Submitted:
24 September 2024
Posted:
24 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. NPS in AD
| NPS | Category | General patients | AD patients |
|---|---|---|---|
| Depression | Prevalence | 5% ~ 8.3% | 14.8% ~ 40% |
| Symptoms | Feeling sad, irritable, empty, disrupted sleep, hopelessness about the future, poor concentration, weight change, low energy, excessive guilt or low self-worth | Persistent feelings of worthlessness, excessive/inappropriate guilt, a pervasive loss of interest or pleasure in all activities | |
| Treatment | SSRIs (citalopram, fluoxetine, paroxetine, sertraline), SNRIs (duloxetine, vanlafaxine, levomilnacipran), Atypical antidepressants (bupropion, mirtazapine, trazodone, vortioxetine), TCAs (imipramine, desipramine, amitriptyline), MAOIs (selegiline,), | SSRIs (fluoxetine, paroxetine, sertraline, citalopram, escitalopram) | |
| Side effects | Nausea and vomiting, agitation, anxiety, indigestion, diarrhea or constipation, loss of appetite and weight loss, dizziness, blurred vision, dry mouth, excessive sweating, insomnia or drowsiness, headaches, and sexual side effects. | No significant effects | |
| Anxiety | Prevalence | 4% | 39% |
| Symptoms | Trouble concentrating or making decisions, feeling irritable, tense or restless, nausea or abdominal distress, heart palpitations, sweating, trembling or shaking, sleep problem | Excessive worry, fear, restlessness, and irritability | |
| Treatment | Benzodiazepines (alprazolam, diazepam, lorazepam), SSRIs, buspirone, TCAs (imipramine, clomipramine), MAOIs | SSRIs, SNRIs (venlafaxine), serotonergic atypical anxiolytics (buspirone), citalopram | |
| Side effects | Problems with balance and memory, drowsiness, confusion, vision problems, headaches, feeling of depression, dizziness, nausea, constipation, urinary retention, constipation | Abnormal bleeding, seizures, headaches, nausea, sleep trouble | |
| Apathy | Prevalence | 26% ~ 82% | 49% |
| Symptoms | Feeling flat, blunted, or numb emotionally, Lack of emotional reaction, low energy and motivation, lack of goal setting, less interest in pleasure activities, hobbies and relationships, anhedonia, lethargy | A decline in motivation and interest across emotional, goal-directed behavior, and cognitive activity | |
| Treatment | Antidepressants (trazodone, deprenyl, fluvoxamine), psychostimulants (methylphenidate, amphetamine), antipsychotics (risperidone), Acetylcholinesterase inhibitors (donepezil, rivastigmine), NMDA receptor antagonist(memantine) | Cholinesterase inhibitors (donepezil, galantamine, rivastigmine), methylphenidate, Ginkgo biloba, modafinil, and SSRIs | |
| Side effects | Modest weight loss, no change of depression score | Weight loss and increased anxiety, no improvement, high blood pressure, cough and osteoarticular pain | |
| Agitation &Aggression | Prevalence | 1% - 30% | 30% ~ 60% |
| Symptoms | Road rage, child abuse, sexual abuse, and domestic violence, verbally (swearing, shouting or threatening), Physically (hitting, punching, scratching or biting) | Emotional distress, excessive psychomotor activity, aggressive behaviors, disruptive irritability, and disinhibition | |
| Treatment | Haloperidol, aripiprazole, droperidol, olanzapine, | Valproic acid, brexpiprazole, Carbamazepine, SSRIs | |
| Side effects | Dizziness and nausea, paradoxical excitation, constipation, dry mouth, problems sleeping | Hepatotoxicity, GI upset, thrombocytopenia, coagulopathies, metabolic disorders, worsening cognitive dysfunction, agranulocytosis, cardiac arrhythmias | |
| Psychosis | Prevalence | 1.5% ~ 3.5% | 50% |
| Symptoms | Delusions, hallucinations, disorganized thought and behavior, poverty of speech, lack of energy, anhedonia, psychomotor retardation, catatonia | Delusions, hallucinations, disorganized thought and speech | |
| Treatment | Antipsychotics (clozapine and olanzapine), Benzodiazepines | Aripiprazole, risperidone, quetiapine, brexpiprazole | |
| Side effects | Drowsiness, dizziness, dry mouth, blurred vision, tiredness, nausea, constipation, weight gain, trouble sleeping or muscle or nervous system problems (anxiety, agitation, jitteriness, drooling, trouble swallowing, restlessness, shaking or stiffness) | Stroke, myocardial infarction | |
| Sleep disturbances | Prevalence | 20% ~ 41.7% | 45% |
| Symptoms | Excessive daytime sleepiness, irregular breathing or increased movement during sleep, depression, weight gain, lack of concentration, daytime fatigue, irritability, anxiety | Insomnia, excessive daytime sleepiness, nighttime awakenings, and alterations in sleep-wake patterns | |
| Treatment | Melatonin, zolpidem, zaleplon, eszopiclone, ramelteon, suvorexant, lamborexant or doxepin. | Melatonin, trazodone, suvorexant, lemborexant | |
| Side effects | Changes in appetite, constipation or diarrhea, dizziness, headache, daytime drowsiness, heartburn, stomach pain, burning or tingling in the hands, arms, feet or legs, mental impairment | Various results, unclear effects | |
| Social processing | Prevalence | 7% ~ 11% | 30-50%, |
| Symptoms | Difficulty using appropriate greetings, changing language and communication style, telling and understanding stories, engaging in conversation, repairing communication breakdowns, using appropriated verbal and nonverbal signals, interpreting the verbal and nonverbal signals of others, making inferences, and forming and maintaining close relationships | Language problems, personality changes and irritability | |
| Treatment | Behavior interventions, social communication treatments (comic strip conversations), social communication intervention, online speech therapy, social skills strengthening activities | N-acetyl cysteine, BDNF, NGF |
2.1. Depression
2.2. Anxiety
2.3. Apathy
2.4. Agitation and Aggression
2.5. Psychosis
2.6. Sleep Disturbances
2.7. Social Processing
2.8. Implication from the NPS in AD
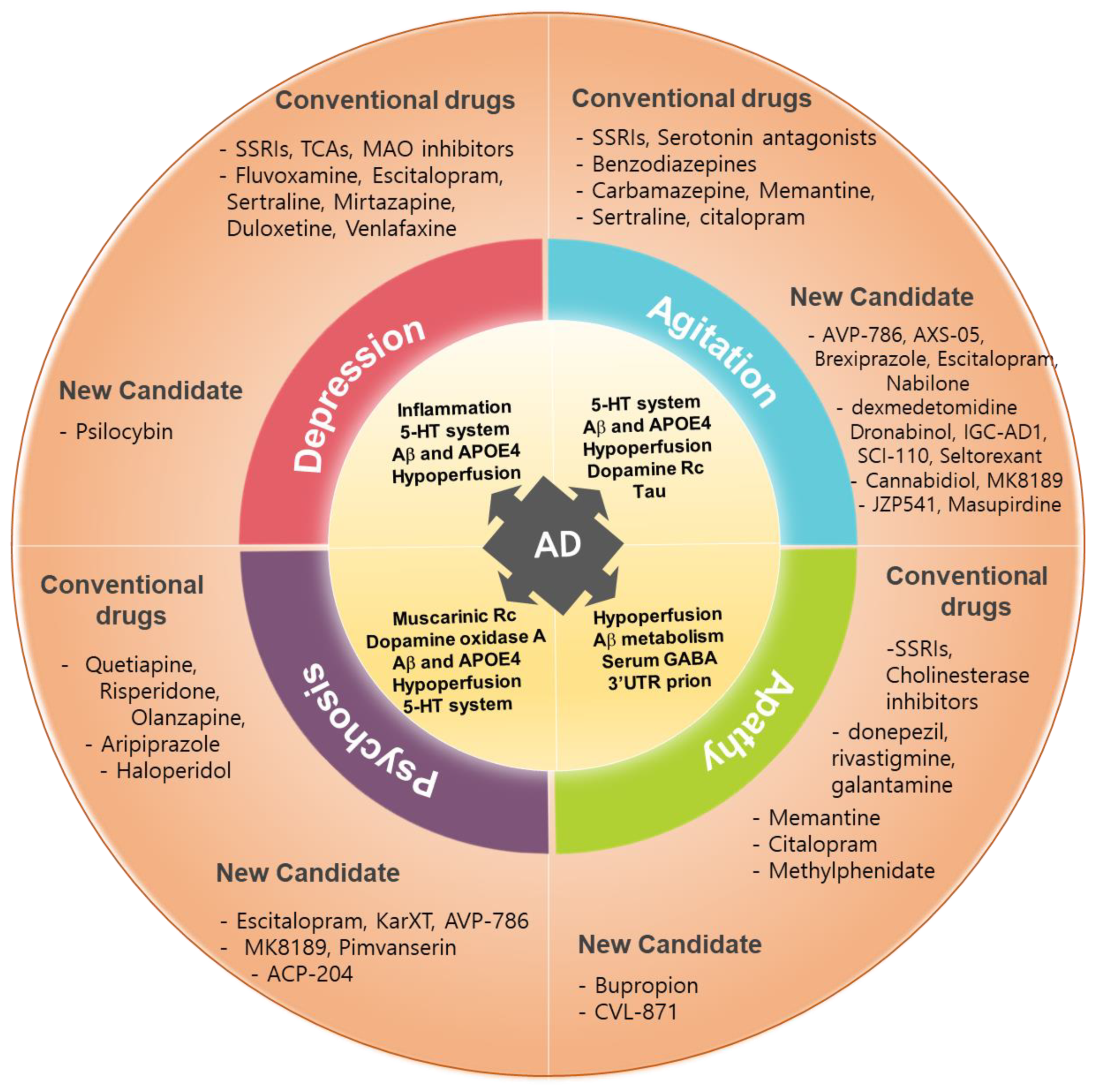
