Submitted:
24 September 2024
Posted:
26 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Genetic Susceptibility or Resistance of Mice to Inhaled Influenza A Virus (FLUAV) Challenge
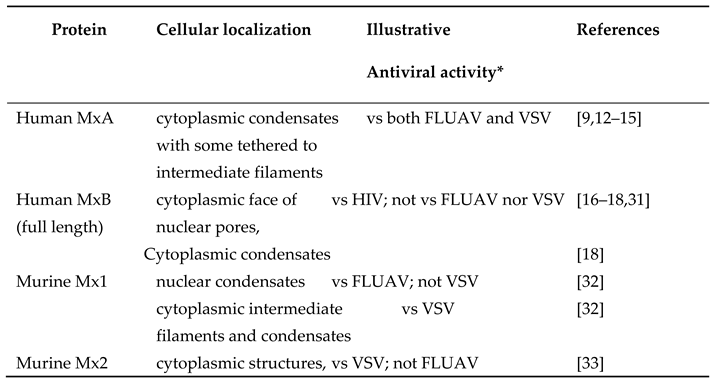
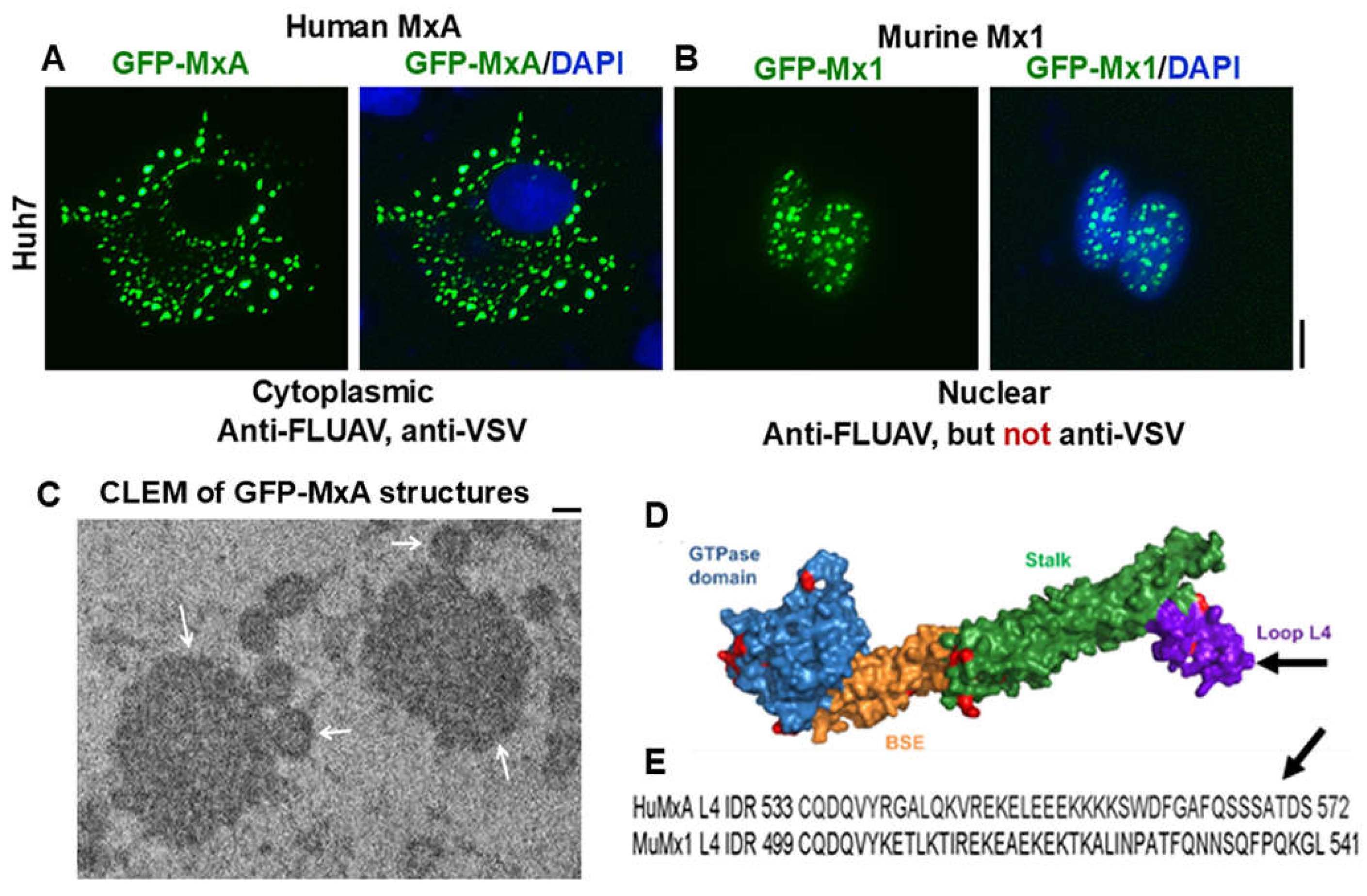
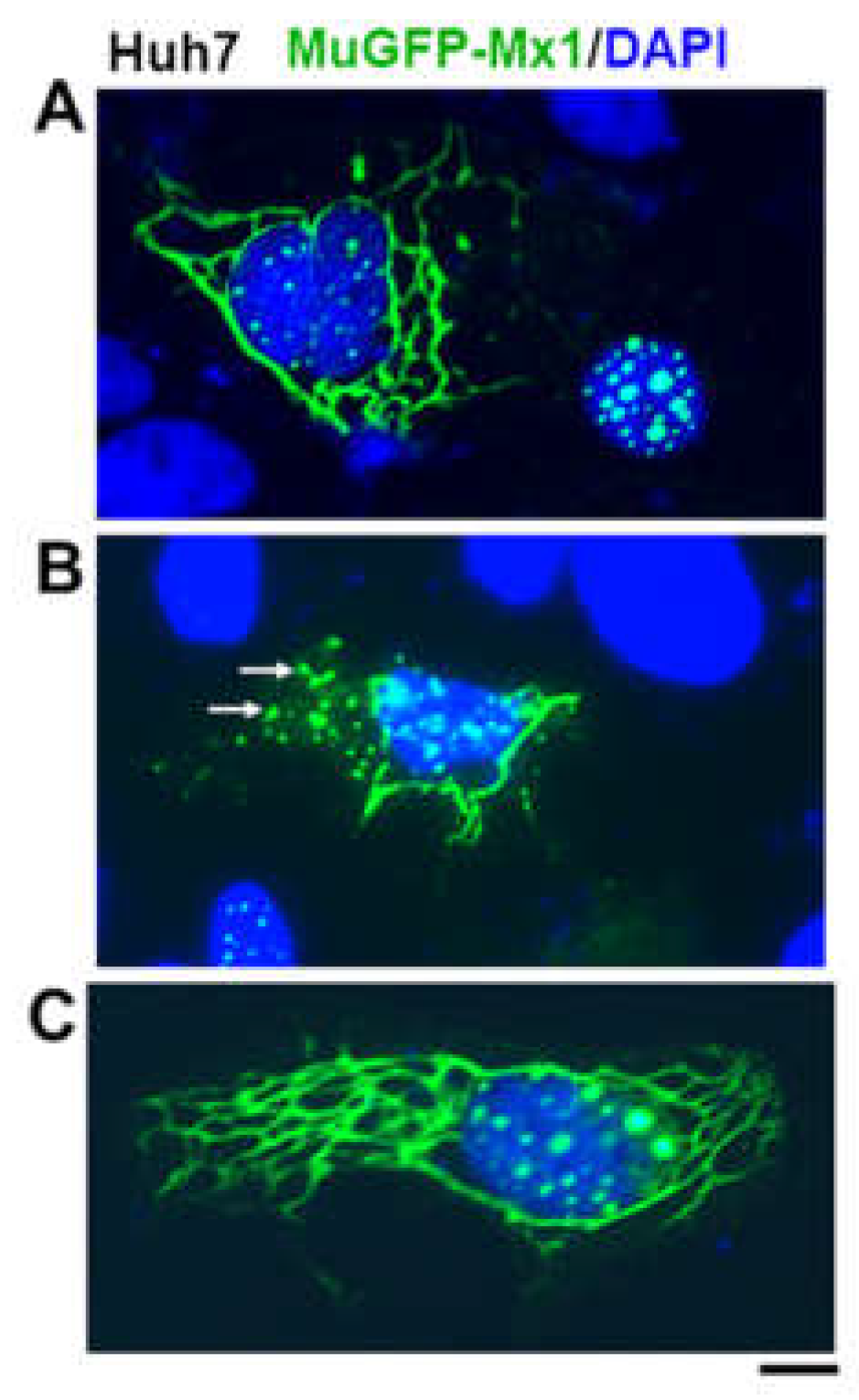
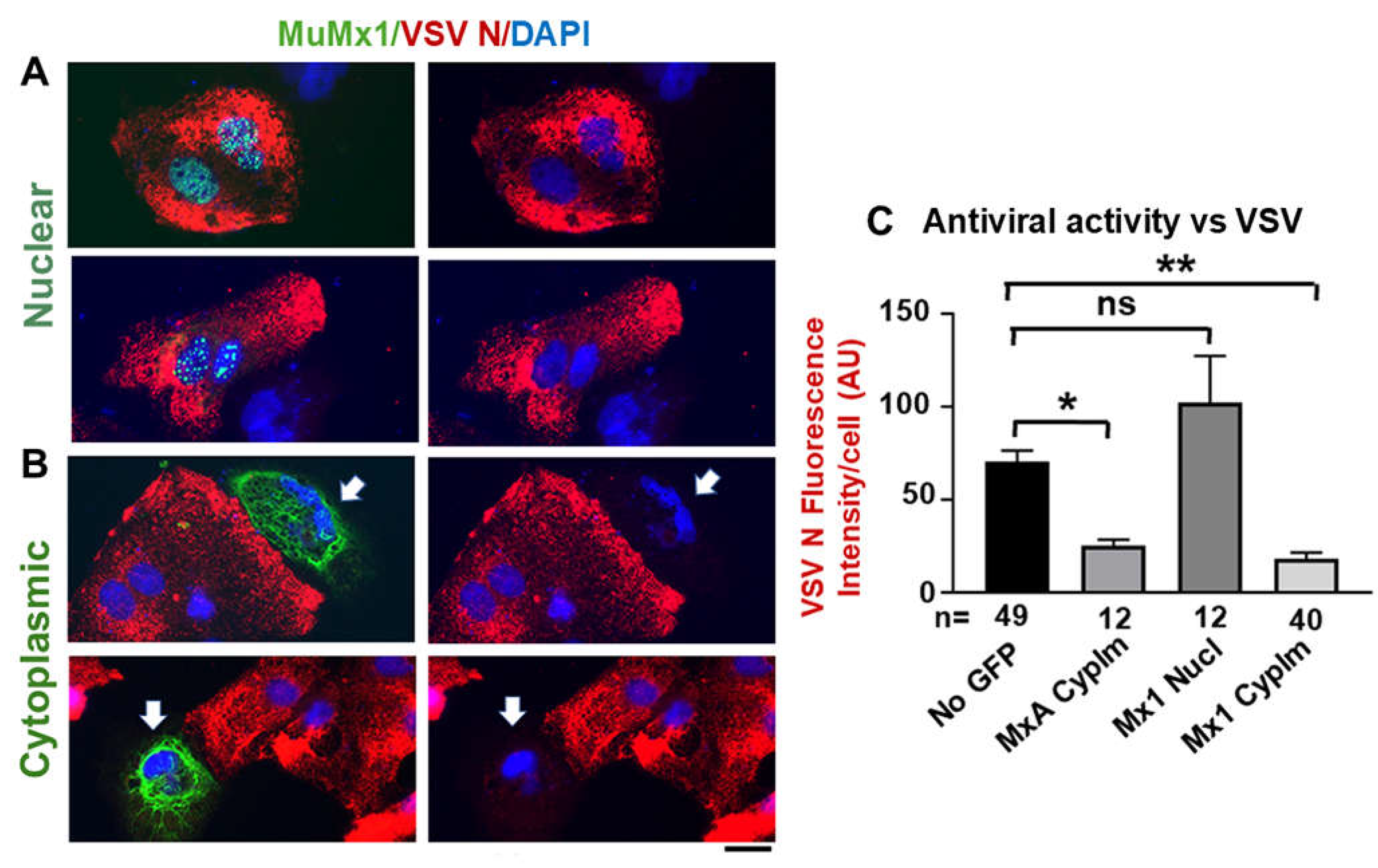
3. Distinct Subcellular Localization of the Major Human and Murine Mx Proteins
4. Overview of MxA Protein Structure
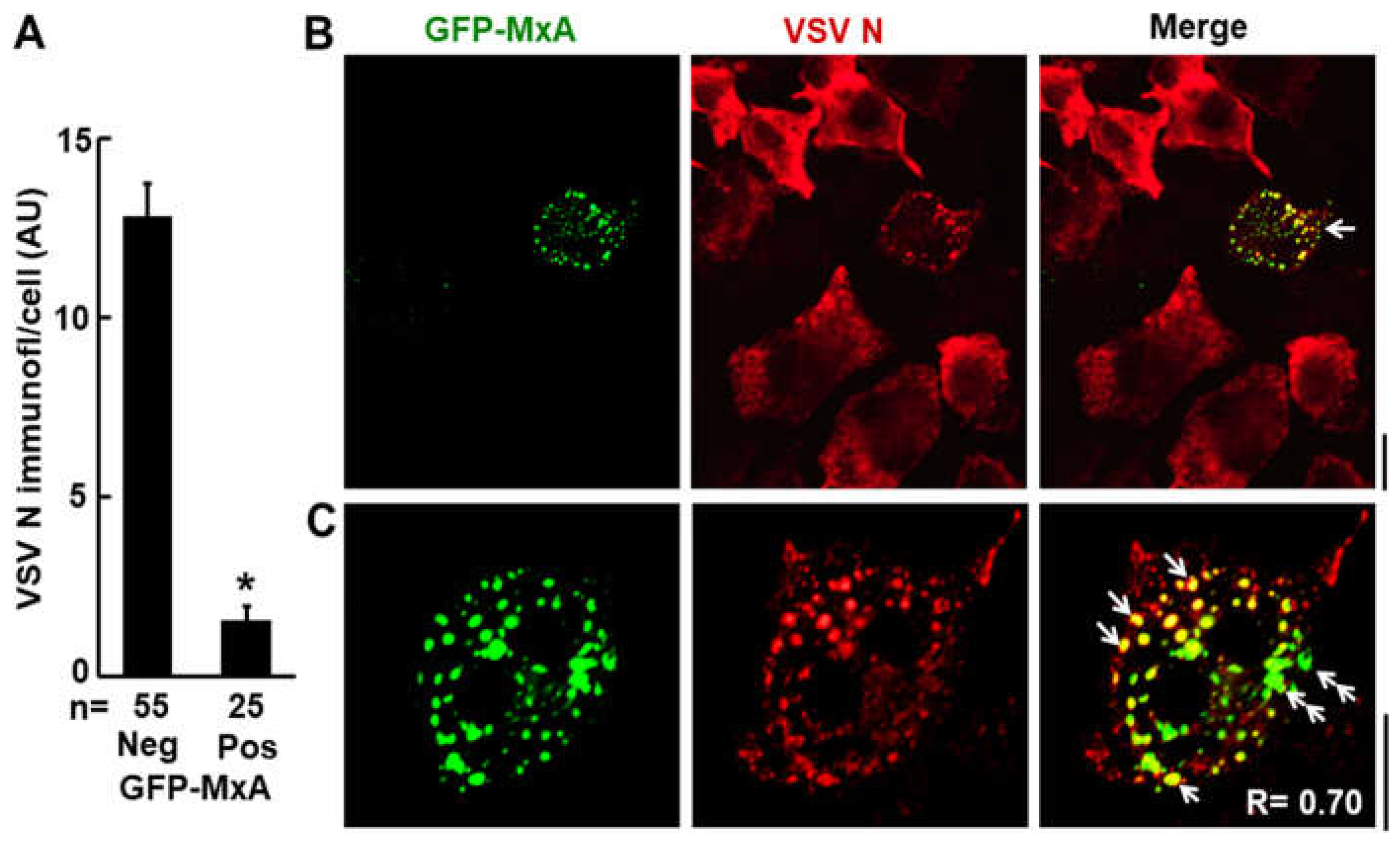
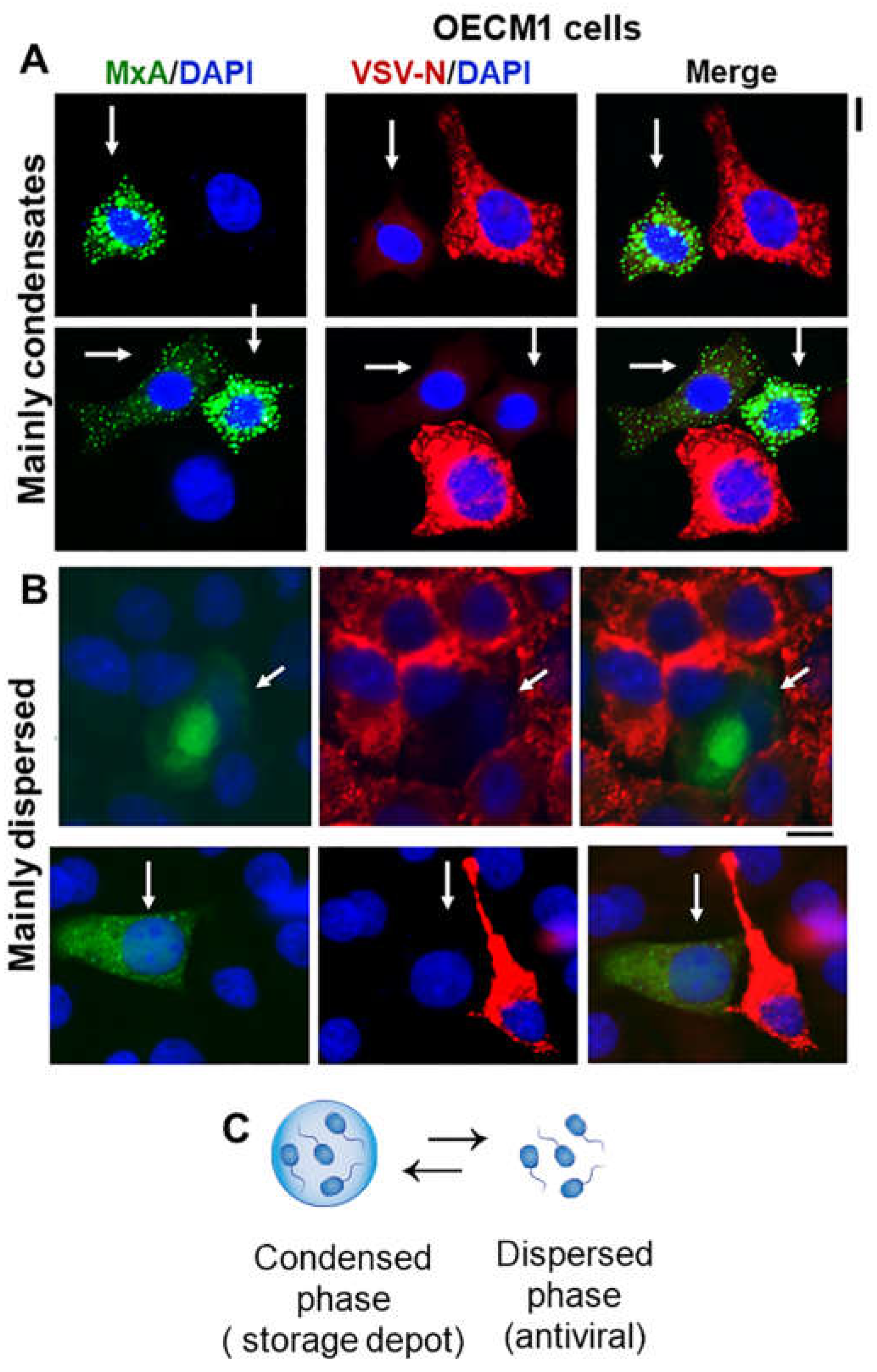
5. Discovery of Cytoplasmic MxA Protein Structures as Membraneless Phase-Separated Biomolecular Condensates
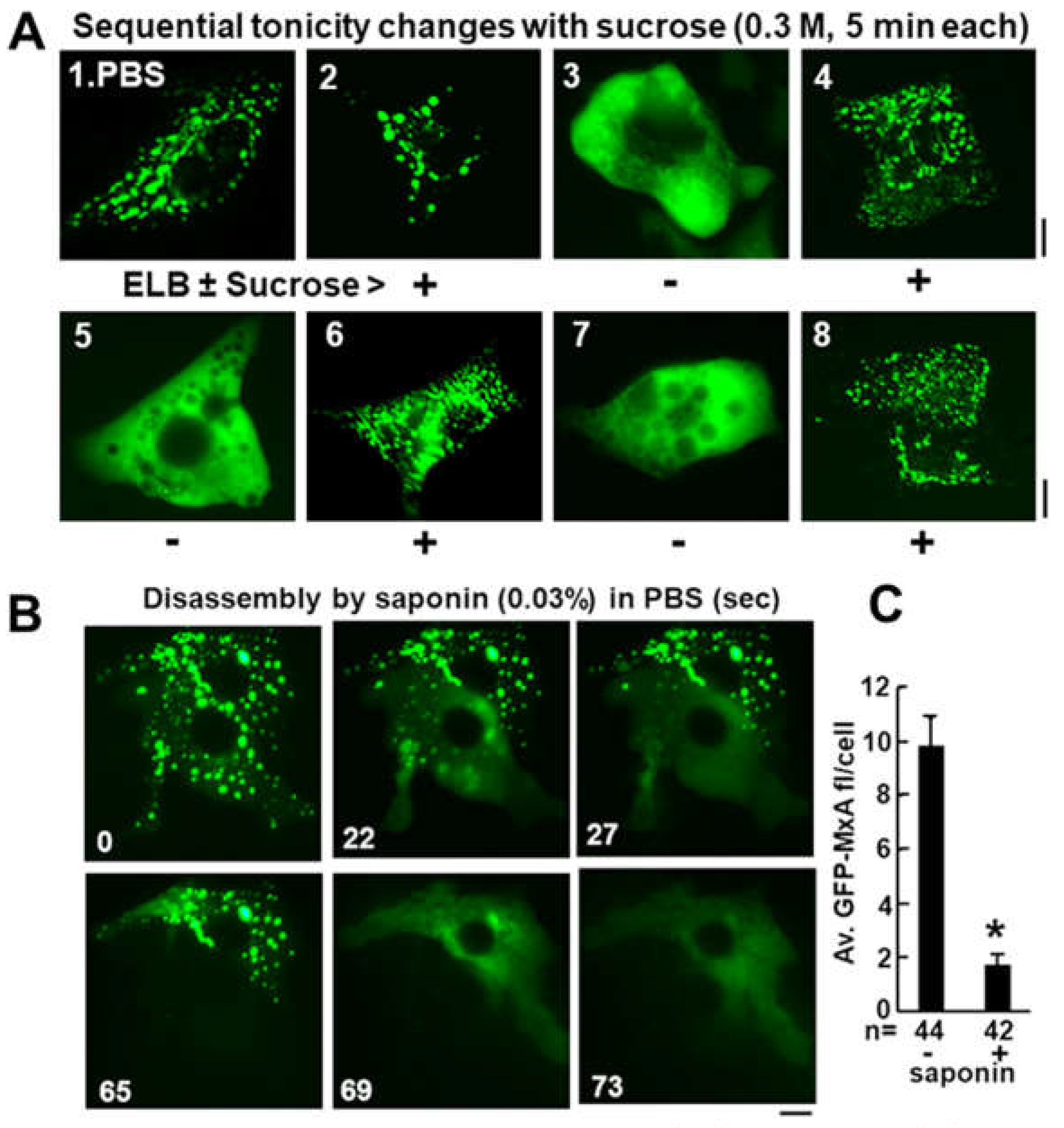
6. Rapid Reversible Metastability of MxA Condensates in Cancer Cells
7. Additional Aspects of Metastability Include the Following
7.1. HA or GFP Tags Affect Appearance of MxA Condensates in the Cytoplasm
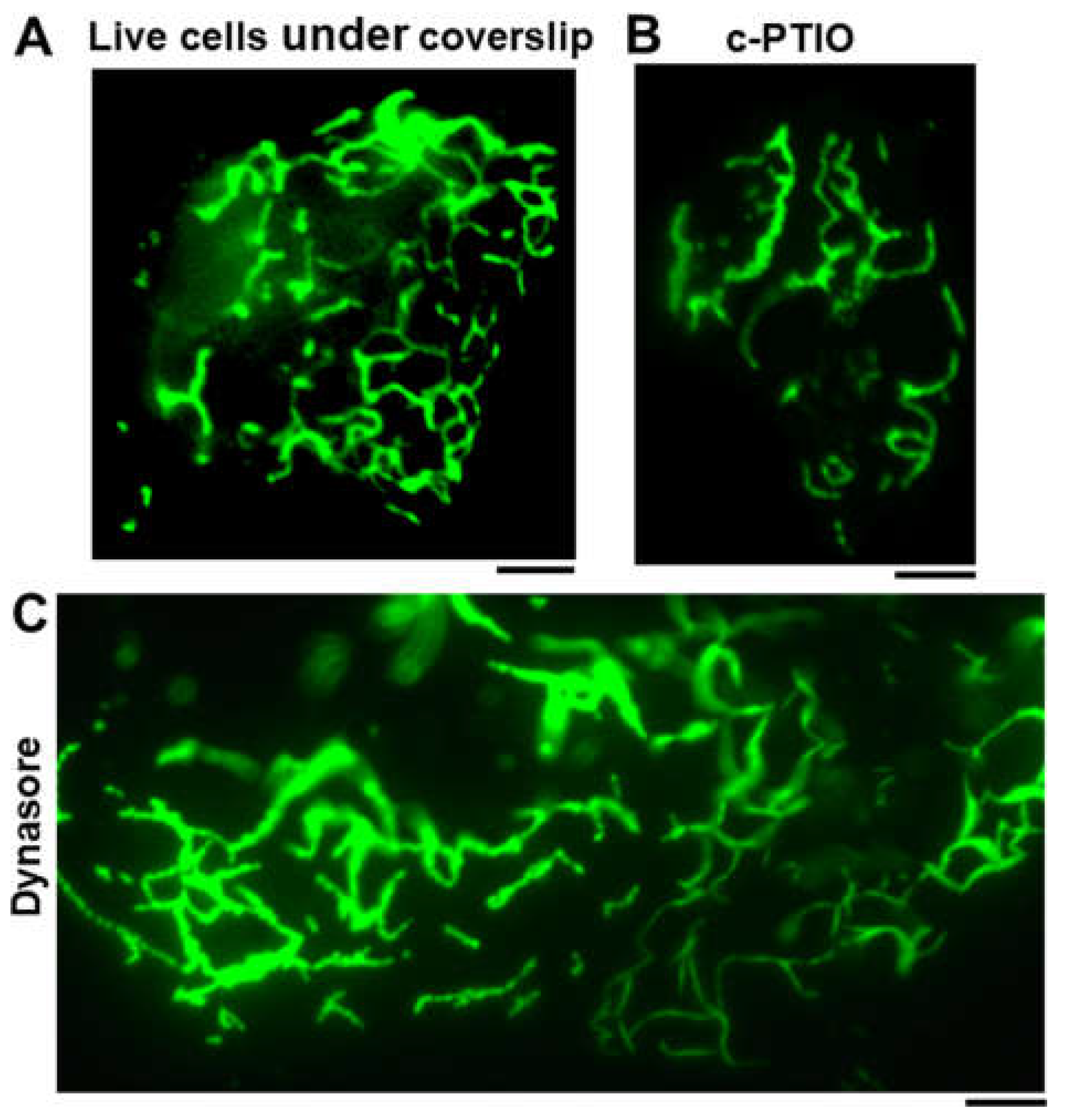
7.2. Integrity of MxA Condensates Requires Intact Cells
7.3. Spheroid to Fibril Transition
7.4. Incomplete Mixing of Proteins within GFP-MxA Condensates
8. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haller, O. and Kochs, G. (2002). Interferon-induced mx proteins: dynamin-like GTPases with antiviral activity. Traffic 3, 710-717. [CrossRef]
- Haller, O., Staeheli, P., Kochs, G. (2007) Interferon-induced Mx proteins in antiviral host defense. Biochimie 89, 812-818. [CrossRef]
- Haller, O., Staeheli, P., Schwemmle, M. and Kochs, G. (2015) Mx GTPases: dynamin- like antiviral machines of innate immunity. Trends Microbiol 23, 154-163. [CrossRef]
- Verhelst, J., Hulpiau, P. and Saelens, X. (2013) Mx proteins: antiviral gatekeepers that restrain the uninvited. Microbiol. Mol. Biol. Rev. 77, 551-566. [CrossRef]
- Staeheli, P., Pavlovic, J. (1991) Inhibition of vesicular stomatitis virus mRNA synthesis by human MxA protein. J Virol 65, 4498-4501. [CrossRef]
- Schwemmle, M.,Weining, K.C., Richter, M.F., Shumacher B., Staeheli, P. (1996) Vesicular stomatitis virus transcription inhibited by purified MxA protein. Virology 206, 545-554. [CrossRef]
- Steiner, F., Pavlovic, J. (2020) Subcellular localization of MxB determines its antiviral potential against influenza virus. J Virol 94, e00125-e220. [CrossRef]
