1. Introduction
The plasma membrane of a cell is a highly dynamic structure with a permanent constitutive turnover, mainly facilitated by endosomes. For example, macrophages and fibroblasts internalize more than 200% of their entire surface area per hour [
1]. A major task of the cellular membrane is to keep contact to the surrounding cells and tissue. In order to maintain environmental contact, cells express receptors at their surface to measure mechanical distortions or pressure to sense circumstances such as pH or temperature and eventually get information or instructions in a ligand mediated way. Those ligands usually find a specialized receptor and binding of both results in specific signaling pathway. Receptor mediated signaling is classified as listed in
Table 1.
In this review, we will concentrate on type III signaling on the example of epithelial growth factor (EGF) and its receptor (epithelial growth factor receptor, EGFR), which involves receptor internalization.
Early endosomes are part of the endocytic trafficking pathways for cargo molecules internalized from the plasma membrane [
2]. Internalization is carried out by diverse mechanisms including clathrin-dependent endocytosis, phago- and pinocytosis or routes utilizing dynamin, caveolae and other [
3]. In all cases, early endosomes serve as the primary sorting platform for diverse cargo, for example receptors and their ligands [
4,
5,
6].
The intention of this graphical review is to provide an overview of early endosomes in particular of key signaling components. In particular, we will address the endosomal sorting and trafficking of EGFR. Eventually, we will delineate the concept of redox-active endosomes (redoxosomes) using the example of EGFR trans-activation by endosomal reactive oxygen species (ROS).
1.1. Models of the Early Endosome
For the description of endocytic traffic (
Figure 1), two basic models have been evolved, namely the “pre-existing compartment model” in contrast to the “maturation model” which are both disputed [
7,
8]. Briefly, the “pre-existing compartment model” aka “Endosomal carrier vesicle hypothesis” states constant endosomal compartments e.g. early endosomes and late with stable marker proteins. Consequently, cargo is shuttled through transport vesicles [
8]. Conversely, in the “maturation model” a single structure gradually transforms into a functional different entity. In the latter case, regulatory proteins are rather recruited from the cytoplasm than resident at the endosome [
6,
9]. Both models agree on initial fusion events between primary endocytic vesicles and early endosomes followed by cargo sorting, but differ in the origin of later endosomal structures and their identity over time [
7].
1.2. Structure of the Early Endosome
Early endosomes are dynamic and morphologically complex structures (
Figure 2) displaying a
cis-trans polarity. On the
cis-side incoming vesicles are received, whereas the
trans-side shows both tubular (~60 nm diameter, pH~6.5) and multivesicular (~300 nm diameter, pH~6.3-6.8) regions as functional subdomains [
2]. Cargo molecules are sorted in the early endosomes either to degrading or recycling routes; however, in most cases the sorting signals are poorly understood [
6].
Functional areas within early endosomes are likely organized by membrane-binding proteins like annexins interacting as well with the actin cytoskeleton and cholesterol-enriched rafts. Apart from shuttling vesicles budded of from the early endosomes, motor protein complexes including myosins, kinesins and dynein, control early endosomes’ intracellular movement [
4,
5].
In spite of the challenges to identify unique molecular markers for each endosomal component, Ras-associated proteins (Rab) and their effectors are essential in regulating endocytic traffic. In the early endosomes, especially Rab4,5,10,14,21,22 are prominent [
5]. In the following section, we will exemplarily consider some key effectors of Rab4 and Rab5, although an exhaustive analysis of the complete signaling network at the early endosomes is beyond the scope of this review.
1.3. Signaling Pathways at Early Endosomes
As mentioned above, Rab proteins are involved in all aspects of vesicle processing (budding, fusion, tethering etc.) (
Figure 2). In their active GTP-bound form, Rabs recruit specific effector proteins. Rab5-GTP promotes its own activation through a positive feedback circuit with Rab GTPase-binding effector protein 1 (Rabaptin-5) and Rab5 GDP/GTP exchange factor 5 (Rabex-5). Additionally, Rabaptin-5 plays a role in vesicle budding from the early endosomes by interacting with the clathrin adapter AP-1 [
4].
Among the Rab5 effectors in early endosomes, the early endosomal antigen-1 (EEA1) is commonly used as an early endosomes marker [
10]. EEA1 and Rab5 also participate in redoxosomes discussed in a later section of this review. In concert with soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs), EEA1 mediates endosome fusions. Furthermore, EEA1 binds to phosphatidylinositol-3-phosphate (PtdIns3P) synthesized by phosphatidylinositol 3-kinase Vps34 complex [
4]. In general, spatially restricted lipid synthesis and enrichment allow the selective recruitment of PtdIns3P-binding proteins like the NADPH oxidase subunit p40
phox [
2]. Similarly, PtdIns3P is converted to phosphatidylinositol-3,5-bisphosphate (PtdIns3,5P2) by the 1-phosphatidylinositol 3-phosphate 5-kinase (PIKFyve) complex. PIKFyve and its counterpart, the lipid phosphatase myotubularin (MTM1) tightly control PtdIns3P levels being crucial for proper EGFR sorting (see later section) [
6]. Contrary to Rab5, Rab4 is not only localized on early endosomes but also on RE, in the first scenario Rab4 and Eps15 homology domain (EHD) proteins coordinate the exit of cargo to the perinuclear endocytic recycling compartment (ERC), which is part of the “slow-recycling” pathway in contrast to the “short loop” transport directly back to the plasma membrane [
5]. Rab4 and Rab5 are linked by dual effectors e.g. Rab Interacting Protein 4 (RabIP4) and Rabenosyn-5 [
2].
Although cargo sorting will not be covered in detail here, notably, the multiple subdomains inside the early endosomes permit cargo sorting to various organelles
inter alia the trans-Golgi network. In this context, the multimeric retrograde transport machinery is initiated at the early endosomes through the action of sorting nexins (SNX) with other components of the retromer[
5].
Interestingly, Rab effectors may partake in signal transduction as well, as it was shown for adapter protein containing PH domain, PTB domain and leucine zipper motif 1,2 (APPL1,2) which remodel nucleosomes and regulate the activity of the kinase Akt [
4,
11].
A growing body of evidence links early endosomes to signal transduction of internalized receptors. Internalization routes, ligand type and concentration determine the signaling outcome. In addition, signaling after endocytosis can be different to the plasma membrane depending on the specific protein equipment of the endosome [
10].
2. The Endosomal Compartment in EGF Signaling
Binding of a ligand to type III receptors triggers internalization and recycling of the receptor itself as well as surrounding portions of the membrane. Internalization of the receptor represents a mechanism to control the dynamic of EGF signaling, as internalization of the EGFR and therefore reduction of the number of receptors at the cells surface at least transiently reduces the ability of its ligand EGF to spur further signaling [
12].