3. Treatment Pipelines, Targets, and Mechanism of Actions
| Candidate drugs | Target | Mechanism of Action | Target NPS | Disease stage of AD | Clinical status | References |
|---|---|---|---|---|---|---|
| Lumateperone (Caplyta) | Neurotransmitter receptors | A 5-HT2A antagonist, a SERT inhibitor, DRD2 antagonist, a GluN2B modulator | Depression | [71,72,73,74] | ||
| Psilocybin | Neurotransmitter receptors | A modulator of 5-HT1A, 5-HT2A and 5-HT2C receptors | Prodormal/Prodromal-Mild | Phase 1 | [75,76,77] | |
| Cannabidiol | Neurotransmitter receptors | Cannabinoid, An agonist against 5-HT1A, A2A and TRP-V1 receptors, Anti-inflammatory action |
Anxiety | Mild-Moderate dementia Prodormal/Prodromal-Mild |
Phase 1 Phase 2 |
[78,79] |
| Dextromethorphan | Neurotransmitter receptors | NMDA receptor antagonist; σ-1 receptor agonist A SERT and NET inhibitor A nicotinic σ3β4 receptor antagonist |
Agitation | [11,80,81,82,83,84] | ||
| AVP-923 (Neudexta) | Neurotransmitter receptors | dextromethorphan/ quinidine |
Mild-Moderate dementia Severe dementia |
[83,85,86] | ||
| AXS-05 | Neurotransmitter receptors | dextromethorphan/ bupropion NMDA receptor antagonist; σ-1 receptor agonist A SERT and NET inhibitor |
Mild-Moderate dementia Severe dementia |
Phase 3 | [87,88,89] | |
| Nabilone | Cannabinoid receptor | A partial agonist against CB1 and CB2 receptor | Mild-Moderate dementia Severe dementia |
Phase 3 | [90,91] | |
| Brexpiprazole | Neurotransmitter receptors | A partial agonist against D2, D3 receptor and 5-HT1A. A serotonin and dopamine modulator |
Mild-Moderate dementia Severe dementia |
[38,92,93] | ||
| AVP-786 | Neurotransmitter receptors | NMDA receptor antagonist; σ-1 receptor agonist A SERT and NET inhibitor |
Mild-Moderate dementia Severe dementia |
Phase 3 | [12,94] | |
| Dexmedetomidine | Neurotransmitter receptors | An α2 adrenergic agonist | Mild-Moderate dementia Severe dementia |
Phase 3 | [95,96,97] | |
| JZP541 | Cannabinoid receptor | An agonist against CB1 and CB2 receptor | Mild-Moderate dementia Severe dementia |
Phase 2 | [12] | |
| Dronabinol | Cannabinoid receptor | A weak partial agonist against CB1 and CB2 receptor | Mild-Moderate dementia | Phase 2 | [98,99,100] | |
| IGC-AD1 | Cannabinoid receptor | Cannabinoid, A partial agonist against CB1 receptor |
Mild-Moderate dementia Severe dementia |
Phase 2 | [12,79] | |
| Prazosin | Neurotransmitter receptors | An α1 adrenergic antagonist | Phase 2b | [101,102] | ||
| SCI-110 | Neurotransmitter receptors | Tetrahydrocannabinol and palmitoylethanolamide | Mild-Moderate dementia | Phase 2 | [103,104] | |
| THC-Free CBD | Cannabinoid receptor | An agonist against CB1 and CB2 receptor | Mild-Moderate dementia Severe dementia |
Phase 2 | [105,106] | |
| Masupirdine | Neurotransmitter receptors | A 5-HT6 receptor antagonist | Mild-Moderate dementia | Phase 3 | [107,108,109,110] | |
| MK-8189 | Phosphodiesterase | A PDE10a inhibitor | Mild-Moderate dementia | phase 1 | [111] | |
| Pimvanserin | Neurotransmitter receptors | A selective inverse agonist of the serotonin 5-HT2A receptor | Psychosis | Phase 3 | [43,112,113] | |
| ACP-204 | Neurotransmitter receptors | A potent and selective antagonist/inverse agonist of 5-HT2A receptor | Mild-Moderate dementia Severe dementia |
Phase 2 Phase 3 |
[11,12,79,114] | |
| KarXT | Cholinergic modulator | A dual M1/M4 muscarinic acetylcholine receptor agonist | Mild-Moderate dementia | Phase 3 | [115,116,117,118,119] | |
| Seltorexant | Orexin system | A selective antagonist of the orexin-2 receptor | Sleep disturbances | Mild-Moderate dementia | Phase 2 | [120,121] |
| SLV | Neurotransmitter receptors | A selective 5-HT6 receptor antagonist | Social processing | [122] | ||
| N-acetyl cysteine | Redox system | An antioxidant and glutathione inducer | [67] | |||
| BDNF | Neurotrophic factor | A member of the neurotrophin family, TrkB activation | [61,68,69] | |||
| Oxytocin | Endocrine system | a nonapeptide hormone, oxytocin receptor activation | [123,124,125] |
3.1. An Antagonist of NMDA Receptor Dextromethorphan
3.1.1. AVP-923
3.1.2. AVP-786
3.1.3. AXS-05
3.2. An Inverse Agonist and Antagonist 5-HT2A Receptor
3.2.1. Pimavanserin
3.2.2. ACP-204
3.3. A Partial Agonist of Dopamine D2 Receptor and 5-HT1A Receptor
3.3.1. Brexpiprazole
3.4. An Antagonist of 5-HT2A and Dopamine (D1, D2 and D4) Receptor
3.4.1. Lumateperone
3.5. Glutamate Receptor Modulator and an Inositol Monophosphatase Inhibitor
3.5.1. Lithium
3.6. Norepinephrine Modulators
3.6.1. Prazosin
3.7. Cannabinoid Receptors
3.7.1. Nabilone
3.7.2. Dronabinol,
3.7.3. JZP541
3.7.4. IGC-AD1
3.7.5. SCI
3.7.6. While Cannabidiol (CBD)
3.8. Cholinergic Modulators
3.8.1. KarXT
3.9. 5-HT6 Receptor Antagonists
3.10. Orexin-2 Receptor Antagonists
3.11. An Agonist of Alpha 2 Adrenergic Receptor
3.12. PDE10 Inhibitors
3.13. Psychedelic Compounds
3.13.1. Psilocybin
3.14. Antioxidants and Anti-Inflammatory Drugs
3.15. Social Deficits in AD
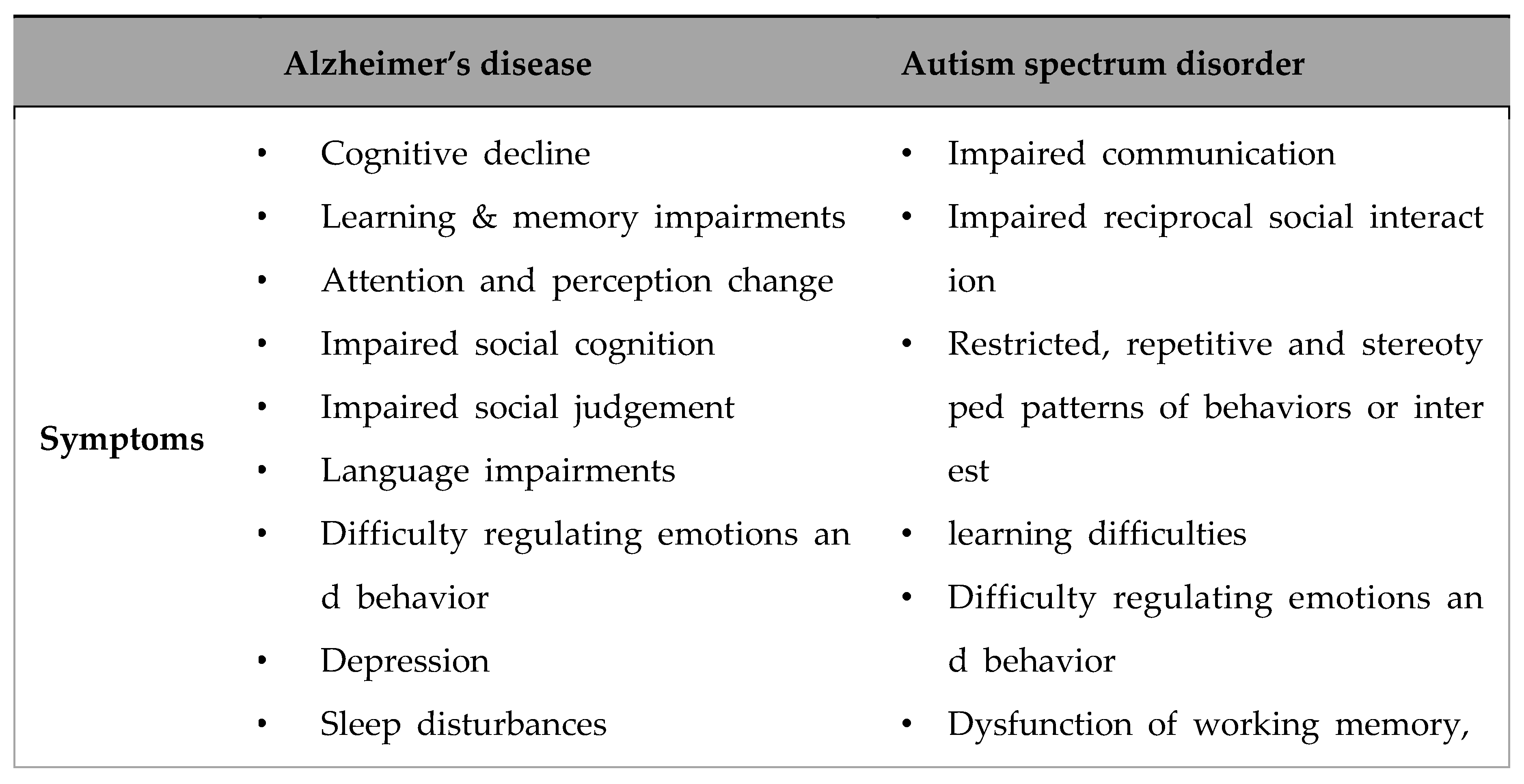
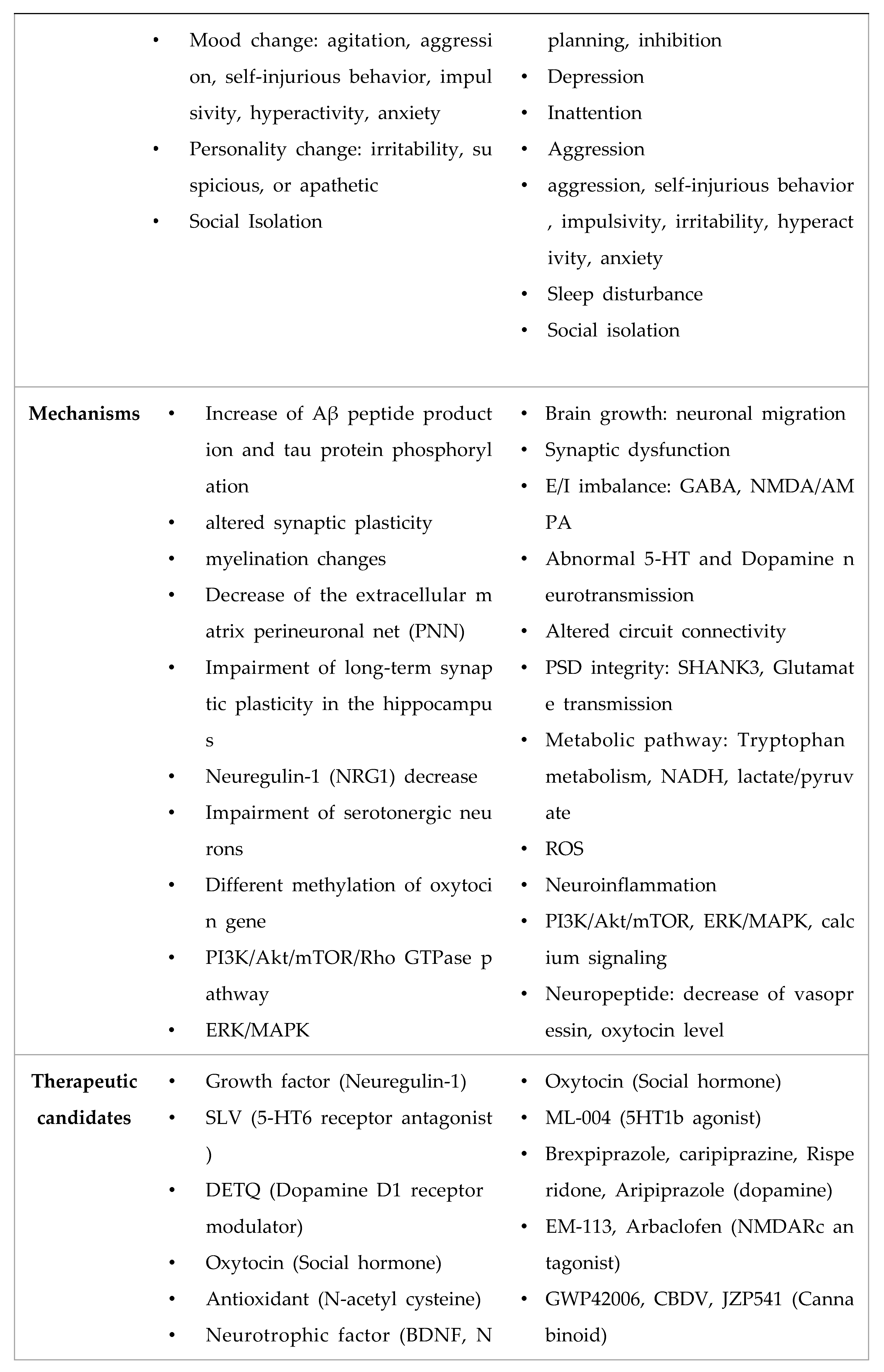
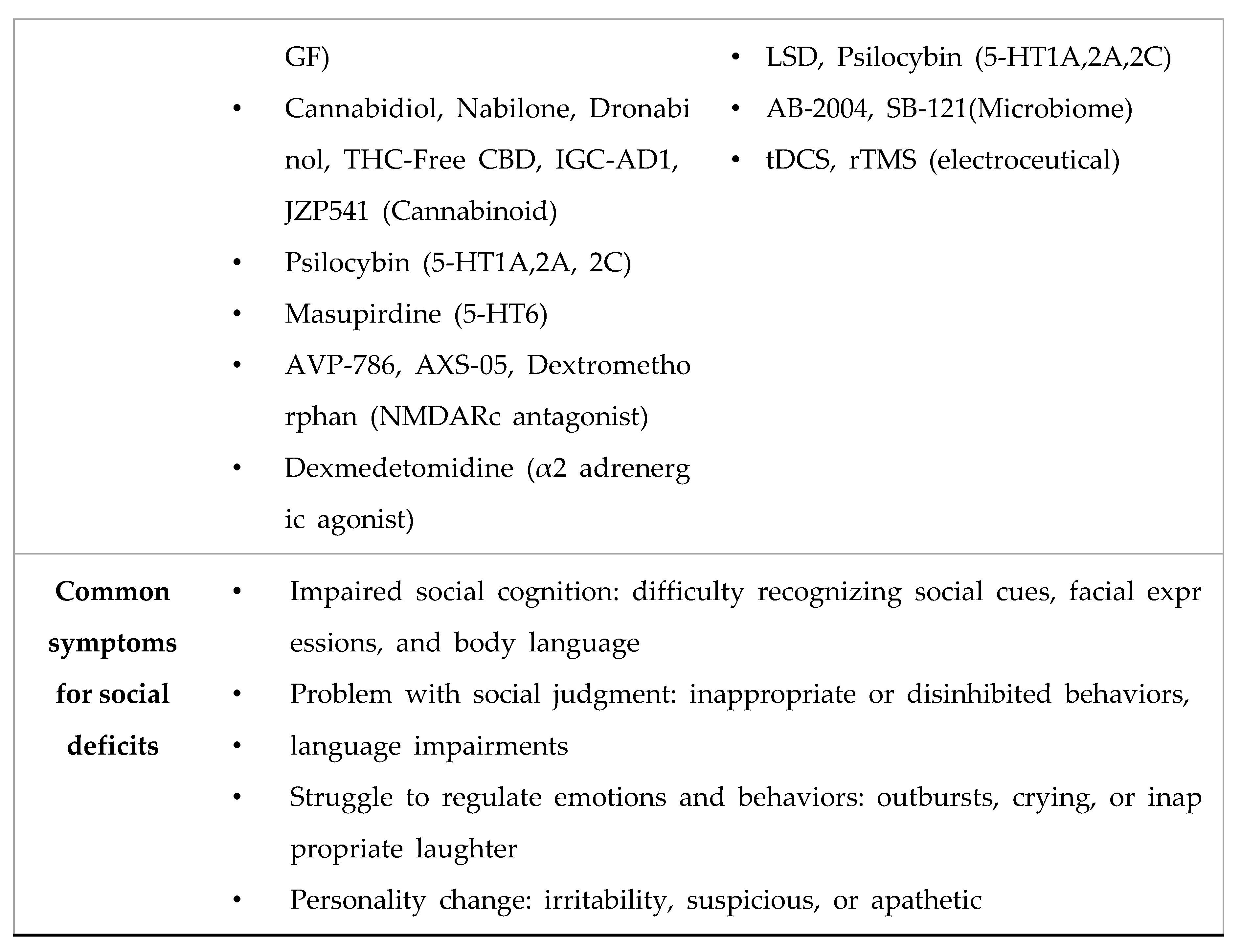
4. Conclusion
Acknowledgments
References
- Tahami Monfared, A.A.; Byrnes, M.J.; White, L.A.; Zhang, Q. The Humanistic and Economic Burden of Alzheimer’s Disease. Neurol Ther 2022, 11, 525–551. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G.; Steinberg, M.; Tschanz, J.T.; Norton, M.C.; Steffens, D.C.; Breitner, J.C. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry 2000, 157, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Jost, B.C.; Grossberg, G.T. The evolution of psychiatric symptoms in Alzheimer’s disease: a natural history study. J Am Geriatr Soc 1996, 44, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G.; Carrillo, M.C.; Ryan, J.M.; Khachaturian, A.S.; Trzepacz, P.; Amatniek, J.; Cedarbaum, J.; Brashear, R.; Miller, D.S. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement 2011, 7, 532–539. [Google Scholar] [CrossRef]
