasche, G. Moser, T. Waldhor, A. Gangl, H. Vogelsang, and R. Knobler, Extracorporeal photochemotherapy in patients with steroid-dependent Crohn's disease: a prospective pilot study. Alimentary Pharmacology and Therapeutics, 2001. 15(9): p. 1313-22.
- Holien, T., O.A. Gederaas, S.R. Darvekar, E. Christensen, and Q. Peng, Comparison between 8-methoxypsoralen and 5-aminolevulinic acid in killing T cells of photopheresis patients ex vivo. Lasers in Surgery and Medicine, 2018. 50(5): p. 469-475. [CrossRef]
- Peng, Q., K. Berg, J. Moan, M. Kongshaug, and J.M. Nesland, 5-Aminolevulinic acid-based photodynamic therapy: principles and experimental research. Photochemistry and Photobiology, 1997. 65(2): p. 235-51. [CrossRef]
- Correia, J.H., J.A. Rodrigues, S. Pimenta, T. Dong, and Z. Yang, Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics, 2021. 13(9). [CrossRef]
- Darvekar, S., P. Juzenas, M. Oksvold, A. Kleinauskas, T. Holien, E. Christensen, T. Stokke, M. Sioud, and Q. Peng, Selective Killing of Activated T Cells by 5-Aminolevulinic Acid Mediated Photodynamic Effect: Potential Improvement of Extracorporeal Photopheresis. Cancers, 2020. 12(2): p. 377. [CrossRef]
- Heerfordt, I.M. and H.C. Wulf, Protoporphyrin IX in the skin measured noninvasively predicts photosensitivity in patients with erythropoietic protoporphyria. British Journal of Dermatology, 2016. 175(6): p. 1284-1289. [CrossRef]
- Stummer, W., U. Pichlmeier, T. Meinel, O.D. Wiestler, F. Zanella, and H.J. Reulen, Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncology, 2006. 7(5): p. 392-401. [CrossRef]
- Shah, H.A., S. Leskinen, H. Khilji, V. Narayan, N. Ben-Shalom, and R.S. D'Amico, Utility of 5-ALA for fluorescence-guided resection of brain metastases: a systematic review. Journal of Neuro-Oncology, 2022. 160(3): p. 669-675. [CrossRef]
- Inoue, K., S. Anai, K. Fujimoto, Y. Hirao, H. Furuse, F. Kai, S. Ozono, T. Hara, H. Matsuyama, and M. Oyama, Oral 5-aminolevulinic acid mediated photodynamic diagnosis using fluorescence cystoscopy for non-muscle-invasive bladder cancer: a randomized, double-blind, multicentre phase II/III study. Photodiagnosis and Photodynamic Therapy, 2015. 12(2): p. 193-200.
- Nakai, Y., K. Inoue, T. Tsuzuki, T. Shimamoto, T. Shuin, K. Nagao, H. Matsuyama, M. Oyama, H. Furuse, and S. Ozono, Oral 5-aminolevulinic acid-mediated photodynamic diagnosis using fluorescence cystoscopy for non-muscle-invasive bladder cancer: a multicenter phase III study. International Journal of Urology, 2018. 25(8): p. 723-729.
- Morton, C.A., R.M. Szeimies, N. Basset-Seguin, P. Calzavara-Pinton, Y. Gilaberte, M. Haedersdal, G.F.L. Hofbauer, R.E. Hunger, S. Karrer, S. Piaserico, C. Ulrich, A.M. Wennberg, and L.R. Braathen, European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 1: treatment delivery and established indications - actinic keratoses, Bowen's disease and basal cell carcinomas. Journal of the European Academy of Dermatology and Venereology, 2019. 33(12): p. 2225-2238. [CrossRef]
- Christensen, E., T. Warloe, S. Kroon, J. Funk, P. Helsing, A.M. Soler, H.J. Stang, O. Vatne, and C. Mørk, Guidelines for practical use of MAL-PDT in non-melanoma skin cancer. Journal of the European Academy of Dermatology and Venereology, 2010. 24(5): p. 505-12. [CrossRef]
- Christensen, E., O.A. Foss, P. Quist-Paulsen, I. Staur, F. Pettersen, T. Holien, P. Juzenas, and Q. Peng, Application of Photodynamic Therapy with 5-Aminolevulinic Acid to Extracorporeal Photopheresis in the Treatment of Patients with Chronic Graft-versus-Host Disease: A First-in-Human Study. Pharmaceutics, 2021. 13(10). [CrossRef]
- Christensen, E., O.A. Foss, T. Holien, P. Juzenas, and Q. Peng, Application of Photodynamic Therapy with 5-Aminolevulinic Acid to Extracorporeal Photopheresis in the Treatment of Cutaneous T-Cell Lymphoma: A First-in-Human Phase I/II Study. Pharmaceutics, 2024. 16(6): p. 815.