- DiPaolo, C., Hefti, H. P., Meli, M., Landis, H., and Pavlovic, J. (1999) Intramolecular backfolding of the carboxyl-terminal end of MxA protein is a prerequisite for its oligomerization. J Biol Chem 274, 32071-32078. [CrossRef]
- Kochs, G., Janzen, C., Hohenberg, H., Haller, O. (2002) Antivirally active MxA protein sequesters La Crosse virus nucleocapsid protein into perinuclear complexes. Proc Natl Acad Sci USA 99, 3153-3158. [CrossRef]
- Accola, M. A., Huang, B., Al Masri, A. and McNiven, M. A. (2002). The antiviral dynamin family member, MxA, tubulates lipids and localizes to the smooth endoplasmic reticulum. J. Biol. Chem. 277, 21829-21835. [CrossRef]
- Stertz, S., Reichelt, M., Krijnse-Locker, J., Mackenzie, J., Simpson, J. C., Haller, O. and Kochs, G. (2006). Interferon-induced, antiviral human MxA protein localizes to a distinct subcompartment of the smooth endoplasmic reticulum. J. Interferon Cytokine Res. 26, 650-660. [CrossRef]
- Davis, D., Yuan, H., Liang, F.X., Yang, Y.M., Westley, J., Petzold, C., Dancel-Manning, K., Deng, Y., Sall, J. and Sehgal, P.B. (2019) Human antiviral protein MxA forms novel metastable membraneless cytoplasmic condensates exhibiting rapid reversible tonicity- driven phase transitions. J Virol 93, e01014-19. [CrossRef]