- Avitan, I.; Halperin, Y.; Saha, T.; Bloch, N.; Atrahimovich, D.; Polis, B.; Samson, A.O.; Braitbard, O. Towards a Consensus on Alzheimer’s Disease Comorbidity? J Clin Med 2021, 10. [Google Scholar] [CrossRef]
- Sinyor, B.; Mineo, J.; Ochner, C. Alzheimer’s Disease, Inflammation, and the Role of Antioxidants. J Alzheimers Dis Rep 2020, 4, 175–183. [Google Scholar] [CrossRef]
- Butler, L.M.; Houghton, R.; Abraham, A.; Vassilaki, M.; Duran-Pacheco, G. Comorbidity Trajectories Associated With Alzheimer’s Disease: A Matched Case-Control Study in a United States Claims Database. Front Neurosci 2021, 15, 749305. [Google Scholar] [CrossRef]
- Olazaran, J.; Carnero-Pardo, C.; Fortea, J.; Sanchez-Juan, P.; Garcia-Ribas, G.; Vinuela, F.; Martinez-Lage, P.; Boada, M. Prevalence of treated patients with Alzheimer’s disease: current trends and COVID-19 impact. Alzheimers Res Ther 2023, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, A.J.; Suemoto, C.K.; Franca Resende, E.P.; Petersen, C.; Leite, R.E.P.; Rodriguez, R.D.; Ferretti-Rebustini, R.E.L.; You, M.; Oh, J.; Nitrini, R.; et al. Neuropathologic Correlates of Psychiatric Symptoms in Alzheimer’s Disease. J Alzheimers Dis 2018, 66, 115–126. [Google Scholar] [CrossRef]
- Lanctot, K.L.; Amatniek, J.; Ancoli-Israel, S.; Arnold, S.E.; Ballard, C.; Cohen-Mansfield, J.; Ismail, Z.; Lyketsos, C.; Miller, D.S.; Musiek, E.; et al. Neuropsychiatric signs and symptoms of Alzheimer’s disease: New treatment paradigms. Alzheimers Dement (N Y) 2017, 3, 440–449. [Google Scholar] [CrossRef]
- Ajenikoko, M.K.; Ajagbe, A.O.; Onigbinde, O.A.; Okesina, A.A.; Tijani, A.A. Review of Alzheimer’s disease drugs and their relationship with neuron-glia interaction. IBRO Neurosci Rep 2023, 14, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s disease drug development pipeline: 2024. Alzheimers Dement (N Y) 2024, 10, e12465. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Hu, N.; Tan, M.S.; Yu, J.T.; Tan, L. Behavioral and psychological symptoms in Alzheimer’s disease. Biomed Res Int 2014, 2014, 927804. [Google Scholar] [CrossRef] [PubMed]
- Evans-Lacko, S.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Benjet, C.; Bruffaerts, R.; Chiu, W.T.; Florescu, S.; de Girolamo, G.; Gureje, O.; et al. Socio-economic variations in the mental health treatment gap for people with anxiety, mood, and substance use disorders: results from the WHO World Mental Health (WMH) surveys. Psychol Med 2018, 48, 1560–1571. [Google Scholar] [CrossRef]
- Pless, A.; Ware, D.; Saggu, S.; Rehman, H.; Morgan, J.; Wang, Q. Understanding neuropsychiatric symptoms in Alzheimer’s disease: challenges and advances in diagnosis and treatment. Front Neurosci 2023, 17, 1263771. [Google Scholar] [CrossRef]
- Asmer, M.S.; Kirkham, J.; Newton, H.; Ismail, Z.; Elbayoumi, H.; Leung, R.H.; Seitz, D.P. Meta-Analysis of the Prevalence of Major Depressive Disorder Among Older Adults With Dementia. J Clin Psychiatry 2018, 79. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.; Wang, C.; Jiang, T.; Zhu, X.C.; Yu, J.T.; Tan, L. The prevalence of depression in Alzheimer’s disease: a systematic review and meta-analysis. Curr Alzheimer Res 2015, 12, 189–198. [Google Scholar] [CrossRef]
- Daly, M.; Sutin, A.R.; Robinson, E. Depression reported by US adults in 2017-2018 and March and April 2020. J Affect Disord 2021, 278, 131–135. [Google Scholar] [CrossRef]
- Wang, J.; Yu, J.T.; Wang, H.F.; Meng, X.F.; Wang, C.; Tan, C.C.; Tan, L. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015, 86, 101–109. [Google Scholar] [CrossRef]
- Simic, G.; Babic Leko, M.; Wray, S.; Harrington, C.R.; Delalle, I.; Jovanov-Milosevic, N.; Bazadona, D.; Buee, L.; de Silva, R.; Di Giovanni, G.; et al. Monoaminergic neuropathology in Alzheimer’s disease. Prog Neurobiol 2017, 151, 101–138. [Google Scholar] [CrossRef]
- Orgeta, V.; Tabet, N.; Nilforooshan, R.; Howard, R. Efficacy of Antidepressants for Depression in Alzheimer’s Disease: Systematic Review and Meta-Analysis. J Alzheimers Dis 2017, 58, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Dudas, R.; Malouf, R.; McCleery, J.; Dening, T. Antidepressants for treating depression in dementia. Cochrane Database Syst Rev 2018, 8, CD003944. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.; Gatchel, J.; Bateman, D.R.; Barcelos-Ferreira, R.; Cantillon, M.; Jaeger, J.; Donovan, N.J.; Mortby, M.E. Affective and emotional dysregulation as pre-dementia risk markers: exploring the mild behavioral impairment symptoms of depression, anxiety, irritability, and euphoria. Int Psychogeriatr 2018, 30, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.F. The Relationship Between Anxiety and Alzheimer’s Disease. J Alzheimers Dis Rep 2021, 5, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Gulpers, B.; Ramakers, I.; Hamel, R.; Kohler, S.; Oude Voshaar, R.; Verhey, F. Anxiety as a Predictor for Cognitive Decline and Dementia: A Systematic Review and Meta-Analysis. Am J Geriatr Psychiatry 2016, 24, 823–842. [Google Scholar] [CrossRef]
- Santabarbara, J.; Lipnicki, D.M.; Olaya, B.; Villagrasa, B.; Bueno-Notivol, J.; Nuez, L.; Lopez-Anton, R.; Gracia-Garcia, P. Does Anxiety Increase the Risk of All-Cause Dementia? An Updated Meta-Analysis of Prospective Cohort Studies. J Clin Med 2020, 9. [Google Scholar] [CrossRef]
- Chen, L.; Sun, Y.; Li, J.; Liu, S.; Ding, H.; Wang, G.; Li, X. Assessing Cannabidiol as a Therapeutic Agent for Preventing and Alleviating Alzheimer’s Disease Neurodegeneration. Cells 2023, 12. [Google Scholar] [CrossRef]
- Drijgers, R.L.; Aalten, P.; Winogrodzka, A.; Verhey, F.R.; Leentjens, A.F. Pharmacological treatment of apathy in neurodegenerative diseases: a systematic review. Dement Geriatr Cogn Disord 2009, 28, 13–22. [Google Scholar] [CrossRef]
- Wolinsky, D.; Drake, K.; Bostwick, J. Diagnosis and Management of Neuropsychiatric Symptoms in Alzheimer’s Disease. Curr Psychiatry Rep 2018, 20, 117. [Google Scholar] [CrossRef]