Internalization of EGFR is stimulated after ligand-binding and
trans-phosphorylation of the receptor tyrosine kinases [
13]. We will not describe the mechanisms of EGFR activation and the downstream signaling cascades in this review, despite it should be noted that some EGFR-signaling is supposed not only be continued in early endosomes, but also several pathways seem to rely on endocytosis [
11,
14].
Internalization requires fusion of membrane cargo vesicles to organelles called endosomes (
Figure 3). Those can be classified as early-, sorting-, recycling- or late endosomes depending on their stage of maturation [
15,
16], eventually lysosomes are considered to be part of the endosomal compartment [
17]. As such early endosomes function as signaling and scaffolding platform to initiate or prolong distinct signaling pathways, or degradation of the internalized ligand and receptor. Accordingly, recognition of early endosomes as part of a degradation, signaling or recycling pathway represents a major step in ligand induced signal transduction.
Endocytosis involves or even depends on the GTPase dynamin 2, which forms helical polymers at lipid rafts at the necks of budding vesicles and its GTP hydrolysis-dependent conformational changes promote fission of the tubular membrane in order to generate a free endocytic vesicle [
18]. According to the various nature of lipid rafts, two major models of endosome formation are proposed: Clathrin-dependent and -independent endocytosis. Clathrin-dependent endocytosis most often is described to build vesicles from 100–200 nm, while clathrin independent endocytosis is mediated by caveolins (caveolae) and lipid rafts, and involves smaller vesicles (40–80 nm) [
17]. In case of EGFR (
Figure 4.1), endocytosis is primarily clathrin-dependent and around 20-25% of clathrin coated pits are loaded with EGF at 37°C, no matter how high an EGF concentration is [
19]. In fact, in different strains of human fibroblasts each cell contains 40,000-100,000 binding sites for EGF [
12]. The data indicates a saturated internalization of EGF determined by the number of EGF binding sites. Interestingly, EGF loaded vesicles lose their clathrin coat as a prerequisite for further sorting and signaling. In general, internalized ligand-receptor complexes after endocytosis via clathrin-coated pits enter the endosomal compartment. EGFR endocytosis is subject to controversy concerning the conditions and proteins involved, anyways, studies suggest that EGFR is internalized via clathrin-dependent and independent pathways [
14,
20]. Besides, the dimerization partners influence the sensitivity to downregulation [
21].
Once in early endosomes membrane proteins are rapidly recycled to the plasma membrane while fluid-phase proteins usually proceed towards the lysosome (
Figure 4) [
22].
A major peculiarity of early endosomes is their ability to facilitate recycling of non-ubiquitinated endocytosed receptors, while ubiquitinated receptors proceed to lysosomal degradation. Recycling endosomes are concentrated at the microtubule organizing center and consist of a largely tubular network [
13]. While late endosomes are mainly spherical, lack tubules, and contain many closely packed intraluminal vesicles, early endosomes consist of a dynamic tubular-vesicular network with vesicles up to 1 µm in diameter and with connected tubules of approx. 50 nm diameter. For maturation of early endosomes into late endosomes, they become increasingly acidic (pH ≈ 6.0–6.8) maintained by an ATP-driven proton pump [
23] or anion transporter chloride channels such as CLC3 [
24], which results in the dissociation of the ligand from its receptor. Sorting of endosomes is mediated by Rab GTPases with Rab5 mainly expressed in early endosomes. Other Rab family members, Rab4 and Rab11 facilitate labelling of early endosomes as they generate recycling vesicles that fuse with plasma membrane, returning mostly non-ubiquitinated receptors directly back to cell surface. In contrast, Rab11-positive but Rab4-negative pericentriolar recycling endosomes appear to be important for membrane domain maintenance and membrane protein mobilization [
25]. Within early endosomes, most receptors disengage from their ligands with subsequent recycling of the receptor and degradation of the ligand. In case of EGFR, disengagement is determined by the ligand bound. TGFα/EGFR is disengaged at endosomal pH, while EGF/EGFR is not. Different to LDL-receptors, EGF and the EGF receptor have a pH-resistant bond that persists until it is delivered to lysosomes for their degradation [
26,
27]. In both cases, adapter proteins like growth factor receptor-bound protein 2 (Grb2) and SH3 domain-containing kinase-binding protein 1 (CIN85) associate with the activated EGFR, leading to recruitment of the E3 ubiquitin (ub) ligase Cbl [
28]. Next, Ub-binding proteins, for example of the clathrin coat, tether to Ub- chains at EGFR. In early endosomes, Cbl proceeds with EGFR ubiquitination, however the exact role for ubiquitination in EGFR endocytosis is not fully elucidated [
29]. Even though many adapter proteins (
Figure 4.1) like Epidermal growth factor receptor substrate 15 (Eps15, found in clathrin-coated pits), have shown to be important for EGFR endocytosis [
30], the molecular mechanisms behind wait to become fully enlightened [
14,
20].
Unoccupied receptor then is quickly recycled back to the membrane, while ligand-bound receptor recycles only slowly or degrades in lysosomes. Sorting into Rab4,35 or Rab8,11 positive early endosomes domains promotes fast or slow recycling (cf. section above) accompanied by continuous deubiquitination (
Figure 4.2). On the other side, if ubiquitinated EGFR is recognized by Rabex-5, hepatocyte growth-factor regulated tyrosine kinase substrate (Hrs) and tumor susceptibility gene 101 (Tsg101), EGFR degradation occurs (
Figure 4.3). Hrs and signal transducing adapter molecule (STAM) cling EGFR in early endosomes, subsequently the whole ESCRT 0-III complexes assemble. Deubiquitinating enzymes (DUBs) remove Ub before EGFR sorting is completed to replenish the free Ub pool. Remarkably, apart from inactivating EGFR, protein tyrosine phosphatase PTP1B co-localizes with the receptor during the endocytic traffic, herby PTP1B dephosphorylates ESCRT members to foster lysosomal degradation [
6,
14]. Recently, autophagy has been shown to regulate EGFR trafficking from early endosomes and Rab11-mediated recycling [
31].
Although initiation of the receptor endocytosis requires the cytoplasmic tail to be present, the process of endosomal retention and progression is independent from intrinsic receptor kinase activity. While in steady state 2-3% of all empty EGFR are internalized per minute, ligand binding accelerated internalization rates and retention of EGF/EGFR within the cell [
32]. Internalized EGF/EGFR proceeds towards lysosomal degradation by maturation of early endosomes. While Rab5 is a component of early endosomes, Rab7 defines the functional identity of late endosomes and makes the compartment competent for fusion with lysosome (degradation competent) [
33]. Accordingly, maturation of early endosome not only includes increasing acidification but also the so-called Rab5–Rab7 conversion. About 35% of Rab5-positive endosomes can be categorized as rapidly maturing, as they accumulate Rab7 with a time constant of 30 seconds, while the remaining 65% mature much slower, not acquiring Rab7 even after 100 seconds [
19]. It is likely that the increase in Rab7 content arises from fusion with preexisting vesicles. EGF is preferentially targeted directly to the dynamic, rapidly maturing population of early endosomes (86%) with only a small fraction (14%) joining the static, slowly maturing endosomes. In contrast, the iron transporter transferrin undergoes none-selective sorting by early endosomes: Since the static, slowly maturing endosomes constitute a larger population than the dynamic, rapidly maturing ones, most of the transferrin molecules are delivered to the static population [
19].