- Davis, D., Yuan, H., Liang, F.X., Sehgal, P.B. (2018) Interferon-α-induced cytoplasmic MxA structures in hepatoma Huh7 and primary endothelial cells. Contemp Oncology (Pozn) 22, 86-94. [CrossRef]
- Sehgal, P. B., Westley, J., Lerea, K. M. DiSenso-Browne, S. and Etlinger, J. D. (2020) Biomolecular condensates in cell biology and virology: phase-separated membraneless organelles (MLOs). Analytical Biochem 597, 113691. [CrossRef]
- Sehgal, P. B. and Lerea, K. L. (2020) Biomolecular condensates and membraneless organelles (MLOs). In Jez. Joseph (Eds) Encyclopedia of Biological Chemistry, 3rd Edn., vol. 1, pp 530-541. Oxford: Elsevier. [CrossRef]
- Goujon, C., Mancorge, O., Bauby, H., Doyle, T., Barclay, W.S., Malim, M.H. (2014) Transfer of the amino-terminal nuclear envelope targeting domain of human MX2 converts MX1 into an HIV-1 resistance factor. J Virol 88, 9017-9026. [CrossRef]
- Dicks, M.D.J., Betancor, G., Jimenez-Guardeno, J.M., Pessel-Vivares, L., Apolonia, I., Goujon, C., Malim, M.H. (2018) Multiple components of the nuclear pore complex onteract with the amino terminus of MX2 to facilitate HIV-1 restriction. PLoS Pathog 14, e1007408. [CrossRef]
- Moschonas, G. D., Delhaye, L., Cooreman, R., Bhat, A., Sutter, D. D., Parthoens, E., Desmer, A, Maciejczak, A, Grzek, H., Lippens, S., Debyser, Z., Eyckerman, S., Saelens, X. (2023) MX2 restricts HIV-1 and herpes simplex virus-1 by forming cytoplasmic biomolecular condensates that mimic nuclear pore complexes. bioArxiv preprint. [CrossRef]
- Banani, S.F., Lee, H.O., Hyman, A.A. and Rosen, M.K. (2017) Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol. 18, 285-298. [CrossRef]
- Shin, Y. and Brangwynne, C.P. (2017) Liquid phase condensation in cell physiology and disease. Science 357(6357) pii: eaaf4382. [CrossRef]
- Alberti, S. (2017) The wisdom of crowds: regulating cell function through condensed states of living matter. J Cell Sci. 130, 2789-2796. [CrossRef]
- Alberti, S., Gladfelter, A. and Mittag, T. (2019) Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 176, 419-434. [CrossRef]