- Costello, H.; Husain, M.; Roiser, J.P. Apathy and Motivation: Biological Basis and Drug Treatment. Annu Rev Pharmacol Toxicol 2024, 64, 313–338. [Google Scholar] [CrossRef]
- Fahed, M.; Steffens, D.C. Apathy: Neurobiology, Assessment and Treatment. Clin Psychopharmacol Neurosci 2021, 19, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ruthirakuhan, M.T.; Herrmann, N.; Abraham, E.H.; Chan, S.; Lanctot, K.L. Pharmacological interventions for apathy in Alzheimer’s disease. Cochrane Database Syst Rev 2018, 5, CD012197. [Google Scholar] [CrossRef]
- Kverno, K.S.; Rabins, P.V.; Blass, D.M.; Hicks, K.L.; Black, B.S. Prevalence and treatment of neuropsychiatric symptoms in advanced dementia. J Gerontol Nurs 2008, 34, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ravyts, S.G.; Perez, E.; Donovan, E.K.; Soto, P.; Dzierzewski, J.M. Measurement of aggression in older adults. Aggress Violent Behav 2021, 57. [Google Scholar] [CrossRef]
- Cummings, J.; Mintzer, J.; Brodaty, H.; Sano, M.; Banerjee, S.; Devanand, D.P.; Gauthier, S.; Howard, R.; Lanctot, K.; Lyketsos, C.G.; et al. Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. Int Psychogeriatr 2015, 27, 7–17. [Google Scholar] [CrossRef]
- Ballard, C.; Orrell, M.; YongZhong, S.; Moniz-Cook, E.; Stafford, J.; Whittaker, R.; Woods, B.; Corbett, A.; Garrod, L.; Khan, Z.; et al. Impact of Antipsychotic Review and Nonpharmacological Intervention on Antipsychotic Use, Neuropsychiatric Symptoms, and Mortality in People With Dementia Living in Nursing Homes: A Factorial Cluster-Randomized Controlled Trial by the Well-Being and Health for People With Dementia (WHELD) Program. Am J Psychiatry 2016, 173, 252–262. [Google Scholar] [CrossRef]
- Safdar, A.; Ismail, F. A comprehensive review on pharmacological applications and drug-induced toxicity of valproic acid. Saudi Pharm J 2023, 31, 265–278. [Google Scholar] [CrossRef]
- Lee, D.; Slomkowski, M.; Hefting, N.; Chen, D.; Larsen, K.G.; Kohegyi, E.; Hobart, M.; Cummings, J.L.; Grossberg, G.T. Brexpiprazole for the Treatment of Agitation in Alzheimer Dementia: A Randomized Clinical Trial. JAMA Neurol 2023, 80, 1307–1316. [Google Scholar] [CrossRef]
- Murray, P.S.; Kumar, S.; Demichele-Sweet, M.A.; Sweet, R.A. Psychosis in Alzheimer’s disease. Biol Psychiatry 2014, 75, 542–552. [Google Scholar] [CrossRef]
- Woodward, M. Aspects of communication in Alzheimer’s disease: clinical features and treatment options. Int Psychogeriatr 2013, 25, 877–885. [Google Scholar] [CrossRef]
- Ismail, Z.; Creese, B.; Aarsland, D.; Kales, H.C.; Lyketsos, C.G.; Sweet, R.A.; Ballard, C. Psychosis in Alzheimer disease - mechanisms, genetics and therapeutic opportunities. Nat Rev Neurol 2022, 18, 131–144. [Google Scholar] [CrossRef]
- Khoury, R.; Marx, C.; Mirgati, S.; Velury, D.; Chakkamparambil, B.; Grossberg, G.T. AVP-786 as a promising treatment option for Alzheimer’s Disease including agitation. Expert Opin Pharmacother 2021, 22, 783–795. [Google Scholar] [CrossRef]
- Kurhan, F.; Akin, M. A New Hope in Alzheimer’s Disease Psychosis: Pimavanserin. Curr Alzheimer Res 2023, 20, 403–408. [Google Scholar] [CrossRef]
- Shi, L.; Chen, S.J.; Ma, M.Y.; Bao, Y.P.; Han, Y.; Wang, Y.M.; Shi, J.; Vitiello, M.V.; Lu, L. Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med Rev 2018, 40, 4–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, R.; Yang, L.; Zhang, H.; Shi, Y.; Okhravi, H.R.; Vitiello, M.V.; Sanford, L.D.; Tang, X. Sleep in Alzheimer’s disease: a systematic review and meta-analysis of polysomnographic findings. Transl Psychiatry 2022, 12, 136. [Google Scholar] [CrossRef]
- Wu, T.T.; Zou, Y.L.; Xu, K.D.; Jiang, X.R.; Zhou, M.M.; Zhang, S.B.; Song, C.H. Insomnia and multiple health outcomes: umbrella review of meta-analyses of prospective cohort studies. Public Health 2023, 215, 66–74. [Google Scholar] [CrossRef]
- Ju, Y.E.; Lucey, B.P.; Holtzman, D.M. Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat Rev Neurol 2014, 10, 115–119. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Z.; Hu, N.; Yang, Y.; Xiong, R.; Fu, Z. Cognition effectiveness of continuous positive airway pressure treatment in obstructive sleep apnea syndrome patients with cognitive impairment: a meta-analysis. Exp Brain Res 2021, 239, 3537–3552. [Google Scholar] [CrossRef]
- Di Meco, A.; Joshi, Y.B.; Pratico, D. Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tangles. Neurobiol Aging 2014, 35, 1813–1820. [Google Scholar] [CrossRef]
- Ooms, S.; Overeem, S.; Besse, K.; Rikkert, M.O.; Verbeek, M.; Claassen, J.A. Effect of 1 night of total sleep deprivation on cerebrospinal fluid beta-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol 2014, 71, 971–977. [Google Scholar] [CrossRef]
- McCurry, S.M.; Reynolds, C.F.; Ancoli-Israel, S.; Teri, L.; Vitiello, M.V. Treatment of sleep disturbance in Alzheimer’s disease. Sleep Med Rev 2000, 4, 603–628. [Google Scholar] [CrossRef]
- Burke, S.L.; Hu, T.; Spadola, C.E.; Burgess, A.; Li, T.; Cadet, T. Treatment of Sleep Disturbance May Reduce the Risk of Future Probable Alzheimer’s Disease. J Aging Health 2019, 31, 322–342. [Google Scholar] [CrossRef] [PubMed]
- McCleery, J.; Sharpley, A.L. Pharmacotherapies for sleep disturbances in dementia. Cochrane Database Syst Rev 2020, 11, CD009178. [Google Scholar] [CrossRef] [PubMed]
- Bediou, B.; Ryff, I.; Mercier, B.; Milliery, M.; Henaff, M.A.; D’Amato, T.; Bonnefoy, M.; Vighetto, A.; Krolak-Salmon, P. Impaired social cognition in mild Alzheimer disease. J Geriatr Psychiatry Neurol 2009, 22, 130–140. [Google Scholar] [CrossRef]
- Moreau, N.; Rauzy, S.; Viallet, F.; Champagne-Lavau, M. Theory of mind in Alzheimer disease: Evidence of authentic impairment during social interaction. Neuropsychology 2016, 30, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Ellis Weismer, S.; Tomblin, J.B.; Durkin, M.S.; Bolt, D.; Palta, M. A preliminary epidemiologic study of social (pragmatic) communication disorder in the context of developmental language disorder. Int J Lang Commun Disord 2021, 56, 1235–1248. [Google Scholar] [CrossRef]