Clinically relevant are EGFR mutants displaying trafficking defects, thereby gaining oncogenic functions. Prominent examples comprise the variants EGFRvIV, EGFRvV, EGFRvIII, and v-ErbB which are supposed to be deficient in endocytic downregulation [
20].
3. Redox Control of the Endosomal Compartment in EGF Signaling
At least a portion of endosomes baring clathrin coats can be sub-grouped into receptosomes (
Figure 1) which enable further signaling of the appropriate receptors upon ligand binding [
34]. Formation of receptosome selectively avoids fusion with lysosomes and thereby further degradation of receptors and ligands. An experiment published in 1996 may shed light on a possible redox mediated sorting procedure of EGF/EGFR complexes. For internalization, uncoated endocytic vesicles fuse with early endosomes as the first line of internalization. After emergence from these vacuoles, EGF/EGFR complexes are delivered exclusively to a preexisting subset of endocytic vacuoles, identified by fluid-phase horseradish peroxidases (HRP) internalized several hours before. Delivery of EGF to these preloaded vacuoles was inhibited by incubating living cells with DAB and H
2O
2. Consequently, EGF degradation was inhibited and late endosomes accumulated, while internalization of EGF proceeded with unaltered kinetics. In contrast, both, internalization and recycling of transferrin, which served as a control was unaffected [
35]. Although we do not know what concentration was used in that study, it cannot be excluded that H
2O
2 had an effect on endosomal fusion. Lysosomes are characterized by markers such as lysosomal-associated membrane protein 1 (LAMP1) and mannose-6-phosphate receptor [
11]. Interestingly, in the paper mentioned above, HRP-loaded vesicles are only positive for LAMP1, indicating, that vesicles arising from endocytosis do not express, but rather fuse to preformed vesicles containing mannose-6-phosphate receptor. The authors compared EGF- and transferrin-internalization in HEp-2 cells. While EGF-loaded late endosomes fused with HRP-preloaded lysosomes, transferrin-loaded vesicles did not [
35]. Together these data underscore the possibility that EGF/EGFR-loaded late endosomes are distinct from transferrin-loaded late endosomes. Transferrin-loaded late endosomes may mature into lysosomes without fusion or fuse only to lysosomes positive for mannose-6-phosphate receptor, while EGF/EGFR-loaded late endosomes fuse with pre-existing lysosomes negative for mannose-6-phosphate receptor that arise from earlier endocytosis. In the context of redox signaling, it is important to note that a FRET probe based analysis of the human transferrin receptor 1 mediated endocytosis, attested a reducing environment in the endosomal compartments and the dynamics of transferrin receptor 1 trafficking [
36].
In contrast, a group of specialized components of the endosomal compartment, termed “redoxosomes” are characterized by redox-active signaling, contain internalized, ligand-bound receptors that need reactive oxygen species (ROS) for signal transduction (
Figure 5) [
37]. The most prominent effector of ROS signaling are cysteines and disulfide bridges within proteins [
38]. It is likely that lysosomes store hydrolases, potentially in an inactive form. Once a substrate has entered a lysosome, the proteolytic steps are probably performed by cysteine endoproteinases [
39]. Reduction of disulfide bonds was discovered as a key step in increasing the access of proteolytic processing enzymes to target proteins in lysosomes. Accordingly, lysosomes where thought to be the vesicular compartment that mediates protein disulfide reduction, while it was clear by 1991 that early, recycling endosomes do not comprise reducing activity [
40]. Later in 2005, Austin
et al. found that recycling endosomes, late endosomes, and lysosomes are not reducing, but oxidizing and comparable with conditions in the endoplasmic reticulum [
41]. These data indicate the existence of two distinct pathways within the endosomal compartment. One of which might be redox independent, while the other one depends on intra-endosomal ROS formation.
3.1. Redoxosomes
By nature, endosomes contain at least a portion of cell membrane and its ROS forming enzymes (
Figure 5), internalized together with the receptor [
42]. The major source of membrane associated ROS is the family of NADPH oxidases, which at present includes seven members (Nox1-5 as well as DUOX1 and 2) [
43]. Especially Nox1 and Nox2 together with their associated subunits, p22
phox for both, and NoxO1 and NoxA1 for Nox1, and p47
phox and p67
phox for Nox2, have been shown to act on redoxosomes [
44,
45]. Both, Nox1 and Nox2, produce •O
2ˉ [
46]. Nox2, the phagocytic NADPH oxidase, usually is recognized in endosomes and lysosomes within the process of unspecific host defense and antigen processing [
47,
48]. However, in SKOV3 cells treated with lysophosphatidic acid (LPA), internalization of the LPA receptor goes along with recruitment of Nox2 into early endosomes and subsequent LPA mediated signaling is abrogated when Nox2 activity is inhibited [
49]. Further, Nox2 derived ROS where described to promote the formation of an active interleukin-1/MyD88 complex in endosomes of MCF-7 cells [
50]. Obviously, endosomes containing Nox2 are composed of internalized plasma membrane and generate •O
2ˉ into the endosomal lumen to initiate signaling at intracellular sites [
51]. A potential mechanism of how the accompanying shift in endosomal charge is prevented was shown in human and murine aortic SMCs. Stimulation of those cells with tumor necrosis factor-alpha and interleukin-1 beta resulted in Nox1 and p22
phox dependent ROS production within intracellular vesicles. ClC3 co-localizes with Nox1 in early endosomes and is required for charge neutralization of the electron flow generated by Nox1 across the membrane of signaling endosomes by transporting Clˉ and H
+ (
Figure 5.2). The importance of this mechanism is certified by the fact that cytokine activation of nuclear factor kappa B in SMCs requires both, Nox1 and ClC3 [
52].
As pointed out above, H
2O
2 is the molecule needed to enable endosomal signaling. Accordingly, endosomes require spontaneous or enzyme-catalyzed dismutation of •O
2ˉ into H
2O
2. Indeed, in addition to intra-endosomal localization of cytosolic SOD1, extracellular superoxide dismutase (ecSOD/ SOD3) is internalized in BSA stimulated murine endothelial cells and co-localizes with early endosome antigen EEA1 [
53]. Interestingly, pH changes in the course of endosome progression may provide a feedback mechanism of SOD activity: Reduction of pH to or below 5 in late endosomes forces evasion of SOD1 from endosomes in human endothelial cells [
54]. Although it is likely that changes in pH also affects endosomal localization of NADPH oxidases, it remains elusive, whether or not they determine processing of early endosomes. NADPH oxidase Nox4 directly produces H
2O
2 and therefore Nox4 mediated signaling is independent from SOD. As Nox4 is mainly expressed in the ER [
55], it is likely that Nox4 stems from intracellular structures fused with early endosomes and accordingly may play a role in late endosomal signaling. In contrast activation of cluster of differentiation (CD)38 in early endosomes appears to be Nox4 dependent [
56]. More research is needed to define the role of Nox4 in endosomal transition and signaling.