- Shin, Y., Berry, J., Pannucci, N., Haataja, N. P. Toettcher, J. E. and Brangwynne, C. P. (2017) Spatiotemporal control of intracellular phase transitions using light-activated optoDroplets. Cell 168, 159-171. [CrossRef]
- Boija, A., Klein, I.A., Young, R.A. (2021) Biomolecular condensates and cancer. Cancer Cell 39, 174-192. [CrossRef]
- Banerjee, P.R., Holehouse, A.S., Kriwacki, R., Robustelli, P., Jiang, H., Sobolevsky, A.I., Hurley, J.M., Mendell, J.T. (2024) Dissecting the biophysics and biology of intrinsically disordered proteins. Trends Biochem Sci 49, 101-104. [CrossRef]
- Shimeki, H.K., Chandra, B., Kriwacki, R.W. (2023) Dissolving fusion oncoprotein condensates to reverse aberrant gene expression. Cancer Res 83, 3324-3326. [CrossRef]
- Heinrich, B.S., Maliga, Z., Stein, D.A., Hyman, A.A. and Whelan, S.P.J. (2018) Phase transitions drive the formation of vesicular stomatitis virus replication compartments. MBio 9, e02290-17. [CrossRef]
- Carlson, C. R., J.B. Asfaha, C.M. Ghent, C.J. Howard, N. Hartooni, D.O. Morgan (2020) Phosphoregulation of phase separation by the SARS-CoV-2 N protein suggests a biophysical basis for its dual functions. Mol Cell 80, 1092-1103.e4.