- Sommerlad, A.; Kivimaki, M.; Larson, E.B.; Rohr, S.; Shirai, K.; Singh-Manoux, A.; Livingston, G. Social participation and risk of developing dementia. Nat Aging 2023, 3, 532–545. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Chang, C.H.; Gean, P.W. Impact of social relationships on Alzheimer’s memory impairment: mechanistic studies. J Biomed Sci 2018, 25, 3. [Google Scholar] [CrossRef]
- Leser, N.; Wagner, S. The effects of acute social isolation on long-term social recognition memory. Neurobiol Learn Mem 2015, 124, 97–103. [Google Scholar] [CrossRef]
- Huang, H.; Wang, L.; Cao, M.; Marshall, C.; Gao, J.; Xiao, N.; Hu, G.; Xiao, M. Isolation Housing Exacerbates Alzheimer’s Disease-Like Pathophysiology in Aged APP/PS1 Mice. Int J Neuropsychopharmacol 2015, 18, pyu116. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Hung, H.C.; Yu, Y.J.; Su, C.L.; Chen, S.H.; Gean, P.W. Co-housing reverses memory decline by epigenetic regulation of brain-derived neurotrophic factor expression in an animal model of Alzheimer’s disease. Neurobiol Learn Mem 2017, 141, 1–8. [Google Scholar] [CrossRef]
- Powell, N.D.; Sloan, E.K.; Bailey, M.T.; Arevalo, J.M.; Miller, G.E.; Chen, E.; Kobor, M.S.; Reader, B.F.; Sheridan, J.F.; Cole, S.W. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A 2013, 110, 16574–16579. [Google Scholar] [CrossRef]
- Azzinnari, D.; Sigrist, H.; Staehli, S.; Palme, R.; Hildebrandt, T.; Leparc, G.; Hengerer, B.; Seifritz, E.; Pryce, C.R. Mouse social stress induces increased fear conditioning, helplessness and fatigue to physical challenge together with markers of altered immune and dopamine function. Neuropharmacology 2014, 85, 328–341. [Google Scholar] [CrossRef]
- Djordjevic, A.; Adzic, M.; Djordjevic, J.; Radojcic, M.B. Chronic social isolation is related to both upregulation of plasticity genes and initiation of proapoptotic signaling in Wistar rat hippocampus. J Neural Transm (Vienna) 2009, 116, 1579–1589. [Google Scholar] [CrossRef]
- Murinova, J.; Hlavacova, N.; Chmelova, M.; Riecansky, I. The Evidence for Altered BDNF Expression in the Brain of Rats Reared or Housed in Social Isolation: A Systematic Review. Front Behav Neurosci 2017, 11, 101. [Google Scholar] [CrossRef]
- Liu, J.; Dietz, K.; DeLoyht, J.M.; Pedre, X.; Kelkar, D.; Kaur, J.; Vialou, V.; Lobo, M.K.; Dietz, D.M.; Nestler, E.J.; et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci 2012, 15, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.H.; Kuo, J.R.; Chen, S.H.; Gean, P.W. Amelioration of social isolation-triggered onset of early Alzheimer’s disease-related cognitive deficit by N-acetylcysteine in a transgenic mouse model. Neurobiol Dis 2012, 45, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, W.; Shao, F.; Wang, W. Cognitive dysfunction and epigenetic alterations of the BDNF gene are induced by social isolation during early adolescence. Behav Brain Res 2016, 313, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Branchi, I.; D’Andrea, I.; Fiore, M.; Di Fausto, V.; Aloe, L.; Alleva, E. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol Psychiatry 2006, 60, 690–696. [Google Scholar] [CrossRef]
- Cosentino, S.; Zahodne, L.B.; Brandt, J.; Blacker, D.; Albert, M.; Dubois, B.; Stern, Y. Social cognition in Alzheimer’s disease: a separate construct contributing to dependence. Alzheimers Dement 2014, 10, 818–826. [Google Scholar] [CrossRef]
- Blair, H.A. Lumateperone: First Approval. Drugs 2020, 80, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.; Marano, G.; Traversi, G.; Sani, G.; Janiri, L. Evidence on the New Drug Lumateperone (ITI-007) for Psychiatric and Neurological Disorders. CNS Neurol Disord Drug Targets 2020, 19, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Vanover, K.E.; Davis, R.E.; Zhou, Y.; Ye, W.; Brasic, J.R.; Gapasin, L.; Saillard, J.; Weingart, M.; Litman, R.E.; Mates, S.; et al. Dopamine D(2) receptor occupancy of lumateperone (ITI-007): a Positron Emission Tomography Study in patients with schizophrenia. Neuropsychopharmacology 2019, 44, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Vyas, P.; Hwang, B.J.; Brasic, J.R. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother 2020, 21, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, J.J.; Wilson, C.; Hannan, A.J.; Renoir, T. Psilocybin as a lead candidate molecule in preclinical therapeutic studies of psychiatric disorders: A systematic review. J Neurochem 2023. [Google Scholar] [CrossRef]
- Kozak, Z.; Johnson, M.W.; Aaronson, S.T. Assessing potential of psilocybin for depressive disorders. Expert Opin Investig Drugs 2023, 32, 887–900. [Google Scholar] [CrossRef]
- Sharma, P.; Nguyen, Q.A.; Matthews, S.J.; Carpenter, E.; Mathews, D.B.; Patten, C.A.; Hammond, C.J. Psilocybin history, action and reaction: A narrative clinical review. J Psychopharmacol 2023, 37, 849–865. [Google Scholar] [CrossRef]
- Chen, K.; Li, H.; Yang, L.; Jiang, Y.; Wang, Q.; Zhang, J.; He, J. Comparative efficacy and safety of antidepressant therapy for the agitation of dementia: A systematic review and network meta-analysis. Front Aging Neurosci 2023, 15, 1103039. [Google Scholar] [CrossRef]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s disease drug development pipeline: 2023. Alzheimers Dement (N Y) 2023, 9, e12385. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chung, C.H.; Chien, W.C.; Chen, H.C. The Association Between Dextromethorphan Use and the Risk of Dementia. Am J Alzheimers Dis Other Demen 2022, 37, 15333175221124952. [Google Scholar] [CrossRef]
- Malar, D.S.; Thitilertdecha, P.; Ruckvongacheep, K.S.; Brimson, S.; Tencomnao, T.; Brimson, J.M. Targeting Sigma Receptors for the Treatment of Neurodegenerative and Neurodevelopmental Disorders. CNS Drugs 2023, 37, 399–440. [Google Scholar] [CrossRef]