3.2. Redox-Dependent EGFR Trans-Activation
In case of the EGF/EGFR, NADPH oxidases may only play a role in transactivation of the receptor (
Figure 5.1) [
57,
58]. For its endosomal signaling, quiescin sulfhydryl oxidase 1 (QSOX1) has been identified as a cellular pro-oxidant, which accelerates ubiquitination-mediated degradation of EGFR and its intracellular endosomal trafficking in hepatocellular carcinoma (HCC) cells [
59]. The longer version of human QSOX1 protein (hQSOX1a) is a transmembrane protein localized primarily to the Golgi apparatus [
60], while QSOX1b lacks the transmembrane helix and therefore is soluble, secreted and accumulates in extracellular fluids [
61]. Importantly, the enzyme produces hydrogen peroxide that can reduce thiols in proteins and forms disulfides. Another reactive oxygen species is superoxide anion (•O
2ˉ). It was suggested that •O
2ˉ and Clˉ are transported through DIDS-sensitive chloride channel(s) out of early endosomes, where •O
2ˉ is converted into H
2O
2 by superoxide dismutase (SOD1). H
2O
2 then enables further signaling outside of the endosome [
62]. Such mechanism would define endosomes as internal sources of ROS, and suggests •O
2ˉ-formation to occur within endosomes. Both cellular and endosomal ROS activate c-Src and Matrix-Metalloproteases (MMP) followed by ligand shedding of EGFR ligands. This EGFR
trans-activation leads as a feed forward mechanism to further ROS creation by Nox. As a result, specific signals are generated for proliferation, neointima formation etc. [
63,
64]
Together, the data indicate a role of H2O2 at least in early endosome signaling, which is terminated by a pH dependent feedback mechanism in the course of endosome progression.
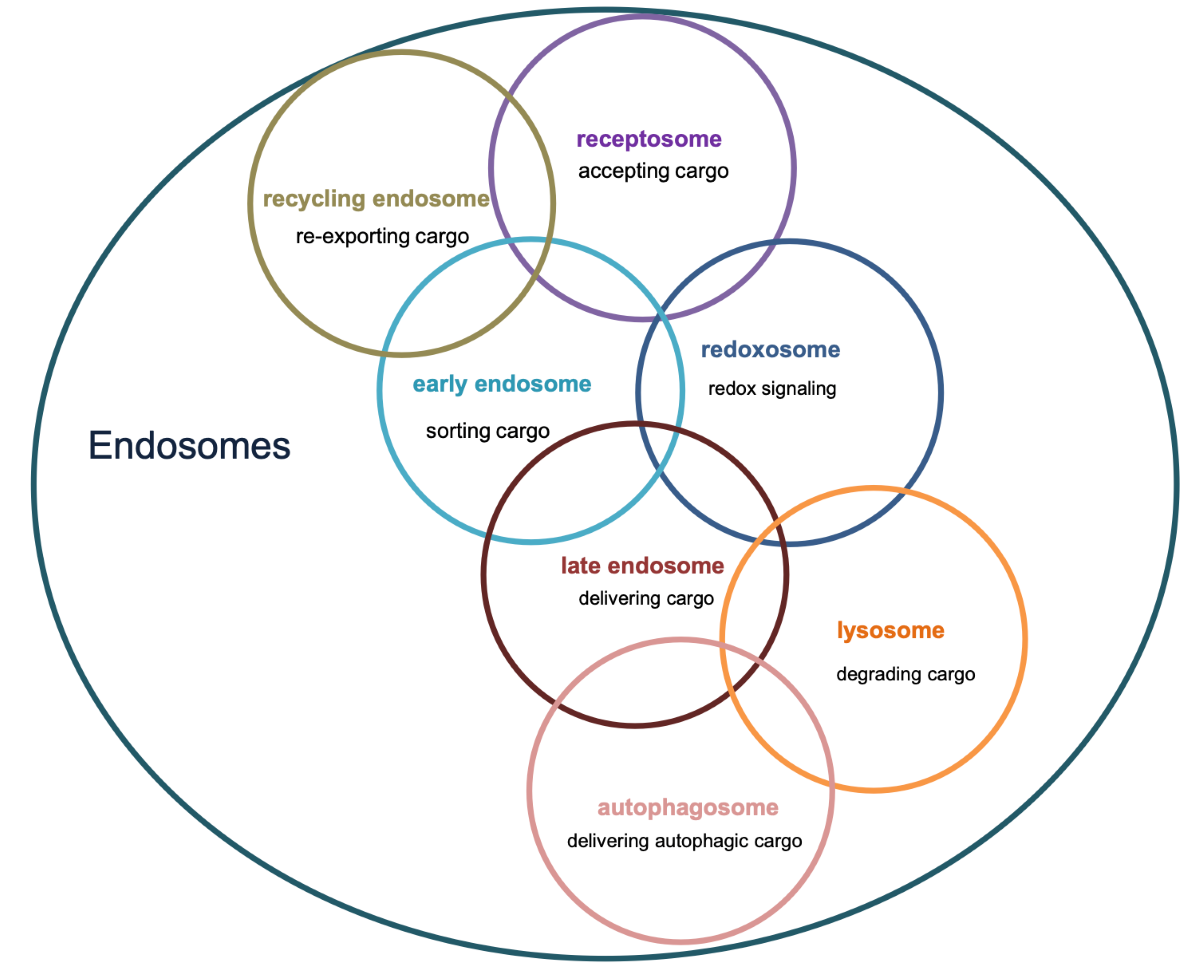
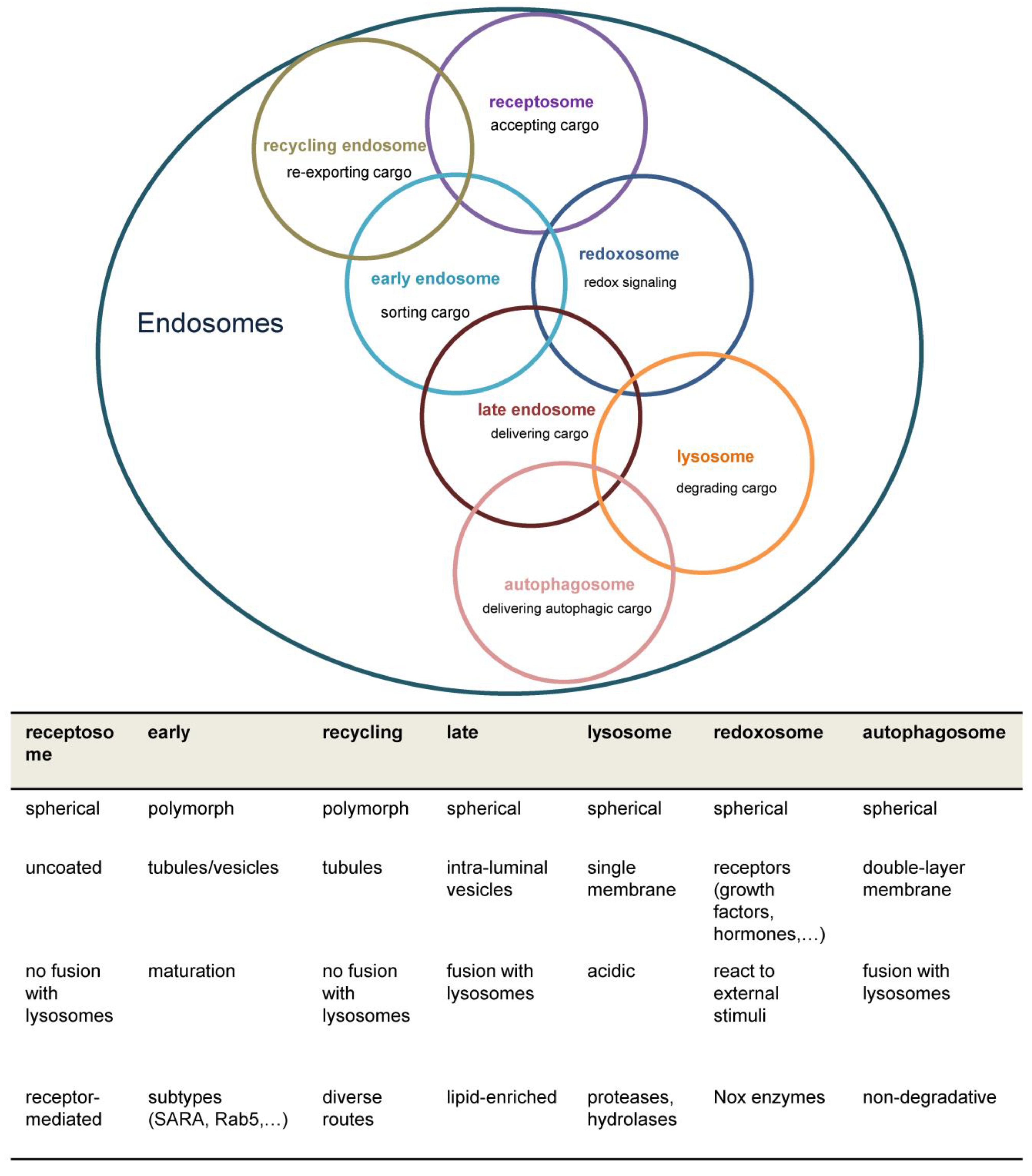
Figure 1.
Overview of major endosomal subtypes and their charactericstic features. Nox=NADPH oxidase, ROS= Reacitve oxygen species , SARA=Smad anchor for receptor activation.
Figure 1.
Overview of major endosomal subtypes and their charactericstic features. Nox=NADPH oxidase, ROS= Reacitve oxygen species , SARA=Smad anchor for receptor activation.
Figure 2.