- Lindenmann, J., Lane, C.A., Hobson, D. (1963) The resistance of A2G mice to myxoviruses. J Immunol 90, 942-951. [CrossRef]
- Haller, O., Arnheiter, H., Gresser, I., Lindenmann, J. (1979) Genetically determined, interferon-dependent resistance to influenza virus in mice. J Exp Med 149, 601-612. [CrossRef]
- Bunasdiego, I., Kane, M., Rihn, S.J., Preugschas, H.F., Hugher, J., Blanco-Melo, D., Strouvelle, V.P., Zang, T.M., Willet, B.J., Boutell, C., Bienlasz, P.D., Wilson, S.J. (2014) Host and viral determinants of Mx2 antiretroviral activity. J Virol 88, 7738-7752. [CrossRef]
- Sehgal, P. B., Yuan, H., Scott, M. F., Deng, Y., Liang, F-X. and Mackiewicz, A. (2020) Murine GFP-Mx1 forms phase-separated nuclear condensates and associates with cytoplasmic intermediate filaments: novel antiviral activity against vesicular stomatitis virus. J Biol Chem 294, 15218-15234.
- Jin, H.K., Takada, A., Kon, Y., Haller, O., Watanabe, T. (1999) Identification of the murine Mx2 gene: interferon-induced expression of Mx2 protein from feral mouse gene confers resistance to vesicular stomatitis virus. J Virol 73, 4925-4930. [CrossRef]
- Dick, A., Graf, L., Olal, D., von der Malsburg, A., Gao, S., Kochs, G. and Daumke, O. (2015) Role of nucleotide binding and GTPase domain dimerization in dynamin-like myxovirus resistance protein A for GTPase activation and antiviral activity. J Biol Chem 290, 12779–12792. [CrossRef]