- Ohi, Y.; Tsunekawa, S.; Haji, A. Dextromethorphan inhibits the glutamatergic synaptic transmission in the nucleus tractus solitarius of guinea pigs. J Pharmacol Sci 2011, 116, 54–62. [Google Scholar] [CrossRef]
- Pioro, E.P.; Brooks, B.R.; Cummings, J.; Schiffer, R.; Thisted, R.A.; Wynn, D.; Hepner, A.; Kaye, R.; Safety, T.; Efficacy Results Trial of, A.V.P.i.P.B.A.I. Dextromethorphan plus ultra low-dose quinidine reduces pseudobulbar affect. Ann Neurol 2010, 68, 693–702. [Google Scholar] [CrossRef]
- Taylor, C.P.; Traynelis, S.F.; Siffert, J.; Pope, L.E.; Matsumoto, R.R. Pharmacology of dextromethorphan: Relevance to dextromethorphan/quinidine (Nuedexta(R)) clinical use. Pharmacol Ther 2016, 164, 170–182. [Google Scholar] [CrossRef]
- Chez, M.; Kile, S.; Lepage, C.; Parise, C.; Benabides, B.; Hankins, A. A Randomized, Placebo-Controlled, Blinded, Crossover, Pilot Study of the Effects of Dextromethorphan/Quinidine for the Treatment of Neurobehavioral Symptoms in Adults with Autism. J Autism Dev Disord 2020, 50, 1532–1538. [Google Scholar] [CrossRef]
- Cummings, J.L.; Lyketsos, C.G.; Peskind, E.R.; Porsteinsson, A.P.; Mintzer, J.E.; Scharre, D.W.; De La Gandara, J.E.; Agronin, M.; Davis, C.S.; Nguyen, U.; et al. Effect of Dextromethorphan-Quinidine on Agitation in Patients With Alzheimer Disease Dementia: A Randomized Clinical Trial. JAMA 2015, 314, 1242–1254. [Google Scholar] [CrossRef]
- Devanand, D.P. Management of neuropsychiatric symptoms in dementia. Curr Opin Neurol 2023, 36, 498–503. [Google Scholar] [CrossRef]
- Lauterbach, E.C. Treatment Resistant Depression with Loss of Antidepressant Response: Rapid-Acting Antidepressant Action of Dextromethorphan, A Possible Treatment Bridging Molecule. Psychopharmacol Bull 2016, 46, 53–58. [Google Scholar]
- Willett, K.C.; Bond, L.R.; Morrill, A.M.; Lorena, D.; Petru, I. Dextromethorphan/Bupropion: A Novel Treatment for Patients With Major Depressive Disorder. Am J Ther 2024, 31, e24–e29. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, N.; Ruthirakuhan, M.; Gallagher, D.; Verhoeff, N.; Kiss, A.; Black, S.E.; Lanctot, K.L. Randomized Placebo-Controlled Trial of Nabilone for Agitation in Alzheimer’s Disease. Am J Geriatr Psychiatry 2019, 27, 1161–1173. [Google Scholar] [CrossRef]
- Ruthirakuhan, M.; Herrmann, N.; Andreazza, A.C.; Verhoeff, N.; Gallagher, D.; Black, S.E.; Kiss, A.; Lanctot, K.L. Agitation, Oxidative Stress, and Cytokines in Alzheimer Disease: Biomarker Analyses From a Clinical Trial With Nabilone for Agitation. J Geriatr Psychiatry Neurol 2020, 33, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Diefenderfer, L.A.; Iuppa, C. Brexpiprazole: A review of a new treatment option for schizophrenia and major depressive disorder. Ment Health Clin 2017, 7, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Eaves, S.; Rey, J.A. Brexpiprazole (Rexulti): A New Monotherapy for Schizophrenia and Adjunctive Therapy for Major Depressive Disorder. P T 2016, 41, 418–422. [Google Scholar] [PubMed]
- Khoury, R. Deuterated dextromethorphan/quinidine for agitation in Alzheimer’s disease. Neural Regen Res 2022, 17, 1013–1014. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.M.; Li, L.Y.; Guo, L.Z.; Wang, L.; Wang, Y.F.; Chen, N.; Wang, E. Dexmedetomidine exerts an anti-inflammatory effect via alpha2 adrenoceptors to alleviate cognitive dysfunction in 5xFAD mice. Front Aging Neurosci 2022, 14, 978768. [Google Scholar] [CrossRef]
- Deiner, S.; Luo, X.; Lin, H.M.; Sessler, D.I.; Saager, L.; Sieber, F.E.; Lee, H.B.; Sano, M.; and the Dexlirium Writing, G.; Jankowski, C.; et al. Intraoperative Infusion of Dexmedetomidine for Prevention of Postoperative Delirium and Cognitive Dysfunction in Elderly Patients Undergoing Major Elective Noncardiac Surgery: A Randomized Clinical Trial. JAMA Surg 2017, 152, e171505. [Google Scholar] [CrossRef]
- Preskorn, S.H.; Zeller, S.; Citrome, L.; Finman, J.; Goldberg, J.F.; Fava, M.; Kakar, R.; De Vivo, M.; Yocca, F.D.; Risinger, R. Effect of Sublingual Dexmedetomidine vs Placebo on Acute Agitation Associated With Bipolar Disorder: A Randomized Clinical Trial. JAMA 2022, 327, 727–736. [Google Scholar] [CrossRef]
- Volicer, L.; Stelly, M.; Morris, J.; McLaughlin, J.; Volicer, B.J. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int J Geriatr Psychiatry 1997, 12, 913–919. [Google Scholar] [CrossRef]
- Woodward, M.R.; Harper, D.G.; Stolyar, A.; Forester, B.P.; Ellison, J.M. Dronabinol for the treatment of agitation and aggressive behavior in acutely hospitalized severely demented patients with noncognitive behavioral symptoms. Am J Geriatr Psychiatry 2014, 22, 415–419. [Google Scholar] [CrossRef]
- Cohen, L.M.; Ash, E.; Outen, J.D.; Vandrey, R.; Amjad, H.; Agronin, M.; Burhanullah, M.H.; Walsh, P.; Wilkins, J.M.; Leoutsakos, J.M.; et al. Study rationale and baseline data for pilot trial of dronabinol adjunctive treatment of agitation in Alzheimer’s dementia (THC-AD). Int Psychogeriatr 2021. [Google Scholar] [CrossRef]
- Fitzgerald, P.J. Norepinephrine May Oppose Other Neuromodulators to Impact Alzheimer’s Disease. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, I.L.; Dello Russo, C.; Novellino, F.; Caso, J.R.; Garcia-Bueno, B.; Leza, J.C.; Madrigal, J.L.M. Noradrenaline in Alzheimer’s Disease: A New Potential Therapeutic Target. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Melck, D.; Orlando, P.; Bisogno, T.; Zagoory, O.; Bifulco, M.; Vogel, Z.; De Petrocellis, L. Palmitoylethanolamide inhibits the expression of fatty acid amide hydrolase and enhances the anti-proliferative effect of anandamide in human breast cancer cells. Biochem J 2001, 358, 249–255. [Google Scholar] [CrossRef]
- Jonsson, K.O.; Vandevoorde, S.; Lambert, D.M.; Tiger, G.; Fowler, C.J. Effects of homologues and analogues of palmitoylethanolamide upon the inactivation of the endocannabinoid anandamide. Br J Pharmacol 2001, 133, 1263–1275. [Google Scholar] [CrossRef]