Structure of the early endosome. Early endosomes display a dynamic, pleiomorphic appearance with a cis site for incoming cargo and a trans region with tubular and multivesicular structures which differ in their pH according to the destination of cargo either for recycling or degradation. The most important Rab proteins and exemplarily one of their effectors are displayed. Cargo exits the early endosome for further intracellular trafficking. Early endosomes are connected to both the microtubule organizing centre (MTOC) of the cytoskeleton through annexins and to several motor proteins which allows movement of Early endosomes themselves and cargo delivery. The endosomal pH gradient is indicated by color. ACAP2= ArfGAP with coiled-coil, ankyrin repeat and PH domains 2, APPL= Adaptor protein, phosphotyrosine interacting with PH domain and leucine zipper 1, EEA1=Early endosomal antigen 1, CRIM1= Cysteine-rich motor neuron 1, MICALL= Mical-like protein , OCRL= Inositol polyphosphate 5-phosphatase, Rab= Ras-related protein, RUFY1= RUN and FYVE domain-containing protein 1.
Figure 2.
Structure of the early endosome. Early endosomes display a dynamic, pleiomorphic appearance with a cis site for incoming cargo and a trans region with tubular and multivesicular structures which differ in their pH according to the destination of cargo either for recycling or degradation. The most important Rab proteins and exemplarily one of their effectors are displayed. Cargo exits the early endosome for further intracellular trafficking. Early endosomes are connected to both the microtubule organizing centre (MTOC) of the cytoskeleton through annexins and to several motor proteins which allows movement of Early endosomes themselves and cargo delivery. The endosomal pH gradient is indicated by color. ACAP2= ArfGAP with coiled-coil, ankyrin repeat and PH domains 2, APPL= Adaptor protein, phosphotyrosine interacting with PH domain and leucine zipper 1, EEA1=Early endosomal antigen 1, CRIM1= Cysteine-rich motor neuron 1, MICALL= Mical-like protein , OCRL= Inositol polyphosphate 5-phosphatase, Rab= Ras-related protein, RUFY1= RUN and FYVE domain-containing protein 1.