- von der Malsburg, A., Abutbul-Ionita, I., Haller, O., Kochs, G. and Danino, D. (2011) Stalk domain of the dynamin-like MxA GTPase protein mediates membrane binding and liposome tubulation via the unstructured L4 loop. J Biol Chem 286, 37858–37865. [CrossRef]
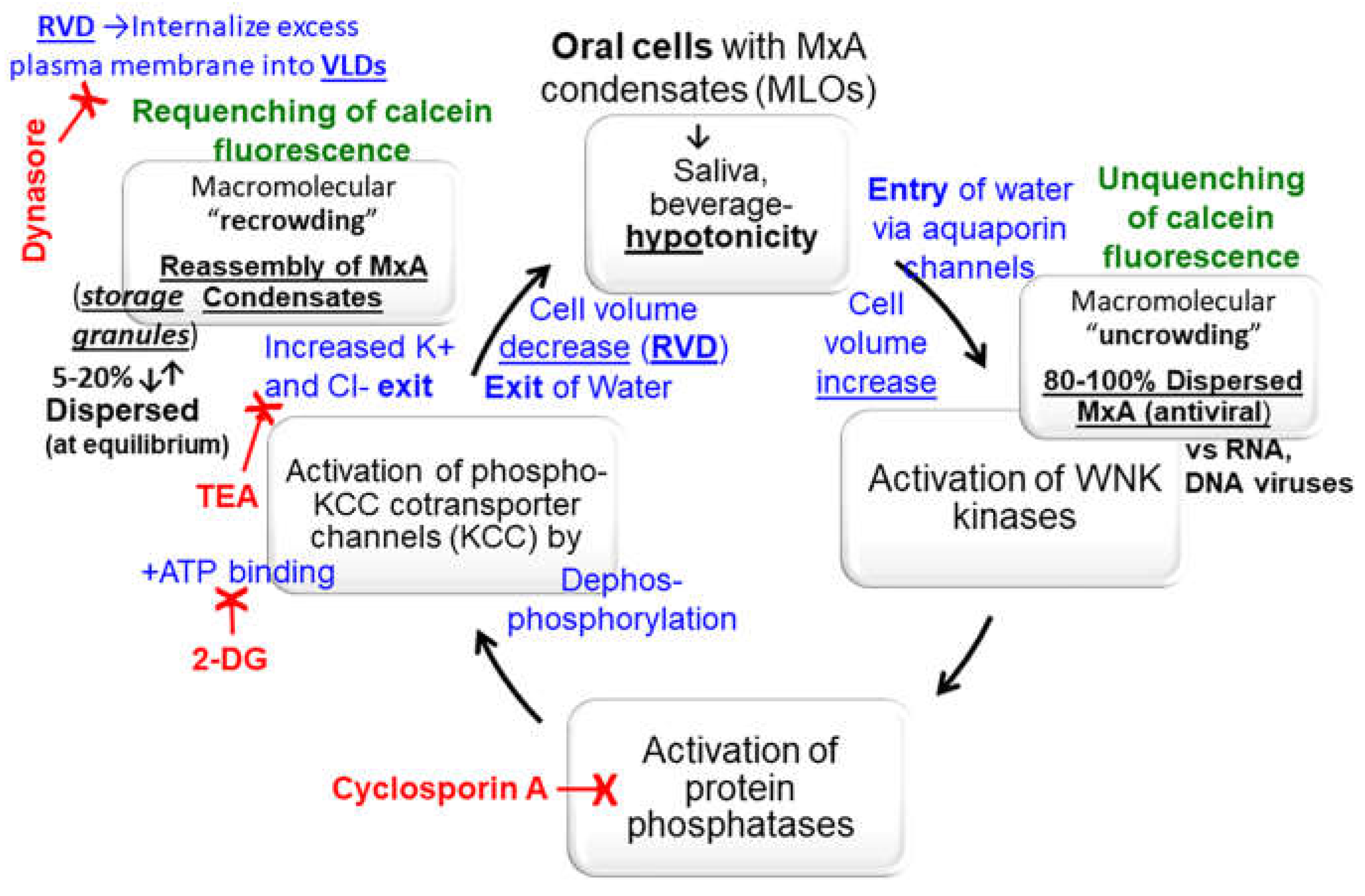
- Sehgal, P. B. (2021) Metastable biomolecular condensates of interferon-inducible antiviral Mx-family GTPases: a paradigm shift in the last three years. J Biosci 46, 72. [CrossRef]
- Sehgal, P.B., Yuan, H., Jin, Y. (2022) Rapid reversible osmoregulation of cytoplasmic biomolecular condensates of human interferon-α-induced antiviral MxA GTPase. Intl J Mol Sci 23, 12739. [CrossRef]
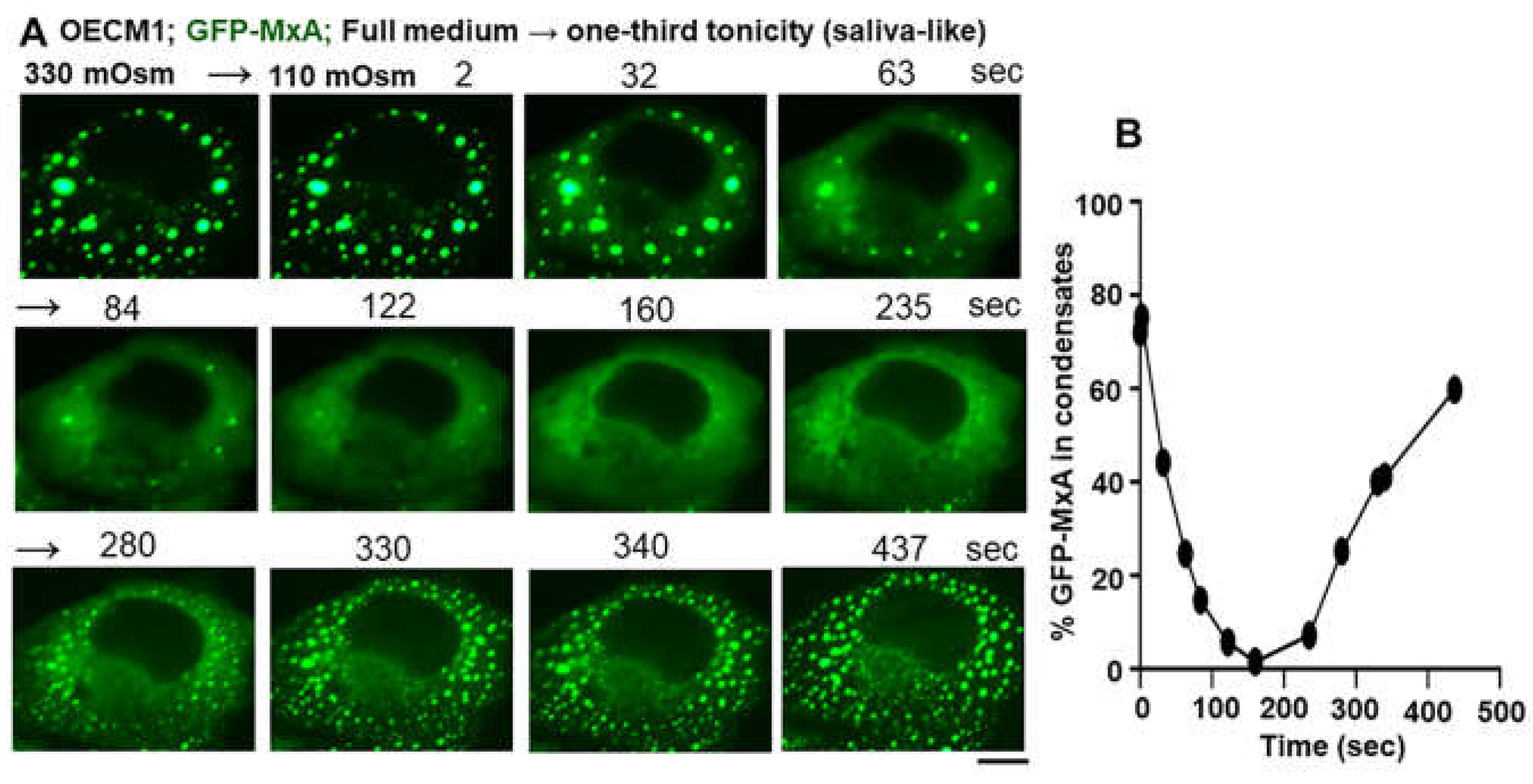
- Sehgal, P.B., Yuan, H., Centone, A., DiSenso-Browne, S. V. (2024) Oral antiviral defense: saliva- and beverage-like hypotonicity dynamically regulate formation of membraneless biomolecular condensates of antiviral human MxA in oral epithelial cells. Cells 13, 590. [CrossRef]
- Mahanonda, R., Sa-Ard-Iam, N., Rerkyen, P., Thitithanyanoni, A., Subbalekha, K., Pichyangkul, S. (2012) MxA expression induced by α-defensin in healthy human periodontal tissue. Eur J Immunol 42, 946-956. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).