- Harvey, R.F. and J.M. Bradshaw, A simple index of Crohn's-disease activity. Lancet, 1980. 1(8167): p. 514.
- Daperno, M., G. D'Haens, G. Van Assche, F. Baert, P. Bulois, V. Maunoury, R. Sostegni, R. Rocca, A. Pera, A. Gevers, J.Y. Mary, J.F. Colombel, and P. Rutgeerts, Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointestinal Endoscopy, 2004. 60(4): p. 505-12. [CrossRef]
- Guyatt, G., A. Mitchell, E.J. Irvine, J. Singer, N. Williams, R. Goodacre, and C. Tompkins, A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology, 1989. 96(3): p. 804-10.
- Irvine, E.J., B. Feagan, J. Rochon, A. Archambault, R.N. Fedorak, A. Groll, D. Kinnear, F. Saibil, and J.W. McDonald, Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn's Relapse Prevention Trial Study Group. Gastroenterology, 1994. 106(2): p. 287-96. [CrossRef]
- EMA, Guideline on the development of new medicinal products for the treatment of Crohn’s Disease. 2018. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-development-new-medicinal-products-treatment-crohns-disease-revision-2_en.pdf.
- Silverberg, M.S., J. Satsangi, T. Ahmad, I.D. Arnott, C.N. Bernstein, S.R. Brant, R. Caprilli, J.F. Colombel, C. Gasche, K. Geboes, D.P. Jewell, A. Karban, E.V. Loftus, Jr., A.S. Peña, R.H. Riddell, D.B. Sachar, S. Schreiber, A.H. Steinhart, S.R. Targan, S. Vermeire, and B.F. Warren, Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Canadian Journal of Gastroenterology, 2005. 19 Suppl A: p. 5a-36a. [CrossRef]
- Cheerva, A., R. Dillard, and S. Bertolone, Extracorporeal photopheresis for the treatment of severe, refractory steroid dependent pediatric Crohn's Disease. Journal of Clinical Apheresis, 2013. 28(5): p. 381-6. [CrossRef]
- Mayer, W., J. Mayr, F. Koch, U. Rechberger, W. Gasser, M. Hermann, A. Kempel, M. Edlinger, and H. Schennach, Increasing the collection flow rate to 2 mL/min is effective and reduces the procedure time in off-line photopheresis. Transfusion, 2023. 63(8): p. 1546-1553. [CrossRef]
- Mayer, W., A. Kontekakis, C. Maas, U. Kuchenbecker, S. Behlke, and H. Schennach, Comparison of procedure times and collection efficiencies using integrated and multistep nonintegrated procedures for extracorporeal photopheresis. Journal of Clinical Apheresis, 2022. 37(4): p. 332-339. [CrossRef]
- Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky, Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunology, 2003. 4(4): p. 330-6. [CrossRef]
- Dominguez-Villar, M. and D.A. Hafler, Regulatory T cells in autoimmune disease. Nature Immunology, 2018. 19(7): p. 665-673. [CrossRef]
- Jalalvand, M., S. Enayati, M. Akhtari, E. Madreseh, A. Jamshidi, E. Farhadi, M. Mahmoudi, and A. Amirzargar, Blood regulatory T cells in inflammatory bowel disease, a systematic review, and meta-analysis. International Immunopharmacology, 2023. 117: p. 109824. [CrossRef]
- Knobler, R., G. Berlin, P. Calzavara-Pinton, H. Greinix, P. Jaksch, L. Laroche, J. Ludvigsson, P. Quaglino, W. Reinisch, J. Scarisbrick, T. Schwarz, P. Wolf, P. Arenberger, C. Assaf, M. Bagot, M. Barr, A. Bohbot, L. Bruckner-Tuderman, B. Dreno, A. Enk, L. French, R. Gniadecki, H. Gollnick, M. Hertl, C. Jantschitsch, A. Jung, U. Just, C.D. Klemke, U. Lippert, T. Luger, E. Papadavid, H. Pehamberger, A. Ranki, R. Stadler, W. Sterry, I.H. Wolf, M. Worm, J. Zic, C.C. Zouboulis, and U. Hillen, Guidelines on the use of extracorporeal photopheresis. Journal of the European Academy of Dermatology and Venereology, 2014. 28 Suppl 1: p. 1-37. [CrossRef]
- Le Berre, C., A. Ricciuto, L. Peyrin-Biroulet, and D. Turner, Evolving Short- and Long-Term Goals of Management of Inflammatory Bowel Diseases: Getting It Right, Making It Last. Gastroenterology, 2022. 162(5): p. 1424-1438. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).