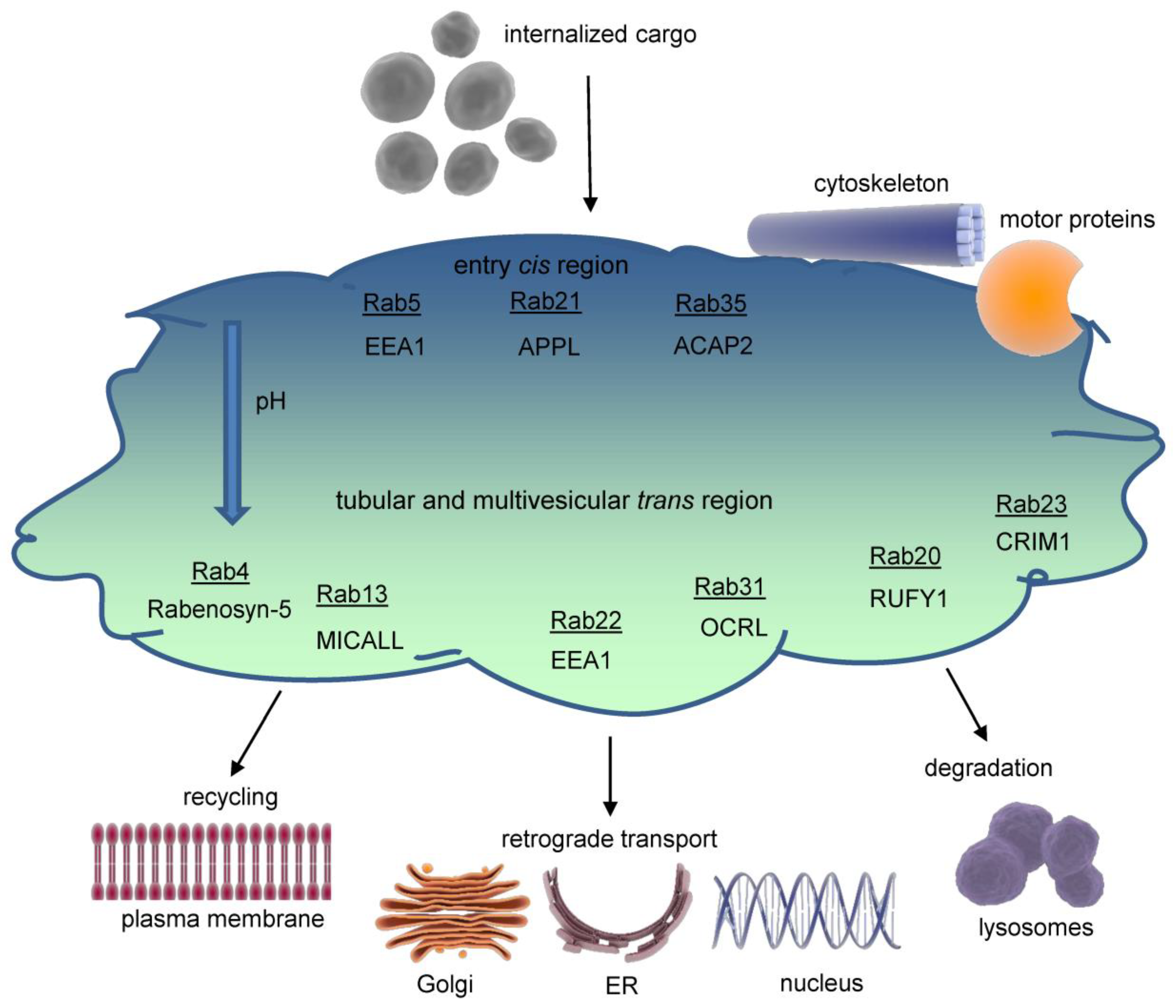
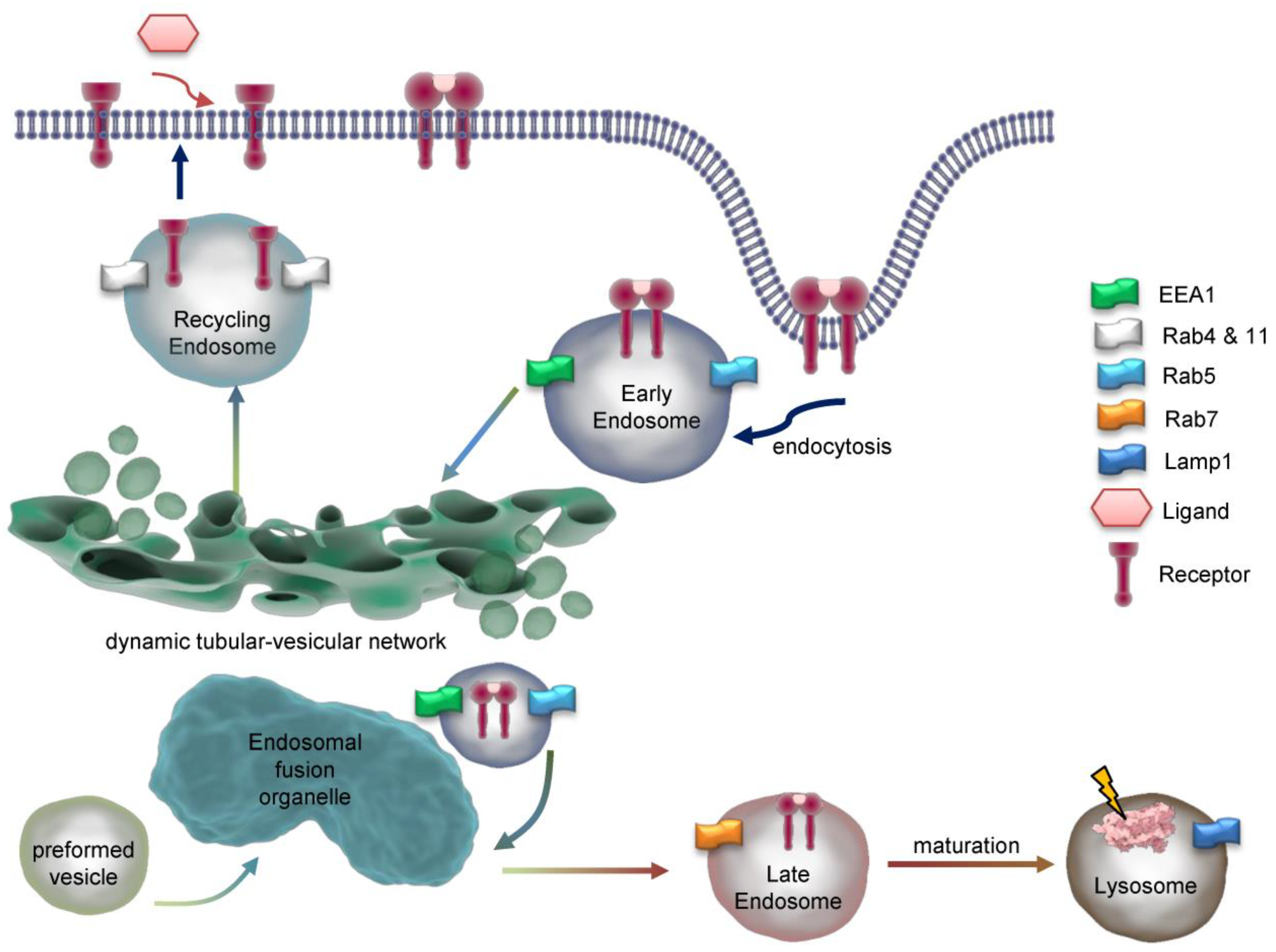
Figure 3.
Dynamics and maturation of endosomes. Exemplarily, endocytosis of a dimeric receptor after ligand binding is shown. At early endosomes, the receptor is sorted for recycling or degradation and travels through the endosomal network. Key marker proteins of endsomal compartments are indicated. EEA1=Early endosome antigen 1, Rab=Ras-related protein, Lamp1=Lysosomal associated membrane protein 1.
Figure 3.
Dynamics and maturation of endosomes. Exemplarily, endocytosis of a dimeric receptor after ligand binding is shown. At early endosomes, the receptor is sorted for recycling or degradation and travels through the endosomal network. Key marker proteins of endsomal compartments are indicated. EEA1=Early endosome antigen 1, Rab=Ras-related protein, Lamp1=Lysosomal associated membrane protein 1.
Figure 4.