- Outen, J.D.; Burhanullah, M.H.; Vandrey, R.; Amjad, H.; Harper, D.G.; Patrick, R.E.; May, R.L.; Agronin, M.E.; Forester, B.P.; Rosenberg, P.B. Cannabinoids for Agitation in Alzheimer’s Disease. Am J Geriatr Psychiatry 2021, 29, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Goveas, J.S. Commentary on “Cannabinoids for Agitation in Alzheimer’s Disease”. Am J Geriatr Psychiatry 2021, 29, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Nirogi, R.; Ieni, J.; Goyal, V.K.; Ravula, J.; Jetta, S.; Shinde, A.; Jayarajan, P.; Benade, V.; Palacharla, V.R.C.; Dogiparti, D.K.; et al. Effect of masupirdine (SUVN-502) on cognition in patients with moderate Alzheimer’s disease: A randomized, double-blind, phase 2, proof-of-concept study. Alzheimers Dement (N Y) 2022, 8, e12307. [Google Scholar] [CrossRef]
- Nirogi, R.; Jayarajan, P.; Benade, V.; Shinde, A.; Goyal, V.K.; Jetta, S.; Ravula, J.; Abraham, R.; Grandhi, V.R.; Subramanian, R.; et al. Potential beneficial effects of masupirdine (SUVN-502) on agitation/aggression and psychosis in patients with moderate Alzheimer’s disease: Exploratory post hoc analyses. Int J Geriatr Psychiatry 2022, 37. [Google Scholar] [CrossRef]
- de Jong, I.E.M.; Mork, A. Antagonism of the 5-HT(6) receptor - Preclinical rationale for the treatment of Alzheimer’s disease. Neuropharmacology 2017, 125, 50–63. [Google Scholar] [CrossRef]
- Ferrero, H.; Solas, M.; Francis, P.T.; Ramirez, M.J. Serotonin 5-HT(6) Receptor Antagonists in Alzheimer’s Disease: Therapeutic Rationale and Current Development Status. CNS Drugs 2017, 31, 19–32. [Google Scholar] [CrossRef]
- Tomaszewski, M.R.; Meng, X.; Haley, H.D.; Harrell, C.M.; McDonald, T.P.; Miller, C.O.; Smith, S.M. Magnetic resonance imaging detects white adipose tissue beiging in mice following PDE10A inhibitor treatment. J Lipid Res 2023, 64, 100408. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.; Song, W.; Fusick, A. Pimavanserin Use in Lewy Body Dementia: A Case Report Demonstrating the Medication’s Efficacy. Cureus 2023, 15, e46356. [Google Scholar] [CrossRef] [PubMed]
- Tariot, P.N.; Ballard, C.; Devanand, D.P.; Cummings, J.L.; Sultzer, D.L. Pimavanserin and dementia-related psychosis. Lancet Neurol 2022, 21, 114–115. [Google Scholar] [CrossRef]
- Ballard, C.; O’Brien, J.; Coope, B.; Fairbairn, A.; Abid, F.; Wilcock, G. A prospective study of psychotic symptoms in dementia sufferers: psychosis in dementia. Int Psychogeriatr 1997, 9, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.A.; Moon, J.; Bourbonais, C.A.; Harms, J.; Edgerton, J.R.; Stark, E.; Steyn, S.J.; Butter, C.R.; Lazzaro, J.T.; O’Connor, R.E.; et al. Striatal, Hippocampal, and Cortical Networks Are Differentially Responsive to the M4- and M1-Muscarinic Acetylcholine Receptor Mediated Effects of Xanomeline. ACS Chem Neurosci 2019, 10, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Kaul, I.; Sawchak, S.; Correll, C.U.; Kakar, R.; Breier, A.; Zhu, H.; Miller, A.C.; Paul, S.M.; Brannan, S.K. Efficacy and safety of the muscarinic receptor agonist KarXT (xanomeline-trospium) in schizophrenia (EMERGENT-2) in the USA: results from a randomised, double-blind, placebo-controlled, flexible-dose phase 3 trial. Lancet 2024, 403, 160–170. [Google Scholar] [CrossRef]
- Sauder, C.; Allen, L.A.; Baker, E.; Miller, A.C.; Paul, S.M.; Brannan, S.K. Effectiveness of KarXT (xanomeline-trospium) for cognitive impairment in schizophrenia: post hoc analyses from a randomised, double-blind, placebo-controlled phase 2 study. Transl Psychiatry 2022, 12, 491. [Google Scholar] [CrossRef]
- Kidambi, N.; Elsayed, O.H.; El-Mallakh, R.S. Xanomeline-Trospium and Muscarinic Involvement in Schizophrenia. Neuropsychiatr Dis Treat 2023, 19, 1145–1151. [Google Scholar] [CrossRef]
- Leber, A.; Ramachandra, R.; Ceban, F.; Kwan, A.T.H.; Rhee, T.G.; Wu, J.; Cao, B.; Jawad, M.Y.; Teopiz, K.M.; Ho, R.; et al. Efficacy, safety, and tolerability of xanomeline for schizophrenia spectrum disorders: a systematic review. Expert Opin Pharmacother 2024. [Google Scholar] [CrossRef]
- Bergamini, G.; Coloma, P.; Massinet, H.; Steiner, M.A. What evidence is there for implicating the brain orexin system in neuropsychiatric symptoms in dementia? Front Psychiatry 2022, 13, 1052233. [Google Scholar] [CrossRef]
- Kron, J.O.J.; Keenan, R.J.; Hoyer, D.; Jacobson, L.H. Orexin Receptor Antagonism: Normalizing Sleep Architecture in Old Age and Disease. Annu Rev Pharmacol Toxicol 2024, 64, 359–386. [Google Scholar] [CrossRef] [PubMed]
- de Bruin, N.; van Loevezijn, A.; Wicke, K.M.; de Haan, M.; Venhorst, J.; Lange, J.H.M.; de Groote, L.; van der Neut, M.A.W.; Prickaerts, J.; Andriambeloson, E.; et al. The selective 5-HT6 receptor antagonist SLV has putative cognitive- and social interaction enhancing properties in rodent models of cognitive impairment. Neurobiol Learn Mem 2016, 133, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Lardenoije, R.; Roubroeks, J.A.Y.; Pishva, E.; Leber, M.; Wagner, H.; Iatrou, A.; Smith, A.R.; Smith, R.G.; Eijssen, L.M.T.; Kleineidam, L.; et al. Alzheimer’s disease-associated (hydroxy)methylomic changes in the brain and blood. Clin Epigenetics 2019, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.T.; Roussos, P.; Garg, P.; Ho, D.J.; Azam, N.; Katsel, P.L.; Haroutunian, V.; Sharp, A.J. Genome-wide DNA methylation profiling in the superior temporal gyrus reveals epigenetic signatures associated with Alzheimer’s disease. Genome Med 2016, 8, 5. [Google Scholar] [CrossRef]
- Koulousakis, P.; Willems, E.; Schepers, M.; Rombaut, B.; Prickaerts, J.; Vanmierlo, T.; van den Hove, D. Exogenous Oxytocin Administration Restores Memory in Female APP/PS1 Mice. J Alzheimers Dis 2023, 96, 1207–1219. [Google Scholar] [CrossRef]
- Journey, J.D.; Agrawal, S.; Stern, E. Dextromethorphan Toxicity. In StatPearls, Treasure Island (FL) ineligible companies. Disclosure: Suneil Agrawal declares no relevant financial relationships with ineligible companies. Disclosure: Evan Stern declares no relevant financial relationships with ineligible companies., 2024.