EGF and MG8 receptor trafficking in detail. Clathrin-mediated endocytosis of EGFR and intracellular trafficking of M6RR are shown. EGFR may be translocated to other cellular compartments like nucleoplasm or mitochondria (not shown). No claim to completeness of molecules showed. 1) Recruting of major adaptor proteins to EGFR during internalization. The activated receptor dimers are internalized by various pathways depending on ligand type and concentration. The adapter protein Grb2 recruits the ubiquitin ligase Cbl to EGFR causing mono- or polyubiquitination. Multiple Ub-binding proteins interact with the ubiquitinated EGFR 2) Association of recycling machinery. Association of Rab4,11,13,35 leads to direct or indirect recycling via the endosomal recycling complex. Deubiquitinating enzymes replenish the free Ub pool. 3) Lysosomal targeting of EGFR. Dephosphorylation of EGF receptor tyrosine kinases by PTPs terminates signaling and promotes formation of intraluminal vesicles / multivesicular bodies. EGFR interaction with Hrs and recruitment of ESCRT complex is necessary for the late endosomal-lysosomal route. AMSH=Associated Molecule with the SH3 domain of STAM, Cbl=Casitas B-lineage Lymphoma protein, CIN-85=Cbl-interacting protein, Eps= Epidermal Growth Factor Receptor Substrate 15, ESCRT=Endosomal Sorting Complex Required for Transport, Hrs=Hepatocyte Growth Factor- Regulated Tyrosine Kinase, PTP1B=Phosphotyrosinephosphatase 1 B, SNX= Sorting Nexin, STAM=Signal Transducing Adaptor Molecule Substrate, Tsg101=Tumor Susceptibility Gene 101, Ub=Ubiquitin,WASH= Wiskott-Aldrich syndrome protein and SCAR homolog.
Figure 4.
EGF and MG8 receptor trafficking in detail. Clathrin-mediated endocytosis of EGFR and intracellular trafficking of M6RR are shown. EGFR may be translocated to other cellular compartments like nucleoplasm or mitochondria (not shown). No claim to completeness of molecules showed. 1) Recruting of major adaptor proteins to EGFR during internalization. The activated receptor dimers are internalized by various pathways depending on ligand type and concentration. The adapter protein Grb2 recruits the ubiquitin ligase Cbl to EGFR causing mono- or polyubiquitination. Multiple Ub-binding proteins interact with the ubiquitinated EGFR 2) Association of recycling machinery. Association of Rab4,11,13,35 leads to direct or indirect recycling via the endosomal recycling complex. Deubiquitinating enzymes replenish the free Ub pool. 3) Lysosomal targeting of EGFR. Dephosphorylation of EGF receptor tyrosine kinases by PTPs terminates signaling and promotes formation of intraluminal vesicles / multivesicular bodies. EGFR interaction with Hrs and recruitment of ESCRT complex is necessary for the late endosomal-lysosomal route. AMSH=Associated Molecule with the SH3 domain of STAM, Cbl=Casitas B-lineage Lymphoma protein, CIN-85=Cbl-interacting protein, Eps= Epidermal Growth Factor Receptor Substrate 15, ESCRT=Endosomal Sorting Complex Required for Transport, Hrs=Hepatocyte Growth Factor- Regulated Tyrosine Kinase, PTP1B=Phosphotyrosinephosphatase 1 B, SNX= Sorting Nexin, STAM=Signal Transducing Adaptor Molecule Substrate, Tsg101=Tumor Susceptibility Gene 101, Ub=Ubiquitin,WASH= Wiskott-Aldrich syndrome protein and SCAR homolog.
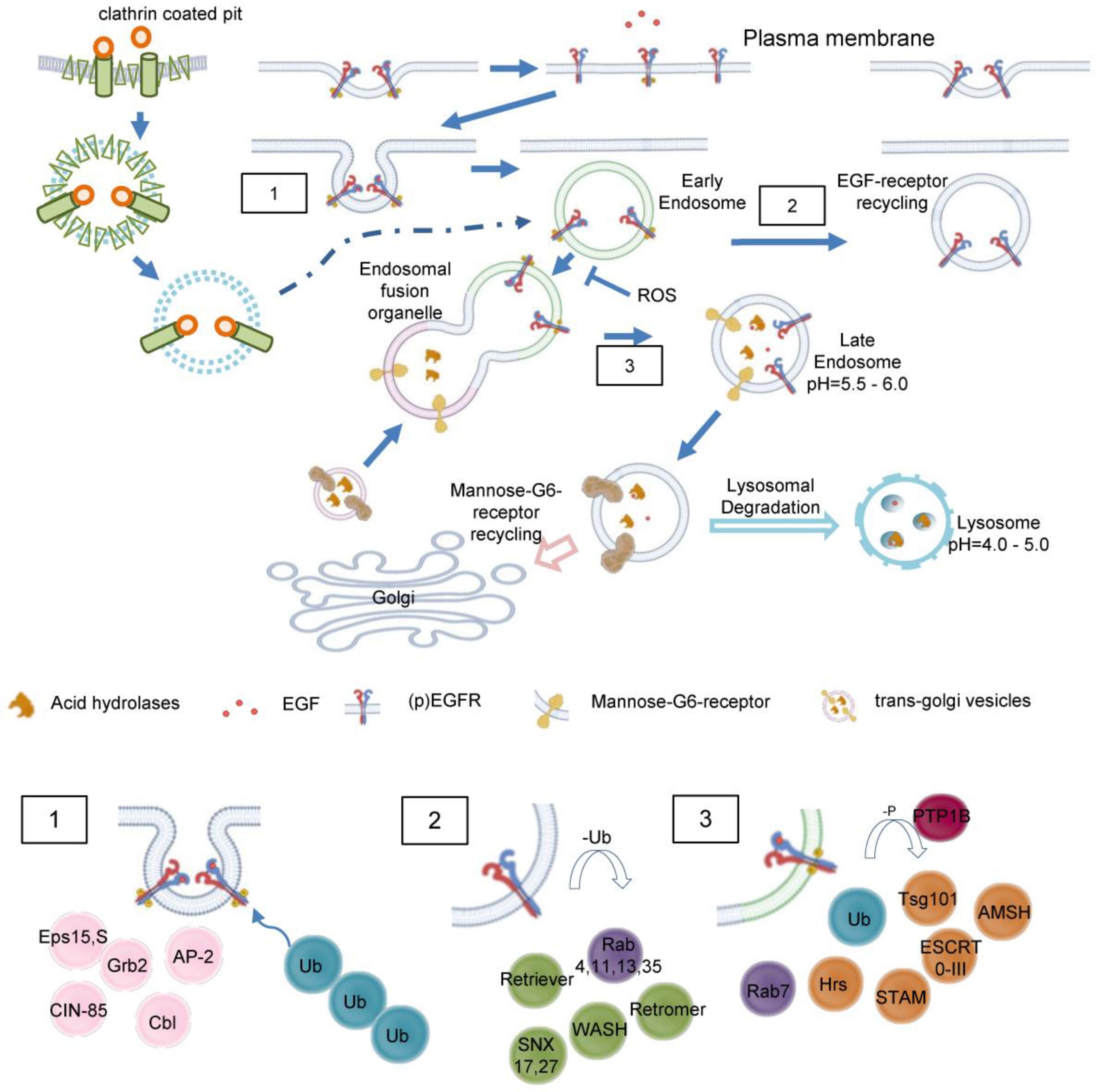
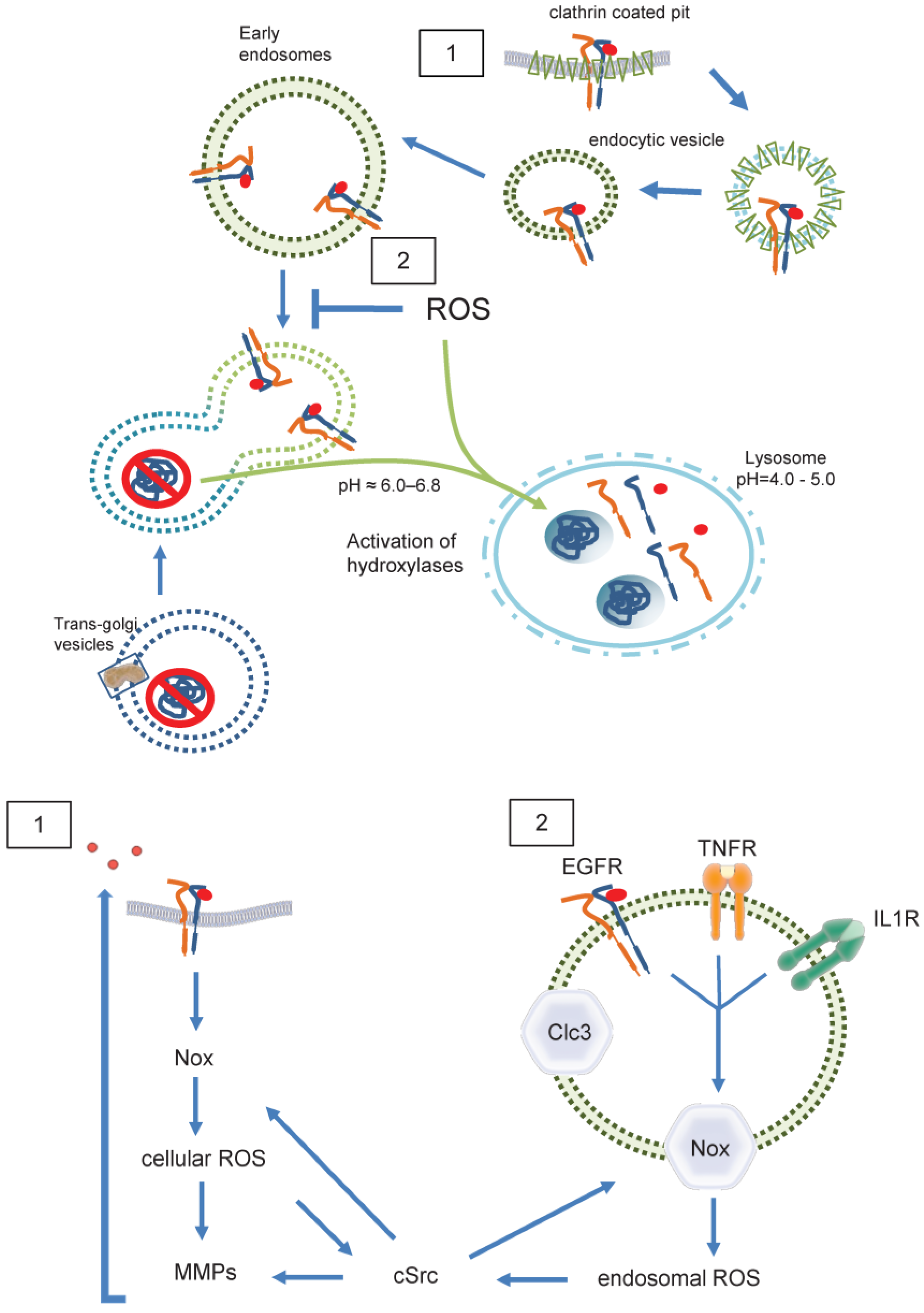
Figure 5.
ROS in endosomal sorting and signaling. Redox signaling is initiated by EGFR 1) at the plasma membrane 2) inside of endosomes. Redox-active endosomes (Redoxosomes) harbor Nox enzymes producing ROS downstream of several signaling cascades. Both cellular and endosomal ROS activate c-Src and MMPs followed by shedding of EGFR ligands. This EGFR trans-activation leads as a feed forward mechanism to further ROS creation by Nox. Termination of the ROS signaling by dismutation or other means is not shown. Clc3 provides charge neutralization. Clc3= Cloride channel 3, cSrc= cellular Sarcoma kinase, EGFR=Epidermal Growth Factor Receptor, IL1R=Interleukin 1 receptor, MMP= Matrixmetalloprotease, Nox= NADPH oxidase, ROS=Reactive oxygen species, TNFR=Tumor necrosis factor receptor.
Figure 5.
ROS in endosomal sorting and signaling. Redox signaling is initiated by EGFR 1) at the plasma membrane 2) inside of endosomes. Redox-active endosomes (Redoxosomes) harbor Nox enzymes producing ROS downstream of several signaling cascades. Both cellular and endosomal ROS activate c-Src and MMPs followed by shedding of EGFR ligands. This EGFR trans-activation leads as a feed forward mechanism to further ROS creation by Nox. Termination of the ROS signaling by dismutation or other means is not shown. Clc3 provides charge neutralization. Clc3= Cloride channel 3, cSrc= cellular Sarcoma kinase, EGFR=Epidermal Growth Factor Receptor, IL1R=Interleukin 1 receptor, MMP= Matrixmetalloprotease, Nox= NADPH oxidase, ROS=Reactive oxygen species, TNFR=Tumor necrosis factor receptor.
Table 1.
Classification of receptor mediated signaling.
Table 1.
Classification of receptor mediated signaling.
| Type I signaling: |
Ligand binding to the receptor results in direct signal transduction |
e.g. Tyrosinkinase receptors |
| Type II signaling |
Upon binding of the ligand, second messengers mediate signal transduction |
e.g. Adrenalin β-receptors |
| Type III signaling |
Binding of the ligand to its receptor induces internalization and formation of endosomes, which transduced the appropriate signaling cascade |
e.g. EGF-receptor |
| IIIA |
recruitment of cytosolic proteins to the receptor, followed by internalization and endosome formation |
e.g. EGF- and TGFβ-receptors |
| IIIB |
Fusion of the endosome with other intracellular vesicles |
e.g. TNFα-receptor fuses with the Golgi to transduce apoptosis signals |