- Tampi, R.R.; Joshi, P.; Marpuri, P.; Tampi, D.J. Evidence for using dextromethorphan-quinidine for the treatment of agitation in dementia. World J Psychiatry 2020, 10, 29–33. [Google Scholar] [CrossRef]
- Garay, R.P.; Grossberg, G.T. AVP-786 for the treatment of agitation in dementia of the Alzheimer’s type. Expert Opin Investig Drugs 2017, 26, 121–132. [Google Scholar] [CrossRef]
- Alva, G.; Cubala, W.J.; Berrio, A.; Coate, B.; Abler, V.; Pathak, S. Safety Profile of Pimavanserin Therapy in Elderly Patients with Neurodegenerative Disease-Related Neuropsychiatric Symptoms: A Phase 3B Study. J Alzheimers Dis 2024, 98, 265–274. [Google Scholar] [CrossRef]
- Quiroz, J.A.; Machado-Vieira, R.; Zarate, C.A., Jr.; Manji, H.K. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology 2010, 62, 50–60. [Google Scholar] [CrossRef]
- Devanand, D.P.; Crocco, E.; Forester, B.P.; Husain, M.M.; Lee, S.; Vahia, I.V.; Andrews, H.; Simon-Pearson, L.; Imran, N.; Luca, L.; et al. Low Dose Lithium Treatment of Behavioral Complications in Alzheimer’s Disease: Lit-AD Randomized Clinical Trial. Am J Geriatr Psychiatry 2022, 30, 32–42. [Google Scholar] [CrossRef]
- Hidding, U.; Mainka, T.; Buhmann, C. Therapeutic use of medical Cannabis in neurological diseases: a clinical update. J Neural Transm (Vienna) 2024, 131, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Lim, C.S. Understanding the Modulatory Effects of Cannabidiol on Alzheimer’s Disease. Brain Sci 2021, 11. [Google Scholar] [CrossRef]
- Dawson, L.A. The central role of 5-HT6 receptors in modulating brain neurochemistry. Int Rev Neurobiol 2011, 96, 1–26. [Google Scholar] [CrossRef]
- Zagorska, A.; Partyka, A.; Bucki, A.; Gawalskax, A.; Czopek, A.; Pawlowski, M. Phosphodiesterase 10 Inhibitors - Novel Perspectives for Psychiatric and Neurodegenerative Drug Discovery. Curr Med Chem 2018, 25, 3455–3481. [Google Scholar] [CrossRef] [PubMed]
- Layton, M.E.; Kern, J.C.; Hartingh, T.J.; Shipe, W.D.; Raheem, I.; Kandebo, M.; Hayes, R.P.; Huszar, S.; Eddins, D.; Ma, B.; et al. Discovery of MK-8189, a Highly Potent and Selective PDE10A Inhibitor for the Treatment of Schizophrenia. J Med Chem 2023, 66, 1157–1171. [Google Scholar] [CrossRef]
- Umbricht, D.; Abt, M.; Tamburri, P.; Chatham, C.; Holiga, S.; Frank, M.J.; Collins, A.G.E.; Walling, D.P.; Mofsen, R.; Gruener, D.; et al. Proof-of-Mechanism Study of the Phosphodiesterase 10 Inhibitor RG7203 in Patients With Schizophrenia and Negative Symptoms. Biol Psychiatry Glob Open Sci 2021, 1, 70–77. [Google Scholar] [CrossRef]
- Murray, A.J.; Rogers, J.C.; Katshu, M.; Liddle, P.F.; Upthegrove, R. Oxidative Stress and the Pathophysiology and Symptom Profile of Schizophrenia Spectrum Disorders. Front Psychiatry 2021, 12, 703452. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Klinedinst, N.J.; Regenold, W.T. A mitochondrial bioenergetic basis of depression. J Bioenerg Biomembr 2015, 47, 155–171. [Google Scholar] [CrossRef]
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular pathways of major depressive disorder converge on the synapse. Mol Psychiatry 2023, 28, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.L.; Weigel, T.K.; Poffenberger, C.N.; Herkenham, M. The Behavioral Sequelae of Social Defeat Require Microglia and Are Driven by Oxidative Stress in Mice. J Neurosci 2019, 39, 5594–5605. [Google Scholar] [CrossRef]
- Elamin, M.; Pender, N.; Hardiman, O.; Abrahams, S. Social cognition in neurodegenerative disorders: a systematic review. J Neurol Neurosurg Psychiatry 2012, 83, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.; de Carvalho, R.L.S.; Nogueira, M.; Baptista, M.A.T.; Kimura, N.; Lacerda, I.B.; Dourado, M.C.N. The Relationship between Social Cognition and Executive Functions in Alzheimer’s Disease: A Systematic Review. Curr Alzheimer Res 2020, 17, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Setien-Suero, E.; Murillo-Garcia, N.; Sevilla-Ramos, M.; Abreu-Fernandez, G.; Pozueta, A.; Ayesa-Arriola, R. Exploring the Relationship Between Deficits in Social Cognition and Neurodegenerative Dementia: A Systematic Review. Front Aging Neurosci 2022, 14, 778093. [Google Scholar] [CrossRef]
- Saris, I.M.J.; Aghajani, M.; Jongs, N.; Reus, L.M.; van der Wee, N.J.A.; Bilderbeck, A.C.; Winter van Rossum, I.; Arango, C.; de la Torre-Luque, A.; Malik, A.; et al. Cross-disorder and disorder-specific deficits in social functioning among schizophrenia and alzheimer’s disease patients. PLoS One 2022, 17, e0263769. [Google Scholar] [CrossRef]
- Legaz, A.; Prado, P.; Moguilner, S.; Baez, S.; Santamaria-Garcia, H.; Birba, A.; Barttfeld, P.; Garcia, A.M.; Fittipaldi, S.; Ibanez, A. Social and non-social working memory in neurodegeneration. Neurobiol Dis 2023, 183, 106171. [Google Scholar] [CrossRef]
- Stam, D.; Rosseel, S.; De Winter, F.L.; Van den Bossche, M.J.A.; Vandenbulcke, M.; Van den Stock, J. Facial expression recognition deficits in frontotemporal dementia and Alzheimer’s disease: a meta-analytic investigation of effects of phenotypic variant, task modality, geographical region and symptomatic specificity. J Neurol 2023, 270, 5731–5755. [Google Scholar] [CrossRef]
- Singleton, E.H.; Fieldhouse, J.L.P.; van ‘t Hooft, J.J.; Scarioni, M.; van Engelen, M.E.; Sikkes, S.A.M.; de Boer, C.; Bocancea, D.I.; van den Berg, E.; Scheltens, P.; et al. Social cognition deficits and biometric signatures in the behavioural variant of Alzheimer’s disease. Brain 2023, 146, 2163–2174. [Google Scholar] [CrossRef]
- Goldberg, T.E.; Choi, J.; Lee, S.; Gurland, B.; Devanand, D.P. Effects of restriction of activities and social isolation on risk of dementia in the community. Int Psychogeriatr 2021, 33, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Drinkwater, E.; Davies, C.; Spires-Jones, T.L. Potential neurobiological links between social isolation and Alzheimer’s disease risk. Eur J Neurosci 2022, 56, 5397–5412. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Savadlou, A.; Park, S.; Siska, P.; Epp, J.R.; Sargin, D. The impact of loneliness and social isolation on the development of cognitive decline and Alzheimer’s Disease. Front Neuroendocrinol 2023, 69, 101061. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Hendrie, K.; Jester, D.J.; Dasarathy, D.; Lavretsky, H.; Ku, B.S.; Leutwyler, H.; Torous, J.; Jeste, D.V.; Tampi, R.R. Social connections as determinants of cognitive health and as targets for social interventions in persons with or at risk of Alzheimer’s disease and related disorders: a scoping review. Int Psychogeriatr 2024, 36, 92–118. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Rey, C.C.; Robert, V.; Bouisset, G.; Loisy, M.; Lopez, S.; Cattaud, V.; Lejards, C.; Piskorowski, R.A.; Rampon, C.; Chevaleyre, V.; et al. Altered inhibitory function in hippocampal CA2 contributes in social memory deficits in Alzheimer’s mouse model. iScience 2022, 25, 103895. [Google Scholar] [CrossRef]
- Rajagopal, L.; Huang, M.; Mahjour, S.; Ryan, C.; Elzokaky, A.; Svensson, K.A.; Meltzer, H.Y. The dopamine D1 receptor positive allosteric modulator, DETQ, improves cognition and social interaction in aged mice and enhances cortical and hippocampal acetylcholine efflux. Behav Brain Res 2024, 459, 114766. [Google Scholar] [CrossRef]
- Michaelian, J.C.; McCade, D.; Hoyos, C.M.; Brodaty, H.; Harrison, F.; Henry, J.D.; Guastella, A.J.; Naismith, S.L. Pilot Randomized, Double-Blind, Placebo-Controlled Crossover Trial Evaluating the Feasibility of an Intranasal Oxytocin in Improving Social Cognition in Individuals Living with Alzheimer’s Disease. J Alzheimers Dis Rep 2023, 7, 715–729. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Hosawi, S.; Alshehri, S.; Ghoneim, M.M.; Imam, S.S.; Murtaza, B.N.; Kazmi, I. Symptomatic, Genetic, and Mechanistic Overlaps between Autism and Alzheimer’s Disease. Biomolecules 2021, 11. [Google Scholar] [CrossRef]
- Steubler, V.; Erdinger, S.; Back, M.K.; Ludewig, S.; Fassler, D.; Richter, M.; Han, K.; Slomianka, L.; Amrein, I.; von Engelhardt, J.; et al. Loss of all three APP family members during development impairs synaptic function and plasticity, disrupts learning, and causes an autism-like phenotype. EMBO J 2021, 40, e107471. [Google Scholar] [CrossRef]
- Sokol, D.K.; Lahiri, D.K. APPlications of amyloid-beta precursor protein metabolites in macrocephaly and autism spectrum disorder. Front Mol Neurosci 2023, 16, 1201744. [Google Scholar] [CrossRef] [PubMed]
- Sokol, D.K.; Lahiri, D.K. Neurodevelopmental disorders and microcephaly: how apoptosis, the cell cycle, tau and amyloid-beta precursor protein APPly. Front Mol Neurosci 2023, 16, 1201723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cai, F.; Lu, R.; Xing, X.; Xu, L.; Wu, K.; Gong, Z.; Zhang, Q.; Zhang, Y.; Xing, M.; et al. CNTNAP2 intracellular domain (CICD) generated by gamma-secretase cleavage improves autism-related behaviors. Signal Transduct Target Ther 2023, 8, 219. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, L.; Bai, Y.; Chen, P.; Xing, M.; Cai, F.; Wu, Y.; Song, W. Intermittent hypoxia-induced enhancement of sociability and working memory associates with CNTNAP2 upregulation. Front Mol Neurosci 2023, 16, 1155047. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.K.; Sokol, D.K.; Erickson, C.; Ray, B.; Ho, C.Y.; Maloney, B. Autism as early neurodevelopmental disorder: evidence for an sAPPalpha-mediated anabolic pathway. Front Cell Neurosci 2013, 7, 94. [Google Scholar] [CrossRef]
- Ray, B.; Sokol, D.K.; Maloney, B.; Lahiri, D.K. Finding novel distinctions between the sAPPalpha-mediated anabolic biochemical pathways in Autism Spectrum Disorder and Fragile X Syndrome plasma and brain tissue. Sci Rep 2016, 6, 26052. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).